Abstract
Our current food system relies on unsustainable practices, which often fail to provide healthy diets to a growing population. Therefore, there is an urgent demand for new sustainable nutrition sources and processes. Microorganisms have gained attention as a new food source solution, due to their low carbon footprint, low reliance on land, water and seasonal variations coupled with a favourable nutritional profile. Furthermore, with the emergence and use of new tools, specifically in synthetic biology, the uses of microorganisms have expanded showing great potential to fulfil many of our dietary needs. In this review, we look at the different applications of microorganisms in food, and examine the history, state-of-the-art and potential to disrupt current foods systems. We cover both the use of microbes to produce whole foods out of their biomass and as cell factories to make highly functional and nutritional ingredients. The technical, economical, and societal limitations are also discussed together with the current and future perspectives.
Subject terms: Applied microbiology, Industrial microbiology
In this Review article, the authors discuss the potential of microorganisms as a solution to the challenges faced by our food system. Engineered microorganisms can be used to produce enhanced foods and ingredients in a sustainable manner. The technical, economical, and societal limitations are also discussed together with the current and future perspectives.
Introduction
The current food systems have been pushed to a crisis, as they struggle to keep up with nutrition and protein demand coupled with population growth1. All our food systems—agriculture, animal husbandry and aquaculture—are grappling with the degradation of land, climate change and climate disasters, which are set to rise in the future2. Although moving towards plant-based foods is less environmentally harmful, it still relies on climate or season and intensive land, water and chemical use3. The time for a microbial revolution in food is ripe as microorganisms have the potential to enhance, improve or even replace the currently available alternatives4,5. They have been proven to be an ecological and resilient food source, especially when compared to traditional protein sources such as meat6,7. Genetic and system design can advance sustainability further when renewable and waste feedstocks are considered8,9. Furthermore, they are highly resilient due to their decentralised nature that does not rely on location limitations, such as temperature or weather10. Finally, they also have a high nutritional profile11, crucial in the face of rising diet-related health epidemics.
Microorganisms are no stranger in the history of food; however, research has lately revealed the vast array of health benefits and ecological savings that can be derived from using microorganisms in food12,13. This has led to an explosion in new applications, improvement in traditional practices using state-of-the-art technology14–16 and a better understanding of their roles and benefits13. Fermentation can be used both directly on foods to improve nutrition, taste or texture17,18, as well as used as a production platform to produce value-added ingredients in the food industry19–21. Moreover, using fermentation to produce microbial biomass as a nutritional food source is starting to be adopted in both animal feed and human foods22–24. However, there are challenges to overcome in each of these applications, including scalability and economic or ecological sustainability. Novel tools can be applied to these fields to enhance and accelerate the development of microbial-based foods and overcome current limitations. This includes high-resolution and high-throughput characterisation of microorganisms14,25, as well as genetic and metabolic engineering tools4. By engineering and selecting strains, it is possible to improve flavour26 and nutrition20,27,28 as well as increase sustainability using waste feed or cheap non-competing carbon sources8,29. This can contribute to increasing applications and uptake to propel a microbial revolution in food.
Due to the high potential and varied applications of microbes in food, there have been numerous recent start-ups in this space, ranging from improving traditional fermentation to creating new products (Table 1). Development is still needed for technical advances and consumer acceptance but the field of single-cell proteins and engineered microbes in food has high potential, as will be explored in this review. This review aims to give an overview of the different applications of microorganisms in food ranging from traditional fermentation techniques to biotech applications of ingredient production (see Fig. 1). It covers the different novel applications of microbes in the food system as well as the role of synthetic biology in advancing this field. Finally, the obstacles and future perspectives will be considered.
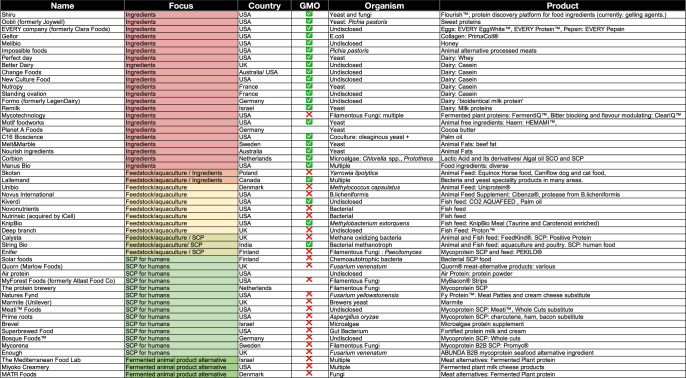
Table 1.
Start-ups and companies developing microbial food either for humans or animal feed as well as individual components or flavourings used
The GMO column has been left blank where unknown.
Different focuses are highlighted with different colour.
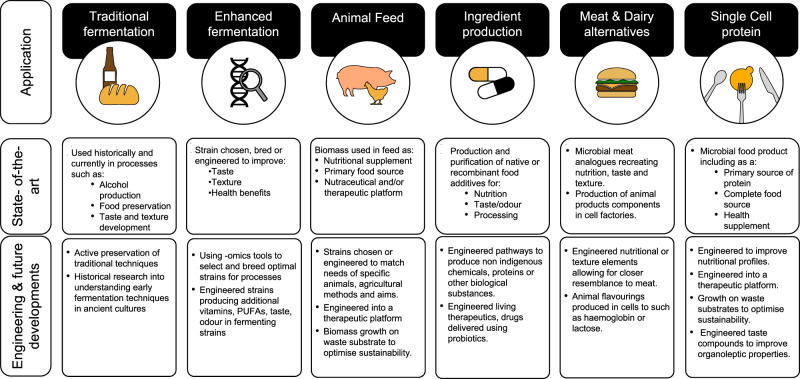
Fig. 1. Timeline of the role of microbes in food.
A view of the various applications that rely on microbial processes. State-of-the-art in each process is explained as well as the current or potential role of genetic engineering and other future developments to enhance the process or use.
The use of microbes in food
Rise of fermentation in history
Microorganisms were first leveraged by humans in the food system for fermentation. Fermentation is one of the earliest known food technologies dating as far back as 7000BC or earlier and arising independently in multiple ancient cultures30,31. Alongside smoking and salting, fermentation was a primary method of food preservation and thus a crucial technology in the rise of human civilisations32. In addition, the process also introduced many new products, flavours and tastes. Different fermented products rose from specific environments and conditions which produced a diversity of edible products32. These include, but are not limited to, dairy products such as cheese and yoghurt, alcoholic products such as beer and wine, fermented bean products such as soy sauce, douchi (豆豉) and natto, other vegetables such as sauerkraut and kimchi and many more32.
The advent of new processing and preservation methods such as refrigeration, the use of natural and artificial preservatives, and freezing and vacuum sealing, among others, have provided alternatives to traditional fermentation. However, more recently, research has brought to our attention the many health benefits offered by a microbial presence in food13,33, causing a resurgence in popularity, and many newly popularised health foods are fermented or have fermented ingredients. This is compounded by the rise of plant-based diets and increasing access to international foods—many of which include traditionally fermented products. A good example is Kombucha, a traditional Manchurian fermented tea drink which was introduced to the international market with many purported health benefits and now is valued at over 1 billion US dollars34. Other well-known examples are Tempeh and Tofu, two fermented soybean products from Indonesia and China, respectively, which are now consumed as meat-alternative protein sources globally35.
Different functions and health benefits of fermented foods
Fermentation, in the context of food, refers to raw material undergoing enzymatic conversions in the presence of microorganisms13,36. These conversions result in alteration in their physicochemical properties. Many of the resulting metabolites play an active role in food preservation, inhibiting the growth of contaminating or spoiling pathogens and increasing shelf life, but others contribute to nutrition, texture, taste and smell13. Depending on their composition, fermented food may also bring health benefits. The list is a brief summary of some of the most relevant benefits, although comprehensive reviews can be found on the topic18,37:
Microbiome enhancing (or probiotic) qualities: The gut microbiome is increasingly proving to be crucial for maintaining health38. The use of probiotics supplements has become widely adopted, although the health benefit and strain formulation remain controversial topics39. The consumption of certain fermented foods themselves has proven to have probiotic and health-promoting effects40.
Increasing bioavailability of nutrients in food: This is due to microorganisms breaking food down for easier digestion and absorption of ingested nutrients. For example, lactic acid fermentation can increase the food’s iron content by optimising pH and acid content for solubility41. Similarly, fermentation can improve the nutritional value of food by interfering with anti-nutritional factors, which impede protein, carbohydrate or phytochemical availability. For example, trypsin inhibitors found abundantly in various cereals, grains and legumes have been shown reduced activities in fermented foods42.
Reducing Glycaemic Index: The Glycaemic Index (GI) measures how quickly carbohydrates in food raise blood glucose levels43. Probiotic and/or fermented cereals, pseudo-cereals and dairy products have been linked to a reduction in the GI of the food and the blood sugar response43,44. Lowering GI intake and response has been shown to reduce risk factors for diseases such as type II diabetes and cardiovascular disease43.
Removing toxins: Microbial consortia can also act by removing toxic compounds and inhibiting the growth of pathogenic species. For example, Aflatoxin, a common toxin found in foods contaminated with Aspergillus flavus, has been shown to be enzymatically reduced in various fermentative processes45. Free radicals in vegetable and fruit products are also reduced during fermentation46.
Biochemical pathways producing health-promoting compounds: Many microorganisms naturally produce nutritionally beneficial chemical compounds including but not limited to antioxidants, polyunsaturated fatty acids, conjugated linoleic acids (CLA), sphingolipids, vitamins and minerals4,47,48.
However, fermentation does not always improve the foods and undesired microorganisms can negatively impact some nutritional aspects. Some examples include the production of toxic biogenic amines by lactic acid bacteria35, including an increase of free histamine due to the high presence of histidine-producing enzymes (l-histidine decarboxylase) in microorganisms49. To counteract this, strategies have been developed to either optimise strain selection50 or use engineered strains to enhance biogenic amine degradation51. Finally, it is also worth noting that many health claims related to fermented foods are yet to be fully verified by randomised controlled trial studies and have often been exaggerated for marketing purposes52.
The nutritional profile of microbes
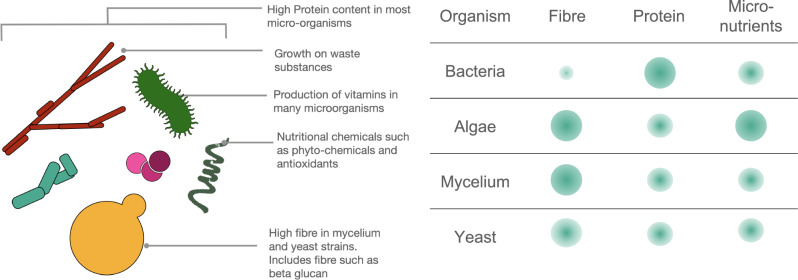
Microbial biomass itself also often has qualities that lend itself to consumption as food, including high protein, fibre and bioactive compound content (see Fig. 2).
Fig. 2. Nutritional profile of microbes.
The left panel shows the various components of microorganisms that are beneficiary for nutritional needs. This includes both macro-molecular elements such as proteins and fibre as well as small bioactive compounds. The right panel shows the relative levels of fibre, protein and micronutrients in four groups of microorganisms commonly used for food applications based on comparisons from the review by Ravindra11.
All microorganisms are generally characterised by high protein content, with algal species averaging between 40–60%, fungi 30–70% and bacteria averaging between 53 to as high as 80%11,12. Furthermore, many species are complete amino acid sources, containing adequate amounts of essential amino acids which humans cannot synthesise and need to acquire from diet53. In addition, many microbes have a high content of essential amino acids that are lacking in plants54.
Fibres, resistant carbohydrates that are key in maintaining gut health55, are also elevated in many microbial species11. Algae, for instance, has a high fibre content that is composed mainly of insoluble fibres, cellulose and other polysaccharides found in their cell walls56. Both filamentous fungi and yeast have potentially beneficial fibres, namely -glucan and mannan-oligosaccharides, both of which are consumed as health supplements for gut health and immune-boosting effects57,58.
Although lipid content is generally low compared to animal products, oleaginous yeasts and algae are a source of high-value dietary lipids, especially long-chain polyunsaturated fatty acids34,59. Interestingly, the overall calorie content can be quite low, such as in commercially available nutritional yeast flakes, which contain 400 calories per 100 g, bringing a high ratio of nutrition to energy. Finally, microorganisms often have high endogenous contents of nutritionally relevant compounds, including vitamins, minerals, antioxidants and other functional ingredients11.
The nutritional profile of microorganisms requires further investigation as their use becomes more widespread. The true digestibility of the elements discussed above has not been fully elucidated11 and the compositions can differ widely based on different species and the environments in which they are grown60. Species need to be carefully selected as some microorganisms also have significant safety and health detriments. An elevated RNA content is often seen in microorganisms which can lead to health issues, such as gout and kidney stones61. Some fungal and bacterial species also produce allergens and toxins and are thus ill-suited as food or require processing before ingestion11. By carefully choosing species, substrates, and conditions, the nutritional aspects of the food can be modulated to suit specific needs.
New technologies and applications for microbes in human food
Enhancing fermentation
Fermentation can be optimised by specially selecting, breeding or engineering strains of microbes to enhance the appearance, taste or health profile of fermented foods18,62,63. Traditionally, breeding and selection techniques were used to select for favourable qualities even before the biology of microbes was discovered, leading to vastly different strains for specific uses30. Using genetic profiling techniques and -omics technology, we are now able to further identify strains with favourable properties14,15. Large-scale analysis has also enabled the identification of strains with desired aromas, which were further improved by hybridisation techniques16.
More recently, fermentation has been enhanced by using genetic engineering, where strains used in traditional fermentation can be manipulated to produce additional beneficial products. Some examples of modifications include the enhanced production of B vitamins in Lactobacilli used in dairy products63,64 or the synthesis of aroma compounds in S. cerevisiae strains for novel and improved beer flavours65.
Genetic engineering has also been used to improve the sustainability of the fermentative food processes, which can be achieved by expanding or improving substrate range and utilisation22,66,67 This furthers the potential to use waste feedstocks8,9 and move towards a fully circular economy.
It is worth noting that many fermentation processes are carried out by microbial communities rather than single strains, which adds an additional layer of complexity to the understanding and limits our capacity to improve them. Advances in sequencing technologies and systems biology have allowed us to improve our knowledge of microbial consortia, including those found naturally in foods, as has been reviewed in previous works68,69. In addition, in the last years, synthetic biology tools specifically developed to engineer microbial communities have been created70, which have the potential to be used to improve food manufacturing. This includes spreading metabolic burden such as when two strategies to reduce browning in soy sauce production were engineered to act synergistically in two microbial species71, or enhancing natural coculture properties, such as increasing quorum sensing mechanisms which reduce food spoilage72.
Use of microbes as a protein source in human food
The use of microbes as a food ingredient is known as single-cell protein (SCP) and usually refers either to dried or processed microbe biomass or to the proteins extracted from it. It can be ingested either as a supplement, ingredient or as a main food source (see Fig. 1). Thanks to its potential for sustainable fermentation8,28 and its favourable nutritional profile11, it has the potential to become a large component of our diet.
SCP has a long and varied history, beginning before the World Wars and continuing into the late and mid 20th century73,74. However, most projects were discontinued in the face of rising energy costs and the success of the green revolution, although some legacies remain75. One of the first of these is Marmite, established in 1902 as a by-product of the beer industry has even been consumed as an army ration as a source of B vitamins61. Since then, there has been development in other, more texturised SCPs—notably that of Quorn. Quorn, established in the 1980s, produces SCP from the filamentous fungi Fusarium venenatum and then treated to remove excess nucleic acid content and finally texturized to create meat replacements76. It is now a widely distributed product sold in 17 countries with a reported revenue of 236 million GBP in 2020. SCPs are also consumed as a health supplement, such as the microalgae Chlorella and Spirulina, which are rich sources of proteins as well as phytonutrients and vitamins77.
Given the ecological and nutritional benefits of SCPs, there is a renewed demand which has resulted in research into new sources of SCPs as well as novel cultivation methods. There is a profusion of start-up companies trying to bring new SCP products to market with some examples listed in Table 1, with many start-ups focussing on meat alternatives.
So far, most research has focused on wild-type (non-engineered) strains, which have been selected based on their protein content and whose production have been optimised manipulating growing conditions. Synthetic biology has the potential to engineer selected strains to further improve protein production, which can be achieved by (1) enhancing and expanding the capacity to efficiently use desirable feedstocks, (2) improving yields for biomass and protein production and (3) adding functionalities to the single-cell protein by the co-production of valuable compounds such as vitamins or antioxidants78. Improving growth and substrate use can greatly improve ecological and economical aspects, for example, by transforming waste into proteins79.
Animal meat alternatives
Microbes are a promising substitute for meat products. This is thanks to their matching protein and nutrient levels, as well as their potential to be modified and texturized to resemble meat.
One of the most established companies is Quorn, which produces SCP derived from filamentous fungi. Quorn has products that resemble meat products from chicken nuggets to beef mince and has a large selection of different textures and forms it comes in refs. 80, 81. To achieve this, the long strands of hyphae are mixed with binding agents and then this fibre–gel complex is freeze texturised which allows for hyphal laminations that recreate the fibrous texture of meat80. Other start-ups including Meati Foods, Mycorena and Nature’s Fynd are also producing meat analogues from filamentous fungi.
Besides mimicking the nutritional profile or protein content, meat flavourings can also be produced by microbes. These products can be extracted and purified, or the whole microbial biomass can be used. For example, in the Impossible burger, Pichia pastoris is engineered to produce soybean leghaemoglobin c226, which recreates part of the flavour profile of meat. The engineered microorganism is then incorporated with other ingredients including soy and potato proteins. Haemoglobin is also being produced as a stand-alone ingredient to add to plant-based meats, such as in the start-up Motif Foodworks. In academia, there is a concentrated effort to produce many variations of haemoglobin proteins which could account for future taste expansions82. Other individual components of meat can also be produced, such as the structural elements gelatine and collagen83,84.
Finally, one main challenge of recreating meat is providing an adequate lipid composition and content. Most plant-based alternatives utilise plant oils, which have a strongly differing taste and mouthfeel. The endogenous contents of lipids in microbes also differ significantly from that of meat; however, there is vast academic research on producing dietary lipids in microbes. Oleaginous species have been found to be a suitable production platform for highly nutritious fatty acids, such as omega-3 fatty acids which are found abundantly in fish27. Furthermore, advancement in the production of microbial oils gives us the potential to not only tune lipid composition but to also modify fatty acids to become more suitable for animal replacement uses85. Little focus has been given to mimick animal fats in academic research, although start-ups such as Melt & Marble and Nourish Ingredients aim to make dietary fats for animal replacements through fermentation.
Other animal product alternatives
Engineering microbes also have the potential to recreate animal products such as dairy and eggs. This is done through precision fermentation, where the pathways of individual components have been engineered into microorganisms.
Milk is composed of oligosaccharides, fats, sugars and proteins, primarily that of casein and whey4. These various components are being reproduced using synthetic biology in microorganisms4. The main milk proteins, namely casein proteins and whey proteins, have been successfully engineered into various organisms, including bacteria and yeasts4. These technologies are being employed by various start-ups developing animal-free milk, such as Perfect day, Better Dairy and Formo, which use purified milk proteins extracted from microbial cell factories and mixed with other fats and sugars.
Human breast milk has also been researched as it is thought to have important effects on the development of the neonatal gut flora and immune system86. Components such as milk fats and milk oligosaccharides have been developed with precision fermentation for human breast milk, both in academia as well as in industry, such as by the SME Conagen. Human milk oligosaccharides (HMOs) have been produced in both S. cerevisiae and B. subtilis87 and human milk fats in the oleaginous yeast Y. lipolytica88. The probiotic effects can also be mimicked by recreating the microbiome of breast milk through the addition of microbial populations to formula89. The actual effects of these supplements would benefit from further studies in humans.
Eggs have a larger and more complex group of proteins that are responsible for their unique texture and taste. However, there have been efforts to recombinantly express different proteins, initially for allergenicity and protein studies90,91, and more recently as food ingredients92,93. Furthermore, there have also commercial efforts to produce egg alternative products made up of multiple egg proteins. This includes the start-up EVERY, which launched an egg white product made from recombinantly produced proteins in 2021.
One animal-based ingredient that has already been largely replaced by precision fermentation is rennet, an enzyme mixture containing chymosin found in the lining of the stomach of young ruminants. Commercial chymosin is now mostly produced in Aspergillus niger, which has allowed many kinds of cheese to become suitable for vegetarians as well as reducing the price, benefiting cheese makers94.
Microorganisms in animal feed
The use of microbes in animal feed first appeared over a century ago when brewery by-products were used to supplement feed by Max Delbruck. More recently, using microorganisms as a main or supplemental nutritional source has become established as an industry norm in both animal agriculture95 and aquaculture23. This is due to an increase in regulatory ease and technological capabilities as well as growing pressures for cost and ecological efficiency96.
Many different microbial species have been investigated for the benefits in both animal health and production output23,24. Different microbial species each have their own limitations and advantages and thus need to be matched to desired functions and livestock23,24. Furthermore, there are different delivery options- including as a sole nutritional source23, as nutritional additive24 or can act as probiotics97,98.
Live microbial supplements can act as probiotics and can either be species delivered to colonise the gut and integrate to improve the existing microflora, or to help balance the existing microbiota by modulating the pH, feed existing microorganisms and to defend against pathogenic species. Using probiotics in animal feed is becoming an industry norm as it has large therapeutic gains while reducing the need for drugs and antibiotics. In addition, the use of probiotics is shown to improve feed uptake, immune response and stress tolerance97–99. It has also been linked to increased growth, biomass and milk production97.
The new generation of SCP-based animal feed uses engineered microorganisms nutritionally tailored to the target animal28,78,100. Moreover, it can be also employed as a nutraceutical and therapeutic platform such as in the previously commercial omega-3 enriched Yarrowia biomass employed in Verlasso® salmon101, and the efforts in the start-ups such as Cyanofeed (see Table 1). Vitamins, fatty acids and phytonutrients have been successfully delivered through feed28. Finally, engineering organisms to utilise waste substances as carbon sources can greatly lower the ecological footprint of highly polluting animal agriculture industries28,29.
Precision fermentation of food ingredients and additives
One of the most developed uses of engineered microbes in our current food ecosystem is the production of ingredients and additives. For decades, microorganisms have been selected and improved to maximise the synthesis of molecules of interest, first by random mutagenesis and selection and then by genetic and metabolic engineering in a practice called precision fermentation16,21. A paradigmatic example is the production of vitamin B2, where chemical synthesis was substituted by fermentation in the 90s102. The yields and productivities of the processes are key to determining economic feasibility and therefore, metabolic engineering is playing an important role not only in increasing yields but also enabling the production of heterologous chemicals22. Interestingly, the use of genetically engineered strains to produce specific compounds is generally well accepted by consumers. This is because, by the end of the fermentation process, the molecules of interest are extracted and purified. They are therefore typically free of recombinant cells or DNA, allowing them to be labelled as natural products103.
While most nutraceuticals and additives with health benefits are still made by chemical synthesis or plant extraction, an increasing number of them are now bio-manufactured by microorganisms4. Some of these nutraceuticals include water-soluble vitamins (vitamin B complex and vitamin C) as well as fat-soluble vitamins (vitamin A/D/E and vitamin K)20. Other nutraceuticals made by engineered microbes have been reviewed elsewhere21, and the list includes omega-3 fatty acids, polyphenols such as resveratrol and naringenin, carotenoids such as beta-carotene or Astaxanthin, and non-proteinogenic amino acids such as GABA and beta-alanine. Other ingredients made by microbes are intended to improve the organoleptic properties of the food to which they are added to, improving taste, odour, colour and feel. Flavour enhancers such as glutamate (MSG), inosine monophosphate (IMP) and guanosine monophosphate (GMP) are made by microbes and contribute to the desired umami flavour104. Microbes have also been engineered to produce sweeteners such as stevia-derived molecules, xylitol or erythritol105–107. More exotic, hoppy flavours have been engineered into yeast to make tastier beer65. Odours and aroma compounds have been made by microbial processes like those of rose (2PE)108, orange/lemon (limonene)109, mint (menthol)110, peach (gamma-decalactone)111, among many others.
In addition, coloured molecules have been synthesised by microbes with the intention to be used as pigments for food and beverages. Some examples include orange (beta-carotene, canthaxanthin), red (lycopene, astaxanthin, prodigiosin), yellow (riboflavin), blue (phycocyanin), purple (violacein) and black (melanin) colourants19.
Obstacles and future perspectives
Technical obstacles
To have a fully incorporated use of microbes in food, there are some technical difficulties that must be overcome. First, one of the main nutritional drawbacks is the high content of nucleic acids—namely RNA content. Ingestion of excessive quantities of nucleic acids particularly purines, increases the quantity of uric acid in the body which is a risk factor for gout and renal calculi as well as a strong risk factor for Metabolic Syndrome and cardiovascular disease112. This can be partially mitigated through processing methods, including heating and purification as employed by current single-cell protein manufacturers113,114. In the future, it would be possible to envisage an inducible method engineered into microbes to self-purify excess nucleic acids.
As a sole food source, the odours and textures of pure microbial cell mass have been postulated to be unsuited to human palate, however this setback could be improved through breeding or engineering in taste with genetic modifications or by creating mixtures or co-cultures to have novel and pleasant tastes16,115.
Many microorganisms, especially yeast, fungal and algal clades also have thick cell walls. In many cases, this is an important contributor of fibre in the diet. However, for some SCP, the thick cell wall can limit the number of nutrients that can be taken up and can itself be indigestible. Therefore, it may be necessary to treat the SCP using heat and/or mechanical and enzymatic processes, improving nutrient bioavailability114.
Food safety
Microbial-based foods and ingredients must go through regulatory approvals, which are stricter when new or engineered species are used. Regulatory bodies assess safety and authorize foods in a country-specific manner. For example, the FDA and EFSA are the main regulatory bodies in the USA and Europe, respectively. Some strategies to facilitate the obtention of approvals for microbial foods include the use of approved organisms and processes, limiting the application to animal feeding, purification of products, and removing foreign DNA and living cells.
The safety of the foods must also be considered for each different species. There has already been extensive investigation into some of the main target species that have confirmed their food safety both for fermentation, ingredient production and SCP use. Special attention must be paid to possible contamination in the process and to the potential production of endo and exotoxins that cause allergic and adverse reactions when ingested. Some toxins may be removed by simple heat or chemical treatments. However, through stringent strain selection116, strain engineering117 and correct fermentation technologies, contamination and toxin production can be prevented or eliminated.
Consumer acceptance
One of the largest challenges of deploying single-cell proteins and genetically engineered microorganisms in food is consumer acceptance. Genetic modification is still under strict regulations, which differ between countries with some being particularly strict on introducing food with modified genetic information. Moreover, a large percentage of people still do not accept the idea of eating genetically modified materials. With the increasing awareness of improving the ecological aspect of diets118, this attitude might be changing as seen with the popularity with lab-grown meats and some synthetic meat and milk alternatives; however, these products are still uncommon in a commercial setting and therefore not incorporated in the average household’s diet.
To promote consumption, it is thus crucial to take the preparation and cultural context of microbial foods into account. Education and marketing can help counteract unfamiliarity and lack of consumption experience119. In addition, the design of microbial foods should consider the need to fulfil religious or cultural values, such as kosher or halal requirements120.
Economic barriers
A large problem of deploying SCP is the capital expenditure needed to expand the technologies and market the new food source. Maintenance costs and substrate usage also limit profitability. Because of the costs incurred for prototype development, one of the initial SCP projects by Imperial Chemical Industries (ICI) was abandoned when it failed to compete with cheap agriculture, especially with modified soybeans10. However, more recent technologies seem to suggest that building a plant for growing microbes could now be economically feasible121, which is facilitated by the optimisation of the growing conditions122, advanced fermentation technologies123, and higher yields achieved by engineered microbes100. Another economic barrier for commercialisation is the lengthy and expensive process associated with obtaining the necessary regulatory and safety approvals. Although dependent on price, variety and transportation, the employment of waste streams also has the potential to lower the process cost and simultaneously increase sustainability10. However, this is harder to introduce to the market as it is not fully understood whether the nutritional qualities of the product would be affected.
Conclusion
Taken together all the information discussed above, there is an obvious interest in developing more microbial-based foods and ingredients, as seen by the increased number of related academic publications, conferences, companies and commercial products. This is in part encouraged by the consumer demand for healthier and more sustainable foods.
Synthetic biology and microbial strain engineering broaden the horizons of microbial foods that can be designed, enabling the creation of desired nutritional profiles, aroma compounds, flavours and textures, all of which can build towards personalised nutrition (Fig. 3). To translate this technological capability into sustainable commercial products, the public perception of microbial foods must continue to change and the legislation must facilitate the implementation of these novel processes while maintaining high safety standards. The expansion and normalisation of microbial foods will increase production volumes, decreasing costs and optimising the efficiency of the technology. Reduced costs can then aid the development of microbial processes in less developed areas of the planet, which often need to improve nutrition. Looking at the future, engineered microbes are expected to play a role in delivering food where traditionally inaccessible, such as in disaster relief, deserts or even in space124,125.
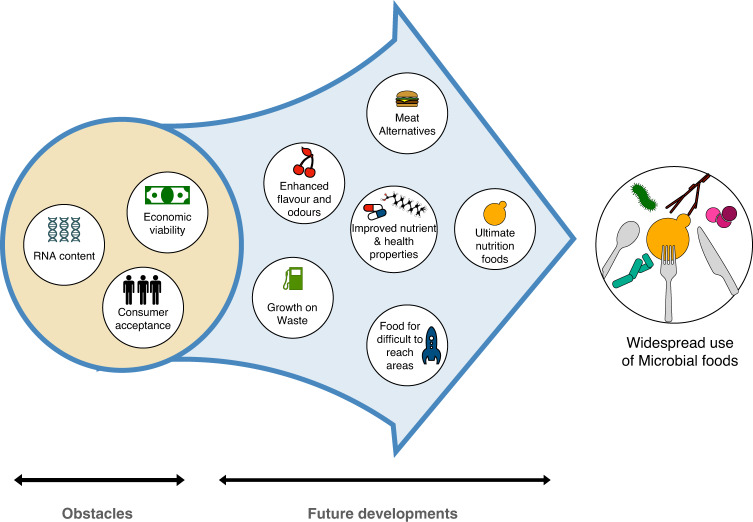
Fig. 3. The future of microorganisms in food.
A schematic showing the obstacles and future developments in the path to adopting widespread use of Microbial foods. In the beige circle the main obstacles are shown, including the economic viability of some processes, the consumer acceptance of some products, especially GMOs and, in some cases, the presence of undesired molecules. Future developments, shown in the blue arrow, aim to improve microbial-based foods and overcome these obstacles, and include producing nutritionally complete whole foods, alternatives to animal products (meat, dairy, eggs), and ingredients (like flavours or nutraceuticals) that can be made in an affordable and sustainable way, perhaps using waste or CO2 as carbon sources.
In conclusion, if there is continued innovation and microbial foods are designed with sustainability and ethics in mind, they have the potential to revolutionise current food systems. This microbial food revolution could be key in designing future-proof strategies to face the health and environmental challenges of the future.
Acknowledgements
R.L.-A. received funding from BBSRC (BB/R01602X/1, BB/T013176/1, BB/T011408/1–19-ERACoBioTech- 33 SyCoLim), British Council 527429894, Newton Advanced Fellowship (NAF\R1\201187), Yeast4Bio Cost Action 18229, European Research Council (ERC) (DEUSBIO–949080) and the Bio-based Industries Joint (PERFECOAT- 101022370) under the European Union’s Horizon 2020 research and innovation programme.
Author contributions
The article was ideated by R.L.A. and A.E.G. A.E.G. wrote the first draft with inputs from R.L.A. R.L.A. supervised, edited and polished the article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rockström J, Edenhofer O, Gaertner J, DeClerck F. Planet-proofing the global food system. Nat. Food. 2020;1:3–5. doi: 10.1038/s43016-019-0010-4. [DOI] [Google Scholar]
- 2.Change, I. C. Land: An IPCC Special Report on Climate Change. Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems 1–864 (IPCC, 2019).
- 3.Sun Z, et al. Dietary change in high-income nations alone can lead to substantial double climate dividend. Nat. Food. 2022;3:29–37. doi: 10.1038/s43016-021-00431-5. [DOI] [PubMed] [Google Scholar]
- 4.Lv X, et al. Synthetic biology for future food: research progress and future directions. Future. Foods. 2021;3:100025. [Google Scholar]
- 5.Choi KR, Yu HE, Lee SY. Microbial food: microorganisms repurposed for our food. Micro. Biotechnol. 2022;15:18–25. doi: 10.1111/1751-7915.13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humpenöder F, et al. Projected environmental benefits of replacing beef with microbial protein. Nature. 2022;605:90–96. doi: 10.1038/s41586-022-04629-w. [DOI] [PubMed] [Google Scholar]
- 7.Järviö N, Maljanen NL, Kobayashi Y, Ryynänen T, Tuomisto HL. An attributional life cycle assessment of microbial protein production: a case study on using hydrogen-oxidizing bacteria. Sci. Total Environ. 2021;776:145764. doi: 10.1016/j.scitotenv.2021.145764. [DOI] [PubMed] [Google Scholar]
- 8.Javourez U, O’Donohue M, Hamelin L. Waste-to-nutrition: a review of current and emerging conversion pathways. Biotechnol. Adv. 2021;53:107857. doi: 10.1016/j.biotechadv.2021.107857. [DOI] [PubMed] [Google Scholar]
- 9.Gao R, et al. Enhanced lipid production by Yarrowia lipolytica cultured with synthetic and waste-derived high-content volatile fatty acids under alkaline conditions. Biotechnol. Biofuels. 2020;13:1–16. doi: 10.1186/s13068-019-1645-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linder T. Making the case for edible microorganisms as an integral part of a more sustainable and resilient food production system. Food Secur. 2019;11:265–278. doi: 10.1007/s12571-019-00912-3. [DOI] [Google Scholar]
- 11.Ravindra P. Value-added food:: single cell protein. Biotechnol. Adv. 2000;18:459–479. doi: 10.1016/S0734-9750(00)00045-8. [DOI] [PubMed] [Google Scholar]
- 12.Sharif M, et al. Single cell protein: Sources, mechanism of production, nutritional value and its uses in aquaculture nutrition. Aquaculture. 2021;531:735885. doi: 10.1016/j.aquaculture.2020.735885. [DOI] [Google Scholar]
- 13.Marco ML, et al. Health benefits of fermented foods: microbiota and beyond. Curr. Opin. Biotechnol. 2017;44:94–102. doi: 10.1016/j.copbio.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Pan M, Barrangou R. Combining omics technologies with CRISPR-based genome editing to study food microbes. Curr. Opin. Biotechnol. 2020;61:198–208. doi: 10.1016/j.copbio.2019.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Palla M, Cristani C, Giovannetti M, Agnolucci M. Identification and characterization of lactic acid bacteria and yeasts of PDO Tuscan bread sourdough by culture dependent and independent methods. Int. J. Food Microbiol. 2017;250:19–26. doi: 10.1016/j.ijfoodmicro.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Steensels J, Meersman E, Snoek T, Saels V, Verstrepen KJ. Large-scale selection and breeding to generate industrial yeasts with superior aroma production. Appl Environ. Microbiol. 2014;80:6965–6975. doi: 10.1128/AEM.02235-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marullo, P. & Dubourdieu, D. Yeast selection for wine flavor modulation. in Managing Wine Quality (ed. Reynolds, A. G.) 371–426 (Elsevier, 2022). https://www.sciencedirect.com/book/9780081020654/managing-wine-quality.
- 18.Tamang JP, Shin D-HH, Jung S-JJ, Chae S-WW. Functional properties of microorganisms in fermented foods. Front. Microbiol. 2016;7:578. doi: 10.3389/fmicb.2016.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sen T, Barrow CJ, Deshmukh SK. Microbial pigments in the food industry—challenges and the way forward. Front. Nutr. 2019;6:7. doi: 10.3389/fnut.2019.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Liu L, Jin Z, Zhang D. Microbial cell factories for green production of vitamins. Front. Bioeng. Biotechnol. 2021;9:473. doi: 10.3389/fbioe.2021.661562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan S-FF, Alper HS. Metabolic engineering of microbial cell factories for production of nutraceuticals. Micro. Cell Fact. 2019;18:1–11. doi: 10.1186/s12934-019-1096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banks M, Johnson R, Giver L, Bryant G, Guo M. Industrial production of microbial protein products. Curr. Opin. Biotechnol. 2022;75:102707. doi: 10.1016/j.copbio.2022.102707. [DOI] [PubMed] [Google Scholar]
- 23.Jones SW, Karpol A, Friedman S, Maru BT, Tracy BP. Recent advances in single cell protein use as a feed ingredient in aquaculture. Curr. Opin. Biotechnol. 2020;61:189–197. doi: 10.1016/j.copbio.2019.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Shurson GC. Yeast and yeast derivatives in feed additives and ingredients: Sources, characteristics, animal responses, and quantification methods. Anim. Feed Sci. Technol. 2018;235:60–76. doi: 10.1016/j.anifeedsci.2017.11.010. [DOI] [Google Scholar]
- 25.Helmy, M., Elhalis, H., Yan, L., Chow, Y. & Selvarajoo, K. Perspective: multi-omics and machine learning help unleash the alternative food potential of microalgae. Adv. Nutr.14, 1–11 (2022). [DOI] [PMC free article] [PubMed]
- 26.Reyes TF, Chen Y, Fraser RZ, Chan T, Li X. Assessment of the potential allergenicity and toxicity of Pichia proteins in a novel leghemoglobin preparation. Regul. Toxicol. Pharmacol. 2021;119:104817. doi: 10.1016/j.yrtph.2020.104817. [DOI] [PubMed] [Google Scholar]
- 27.Xue Z, et al. Production of omega-3 eicosapentaenoic acid by metabolic engineering of Yarrowia lipolytica. Nat. Biotechnol. 2013;31:734–740. doi: 10.1038/nbt.2622. [DOI] [PubMed] [Google Scholar]
- 28.Yan J, et al. Engineering Yarrowia lipolytica to simultaneously produce lipase and single cell protein from agro-industrial wastes for feed. Sci. Rep. 2018;8:1–10. doi: 10.1038/s41598-018-19238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gleizer S, et al. Conversion of Escherichia coli to generate all biomass carbon from CO2. Cell. 2019;179:1255–1263.e12. doi: 10.1016/j.cell.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Legras J-L, Merdinoglu D, Cornuet J-M, Karst F. Bread, beer and wine: Saccharomyces cerevisiae diversity reflects human history. Mol. Ecol. 2007;16:2091–2102. doi: 10.1111/j.1365-294X.2007.03266.x. [DOI] [PubMed] [Google Scholar]
- 31.Dudley R. Ethanol, fruit ripening, and the historical origins of human alcoholism in primate frugivory. Integr. Comp. Biol. 2004;44:315–323. doi: 10.1093/icb/44.4.315. [DOI] [PubMed] [Google Scholar]
- 32.Tamang JP, et al. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf. 2020;19:184–217. doi: 10.1111/1541-4337.12520. [DOI] [PubMed] [Google Scholar]
- 33.Taylor BC, et al. Consumption of fermented foods is associated with systematic differences in the gut microbiome and metabolome. mSystems. 2020;5:e00901–e00919. doi: 10.1128/mSystems.00901-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J, Adhikari K. Current trends in kombucha: marketing perspectives and the need for improved sensory research. Beverages. 2020;6:15. doi: 10.3390/beverages6010015. [DOI] [Google Scholar]
- 35.He J, Evans NM, Liu H, Shao S. A review of research on plant-based meat alternatives: driving forces, history, manufacturing, and consumer attitudes. Compr. Rev. Food Sci. Food Saf. 2020;19:2639–2656. doi: 10.1111/1541-4337.12610. [DOI] [PubMed] [Google Scholar]
- 36.Singh S, Yap WS, Ge XY, Min VLX, Choudhury D. Cultured meat production fuelled by fermentation. Trends Food Sci. Technol. 2022;120:48–58. doi: 10.1016/j.tifs.2021.12.028. [DOI] [Google Scholar]
- 37.Şanlier N, Gökcen BB, Sezgin AC. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2017;59:506–527. doi: 10.1080/10408398.2017.1383355. [DOI] [PubMed] [Google Scholar]
- 38.Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019;25:716–729. doi: 10.1038/s41591-019-0439-x. [DOI] [PubMed] [Google Scholar]
- 40.Wastyk HC, et al. Gut-microbiota-targeted diets modulate human immune status. Cell. 2021;184:4137–4153. doi: 10.1016/j.cell.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergqvist SW, Sandberg A-S, Carlsson N-G, Andlid T. Improved iron solubility in carrot juice fermented by homo-and hetero-fermentative lactic acid bacteria. Food Microbiol. 2005;22:53–61. doi: 10.1016/j.fm.2004.04.006. [DOI] [Google Scholar]
- 42.Osman MA. Changes in sorghum enzyme inhibitors, phytic acid, tannins and in vitro protein digestibility occurring during Khamir (local bread) fermentation. Food Chem. 2004;88:129–134. doi: 10.1016/j.foodchem.2003.12.038. [DOI] [Google Scholar]
- 43.Andreasen AS, et al. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br. J. Nutr. 2010;104:1831–1838. doi: 10.1017/S0007114510002874. [DOI] [PubMed] [Google Scholar]
- 44.de Angelis M, et al. Use of sourdough lactobacilli and oat fibre to decrease the glycaemic index of white wheat bread. Br. J. Nutr. 2007;98:1196–1205. doi: 10.1017/S0007114507772689. [DOI] [PubMed] [Google Scholar]
- 45.Shetty PH, Jespersen L. Saccharomyces cerevisiae and lactic acid bacteria as potential mycotoxin decontaminating agents. Trends Food Sci. Technol. 2006;17:48–55. doi: 10.1016/j.tifs.2005.10.004. [DOI] [Google Scholar]
- 46.Nkhata SG, Ayua E, Kamau EH, Shingiro J-B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018;6:2446–2458. doi: 10.1002/fsn3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fang H, et al. Metabolic engineering of Escherichia coli for de novo biosynthesis of vitamin B12. Nat. Commun. 2018;9:1–12. doi: 10.1038/s41467-018-07412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen B, et al. Fermentative production of vitamin E tocotrienols in Saccharomyces cerevisiae under cold-shock-triggered temperature control. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-020-18958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maintz L, Novak N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007;85:1185–1196. doi: 10.1093/ajcn/85.5.1185. [DOI] [PubMed] [Google Scholar]
- 50.Barbieri F, Montanari C, Gardini F, Tabanelli G. Biogenic amine production by lactic acid bacteria: a review. Foods. 2019;8:17. doi: 10.3390/foods8010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li B, Lu S. The importance of amine-degrading enzymes on the biogenic amine degradation in fermented foods: a review. Process Biochem. 2020;99:331–339. doi: 10.1016/j.procbio.2020.09.012. [DOI] [Google Scholar]
- 52.Marco ML, et al. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021;18:196–208. doi: 10.1038/s41575-020-00390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jach ME, Serefko A, Ziaja M, Kieliszek M. Yeast protein as an easily accessible food source. Metabolites. 2022;12:63. doi: 10.3390/metabo12010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamada EA, Sgarbieri VC. Yeast (Saccharomyces cerevisiae) protein concentrate: preparation, chemical composition, and nutritional and functional properties. J. Agric Food Chem. 2005;53:3931–3936. doi: 10.1021/jf0400821. [DOI] [PubMed] [Google Scholar]
- 55.Wastyk HC, et al. Gut-microbiota-targeted diets modulate human immune status. Cell. 2021;184:4137–4153. doi: 10.1016/j.cell.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moreira, J. B. et al. Microalgae polysaccharides: an alternative source for food production and sustainable agriculture. Polysaccharides3, 441–457 (2022).
- 57.McFarlin BK, Carpenter KC, Davidson T, McFarlin MA. Baker’s yeast beta glucan supplementation increases salivary iga and decreases cold/flu symptomatic days after intense exercise. J. Diet. 2013;10:171–183. doi: 10.3109/19390211.2013.820248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cuskin F, et al. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature. 2015;517:165–169. doi: 10.1038/nature13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ratledge C. Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie. 2004;86:807–815. doi: 10.1016/j.biochi.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 60.Jach ME, Serefko A, Ziaja M, Kieliszek M. Yeast protein as an easily accessible food source. Metabolites. 2022;12:63. doi: 10.3390/metabo12010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ritala A, Häkkinen ST, Toivari M, Wiebe MG. Single cell protein—state-of-the-art, industrial landscape and patents 2001–2016. Front. Microbiol. 2017;8:2009. doi: 10.3389/fmicb.2017.02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arendt EK, Moroni A, Zannini E. Medical nutrition therapy: use of sourdough lactic acid bacteria as a cell factory for delivering functional biomolecules and food ingredients in gluten free bread. Micro. Cell Fact. 2011;10:1–9. doi: 10.1186/1475-2859-10-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waters DM, Mauch A, Coffey A, Arendt EK, Zannini E. Lactic acid bacteria as a cell factory for the delivery of functional biomolecules and ingredients in cereal-based beverages: a review. Crit. Rev. Food Sci. Nutr. 2015;55:503–520. doi: 10.1080/10408398.2012.660251. [DOI] [PubMed] [Google Scholar]
- 64.Leroy F, de Vuyst L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004;15:67–78. doi: 10.1016/j.tifs.2003.09.004. [DOI] [Google Scholar]
- 65.Denby CM, et al. Industrial brewing yeast engineered for the production of primary flavor determinants in hopped beer. Nat. Commun. 2018;9:1–10. doi: 10.1038/s41467-018-03293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ledesma-Amaro R, Nicaud JM. Metabolic engineering for expanding the substrate range of Yarrowia lipolytica. Trends Biotechnol. 2016;34:798–809. doi: 10.1016/j.tibtech.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 67.Malav, A., Meena, S., Sharma, M., Sharma, M. & Dube, P. A critical review on single cell protein production using different substrates. Int. J. Dev. Res.7, 16682–16687 (2017).
- 68.Abram F. Systems-based approaches to unravel multi-species microbial community functioning. Comput Struct. Biotechnol. J. 2015;13:24–32. doi: 10.1016/j.csbj.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sabra W, Dietz D, Tjahjasari D, Zeng AP. Biosystems analysis and engineering of microbial consortia for industrial biotechnology. Eng. Life Sci. 2010;10:407–421. doi: 10.1002/elsc.201000111. [DOI] [Google Scholar]
- 70.McCarty, N. & Ledesma-Amaro, R. Synthetic biology tools to engineer microbial communities for biotechnology. Trends Biotechnol.37, 181–197 (2019). [DOI] [PMC free article] [PubMed]
- 71.Det-Udom R, et al. Towards semi-synthetic microbial communities: enhancing soy sauce fermentation properties in B. subtilis co-cultures. Micro. Cell Fact. 2019;18:1–8. doi: 10.1186/s12934-019-1149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li J, et al. Cooperation of lactic acid bacteria regulated by the AI-2/LuxS system involve in the biopreservation of refrigerated shrimp. Food Res. Int. 2019;120:679–687. doi: 10.1016/j.foodres.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 73.Jenkins, G. in SCP—The BP Protein Process. Resources and Applications of Biotechnology (ed. Greenshields, R.) 141–149 (Springer, 1988). https://link.springer.com/book/10.1007/978-1-349-09574-2.
- 74.Hamdan, I. & Senez, J. Protein (SCP) production in the twenty-first century. in Biotechnology: Economic and Social Aspects: Issues for Developing Countries. (eds DaSilva, E. J. & Ratledge, C.) 142–164 (Cambridge University Press, 1992). https://www.cambridge.org/core/books/biotechnology-economic-and-social-aspects/1EE6FBE4E3C1028E2CF7D0C47640D7BD.
- 75.Ritala A, Häkkinen ST, Toivari M, Wiebe MG. Single cell protein-state-of-the-art, industrial landscape and patents 2001-2016. Front. Microbiol. 2017;8:2009. doi: 10.3389/fmicb.2017.02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Finnigan TJA, et al. Mycoprotein: the future of nutritious nonmeat protein, a symposium review. Curr. Dev. Nutr. 2019;3:nzz021. doi: 10.1093/cdn/nzz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Janssen M, Wijffels RH, Barbosa MJ. Microalgae based production of single-cell protein. Curr. Opin. Biotechnol. 2022;75:102705. doi: 10.1016/j.copbio.2022.102705. [DOI] [PubMed] [Google Scholar]
- 78.Balagurunathan B, Ling H, Choi WJ, Chang MW. Potential use of microbial engineering in single-cell protein production. Curr. Opin. Biotechnol. 2022;76:102740. doi: 10.1016/j.copbio.2022.102740. [DOI] [PubMed] [Google Scholar]
- 79.Zha X, et al. Bioconversion of wastewater to single cell protein by methanotrophic bacteria. Bioresour. Technol. 2021;320:124351. doi: 10.1016/j.biortech.2020.124351. [DOI] [PubMed] [Google Scholar]
- 80.Whittaker, J. A., Johnson, R. I., Finnigan, T. J. A. A., Avery, S. V. & Dyer, P. S. The biotechnology of quorn mycoprotein: past, present and future challenges. in Grand Challenges in Fungal Biotechnology (ed. Nevalainen, H.) 59–79 (Springer, 2020).
- 81.Vegetarian & Vegan Products, Meat Free Recipes & News | Quorn. https://www.quorn.co.uk/.
- 82.Zhao XR, Choi KR, Lee SY. Metabolic engineering of Escherichia coli for secretory production of free haem. Nat. Catal. 2018;1:720–728. doi: 10.1038/s41929-018-0126-1. [DOI] [Google Scholar]
- 83.Nokelainen M, et al. High-level production of human type I collagen in the yeast Pichia pastoris. Yeast. 2001;18:797–806. doi: 10.1002/yea.730. [DOI] [PubMed] [Google Scholar]
- 84.Williams KE, Olsen DR. Gelatin expression from an engineered Saccharomyces cerevisiae CUP1 promoter in Pichia pastoris. Yeast. 2021;38:382–387. doi: 10.1002/yea.3554. [DOI] [PubMed] [Google Scholar]
- 85.Ledesma-Amaro R. Microbial oils: a customizable feedstock through metabolic engineering. Eur. J. Lipid Sci. Technol. 2015;117:141–144. doi: 10.1002/ejlt.201400181. [DOI] [Google Scholar]
- 86.Duale, A., Singh, P. & Al Khodor, S. Breast milk: a meal worth having. Front Nutr.8, 800927 (2022). [DOI] [PMC free article] [PubMed]
- 87.Lu M, Mosleh I, Abbaspourrad A. Engineered microbial routes for human milk oligosaccharides synthesis. ACS Synth. Biol. 2021;10:923–938. doi: 10.1021/acssynbio.1c00063. [DOI] [PubMed] [Google Scholar]
- 88.Bhutada G, et al. Production of human milk fat substitute by engineered strains of Yarrowia lipolytica. Metab. Eng. Commun. 2022;14:e00192. doi: 10.1016/j.mec.2022.e00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Braegger C, et al. Supplementation of infant formula with probiotics and/or prebiotics: a systematic review and comment by the ESPGHAN committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2011;52:238–250. doi: 10.1097/MPG.0b013e3181fb9e80. [DOI] [PubMed] [Google Scholar]
- 90.Rupa P, Mine Y. Structural and immunological characterization of recombinant ovomucoid expressed in Escherichia coli. Biotechnol. Lett. 2003;25:427–433. doi: 10.1023/A:1022489724910. [DOI] [PubMed] [Google Scholar]
- 91.Upadhyay V, Singh A, Panda AK. Purification of recombinant ovalbumin from inclusion bodies of Escherichia coli. Protein Expr. Purif. 2016;117:52–58. doi: 10.1016/j.pep.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 92.Järviö N, et al. Ovalbumin production using Trichoderma reesei culture and low-carbon energy could mitigate the environmental impacts of chicken-egg-derived ovalbumin. Nat. Food. 2021;2:1005–1013. doi: 10.1038/s43016-021-00418-2. [DOI] [PubMed] [Google Scholar]
- 93.Aro N, et al. Production of bovine beta-lactoglobulin and hen egg ovalbumin by Trichoderma reesei using precision fermentation technology and testing of their techno-functional properties. Food Res. Int. 2023;163:112131. doi: 10.1016/j.foodres.2022.112131. [DOI] [PubMed] [Google Scholar]
- 94.Dunn-Coleman NS, et al. Commercial levels of chymosin production by Aspergillus. BioTechnology. 1991;9:976–981. doi: 10.1038/nbt1091-976. [DOI] [PubMed] [Google Scholar]
- 95.Giec A, Skupin J. Single cell protein as food and feed. Food/Nahr. 1988;32:219–229. doi: 10.1002/food.19880320302. [DOI] [PubMed] [Google Scholar]
- 96.Kim SW, et al. Meeting global feed protein demand: challenge, opportunity, and strategy. Annu. Rev. Anim. Biosci. 2019;7:221–243. doi: 10.1146/annurev-animal-030117-014838. [DOI] [PubMed] [Google Scholar]
- 97.Chaucheyras-Durand F, Durand H. Probiotics in animal nutrition and health. Benef. Microbes. 2010;1:3–9. doi: 10.3920/BM2008.1002. [DOI] [PubMed] [Google Scholar]
- 98.Rollo A, et al. Live microbial feed supplement in aquaculture for improvement of stress tolerance. Fish. Physiol. Biochem. 2006;32:167–177. doi: 10.1007/s10695-006-0009-2. [DOI] [Google Scholar]
- 99.Dawson KA, Newman KE, Boling JA. Effects of microbial supplements containing yeast and lactobacilli on roughage-fed ruminal microbial activities. J. Anim. Sci. 1990;68:3392–3398. doi: 10.2527/1990.68103392x. [DOI] [PubMed] [Google Scholar]
- 100.Balabanova L, et al. Engineered fungus Thermothelomyces thermophilus producing plant storage proteins. J. Fungi. 2022;8:119. doi: 10.3390/jof8020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xie D, Jackson EN, Zhu Q. Sustainable source of omega-3 eicosapentaenoic acid from metabolically engineered Yarrowia lipolytica: from fundamental research to commercial production. Appl. Microbiol. Biotechnol. 2015;99:1599–1610. doi: 10.1007/s00253-014-6318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Revuelta JL, et al. Bioproduction of riboflavin: a bright yellow history. J. Ind. Microbiol. Biotechnol. 2017;44:659–665. doi: 10.1007/s10295-016-1842-7. [DOI] [PubMed] [Google Scholar]
- 103.Hanlon P, Sewalt V. GEMs: genetically engineered microorganisms and the regulatory oversight of their uses in modern food production. Crit. Rev. Food Sci. Nutr. 2020;61:959–970. doi: 10.1080/10408398.2020.1749026. [DOI] [PubMed] [Google Scholar]
- 104.Ledesma-Amaro R, Jiménez A, Santos MA, Revuelta JL. Biotechnological production of feed nucleotides by microbial strain improvement. Process Biochem. 2013;48:1263–1270. doi: 10.1016/j.procbio.2013.06.025. [DOI] [Google Scholar]
- 105.Moon H-J, Jeya M, Kim I-W, Lee J-K. Biotechnological production of erythritol and its applications. Appl. Microbiol. Biotechnol. 2010;86:1017–1025. doi: 10.1007/s00253-010-2496-4. [DOI] [PubMed] [Google Scholar]
- 106.Mohamad NL, Mustapa Kamal SM, Mokhtar MN. Xylitol biological production: a review of recent studies. Food Rev. Int. 2015;31:74–89. doi: 10.1080/87559129.2014.961077. [DOI] [Google Scholar]
- 107.Xu Y, et al. De novo biosynthesis of rubusoside and rebaudiosides in engineered yeasts. Nat. Commun. 2022;13:1–12. doi: 10.1038/s41467-022-30826-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Celińska E, et al. Genetic engineering of Ehrlich pathway modulates production of higher alcohols in engineered Yarrowia lipolytica. FEMS Yeast Res. 2019;19:foy122. doi: 10.1093/femsyr/foy122. [DOI] [PubMed] [Google Scholar]
- 109.Li J, et al. Simultaneous improvement of limonene production and tolerance in Yarrowia lipolytica through tolerance engineering and evolutionary engineering. ACS Synth. Biol. 2021;10:884–896. doi: 10.1021/acssynbio.1c00052. [DOI] [PubMed] [Google Scholar]
- 110.Toogood HS, et al. Enzymatic menthol production: one-pot approach using engineered Escherichia coli. ACS Synth. Biol. 2015;4:1112–1123. doi: 10.1021/acssynbio.5b00092. [DOI] [PubMed] [Google Scholar]
- 111.Ledesma-Amaro R, Nicaud J-M. Yarrowia lipolytica as a biotechnological chassis to produce usual and unusual fatty acids. Prog. Lipid Res. 2016;61:40–50. doi: 10.1016/j.plipres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 112.de Oliveira EP, Burini RC. High plasma uric acid concentration: causes and consequences. Diabetol. Metab. Syndr. 2012;4:1–7. doi: 10.1186/1758-5996-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.O’Donnell K, Cigelnik E, Casper HH. Molecular phylogenetic, morphological, and mycotoxin data support reidentification of the quorn mycoprotein fungus as Fusarium venenatum. Fungal Genet. Biol. 1998;23:57–67. doi: 10.1006/fgbi.1997.1018. [DOI] [PubMed] [Google Scholar]
- 114.Nasseri, A. T., Rasoul-Amini, S., Morowvat, M. H. & Ghasemi, Y. Single cell protein: production and process. Am. J. Food Technol.6, 103–116 (2011).
- 115.Hansen EH, et al. De novo biosynthesis of vanillin in fission yeast (Schizosaccharomyces pombe) and baker’s yeast (Saccharomyces cerevisiae) Appl. Environ. Microbiol. 2009;75:2765–2774. doi: 10.1128/AEM.02681-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kovac J, den Bakker H, Carroll LM, Wiedmann M. Precision food safety: a systems approach to food safety facilitated by genomics tools. TrAC Trends Anal. Chem. 2017;96:52–61. doi: 10.1016/j.trac.2017.06.001. [DOI] [Google Scholar]
- 117.Liu W, et al. A dual-plasmid CRISPR/Cas system for mycotoxin elimination in polykaryotic industrial fungi. ACS Synth. Biol. 2020;9:2087–2095. doi: 10.1021/acssynbio.0c00178. [DOI] [PubMed] [Google Scholar]
- 118.Grasso AC, Hung Y, Olthof MR, Verbeke W, Brouwer IA. Older consumers’ readiness to accept alternative, more sustainable protein sources in the European Union. Nutrients. 2019;11:1904. doi: 10.3390/nu11081904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Onwezen MC, Bouwman EP, Reinders MJ, Dagevos H. A systematic review on consumer acceptance of alternative proteins: pulses, algae, insects, plant-based meat alternatives, and cultured meat. Appetite. 2021;159:105058. doi: 10.1016/j.appet.2020.105058. [DOI] [PubMed] [Google Scholar]
- 120.Sultana, S., Ali, M. E. & Ahamad, M. N. U. Gelatine, collagen, and single cell proteins as a natural and newly emerging food ingredients. in Preparation and Processing of Religious and Cultural Foods (eds Ali, E., Naquiah, N. & Nizar, A.) 215–239 (Elsevier, 2018).
- 121.Voutilainen E, Pihlajaniemi V, Parviainen T. Economic comparison of food protein production with single-cell organisms from lignocellulose side-streams. Bioresour. Technol. Rep. 2021;14:100683. doi: 10.1016/j.biteb.2021.100683. [DOI] [Google Scholar]
- 122.Jach ME, et al. Statistical evaluation of growth parameters in biofuel waste as a culture medium for improved production of single cell protein and amino acids by Yarrowia lipolytica. AMB Express. 2020;10:1–12. doi: 10.1186/s13568-020-00968-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Linder T. Edible microorganisms—an overlooked technology option to counteract agricultural expansion. Front. Sustain Food Syst. 2019;3:32. doi: 10.3389/fsufs.2019.00032. [DOI] [Google Scholar]
- 124.Menezes AA, Montague MG, Cumbers J, Hogan JA, Arkin AP. Grand challenges in space synthetic biology. J. R. Soc. Interface. 2015;12:20150803. doi: 10.1098/rsif.2015.0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.García Martínez JB, et al. Potential of microbial protein from hydrogen for preventing mass starvation in catastrophic scenarios. Sustain Prod. Consum. 2021;25:234–247. doi: 10.1016/j.spc.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]