Version Changes
Revised. Amendments from Version 1
1)We added a text to the 6 th paragraph in discussion to address the issue of “lost-to-follow-up”. 2)Moreover, we added a sentence in the 8 th paragraph in the discussion to address safety surveillance system could inform policy to mitigate higher AEs rate.
Abstract
Background: Since the recommendation of voluntary medical male circumcision (VMMC) to reduce the risk of heterosexually acquired HIV, a number of adolescent boys and men in 15 priority countries in Africa have been circumcised. Our primary goal was to identify the incidence of adverse events (AEs) associated with VMMC and to assess the safety profile among adolescent boys 10 – 14 years.
Methods: We searched the databases MEDLINE and Embase, WHO, and conference abstracts from 2005 to 2019. The incidence of AEs was estimated by type of AE, size of study and age.
Results: We retained 40 studies. Severe and moderate AEs overall were estimated at 0.30 per 100 VMMC clients with wide variability per study type. A higher rate was noted in small and moderate scale programmes and device method research studies compared with larger scale programmes. There was a limited number of studies reporting AEs among younger adolescent boys and they had higher infection-related AEs than those aged 20 years and older. Case studies noted rare AEs such as necrotizing fasciitis, tetanus, and glans injury.
Conclusions: AE rates were comparable to those from the randomized controlled trials (RCTs) that led to recommendations and implementation of VMMC in high HIV burden countries, despite being implemented in low resource settings. Clients over time have increasingly included adolescents under the age of 15 years. Studies suggest potentially higher risks in this age group. As VMMC services are sustained, patient safety surveillance systems and promoting a patient safety culture are crucial to identify and mitigate potential harms from medical male circumcision.
Keywords: voluntary medical male circumcision, male urologic surgical procedures, adverse event, human immunodeficiency virus infection, Africa, adolescent
Introduction
Three randomized controlled trial (RCT)s and multiple observational studies demonstrated that medical male circumcision reduced the risk of female-to-male HIV transmission by about 60 % 1– 3 . This evidence led WHO and Joint United Nations Programme on HIV/AIDS(UNAIDS) to recommend in 2007 that voluntary medical male circumcision (VMMC) be implemented as part of HIV prevention programmes in settings with high HIV prevalence and low male circumcision prevalence 4 . By the end of 2019 an estimated 27 million adolescent boys and men in 15 priority countries in east and southern Africa had been circumcised and provided with other HIV and STI prevention and health services through public health programmes 5, 6 . This population is often not reached by health care services.
Medical male circumcision is regarded as a safe procedure when performed by a trained and experienced operator. At the time of the 2007 recommendation, the only systematic compilation on male circumcision safety was from the three well-resourced RCTs of immediate or delayed circumcision, which reported a total of 168 adverse events (AEs) in 5230 surgical procedures (3.2%), though the circumstances in which male circumcision would be provided in VMMC programmes would be expected to differ from the research settings. Hence, care was taken to recognize, mitigate and prevent the risks associated with performing a minor surgical procedure at large scale for long-term prevention against HIV infection in the initial programme implementation plans 7 . Guidance was developed for training, quality standards and assurance, and safety monitoring for countries and other implementers to apply in their programmes, a large proportion of which were supported by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) 8 . Moreover, as VMMC has been scaled up rapidly in priority countries, clinical cadres other than qualified surgeons or medical officers were crucial to implement programmes in countries with limited surgical human resource capacity. VMMC programmes led by trained mid-level providers with recourse to skilled surgical backup has been shown to be safe and has become a standard practice 9 . Over the past decade there have been on-going efforts to address barriers and facilitators of uptake of VMMC programmes among adults and adolescents, which have accelerated implementations of VMMC programmes. Hence, it was considered timely to describe systematically the current status of safety, measured by adverse events to further support implementation of safe VMMC for adolescent boys and men in the prioritized countries.
The purpose of this systematic review was to compile all information from the published literature on the safety of surgical male circumcision performed in research studies and VMMC for HIV prevention programmes in African countries, considering, where available, specific surgical methods and client age.
Methods
Our primary outcome of interest was the incidence of AEs, overall, stratified by type of AE, size of study, and age. A secondary objective was to assess specifically the safety profile among boys ages 10 – 14 years. This review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines 10 .
Literature review and search strategy
A comprehensive MEDLINE and Embase search in June 2019 on general male circumcision safety and methods was supplemented with a search for specific types of AEs and by device-based methods ( Extended data, Table 1 10 ). We restricted inclusion to publications since 2005 and excluded those with no primary data on circumcision safety, which referred exclusively to male circumcision in infants or children, or which reported on the safety of male circumcisions performed for therapeutic reasons.
Titles and abstracts of retained publications were independently assessed by two reviewers to exclude those with no English language abstract and no primary data on safety, efficacy, healing or acceptability of specific circumcision methods in adults or adolescents, and to identify duplicate or overlapping publications. Discrepancies were resolved by discussion including a third reviewer as needed. Additional publications were identified by scanning references in reviews, abstracts, relevant conferences since 2015 (International AIDS Society, Conference on Retroviruses and Opportunistic Infections), and by correspondence with investigators known to be engaged in male circumcision for HIV prevention programmes and assessment of novel male circumcision devices for information on new studies or forthcoming publications.
Retained studies were grouped into broad categories reflecting their context – well-resourced and closely monitored facilities which implemented the three RCTs of impact of circumcision on HIV incidence, surgical arms in research studies investigating new circumcision devices, pilot VMMC programmes with under 1000 clients, medium sized implementation programmes (more than 1000 but under 10,000 clients), and larger programmes (10,000 clients and more).
Terminology and outcomes
Circumcision safety was assessed by the number and severity of AEs reported in clients, excluding events which were definitely not related to the circumcision procedure.
We assessed how each included study reported on the ascertainment of AEs. As we included case reports and case series, we preferred narrative descriptions on the AEs reported in those papers 11 . The majority of clinical studies followed a common classification of AE severity. That classification was based on definitions adopted in 2013 12 and 2014 by the WHO Technical Advisory Group on Innovations in Male Circumcision 13 , which were aligned with the terminology of the Global Harmonization Task Force 14 and in the Adverse Event Action Guide for VMMC by Surgery or Device 15 . Moreover, AEs are classified as severe when an AE required intervention by a skilled surgeon 14 . Moderate AEs included any AE not classified as severe but which required intervention by a trained mid-level health care provider or medication (parenteral, oral or topical). Other AEs were classified as mild 13 . Similar definitions of AEs were adopted by PEPFAR for notifiable adverse events and in the WHO quality assurance guidance 16, 17 .
We computed the proportion of clients with AEs and corresponding 95% confidence intervals (CIs) as long as studies included denominator information using Microsoft Excel (ver. 16.65).
Results
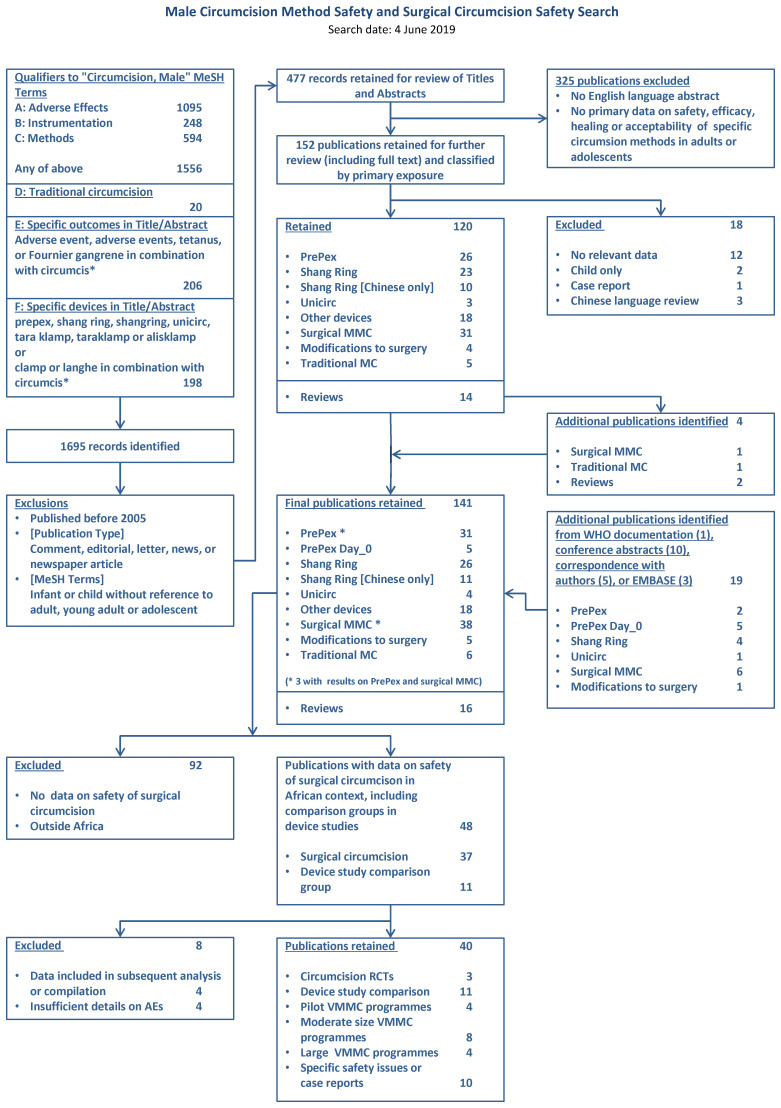
The search identified 1695 records ( Figure 1). After we removed duplicates and reviewed titles and abstracts, 141 were retained for full text review. Restriction to publications with data on the safety of surgical circumcision in Africa left 48, of which eight were excluded because of duplicate or overlapping publication (four) or insufficient data (four) ( Extended data, Table 2 10 ). Extended data Table 3 10 summarizes key information from 31 studies with sufficient data to compute proportions of clients with AEs; and also describes country, study design, the type of procedure, the definition of AEs, age of the participants, providers and settings where male circumcision procedures were performed. The time periods covered by the different studies are shown in Figure 2 together with country of implementation. Within each type, studies were ordered chronologically according to the approximate time period when the circumcisions were performed.
Figure 1. PRISMA flowchart.
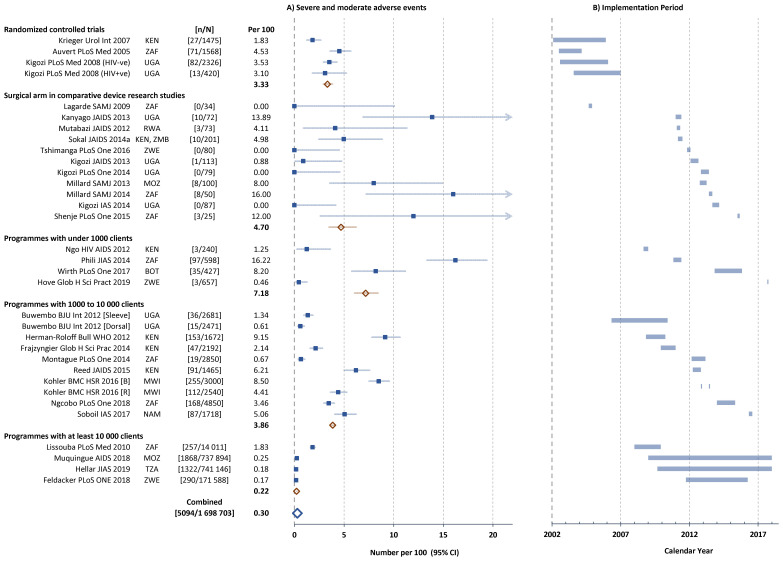
Figure 2.
A) Severe and moderate adverse events (AEs) in individual studies and B) Implementation period of studies reporting circumcision safety (approximate dates of first and last circumcision). Filled squares individual cohorts, open red lozenge all cohorts in subgroup, large open blue lozenge all cohorts combined, lines 95% confidence intervals (truncated line indicated by arrow). ISO-3166 three-letter country codes were use used to represent country. https://www.iso.org/obp/ui/#search/code/
The first three publications 1, 18, 19 referred to the safety of surgical circumcision when performed under well-resourced research conditions (RCTs) by trained medical doctors including surgeons. Similarly, 11 studies compared a device-based method to conventional surgical circumcision within well controlled settings, close follow-up and experienced providers (‘Surgical arm’ in comparative device research studies) 20– 30 .
Within a programme implementation context, 16 studies were undertaken. Many of the ‘smaller-sized studies’ (under 1000 study participants) were part of pilot implementation projects as VMMC was being established within HIV programmes 31– 34 or ‘medium sized’ during expansion to new areas or facilities (1000 to 10,000 participants) 35– 42 . This group included two research studies conducted within VMMC programmes. One study in Uganda started implementation soon after the completion of the Rakai RCT and took the opportunity to compare the dorsal slit and sleeve methods of circumcision which were provided on alternate days of the week 35 . While not an ideal method of assignment, this ‘randomisation’ was done for logistical simplicity and ensured that client characteristics were reasonably balanced across the two groups. A second publication described the implementation of a quality improvement programme within the VMMC service facility in Malawi, and presented data on AEs during the baseline review of a relatively small number of clinic records and a repeat review conducted six months later 40 .
The four largest studies (over 10,000 participants) 43– 46 cover a similarly mixed variety of settings. One study was a description of the implementation of the post-trial implementation of a VMMC programme within the Orange Farm community, South Africa, which had been the site of the first RCT in South Africa 1 . Only the total number of Aes were reported with no detail on AE types. The second study was from Mozambique and provided information on the number and types of adverse events in 740,000 circumcision clients 44 . Data reported to WHO showed that approximately 1.3 million circumcisions were performed in the country over the same period, so this report covered the safety of just over half the total VMMC programme. Similarly, the third study reported from the ZAZIC consortium of partners implementing the Zimbabwe VMMC programme over the period October 2011 to March 2014 and included information on the safety of 171,000 surgical circumcisions, representing approximately 28% of the estimated 610,000 circumcisions performed over the same period 6, 46 .
The majority of the circumcision providers were specially trained physicians, medical officers (physician assistant equivalent), or mid-level clinical officers or nurses ( Extended data, Table 3 10 ). Experienced urological surgeons performed the circumcisions only in three studies. The settings ranged from minor surgical procedure facilities established for the clinical research studies, general practitioner’s offices equipped for minor surgical procedures, to dedicated high volume fixed or mobile outreach facilities ( Extended data, Table 3 10 ).
Reported severe and moderate adverse events
Adverse events were considered first by the number of clients with severe and moderate Aes irrespective of type or cause, and then by main type (bleeding, infection, wound dehiscence). Multiple Aes occurring in the same client were classified where possible according to the most severe or with the greatest potential for permanent injury or sequalae if not treated. Apart from the earliest studies, all studies followed the mild-moderate-severe schema first introduced by WHO and PEPFAR in 2009, but there was insufficient detail to distinguish severe and moderate Aes in all studies reliably.
Figure 2 shows the proportion of clients with reported severe or moderate Aes for each study stratified by study type. RCTs showed AE rates between 1.8 and 4.5 per 100 (mean 3.3 per 100). The comparative male circumcision method studies showed considerable variability (range 0 to 13.9 per 100, mean 4.7 per 100). In the comparison between sleeve and dorsal slit methods performed on alternate days, somewhat lower AE rates were noted with the dorsal slit than sleeve method (0.6 compared with 1.3 per 100) 35 .
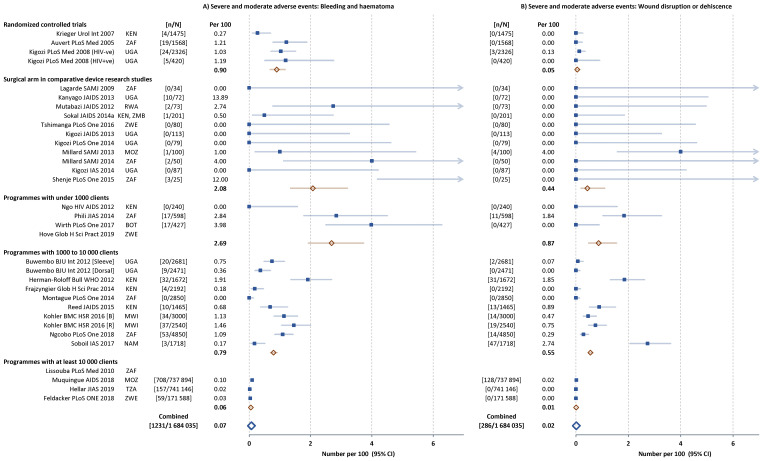
In the smaller sized pilot studies implemented as part of HIV prevention through VMMC programmes, the rates were quite variable (range 1.3 to 16.2 per 100, mean 7.2 per 100), while the medium-sized studies were more homogeneous with overall 3.9 per 100. The quality improvement study 40 showed rates before and after implementation of the quality assessment – ‘B’ baseline study, ‘R’ repeat survey after 6 months implementation. Overall, the AE rate decreased from 8.5 to 4.4 per 100, mainly due to a reduction in the infection rate (decreased from 5.4 to 1.8 per 100), but small increases were noted with bleeding AEs (increased from 0.3 to 1.5 per 100) and with wound healing AEs (increased from 0.5 to 0.8 per 100).
Programmes with at least 10,000 clients in Mozambique, Tanzania and Zimbabwe reported very low total AE rates (range 0.17 to 0.25 per 100), while the Orange Farm follow-on VMMC programme in the community after the RCT reported 1.83 AEs per 100 43 .
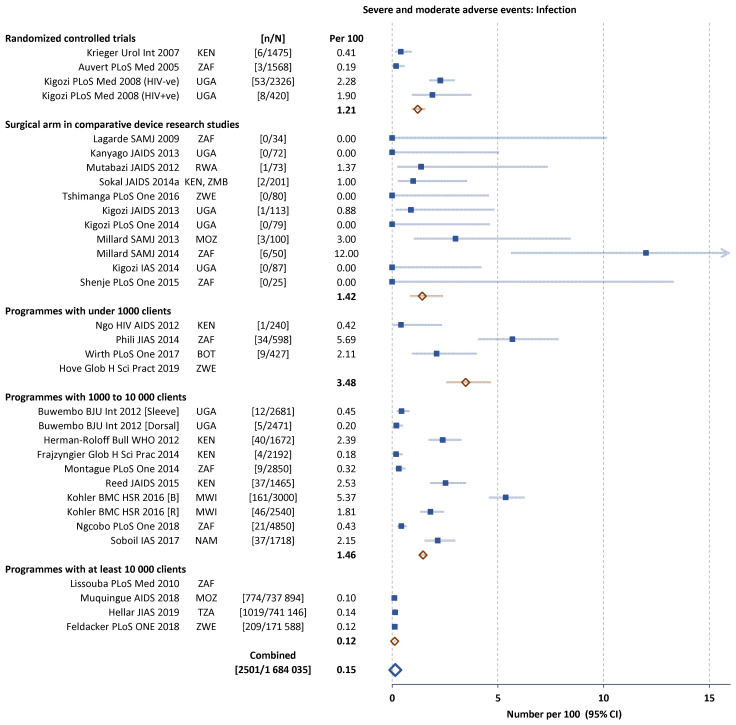
Infection-related AEs represented 50% (2502 of 5114) of all AEs reported, followed by bleeding and haematoma (1237 or 24%) and wound disruption or dehiscence (291 or 6%). The frequency of the main subcategories of AEs were similar across the five types of study ( Figure 3 and Figure 4). In the study comparing dorsal slit and sleeve resection there were fewer AEs of each subcategory with the dorsal slit method.
Figure 3. Severe and moderate infection-related adverse events (AEs) in individual studies.
Filled squares individual cohorts, open red lozenge all cohorts in subgroup, large open blue lozenge all cohorts combined, lines 95% confidence intervals (truncated line indicated by arrow).
Figure 4.
Severe and moderate bleeding- ( A) and wound-related ( B) adverse events (AEs) in individual studies. Filled squares individual cohorts, open red lozenge all cohorts in subgroup, large open blue lozenge all cohorts combined, lines 95% confidence intervals (truncated line indicated by arrow).
Rare adverse events
We also focused on published information on rare AEs considered life-threatening or with long-term consequences. We identified 10 case series or case reports ( Extended data, Table 4 10 ). According to our search strategy, we found serious and rare AEs of the following types and numbers. There were 13 tetanus cases during 2012 through 2016 in five countries among men ages 11–47 years; cases occurred after use of PrePex™ device and conventional surgery, eight of which were adolescents 47 . Necrotizing fasciitis of the perineum was reported from Kenya 48 and Uganda (two cases in 3 years) 49 . There were 19 bleeding AEs requiring prolonged hospital stay that were reported to the PEPFAR from programmes in multiple countries between 2015 and 2016 (among which five patients developed secondary infection, including one necrotizing fasciitis) 50 . In Tanzania between October 2014 and September 2016, three glans injury cases were reported 51 .
Age specific findings in the age group 10–14 years
Over time, the proportion of VMMC clients shifted to the younger adolescent boys. In the large-scale programme study in Tanzania, over a nine year period, 741,146 clients were circumcised, 51.6% of these clients were in the 10–14 age group 45 . Similarly, in Mozambique during 2009–2017, where 737,854 sought the service, 52.6% were in the 10–14 age group 44 .
VMMC clients within the 10–14 year age group were included in 12 of the papers reviewed (one RCT; two in the <1000 category; five in the 1000 – 10,000 category and all four in the >10,000 category). There were varying levels of age disaggregation, type and severity of AEs which limited the compilation of data. More often, the age distribution was given, but not the corresponding breakdown of AEs for each age group. Findings specific to the 10–14 year age group could be found from only two publications on the ZAZIC programme in Zimbabwe.
In the period October 2014 – September 2015 a total of 156 severe and moderate AEs were reported in 44,868 circumcision clients (0.35 per 100, 95% CI 0.3 to 0.41 per 100). The 156 AEs reported represented 25% of total AEs reported over the three-year period. While there was little difference by age group in the incidence of all severe and moderate AEs combined, the incidence of infection-related AEs was approximately 2-fold higher in the 15–19 year age group compared with those 20 years and older, and 3-fold higher in the 10–14 year age group 52 .
Feldacker and colleagues 46 analysed the number and timing of severe and moderate AEs by age group and MC method from the same cohort over the period March 2014 – March 2017 (3 years), but were unable to compute AE rates as the number of procedures performed was not available in sufficient detail over the full time period. They reported a total 617 AEs of which 421 (68%) had complete information on risk factors and 290 had occurred following surgical and 131 following PrePex™ circumcision, which had only been offered to clients aged at least 18 years from April 2014 and aged at least 15 years from July 2016. The majority of the 290 AEs were due to infection (209, 72%) and the remainder were bleeding (59, 20%) or oedema, injury, pain or anaesthesia related (total 22, 8%). The number of AEs by type, days since circumcision and age group showed the largest proportion of infection related AEs were among adolescents with the largest proportion among those 10 – 14 years.
Discussion
HIV prevention through VMMC remains a priority public health intervention in countries where heterosexually acquired HIV infection is common. We sought to better understand the safety of VMMC since the time period when it was introduced in 2007 and through 2019 when over 27 million men and adolescent boys had been reached. We conducted a systematic review on the prevalence of patient harms, specifically adverse events, associated with surgical male circumcision procedures as recommended for use in public health programmes.
Data from 40 studies with sufficient data showed severe and moderate AEs occurred overall at 0.3 per 100 VMMC clients, whereas the rate from the original three RCTs was at 3.33 per 100 VMMC clients, which suggests an acceptable level of safety during programme implementation. The lower overall AE rates is dominated by the very low AE rates in the large programmes. Higher rates were noted in the smaller-scale pilot type studies and device method comparative studies than in the larger sized studies which used mostly programme data. This difference in rates possibly reflects closer follow up of participants in smaller-scale pilot type studies and underreporting in large programmes. Accuracy of rates is also affected by correct identification, classification and reporting of AEs, as noted in the quality improvement study. The post-training results showed a shift to lower rates from the pre-training results likely due to improved classification of symptoms associated with normal healing, as well as improved surgical technique.
Infection-related or bleeding-related AEs were also reported with higher occurrence in the smaller-size studies. Tetanus, Fournier’s gangrene, glans injuries and urethral fistula, all serious adverse events, were reported in case series. Such events were rare and most can be prevented. For instance, WHO advised that all patients be assessed for adequate tetanus protection prior to male circumcision procedures, that enhanced attention be given to standard protocols for skin preparation, cleanliness and wound care education 53 . Fournier's gangrene develops quickly with formidable consequences, and clinical suspicion, urgent care and treatment are the priority.
Regarding adolescents, a limited number of studies, each with method limitations, make interpretation of risk by age group difficult. Younger age adolescents, 10–14 years, were not included in the initial RCTs (18 years and older in two RCTs and 15 years and older in the third). Infection-related AE rates might be higher in the adolescent age group (10–19 years), and more so among younger adolescents, compared with men aged 20 years and older. More recent reports suggested 36 glans injuries and 41 urethral fistulas, nearly always among younger adolescents 54, 55 .
Glans injury and the risk of urethral fistula can be prevented by using techniques directly visualizing the glans penis and by delaying the procedure until an adolescent boy has a more mature penile anatomy. The value of subregional reporting and response is shown by the reported cases of glans injuries with use of forceps-guided method in young boys. This served as a safety signal that led to the WHO Technical Advisory Group on Innovations in Male Circumcision recommending in 2014 that the method not be used in younger adolescents (particularly boys aged below 15 yeas) 13 . The reporting at a subregional level of cases of urethral fistula was one reason that WHO recommended VMMC for adults and adolescents aged 15 years and older, seeking to reduce this rare event from conventional surgical methods 54, 55 . This information comes at a time when a large and increasing proportion of circumcisions performed in national VMMC programmes have been in adolescents, including nearly half in the age group 10 – 14 years (up to 70% for all adolescents) 56 . Decision makers in national programmes will be determining which age group to focus on as they move to maintain high VMMC coverage levels. Thus, surgical safety is a key consideration on offering VMMC to younger adolescents. To better inform the type, severity and magnitude of adverse events among adolescents, programmes should disaggregate the reported VMMCs and AEs into the smaller band age-groups or by individual ages.
According to a 2015 report from a PEPFAR supported VMMC programmes, in 2012, ~85% of patients returned for at least one follow-up visit within 14 days of circumcision 8 . Moderate or severe AEs were more common clients among patients who did not return for a follow-up visit than among clients who did 39 . Active client follow up is important for more accurate AE reporting and response 36 . The VMMC programmes should be supported to strengthen the VMMC safety surveillance system to identify, at least on a periodic basis, AEs among those clients who do not return to services even outside the VMMC programmes.
Our findings also suggest policy implications to further promote the safety of VMMC programmes, knowing that access to safe surgery in general is greatly limited in low-income and lower-middle-income countries, where 90% of people cannot access basic surgical care 57 .
First, VMMC programmes should enhance patient safety surveillance systems, including the reporting of severe and moderate AEs that occur within 30 days after circumcision procedures. Post-surgical follow-up should be assessed for return contact rates. A positive health-care seeking culture should be enhanced with male populations who tend to have poorer health care seeking behaviours 58 . Using communication technology and community workers could also be important tactics to ensure post-surgical follow-up 57 . At the national and sub-regional level, it is important to develop or enhance standardized national and regional surveillance systems for reporting moderate and severe (and serious) AEs including clearly defined protocols and terms of reference for safety monitoring groups. Rare events can best be understood when assessed across the region, so that the number of cases is sufficiently large to identify risks and mitigation factors. Moreover, surveillance of AEs permits to inform relevant responses that could lower higher rates of AEs including for type-specific events (such as tetanus, urethral fistula).
Second, VMMC programmes should work closely with broader patient safety programmes towards an integrated systems approach, supporting development and implementation of patient safety policies at different health administration and care 59 .
Third, cultivating a patient safety environment (‘culture of safety’) within a health care system is important to increase the reporting of AEs and to use that information for learning and improving/revising service delivery. Punitive cultures of blaming providers and perhaps patients impede learning and can prevent reporting of safety related incidents and limit effective responses 59 . A learning culture must be enhanced. Further studies should investigate strategies drawn from other patient safety interventions and participatory learning approaches with community and patient engagement, that build a positive patient safety culture for reporting, learning and responding to moderate and severe AEs in the VMMC settings.
Several limitations of the current study are worth noting. First, AEs can be reported through non-RCT studies including case series/case reports and national surveillance systems that collect AEs during the perioperative period. The quality of such surveillance systems and reporting of ad hoc events is likely variable and heterogeneous across countries. Our paper therefore could underestimate moderate and severe AEs; and it may have missed rare but serious AEs that were not detected and reported in the literature within the study period. Secondly, reported AE rates vary. There was wide diversity across studies in how AEs were defined, ascertained, analysed and reported according to setting and intensity of follow-up. This variability makes comparison between studies and programmes difficult. Clients may have a moderate or severe AE but prefer not to return the health service linked with the programme or may manage the complications at home or in another health care facility. Thirdly, we included case reports and case series to identify signals that could potentially further inform about serious AEs. This approach can be inclusive but the risk of bias is high 11, 60 . Fourthly, variable disaggregation of the AEs either by age-group or type (and severity) makes it challenging to comparatively analyse the study results from these perspectives. As sustaining VMMC for HIV prevention will focus on adolescent boys, such granularity of the data is important.
These limitations notwithstanding, our findings provide insights into the safety of the VMMC programmes that have been implemented over the previous decade. The VMMC programmes over the subregion are generally safe and it is plausible to state that the implementation of VMMC programmes have contributed to improved health with limited harm. Patient safety activities of VMMC programmes need to be sustained and regularly evaluated including as part of programme quality assurance, cultivating a patient safety, and a patient safety surveillance system.
Acknowledgement
The research and views represented in this article are those of the authors alone and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated
Funding Statement
This work was supported by the Bill and Melinda Gates Foundation [OPP1165122 and INV005555]. Funding sources had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
Data availability
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
Extended data
Figshare: Systematic review: Safety of surgical male circumcision in context of HIV prevention public health programmes. https://doi.org/10.6084/m9.figshare.21541392 10 .
This project contains the following extended data:
Reference_gates.docx
Supplement_tables.docx
Surgical MC Safety Charts & AEs.xlsx
Reporting guidelines
Repository: PRISMA checklist and flowchart for Systematic review: Safety of surgical male circumcision in context of HIV prevention public health programmes. https://doi.org/10.6084/m9.figshare.21541392 10 .
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
References
- 1. Auvert B, Taljaard D, Lagarde E, et al. : Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: The ANRS 1265 Trial. PLoS Med. 2005;2(11):e298. 10.1371/journal.pmed.0020298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bailey RC, Moses S, Parker CB, et al. : Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369(9562):643–56. 10.1016/S0140-6736(07)60312-2 [DOI] [PubMed] [Google Scholar]
- 3. Gray RH, Kigozi G, Serwadda D, et al. : Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369(9562):657–66. 10.1016/S0140-6736(07)60313-4 [DOI] [PubMed] [Google Scholar]
- 4. WHO/UNAIDS: New Data on Male Circumcision and HIV Prevention: Policy and Programme Implications.Geneva, Switzerland: World Health Organization and Joint United Nations Programme on HIV/AIDS; 2007. Reference Source [Google Scholar]
- 5. UNAIDS: Global AIDS Update: Communities at the Centre.Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; Report No.: UNAIDS/JC2956, 2019. Reference Source [Google Scholar]
- 6. World Health Organization: WHO Progress Brief: Voluntary medical male circumcision for HIV prevention.Brazzaville, Republic of Congo: World Health Organization Regional Office for Africa, HIV/AIDS Do; Report No.: WHO/AF/CDS/HIV/03/2018, 2018. [Google Scholar]
- 7. WHO/UNAIDS/Jhpiego: Technical Manual for Male Circumcision under Local Anaesthesia.Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 8. Davis SM, Hines JZ, Habel M, et al. : Progress in voluntary medical male circumcision for HIV prevention supported by the US President's Emergency Plan for AIDS Relief through 2017: longitudinal and recent cross-sectional programme data. BMJ Open. 2018;8(8):e021835. 10.1136/bmjopen-2018-021835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davis SM, Baker H, Gross JM, et al. : The Role of Nurses and Midwives in Expanding and Sustaining Voluntary Medical Male Circumcision Services for HIV Prevention: A Systematic and Policy Review. J Assoc Nurses AIDS Care. 2021;32(1):3–28. 10.1097/JNC.0000000000000222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jindai K, Farley T, Awori Q, et al. : Systematic review: Safety of surgical male circumcision in context of HIV prevention public health programmes. figshare. 2022. 10.6084/m9.figshare.21541392.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murad MH, Sultan S, Haffar S, et al. : Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–3. 10.1136/bmjebm-2017-110853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization: WHO Technical Advisory Group on Innovations in Male Circumcision: Evaluation of Two Adult Devices.Geneva, Switzerland: World Health Organization; Report No.: ISBN 978 92 4 150563 5, 2013. Reference Source [Google Scholar]
- 13. World Health Organization: Technical Advisory Group on Innovations in Male Circumcision.Report of Meeting 30 September - 2 October 2014. Geneva, Switzerland: World Health Organization; Report No.: ISBN 978 92 4 150880 3, 2015. Reference Source [Google Scholar]
- 14. Global Harmonization Task Force: Reportable Events During Pre-Market Clinical Investigations.Report No.: GHTF/SG5/N5: 2012, 2012. [Google Scholar]
- 15. Population Services International, College of Surgeons of East, Central, and Southern Africa: Adverse Event Action Guide for VMMC by Surgery or Device.Washington, DC: Population Services International; 2018. [Google Scholar]
- 16. World Health Organization: Male circumcision quality assurance: a guide to enhancing the safety and quality of services.Geneva, Switzerland: World Health Organization; Report No.: ISBN 978 92 4 159731 9, 2008. Reference Source [Google Scholar]
- 17. World Health Organization: Male circumcision quality assessment toolkit.Geneva, Switzerland: World Health Organization; Report No.: ISBN 978 92 4 159751 7, 2009. Reference Source [Google Scholar]
- 18. Krieger JN, Bailey RC, Opeya JC, et al. : Adult male circumcision outcomes: experience in a developing country setting. Urol Int. 2007;78(3):235–40. 10.1159/000099344 [DOI] [PubMed] [Google Scholar]
- 19. Kigozi G, Gray RH, Wawer MJ, et al. : The safety of adult male circumcision in HIV-infected and uninfected men in Rakai, Uganda. PLoS Med. 2008;5(6):e116. 10.1371/journal.pmed.0050116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lagarde E, Taljaard D, Puren A, et al. : High rate of adverse events following circumcision of young male adults with the Tara KLamp technique: a randomised trial in South Africa. S Afr Med J. 2009;99(3):163–9. [PubMed] [Google Scholar]
- 21. Kanyago S, Riding DM, Mutakooha E, et al. : Shang Ring versus forceps-guided adult male circumcision: a randomized, controlled effectiveness study in southwestern Uganda. J Acquir Immune Defic Syndr. 2013;64(2):130–3. 10.1097/QAI.0b013e3182965d67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mutabazi V, Kaplan SA, Rwamasirabo E, et al. : HIV prevention: male circumcision comparison between a nonsurgical device to a surgical technique in resource-limited settings: a prospective, randomized, nonmasked trial. J Acquir Immune Defic Syndr. 2012;61(1):49–55. 10.1097/QAI.0b013e3182631d69 [DOI] [PubMed] [Google Scholar]
- 23. Sokal DC, Li PS, Zulu R, et al. : Randomized controlled trial of the shang ring versus conventional surgical techniques for adult male circumcision: safety and acceptability. J Acquir Immune Defic Syndr. 2014;65(4):447–55. 10.1097/QAI.0000000000000061 [DOI] [PubMed] [Google Scholar]
- 24. Tshimanga M, Mangwiro T, Mugurungi O, et al. : A Phase II Randomized Controlled Trial Comparing Safety, Procedure Time, and Cost of the PrePex™ Device to Forceps Guided Surgical Circumcision in Zimbabwe. PLoS One. 2016;11(5):e0156220. 10.1371/journal.pone.0156220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kigozi G, Musoke R, Watya S, et al. : The acceptability and safety of the Shang Ring for adult male circumcision in Rakai, Uganda. J Acquir Immune Defic Syndr. 2013;63(5):617–21. 10.1097/QAI.0b013e3182968dda [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kigozi G, Musoke R, Watya S, et al. : The safety and acceptance of the PrePex device for non-surgical adult male circumcision in Rakai, Uganda. A non-randomized observational study. PLoS One. 2014;9(8):e100008. 10.1371/journal.pone.0100008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Millard PS, Wilson HR, Veldkamp PJ, et al. : Rapid, minimally invasive adult voluntary male circumcision: A randomised trial. S Afr Med J. 2013;103(10):736–42. 10.7196/samj.6856 [DOI] [PubMed] [Google Scholar]
- 28. Millard PS, Wilson HR, Goldstuck ND, et al. : Rapid, minimally invasive adult voluntary male circumcision: a randomised trial of Unicirc, a novel disposable device. S Afr Med J. 2014;104(1):52–7. 10.7196/samj.7357 [DOI] [PubMed] [Google Scholar]
- 29. Kigozi G, Musoke R, Kighoma N, et al. : The acceptability and safety of the Shang Ring for adolescent male circumcision in Rakai, Uganda (TUPE148).20th International AIDS Conference; Melbourne, Australia, 2014. [Google Scholar]
- 30. Shenje J, Millard PS: Sutureless adult voluntary male circumcision with topical anesthetic: A randomized field trial of Unicirc, a single-use surgical instrument. PLoS One. 2016;11(6):e0157065. 10.1371/journal.pone.0157065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ngo TD, Obhai G: Male circumcision uptake, postoperative complications, and satisfaction associated with mid-level providers in rural Kenya. HIV AIDS (Auckl). 2012;4:37–43. 10.2147/HIV.S30357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Phili R, Abdool-Karim Q, Ngesa O: Low adverse event rates following voluntary medical male circumcision in a high HIV disease burden public sector prevention programme in South Africa. J Int AIDS Soc. 2014;17(1):19275. 10.7448/IAS.17.1.19275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wirth KE, Semo BW, Spees LP, et al. : A prospective cohort study of safety and patient satisfaction of voluntary medical male circumcision in Botswana. PLoS One. 2017;12(11):e0185904. 10.1371/journal.pone.0185904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hove J, Masimba L, Murenje V, et al. : Incorporating Voluntary Medical Male Circumcision into traditional circumcision contexts: Experiences of a local consortium in Zimbabwe collaborating with an ethnic group. Glob Health Sci Pract. 2019;7(1):138–46. 10.9745/GHSP-D-18-00352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Buwembo DR, Musoke R, Kigozi G, et al. : Evaluation of the safety and efficiency of the dorsal slit and sleeve methods of male circumcision provided by physicians and clinical officers in Rakai, Uganda. BJU Int. 2012;109(1):104–8. 10.1111/j.1464-410X.2011.10259.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Herman-Roloff A, Bailey RC, Agot K: Factors associated with the safety of voluntary medical male circumcision in Nyanza province, Kenya. Bull World Health Organ. 2012;90(10):773–81. 10.2471/BLT.12.106112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Frajzyngier V, Odingo G, Barone M, et al. : Safety of adult medical male circumcision performed by non-physician clinicians in Kenya: a prospective cohort study. Glob Health Sci Pract. 2014;2(1):93–102. 10.9745/GHSP-D-13-00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Montague C, Ngcobo N, Mahlase G, et al. : Implementation of adolescent-friendly voluntary medical male circumcision using a school based recruitment program in rural KwaZulu-Natal, South Africa. PLoS One. 2014;9(5):e96468. 10.1371/journal.pone.0096468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reed JB, Grund J, Liu Y, et al. : Implementation and Operational Research: Evaluation of Loss-to-Follow-up and Postoperative Adverse Events in a Voluntary Medical Male Circumcision Program in Nyanza Province, Kenya. J Acquir Immune Defic Syndr. 2015;69(1):e13–23. 10.1097/QAI.0000000000000535 [DOI] [PubMed] [Google Scholar]
- 40. Kohler PK, Namate D, Barnhart S, et al. : Classification and rates of adverse events in a Malawi male circumcision program: impact of quality improvement training. BMC Health Serv Res. 2016;16:61. 10.1186/s12913-016-1305-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ngcobo S, Wolvaardt JE, Bac M, et al. : The quality of voluntary medical male circumcision done by mid-level workers in Tshwane District, South Africa: A retrospective analysis. PLoS One. 2018;13(1):e0190795. 10.1371/journal.pone.0190795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Soboil N, Laube C, Mwinyi A, et al. : Monitoring adverse events in a new mature male circumcision client cohort in Namibia [WEPEC0909].IAS; Paris,2017. [Google Scholar]
- 43. Lissouba P, Taljaard D, Rech D, et al. : A model for the roll-out of comprehensive adult male circumcision services in African low-income settings of high HIV incidence: the ANRS 12126 Bophelo Pele Project. PLoS Med. 2010;7(7):e1000309. 10.1371/journal.pmed.1000309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muquingue H, Ndimande S, Necochea E, et al. : Profile of adverse events in a national VMMC program in Mozambique (2009 to 2017): Reduction in AE with a national scale-up, but three events require further attention [TUAC0204].AIDS; Amsterdam,2018. [Google Scholar]
- 45. Hellar A, Plotkin M, Lija G, et al. : Adverse events in a large-scale VMMC programme in Tanzania: findings from a case series analysis. J Int AIDS Soc. 2019;22(7):e25369. 10.1002/jia2.25369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Feldacker C, Bochner AF, Murenje V, et al. : Timing of adverse events among voluntary medical male circumcision clients: Implications from routine service delivery in Zimbabwe. PLoS One. 2018;13(9):e0203292. 10.1371/journal.pone.0203292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dalal S, Samuelson J, Reed J, et al. : Tetanus disease and deaths in men reveal need for vaccination. Bull World Health Organ. 2016;94(8):613–21. 10.2471/BLT.15.166777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Odoyo-June E, Agot K, Owuor N, et al. : Notifiable adverse events associated with medical male circumcision in Kenya [Abstract 1066].CROI; Boston,2018. [Google Scholar]
- 49. Galukande M, Sekavuga DB, Muganzi A, et al. : Fournier's gangrene after adult male circumcision. Int J Emerg Med. 2014;7:37. 10.1186/s12245-014-0037-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hinkle LE, Toledo C, Grund JM, et al. : Bleeding and Blood Disorders in Clients of Voluntary Medical Male Circumcision for HIV Prevention - Eastern and Southern Africa, 2015-2016. MMWR Morb Mortal Wkly Rep. 2018;67(11):337–9. 10.15585/mmwr.mm6711a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hellar A, Christensen A, Reed J, et al. : Switching from the forceps-guided to the dorsal slit technique in a Voluntary Medical Male Circumcision (VMMC) program: Experience from Tanzania.22nd International AIDS Conference; Amsterdam,2018. Reference Source [Google Scholar]
- 52. Bochner AF, Feldacker C, Makunike B, et al. : Adverse event profile of a mature voluntary medical male circumcision programme performing PrePex and surgical procedures in Zimbabwe. J Int AIDS Soc. 2017;19(1):21394. 10.7448/IAS.20.1.21394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. World Health Organization: WHO Informal Consultation on Tetanus and Voluntary Medical Male Circumcision: Report of meeting convened in Geneva, Switzerland, 9-10 March 2015.Geneva, Switzerland: World Health Organization; Report No.: ISBN 978 92 4 150923 7,2015. Reference Source [Google Scholar]
- 54. Lucas TJ, Toledo C, Davis SM, et al. : Case series of glans injuries during voluntary medical male circumcision for HIV prevention - eastern and southern Africa, 2015-2018. BMC Urol. 2020;20(1):45. 10.1186/s12894-020-00613-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lucas T, Hines JZ, Samuelson J, et al. : Urethrocutaneous fistulas after voluntary medical male circumcision for HIV prevention-15 African Countries, 2015-2019. BMC Urol. 2021;21(1):23. 10.1186/s12894-021-00790-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. World Health Organization: WHO progress brief: voluntary medical male circumcision for HIV prevention in 14 priority countries in Eastern and Southern Africa. 2017. Reference Source [Google Scholar]
- 57. Meara JG, Leather AJM, Hagander L, et al. : Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet. 2015;386(9993):569–624. 10.1016/S0140-6736(15)60160-X [DOI] [PubMed] [Google Scholar]
- 58. World Health Organization: A framework for voluntary medical male circumcision: Effective HIV prevention and a gateway to improved adolescent boys’ & men’s health in eastern and southern Africa by 2021.Geneva, Switzerland: World Health Organization, HIV/AIDS Do; Report No.: WHO/HIV/2016.17,2016. Reference Source [Google Scholar]
- 59. Executive Board, 144: Patient safety: global action on patient safety: report by the Director-General.World Health Organization,2018. Reference Source [Google Scholar]
- 60. Zorzela L, Golder S, Liu Y, et al. : Quality of reporting in systematic reviews of adverse events: systematic review. BMJ. 2014;348:f7668. 10.1136/bmj.f7668 [DOI] [PMC free article] [PubMed] [Google Scholar]