Abstract
Depression is a major neuropsychiatric disease that considerably impacts individuals’ psychosocial function and life quality. Neurotrophic factors are now connected to the pathogenesis of depression, while the definitive neurotrophic basis remains elusive. Besides, phytotherapy is alternative to conventional antidepressants that may minimize undesirable adverse reactions. Thus, further research into the interaction between neurotrophic factors and depression and phytochemicals that repair neurotrophic factors deficit is highly required. This review highlighted the implication of neurotrophic factors in depression, with a focus on the brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), vascular endothelial growth factor (VEGF), and nerve growth factor (NGF), and detailed the antidepressant activities of various phytochemicals targeting neurotrophic factors. Additionally, we presented future opportunities for novel diagnostic and therapeutic strategies for depression and provided solutions to challenges in this area to accelerate the clinical translation of neurotrophic factors for the treatment of depression.
Keywords: depression, neurotrophic factors, pathogenesis, neurotrophic basis, phytotherapy, phytochemicals, antidepressant
1 Introduction
Depression is one of the most common and serious neuropsychiatric disorders, affecting people’s thoughts, behaviors, interests, and feelings. Clinical patients with depression are characterized by several manifestations such as gloomy mood, loss of interest, sleep disturbances, etc. (Malhi and Mann, 2018; Wang et al., 2021a). While the pathogenesis of depression is multifactorial and poorly understood. Its diverse manifestations, erratic course and prognosis, and inconsistent responsiveness to therapy pose a challenge to its detection, diagnosis, and management (Leung et al., 2022). Therefore, it is necessary to investigate theoretical underpinnings and novel targets for early prevention and accurate diagnosis of depression. Additionally, conventional antidepressants display remarkable limitations, such as the delayed onset of action, low response rates, and relapse following medication discontinuation, impeding treatment compliance in patients with depression (Sabella, 2018). Accordingly, identifying non-adverse and side-effect-free alternatives to traditional antidepressants is vital to improving drug adherence in depressed individuals.
2 Neurotrophic basis of depression
Neuroplasticity is responsible for neurogenesis and the modification of mature neuronal morphology (Allen and Lyons, 2018). Limiting neurogenesis prevents antidepressant action and has been substantiated to depression-like syndromes, especially under stressful situations (Castreń, 2013). Therefore, neurogenesis has been proposed to facilitate stress resilience, which might be the foundation of antidepressant therapeutic benefits. Neurotrophic factors are essential mediators of neuroplasticity among several candidates (Song et al., 2017), to boost neuroplasticity, particularly synaptic plasticity, neurotransmission, and neuronal survival, growth, and differentiation (Thoenen, 1995; Wang et al., 2022). Furthermore, the increase in neuroplasticity is expected to attract antidepressant benefits (Figure 1). The secretion of neurotrophic production increased after antidepressant treatment, promoting the survival of neurons and shielding them from stress-related damage. As a result, the onset of depression is implicated in the impairment of neurotrophic factor signaling (Table 1). Although efforts have been made to understand the neurotrophic basis of the pathogenesis of depression, many fundamental questions regarding their mechanisms of action remain to be addressed systematically to better understand the complicated neurotrophic basis in depression treatment.
FIGURE 1.
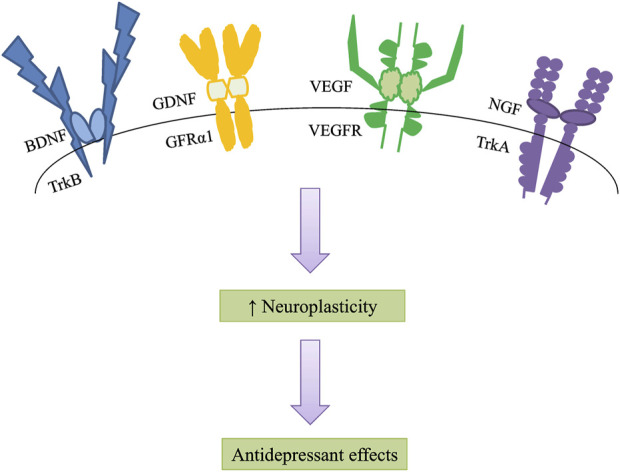
Neurotrophic factors increase neuroplasticity, especially synaptic plasticity, neurotransmission, and neuronal survival, growth, and differentiation. An increase in neuroplasticity is likely to induce antidepressant effects. BDNF, brain-derived neurotrophic factor; TrkB, tropomyosin-related kinase receptor B; GDNF, glial cell line-derived neurotrophic factor; GFRα1, GDNF-family receptor-α1; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; NGF, nerve growth factor; TrkA, tropomyosin-related kinase receptor A.
TABLE 1.
Relationship between neurotrophins and the pathogenesis of depression.
| Neurotrophins | First discovered | Changes in MDD | References |
|---|---|---|---|
| BDNF | 1980s | ↓Amygdala | Hofer and Barde, (1988), Leibrock et al., 1989, Guilloux et al., 2012, Wook Koo et al., 2016, Zhang, (2011) |
| Barde | ↓Plasma | ||
| ↓Serum | |||
| ↓DG | |||
| ↑NAc | |||
| GDNF | 1993 | ↓Serum | Lin et al., 1993, Zhang et al., 2009 |
| Lin | |||
| VEGF | 1989 | ↓Plasma | Leung et al., 1989, Isung et al., 2012, Castillo et al., 2020, Lee and Kim, (2012) |
| Ferrara | ↑Plasma | ||
| NGF | 1956 | ↓Serum | Levi-Montalcini and Angeletti, (1968), Wiener et al., 2015 |
| Levi-Montalcini |
MDD, major depressive disorder; BDNF, brain-derived neurotrophic factor; GDNF, glial cell-derived neurotrophic factor; VEGF, vascular endothelial growth factor; NGF, nerve growth factor; ↓, decrease; ↑, increase; DG, dentate gyrus; NAc, nucleus accumbens.
2.1 Brain-derived neurotrophic factor and depression
Brain-derived neurotrophic factor (BDNF), an essential member of the neurotrophic factor family, was initially discovered in the brain of a pig by Barde in the 1980s (Leibrock et al., 1989). BDNF, primarily synthesized in neurons, is ubiquitously distributed throughout the central nervous system (CNS). It is involved in the repair of synaptic plasticity, the transduction of 5-hydroxytryptamine (5-HT) signaling, and the level of 5-HT in the brain (Bhattarai et al., 2020; Costa et al., 2022). Consistent reports have certified that BDNF is associated with the occurrence, development, and management of depression, and it has received the most attention in the neurobiology of depression among any neurotrophic factors.
Researchers are constantly investigating the relationship between variations in activity and content of BDNF and the occurrence or outcome of depression. BDNF deficiency in the amygdala is visible in women with major depressive disorder (MDD) (Guilloux et al., 2012). Postmortem analysis revealed that plasma BDNF levels are lower in depressed patients than that in controls (Gadad et al., 2021). Moreover, a series of experiments have confirmed that intracerebral administration of BDNF has antidepressant efficacy in depressive animal models (Deltheil et al., 2009). Antidepressant studies targeting BDNF have the potential to be one of the most valid strategies for the development of novel antidepressant medications. Subsequently, Fukumoto et al. demonstrated that the antidepressant effect of (2R, 6R)-Hydroxynorketamine [(2R, 6R)-HNK], a ketamine metabolite that can produce rapid and sustained antidepressant actions in animal models without side effects of ketamine, was mediated through active-dependent release of BDNF in the medial prefrontal cortex (mPFC), sufficiently demonstrating the indispensable role of BDNF in antidepressant treatment (Fukumoto et al., 2019).
However, the association between BDNF and depression has not yielded conclusive results. Tropomyosin-related kinase B (TrkB), a specific BDNF receptor, has been pointed to activate BDNF-TrkB signaling to exert the antidepressant action (Rantamäki et al., 2007), and ketamine improves postoperative depression symptoms by upregulating BDNF-TrkB signaling as well. However, Wook Koo et al. pointed out that chronic social defeat stress increased BDNF expression level in the nucleus accumbens, and local knockout of the BDNF gene in the ventral tegmental area reduced depression-like phenotypes, demonstrating that BDNF signaling induces depression susceptibility (Wook Koo et al., 2016). The role of BDNF acts variably in diverse brain regions, warranting additional study of individual mechanisms. Besides, a substantial reduction in BDNF levels in rheumatoid arthritis patients with depression were detected (Cheon et al., 2018; Nerurkar et al., 2019), and the severity of depression is related to fatigue, poor BDNF expression, and serious state of rheumatoid arthritis. Therefore, BDNF levels might be potential biomarkers for the prediction or monitoring of depression.
Much work on BDNF has recently been reported in this field, while the following issues should be highlighted: there are differences in the stability of BDNF levels measured by different laboratories in whole blood, serum, and plasma (Karege et al., 2005; Suwalska et al., 2010; Arosio et al., 2021), which may be attributed to differences in enzyme-linked immunosorbent assay methods or sampling tubes; the discrepant level and mechanism of BDNF in various brain regions are different, which deserves further study; the more stable and accurate BDNF measurements should be determined and find out which source of BDNF is the most reliable biomarker of MDD, as concentrations of BDNF markers in the circulation do not always reflect the CNS concentrations.
2.2 Glial cell line-derived neurotrophic factor and depression
Glial cell line-derived neurotrophic factor (GDNF) is a neurotrophic factor of the β family that is widely distributed throughout the brain and regulates the noradrenergic and GABAergic systems. It was first purified and named in 1993 by Lin et al. (Lin et al., 1993). GDNF is one of the most efficient neurotrophins, influencing the growth, survival, and activity of midbrain dopaminergic neurons, protecting neurons from oxidative stress, and constituting major players in the development and function of hippocampal neurons (Yang et al., 2001; Bonafina et al., 2019).
A postmortem study on characters with MDD found that the level of GDNF decreased in PFC and the concentration of GDNF in the amygdala reduced as well (Michel et al., 2008; Järvelä et al., 2011; Tang et al., 2023), implying that lower serum GDNF may be involved in the pathophysiology of MDD. Zhang et al. investigated whether the serum GDNF of patients with MDD differed from that of the healthy control group before antidepressant treatment and whether it could affect serum GDNF expression in patients with MDD after antidepressant treatment (Zhang et al., 2010). The results revealed that serum GDNF levels were conspicuously lower in MDD patients before treatment than that in healthy volunteers. Antidepressants could increase GDNF mRNA and protein levels, suggesting the increased GDNF might contribute to the improvement of depression (Maheu et al., 2015). Furthermore, central GDNF signaling may also be a potential antidepressant target. High plasma GDNF levels may be implicated in the pathophysiology of late-onset depression and cognitive impairment in late-onset depression patients (Wang et al., 2011). Consequently, a reduction in GDNF levels might be a biomarker of depressed status.
Based on the above studies on the interaction between GDNF and depression, researchers can recognize that: whether the influence of peripheral and central GDNF on the pathogenesis of depression is not completely clear; supplementation of exogenous GDNF has an antidepressant effect. When it comes to exogenous GDNF supplied to serum, plasma, and whole blood, the optimal strategy must be determined.
2.3 Vascular endothelial growth factor and depression
Vascular endothelial growth factor (VEGF) is an effective mitogen and survival factor for endothelial cells and neurons, as well as a modulator of synaptic transmission (Vargish et al., 2017). In 1989, Ferrara et al. isolated and cloned this substance and named it (Leung et al., 1989). In addition to angiogenic action (Apte et al., 2019), current research has revealed the neurotrophic and neuroprotective potentiality of VEGF in the CNS (Jin et al., 2002; Sene et al., 2015). For example, VEGF influences the pathophysiology of hippocampal neurogenesis and depression, contributes to the occurrence of hippocampal neurons, and shields stress-related neurons from damage (Cao et al., 2004; Kirby et al., 2015), which is essential for antidepressant therapy. Inhibiting the expression of VEGF receptor 2 in nerve cells impairs hippocampal-dependent synaptic plasticity and emotional memory consolidation (De Rossi et al., 2016).
Current clinical research on the correlation between VEGF and the onset of depression has not yielded consistent results. When compared to that in healthy volunteers, the expression of VEGF in patients with depression tends to increase in serum and plasma (Castillo et al., 2020), while quite a few studies have detected an average decrease in VEGF levels in patients with depression (Du Preez et al., 2021), which may be due to inadequate assessment of environmental factors such as gender, age, and body mass index. VEGF can predict the response of antidepressant treatment, suggesting that it is a possible biomarker and mediator engaged in neuroplastic processes (Castillo et al., 2020).
The findings make an important contribution to this expanding field of VEGF research, which can be emphasized as follows: even though BDNF is currently the most studied neurotrophic factor in neurobiology in MDD, the effects of VEGF on the pathogenesis of depression should not be underestimated, which means that the relationship between VEGF and depression should be investigated thoroughly; the correlation between VEGF and depression remains inconsistent, so the effect of VEGF on depression should be designed to combine with environmental variables.
2.4 Nerve growth factor and depression
Nerve growth factor (NGF), an essential member of the neurotrophic factor family, was first isolated in 1956 by Levi-Montalcini (Levi-Montalcini and Angeletti, 1968). It is primarily generated in the cortex, hippocampus, and hypothalamus, but it is also found in the peripheral nervous system and the immune system (Meng et al., 2022). NGF has a strong affinity for TrkA (Riccio et al., 1997; Deppmann et al., 2008). Owing to its participation in neuroplasticity, learning, and memory, NGF is essential for the response to stress and the regulation of the neuro-endocrine-immunity system (Mohammadi et al., 2018).
NGF plays an important role in the pathogenesis of depressive symptoms and the response to antidepressant treatment, which can be seen from that exogenous NGF could induce antidepressant-like effects in rodent depression models (Mezhlumyan et al., 2022). In a study examining the effects of NGF on depression, NGF improved depression-like behaviors like fluoxetine and amitriptyline (McGeary et al., 2011), suggesting NGF is involved in the pathogenesis of depressive symptoms and the response to antidepressant treatment. To test whether NGF is associated with the etiology of depression or suicide risk, Wiener et al. examined changes in serum NGF levels in MDD patients with or without suicidal risk (Wiener et al., 2015). The results showed that the serum levels of NGF in the MDD group and MDD along with suicide risk group were significantly reduced, however, there was no difference between the MDD group and MDD along with suicide risk group, from where we could point out that NGF was a biomarker of MDD. It may be associated with the diagnosis of MDD but not with the severity of symptoms. Early adverse experiences in humans, for instance, maternal deprivation, are linked to an increased risk of mental illnesses such as anxiety and MDD, and data from Cirulli et al. showed that NGF was a potential candidate for adverse events in brain dysfunction and a neuroendocrine marker for the different responses of male and female rhesus monkeys suffering from maternal deprivation (Cirulli et al., 2009).
Based on the above NGF and depression studies, researchers can find that the presence of suicide risk does not affect the serum levels of NGF, suggesting NGF may be associated with the diagnosis of MDD but not with the severity of symptoms.
3 Phytotherapy on depression targeting neurotrophic factors
Despite the fact that conventional antidepressant therapy can help relieve symptoms of depression, concerns have been raised regarding complementary therapies due to the drawbacks of the current medications. Phytochemical constituents, a ubiquitous class of plant secondary metabolites, have revealed their therapeutic benefits in many indications, including mental disorders (Raimundo et al., 2022). The use of phytochemicals is a complementary method to conventional antidepressants to provide therapeutic advantages and avoid unwanted adverse reactions. To date, subsequent evidence indicates that impairment in neurotrophic basis is associated with depression, and phytochemicals targeting neurotrophic factors exert antidepressant properties. It is thus not surprising that the focus of the pharmacological study on phytochemicals for the treatment of depression has been targeting neurotrophic factors, among which BDNF, GDNF, VEGF, and NGF are the most relevant neurotrophins. For example, curcumin, one of the few phytochemicals that have found its way into human studies, exerts antidepressant effects by improving the levels of hippocampal BDNF (Sanmukhani et al., 2014; Fusar-Poli et al., 2020). Besides, resveratrol is a natural polyphenol that could improve the reduction in sucrose preference in rats by promoting BDNF and GDNF levels (Liu et al., 2014; Couteur et al., 2021). GDNF and NGF could be inducted by olive polyphenol administration in the hippocampus and olfactory bulbs of mice (De Nicoló et al., 2013). Naringin increased the expression of BDNF and VEGF in rat models (Rong et al., 2012; Viswanatha et al., 2022). Table 2 manifested other phytochemicals targeting neurotrophic factors for depression treatment.
TABLE 2.
Antidepressant effects induced by phytochemicals based on neurotrophic factors.
| Phytochemicals | Behavioral effects | Neurotrophic mechanisms | References |
|---|---|---|---|
| Auraptene | ↓Immobility time in FST and TST | ↑GDNF mRNA | Amini-Khoei et al., 2022, Furukawa et al., 2020 |
| Baicalein | ↓Immobility time in FST and TST | ↑BDNF/TrkB/CREB pathway | Zhao et al., 2021, Liu et al., 2022 |
| ↑Sucrose preference in SPT | ↓Inflammatory cytokines | ||
| ↑OFT | ↑BDNF | ||
| Catalpol | ↓Immobility time in FST | ↓NLRP3 inflammasome and neuroinflammation | Wang et al., 2021d, Wang et al., 2015, Xu et al., 2010 |
| ↑OFT | ↑BDNF expression and TrkB, ↓COX-2 expression and PGE2 | ||
| ↑GDNF | |||
| Chrysin | ↓Immobility time in FST | ↑BDNF | Filho et al., 2016, Ma et al., 2020 |
| ↓Cytokines and 5-HT | |||
| ↑OFT | ↓Ca2+ availability | ||
| ↑cAMP/PKA and NO/cGMP signaling pathways | |||
| Curcumin | ↓Immobility time in FST | ↑Hippocampal synaptic plasticity | Fan et al. (2021) |
| ↑Sucrose preference in SPT | ↑Hippocampal BDNF | ||
| Dimethyl fumarate | ↓Immobility time in FST | ↑Hippocampal BDNF and β-catenin | Abd El-Fattah et al. (2018) |
| ↑Sucrose preference in SPT | |||
| ↑Sucrose preference in SPT | |||
| Emodin | ↓Immobility time in FST and TST | ↑BDNF | Ahn et al., 2016, Zhang et al., 2021b |
| ↑Sucrose intake in SPT | ↓Inflammatory responses | ||
| Eugenol | ↓Immobility time in FST | ↑BDNF | Irie et al. (2004), Norte et al., 2005 |
| Genipin | ↓Immobility time in FST and TST | ↑Hippocampal BDNF | Ye et al. (2018) |
| Ginsenosides Rb1 | ↑social interaction | ↑BDNF | Jiang et al., 2022, Zhang et al., 2021a, Liang et al., 2010 |
| ↓Immobility time in FST and TST | ↑NGF | ||
| Ginsenoside Rg1 | ↓Immobility time in FST and TST | ↑Hippocampal BDNF | Wang et al., 2021c, Jiang et al., 2012, Liang et al., 2010, Wang et al., 2021b |
| ↑Sucrose preference in SPT | ↑NGF | ||
| ↑OFT | ↑Cx43-based gap junction | ||
| Hesperidin | ↑Sucrose preference in SPT | ↑BDNF | Sharma et al., 2021, Li et al., 2016, Zhu et al., 2020 |
| ↓Immobility time in FST and TST | ↑GDNF | ||
| Hyperforin | ↓Immobility time in TST | ↑BDNF | Pochwat et al. (2018) |
| Hypericin | ↑Sucrose preference in SPT | ↓PI3K/Akt pathway | Zhai et al., 2015, Zhang et al., 2015, Thong et al., 2006, Lavie et al., 2005 |
| ↑Body weight | ↑VEGF | ||
| ↓Immobility time in TST | ↓Phosphorylation of ERK1/2 | ||
| Macranthol | ↑Sucrose preference in SPT | ↑BDNF | Luo et al., 2015, Weng et al., 2019 |
| Magnolol | ↑Sucrose preference in SPT | ↓M1 polarization | Tao et al. (2021) |
| Naringenin | ↑Sucrose preference in SPT | ↑BDNF | Bansal et al., 2018, Eraky et al., 2023 |
| ↓Inflammatory cytokines | |||
| Naringin | ↓Immobility time in FST and TST | ↑BDNF/TrkB/CREB pathway | Gao et al., 2022, Rong et al., 2012, Viswanatha et al., 2022 |
| ↑VEGF | |||
| Oleanolic acid | ↓Immobility time in FST and TST | ↑Hippocampal BDNF | Fajemiroye et al. (2014) |
| Olive polyphenol | _ | ↑GDNF and NGF in the hippocampus and olfactory bulbs | De Nicoló et al. (2013) |
| Orcinol glucoside | ↓Immobility time in FST and TST | ↑BDNF/TrkB/CREB pathway | Li et al. (2021) |
| ↑Sucrose preference in SPT | |||
| ↑OFT | |||
| Paeoniflorin | ↑Sucrose preference in SPT | ↑ERK1/2 pathway | Tang et al., 2021, Tian et al., 2021 |
| ↑function of balance control and motor coordination in the BBT | ↓Pyroptosis CASP-11/GSDMD pathway | ||
| Piperine | ↑Sucrose preference in SPT | ↑BDNF | Ren and Zuo, (2019), Mao et al., 2014, Wattanathorn et al., 2008 |
| ↑Spontaneous locomotor behavior | |||
| ↓Immobility time in FST | |||
| Quercetin | ↑Bodyweight gain | ↑BDNF in both the hippocampus and PFC | Ke et al. (2020) |
| ↑Saccharin preference index | |||
| ↓Immobility time in FST | |||
| Resveratrol | ↑Sucrose preference in SPT | ↑Peroxisome proliferator–activated receptor-γ coactivator | Abd El-Fattah et al., 2018, Smeding et al., 2012 |
| 1α abundance and function | |||
| BDNF in both the hippocampus and PFC | |||
| Tetrandrine | ↓Immobility time in TST and FST | ↑BDNF | Gao et al. (2013) |
FST, forced swimming test; TST, tail suspension test; SPT, sucrose preference test; OFT, open field test; BBT, beam balance test; GDNF, glial cell-derived neurotrophic factor; BDNF, brain-derived neurotrophic factor; VEGF, vascular endothelial growth factor; NGF, nerve growth factor; TrkB, tyrosine kinase receptor B; cAMP, cyclic adenosine monophosphate; CREB, cAMP-response element binding protein; NLRP3, NOD-like receptor thermal protein domain associated protein 3; COX-2, cyclooxygenase-2; PGE2, prostaglandin E2; GDNF, PKA, protein kinase A; CASP-11, caspase-11; GSDMD, pore-forming protein gasdermin D; PFC, prefrontal cortex.
In conclusion, it suggests that antidepressant-like effects of phytochemicals may be mediated, at least in part, by enhanced neurotrophic factors produced in the brain. Phytochemicals targeting neurotrophic factors are the potential to be profoundly developed and used in the future. Research into the biochemical and pharmacological effects of these bioactive constituents may uncover novel treatments for psychiatric illness or yield fresh insights into basic disease mechanisms.
4 Conclusion and perspectives
Depression is one of the most serious health challenges that affect the quality and duration of life substantially and disastrously. In terms of the therapeutic efficacy of depression, the limitations of traditional antidepressants remain notable. For example, a significant portion of patients with depression is prone to recurrence or unresponsive to various antidepressants (Daly et al., 2019). Additionally, it delays several weeks for 5-HT reuptake inhibitors, the mainstream antidepressants, to take action. Nevertheless, innovative therapeutics are still rare, owing in part to the difficulty of uncovering the underlying biological mechanisms of depression. As a result, the development of identifying novel therapeutic targets for depression is urgently required.
The expression and levels of BDNF, GDNF, VEGF, and NGF appear to be differentially altered in MDD patients compared to healthy persons, indicating that these molecules may constitute crucial roles in the pathophysiology of depression and antidepressant activity of treatment interventions. Coupled with new insights into the underlying mechanisms of depression, the rich abundance of chemical entities derived from herbs is proving to be an enticing resource in the search for effective therapy. Phytotherapy with a long history of useful applications is gaining popularity in pharmaceutical research. The active ingredients operating on multiple neurotrophic factors have been identified and extensively evaluated for therapeutic efficacies. Phytochemical components are more broadly available, tolerable, and presumably possess fewer negative effects in comparison to synthetic pharmaceutical medications, making them especially appealing for further exploitation and characterization for potential application in depression. Although animal research has yielded a plethora of candidates for phytotherapy, only a limited number of these compounds have made it into clinical trials. It is necessary to perform clinical trials to establish the therapeutic potential and validate the efficacy and safety of natural antidepressants.
Funding Statement
This work was supported by the National Natural Science Foundation of China (82130109, 81773924), Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (2021-I2M-1-020), Natural Science Foundation of Hunan (2021JJ30512), Natural Science Foundation of Changsha (kq2014091), and General Projects of Education Department of Hunan Province (No 19C1406), The Hunan University of Chinese Medicine First-class Disciple Construction Project of Chinese Material Medica (No 201803).
Author contributions
HW and NC designed this review. HW wrote the first draft of the manuscript; YY and GP participated in writing; ZW and NC supervised and revised the draft. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abd El-Fattah A. A., Fahim A. T., Sadik N. A. H., Ali B. M. (2018). Resveratrol and dimethyl fumarate ameliorate depression-like behaviour in a rat model of chronic unpredictable mild stress. Brain Res. 1701, 227–236. 10.1016/j.brainres.2018.09.027 [DOI] [PubMed] [Google Scholar]
- Ahn S. M., Kim H. N., Kim Y. R., Choi Y. W., Kim C. M., Shin H. K., et al. (2016). Emodin from Polygonum multiflorum ameliorates oxidative toxicity in HT22 cells and deficits in photothrombotic ischemia. J. Ethnopharmacol. 188, 13–20. 10.1016/j.jep.2016.04.058 [DOI] [PubMed] [Google Scholar]
- Allen N. J., Lyons D. A. (2018). Glia as architects of central nervous system formation and function. Sci. (80-. ) 362, 181–185. 10.1126/science.aat0473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini-Khoei H., Nasiri Boroujeni S., Maghsoudi F., Rahimi-Madiseh M., Bijad E., Moradi M., et al. (2022). Possible involvement of l-arginine-nitric oxide pathway in the antidepressant activity of Auraptene in mice. Behav. Brain Funct. 18, 4–9. 10.1186/s12993-022-00189-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte R. S., Chen D. S., Ferrara N. (2019). VEGF in signaling and disease: Beyond discovery and development. Cell 176, 1248–1264. 10.1016/j.cell.2019.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arosio B., Guerini F. R., Voshaar R. C. O., Aprahamian I. (2021). Blood brain-derived neurotrophic factor (BDNF) and major depression: Do we have a translational perspective? Front. Behav. Neurosci. Feb 15, 626906. 10.3389/fnbeh.2021.626906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal Y., Singh R., Saroj P., Sodhi R. K., Kuhad A. (2018). Naringenin protects against oxido-inflammatory aberrations and altered tryptophan metabolism in olfactory bulbectomized-mice model of depression. Toxicol. Appl. Pharmacol. 355, 257–268. 10.1016/j.taap.2018.07.010 [DOI] [PubMed] [Google Scholar]
- Bhattarai P., Cosacak M. I., Mashkaryan V., Demir S., Popova S. D., Govindarajan N., et al. (2020). Neuron-glia interaction through Serotonin-BDNF-NGFR axis enables regenerative neurogenesis in Alzheimer’s model of adult zebrafish brain. PLoS Biol. 18, e3000585–e3000627. 10.1371/journal.pbio.3000585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonafina A., Trinchero M. F., Ríos A. S., Bekinschtein P., Schinder A. F., Paratcha G., et al. (2019). GDNF and GFRα1 are required for proper integration of adult-born hippocampal neurons. Cell Rep. 29, 4308–4319.e4. 10.1016/j.celrep.2019.11.100 [DOI] [PubMed] [Google Scholar]
- Cao L., Jiao X., Zuzga D. S., Liu Y., Fong D. M., Young D., et al. (2004). VEGF links hippocampal activity with neurogenesis, learning and memory. Nat. Genet. 36, 827–835. 10.1038/ng1395 [DOI] [PubMed] [Google Scholar]
- Castillo M. F. R., Cohen A., Edberg D., Hoppensteadt D., Fareed J., Martin B., et al. (2020). Vascular endothelial growth factor in bipolar depression: A potential biomarker for diagnosis and treatment outcome prediction. Psychiatry Res. 284, 112781. 10.1016/j.psychres.2020.112781 [DOI] [PubMed] [Google Scholar]
- Castreń E. (2013). Neuronal network plasticity and recovery from depression. JAMA Psychiatry 70, 983–989. 10.1001/jamapsychiatry.2013.1 [DOI] [PubMed] [Google Scholar]
- Cheon Y. H., Lee S. G., Kim M., Kim H. O., Sun Suh Y., Park K. S., et al. (2018). The association of disease activity, pro-inflammatory cytokines, and neurotrophic factors with depression in patients with rheumatoid arthritis. Brain. Behav. Immun. 73, 274–281. 10.1016/j.bbi.2018.05.012 [DOI] [PubMed] [Google Scholar]
- Cirulli F., Francia N., Branchi I., Antonucci M. T., Aloe L., Suomi S. J., et al. (2009). Changes in plasma levels of BDNF and NGF reveal a gender-selective vulnerability to early adversity in rhesus macaques. Psychoneuroendocrinology 34, 172–180. 10.1016/j.psyneuen.2008.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R. O., Martins L. F., Tahiri E., Duarte C. B. (2022). Brain-derived neurotrophic factor-induced regulation of RNA metabolism in neuronal development and synaptic plasticity. Wiley Interdiscip. Rev. RNA 13, e1713. 10.1002/wrna.1713 [DOI] [PubMed] [Google Scholar]
- Couteur D. G. L., Samantha M., Solon-Biet B. L. P., Pulpite T., Brandon A. E., Hunt N. J., et al. (2021). Nutritional reprogramming of mouse liver proteome is dampened by metformin, resveratrol, and rapamycin. Cell Metab. 33, 2367–2379.e4. 10.1016/j.cmet.2021.10.016 [DOI] [PubMed] [Google Scholar]
- Daly E. J., Trivedi M. H., Janik A., Li H., Zhang Y., Li X., et al. (2019). Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry 76, 893–903. 10.1001/jamapsychiatry.2019.1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nicoló S., Tarani L., Ceccanti M., Maldini M., Natella F., Vania A., et al. (2013). Effects of olive polyphenols administration on nerve growth factor and brain-derived neurotrophic factor in the mouse brain. Nutrition 29, 681–687. 10.1016/j.nut.2012.11.007 [DOI] [PubMed] [Google Scholar]
- De Rossi P., Harde E., Dupuis J. P., Martin L., Chounlamountri N., Bardin M., et al. (2016). A critical role for VEGF and VEGFR2 in NMDA receptor synaptic function and fear-related behavior. Mol. Psychiatry 21, 1768–1780. 10.1038/mp.2015.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deltheil T., Tanaka K., Reperant C., Hen R., David D. J., Gardier A. M. (2009). Synergistic neurochemical and behavioural effects of acute intrahippocampal injection of brain-derived neurotrophic factor and antidepressants in adult mice. Int. J. Neuropsychopharmacol. 12, 905–915. 10.1017/S1461145709000017 [DOI] [PubMed] [Google Scholar]
- Deppmann C. D., Mihalas S., Sharma N., Lonze B. E., Niebur E., Ginty D. D. (2008). A model for neuronal competition during development. Sci. (80-. ) 320, 369–373. 10.1126/science.1152677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Preez A., Onorato D., Eiben I., Musaelyan K., Egeland M., Zunszain P. A., et al. (2021). Chronic stress followed by social isolation promotes depressive-like behaviour, alters microglial and astrocyte biology and reduces hippocampal neurogenesis in male mice. Brain. Behav. Immun. 91, 24–47. 10.1016/j.bbi.2020.07.015 [DOI] [PubMed] [Google Scholar]
- Eraky S. M., El-Kashef D. H., El-Sherbiny M., El-Magd N. F. A. (2023). Naringenin mitigates thioacetamide-induced hepatic encephalopathy in rats: Targeting the JNK/Bax/caspase-8 apoptotic pathway. Food Funct. 14, 1248–1258. 10.1039/d2fo03470k [DOI] [PubMed] [Google Scholar]
- Fajemiroye J. O., Galdino P. M., Florentino I. F., Da Rocha F. F., Ghedini P. C., Polepally P. R., et al. (2014). Plurality of anxiety and depression alteration mechanism by oleanolic acid. J. Psychopharmacol. 28, 923–934. 10.1177/0269881114536789 [DOI] [PubMed] [Google Scholar]
- Fan C., Li Y., Lan T., Wang W., Mao X., Yu S. Y. (2021). Prophylactic treatment of curcumin in a rat model of depression by attenuating hippocampal synaptic loss. Food Funct. 12, 11202–11213. 10.1039/d1fo02676c [DOI] [PubMed] [Google Scholar]
- Filho C. B., Jesse C. R., Donato F., Del Fabbro L., Gomes de Gomes M., Rossito Goes A. T., et al. (2016). Chrysin promotes attenuation of depressive-like behavior and hippocampal dysfunction resulting from olfactory bulbectomy in mice. Chem. Biol. Interact. 260, 154–162. 10.1016/j.cbi.2016.11.005 [DOI] [PubMed] [Google Scholar]
- Fukumoto K., Fogaca M. V., Liu R. J., Duman C., Kato T., Li X. Y., et al. (2019). Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)-hydroxynorketamine. Proc. Natl. Acad. Sci. U. S. A. 116, 297–302. 10.1073/pnas.1814709116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y., Hara R. I., Nakaya M., Okuyama S., Sawamoto A., Nakajima M. (2020). Citrus auraptene induces glial cell line-derived neurotrophic factor in C6 cells. Int. J. Mol. Sci. 21, 253. 10.3390/ijms21010253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli L., Vozza L., Gabbiadini A., Vanella A., Concas I., Tinacci S., et al. (2020). Curcumin for depression: A meta-analysis. Crit. Rev. Food Sci. Nutr. 60, 2643–2653. 10.1080/10408398.2019.1653260 [DOI] [PubMed] [Google Scholar]
- Gadad B. S., Vargas-Medrano J., Ramos E. I., Najera K., Fagan M., Forero A., et al. (2021). Altered levels of interleukins and neurotrophic growth factors in mood disorders and suicidality: An analysis from periphery to central nervous system. Transl. Psychiatry 11, 341. 10.1038/s41398-021-01452-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C., Wu M., Du Q., Deng J., Shen J. (2022). Naringin mediates adult hippocampal neurogenesis for antidepression via activating CREB signaling. Front. Cell Dev. Biol. 10, 731831. 10.3389/fcell.2022.731831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S., Cui Y. L., Yu C. Q., Wang Q. S., Zhang Y. (2013). Tetrandrine exerts antidepressant-like effects in animal models: Role of brain-derived neurotrophic factor. Behav. Brain Res. 238, 79–85. 10.1016/j.bbr.2012.10.015 [DOI] [PubMed] [Google Scholar]
- Guilloux J. P., Douillard-Guilloux G., Kota R., Wang X., Gardier A. M., Martinowich K., et al. (2012). Molecular evidence for BDNF-and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol. Psychiatry 17, 1130–1142. 10.1038/mp.2011.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer M. M., Barde Y. A. (1988). Brain-derived neurotrophic factor prevents neuronal death in vivo . Nature 331, 261–262. 10.1038/331261a0 [DOI] [PubMed] [Google Scholar]
- Irie Y., Itokazu N., Anjiki N., Ishige A., Watanabe K., Keung W. M. (2004). Eugenol exhibits antidepressant-like activity in mice and induces expression of metallothionein-III in the hippocampus. Brain Res. 1011, 243–246. 10.1016/j.brainres.2004.03.040 [DOI] [PubMed] [Google Scholar]
- Isung J., Mobarrez F., Nordström P., Åsberg M., Jokinen J. (2012). Low plasma vascular endothelial growth factor (VEGF) associated with completed suicide. World J. Biol. Psychiatry 13, 468–473. 10.3109/15622975.2011.624549 [DOI] [PubMed] [Google Scholar]
- Järvelä J. T., Lopez-Picon F. R., Plysjuk A., Ruohonen S., Holopainen I. E. (2011). Temporal profiles of age-dependent changes in cytokine mRNA expression and glial cell activation after status epilepticus in postnatal rat hippocampus. J. Neuroinflammation 8, 29–12. 10.1186/1742-2094-8-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B., Xiong Z., Yang J., Wang W., Wang Y., Hu Z. L., et al. (2012). Antidepressant-like effects of ginsenoside Rg1 are due to activation of the BDNF signalling pathway and neurogenesis in the hippocampus. Br. J. Pharmacol. 166, 1872–1887. 10.1111/j.1476-5381.2012.01902.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N., Zhang Y., Yao C., Huang H., Wang Q., Huang S., et al. (2022). Ginsenosides Rb1 attenuates chronic social defeat stress-induced depressive behavior via regulation of SIRT1-NLRP3/nrf2 pathways. Front. Nutr. 9, 868833. 10.3389/fnut.2022.868833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K., Zhu Y., Sun Y., Mao X. O., Xie L., Greenberg D. A. (2002). Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo . Proc. Natl. Acad. Sci. U. S. A. 99, 11946–11950. 10.1073/pnas.182296499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F., Bondolfi G., Gervasoni N., Schwald M., Aubry J. M., Bertschy G. (2005). Low Brain-Derived Neurotrophic Factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol. Psychiatry 57, 1068–1072. 10.1016/j.biopsych.2005.01.008 [DOI] [PubMed] [Google Scholar]
- Ke F., Li H. R., Chen X. X., Gao X. R., Huang L. L., Du A. Q., et al. (2020). Quercetin alleviates LPS-induced depression-like behavior in rats via regulating BDNF-related imbalance of copine 6 and TREM1/2 in the hippocampus and PFC. Front. Pharmacol. 10, 1544. 10.3389/fphar.2019.01544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby E. D., Kuwahara A. A., Messer R. L., Wyss-Coray T. (2015). Adult hippocampal neural stem and progenitor cells regulate the neurogenic niche by secreting VEGF. Proc. Natl. Acad. Sci. U. S. A. 112, 4128–4133. 10.1073/pnas.1422448112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie G., Mandel M., Hazan S., Barliya T., Blank M., Grunbaum A., et al. (2005). Anti-angiogenic activities of hypericin in vivo: Potential for ophthalmologic applications. Angiogenesis 8, 35–42. 10.1007/s10456-005-3828-3 [DOI] [PubMed] [Google Scholar]
- Lee B. H., Kim Y. K. (2012). Increased plasma VEGF levels in major depressive or manic episodes in patients with mood disorders. J. Affect. Disord. 136, 181–184. 10.1016/j.jad.2011.07.021 [DOI] [PubMed] [Google Scholar]
- Leibrock J., Lottspeich F., Hohn A., Hofer M., Hengerer B., Masiakowski P., et al. (1989). Molecular cloning and expression of brain-derived neurotrophic factor. Nature 341, 149–152. 10.1038/341149a0 [DOI] [PubMed] [Google Scholar]
- Leung D. W., Cachianes G., Kuang W., Goeddel D. V., Ferrara N. (1989). Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246, 1306–1309. 10.1126/science.2479986 [DOI] [PubMed] [Google Scholar]
- Leung L. B., Chu K., Rose D., Stockdale S., Post E. P., Wells K. B., et al. (2022). Electronic population-based depression detection and management through universal screening in the veterans health administration. JAMA Netw. Open 5, e221875. 10.1001/jamanetworkopen.2022.1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. (1968). Nerve growth factor. Physiol. Rev. 48, 534–569. 10.1152/physrev.1968.48.3.534 [DOI] [PubMed] [Google Scholar]
- Li C. F., Chen S. M., Chen X. M., Mu R. H., Wang S. S., Geng D., et al. (2016). ERK-dependent brain-derived neurotrophic factor regulation by hesperidin in mice exposed to chronic mild stress. Brain Res. Bull. 124, 40–47. 10.1016/j.brainresbull.2016.03.016 [DOI] [PubMed] [Google Scholar]
- Li J., He P., Zhang J., Li N. (2021). Orcinol glucoside improves the depressive-like behaviors of perimenopausal depression mice through modulating activity of hypothalamic–pituitary–adrenal/ovary axis and activating BDNF- TrkB-CREB signaling pathway. Phyther. Res. 35, 5795–5807. 10.1002/ptr.7237 [DOI] [PubMed] [Google Scholar]
- Liang W., Ge S., Yang L., Yang M., Ye Z., Yan M., et al. (2010). Ginsenosides Rb1 and Rg1 promote proliferation and expression of neurotrophic factors in primary Schwann cell cultures. Brain Res. 1357, 19–25. 10.1016/j.brainres.2010.07.091 [DOI] [PubMed] [Google Scholar]
- Lin L. F. H., Doherty D. H., Lile J. D., Bektesh S., Collins F. (1993). Gdnf: A glial cell line - derived neurotrophic factor for midbrain dopaminergic neurons. Sci. (80-. ) 260, 1130–1132. 10.1126/science.8493557 [DOI] [PubMed] [Google Scholar]
- Liu D., Zhang Q., Gu J., Wang X., Xie K., Xian X., et al. (2014). Resveratrol prevents impaired cognition induced by chronic unpredictable mild stress in rats. Prog. Neuro-Psychopharmacology Biol. Psychiatry 49, 21–29. 10.1016/j.pnpbp.2013.10.017 [DOI] [PubMed] [Google Scholar]
- Liu H.-T., Lin Y.-N., Tsai M.-C., Wu Y.-C., Lee M.-C. (2022). Baicalein exerts therapeutic effects against endotoxin-induced depression-like behavior in mice by decreasing inflammatory cytokines and increasing brain-derived neurotrophic factor levels. Antioxidants (Basel) 11, 947. 10.3390/antiox11050947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Liu X. L., Li J., Mu R. H., Liu Q., Yi L. T., et al. (2015). Macranthol promotes hippocampal neuronal proliferation in mice via BDNF-TrkB-PI3K/Akt signaling pathway. Eur. J. Pharmacol. 762, 357–363. 10.1016/j.ejphar.2015.05.036 [DOI] [PubMed] [Google Scholar]
- Ma G., Zhang J., Yang X., Guo P., Hou X., Fan Y., et al. (2020). TMEM16A-encoded anoctamin 1 inhibition contributes to chrysin-induced coronary relaxation. Biomed. Pharmacother. 131, 110766. 10.1016/j.biopha.2020.110766 [DOI] [PubMed] [Google Scholar]
- Maheu M., Lopez J. P., Crapper L., Davoli M. A., Turecki G., Mechawar N. (2015). MicroRNA regulation of central glial cell line-derived neurotrophic factor (GDNF) signalling in depression. Transl. Psychiatry 5, e511. 10.1038/tp.2015.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi G. S., Mann J. J. (2018). Depression. Lancet 392, 2299–2312. 10.1016/S0140-6736(18)31948-2 [DOI] [PubMed] [Google Scholar]
- Mao Q. Q., Huang Z., Zhong X. M., Xian Y. F., Ip S. P. (2014). Brain-derived neurotrophic factor signalling mediates the antidepressant-like effect of piperine in chronically stressed mice. Behav. Brain Res. 261, 140–145. 10.1016/j.bbr.2013.12.020 [DOI] [PubMed] [Google Scholar]
- McGeary J. E., Gurel V., Knopik V. S., Spaulding J., McMichael J. (2011). Effects of nerve growth factor (NGF), fluoxetine, and amitriptyline on gene expression profiles in rat brain. Neuropeptides 45, 317–322. 10.1016/j.npep.2011.06.002 [DOI] [PubMed] [Google Scholar]
- Meng X., Qian X., Ding X., Wang W., Yin X., Zhuang G., et al. (2022). Eosinophils regulate intra-adipose axonal plasticity. Proc. Natl. Acad. Sci. U. S. A. 119, e2112281119. 10.1073/pnas.2112281119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezhlumyan A. G., Tallerova A. V., Povarnina P. Y., Tarasiuk A. V., Sazonova N. M., Tatiana A Gudasheva S. B. S., et al. (2022). Antidepressant-like effects of BDNF and NGF individual loop dipeptide mimetics depend on the signal transmission patterns associated with trk. Pharm 15, 284. 10.3390/ph15030284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel T. M., Frangou S., Camara S., Thiemeyer D., Jecel J., Tatschner T., et al. (2008). Altered glial cell line-derived neurotrophic factor (GDNF) concentrations in the brain of patients with depressive disorder: A comparative post-mortem study. Eur. Psychiatry 23, 413–420. 10.1016/j.eurpsy.2008.06.001 [DOI] [PubMed] [Google Scholar]
- Mohammadi A., Rashidi E., Amooeian V. G. (2018). Brain, blood, cerebrospinal fluid, and serum biomarkers in schizophrenia. Psychiatry Res. 265, 25–38. 10.1016/j.psychres.2018.04.036 [DOI] [PubMed] [Google Scholar]
- Nerurkar L., Siebert S., McInnes I. B., Cavanagh J. (2019). Rheumatoid arthritis and depression: An inflammatory perspective. Lancet Psychiatry 6, 164–173. 10.1016/S2215-0366(18)30255-4 [DOI] [PubMed] [Google Scholar]
- Norte M. C. B., Cosentino R. M., Lazarini C. A. (2005). Effects of methyl-eugenol administration on behavioral models related to depression and anxiety, in rats. Phytomedicine 12, 294–298. 10.1016/j.phymed.2003.12.007 [DOI] [PubMed] [Google Scholar]
- Pochwat B., Szewczyk B., Kotarska K., Rafało-Ulińska A., Siwiec M., Sowa J. E., et al. (2018). Hyperforin potentiates antidepressant-like activity of lanicemine in mice. Front. Mol. Neurosci. 11, 456. 10.3389/fnmol.2018.00456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimundo V. D., Carvalho R. P. R., Machado-Neves M., Marques-da-Silva E. de A. (2022). Effects of terpenes in the treatment of visceral leishmaniasis: A systematic review of preclinical evidence. Pharmacol. Res. 177, 106117. 10.1016/j.phrs.2022.106117 [DOI] [PubMed] [Google Scholar]
- Rantamäki T., Hendolin P., Kankaanpää A., Mijatovic J., Piepponen P., Domenici E., et al. (2007). Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology 32, 2152–2162. 10.1038/sj.npp.1301345 [DOI] [PubMed] [Google Scholar]
- Ren T., Zuo Z. (2019). Role of piperine in CNS diseases: Pharmacodynamics, pharmacokinetics and drug interactions. Expert Opin. Drug Metab. Toxicol. 15, 849–867. 10.1080/17425255.2019.1672658 [DOI] [PubMed] [Google Scholar]
- Riccio A., Pierchala B. A., Ciarallo C. L., Ginty D. D. (1997). An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Sci. (80- 277, 1097–1100. 10.1126/science.277.5329.1097 [DOI] [PubMed] [Google Scholar]
- Rong W., Wang J., Liu X., Jiang L., Wei F., Hu X., et al. (2012). Naringin treatment improves functional recovery by increasing BDNF and VEGF expression, inhibiting neuronal apoptosis after spinal cord injury. Neurochem. Res. 37, 1615–1623. 10.1007/s11064-012-0756-7 [DOI] [PubMed] [Google Scholar]
- Sabella D. (2018). Antidepressant medications. Am. J. Nurs. 118, 52–59. 10.1097/01.NAJ.0000544978.56301.f6 [DOI] [PubMed] [Google Scholar]
- Sanmukhani J., Satodia V., Trivedi J., Patel T., Tiwari D., Panchal B., et al. (2014). Efficacy and safety of curcumin in major depressive disorder: A randomized controlled trial. Phyther. Res. 28, 579–585. 10.1002/ptr.5025 [DOI] [PubMed] [Google Scholar]
- Sene A., Chin-Yee D., Apte R. S. (2015). Seeing through VEGF: Innate and adaptive immunity in pathological angiogenesis in the eye. Trends Mol. Med. 21, 43–51. 10.1016/j.molmed.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Kumari S., Sharma J., Purohit R., Singh D. (2021). Hesperidin interacts with CREB-BDNF signaling pathway to suppress pentylenetetrazole-induced convulsions in zebrafish. Front. Pharmacol. Pharmacol. 11, 607797. 10.3389/fphar.2020.607797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeding L., Leong-Poi H., Hu P., Shan Y., Haitsma J. J., Horvath E., et al. (2012). Salutary effect of resveratrol on sepsis-induced myocardial depression. Crit. Care Med. 40, 1896–1907. 10.1097/CCM.0b013e31824e1370 [DOI] [PubMed] [Google Scholar]
- Song M., Martinowich K., Lee F. S. (2017). BDNF at the synapse: Why location matters. Mol. Psychiatry 22, 1370–1375. 10.1038/mp.2017.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwalska A., Sobieska M., Rybakowski J. K. (2010). Serum brain-derived neurotrophic factor in euthymic bipolar patients on prophylactic lithium therapy. Neuropsychobiology 62, 229–234. 10.1159/000319949 [DOI] [PubMed] [Google Scholar]
- Tang C.-X., Chen J., Shao K.-Q., Liu Y.-H., Zhou X.-Y., Ma C.-C., et al. (2023). Blunt dopamine transmission due to decreased GDNF in the PFC evokes cognitive impairment in Parkinson’s disease. Neural Regen. Res. 18, 1107–1117. 10.4103/1673-5374.355816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M., Chen M., Li Q. (2021). Paeoniflorin ameliorates chronic stress-induced depression-like behavior in mice model by affecting ERK1/2 pathway. Bioengineered 12, 11329–11341. 10.1080/21655979.2021.2003676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W., Hu Y., Chen Z., Dai Y., Hu Y., Qi M. (2021). Magnolol attenuates depressive-like behaviors by polarizing microglia towards the M2 phenotype through the regulation of Nrf2/HO-1/NLRP3 signaling pathway. Phytomedicine 91, 153692. 10.1016/j.phymed.2021.153692 [DOI] [PubMed] [Google Scholar]
- Thoenen H. (1995). Neurotrophins and neuronal plasticity. Sci. (80- 270, 593–598. 10.1126/science.270.5236.593 [DOI] [PubMed] [Google Scholar]
- Thong P. S. P., Watt F., Ren M. Q., Tan P. H., Soo K. C., Olivo M. (2006). Hypericin-photodynamic therapy (PDT) using an alternative treatment regime suitable for multi-fraction PDT. J. Photochem. Photobiol. B Biol. 82, 1–8. 10.1016/j.jphotobiol.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Tian D. D., Wang M., Liu A., Gao M. R., Qiu C., Yu W., et al. (2021). Antidepressant effect of paeoniflorin is through inhibiting pyroptosis CASP-11/GSDMD pathway. Mol. Neurobiol. 58, 761–776. 10.1007/s12035-020-02144-5 [DOI] [PubMed] [Google Scholar]
- Vargish G. A., Pelkey K. A., Yuan X., Chittajallu R., Collins D., Fang C., et al. (2017). Co-activation of VEGF and NMDA receptors promotes synaptic targeting of AMPA receptors. Mol. Psychiatry 22, 1. 10.1038/mp.2016.245 [DOI] [PubMed] [Google Scholar]
- Viswanatha G. L., Shylaja H., Keni R., Krishnadas Nandakumar S. R., Rajesh S. (2022). A systematic review and meta-analysis on the cardio-protective activity of naringin based on pre-clinical evidences. Phytother. Res. 36, 1064–1092. 10.1002/ptr.7368 [DOI] [PubMed] [Google Scholar]
- Wang C. S., Kavalali E. T., Monteggia L. M. (2022). BDNF signaling in context: From synaptic regulation to psychiatric disorders. Cell 185, 62–76. 10.1016/j.cell.2021.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. Q., Wang Z. Z., Chen N. H. (2021a). The receptor hypothesis and the pathogenesis of depression: Genetic bases and biological correlates. Pharmacol. Res. 167, 105542. 10.1016/j.phrs.2021.105542 [DOI] [PubMed] [Google Scholar]
- Wang H. Q., Yang S. W., Gao Y., Liu Y. J., Li X., Ai Q. D., et al. (2021b). Novel antidepressant mechanism of ginsenoside Rg1: Regulating biosynthesis and degradation of connexin43. J. Ethnopharmacol. 278, 114212. 10.1016/j.jep.2021.114212 [DOI] [PubMed] [Google Scholar]
- Wang H., Yang Y., Yang S., Ren S., Feng J., Liu Y., et al. (2021c). Ginsenoside Rg1 ameliorates neuroinflammation via suppression of Connexin43 ubiquitination to attenuate depression. Front. Pharmacol. 12, 709019. 10.3389/fphar.2021.709019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. M., Yang L. H., Zhang Y. Y., Niu C. L., Cui Y., Feng W. S., et al. (2015). BDNF and COX-2 participate in anti-depressive mechanisms of catalpol in rats undergoing chronic unpredictable mild stress. Physiol. Behav. 151, 360–368. 10.1016/j.physbeh.2015.08.008 [DOI] [PubMed] [Google Scholar]
- Wang X., Hou Z., Yuan Y., Hou G., Liu Y., Li H., et al. (2011). Association study between plasma GDNF and cognitive function in late-onset depression. J. Affect. Disord. 132, 418–421. 10.1016/j.jad.2011.03.043 [DOI] [PubMed] [Google Scholar]
- Wang Y. L., Wu H. R., Zhang S. S., Xiao H. L., Yu J., Ma Y. Y., et al. (2021d). Catalpol ameliorates depressive-like behaviors in CUMS mice via oxidative stress-mediated NLRP3 inflammasome and neuroinflammation. Transl. Psychiatry 11, 353. 10.1038/s41398-021-01468-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattanathorn J., Chonpathompikunlert P., Muchimapura S., Priprem A., Tankamnerdthai O. (2008). Piperine, the potential functional food for mood and cognitive disorders. Food Chem. Toxicol. 46, 3106–3110. 10.1016/j.fct.2008.06.014 [DOI] [PubMed] [Google Scholar]
- Weng L., Dong S., Wang S., Yi L., Geng D. (2019). Macranthol attenuates lipopolysaccharide-induced depressive-like behaviors by inhibiting neuroinflammation in prefrontal cortex. Physiol. Behav. 204, 33–40. 10.1016/j.physbeh.2019.02.010 [DOI] [PubMed] [Google Scholar]
- Wiener C. D., De Mello Ferreira S., Pedrotti Moreira F., Bittencourt G., De Oliveira J. F., Lopez Molina M., et al. (2015). Serum levels of nerve growth factor (NGF) in patients with major depression disorder and suicide risk. J. Affect. Disord. 184, 245–248. 10.1016/j.jad.2015.05.067 [DOI] [PubMed] [Google Scholar]
- Wook Koo J., Labonté B., Engmann O., Calipari E. S., Juarez B., Lorsch Z., et al. (2016). Essential role of mesolimbic brain-derived neurotrophic factor in chronic social stress–induced depressive behaviors. Biol. Psychiatry 80, 469–478. 10.1016/j.biopsych.2015.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G., Xiong Z., Yong Y., Wang Z., Ke Z., Xia Z., et al. (2010). Catalpol attenuates MPTP induced neuronal degeneration of nigral-striatal dopaminergic pathway in mice through elevating glial cell derived neurotrophic factor in striatum. Neuroscience 167, 174–184. 10.1016/j.neuroscience.2010.01.048 [DOI] [PubMed] [Google Scholar]
- Yan Z., Rein B. (2022). Mechanisms of synaptic transmission dysregulation in the prefrontal cortex: Pathophysiological implications. Mol. Psychiatry 27, 445–465. 10.1038/s41380-021-01092-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Feng L., Zheng F., Yang F., Wu C. P., Shen L., et al. (2001). GDNF acutely modulates excitability and A-type K+ channels in midbrain dopaminergic neurons. Nat. Neurosci. 4, 1071–1078. 10.1038/nn734 [DOI] [PubMed] [Google Scholar]
- Ye D., Zhang L., Fan W., Zhang X., Dong E. (2018). Genipin normalizes depression-like behavior induced by prenatal stress through inhibiting DNMT1. Epigenetics 13, 310–317. 10.1080/15592294.2018.1450033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai X. J., Chen F., Chen C., Zhu C. R., Lu Y. N. (2015). LC-MS/MS based studies on the anti-depressant effect of hypericin in the chronic unpredictable mild stress rat model. J. Ethnopharmacol. 169, 363–369. 10.1016/j.jep.2015.04.053 [DOI] [PubMed] [Google Scholar]
- Zhang H. L., Bragatti J. A., Torres C. M., Nuernberg G. L., Rieder C. R. (2011). Decreased serum BDNF levels in patients with epileptic and psychogenic nonepileptic seizures. Neurology 76, 1772-1773. 10.1212/WNL.0b013e318219a086 [DOI] [PubMed] [Google Scholar]
- Zhang J.-H., Yang H.-Z., Su H., Song J., Bai Y., Deng L., et al. (2021a). Berberine and ginsenoside Rb1 ameliorate depression-like behavior in diabetic rats. Am. J. Chin. Med. 49, 1195–1213. 10.1142/S0192415X21500579 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Li Z. H., Li Y. Y., Shi S. J., Zhou S. W., Fu Y. Y., et al. (2015). Hypericin-photodynamic therapy induces human umbilical vein endothelial cell apoptosis. Sci. Rep. 5, 18398. 10.1038/srep18398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Yang C., Chu J., Ning L.-N., Zeng P., Wang X.-M., et al. (2021b). Emodin prevented depression in chronic unpredicted mild stress-exposed rats by targeting miR-139-5p/5-lipoxygenase. Front. Cell Dev. Biol. 9, 696619. 10.3389/fcell.2021.696619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhang Z., Sha W., Xie C., Xi G., Zhou H., et al. (2010). Effect of treatment on serum glial cell line-derived neurotrophic factor in bipolar patients. J. Affect. Disord. 126, 326–329. 10.1016/j.jad.2010.03.003 [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhang Z., Sha W., Xie C., Xi G., Zhou H., et al. (2009). Electroconvulsive therapy increases glial cell-line derived neurotrophic factor (GDNF) serum levels in patients with drug-resistant depression. Psychiatry Res. 170, 273–275. 10.1016/j.psychres.2009.01.011 [DOI] [PubMed] [Google Scholar]
- Zhao X., Kong D., Zhou Q., Wei G., Song J., Liang Y., et al. (2021). Baicalein alleviates depression-like behavior in rotenone-induced Parkinson’s disease model in mice through activating the BDNF/TrkB/CREB pathway. Biomed. Pharmacother. 140, 111556. 10.1016/j.biopha.2021.111556 [DOI] [PubMed] [Google Scholar]
- Zhu X., Liu H., Liu Y., Chen Y., Liu Y., Yin X. (2020). The antidepressant-like effects of hesperidin in streptozotocin‐induced diabetic rats by activating Nrf2/ARE/glyoxalase 1 pathway. Front. Pharmacol. 11, 1325. 10.3389/fphar.2020.01325 [DOI] [PMC free article] [PubMed] [Google Scholar]


