Abstract
The COVID-19 pandemic has accelerated the deployment of telehealth services in many countries around the world. It also revealed many barriers and challenges to the use of digital health technologies in health organisations and systems that have persisted for decades. One of these barriers is what is known as the ‘wrong pocket’ problem – where an organisation or sector makes expenditures and investments to address a given problem, but the benefits (return on investment) are captured by another organisation or sector (the wrong pocket). This problem is the origin of many difficulties in public policies and programmes (e.g. education, environment, justice and public health), especially in terms of sustainability and scaling-up of technology and innovation. In this essay/perspective, we address the wrong pocket problem in the context of a major telehealth project in Canada. We show how the problem of sharing investments and expenses, as well as the redistribution of economies among the different stakeholders involved, may have threatened the sustainability and scaling-up of this project, even though it has demonstrated the clinical utility and contributed to improving the health of populations. In conclusion, the wrong pocket problem may be decisive in the reduced take-up, and potential failure, of certain telehealth programmes and policies. It is not enough for a telehealth service to be clinically relevant and ‘efficient’, it must also be mutually beneficial to the various stakeholders involved, particularly in terms of the equitable sharing of costs and benefits (return on investment) associated with the implementation of this new service model. Finally, the wrong pocket concept offers a helpful lens for studying the success, sustainability, and scale-up of digital transformations in health organisations and systems. This needs to be considered in future research and evaluations in the field.
Keywords: Wrong pocket, telehealth, digital health, telemedicine, health systems, health organisations, sustainability, scaling-up, business model, evaluation
Introduction
The ‘wrong pocket’ phenomenon refers to a situation where an organisation or sector makes expenditures and investments to address a given problem, but the benefits (i.e. the return on that investment) are captured by another organisation or sector. 1 With a stakeholder incurring costs but not gaining at least some of the value generated, it is likely that they will then underinvest, not invest or even disinvest. 1 The wrong pocket is a potentially major barrier to convincing stakeholders to assume costs to improve healthcare (Box 1). 2 It is also a major barrier to interorganisational or intersectoral collaboration.1,2 This phenomenon is widely acknowledged in healthcare. To date, it has received limited attention in the scientific literature and public policy.
Box 1.
Addressing social determinants of health – examples of the wrong pocket problem.
The following are two examples of potential investment in health promotion and non-medical preventive services that could address social determinants (e.g. education, housing and active ageing) and improve the health of the population,3,4 as well as avoid unnecessary medical care and the costs associated with it. 2
Falls prevention: more than 800,000 elderly people are hospitalised for fall injuries each year in the USA.1,2 This results in an annual cost to the healthcare system of more than US$ 50 billion. These accidents are mainly due to damaged carpets, irregular stair treads or bathrooms not adapted to the needs of the elderly.1,2 Improving and securing these environments would help to avoid these accidents, thus saving the costs of hospitalisations. However, it is not in the interest of the building owners to spend money on such improvements, as the healthcare system will recover the savings.
Smoking cessation campaigns: these are financed by public health departments or municipalities, with the intention of reducing smoking and capturing health benefits. However, part of the benefits (e.g. cancer and/or lung disease avoided) are captured by insurance companies/agencies (fewer medical costs) that have limited (if any) investment in such campaigns. 5
During the COVID-19 crisis, telehealth has been an important lever in policies and actions aimed at improving access and continuity in healthcare and services (including social, preventive and curative services). Telehealth involves health and care services being ‘delivered remotely by means of a telecommunication, including audiovisual exchanges for information, education and research, and treatment of clinical and administrative data purposes’. 6 The pandemic also highlighted the inherent complexity of integrating telehealth into health organisations and systems.7–9 Even pre-pandemic, telehealth was mainly viewed as a ‘plague of pilots where innovations fail to become part of routine practice because of limited funding or inability to scale to broader sectors of the health care system’. 10 Reasons for this include the persistence of socio-political and financial barriers to moving from a successful pilot project to a widespread routine service (e.g. recurrent and adequate funding, appropriate regulatory and governance frameworks for virtual clinical networks).10–14
In this ‘essay/perspective’, we discuss the wrong pocket problem in relation to telehealth. Drawing on the experience of a large telehealth network in Quebec (Canada), evaluated by our team (see Alami et al. 14 for full details of the evaluation approach, methods and results). We show that the wrong pocket problem was a source of significant difficulties experienced by the network regarding the sustainability and integration of telehealth as a routine service. To the best of our knowledge, this is the first time that the wrong pocket problem has been articulated in relation to telehealth.
Introducing the Eastern Quebec Telepathology Network
The province of Quebec (Canada) is characterised by a vast territory (1,667,441 km²) and an uneven geographic distribution of its population. Rural and remote areas face major problems in accessing healthcare and services, including in relation to anatomopathology services. 15 At the time of our evaluation (2016–2018), these problems intensified in Eastern Quebec (1.7 million inhabitants, over 410,000 km2) where approximately 61% of pathologist physicians were concentrated in the ‘Capitale Nationale’ (Quebec City). The other 39% were unevenly distributed over the rest of the territory. This situation came about due to: (1) problems with the recruitment and retention of pathologists in the regions; (2) difficult working conditions for pathologists who struggle to ensure continuity of services due to a lack of replacements during vacations and/or difficult travel in winter; (3) several pathologists being close to retirement and will not be automatically replaced due to lack of succession; and (4) overspecialisation of anatomopathology with a risk of underutilisation of cutting-edge expertise makings it difficult to practice in small localities.14,16–18 All of the above meant that it was challenging to recruit and retain surgeons and other clinicians who require anatomopathology services.
The Eastern Quebec Telepathology Network (EQTN) was formed with the explicit aim of addressing the above issues. Telepathology is a specific area of telehealth, defined as ‘the electronic transmission of pathological images, usually derived from microscopes, from one location to another, for the purpose of interpretation and diagnosis’. 19 The objective of the EQTN was to install a viable and efficient solution that would, not only avoid disruptions in anatomopathology services in the regions, but also expand the range of services offered, mainly in oncology (see Têtu et al. 20 and Alami et al. 14 for more details on the functioning of the EQTN).
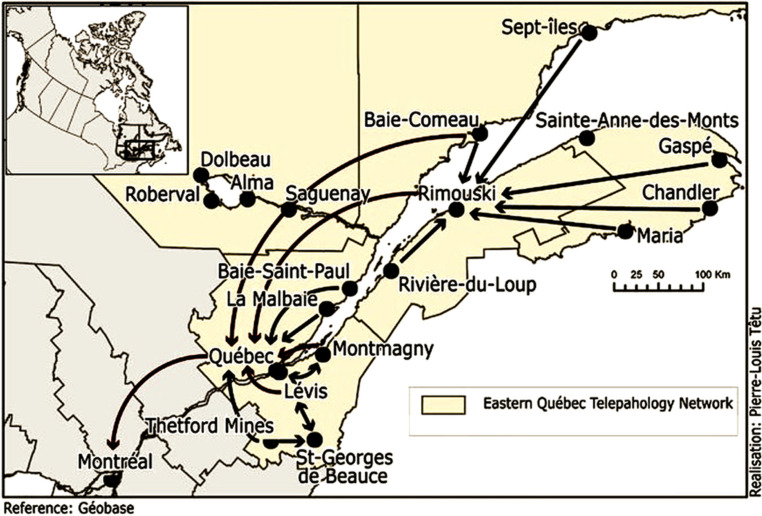
The EQTN initially had 22 hospitals operating as a virtual clinical network, with one University Hospital in Quebec City and at least two regional hospitals responsible for providing telepathology services to small rural and remote hospitals. This number varied over time as participating hospitals joined or withdrew, for example, due to clinicians declining to participate in the network, tensions over funding, technological and organisational interoperability problems, or fear of losing local expertise (Figure 1). The Network was jointly funded by the Quebec Ministry of Health and Social Services (known as MSSS) and Canada Health Infoway (a federal agency) with a non-recurring budget of more than CA$6.2 million. This funding was mainly for the purchase of telepathology equipment and other related technologies (e.g. scanners and slide digitisers, digital pathology software, adaptation and harmonisation of anatomopathology techniques). 14
Figure 1.
The territory covered by the Eastern Quebec Telepathology Network (EQTN), with examples of interactions between the different participating organisations. The arrows indicate the sites of origin and destination of the requests. 20
The EQTN and the wrong pocket problem
Initial and recurring expenditures and investments
The 22 hospitals initially participating in EQTN varied in their technological readiness and maturity to adequately connect to the network. Some needed to upgrade their technological systems and infrastructure and recruit technical staff. Since funders only financed telepathology equipment and related technologies, organisations had to cover the remaining costs from their own operating budgets (e.g. updating or replacing local electronic records and other ad hoc technologies, interoperability with other organisations’ systems, upgrades and maintenance of telepathology technologies once installed, recruitment of necessary human resources). These costs were significant for small rural hospitals that were known to have outdated technological systems and infrastructure and limited on-site technical expertise. In contrast, urban hospitals had more modern systems and infrastructure plus technical expertise and did not therefore incur costs of the same magnitude.
Savings, return on investment and/or losses
The EQTN quickly demonstrated the potential of telepathology to generate considerable savings. This included avoiding patient transfers to urban hospitals and over long distances (in some cases over 600 km), two-stage surgeries, pathologist travel from urban to rural/remote hospitals, and the costs of sending pathology specimens to urban hospitals. This was combined with savings related to the early diagnosis and management of certain cancers, where long waits for anatomopathology services had previously risked diagnostic and/or surgical delays. 14
It was apparent early on that these costs and benefits would not be shared equitably among participating organisations, thereby jeopardising the sustainability of the EQTN. Any savings generated from the use of telepathology were mainly captured by the MSSS and the Quebec Health Insurance Agency (known as RAMQ). Both organisations reimburse and fund healthcare and services, including the costs of transferring patients and their families' travel expenses, and the costs and incentives (potentially exceeding 30% of salary) for physicians to travel to rural and remote hospitals. Urban hospitals are partially paid for the services provided by their pathologists via telepathology. However, part of the financing of Quebec hospitals is dependent on the number of services provided by physicians. With the delivery of telepathology services, this meant that small hospitals receiving telepathology services were not paid, in part because of the absence of ‘double billing’. This is despite their being responsible for costs associated with physically caring for patients on site (i.e. those patients who would normally be transferred to the urban hospital). This resulted in a double benefit to the urban hospitals, as they were paid for telepathology services without having to pay for the physical management of patients. In the words of one manager of a small rural hospital: ‘It's nice to not transfer the patient, but then we are stuck with him/her in our hospital’. 14
Telepathology undoubtedly brings benefits, allowing hospitals to avoid transferring patients. However, in Eastern Quebec at least, this relatively new service model also implies that small hospitals should take care of increasingly complex patients locally. In addition, in the case of intraoperative consultations (i.e. when a pathologist accompanies a surgeon during a surgery), telepathology makes it possible to extend the time of use of the operating room. However, small hospitals do not always have the infrastructure and human resources to do this, as they usually transfer patients to specialised urban hospitals. Extending the time for use of the operating room entails additional costs, mainly due to extended clinical time. This brought additional pressures due to the lack of clinical staff, with local teams under pressure (e.g. nurses and pathology technologists) and at risk of exacerbating their working conditions (e.g. mandatory overtime). In a context of increasing burnout among healthcare providers,21,22 this heightened the risks of resignation and difficulties of recruitment and retention in rural and remote areas. As one clinician stated: ‘If I leave tomorrow morning, the patients would not have the service. It's very difficult to take a vacation. I'm managing a significant portion of the complex cases (…). I'm starting to feel tired’.
In some small hospitals, it appears that telepathology may have encouraged the departure of certain pathologists who were working on-site. According to one pathologist: ‘Telepathology was an encouraging and catalytic element in my leaving the (…) [rural hospital]. For me, by joining (…) [an urban hospital], there was the possibility of covering all the regions through this regional hub where telepathology would have a role to play’.14
Some pathologists also left small hospitals where they were working for fear of using telepathology and being forced to provide the service to other hospitals. These departures were sometimes considered to have contributed, directly or indirectly, to the closure of anatomopathology and medical biology laboratories in small hospitals, as well as difficulties in recruiting and retaining surgeons and other clinicians who require anatomopathology services and a pathologist on site. This situation had raised concerns that small hospitals might become dependent on the goodwill of urban hospitals, and face difficulties with medium- and long-term service planning. These were justified concerns because some urban hospitals required their pathologists to give priority to any in-house request for services (both urgent and non-urgent), before accepting requests for services from other hospitals. With this balance of power to their advantage, certain urban hospitals required other incentives to agree to treat external service requests at the same level of priority as internal requests.
In our evaluation of the EQTN, the fears of small hospitals were well-founded, as at least one urban hospital had clearly expressed its desire to become a regional reference centre for telepathology. 14 Such a status allows them to increase their volume of activity (and therefore the funding they receive from the MSSS and RAMQ) and to attract more clinicians, including pathologists.
In sum, for some smaller hospitals, telepathology has been associated with financial losses and exacerbated difficulties in recruiting and retaining clinical staff.
Discussion and conclusion
We have shown that, in the EQTN context, the wrong pocket problem has significantly impacted small rural and remote hospitals. While these sites incurred considerable expenses to be able to use the technology effectively, the subsequent return on investment was not always there. Most of the savings and benefits from the telepathology service were captured by MSSS and RAMQ and the urban hospitals that provided services. There is also a potential knock-on effect of the wrong pocket problem with increased expenditure and service re-design bringing challenges for recruiting and/or retaining clinicians and a deterioration of working conditions.
Our analysis of the EQTN experience highlights that improving services for the population alone is not sufficient to ensure the commitment of organisations, especially when their own survival is threatened by the adoption of innovation. This is in line with the wider literature, for instance, Lehoux et al. 23 reporting that: ‘The technology was designed as if the patient's best interest was a sufficient incentive for hospitals and (…) providers to work collaboratively and thus, together ‘buy in’ the new technology. This proved very hard to achieve in practice’. The EQTN mirrors many public programmes and policies that are shaped by quantitative and cost-effective measures, where organisations are conceptualised as entrepreneurs seeking to maximise their own profits. 24 In a context where the government (e.g. MSSS and RAMQ) is more focused on cost reduction and savings alone, organisations tended to prioritise their objectives and serve their performance indicators. The objective of improving services for the population was undermined as soon as some organisations adopted a logic of competition, not cooperation, in response to the requirements of the funding mechanism. This then limited the ability of organisations or sectors to collaborate beyond their core services. 25 Through this lens, the systemic objectives of the EQTN were in conflict with the local objectives and priorities of some of the participating organisations. This reinforces what we already know about the difficulty of balancing national (MSSS level) vision and priorities with local organisational needs and contexts/factors, and the impact on successful digital health transformations. 26 As Cresswell et al. 26 pointed out, this is ‘likely to lead to disillusionment among those involved as health-care organisations might become frustrated with their local needs not being met and policy-makers might become frustrated by mandated targets not being met’.
The literature has previously reported that the implementation of new telehealth services may be more costly for small health organisations. 27 They have limited capacity to absorb the costs of infrastructure, new workflows, logistics, as well as the training needed to ensure clinician adoption of new practices. 27 We might think of this as a ‘digital divide’ between small and large organisations. For some organisations, the opportunity cost was high enough to dissuade them from joining the EQTN or cause them to leave afterwards. This serves as a reminder that the implementation of a project, no matter how clinically relevant, is doomed if one stakeholder assumes a portion of the initial, or recurring, costs without being able to capture the offsetting benefits. 28 According to Katz et al., 24 the requirements of funding mechanisms play an important role when it comes to whether a programme could go forward (or not). This is particularly the case in public funding structures where costs and benefits are consumed so that one entity (e.g. organisation, sector and department) bears the costs and another reaps the benefits. 28
Digital transformations are nested within a broader socio-political environment and local contexts that should adapt and evolve to ensure the successful integration of innovation. 29 Socio-political and financial conditions are expected to evolve to reflect the nature of innovation, especially to enable the alignment of health system goals with organisational goals. 10 It is then possible to build a clear vision of what is expected of a project, while being able to manage and resolve conflicts and tensions that may emerge from competing practices and/or objectives, and which may threaten the sustainability of the change. 10 Through this lens, remuneration for telepathology services should equitably benefit the organisations requesting the services, not least to offset the costs of physical patient care. In practice, however, this is clearly not the case. Returning to the example of the EQTN, to ensure the sustainability of the network smaller hospitals called for any savings realised, mainly by the MSSS and the RAMQ, to be redistributed to the participating organisations. The idea is that resources could be reinvested in the modernisation of technology systems and infrastructure, as well as in the recruitment and retention of on-site personnel. This proposition was not seriously considered by the MSSS, largely due to the province undergoing major austerity reforms at the time.
As observed in the case of the EQTN, the wrong pocket problem could be decisive in the success or failure of certain innovations, even those with a real added value for the population. A handful of other studies have shown similar results.30–32 For instance, Kelley et al. 30 reported that one of the barriers to implementing sustainable digital health business models in health systems is that the benefits/savings accrue at the system level (e.g. avoiding transfers and travel, improved patient health outcomes), without being reflected in organisational performance outcomes. This issue should be further explored in future research, especially to be able to identify the business models best suited to the specificity of digital transformations in health organisations and systems. The wrong pocket concept offers a lens of interest for studying the success and sustainability issues of digital transformations in health organisations and systems. To date, very little work has explicitly addressed this in digital health, although the problem of funding and reimbursement is now recognised as one of the major challenges to the success and sustainability of digital health initiatives.11,13,30–34 This is surprising given that these initiatives involve a diversity of stakeholders and levels of governance with objectives, interests, visions, and expectations that may be divergent or even antagonistic. In this regard, with some hindsight from our previous work on digital health, we can state that the wrong pocket problem was a significant factor in the difficulties, and even failures, that some projects have faced.6–8,13,31,35,36 In some cases, it appeared as critical to success as technological (e.g. interoperability and confidentiality), clinical (e.g. clinical appropriateness and safety), or organisational (e.g. clinical-administrative flows) issues. Our hope is that this paper will build on this work and serve as a starting point for future research, policy and practice discussions oriented toward addressing the wrong pocket problem.
To begin, the literature from other public policy areas (e.g. education, environment, justice, public health) offers avenues of interest for overcoming the wrong pocket problem, which could be applied to the EQTN and other digital health initiatives.1,37,38 Paraphrasing McCullough, 38 there is no magic bullet to address the wrong pocket in all contexts and programmes, but there are several strategies and avenues that could inform more appropriate funding models for digital health and enable collaboration across a diversity of organisations. These innovative funding structures should encourage cross-organisational engagement and avoid additional inequities among stakeholders, including ensuring a fair redistribution of benefits for impact. 25 For example, breaking down budget and funding silos between participating organisations, and between some government agencies that are involved in financing healthcare: ‘common budgeting models (pocket alignment)’.38,39 As in the case of the EQTN, it would have been more appropriate for funding and cost/benefit to adopting a ‘network organisation’ perspective, rather than the historical logic of outcomes and performance by facility. 28 Such a cultural change requires a relationship of trust that could capitalise on the idea of managing common resources and sharing results in order to maximise collective value. According to Nichols et al., 40 ‘the investment is worth undertaking if the collective value is greater than the total cost of [the global investment] at scale’. In the same vein as McCullough, 38 if one of the obstacles to addressing the wrong pocket problem is the lack of political will or the reluctance of actors who benefit from the current situation, this is a clear opportunity to produce further evidence that alternative financing models (e.g. value-based financing and common budgeting models) contribute to the success and sustainability of digital health projects and programmes.
Acknowledgements
The views expressed are those of the authors and not necessarily those of their organisations or funding agencies.
Footnotes
Contributorship: HA produced the first draft of this manuscript and received input from SES, JPF, MS, RF, and BT. All authors read and approved the final manuscript.
Data availability statement: All data is from public articles and documents. Sources are cited in the references. The authors are available to answer any reasonable request related to the article's content if necessary.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval and consent to participate: Not applicable.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: HA was supported by the In Fieri research programme (Prof. Pascale Lehoux - Montreal University, Canada L.). SES was supported by the NIHR-funded Remote by Default 2 study (ES/V010069/1), and a BRC/ARC Fellowship focused on sustainable health care.
Guarantor: HA.
ORCID iD: Hassane Alami https://orcid.org/0000-0002-5461-7693
References
- 1.Butler SM.How “wrong pockets” hurt health. JAMA Health Forum 2018, https://jamanetwork.com/channels/health-forum/fullarticle/2760141 (2018, accessed 24 October 2022). [Google Scholar]
- 2.Butler S.An antidote to the “wrong pockets” problem?The Brookings Institution. , https://pfs.urban.org/pay-success/pfs-perspectives/antidote-wrong-pockets-problem (2018, accessed 24 October 2022).
- 3.McGinnis JM, Williams-Russo P, Knickman JR.The case for more active policy attention to health promotion. Health Aff 2002; 21: 78–93. [DOI] [PubMed] [Google Scholar]
- 4.Braveman P, Gottlieb L.The social determinants of health: It's time to consider the causes of the causes. Public Health Rep 2014; 129: 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gondi S, Chokshi DA.Public health and payers—bridging the gap to boost public health investment. JAMA Health Forum 2022; 3: e222750-e. [DOI] [PubMed] [Google Scholar]
- 6.Alami H, Gagnon M-P, Fortin J-P.Some multidimensional unintended consequences of telehealth utilization: A multi-project evaluation synthesis. Int J Health Policy Manag 2019; 8: 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alami H, Lehoux P, Attieh R, et al. A “not so quiet” revolution: Systemic benefits and challenges of telehealth in the context of COVID-19 in Quebec (Canada). Front Digit Health 2021; 133: 1–13. [Google Scholar]
- 8.Shaw SE, Hughes G, Wherton J, et al. Achieving spread, scale up and sustainability of video consulting services during the COVID-19 pandemic? Findings from a comparative case study of policy implementation in England, Wales, Scotland and Northern Ireland. Front Digit Health 2021; 3: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenhalgh T, Ladds E, Hughes G, et al. Why do GPs rarely do video consultations? Qualitative study in UK general practice. Br J Gen Pract 2022; 72: e351–ee60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desveaux L, Soobiah C, Bhatia RS, et al. Identifying and overcoming policy-level barriers to the implementation of digital health innovation: Qualitative study. JMIR 2019; 21: e14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenhalgh T, Wherton J, Papoutsi C, et al. Beyond adoption: A new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. JMIR 2017; 19: e8775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenhalgh T, Rosen R, Shaw SE, et al. Planning and evaluating remote consultation services: A new conceptual framework incorporating complexity and practical ethics. Front Digit Health 2021; 103: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alami H, Lamothe L, Fortin J-P, et al. L’implantation de la télésanté et la pérennité de son utilisation au Canada: quelques leçons à retenir. Rech Eur Télém 2016; 5: 105–117. [Google Scholar]
- 14.Alami H, Fortin J-P, Gagnon M-P, et al. The challenges of a complex and innovative telehealth project: A qualitative evaluation of the Eastern Quebec Telepathology Network. Int J Health Policy Manag 2018; 7: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tetu B, Boulanger J, Houde C, et al. The Eastern Quebec Telepathology Network: A real collective project. Med Sci 2012; 28: 993–999. [DOI] [PubMed] [Google Scholar]
- 16.Bellis M, Metias S, Naugler C, et al. Digital pathology: Attitudes and practices in the Canadian pathology community. J Pathol Inf 2013; 4: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer J, Paré G, Trudel M-C, et al. Télémédecine et accessibilité aux soins de santé spécialisés en régions éloignées. Gestion. 2014; 39: 29–37. [Google Scholar]
- 18.Bernard C, Chandrakanth S, Cornell IS, et al. Guidelines from the Canadian Association of Pathologists for establishing a telepathology service for anatomic pathology using whole-slide imaging: The Canadian Association of Pathologists Telepathology Guidelines Committee. J Pathol Inf 2014; 5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowe J.Telepathology: Guidance from the Royal College of Pathologists. The Royal College of Pathologists, https://www.rcpath.org/uploads/assets/6b748863-cf1a-4693-970ab7a28fba11c4/telepathology-guidance-from-the-royal-college-of-pathologists.pdf (2013, accessed 24 October 2022).
- 20.Têtu B, Paré G, Trudel M-C, et al. Whole-slide imaging-based telepathology in geographically dispersed healthcare networks. The Eastern Québec Telepathology project. Diagn Histopathol 2014; 20: 462–469. [Google Scholar]
- 21.Bodenheimer T, Sinsky C.From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med 2014; 12: 573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alami H, Lehoux P, Gagnon M-P, et al. Rethinking the electronic health record through the quadruple aim: time to align its value with the health system. BMC Med Inf Decis Mak 2020; 20: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehoux P, Miller FA, Daudelin G, et al. Providing value to new health technology: the early contribution of entrepreneurs, investors, and regulatory agencies. Int J Health Policy Manag 2017; 6: 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz AS, Brisbois B, Zerger S, et al. Social impact bonds as a funding method for health and social programs: potential areas of concern. Am J Public Health 2018; 108: 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu PY, Beck AF, Lindau ST, et al. A framework for cross-sector partnerships to address childhood adversity and improve life course health. Pediatrics 2022; 149: e2021053509O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cresswell K, Sheikh A, Krasuska M, et al. Reconceptualising the digital maturity of health systems. Lancet Digit Health 2019; 1: e200–e2e1. [DOI] [PubMed] [Google Scholar]
- 27.Chang JE, Lai AY, Gupta A, et al. Rapid transition to telehealth and the digital divide: implications for primary care access and equity in a post-COVID era. Milbank Q 2021; 99: 340–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roman J.Solving the wrong pockets problem. Urban Institute, https://www.urban.org/sites/default/files/publication/71501/2000427-solving-the-wrong-pockets-problem_0.pdf (2015, accessed 24 October 2022).
- 29.Cripps M, Scarbrough H.Making digital health “solutions” sustainable in healthcare systems: a practitioner perspective. Front Digit Health 2022; 4: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelley LT, Fujioka J, Liang K, et al. Barriers to creating scalable business models for digital health innovation in public systems: Qualitative case study. JMIR Public Health Surveill 2020; 6: e20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alami H, Gagnon M-P, Wootton R, et al. Exploring factors associated with the uneven utilization of telemedicine in Norway: A mixed methods study. BMC Med Inf Decis Mak 2017; 17: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rakers MM, van Os HJ, Recourt K, et al. Perceived barriers and facilitators of structural reimbursement for remote patient monitoring, an exploratory qualitative study. Health Policy Technol 2023; 12: 100718. [Google Scholar]
- 33.de Brantes F, Rosenthal MB, Struijs J.Ensuring the adoption of the health care warranty: A well-defined model to resolve issues with risk and uncertainty. NEJM Catal Innov Care Deliv 2020; 1: 1–8. [Google Scholar]
- 34.van Limburg M, van Gemert-Pijnen JE, Nijland N, et al. Why business modeling is crucial in the development of eHealth technologies. JMIR 2011; 13: e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenhalgh T, Shaw S, Wherton J, et al. Real-world implementation of video outpatient consultations at macro, meso, and micro levels: Mixed-method study. JMIR 2018; 20:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw SE, Wherton J, Vijayaraghavan S, et al. Advantages and limitations of virtual online consultations in a NHS acute trust: The VOCAL mixed-methods study. Health Serv Del Res 2018; 6: 1–162. [PubMed] [Google Scholar]
- 37.Taylor L.Housing and health: An overview of the literature. Health Aff 2018, https://www.healthaffairs.org/do/10.1377/hpb20180313.396577/ (2018, accessed 24 October 2022). [Google Scholar]
- 38.McCullough JM.Declines in spending despite positive returns on investment: understanding public health's wrong pocket problem. Front Public Health 2019; 7: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao GJ, Lee CI, Liao JM.Right pockets, right solutions: Aligning investments to address breast cancer screening disparities. J Am Coll Radiol 2019; 16: 586–589. [DOI] [PubMed] [Google Scholar]
- 40.Nichols LM, Taylor LA.Social determinants as public goods: A new approach to financing key investments in healthy communities. Health Aff 2018; 37: 1223–1230. [DOI] [PubMed] [Google Scholar]