Abstract
Fungi can produce many compounds, such as proteins, enzymes, amino acids, and polysaccharides, which are internalised and enriched for metals, and are widely used as reducing and stabilising agents for the biosynthesis of gold nanoparticles (Au NPs). Almost all fungal sources used in the synthesis of the Au NPs are in the form of cell filtrates or mycelial suspensions. However, the culture of cell‐free fungal filtrate and mycelium is not comparable to the propagation of fungal substrates in input and operation. Here, we evaluated in vivo biosynthesis of Au NPs in enoki mushrooms (Flammulina velutipes). HAuCl4 was reduced in the fruiting body of the enoki mushrooms via induction by Pb2+, resulting in the generation of Au NPs. We then employed UV‐Vis absorption spectroscopy, Transmission Electron Microscope, and Energy Dispersive Spectrometer to characterise various shapes of the Au NPs. The elemental analysis indicated that the Au NPs were mainly concentrated in organelles of the stalk and cap cells. We also demonstrated that 0.3–0.5 mM HAuCl4 was the optimal stress treatment concentration based on the changes in physiological indicators of the enoki mushrooms. This work reveals that fungi can be utilised well as nanomaterial bioreactors.

HAuCl4 was reduced by fruiting body of the enoki mushrooms via induction by Pb2+, which resulted in production of Au NPs.
1. INTRODUCTION
Au NPs are frequently used in the domains of optics, electronics, and catalysis because of their distinct physical and chemical characteristics. The current standard physical and chemical synthesis methods are associated with high energy consumption, intense pollution, and slow speed [1]. As a result, there is a lot of interest in the development of green and sustainable synthesis processes. Due to their high tolerance and prolific enzyme production, microbial organisms like fungus have been the subject of several studies evaluating their biomineralisation mechanisms [2, 3, 4].
The synthesis of fungal nanomaterials is achieved because fungi can metabolically secrete many active substances, such as extracellular proteins, and that the synthesised nanomaterials can easily be separated from the organism. Previous researchers have reported the synthesis of Au NPs using Verticillium sp., Colletotrichum sp., Aspergillus niger, Helminthosporium solani, Trichoderma koningii, Rhizopus stolonifer, Penicillium sp., Epicoccum nigrum, Neurospora crassa and Cylindrocladium floridanum. The Au NPs are of various shapes, including spherical, rod‐shaped, triangular, pentagonal, star‐shaped regular nanoplates, helical nanoplates, layered nanoclusters agglomerates and spherical nano‐agglomerates, and range between 1 and 50 nm [5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16].
Almost all fungal resources synthesising Au NPs are in the form of cell filtrates or mycelial suspensions [17, 18]. Although it is theoretically simple to control the synthesis of extracellular Au NPs, the input and operation of the growth of cell‐free fungal filtrate and mycelium are not comparable to those of the propagation of fungal substrates. Inspired by the previous work on mycosynthesis of Au NPs using the extract of Flammulina velutipes [19], we exploited the super heavy metal enrichment ability of the fruiting body of enoki mushrooms to allow direct reduction of HAuCl4 for in vivo biosynthesis of Au NPs. In addition, we explored the optimised conditions under Pb2+ induction, characterised the Au NPs particle size, morphology, and surface state, and investigated changes in physiological indicators of enoki mushrooms. The yield of Au NPs obtained from enoki mushrooms has not yet achieved the acceptable level; however, in terms of the properties of as‐prepared Au NPs, it can be employed in a manner quite similar to the method of obtaining Au NPs using fungal extracts.
2. MATERIALS AND METHODS
2.1. Optimisation of growth conditions of the fruiting body of enoki mushrooms
Enoki mushroom roots were cleaned three times with distilled water before being blotted on filter paper. A petri dish with a diameter of 10 cm was filled with two filter sheets, and roots weighing 25 g and 2 cm in length were planted on the filter papers. The roots were wrapped in plastic and incubated for 15 days at various temperatures: 15, 20, 25, and 30℃. Periodically, the substrate root's length was measured.
2.2. Biosynthesis of Au NPs
The fungi culture is carried out in a culture room with fungal culture shelves, temperature control devices, and ventilation equipment. The culture temperature was 25℃ in the early stages to enhance the germination of the mycelium, 20℃ in the middle, and 15℃ in the late stages. The mycelium was rejuvenated to maturation. Culture humidity was kept at 60%, with a CO2 concentration of 3000–4000 ml/L, which is optimal for mycelium growth.
After the mycelium grew to the full size of the vial, 7–8 mm of the ageing mycelium was removed and promptly replenished with water. The culture temperature was kept at 15℃, with a relative humidity of 90%–95%, and a CO2 concentration of 2000–3000 ml/L. The vials were sprayed with water twice a day and placed under 100% light after 3 days. When the mycelium grew to the formative stage, they underwent treatment with HAuCl4 solution (0.01, 0.05, 0.1, 0.3, 0.5, 1 mM HAuCl4 solution) with or without 0.1 mmol/L Pb2+ and then incubated for 10 days.
2.3. Extraction of Au NPs
The Enoki mushroom substrates grown and enriched with Au NPs were placed in a mortar. Liquid nitrogen and Phosphate Buffered Saline buffer were added, and then the substrates were fully ground. The ground homogenate was centrifuged at 8000 rpm for 20 min, and then the supernatant was placed in a refrigerator (4℃) for analysis.
3. RESULTS AND DISCUSSION
3.1. Analysis of growth conditions for enoki mushrooms
Although it has been shown that the temperature for the formation of enoki mushrooms was 5–19℃, with an optimum temperature of 13–14℃ [20], the growth temperature of enoki mushroom substrates also varies on the species. The results of the pre‐experiments showed that the enoki mushroom's growth hardly continued in conditions above 25℃ (Figures S1 and S2). The higher fresh weight of golden mushroom was obtained at 20℃ and 15℃ respectively. Eventually, the 15℃ culture condition was selected as a more suitable temperature for the growth of the enoki mushrooms.
3.2. UV‐vis spectral characterisation
The HAuCl4 concentrations we used were optimised and it was found that from the colour and absorption spectra of the enoki mushrooms extracts (Figures S3 and S4), the 0.3 and 0.5 mM HAuCl4 treatment groups were preferable, so what follows focuses on the former and the latter as a reference.
0.3 and 0.5 mM HAuCl4, with or without Pb2+ induction, had characteristic absorption peaks around 550 nm, with the synthesised Au NPs having subspherical irregular shapes, and other non‐spherical shapes based on the peaks (Figure 1) [21]. A range of concentrations of the HAuCl4 solution is reducible to synthesise Au NPs, as shown in Table 1.
FIGURE 1.

The UV‐vis of gold nanoparticles (Au NPs) synthesised under (a, c) 0.3 and 0.5 mM HAuCl4, (b, d) Pb2+ induced.
TABLE 1.
UV‐vis characteristic absorption peaks of gold nanoparticles (Au NPs) synthesised under different conditions
| Synthetic conditions | UV‐visible characteristic absorption peak (nm) | |
|---|---|---|
| HAuCl4 concentration (mM) | With (+) or without (−) Pb2+ induction | |
| 0.01 | − | 548 |
| + | 544 | |
| 0.05 | − | 550 |
| + | 545 | |
| 0.1 | − | 552 |
| + | 545 | |
| 0.3 | − | 550 |
| + | 542 | |
| 0.5 | − | 550 |
| + | 542 | |
| 1 | − | 550 |
| + | 542 | |
3.3. TEM characterisation
As shown in Figure 2a, near‐spherical and irregularly shaped Au NPs were synthesised under 0.3 mM HAuCl4, and the Energy Dispersive Spectrometer further demonstrated the formation of Au NPs. The results showed that their agglomeration was apparent, which was in sync with the UV‐vis spectra. The maximum particle size was 115.386 nm, an aggregate of Au NPs, while the minimum particle size was 17.871 nm and the average particle size was 50.43 nm. Figure 2b,d shows that Pb2+ synthesised subspherical, hexagonal Au NPs and large aggregates under 0.3 and 0.5 mM HAuCl4. In addition, Pb2+ regulated the formation and secretion of extracellular polymers (glyoxalate and nitrate reductase), which affected the assembly and growth process of the Au NPs, further affecting the particle size of the nanomaterials [22]. The morphology and particle size of the produced Au NPs are shown in Table 2.
FIGURE 2.

The Transmission Electron Microscope and Energy Dispersive Spectrometer of the gold nanoparticles (Au NPs) synthesised under (a) 0.3 mM HAuCl4, (b) 0.3 mM HAuCl4 induced by Pb2+, (c) 0.5 mM HAuCl4, (d) 0.5 mM HAuCl4 induced by Pb2+.
TABLE 2.
Morphology and particle aggregates size of the gold nanoparticles (Au NPs) synthesised under different conditions
| Synthetic conditions | Characteristics of Au NPs | ||||
|---|---|---|---|---|---|
| HAuCl4 concentration (mM) | With (+) or without (−) Pb2+ induction | Maximum particle size (nm) | Minimum particle size (nm) | Average particle size (nm) | Morphology |
| 0.3 | − | 115.386 | 17.871 | 50.43 | Irregularly shaped, subspherical |
| + | 536.122 | 11.616 | 321.516 | Subspherical, hexagonal, quadrilateral | |
| 0.5 | − | 71.775 | 12.346 | 38.884 | Irregularly shaped, subspherical |
| + | 154.087 | 3.659 | 41.323 | Irregularly shaped, subspherical | |
3.4. Zeta potential and FTIR characterisation
Due to the limitations of the purification process and reaction conditions, we have not yet been able to obtain Au NPs with excellent dispersion. Therefore, such a large value of positive charge for Zeta potential results is most likely due to the surface charge of the Au NPs aggregates (Table 3). Although we do not have access to the surface charge of individual Au NPs at present, it can be assumed from the extent of Au NPs aggregates in Transmission Electron Microscope that the exact value of the surface charge should be much less than +30 mV.
TABLE 3.
Zeta potential of gold nanoparticles aggregates under different conditions
| HAuCl4 concentration (mM) | With (+) or without (−) Pb2+ induction | Zeta potential (mV) |
|---|---|---|
| 0.3 | − | 478.7 |
| + | 639.2 | |
| 0.5 | − | 527.2 |
| + | 715.5 |
It was shown that the sugars contained in the enoki mushrooms (glucose, rhamnose, lactose, maltose, fructose, galactose, mannose, xylose, arabinose, and fucose) might be involved in the synthesis of the Au NPs [23]. This was corroborated by the characteristic absorption peak maps of the infrared spectra of the Au NPs. The IR absorption peaks at 1636.304 cm−1 were due to the stretching vibration of –C = C–, 2018.623, 2160.365 cm−1 were due to the stretching vibration of –C≡C–, while 3272.609, 3367.586 cm−1 were due to the stretching vibration of alcohols and phenols with hydroxy‐OH (Figure 3). These data indicated that –C = C–, –C≡C–, and –OH play a role in the synthesis of the Au NPs.
FIGURE 3.
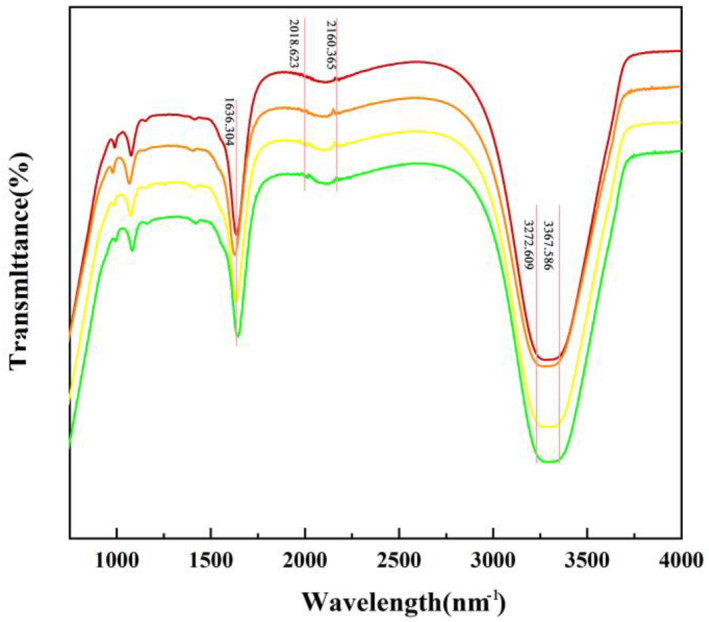
Fourier Transform infrared spectroscopy of the gold nanoparticles (Au NPs) synthesised under different conditions (a) 0.3 mM HAuCl4, (b) 0.3mM HAuCl4 induced by Pb2+, (c) 0.5 mM HAuCl4, (d) 0.5mM HAuCl4 induced by Pb2+.
In contrast to the manufacture of plant extracts, the Au NPs so generated are challenging to identify and purify from biological matrices [24], resulting in a weak signal for characterisation of properties. However, there is still a lot of application potential for this in situ synthesis. The body of the enoki mushroom held the produced Au NPs, allowing for easy control of the size and concentration of the metal nanoparticles immobilised on the biological matrix. This was accomplished by physically breaking the fungal body into tiny pieces. These metal nanoparticles are anticipated to serve as catalysts for catalytic reactions, similar to immobilised enzymes. This work indicates the viability of using fungi as nanomaterial bioreactors.
3.5. Distribution of Au NPs in the cotyledons of enoki mushrooms
As seen in Figure 4, the enoki mushrooms' colour gradually turned purple‐black, and the morphological change was not noticeable after 7 days. The substrates were now enriched with Au NPs while still being in a growing state.
FIGURE 4.

The process of synthesising ‘real gold’ mushrooms.
Under Au3+ single stress treatment, the Au content in the stalk was greater than that in the cap at low concentrations (0.01 mM). The difference in the Au content was not significant at 0.05–0.3 mM, and the Au content in the cap was significantly greater than that in the stalk at high concentrations (0.5–1 mM). Under Pb2+ induction, the difference in the Au content between the stalk and the cap of the ascomata was not significant at all concentrations, but only at 0.05 mM, where the Au content in the cap was significantly higher than that in the stalk (Figure 5).
FIGURE 5.

Effect of (a) HAuCl4 solution and (b) Pb2+ on Au content in different parts of fruiting body. The comparisons within groups are indicated by lowercase letters, with different letters indicating significant differences (p < 0.05).
Previous physiological and biochemical analysis showed that the detoxification mechanism of golden mushroom for Au3+ may be associated with converting highly toxic heavy metal ions into less toxic ones through intracellular redox and methylation [25, 26, 27]. Besides, it can be detoxified by metabolising specific proteins, such as metallothioneins, which directly interact with heavy metal ions through regionalisation [28, 29]. Au elements were found in the stalk and cap of the organelle and cell wall after they were subjected to Au3+ single stress treatment and Pb2+ induction (Figure 6). Similar to how plants detoxify heavy metals, this process uses cellular compartmentalisation [30, 31].
FIGURE 6.

Effects of HAuCl4 of (a) stipe and (b) cap and (c) stipe and (d) cap induced by Pb2+ on the content of Au in subcells. The comparisons within groups are indicated by lowercase letters, with different letters indicating significant differences (p < 0.05).
The stalk's cytoplasm displayed the typical absorption peaks at 550–570 nm at concentrations of 0.05, 0.5, and 1 mM HAuCl4. The stalk's cytoplasm displayed the typical absorption peaks at about 530–535 nm under Pb2+ induction, showing that small amounts of Au were present. In the cytoplasm of the cap and stalk, the presence or absence of Au NPs is indicated by the absence of a distinctive absorption peak in the range of 600 nm (Figure 7).
FIGURE 7.

UV‐vis absorption spectra of cytoplasmic extracts of fungal caps and stalks induced by different concentrations of HAuCl4 and Pb2+.
3.6. Changes in the antioxidant system of the enoki mushrooms
Under Au3+ single stress treatment, Figure 8 demonstrates a substantial overall pattern of ‘promotion followed by inhibition’ of catalase (CAT) activity, with 0.01 mM producing the maximum promotion and 0.3 mM exerting the maximum inhibition. Peroxidase (POD) activity was generally insignificantly promoted, and the maximum promotion was attained at 0.5 mM. However, Superoxide dismutase (SOD) activity was decreased, with the highest level of inhibition occurring at 0.3 mM. Malonic dialdehyde (MDA) content was generally not promoted, and the greatest promotion was attained at 0.05 mM. Previous research indicated that the HAuCl4 solution's foreign stress has an impact on the antioxidant enzyme system's activity, making the organism less able to withstand stress and increasing MDA [32]. The MDA level rises with an increase in Au3+ stress concentration, harming the organism. Furthermore, stress from the environment may increase the organism's resilience, reducing the MDA content [33]. It has been demonstrated that Chlorella pyrenoidosa's MDA concentration keeps rising in order to withstand heavy metal Zn2+ stress. A drop in the MDA level occurs as a result of cellular damage that occurs after a period of stress, which prevents the organism from continuing to fight the stress [34]. While low doses have little impact, high amounts of heavy metal stress enhance the MDA content in Halamphora veneta (Kützing) Levkov [35]. When enoki mushrooms were under heavy metal Au3+ stress, this was similar to the overall trend in the MDA level of the mushrooms.
FIGURE 8.

Effect of HAuCl4 solution on the antioxidant system (a) catalase (CAT); (b) peroxidase (POD); (c) superoxide dismutase (SOD) and (d) malonic dialdehyde (MDA) content of the enoki mushrooms. The comparisons within groups are indicated by lowercase letters, with different letters indicating significant differences (p < 0.05).
Studies have shown that heavy metal‐induced conditions such as Cd2+, Co2+, Pb2+, Cu2+, Hg2+, Bi3+, Fe3+, Mn2+, and Zn2+ can promote the synthesis of nanomaterials such as Se NPs [36]. For instance, Co2+ causes clustering of Au NPs synthesised by Trichoderma sp., Pb2+ and Sn2+ fuel the synthesis rate of Trichoderma sp., while Pb2+ induces synthesis of homogeneous nanorods with uniform morphology by Aspergillus sp. [37, 38]. However, adding Pb2+ triggers strong oxidative stress effects, while free radicals trigger thermal excitation effects and redox atmospheres [39]. In this study, under Pb2+ induction, there was an overall trend of ‘promotion followed by inhibition’ of CAT activity. The effect on the trend was more significant with increased Au3+ concentration, which saw a maximum promotion at 0.05 mM while 0.5 mM exerted maximum inhibition. The effect on POD activity showed a non‐significant ‘promotion followed by inhibition’ trend, and the degree of enhancement reached the maximum at 0.1 mM, while 0.5 mM had the maximum degree of inhibition. In addition, SOD activity was inhibited, and the maximum degree of inhibition was achieved at 0.3 mM. The overall ‘promotion followed by inhibition’ trend for the MDA content was not significant. The maximum promotion and inhibition were achieved at 0.05 and 1 mM respectively.
3.7. Effect of osmoregulatory substances in enoki mushrooms
Living things and biofilms are protected by osmoregulatory and nutritive components such soluble sugars, soluble proteins, and total sugars. They are crucial to understanding the overall metabolism of the plant body because their contents can predict whether an organism will experience heavy metal stress, drought, or salt resistance under environmental conditions [40]. There was a non‐significant trend towards inhibiting soluble protein content and total soluble sugar content under Au3+ single stress treatment settings, with the maximum promotion reported at 0.01 mM and inhibition at 0.1 and 0.5 mM. Additionally, there was a propensity to increase the concentration of total sugar, with the biggest increase occurring at 1 mM (Figure 9).
FIGURE 9.

Effect of HAuCl4 solution on osmotic substances (a) soluble protein; (b) soluble sugar; (c) total sugar of enoki mushrooms.
Under Pb2+ induction, there was an overall but non‐significant promotion of soluble protein content and effect of soluble sugar content, with 0.5 mM yielding the most significant promoting effect, and 0.1 mM exerting most significant inhibitory effect. Besides, there was overall enhancement of total sugar concentration, with the highest promoting effect observed at 0.01 mM while the best inhibitory effect was achieved at 0.5 mM. It was presumed that with the changes in the activity of various enzymes in the cells, the rate of metabolism of substances in the organism slows down, leading to their elevated levels [41]. Overall, Pb2+ induction did not exert any significant difference on the soluble protein content, soluble sugar content, or total sugar concentration.
3.8. Scavenging of DPPH· and ·OH in the enoki mushrooms
Our results demonstrated that the diphenyl picryl hydrazinyl radical (DPPH·) clearance rate was significantly increased under Au3+ single stress treatment. The maximum DPPH· clearance rate (76.39%) was achieved at 1 mM (Figure 10a). Under Pb2+ induction, DPPH· clearance showed an ‘increase followed by inhibition’ trend, with 0.3 mM yielding the highest DPPH· clearance (79.68%), while the lowest DPPH· clearance (32.09%) was achieved at 1 mM. Longitudinally, this data demonstrated that Pb2+‐induction was associated with higher DPPH‐clearance compared to Au3+, at the same concentration (0.1–0.3 mM), while DPPH· clearance was higher for Au3+ under other conditions.
FIGURE 10.

Effect of HAuCl4 solution on (a) DPPH· scavenging ability and (b) ·OH scavenging ability of enoki mushrooms. The comparisons within groups are indicated by lowercase letters, with different letters indicating significant differences (p < 0.05).
The ·OH scavenging rate was significantly higher under Au3+ single stress treatment. The ·OH scavenging rate was as high as 97.86% at 0.5 mM. Under Pb2+‐induced conditions, the ·OH scavenging rate was significantly higher in all cases, and the highest scavenging rate (86.58%) was achieved at 0.05 mM. Longitudinally, our data showed that at low concentrations (0.01–0.3 mM), Pb2+ induced higher ·OH clearance compared to Au3+, whereas, at high concentrations (0.5–1 mM), the ·OH clearance was higher for Au3+ (Figure 10b). This is related to the substrates' structure and type of antioxidants [42].
3.9. Change of active oxygen (O2 −) content in enoki mushrooms
Au3+ single stress treatment exerted an inhibitory effect on O2 − production rate and a facilitative effect on H2O2 content. The inhibitory effect on O2 − production rate reached the maximum at 0.1 mM, while the H2O2 content reached the maximum at 0.1 mM (Figure 11). Pb2+ induction had an inhibitory effect on the O2 − production rate and a facilitative effect on the H2O2 content. The inhibitory effect on the O2 − production rate reached the maximum at 0.3 mM, while the H2O2 content reached the maximum at 0.05 mM. Longitudinally, our data showed that the O2 − production rate was higher under Pb2+ induction than Au3+ at 0.1 mM, while Pb2+ induction showed lower performance at other concentrations of Au3+. Besides, Pb2+ induction led to higher H2O2 content than Au3+ at the same concentration. This outcome was attributed to the disruption of the intracellular membrane structure due to HAuCl4 solution stress, which alters the structure and type of antioxidants in the organism, leading to differences in the scavenging capacity of ·OH as well as the H2O2 and O2 − content [43].
FIGURE 11.

Effects of HAuCl4 solution on (a) superoxide anion and (b) hydrogen peroxide content in enoki mushrooms. The comparisons within groups are indicated by lowercase letters, with different letters indicating significant differences (p < 0.05).
4. CONCLUSION
By reducing Au3+ ions in the cotyledon cells of enoki mushrooms, we were able to create inorganic Au NPs in a safe and controlled manner. This process took advantage of the effective metal ion detoxification system that macrofungi have by nature. The Au NPs were reduced using –C=C, –CC, –OH, and other functional groups. The resulting particles were irregular hexagonal, tetragonal, subspherical, or subspherical aggregates with positively charged surfaces. They were primarily present in the stalk and cap of the Enoki mushrooms, with subcellular distribution in the organelles and cell walls but almost absent in the cytoplasm. The data showed an increase in soluble sugar content, total sugar concentration, DPPH· and ·OH scavenging capacity, and reactive oxygen species content, and there was antioxidant enzyme system activity in the enoki mushroom substrates.
AUTHOR CONTRIBUTIONS
Jingang Mo: Writing – original draft. Jun Jin: Data curation. Han Yu: Data curation. Mingjun Ai: Investigation. Die Hu: Methodology. Linlin Li: Methodology. Kai Song: Conceptualisation.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supporting Information S1
ACKNOWLEDGEMENTS
The authors appreciate the financial supports by the National Natural Science Foundation of China (31870486), the Natural Science Foundation of Jilin Province (YDZJ202101ZYTS092), the 17th batch of innovative entrepreneurial talent projects of Jilin Province (2021Y032) and the Natural Science Foundation of Changchun Normal University (2017017, KXK2020002).
Mo, J. , et al.: Biosynthesis of gold nanoparticles in the fruiting body of enoki mushrooms (Flammulina velutipes) under Pb2+ induction. IET Nanobiotechnol. 17(2), 61–68 (2023). 10.1049/nbt2.12104
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Jeevanandam, J. , et al.: Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J. Nanotechnol. 9, 1050–1074 (2018). 10.3762/bjnano.9.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kitching, M. , Ramani, M. , Marsili, E. : Fungal biosynthesis of gold nanoparticles: mechanism and scale up. Microb. Biotechnol. 8(6), 904–917 (2015). 10.1111/1751-7915.12151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siddiqi, K.S. , Husen, A. : Fabrication of metal nanoparticles from fungi and metal salts: scope and application. Nanoscale Res. Lett. 11(1), 98 (2016). 10.1186/s11671-016-1311-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prasad, R. , Pandey, R. , Barman, I. : Engineering tailored nanoparticles with microbes: quo vadis? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 8(2), 316–330 (2016). 10.1002/wnan.1363 [DOI] [PubMed] [Google Scholar]
- 5. Mukherjee, P. , et al.: Bioreduction of AuCl4 − ions by the fungus, Verticillium Sp. and surface trapping of the gold nanoparticles formed. Angew. Chem. Int. Ed. 11(1), 1–15 (2001) [DOI] [PubMed] [Google Scholar]
- 6. Shankar, S.S. , et al.: Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J. Mater. Chem. A. 13(7), 1822 (2003). 10.1039/b303808b [DOI] [Google Scholar]
- 7. Xie, J. , et al.: High‐yield synthesis of complex gold nanostructures in a fungal system. J. Phys. Chem. C. 111(45), 16858–16865 (2007). 10.1021/jp0752668 [DOI] [Google Scholar]
- 8. Dasaratrao Sawle, B. , et al.: Biosynthesis and stabilization of Au and Au‐Ag alloy nanoparticles by fungus, Fusarium semitectum . Sci. Technol. Adv. Mater. 9(3), 035012 (2008). 10.1088/1468-6996/9/3/035012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumar, S.A. , Peter, Y.A. , Nadeau, J.L. : Facile biosynthesis, separation and conjugation of gold nanoparticles to doxorubicin. Nanotechnology. 19(49), 495101 (2008). 10.1088/0957-4484/19/49/495101 [DOI] [PubMed] [Google Scholar]
- 10. Maliszewska, I. , Aniszkiewicz, L. , Sadowski, Z. : Biological synthesis of gold nanostructures using the extract of Trichoderma koningii . Acta Phys. Pol. A. 116(Supplement), S163–S165 (2009). 10.12693/aphyspola.116.s-163 [DOI] [Google Scholar]
- 11. Binupriya, A.R. , Sathishkumar, M. , Yun, S.I. : Biocrystallization of silver and gold ions by inactive cell filtrate of Rhizopus stolonifer . Colloids Surf. B Biointerfaces. 79(2), 531–534 (2010). 10.1016/j.colsurfb.2010.05.021 [DOI] [PubMed] [Google Scholar]
- 12. Du, L. , Xian, L. , Feng, J.‐X. : Rapid extra‐/intracellular biosynthesis of gold nanoparticles by the fungus Penicillium Sp. J. Nanopart. Res. 13(3), 921–930 (2010). 10.1007/s11051-010-0165-2 [DOI] [Google Scholar]
- 13. Sheikhloo, Z. , Salouti, M. , Katiraee, F. : Biological synthesis of gold nanoparticles by fungus Epicoccum nigrum . J. Clust. Sci. 22(4), 661–665 (2011). 10.1007/s10876-011-0412-4 [DOI] [Google Scholar]
- 14. Castro‐Longoria, E. , Vilchis‐Nestor, A.R. , Avalos‐Borja, M. : Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora crassa . Colloids Surf. B. 83(1), 42–48 (2011). 10.1016/j.colsurfb.2010.10.035 [DOI] [PubMed] [Google Scholar]
- 15. Mishra, A. , et al.: Microbial synthesis of gold nanoparticles using the fungus Penicillium brevicompactum and their cytotoxic effects against mouse Mayo blast cancer C2C12 cells. Appl. Microbiol. Biotechnol. 92(3), 617–630 (2011). 10.1007/s00253-011-3556-0 [DOI] [PubMed] [Google Scholar]
- 16. Narayanan, K.B. , Sakthivel, N. : Mycocrystallization of gold ions by the fungus Cylindrocladium floridanum . World J. Microbiol. Biotechnol. 29(11), 2207–2211 (2013). 10.1007/s11274-013-1379-0 [DOI] [PubMed] [Google Scholar]
- 17. Salem, S.S. , Fouda, A. : Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol. Trace Elem. Res. 199(1), 344–370 (2021). 10.1007/s12011-020-02138-3 [DOI] [PubMed] [Google Scholar]
- 18. Ovais, M. , et al.: Biosynthesis of metal nanoparticles via microbial enzymes: a mechanistic approach. Int. J. Mol. Sci. 19(12), 4100 (2018). 10.3390/ijms19124100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rabeea, M.A. , et al.: Mycosynthesis of gold nanoparticles using the extract of Flammulina velutipes, Physalacriaceae, and their efficacy for decolorization of methylene blue. J. Environ. Chem. Eng. 8(3), 103841 (2020). 10.1016/j.jece.2020.103841 [DOI] [Google Scholar]
- 20. Sharma, V.P. , et al.: Enoki mushroom (Flammulina velutipes (Curtis) Singer) breeding. In: Advances in Plant Breeding Strategies: Vegetable Crops, pp. 423–441. Cham: Springer; (2021) [Google Scholar]
- 21. Deol, S. , Weerasuriya, N. , Shon, Y.S. : Stability, cytotoxicity and cell uptake of water‐soluble dendron‐conjugated gold nanoparticles with 3, 12 and 17 nm cores. J. Mater. Chem. B. 3(29), 6071–6080 (2015). 10.1039/c5tb00608b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang, P. , et al.: Extracellular polymeric substances dependence of surface interactions of bacillus subtilis with Cd2+ and Pb2+: an investigation combined with surface plasmon resonance and infrared spectra. Colloids Surf. B. 154, 357–364 (2017). 10.1016/j.colsurfb.2017.03.046 [DOI] [PubMed] [Google Scholar]
- 23. Leong, Y.K. , Yang, F.C. , Chang, J.S. : Extraction of Polysaccharides from edible mushrooms: emerging technologies and recent advances. Carbohydr. Polym. 251, 117006 (2021). 10.1016/j.carbpol.2020.117006 [DOI] [PubMed] [Google Scholar]
- 24. Narayanan, K.B. , Park, H.H. , Han, S.S. : Synthesis and characterization of biomatrixed‐gold nanoparticles by the mushroom Flammulina velutipes and its heterogeneous catalytic potential. Chemosphere. 141, 169–175 (2015). 10.1016/j.chemosphere.2015.06.101 [DOI] [PubMed] [Google Scholar]
- 25. Bahar, M.M. , Megharaj, M. , Naidu, R. : Oxidation of arsenite to arsenate in growth medium and groundwater using a novel arsenite‐oxidizing diazotrophic bacterium isolated from soil. Int. Biodeterior. Biodegrad. 106, 178–182 (2016). 10.1016/j.ibiod.2015.10.019 [DOI] [Google Scholar]
- 26. Santos‐Gandelman, J.F. , et al.: Potential application in mercury bioremediation of a marine sponge‐isolated bacillus cereus strain Pj1. Curr. Microbiol. 69(3), 374–380 (2014). 10.1007/s00284-014-0597-5 [DOI] [PubMed] [Google Scholar]
- 27. Rahman, Z. , Singh, V.P. : Cr (VI) reduction by Enterobacter Sp. Du17 isolated from the tannery waste dump site and characterization of the bacterium and the Cr (VI) reductase. Int. Biodeterior. Biodegrad. 91, 97–103 (2014). 10.1016/j.ibiod.2014.03.015 [DOI] [Google Scholar]
- 28. Sinha, A. , Kumar, S. , Khare, S.K. : Biochemical basis of mercury remediation and bioaccumulation by Enterobacter Sp. Emb21. Appl. Biochem. Biotechnol. 169(1), 256–267 (2013). 10.1007/s12010-012-9970-7 [DOI] [PubMed] [Google Scholar]
- 29. Ward, S.K. , et al.: CtpV: a putative copper exporter required for full virulence of Mycobacterium tuberculosis . Mol. Microbiol. 77(5), 1096–1110 (2010). 10.1111/j.1365-2958.2010.07273.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khan, A. , et al.: The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: a review. Environ. Sci. Pollut. Res. Int. 22(18), 13772–13799 (2015). 10.1007/s11356-015-4881-0 [DOI] [PubMed] [Google Scholar]
- 31. Alia, N. , et al.: Toxicity and bioaccumulation of heavy metals in spinach (Spinacia oleracea) grown in a controlled environment. Int. J. Environ. Res. Public Health. 12(7), 7400–7416 (2015). 10.3390/ijerph120707400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ghori, N.H. , et al.: Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 16(3), 1807–1828 (2019). 10.1007/s13762-019-02215-8 [DOI] [Google Scholar]
- 33. Dhankhar, R. , Hooda, A. : Fungal biosorption – an alternative to meet the challenges of heavy metal pollution in aqueous solutions. Environ. Technol. 32(5–6), 467–491 (2011). 10.1080/09593330.2011.572922 [DOI] [PubMed] [Google Scholar]
- 34. Mo, L.Y. , et al.: Joint toxicity of six common heavy metals to Chlorella pyrenoidosa . Environ. Sci. Pollut. Res. Int. 26(30), 30554–30560 (2019). 10.1007/s11356-017-0837-x [DOI] [PubMed] [Google Scholar]
- 35. Mu, W. , et al.: Toxicological effects of cadmium and lead on two freshwater diatoms. Environ. Toxicol. Pharmacol. 59, 152–162 (2018). 10.1016/j.etap.2018.03.013 [DOI] [PubMed] [Google Scholar]
- 36. Zhang, H. , et al.: Biosynthesis of selenium nanoparticles mediated by fungus Mariannaea Sp. HJ and their characterization. Colloids Surf. A. 571, 9–16 (2019). 10.1016/j.colsurfa.2019.02.070 [DOI] [Google Scholar]
- 37. Prakash, S. , Soni, N. : Synthesis of Gold Nanoparticles by the Fungus Aspergillus niger and Its Efficacy against Mosquito Larvae. Reports in Parasitology (2012)
- 38. Qu, Y. , et al.: Biosynthesis of gold nanoparticles by Trichoderma Sp. Wl‐Go for azo dyes decolorization. J. Environ. Sci. (China). 56, 79–86 (2017). 10.1016/j.jes.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 39. Prasad, K. , Jha, A.K. : Biosynthesis of CdS nanoparticles: an improved green and rapid procedure. J. Colloid Interface Sci. 342(1), 68–72 (2010). 10.1016/j.jcis.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 40. Tari, D.B. , Fathi, A. : Effect of drought stress and its mechanism in plants. Int. J. Life Sci. 10(1), 1–6 (2016). 10.3126/ijls.v10i1.14509 [DOI] [Google Scholar]
- 41. Mocan, A. , et al.: Chemical composition and bioactive properties of the wild mushroom Polyporus squamosus (Huds.) Fr: a study with samples from Romania. Food Funct. 9(1), 160–170 (2018). 10.1039/c7fo01514c [DOI] [PubMed] [Google Scholar]
- 42. Yeh, M.Y. , Ko, W.C. , Lin, L.Y. : Hypolipidemic and antioxidant activity of enoki mushrooms (Flammulina velutipes). BioMed Res. Int. 2014, 352385–6 (2014). 10.1155/2014/352385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shabnam, N. , Pardha‐Saradhi, P. , Sharmila, P. : Phenolics impart Au3+‐stress tolerance to cowpea by generating nanoparticles. PLoS One. 9(1), e85242 (2014). 10.1371/journal.pone.0085242 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


