The incidence of chronic thromboembolic pulmonary hypertension (CTEPH) in coronavirus disease 2019 (COVID-19) survivors who were diagnosed with acute pulmonary embolism (PE) is currently unknown. Considering the high PE incidence reported in COVID-19 and its potentially unique pathophysiology, it may be hypothesised that thrombus resolution occurs to a lesser extent after COVID-19-associated PE, and that the prevalence of CTEPH is higher compared to non-COVID-19-associated PE populations. CTEPH could therefore be a treatable cause of long COVID, which captures a broad range of post-acute COVID-19 sequelae, in those with PE during acute COVID-19 [1]. In this multicentre cross-sectional study, we aimed to establish the prevalence of CTEPH and recurrent venous thromboembolism (VTE), and evaluate thrombus resolution in COVID-19-associated PE survivors.
Short abstract
The results of this study suggest that CTEPH is not a more common long-term complication after COVID-19-associated PE than after PE in non-COVID-19 patients, and thrombus resolution did not seem to be different from non-COVID-19-associated PE https://bit.ly/3IjvWL3
To the Editor:
The incidence of chronic thromboembolic pulmonary hypertension (CTEPH) in coronavirus disease 2019 (COVID-19) survivors who were diagnosed with acute pulmonary embolism (PE) is currently unknown. Considering the high PE incidence reported in COVID-19 and its potentially unique pathophysiology, it may be hypothesised that thrombus resolution occurs to a lesser extent after COVID-19-associated PE, and that the prevalence of CTEPH is higher compared to non-COVID-19-associated PE populations. CTEPH could therefore be a treatable cause of long COVID, which captures a broad range of post-acute COVID-19 sequelae, in those with PE during acute COVID-19 [1]. In this multicentre cross-sectional study, we aimed to establish the prevalence of CTEPH and recurrent venous thromboembolism (VTE), and evaluate thrombus resolution in COVID-19-associated PE survivors.
Adult hospitalised COVID-19 patients, who had been diagnosed with and treated for acute PE during or after admission, were eligible if they received follow-up procedures aimed at detection of long-term complications after COVID-19-associated PE, including assessment of pulmonary hypertension (PH) or PE resolution, in any of 13 Dutch university and non-university hospitals that are part of the Dutch COVID & Thrombosis Coalition [2]. The study was approved by the institutional review board of the Leiden University Medical Center for observational studies. In each participating site, either informed consent was obtained or an opt-out procedure was applied. Data were extracted from the patients’ medical charts.
The primary outcome was the prevalence of CTEPH. CTEPH was defined as mean pulmonary arterial pressure (mPAP) ≥25 mmHg and pulmonary capillary wedge pressure ≤15 mmHg in the presence of multiple chronic, organised occlusive thrombi after ≥3 months of anticoagulation. The new definition of pre-capillary PH according to the European Society of Cardiology/European Respiratory Society guideline, i.e. mPAP >20 mmHg and pulmonary vascular resistance >2 Wood Units, was post hoc included in the study protocol [3]. CTEPH was considered ruled out based on ≥1 of the following criteria: 1) low pre-test probability (CTEPH prediction score ≤6 points) without symptoms suggestive of PH after 3 months of follow-up; 2) normal electrocardiogram (ECG) and age- and sex-dependent N-terminal-prohormone of brain natriuretic peptide (NT-proBNP) concentration (CTEPH rule-out criteria); 3) low probability of PH on echocardiography; 4) no persistent perfusion defects on ventilation–perfusion scintigraphy (VQ scan); 5) complete thrombus resolution and/or normal perfusion and no signs of PH on computed tomography pulmonary angiography (CTPA); or 6) right heart catheterisation ruling out PH [3–5]. Secondary outcomes were the prevalence of VTE recurrence, and PE resolution based on CTPA performed during follow-up. Two experts adjudicated all suspected recurrent VTE events.
For the primary outcome, the proportion of patients with CTEPH was calculated with corresponding 95% confidence interval (CI), as was the proportion of patients with recurrent VTE and radiographic PE resolution. Missing data were not imputed. Analyses were performed in SPSS version 25.0.
A total of 299 patients who had been diagnosed with COVID-19-associated PE between March 2020 and December 2021 and received follow-up were included. Mean age was 60 years, and 71% (213/299) were male. PE was diagnosed during admission in 265 (89%) patients (257 confirmed by imaging; eight based on high clinical suspicion when imaging could not be obtained due to clinical status) and after discharge in 34 (11%) patients, after a median of 6.5 days (interquartile range (IQR) 3–20 days). All patients were treated with anticoagulation for at least 3 months.
The median follow-up after PE diagnosis was 19 months (IQR 15–22 months). All PEs were considered COVID-19-related with diagnostic delay of less than 14 days, indicating low pre-test probability based on the CTEPH prediction score. After 3 months, 184 (62%) patients did not report any persistent dyspnoea or symptoms compatible with CTEPH, of whom 43 were also subjected to the CTEPH rule-out criteria (ECG and NT-proBNP) and 54 to echocardiography, which did not show any signs of CTEPH. CTEPH was considered absent in these 184 patients. Of the 115 (38%) patients who reported persistent dyspnoea or symptoms, CTEPH was considered ruled out in all (prevalence 0%, upper limit of 95% CI 1.3%): by the CTEPH rule-out criteria in 10, by echocardiography in 62, by VQ scan in 26, and by CTPA in 17. Hence, no patients were subjected to right heart catheterisation or referred to a CTEPH expertise centre.
Five patients were evaluated for recurrent VTE (three suspected deep vein thrombosis; two suspected PE) after a median follow-up of 117 days (IQR 43–216 days). Recurrent VTE was ruled out in all five with appropriate diagnostic tests (recurrent VTE 0%, upper limit of 95% CI 1.3%).
Residual thrombotic obstruction was observed in six of the 51 patients who were subjected to follow-up CTPA after the first 3 months of follow-up (median 127 days to CTPA; IQR 113–293 days), all within the first 6 months of follow-up. The estimated PE resolution over time is shown in figure 1. After 6 months, an estimated 4.4% (95% CI 1.2–15%) of patients had residual thrombotic obstruction.
FIGURE 1.
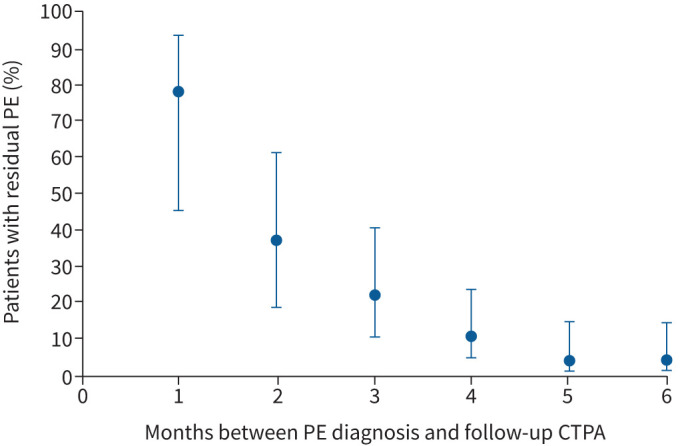
Trend of resolution of COVID-19-associated pulmonary embolism (PE) over time revealed by follow-up computed tomography pulmonary angiography (CTPA) performed during the first 6 months after diagnosis. To estimate the proportion of patients with residual PE, based on evaluation of follow-up CTPA, at each time-point (number of months between PE diagnosis and follow-up CTPA), the patients who were subjected to CTPA at that time-point were taken into account. The assumption was made that patients with residual thrombosis observed at follow-up CTPA would have residual thrombosis in the months prior to the month in which follow-up CTPA was performed. Patients with resolution of PE, revealed by follow-up CTPA, were assumed to have no signs of residual thrombosis in the months following the month in which their follow-up CTPA was performed.
Several previous small studies investigated resolution of COVID-19-associated PE based on repeat imaging, with varying findings: thrombus resolution in 72% of patients after 44±48 days [6], and 60% after 105 days [7]; perfusion defects in 57% after 90 days [8], and 10% after 6 months [9]. In the non-COVID-19-associated PE population, complete resolution occurs in 84% of patients after 6-month follow-up (95% CI 77–89%), which is not higher than observed in the current study [10].
A higher than expected prevalence of PH has been reported in COVID-19 survivors; however, specific data on CTEPH after COVID-19 are very limited. In a series of 77 patients with COVID-19-associated acute PE, three (4%) met the criteria for CTEPH [11]. Data from the CTEPH quaternary centre in the UK, however, showed a decrease in referrals during the pandemic to 228 referred CTEPH cases, of whom none had a history of COVID-19 [12]. Our findings are in line with the latter as we did not find any CTEPH cases, which does not suggest that CTEPH cannot occur after COVID-19-associated PE, but rather suggests that the prevalence is likely not higher than the 2–3% observed after non-COVID-19-associated PE [13, 14]. As reported in other studies, we observed a low number of patients with (suspected) recurrent VTE [7, 9, 15].
Based on a large nationwide population, this study provides data on the occurrence of CTEPH based on long-term follow-up after COVID-19-associated PE: information that was not previously available. One of the limitations is that the findings are based on a selected group of patients who survived COVID-19-associated PE and received follow-up appropriate to detect long-term complications, and may therefore not be fully generalisable to the general COVID-19 population. We did not follow all patients for 2 years, which may have led to an underestimation of the CTEPH prevalence. Of note, in recent (non-COVID-19) PE follow-up studies, most CTEPH cases (69–77%) were identified within 6 months of follow-up [4, 14]. Lastly, the follow-up strategies in the participating hospitals were not fully harmonised. As a result, not all patients were subjected to follow-up CTPA and therefore thrombus resolution was not evaluated in the entire study population.
In conclusion, CTEPH was absent in our study population of COVID-19-associated PE survivors, suggesting that CTEPH is not a more common long-term complication after COVID-19-associated PE than after non-COVID-19-associated PE. None of the patients had recurrent symptomatic VTE during median follow-up of 19 months, and thrombus resolution did not seem to be different than after non-COVID-19-associated PE. Hence, typical long-term PE sequelae may not be important determinants of long COVID in COVID-19-associated PE survivors.
Shareable PDF
Acknowledgements
We thank Jeannette B. Peters (Department of Pulmonary Diseases, Radboud University Medical Center, Nijmegen, the Netherlands) for involvement in data management. This study was performed on behalf of the Dutch COVID & Thrombosis Coalition.
Footnotes
Data sharing statement: Deidentified participant data collected for this study will be made available after publication to researchers whose proposed use of the data has been approved with a signed data access agreement. Requests for access to the clinical study data can be submitted via e-mail to f.a.klok@lumc.nl.
Author contributions: C.M.M. de Jong and C. Visser collected data and performed analyses. The manuscript was drafted by C.M.M. de Jong and F.A. Klok. All authors provided important intellectual content, reviewed and edited the manuscript, and all have approved the manuscript's final version.
Conflict of interest: M.E. Hellemons reports honoraria for lectures (unrestricted) from Boehringer Ingelheim and Pfizer. M.V. Huisman reports grants or contracts from the Dutch Heart Foundation, ZonMW, Bayer Health Care, Pfizer-BMS and Leo Pharma, all outside this work. J. Leentjens reports payments from BMS-Pfizer, consulting fees from Viatris, contracts from Synapse, all unrelated to this work and paid to her institution. K. Meijer reports speaker fees from Alexion, Bayer and CSL Behring, participation in trial steering committees for Bayer and AstraZeneca, consulting fees from Uniqure, participation in data monitoring and endpoint adjudication committee for Octapharma, all unrelated to this work and paid to her institution. M.J.H.A. Kruip reports grants from The Netherlands Organisation for Health Research and Development, the Dutch Thrombosis Association and Sobi, speaker fees from Sobi, Roche and BMS, all paid to her institution. F.A. Klok reports grants or contracts from Bayer, BMS, BSCI, MSD, Leo Pharma, Actelion, Pharm-X, The Netherlands Organisation for Health Research and Development, the Dutch Thrombosis Association, The Dutch Heart Foundation and the Horizon Europe Program, all unrelated to this work and paid to his institution. C.M.M. de Jong, C. Visser, R.H.H. Bemelmans, W.G. Boersma, B. van den Borst, J.L.I. Burggraaf, S.C. Cannegieter, A.J. ten Cate-Hoek, F.N. Croles, H.J. Faber, L.M. Faber, L.M. Hessels, P.W. Kamphuisen, S.C.E. Koster, L.J.M. Kroft, I. van der Lee, M.K. Ninaber, B.M. Sondermeijer, S. Stads, A. Vonk Noordegraaf and K. Winckers report no conflicts of interest related to this project.
Support statement: The Dutch COVID & Thrombosis Coalition is supported by The Netherlands Organisation for Health Research and Development (ZonMw project number 10430012010004) and the Dutch Thrombosis Association (project number 2020_A). This study was supported by an unrestricted grant from Johnson & Johnson. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Nalbandian A, Sehgal K, Gupta A, et al. . Post-acute COVID-19 syndrome. Nat Med 2021: 27: 601–615. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kruip M, Cannegieter SC, Ten Cate H, et al. . Caging the dragon: research approach to COVID-19-related thrombosis. Res Pract Thromb Haemost 2021: 5: 278–290. doi: 10.1002/rth2.12470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humbert M, Kovacs G, Hoeper MM, et al. . 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022; 43: 3618–3731. doi: 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 4.Boon G, Ende-Verhaar YM, Bavalia R, et al. . Non-invasive early exclusion of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: the InShape II study. Thorax 2021; 76: 1002–1009. doi: 10.1136/thoraxjnl-2020-216324 [DOI] [PubMed] [Google Scholar]
- 5.Delcroix M, Torbicki A, Gopalan D, et al. . ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J 2021; 57: 2002828. doi: 10.1183/13993003.02828-2020 [DOI] [PubMed] [Google Scholar]
- 6.Ritchie CA, Johnson MM, Stowell JT, et al. . Resolution of acute pulmonary embolism using anticoagulation therapy alone in coronavirus disease 2019. J Vasc Surg Venous Lymphat Disord 2022; 10: 578–584.e2. doi: 10.1016/j.jvsv.2021.12.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whyte MB, Barker R, Kelly PA, et al. . Three-month follow-up of pulmonary embolism in patients with COVID-19. Thromb Res 2021; 201: 113–115. doi: 10.1016/j.thromres.2021.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calabrese C, Annunziata A, Flora M, et al. . Three month follow-up of patients with COVID-19 pneumonia complicated by pulmonary embolism. Front Mol Biosci 2021; 8: 809186. doi: 10.3389/fmolb.2021.809186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berghaus TM, Bader S, Faul C, et al. . Lung perfusion assessed by SPECT/CT after a minimum of three months anticoagulation therapy in patients with SARS-CoV-2-associated acute pulmonary embolism: a retrospective observational study. Respir Res 2022; 23: 296. doi: 10.1186/s12931-022-02188-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.den Exter PL, van Es J, Kroft LJ, et al. . Thromboembolic resolution assessed by CT pulmonary angiography after treatment for acute pulmonary embolism. Thromb Haemost 2015; 114: 26–34. doi: 10.1160/TH14-10-0842 [DOI] [PubMed] [Google Scholar]
- 11.Cueto-Robledo G, Roldan-Valadez E, Graniel-Palafox LE, et al. . Chronic thromboembolic pulmonary hypertension (CTEPH): a review of another sequel of severe post-Covid-19 pneumonia. Curr Probl Cardiol 2022; in press [ 10.1016/j.cpcardiol.2022.101187]. doi: 10.1016/j.cpcardiol.2022.101187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman J, Boubriak I, Jenkins D, et al. . Rising COVID-19 related acute pulmonary emboli but falling national chronic thromboembolic pulmonary hypertension referrals from a large national dataset. ERJ Open Res 2021; 7: 00431-2021. doi: 10.1183/23120541.00431-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ende-Verhaar YM, Cannegieter SC, Vonk Noordegraaf A, et al. . Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J 2017; 49: 1601792. doi: 10.1183/13993003.01792-2016 [DOI] [PubMed] [Google Scholar]
- 14.Valerio L, Mavromanoli AC, Barco S, et al. . Chronic thromboembolic pulmonary hypertension and impairment after pulmonary embolism: the FOCUS study. Eur Heart J 2022: 43: 3387–3398. doi: 10.1093/eurheartj/ehac206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jara-Palomares L, Bikdeli B, Jiménez D, et al. . Rate of recurrence after discontinuing anticoagulation therapy in patients with COVID-19-associated venous thromboembolism. JAMA Intern Med 2022; 182: 1326–1328. doi: 10.1001/jamainternmed.2022.4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00171-2023.Shareable (826.6KB, pdf)


