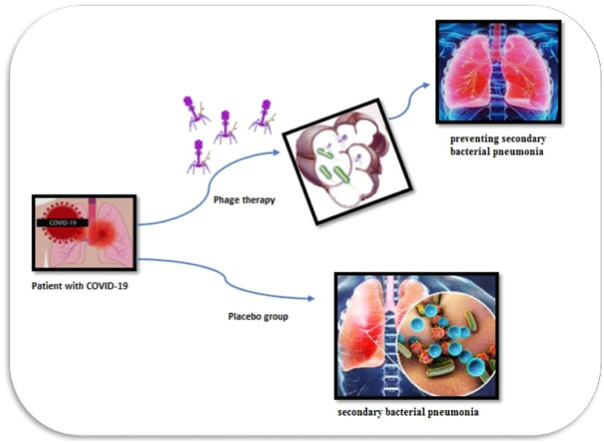
Abstract
Inhalation phage therapy is proposed as a replacement approach for antibiotics in the treatment of pulmonary bacterial infections. This study investigates phage therapy on bacterial pneumonia in patients with moderate to severe COVID-19 via the inhalation route. In this double-blind clinical trial, 60 patients with positive COVID-19 hospitalized in three central Mazandaran hospitals were chosen and randomly divided into two intervention and control groups. Standard country protocol drugs plus 10 mL of phage suspension every 12 h with a mesh nebulizer was prescribed for 7 days in the intervention group. The two groups were compared in terms of O2Sat, survival rate, severe secondary pulmonary bacterial infection and duration of hospitalization. Comparing the results between the intervention and control group, in terms of the trend of O2Sat change, negative sputum culture, no fever, no dyspnea, duration of hospitalization, duration of intubation and under ventilation, showed that the difference between these two groups was statistically different (P value < 0.05). In conclusion, inhalation phage therapy may have a potential effect on secondary infection and in the outcome of COVID-19 patients. However, more clinical trials with control confounding factors are needed to further support this concept.
Keywords: Phage therapy, Nebulizer, Secondary infection, COVID-19
Graphical abstract
1. Introduction
Coronavirus (COVID-19) started in 2019 and spread all over the world. This virus, with its main route of entry being through the human respiratory system, has spread at an unprecedented rate, causing the dysfunction of the respiratory system, gastrointestinal tract (GIT) and inflammation of several organs [[1], [2], [3], [4]] and thus threatening the health and economy of the world.
It has been documented that the growth of the virus stimulates the immune system, with the immune response causing the secretion of inflammatory substances (fluids and inflammatory cells) and a storm of cytokines. The damage and death of lung tissue cells caused by the viruses are therefore provided for the growth of bacteria. Infection stimulates immunity and hence exacerbates respiratory issues such as micro thrombosis in lung vessels. Secondary bacterial infection has also been reported to cause high mortality in patients with COVID-19 [5,6]. Recently, 50% of mortality was reported in patients with COVID-19 secondary bacterial infection [5]. Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumonia, and Acinetobacter baumannii are common causes of secondary nosocomial infections in these patients [[5], [6], [7]]. At present, these bacteria have become resistant to many antibiotics. This is a result of the inappropriate use of antibiotics, genetic changes, and the production of various enzymes that destroy the structure of antibiotics. Due to the emergence and spread of antibiotic resistance, increasing treatment costs and mortality of patients with respiratory infections require a suitable option for prevention and treatment. Empirical treatment of lung infections with various antibiotics has resulted in resistant strains of vancomycin-resistant Staphylococcus aureus (VRE) and methicillin-resistant Staphylococcus aureus (MRSA), and gram-negative extended β-lactamase [5,6].
Phage therapy is a safe and substitute treatment option for eradicating infection. Bacteriophages, especially lytic bacteria and do not affect the normal flora and eukaryotic cells. They are multiplied at the site of infection and, unlike antibiotics, do not require dose adjustment. Phages are self-replicating and their production is fast and economically viable [[7], [8], [9]]. In Georgia, Herelle'd Félix with the collaboration of Eliava commercially produced the first phage product against gram-negative bacteria that caused gastrointestinal infections in 1931. Phage therapy was activated in Georgia, Russia, Ukraine, Belarus, and Azerbaijan in 1940. Currently, monophage and polyphage products are used against infections such as gastrointestinal, sepsis, urinary tract, burn ulcer, and cystic fibrosis in the Hirszfeld Institute of Immunology and experimental therapy (HIIET) in the Netherlands, Queen Astrid Military Hospital in the Brussels, Deutsche Sammlung vonMikroorganismen und Zellkulturen in Germany, and the Eliava Institute of Bacteriophage, Microbiology, and Virology in Georgia [7].
This study is designed and conducted to investigate the influence of inhalation phage therapy on bacterial pneumonia in patients with moderate to severe COVID-19. The potential effects of phage therapy to prevent secondary bacterial pneumonia and reduce mortality and morbidity owing to secondary bacterial infection in patients with COVID-19 is of great importance.
2. Material and methods
2.1. Preparation of bacteria strains
Conventional microbial methods were employed to identify Pseudomonas aeruginosa (ATCC No.27853), Acinetobacter baumannii (ATCC No. BAA-1605) and Methicillin-resistant Staphylococcus aureus (ATCC No.33591). The sensitivity of the mentioned bacteria to the antibiotics was measured by the Kirby–Bauer test with antibiotic discs (vancomycin (30 μg), Oxacillin (1 μg), kanamycin (30 μg), amikacin (30 μg), ampicillin (10 μg), nitrofurantoin (300 μg), tetracycline (30 μg) meropenem (10 μg), cefotaxime (30 μg), gentamicin (10 μg), cefixime (30 μg) and ciprofloxacin (10 μg)), according to the Clinical & Laboratory Standards Institute (CLSI) guidelines [10,11].
2.2. Isolation of bacteriophage
The sewage samples (1000 mL) were gathered from sewage at Bou Ali Sina Hospital in Sari, Mazandaran, Iran (the tertiary pediatric). The samples were transferred to the research laboratory and were kept at 4 °C. The LB broths (200 mL) (Quelab, USA) were transferred to the sewage sample (200 mL) and mixed. Then the Pseudomonas aeruginosa, Acinetobacter, and Methicillin-resistant Staphylococcus aureus strains were added in separate vials. The three samples were placed in an incubator (150 rpm) for 24 h with the temperature kept constant at 37 °C. The specimens were then centrifuged at 10,000×g (15 min). The supernatants were filtrated (using a 0.22-μm filter) under sterile conditions, followed by mixing to obtain a phage cocktail. The phage cocktail was kept at a temperature of 4 °C before use [10,11].
2.3. Determining the titer of bacteriophage
Double Layer Agar (DAL assay) was used to calculate the titer of phage. A sterilized solution of sodium chloride-magnesium sulfate buffer (900 μL, 100 mmol/L sodium chloride, 8 mmol/L magnesium sulfate, 2% gelatin, and 50 mmol/L Tris–HCl [pH 7.5]) was incorporated into 10 sterile tubes (labelled as tubes no. 1 to 10). The phage cocktail (100 μL) was transferred to tube no. 1 and was vortexed. Then from tube no. 1, 100 μL of the solution was removed and transferred to tube no. 2. This process was recurrent until tube no. 8. The two final tubes (tubes no. 9 and 10) were chosen as the positive and negative control respectively. When the phage cocktails were diluted, 200 μL from each was shifted to 200 μL of Pseudomonas aeruginosa (1.5 × 108 CFU/mL). The final blends were transferred to the top agar (0.8% agar) followed by the introduction of the top agar to the bottom agar (1% agar) after which the plates were incubated for 20 h at 37 °C. Plaque-forming unit (PFU) was computed per mL by determining the number of plaques × 10 × the inverse of the dilution factor [10,11].
2.4. Determination of the host range of phage cocktail
To detect the phage cocktail host cell, the spot test was carried out. The cultured Pseudomonas aeruginosa, Acinetobacter and methicillin-resistant Staphylococcus aureus that were previously incubated for 24 h at 37 °C, in top agar were inoculated and poured into the bottom agar. The phage cocktail supernatant (50 μl) was emptied on the surface of solidified agar. The plates were incubated for 24 h at 37 °C after which the formation of the inhibition zone was tested [10,11].
2.5. Transmission electron microscopy (TEM)
Before visual observation of the phage by TEM, the phage cocktail was centrifuged at 10,000×g for 60 min. After centrifuging, the obtained supernatant was placed on a carbon-coated copper grid and stained using 2% uranyl acetate. The phage cocktail was evaluated using the Zeiss EM 900 TEM at 120 kV [10,11].
2.6. Endotoxin removal method from phage cocktail supernatant
Triton X-100 (3% v/v) was transferred to the supernatant of the phage obtained after centrifugation (samples were incubated for 30 min at 25 °C under shaking conditions). To eliminate the Triton X-100 from the incubated phage supernatant, 12% activated carbon was introduced and incubated for 30 min at 25 °C. The solution was then centrifuged at 5000×g for 10 min. To eliminate the residual activated carbon, the produced supernatant was purified via a 0.45 μm filter membrane. The filtered and purified phage cocktail was kept overnight at 4 °C. The phage titer was measured by the double-layer agar (DLA) assay that was defined elsewhere [12].
2.7. Limulus Amebocyte Lysate (LAL) test
To examine the elimination of endotoxin from the phage cocktail supernatant, the LAL test was carried out according to clotting response based on the protocol kit (ENDOSAFE, USA) with a strength of 0.06 EU/mL and 5 EU/mL endotoxin limit. Escherichia coli 055: B5 was employed as a positive control [10,12].
2.8. Participants in the clinical phase
This double-blind, placebo-controlled, randomized study was executed from April 2021 to June 2021 in the 3 referral centre hospitals in Northern Iran. The study was approved by the Ethics Committee at Mazandaran University of Medical Sciences IR.MAZUMS.REC.1399.819 and was registered at www.clinicaltrials.gov with the code number IRCT20111224008507N6. An agreement was acquired from all patients involved in the study.
A total of 60 COVID-19 patients with “inclusion criteria” were included in the study. Inclusion criteria were children and adults with moderate to severe COVID-19, moderate disease (people who have signs of pulmonary involvement or symptoms on imaging, oxygen saturation level> 93% O2sat > in room air and sea-level areas), severe disease (people breathing more than 30 times per minute, O2Sat <93% in room air and sea-level areas, the partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) < 300 and lung infiltration of more than 50%. Patients showing any of the subsequent symptoms such as dry cough, severe weakness and fatigue, dyspnea (shortness of breath), a feeling of pain and pressure in the chest, with or without fever (greater than 38 °C) were also part of the inclusion criteria. Patients less than 3 days after the onset of symptoms, a conclusive diagnosis of COVID-19 based on a RT-PCR test or involvement of a maximum of 3 or 4 pulmonary lobes with an area less than 1/3 of the volume of each lobe or infection of one or two lobes with a larger area on CT scan and O2Sat< 94% were included in this study. Exclusion criteria were a history of COVID-19 (a patient who has previously been hospitalized for coronary heart disease), participation in any other clinical trial for the treatment of COVID-19, bradycardia, active cancer, immune system compromised or immune-compromised, abnormal primary ECG, underlying liver and kidney disease, re-admission for treatment of COVID-19.
2.9. Intervention
Individuals with inclusion criteria were coded using software and randomly divided into two arms of intervention and control with 30 patients allocated to each group in the study. The researchers had no role in assigning patients to the two groups of control and intervention. The selection of the participants was conducted by randomization software. Phage and placebo vials were identical in shape and size, and a specific code was assigned to each vial. The participants were then given the phage (intervention group) and placebo (control group) samples.
The control group received 10 mL of phage-free suspension (placebo) every 12 h with a mesh nebulizer, whereas in the case of the intervention group, 10 mL of phage cocktail with a titer of 1012 PFU/ml was given via the same method as the control group. Phage cocktails and placebos were manufactured in uniform packages for nebulization and were labelled as group A (cocktails) and group B (placebo) by the co-producer.
2.10. Clinical data collection
Clinical manifested characteristics, respiratory rate per minute and blood oxygen level (O2Sat) and lab tests containing CT scan, c-reactive protein (CRP), erythrocyte sedimentation rate (ESR), lactate dehydrogenase (LDH), cholesterol, triglyceride (TG), low-density lipoprotein(LDL), high-density lipoprotein (HDL), alkaline phosphatase (ALT), aspartate transaminase (AST), blood urea nitrogen (BUN), creatinine (Cr), potassium (K), magnesium (Mg), sodium (Na), red blood cell (RBC), white blood cell (WBC), neutrophils (Neut), lymphocyte (Lymph), monocytes (Mono), eosinophils (Eo), haemoglobin (Hb) and urea were recorded.
Upper and lower respiratory samplings were performed for RT-PCR before the start of the study and 7 days after phage therapy. Daily respiration rate, O2Sat, clinical signs and side effects of patients were evaluated and recorded by a clinical pharmacist.
2.11. Patient sample collection and analysis
Before and after treatment, patients' sputum samples were sent for microbial culture, radiological images taken from patients, vital respiratory symptoms recorded, evaluated and compared with pre-treatment results. Also, the type of ventilation, respiration rate, and O2Sat were recorded daily. CRP, WBC, PMN, and Lymph levels were also evaluated.
The intervention was stopped if there was a significant disorder related to drug treatment. Side effects were checked daily and entered in the Important Event Form if they occurred. Monitoring the primary and secondary outcomes of patients was also the responsibility of the clinical pharmacist.
2.12. Primary and secondary outcome assessment
Primary outcomes included recovery within ten days of starting treatment. Improvement was defined as O2Sat> 95%, no fever, no dyspnea, no cough, no fatigue and no recurrences of secondary bacterial lung infection. Secondary outcomes included recovery within 14 days after starting medication, survival rate, hospitalization duration, intubation duration or under ventilation, number of days in the ICU, and no severe secondary pulmonary bacterial infection.
2.13. Sample size calculation and statistical analysis
The distribution of quantitative variables was first investigated by executing the non-parametric Kolmogorov-Smirnov test and generating a histogram. In order to portray quantitative variables, the mean ± SD (standard deviation), median (mid-quarter range), qualitative variables and frequency (percentage) were determined. Depending on the distribution of data, the mean of the variables was tested with an independent t-test or its non-parametric equivalent and a comparison of the frequency of outcomes while performing a Chi-square test conducted. It should be noted that the investigation of the trial results was performed by the intention-to-treat (ITT) method. The description and analysis findings were extracted from the outputs of the IBM SPSS 25 software. In all cases, a two-sided value of P less than 0.05 was used as the criterion for statistical prominence.
3. Results and discussion
3.1. Preparation of bacteria strains
The Kirby–Bauer test demonstrated that Pseudomonas aeruginosa and Acinetobacter were susceptible to nitrofurantoin, cefotaxime, cefixime and colistin but resistant to ampicillin, tetracycline, meropenem, gentamicin and ciprofloxacin. Methicillin-resistant Staphylococcus aureus was sensitive to vancomycin (30 μg) and resistant to oxacillin (30 μg), kanamycin (30 μg), amikacin (30 μg), ampicillin (10 μg), nitrofurantoin (300 μg).
3.2. Characterization of bacteriophage
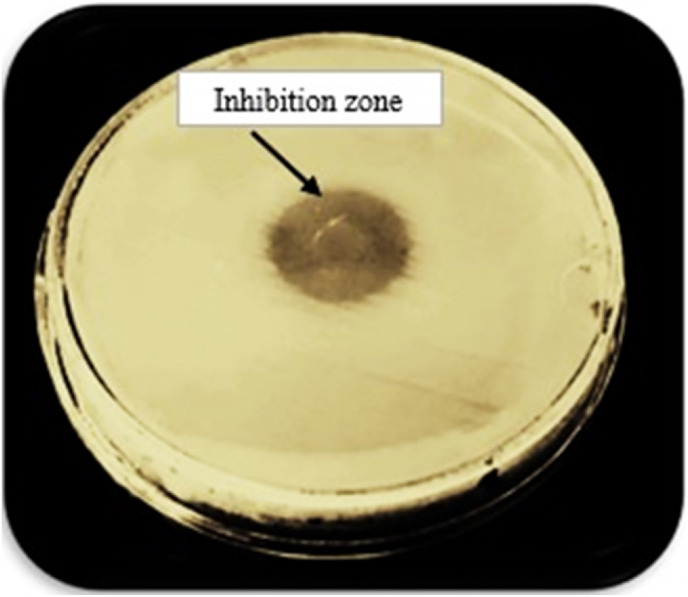
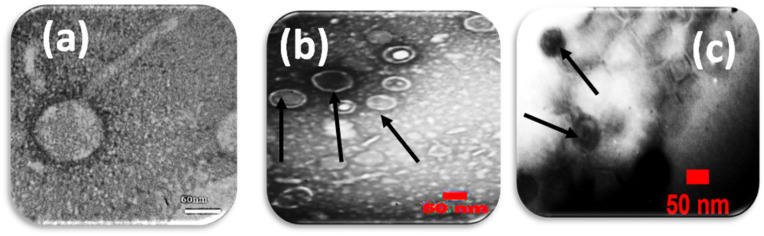
The lytic activity and host range of the phage cocktail detected by the creation of an inhibition zone using a spot test was presented in Fig. 1 . The phage titer for the isolated phage cocktail was determined (1012 PFU/mL). The shape of the phage cocktail was determined using TEM as represented in (Fig. 2 ). It was obvious from this Figure that there were three phages in the phage cocktail supernatant. The three phages include the Siphoviridae with an icosahedral head (50–80 nm) and a long non-contractile tail (400 nm) (Fig. 2a), Cystoviridae with a spherical shape (80–100 nm) with a lipid membrane around the capsomere (Fig. 2b) and Podoviridae with an icosahedral head (45–50 nm) (Fig. 2c).
Fig. 1.
The spot test shows the formation of the inhibition zone and lytic activity of the phage cocktail against bacteria.
Fig. 2.
(a) Microscopic images of phage belonging to the Siphoviridae family (b) The Cystoviridae family and (c) Podoviridae family.
In a previous study, the vast majority of known methicillin-resistant Staphylococcus aureus phages were reported to belong to the Siphoviridae family [13,14], with only a small number, including phage SAP-2, belonging to the Podoviridae family [15]. Tai et al., 2021 isolated a Siphoviridae and Podoviridae phage cocktail against Pseudomonas aeruginosa [16].
Generally, patients with COVID-19 suffer from pneumonia and secondary respiratory infection with high hospitalization and mortality rates [[17], [18], [19], [20]]. The development of multi-drug-resistant (MDR) bacteria recommends that phage therapy could be another option to treat pulmonary infections [8,[21], [22], [23], [24]]. Phage therapy through the inhalation route has long been employed and is in practice in Eastern European countries [25]. Lin et al. [26] and Doss et al. [21] reported that phage therapy is useful in the treatment of multidrug-resistant bacterial infections and it is possible to be employed as either another or a supplement to antibiotic treatments. Nicholas et al. (2019) confirmed the effect of phage prophylaxis on the infected lungs of mice with Mycobacterium tuberculosis [27]. The mice were nebulized with bacteriophage D29 using a mesh nebulizer. Lytic activity against bacteria prevents lung bacterial uptake by macrophages and granuloma formation [27]. The limitation of the administration of mono-phage in phage therapy is the bacterial's resistance to the mono-phage. The use of cocktail phages (more than two phages) therefore reduces the likelihood of resistance [26,[28], [29], [30], [31]].
3.3. Limulus Amebocyte Lysate (LAL) test
The LAL test revealed that the supernatant of phage with the strength of 0.06 EU/mL and 5 EU/mL endotoxin was unable to make a clot after 1 h at 37 °C. In the case of the positive control, the clot was detected under the same conditions used for the phage supernatant. It was found that after detoxification, there was no drop in the titer of phages and their validity was observed.
3.4. Participants in the clinical phase
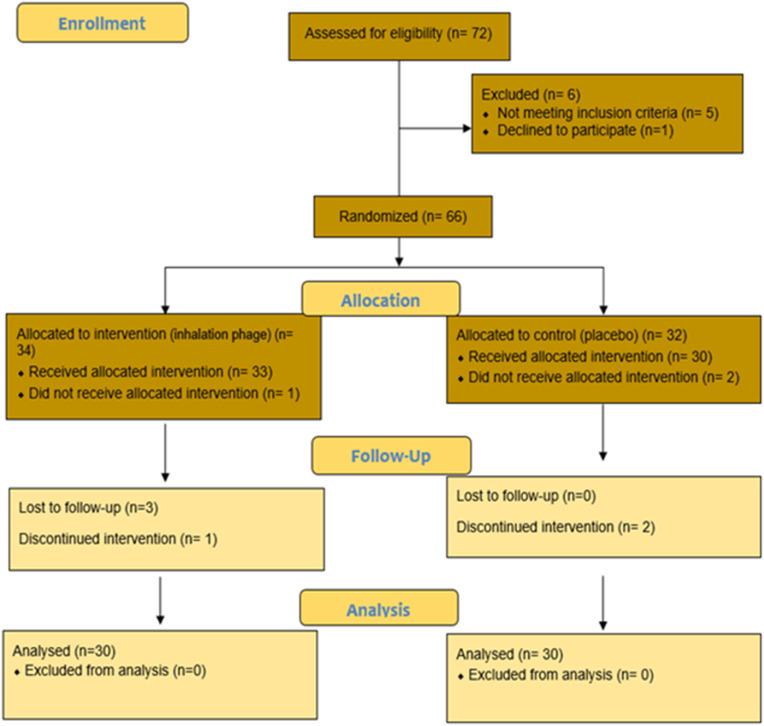
Sixty patients with COVID-19 were monitored from April 2021 to June 2021 (Fig. 3 ). The selected patients were randomized and divided into two arms: the inhalation phage therapy (n = 30) and the placebo groups (n = 30). All patients were diagnosed with moderate to severe COVID-19. Patient characteristics (age, sex, BMI, smoking status, addiction, medical histories such as diabetes, hypertension, high lipid profile, COPD, IHD and drug history) are described in Table 1 which shows that the 2 groups were not significantly different in this regard (P > 0.05).
Fig. 3.
Consort flow diagram.
Table 1.
Baseline characteristics of patients in inhalation phage and placebo groups.
| Inhalation phage group (N = 30) | Placebo (N = 30) | P value | ||
|---|---|---|---|---|
| Gender (%) | Female | 43.3 | 53.3 | 0.67 |
| Male | 56.7 | 46.7 | ||
| Age (years) mean± SD | 62.43 ± 13.31 | 63.77 ± 14.76 | 0.65 | |
| BMI (kg/m2) mean± SD | 25.98 ± 3.8 | 27.08 ± 5.95 | 0.27 | |
| Smoking (%) | Yes | 22.5 | 17 | 0.59 |
| No | 77.5 | 83 | ||
| Drug addiction (%) | Yes | 10 | 13.2 | 0.6 |
| No | 90 | 84.9 | ||
| Medical history (%) | DM | 40 | 40 | 1 |
| HTN | 10 | 40 | 0.08 | |
| HLP | 2.5 | 5.7 | 0.11 | |
| COPD | 0 | 3.8 | 0.10 | |
| CHD | 16.7 | 30 | 0.18 | |
| Anemia | 10 | 10 | 1 | |
| Malignancy | 0 | 5.7 | 0.28 | |
| TD | 3.3 | 0 | 1 | |
| Asthma | 0 | 0 | 1 | |
DM: Diabetes mellitus, HTN: hypertension, HLP: High lipid profile, COPD: chronic obstructive pulmonary disease, CHD: chronic heart disease, TD: Thyroid disease.
Table 1 shows the inhalation phage group included 43.3% males and 56.7% females. The placebo group included 53.3% males and 46.7% females. In these 2 groups BMI, smoking status, addiction, and medical histories such as diabetes, hypertension, high lipid profile, COPD, IHD, and drug history were not considerably different in this regard (P > 0.05).
The primary signs and symptoms of patients in the inhalation phage therapy and placebo groups were, fever (66.7%, 73.3%), shivering (53.3%, 56.7%), sore throat (3.3%, 3.3%), dry cough (40%, 36.7%), mucous cough (13.3%, 3.3%), dyspnea (83.3%, 80%), tachypnea (23.3%, 43.3%), rales (13.3%, 10%), wheezing (13.3%, 6.7%), retraction (13.3%, 6.7%), chest pain (13.3%, 16.7%), abdominal pain (13.3%, 0%), fatigue (70%, 53.3%), myalgia (26.7%, 30%), dyspepsia (36.7%, 30%), diarrhoea (6.7%, 10%), nausea (30%, 16.7%), vomiting (23.3%, 6.7%), dizziness (0%, 6.7%) and headache (13.3%, 6.7%). It is also important to note that the level of consciousness declined in 6.7% of all participants in each group.
3.5. Measures to improve patient's primary and secondary outcomes
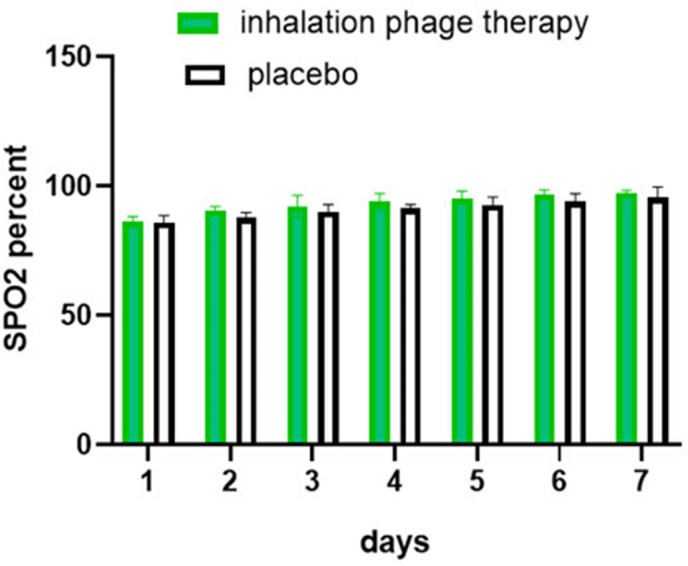
The trend of O2Sat change in 7 days of treatment is depicted in (Fig. 4 ). The results suggest a remarkable difference between the inhalation phage therapy and placebo groups (P > 0.001). As shown in Table 2 , the difference between the placebo and phage cocktail in terms of no fever (P = 0.04), no dyspnea after the duration of treatment (P = 0.008) and negative sputum culture (P value = 0.02) were statistically different. In addition, a remarkable difference between the two groups (control group and phage receiving group) was observed in the secondary outcomes namely with the duration of hospitalization (P = 0.04), duration of intubation and under ventilation (P = 0.02) and no severe secondary infection (P = 0.03). The survival rate (P value = 0.5) and duration of stay in the ICU however (P = 0.9) were not statistically different. Possible adverse events such as rash, edema, itching, hives and hypotension were not observed in any of the patients in inhalation phage therapy and control groups.
Fig. 4.
The trend of SPO2 change in patients in inhalation phage therapy and placebo.
Table 2.
Primary and secondary outcomes in patients in inhalation phage therapy and placebo groups.
| Outcome | Inhalation phage therapy N = 30 |
Placebo N = 30 |
P-value |
|---|---|---|---|
| No cough or improvement | 28 | 25 | 0.6 |
| No fever | 28 | 22 | 0.04 |
| No dyspnea | 21 | 12 | 0.008 |
| No fatigue or improvment | 20 | 22 | 0.38 |
| Negative sputum culture | 26 | 15 | 0.02 |
| Survival rate | 29 | 28 | 0.5 |
| No severe secondary infection | 28 | 21 | 0.03 |
| Duration of hospitalization | 5.1 ± 1.01 | 9.53 ± 2.07 | 0.04 |
| Duration of incubation or under ventilation | 3 ± 0.05 | 6 ± 2.01 | 0.02 |
| Duration of stay in ICU | 9.1 ± 1.01 | 9.53 ± 0.679 | 0.9 |
In the phage group, the phage cocktail solution (1012 PFU/mL) was nebulized using a mesh nebulizer for patients with COVID-19 every 12 h. The results presented in Fig. 4 and Table 2 showed that the phage cocktail solution (1012 PFU/mL) could improve and reduce the adverse reactions of coronavirus in patients with COVID-19 such as an increase in O2Sat. This increase in O2Sat in COVID-19 patients having inhalation phage therapy was significantly different in comparison to the placebo groups (P > 0.001). Table 2 showed patients with inhalation phage therapy had no fever (P = 0.04), dyspnea after the duration of treatment (P = 0.008), and negative sputum culture (P value = 0.02). A considerable difference was also found among the two groups (control group and phage receiving group) in the secondary outcomes namely in the duration of hospitalization (P = 0.04), intubation duration, under ventilation (P = 0.02) and no severe secondary infection (P = 0.03). Hoyle et al. reported a case in 2018, where a female patient (17-year-old) with cystic fibrosis and chronic infection was taking phages via the inhalation route using a compression nebulizer once daily (3 × 108 PFU/ml). Phages were also taken orally twice daily for twenty days. After the preliminary round of phage treatment, the patient's circumstances remarkably got better, dyspnea was eliminated and cough decreased [32].
In another case reported by Tan et al., an older man (88-year-old) already suffering from COPD developed hospital-acquired pneumonia (HAP) with carbapenem-resistant acinetobacter baumannii. The treatment led to the clearance of the infection from the patient's lungs with clinical enhancement in lung function [33].
In this current research, cocktail phage (1012 PFU/mL) was nebulized every 12 h for 7 days and adverse events were not observed such as rash, edema, itching, hives and hypotension. Also, enhancing O2Sat from day 2 to day 7 may be considered as a reason for the effect of phage on reducing infection and inflammation in lung tissue thereby improving alveolar oxygen uptake. Debarbieux et al. (2010) investigated the effect of intranasal administration of anti-somonas phage on lung infection in a mouse model [22]. The authors showed a decrease in the IL-6 and TNF-α levels in the lungs of phage-treated animals [22]. Hua et al. (2017) and Kvachadze et al. (2011) reported that in clinical trials, no side effects were observed during the treatment of pulmonary infections by bacteriophage nebulization [34,35]. Yajun et al. (2021) evaluated phage therapy for a lung infection in mice by liquid aerosol-exposure Pseudomonas aeruginosa [36]. The authors reported no obvious alterations in the lung tissue of the phage group in comparison to the control group. This produced more safety evidence for the phage therapy by the intratracheal aerosol delivery route and supported the feasibility of phage therapy in lung infection [36]. Nannan et al. investigated 4 patients with critical COVID-19 and pulmonary carbapenem-resistant Acinetobacter baumannii (CRAB) infections under phage therapy where one patient experienced an atypical cytokine storm (IL 6 and 8) and fever at 4 h post ɸAb124 administration phage. However, one day later the IL-6 and IL-8 patients returned to their typical levels [9].
A mesh nebulizer was used for inhalation phage therapy a nebulization is a more efficient way of administering phage particles to the lung. Liu et al. (2016) reported that 10% of D29 phage was capable of reaching the mice's lungs after nebulization and the whole elimination of phage was recorded at 72 h. Only 0.1% of the phage was able to reach the lung by intraperitoneal (i.p) injection and no phage was spotted after 12 h. Also, no inflammation was observed in the lungs of mice receiving the phage [37]. Furthermore, Chang et al. (2018) showed that nebulization was an efficient way of carrying phage particles to the lung than intranasal instillation [38]. The bacterial load was dropped by 0.5 log in mice that administered phage via i.p. route while 2-log bacterial decrease was observed in the group treated via the inhalation route [38].
It could have been ideal to measure bacteriophage load in the lung by taking samples and also assaying inflammatory factors, but this was extremely challenging in the current study and could be considered one of the limitations of the present investigation. Also, this study was conducted only in the North of Iran. To generalize the findings, more regions should be included.
4. Conclusion
In the current study, an attempt was made to investigate whether inhalation phage therapy could be used as an alternative approach to antibiotics in the treatment of pulmonary bacterial infections. The results of the current study demonstrated that inhalation phage therapy could have potential effects on secondary infection and the outcome of COVID-19 patients. Furthermore, the current inhalation phage therapy did not show any complications after the phage therapy through the inhalation route. It can therefore, it can be considered a safe treatment to be used in COVID-19 patients. Although the inhalation phage therapy showed that this approach can be a better replacement for antibiotics in the treatment of COVID-19 patients, it should be noted that it is still too early to apply this treatment to all Covid-19 patients worldwide. The authors therefore suggest an extensive study into the efficacy of the inhalation phage therapy in COVID-19 patients and patients with pneumonia in various regions of the country or perhaps different countries with a larger number of patient participants to draw out these conclusions.
Ethical approval
The study was confirmed by the Ethics Committee at Mazandaran University of Medical Sciences IR.MAZUMS.REC.1399.819 and was registered at www.clinicaltrials.gov with the code number IRCT20111224008507N6.
Credit author statement
Hamid Reza Samaee: Methodology, Validation, Investigation.
Gohar Eslami: Validation, Investigation, Resources.
Golnar Rahimzadeh: Supervision, Project administration, Conceptualization, Methodology, Validation, Investigation, Writing - Original Draft.
Majid Saeedi: Resources.
Alireza Davoudi Badabi: Resources.
Kofi Asare-Addo: Writing - Review & Editing.
Ali Nokhodchi: Writing - Review & Editing.
Fatemeh Roozbeh: Resources.
Mahmood Moosazadeh: Formal Analysis.
Roya Ghasemian: Resources.
Ahmad Alikhani: Resources.
Mohammad Sadegh Rezai: Supervision, Project administration, Conceptualization, Methodology, Validation, Investigation, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank the Pediatric Infectious Diseases Research Center lab at Bou-Ali Sina Hospital in Sari for performing laboratory tests and for their cooperation in the use of equipment and materials in their laboratory.
Data availability
Data will be made available on request.
References
- 1.Cevik M., Bamford C., Ho A. COVID-19 pandemic A focused review for clinicians. Clin. Microbiol. Infect. 2020;26:842–847. doi: 10.1016/j.cmi.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jahirul Islam Md, Nawal Islam N., Siddik Alom Md, Kabir M. A review on structural, non-structural, and accessory proteins of SARS-CoV-2: highlighting drug target sites. Immunobiology. 2023;228 doi: 10.1016/j.imbio.2022.152302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J.J., Dong X., Liu G.H., Gao Y.D. Risk and protective factors for COVID-19 morbidity, severity, and mortality. Clin. Rev. Allergy Immunol. 2023;64:90–107. doi: 10.1007/s12016-022-08921-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang H., Sun Y., Wang Y., Yazici D., et al. Recent developments in the immunopathology of COVID-19, REVIEW. EAACI. 2023;78:369–388. doi: 10.1111/all.15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah M., Patel K., Milucky J., Taylor C.A., et al. Bacterial and viral infections among adults hospitalized with COVID-19. Influenza Other Respir Viruses. 2023;17 doi: 10.1111/irv.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iacovelli A., Oliva A., Siccardi G., Tramontano A., et al. Risk factors and effect on mortality of superinfections in a newly established COVID-19 respiratory sub-intensive care unit at University Hospital in Rome. BMC Pulm. Med. 2023;20:30–43. doi: 10.1186/s12890-023-02315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nir-Paz R. In: Clin Microbiol Infect. Kuijper J., editor. 2023. Bacteriophage therapy in humans; pp. 125–128. 1198-743X. [DOI] [PubMed] [Google Scholar]
- 8.Willy Ch, Bugert J., Classen A., Deng L., et al. Phage therapy in Germany-update 2023. Viruses. 2023;15:588–611. doi: 10.3390/v15020588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nannan W., Chen L.K., Zhu T. Phage therapy for secondary bacterial infections with COVID-19. Curr Opin Virol. 2022;52:9–14. doi: 10.1016/j.coviro.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahimzadeh G., Saeedi G., Moosazadeh M., Hashemi M.M., et al. Encapsulation of bacteriophage cocktail into chitosan for the treatment of bacterial diarrhea. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-95132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahimzadeh G., Gill P., Rezai M.S. Ultra structural characteristics of methicillin resistant Staphylococcus aureus cell wall after affecting with lytic bacteriophages using atomic force microscopy. IJBMS. 2019;22:290–295. doi: 10.22038/ijbms.2019.31226.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Putsch D., Anspach F. Endotoxin removal from protein solutions. J. Biotechnol. 2000;76:97–119. doi: 10.1016/s0168-1656(99)00185-6. [DOI] [PubMed] [Google Scholar]
- 13.Rahimzadeh G., Gill P., Rezai M.S. Characterization and lytic activity of methicillin-resistant Staphylococcus aureus (MRSA) phages isolated from NICU. Australas. Med. J. 2016;9:169. [Google Scholar]
- 14.Rahimzadeh G., Gill P., Rezai M.S. Characterization of methicillin-resistant Staphylococcus aureus (MRSA) phages from sewage at a tertiary pediatric hospital. Arch Pediatr Infect Dis. 2017;5 [Google Scholar]
- 15.Son J.S., Lee S.J., Youn Jun S., Jun Yoon S., et al. Antibacterial and biofilm removal activity of a Podoviridae Staphylococcus aureus bacteriophage SAP-2 and a derived recombinant cell-wall-degrading enzyme. Appl. Microbiol. Biotechnol. 2010;86:1439–1449. doi: 10.1007/s00253-009-2386-9. [DOI] [PubMed] [Google Scholar]
- 16.The Tai D., Anh Quang N., Thi Ngoc Nhi N., Ha Duc Anh L. Application of bacteriophage cocktail to control multi-drug resistant Pseudomonas aeruginosa. J Microbiol Exp. 2021;9:72–76. [Google Scholar]
- 17.Fernández L., Cima-Cabal M., Catarina Duarte A., Rodríguez A., et al. Gram-positive pneumonia: possibilities offered by phage therapy. Antibiotics. 2021;10:1000. doi: 10.3390/antibiotics10081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldman C., Anderson R. The role of co-infections and secondary infections in patients with COVID-19. Pneumonia. 2021;25:5. doi: 10.1186/s41479-021-00083-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv Z., Cheng S., Le J. Clinical characteristics and co-infections of 354 hospitalized patients with COVID-19 in Wuhan, China: a retrospective cohort study. Microb. Infect. 2020;22:195–199. doi: 10.1016/j.micinf.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J., et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doss J. A review of phage therapy against bacterial pathogens of aquatic and terrestrial organisms. Viruses. 2017;18:50. doi: 10.3390/v9030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Debarbieux L., Leduc D., Maura D., Morello E., et al. Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J. Infect. Dis. 2010;201:1096–1104. doi: 10.1086/651135. [DOI] [PubMed] [Google Scholar]
- 23.Torres-Barceló C. The disparate effects of bacteriophages on antibiotic-resistant bacteria. Emerg. microbes & infect. 2018;7:1–12. doi: 10.1038/s41426-018-0169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raza A. Bacteriophage therapy: recent development and applications. Sch Bull. 2021;7:27–37. [Google Scholar]
- 25.Abaddon S.T. Phage therapy of pulmonary infections. Bacteriophage. 2015;5 doi: 10.1080/21597081.2015.1020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin D.M., Koskella B., Lin H.C. Phage therapy: an alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Therapeut. 2017;8:162–173. doi: 10.4292/wjgpt.v8.i3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholas B., Sasha E., Valerie R., Tiffany P. Prophylaxis of Mycobacterium tuberculosis H37Rv infection in a preclinical mouse model via inhalation of nebulized bacteriophage D29. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.00871-19. e00871-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qadir M.I., Sajjad S. Phage therapy against Streptococcus pneumoniae: modern tool to control pneumonia. Crit. Rev. Eukaryot. Gene Expr. 2017;27:289–295. doi: 10.1615/CritRevEukaryotGeneExpr.2017019527. [DOI] [PubMed] [Google Scholar]
- 29.Josef Praza k. Benefits of aerosolized phages for the treatment of pneumonia due to methicillin-resistant Staphylococcus aureus: an experimental study in rats. J. Infect. Dis. 2022;225:1452–1459. doi: 10.1093/infdis/jiab112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luong T., Calabria A., Roach D. Phage therapy in the resistance era: where do we stand and where are we going? Clin. Therapeut. 2020;42:1659–1680. doi: 10.1016/j.clinthera.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Dufour N., Delattre R., Chevallereau A., Ricard J.D., Debarbieux L. Phage therapy of pneumonia is not associated with an overstimulation of the inflammatory response compared to antibiotic treatment in mice. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.00379-19. e00379-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoyle N., Shaniya P., Balarjishvili N., Bolkvadze D., et al. Phage therapy against achromobacter xylosoxidans lung infection in a patient with cystic fibrosis: a case report. Res. Microbiol. 2018;169:540–542. doi: 10.1016/j.resmic.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Tan X., Chen H., Zhang M., Zhao Y., Jiang Y., et al. Clinical experience of personalized phage therapy against carbapenem-resistant acinetobacter baumannii lung infection in a patient with chronic obstructive pulmonary disease. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.631585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kvachadze L., Balarjishvili N., Meskhi T., et al. Evaluation of lytic activity of staphylococcal bacteriophage Sb‐1 against freshly isolated clinical pathogens. Microb. Biotechnol. 2011;4:643–650. doi: 10.1111/j.1751-7915.2011.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hua Y., Luo T., Yang Y., et al. Phage therapy as a promising new treatment for lung infection caused by carbapenem-resistant Acinetobacter baumannii in mice. Front. Microbiol. 2017;8:2659. doi: 10.3389/fmicb.2017.02659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yajun Z., Biao M., Xiao W., Yan L., et al. Evaluation of phage therapy for pulmonary infection of mouse by liquid aerosol-exposure Pseudomonas aeruginosa. Infect. Drug Resist. 2021;14:4457–4469. doi: 10.2147/IDR.S326230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu K., Yang W., Dong X., Cong L., et al. Inhalation study of mycobacteriophage D29 aerosol for mice by endotracheal route and nose-only exposure. J. Aerosol Med. Pulm. Drug Deliv. 2016;29:393–405. doi: 10.1089/jamp.2015.1233. [DOI] [PubMed] [Google Scholar]
- 38.Chang R., Wallin M., Lin Y., Leung S., et al. Phage therapy for respiratory infections. Adv. Drug Deliv. Rev. 2018;133:76–86. doi: 10.1016/j.addr.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.