Highlights
-
•
In treating COVID-19 patients, it is crucial to triage patients who are likely to become severe disease appropriately.
-
•
Patients with oxygen demand are older, obese, have numerous comorbidities, and have elevated D-dimer.
-
•
The liver-to-spleen ratio, lymphocyte count, D-dimer, and abdominal circumference are predictors of oxygen demand in COVID-19 patients.
Keywords: COVID-19, SARS-CoV-2, Oxygen demand, The liver-to-spleen ratio, Abdominal circumference
Abstract
Background
We aimed to investigate risk factors predicting oxygen demand in COVID-19 patients.
Methods
Patients admitted to Shizuoka General Hospital with COVID-19 from August 2020 to August 2021 were included. First, we divided patients into groups with and without oxygen demand. Then, we compared patients' clinical characteristics and laboratory and radiological findings to determine factors predicting oxygen demand.
Results
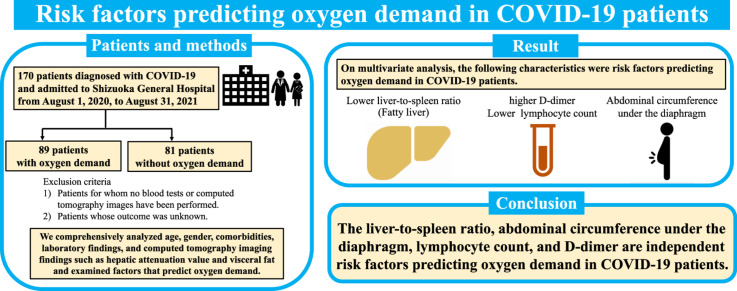
One hundred seventy patients with COVID-19 (aged 58±15 years, 57 females) were enrolled. Common comorbidities were cardiovascular diseases (47.6%), diabetes mellitus (28.8%), and dyslipidemia (26.5%). Elder age, higher body mass index, cardiovascular diseases, diabetes mellitus, lower lymphocyte count, albumin, hepatic attenuation value, and the liver-to-spleen ratio (L/S), higher D-dimer, aspartate aminotransferase, lactate dehydrogenase, troponin-T, C-reactive protein, KL-6, chest and abdominal circumference, and visceral fat were found in patients with oxygen demand. According to the multivariate logistic regression analysis, L/S, lymphocyte count, D-dimer, and abdominal circumference under the diaphragm were independent risk factors predicting oxygen demand in COVID-19 patients.
Conclusions
On admission, L/S, lymphocyte count, D-dimer, and abdominal circumference were predictive factors for oxygen demand. These factors may help in the appropriate triage of COVID-19 patients in the decision to admit them to the hospital.
Graphical abstract
1. Introduction
In late 2019, a novel coronavirus was identified as the cause of pneumonia, which spread rapidly, resulting in the global pandemic. The number of infections is increasing worldwide, and the clinical manifestations range from asymptomatic cases to patients with mild and severe symptoms, with or without pneumonia [1]. Among patients with COVID-19, 81.4% remained in mild disease, while 13.9% reported moderate disease and 4.7% progressed to severe disease [2]. However, with the rapid increase in COVID-19 patients, most patients were treated at home rather than hospitalized in Japan. Therefore, it is crucial to appropriately triage patients who are most likely to become severe disease.
The risk of severe disease from COVID-19 is being studied. Many studies have examined risk factors for severe disease based on age, gender, comorbidity, and laboratory examination findings [3]. In comorbidity, cardiovascular diseases, diabetes, and hypertension have been associated with oxygen demand and death [4]. However, there are few studies on risk factors for severe disease related to computerized tomography (CT) images, and those analyses focused on lung field findings. Although some studies have focused on hepatic attenuation value and visceral fat, prognostic factors have been analyzed for hepatic attenuation value alone or visceral fat alone [5,6]. Therefore, we comprehensively analyzed age, gender, comorbidities, laboratory findings, and CT imaging findings such as hepatic attenuation value and visceral fat and examined factors that predict oxygen demand.
2. Patients and methods
2.1. Study design and participants
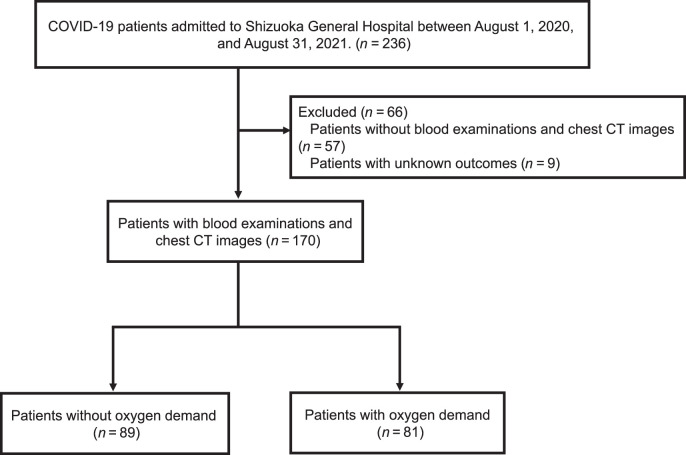
This was a retrospective single-center study of adult patients. Patients included in the study had been diagnosed with COVID-19 and admitted to Shizuoka General Hospital from August 1, 2020, to August 31, 2021. Specific inclusion criteria were: (1) patients whose blood examinations had been performed; (2) patients whose computed tomography (CT) images could be analyzed using SYNAPSE VINCENT and (3) patients whose outcome was known. We set the target SpO2 at 93–96% and started supplemental oxygen when the oxygen saturation was less than 93% at rest. Supplemental oxygen was adjusted to maintain this range. According to the oxygen demand on admission or during hospitalization, we divided patients into groups with and without oxygen demand (Fig. 1). Even if there was no oxygen demand at the time of admission, if oxygen demand emerged during hospitalization, the patient was included in the group with oxygen demand. In other words, the group without oxygen demand was defined as patients who did not require oxygen on admission and during hospitalization. Treatment was based on the severity of COVID-19 according to the guidelines issued by the Ministry of Health, Labour and Welfare (Japan).
Fig. 1.
Patient flow through this study.
2.2. Data collection
We extracted patients' personal and clinical data included in the study from electronic medical records. Personal data included sex, age, body mass index (BMI), epidemiological history, history of smoking, and comorbidities (e.g., cardiovascular diseases, diabetes, dyslipidemia, respiratory disease, chronic renal failure, immunodeficiency, and malignancy). Clinical data included days from onset to hospitalization, respiratory support, laboratory data, CT images, treatment, and outcome.
2.3. Laboratory examinations and CT images
We performed laboratory examinations and chest CT images on admission. Laboratory examinations included complete blood count, coagulation profile, serum biochemical tests (including liver function, creatine kinase, lactate dehydrogenase, and electrolytes), myocardial enzymes, serum ferritin, immunoglobulin, KL-6, SP-D, and procalcitonin. CT was performed without contrast material, from lung apex to upper abdomen, and in a single breath at maximum inspiration after the patient received breathing instructions.
2.4. CT images analysis
CT images comprised 1 mm slice thickness throughout the entire lung. The chest CT images analyzed the visceral fat, subcutaneous fat, chest circumference, abdominal circumference, hepatic attenuation value, and splenic attenuation values. Hepatic and splenic attenuation values were measured using area region of interest (ROI) greater than 100 mm2. Hepatic attenuation values were calculated by placing 2 ROIs greater than 100 mm2 in the liver while excluding regions of nonuniform parenchymal attenuation, including hepatic vessels. Splenic attenuation was obtained by placing 1 ROI greater than 100mm2 in the spleen. The liver-to-spleen ratio (L/S) was calculated by taking the average Hounsfield unit (HU) measurement of both hepatic ROIs and dividing it by the spleen HU value [7]. Additionally, we measured the visceral fat, subcutaneous fat, and abdominal circumference in CT cross-sections under the diaphragm and the subcutaneous fat and chest circumference at the fourth thoracic vertebra using the SYNAPSE VINCENT image analysis system (Fujifilm Inc., Tokyo, Japan). We set the lower and upper thresholds of visceral and subcutaneous fat analysis to −200 HU and −50 HU, respectively.
2.5. Statistical analysis
Categorical variables were presented as numbers (percentages) and analyzed using Fisher's exact test. Continuous variables were expressed as mean±standard deviation and analyzed using t test. The logistic regression model was used for univariate and multivariate analyses to identify oxygen demand associated variables. p < 0.05 was considered statistically significant. All analyses were performed with EZR software (version 1.27, Saitama Medical Center, Jichi Medical University, Saitama, Japan) [8].
2.6. Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Shizuoka General Hospital (SGHIRB#2022017).
3. Results
3.1. Baseline clinical characteristics
Table 1 indicates the subjects' clinical characteristics, comorbidity, and therapeutic drugs. This study included 170 patients, including 113 males and 57 females. The mean age and BMI were 58 and 24.7, relatively. Twenty-five patients were current smokers, 57 were ex-smokers, and 88 were never smokers. The most common comorbidity was cardiovascular diseases (81, 47.6%), followed by diabetes mellitus (49, 28.8%) and dyslipidemia (45, 26.5%). One hundred seventeen patients received dexamethasone, and 73 patients received remdesivir. Twenty-nine patients with severe disease or a high likelihood of developing severe disease received tocilizumab or baricitinib.
Table 1.
Clinical characteristics of the subjects.
| All n = 170 |
Oxygen demand (−) n = 89 |
Oxygen demand (+) n = 81 |
p value | |
|---|---|---|---|---|
| Age, years | 58.36±15.06 | 55.76±15.21 | 61.22±14.46 | 0.002 |
| Sex, male/female | 113/57 | 54/35 | 59/22 | 0.106 |
| BMI, kg/m2 | 24.71±4.82 | 23.94±4.07 | 25.55±5.43 | 0.003 |
| Smoking history, never/ex/current smoker | 88/57/25 | 48/24/17 | 40/33/8 | 0.082 |
| Pack-years | 13.92±22.10 | 10.81±17.20 | 17.33±26.15 | 0.054 |
| Days from onset to hospitalization, days | 6.54±3.03 | 6.48±3.18 | 6.61±2.88 | 0.792 |
| Comorbidity (%) | ||||
| Cardiovascular diseases | 81 (47.6) | 32 (36.0) | 49 (50.5) | 0.002 |
| Diabetes mellitus | 49 (28.8) | 19 (21.3) | 30 (37.0) | 0.028 |
| Dyslipidemia | 45 (26.5) | 20 (22.4) | 25 (30.9) | 0.228 |
| Respiratory diseases | 24 (14.1) | 9 (10.1) | 15 (18.5) | 0.128 |
| Chronic kidney failure | 24 (14.1) | 14 (15.7) | 10 (12.3) | 0.660 |
| Immunodeficiency | 8 (4.7) | 4 (4.5) | 4 (4.9) | >0.999 |
| Malignant tumor | 5 (2.9) | 2 (2.2) | 3 (3.7) | 0.670 |
| Treatment (%) | ||||
| Dexamethasone | 117 (68.8) | 39 (43.8) | 78 (96.3) | <0.001 |
| Remdesivir | 73 (42.9) | 21 (23.6) | 52 (64.2) | <0.001 |
| Anticoagulant medicines | 27 (15.9) | 0 (0) | 27 (33.3) | <0.001 |
| Favipiravir | 25 (14.7) | 6 (6.7) | 19 (23.5) | 0.002 |
| Baricitinib | 19 (11.1) | 0 (0) | 19 (23.5) | <0.001 |
| Ciclesonide | 12 (7.1) | 7 (7.9) | 5 (6.2) | 0.769 |
| Tocilizumab | 10 (5.9) | 0 (0) | 10 (12.3) | <0.001 |
| Casirivimab and Imdevimab | 5 (2.9) | 5 (5.6) | 0 (0) | 0.060 |
Continuous variables were expressed as mean±SD and categorical variables were expressed as number (percentage). Data were compared using t test or Fisher's exact test.
BMI, body mass index.
3.2. Comparison of groups with and without oxygen demand
The comparisons of laboratory results and CT images between groups with oxygen demand and without oxygen demand are shown in Table 1, Table 2, Table 3. Compared with a group without oxygen demand, the group with oxygen demand was older in age (p = 0.002) and had higher BMI (p = 0.003), had a higher prevalence of cardiovascular diseases (p = 0.002) and diabetes mellitus (p = 0.028) (Table 1). Also, a group with oxygen demand had lower lymphocyte count (p = 0.031) and albumin (p < 0.001), higher D-dimer (p < 0.001), aspartate aminotransferase (AST) (p = 0.001), lactate dehydrogenase (p < 0.001), troponin-T (p = 0.046), C-reactive protein (CRP) (p < 0.001), KL-6 (p < 0.001). The differences between the other parameters were not statistically significant (p > 0.05) (Table 2). In comparison to a group without oxygen demand, the group with oxygen demand was lower hepatic attenuation value (p < 0.001) and L/S (p < 0.001), higher chest circumference at the 4th thoracic vertebra, the visceral fat, visceral/subcutaneous fat ratio, and abdominal circumference under the diaphragm (Table 3).
Table 2.
Laboratory results of the subjects.
| Oxygen demand (−) n = 89 |
Oxygen demand (+) n = 81 |
p value | |
|---|---|---|---|
| WBC, ×102/μL | 57.66±25.48 | 58.20±26.96 | 0.894 |
| Hb, g/dL | 14.51±1.78 | 14.76±1.88 | 0.364 |
| Neutrophil count, /μL | 4284.80±2455.02 | 4540.38±2639.49 | 0.515 |
| Lymphocyte count, /μL | 1052.49±382.46 | 919.26±412.38 | 0.031 |
| D-dimer, μg/mL | 0.93±0.38 | 1.40±1.07 | <0.001 |
| Albumin, g/dL | 3.76±0.42 | 3.49±0.43 | <0.001 |
| Total cholesterol, mg/dL | 166.38±30.49 | 162.67±31.27 | 0.485 |
| HDL cholesterol, mg/dL | 48.68±17.62 | 46.73±12.61 | 0.462 |
| Neutral fat, mg/dL | 117.81±45.92 | 122.44±44.28 | 0.542 |
| AST, IU/L | 39.65±33.17 | 56.21±32.61 | 0.001 |
| ALT, IU/L | 39.16±40.49 | 44.23±29.17 | 0.355 |
| LDH, IU/L | 299.45±84.53 | 411.21±155.32 | <0.001 |
| BNP, pg/mL | 59.23±171.22 | 86.40±411.00 | 0.574 |
| Troponin T, ng/mL | 0.011±0.012 | 0.018±0.032 | 0.046 |
| CRP, mg/dL | 4.56±5.73 | 7.99±6.53 | <0.001 |
| IgG, mg/dL | 1209.59±270.86 | 1169.52±322.83 | 0.428 |
| IgA, mg/dL | 267.04±101.14 | 240.48±84.11 | 0.091 |
| IgM, mg/mL | 86.76±40.13 | 77.94±36.63 | 0.172 |
| Procalcitonin, ng/mL | 0.12±0.26 | 0.30±1.06 | 0.169 |
| Ferritin, ng/mL | 684.57±763.12 | 698.00±1141.51 | 0.928 |
| HbA1c, % | 6.23±1.01 | 6.36±0.92 | 0.527 |
| KL-6, IU/mL | 227.90±77.43 | 346.93±211.51 | <0.001 |
| SP-D, ng/mL | 47.19±60.40 | 32.89±26.47 | 0.068 |
The variables were expressed as mean±SD and compared using t test.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BNP, brain natriuretic hormone; CRP, C-reactive protein; Hb, hemoglobin; HDL, high-density lipoprotein; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; WBC, white blood cell.
Table 3.
Image findings of the subjects.
| Oxygen demand (−) n = 89 |
Oxygen demand (+) n = 81 |
p value | |
|---|---|---|---|
| Hepatic attenuation value, HU | 52.65±10.99 | 45.12±13.72 | <0.001 |
| Splenic attenuation value, HU | 50.40±3.45 | 49.94±3.10 | 0.364 |
| The liver-to-spleen ratio | 1.05±0.22 | 0.91±0.29 | <0.001 |
| The subcutaneous fat of the 4th thoracic vertebra, cm2 | 115.15±65.61 | 135.73±77.28 | 0.062 |
| Chest circumference of the 4th thoracic vertebra | 94.17±12.50 | 97.71±8.52 | 0.034 |
| The subcutaneous fat area under the diaphragm | 80.19±49.98 | 88.71±54.12 | 0.287 |
| The visceral fat area under the diaphragm | 109.71± 72.97 | 144.98±88.37 | 0.005 |
| The visceral/subcutaneous fat ratio under the diaphragm | 1.63±1.08 | 2.10±1.56 | 0.022 |
| abdominal circumference under the diaphragm | 89.27±9.67 | 93.94±9.34 | 0.002 |
The variables were expressed as mean±SD and compared using t test.
HU, Hounsfield unit.
3.3. Univariate and multivariate analyses of risk factors predicting oxygen demand in COVID-19 patients
3.3.1. Univariate analysis
On univariate analysis, the following characteristics were associated with an increased odds of oxygen demand: age of patients (odds ratio (OR) 1.03, CI 1.00–1.05), BMI (OR 1.08, CI 1.01–1.16), diabetes mellitus (OR 2.17, CI 1.10–4.27), cardiovascular diseases (OR 2.73, CI 1.47–5.08), D-dimer (OR 5.52, CI 2.18–14.00), abdominal circumference under the diaphragm (OR 1.05, CI 1.02–1.09), the visceral fat area under the diaphragm (OR 1.01, CI 1.00–1.02). On the other hand, the following factors were associated with decreased odds ratios: L/S (OR 0.10, CI 0.27–0.39), lymphocyte count (OR 0.999, CI 0.998–1.000) (Table 4).
Table 4.
Univariate and multivariate linear regression analysis of factors predicting oxygen demand in COVID-19 patients.
| Variable | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age, years | 1.03 | (1.00, 1.05) | 0.020 | 1.02 | (0.99, 1.05) | 0.223 |
| Sex, male | 1.74 | (0.91, 3.32) | 0.095 | |||
| BMI, kg/m2 | 1.08 | (1.01, 1.16) | 0.034 | 0.95 | (0.84, 1.06) | 0.348 |
| Smoking history, yes | 1.20 | (0.66, 2.19) | 0.553 | |||
| Diabetes mellitus | 2.17 | (1.10, 4.27) | 0.026 | 0.93 | (0.38, 2.27) | 0.869 |
| Cardiovascular diseases | 2.73 | (1.47, 5.08) | 0.002 | 1.72 | (0.76, 3.90) | 0.191 |
| The liver-to-spleen ratio | 0.10 | (0.27, 0.39) | <0.001 | 0.14 | (0.02, 0.93) | 0.042 |
| Lymphocyte count, /μL | 0.999 | (0.998, 1.00) | 0.003 | 0.999 | (0.998, 1.00) | 0.045 |
| D-dimer, μg/mL | 5.52 | (2.18, 14.00) | <0.001 | 4.64 | (1.60, 13.40) | 0.043 |
| Abdominal circumference under the diaphragm, cm | 1.05 | (1.02, 1.09) | 0.003 | 1.09 | (1.01, 1.18) | 0.024 |
| The visceral fat area under the diaphragm, cm2 | 1.01 | (1.00, 1.02) | 0.007 | 0.99 | (0.99, 1.00) | 0.340 |
BMI, body mass index.
3.3.2. Multivariable analysis
Multivariate analysis indicated that L/S (OR 0.14, CI 0.02–0.93), lymphocyte count (OR 0.999, CI 0.998–1.000), D-dimer (OR 4.46, CI 1.60–13.40), abdominal circumference under the diaphragm (OR 1.09, CI 1.01–1.18) were risk factors predicting oxygen demand in COVID-19 patients (Table 4).
4. Discussion
This study described abdominal circumference under the diaphragm, L/S, lymphocyte count, and D-dimer as independent risk factors predicting oxygen demand in COVID-19 patients.
Previous meta-analyses have reported that high D-dimer and low lymphocyte counts are poor prognostic factors [9], [10], [11]. This known finding is consistent with our study. The postulated mechanisms for lymphocytopenia in patients with severe COVID-19 include inflammatory cytokine storms such as TNF-α and IL-6 [12], cell exhaustion due to COVID-19 infection [13], infection of T cells with the SARS-CoV-2 [14], and downregulation of genes involved in T cell activation and function due to SARS-CoV-2 infection [15]. This study did not examine inflammatory cytokines such as IL-6 and TNF-α or T-cell counts. However, CRP is a nonspecific acute-phase protein induced by IL-6 [16], and CRP was significantly higher in the group with oxygen demand in this study. Therefore, it is highly likely that inflammatory cytokines are involved in the appearance of oxygen demand.
Liver injury is a well-known pathologic feature of COVID-19, with systematic reviews reporting the incidence of elevations in AST from 2.5% to 50.0% and alanine aminotransferase from 2.5% to 61.1% [17]. According to a previous report, a liver biopsy specimen from a patient who died due to severe COVID-19 showed hepatic steatosis, mild lobular and portal inflammation [18,19]. Concerning liver injury in COVID-19, immune-mediated damage as a result of the severe inflammatory response following SARS-CoV-2 infection [20], direct cytotoxicities as a result of active viral replication in hepatic cells [21], and anoxia [22] are thought to be involved. A previous report showed that L/S was reduced in cases that led to severe disease than in those that did not [23]. Therefore, it is possible that a decreased L/S indirectly reflects that hepatic steatosis and mild lobular and portal inflammation result from liver damage due to the above. On the other hand, underlying preexisting liver disease is a risk factor for increased severity of COVID-19 [24]. In our study, L/S before SARS-CoV-2 infection was not evaluated, and it is unclear whether the decrease in L/S was present before SARS-CoV-2 infection. The relationship between L/S and oxygen demand is not a clear conclusion, but the decrease in L/S is an objective indicator of the presence of fatty liver. Chang et al. demonstrated that fatty liver was positively associated with an increased risk of severe COVID-19, requiring mechanical ventilation, admission to the intensive care unit (ICU), and high-flow oxygen therapy [25]. Moreover, a previous study reported increased hepatic expression of the SARS-CoV-2 entry points angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2) in individuals with fatty liver [26]. Therefore, it has been noted that more ACES and TMPRSS2 in patients with fatty liver may facilitate SARS-CoV-2 entry into cells and lead to the progression to severe COVID-19. Hence, we should consider the possibility of progression to severe disease in patients with decreased L/S.
Abdominal circumference was also an independent predictor of oxygen demand in this present study. Obesity has been a risk factor for severe disease in previous studies [27,28], and abdominal circumference is an objective value that reflects obesity. However, it is noteworthy that the group with oxygen demand had significantly more visceral fat than those without oxygen demand. Previous studies have reported that visceral fat, but not subcutaneous fat, is a factor in the severity of COVID-19 [29], [30], [31], which is consistent with the results of this study. Furthermore, visceral fat was more strongly correlated with COVID-19 severity than BMI and subcutaneous fat [6,32], suggesting that visceral fat has some relationship to COVID-19 severity. In obese patients, visceral adipocytes secrete IL-6 and increase the production and release of leptin, which enhances the pro-inflammatory state [33,34]. Conversely, subcutaneous fat produces and secretes adiponectin, which acts against inflammatory conditions by suppressing the secretion of IL-6 and TNF-α and increasing the production of anti-inflammatory cytokines [35]. It is known that adiponectin decreases as visceral fat increases, and furthermore, it has been reported that adiponectin is decreased in COVID-19 compared to healthy controls, even when BMI matched [36]. Therefore, high visceral fat may be one of the key triggers for the underlying exacerbated inflammatory state associated with severe COVID-19.
It should be noted here that the present study measured visceral fat under the diaphragm. Previous studies were measured between the third and fourth lumbar vertebrae or at the first lumbar vertebrae. However, visceral fat remained significantly associated with metabolic syndrome regardless of measurement sites between T10–T11 and L5–S1 [37]. Thus, there is a certain validity to the association between visceral fat under the diaphragm and oxygen demand.
Managing all COVID-19 patients in the hospital is impossible, and we need to triage patients progressing to severe disease appropriately. In this study, patients with oxygen demand had larger abdominal circumference under the diaphragm and D-dimer, lower L/S and lymphocyte count. Therefore, if those patients are infected with COVID-19, they are more likely than those without to experience the emergence of oxygen demand. Consequently, they should be managed with pharmacotherapeutic intervention or strictly inpatient management from earlier when there is no oxygen demand.
This study has several limitations. First, this is a retrospective design and a small number of patients, leading to biased results. Thus, a large-scale multicenter survey with additional researchers is required. Second, since the study was limited to inpatients and outpatients were excluded, the proportion of severe patients and fatality rates may differ from the total infected population. Third, we did not evaluate the thoracic imaging characteristics of COVID-19. Fourth, we only assessed CT images on admission. The previous report demonstrated the changes in hepatic attenuation during the course of the disease in COVID-19 patients [23]. Therefore, the CT attenuation value may have changed with the time elapsed since the onset of the disease, which may have affected the results.
5. Conclusion
L/S, lymphocyte count, D-dimer, and abdominal circumference may predict the appearance of oxygen demand. Therefore, paying attention to L/S as well as laboratory examinations and comorbidities may be possible to extract patients with the more severe disease with greater accuracy.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
H.N.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing - original draft; S.S (Shogo Sakurai): Data curation, Formal analysis, Investigation, Methodology, Writing - review & editing; S.S (Syuichi Sugiyama): Data curation. K.S.: Data curation. K.O.: Data curation. Y.K. (Yoshihiro Kitahara): Conceptualization, Data curation. S.T.: Conceptualization, Data curation. T.M.: Conceptualization, Data curation. M.S.: Conceptualization, Data curation. A.Y.: Conceptualization, Data curation. T.A.: Conceptualization, Data curation, Formal analysis, Methodology. S.M.: Conceptualization, Data curation. K.A.: Conceptualization, Data curation, Formal analysis, Methodology. T.S.: Conceptualization, Supervision, Writing - review & editing.
Data available statement
Research data are not shared.
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Shizuoka General Hospital (SGHIRB#2022017).
Informed consent
The Board waived patient approval or informed consent because the study was a retrospective review of patient records.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
References
- 1.Dong X., Cao Y.Y, Lu X.X., et al. Eleven faces of coronavirus disease 2019. Allergy. 2020;75(7):1699–1709. doi: 10.1111/all.14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Peckham H., de Gruijter N.M., Raine C., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ICU admission. Nat. Commun. 2020;11(1):6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terada M., Ohtsu H., Saito S., et al. Risk factors for severity on admission and the disease progression during hospitalization in a large cohort of patients with COVID-19 in Japan. BMJ Open. 2021;11(6) doi: 10.1136/bmjopen-2020-047007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uchida Y., Uemura H., Yamaba S., et al. Significance of liver dysfunction associated with decreased hepatic CT attenuation values in Japanese patients with severe COVID-19. J. Gastroenterol. 2020;55(11):1098–1106. doi: 10.1007/s00535-020-01717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Favre G., Legueult K., Pradier C., et al. Visceral fat is associated to the severity of COVID-19. Metabolism. 2021;115 doi: 10.1016/j.metabol.2020.154440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeb I., Li D., Nasir K., et al. Computed tomography scans in the evaluation of fatty liver disease in a population based study: the multi-ethnic study of atherosclerosis. Acad. Radiol. 2012;19(7):811–818. doi: 10.1016/j.acra.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danwang C., Endomba F.T., Nkeck J.R., et al. A meta-analysis of potential biomarkers associated with severity of coronavirus disease 2019 (COVID-19) Biomark. Res. 2020;8:37. doi: 10.1186/s40364-020-00217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ou M., Zhu J., Ji P., et al. Risk factors of severe cases with COVID-19: a meta-analysis. Epidemiol. Infect. 2020;148 doi: 10.1017/S095026882000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghahramani S., Tabrizi R., Lankarani K.B., et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur. J. Med. Res. 2020;25(1):30. doi: 10.1186/s40001-020-00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzoni A., Salvati L., Maggi L., et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J. Clin. Invest. 2020;130(9):4694–4703. doi: 10.1172/JCI138554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diao B., Wang C., Tan Y., et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tavakolpour S., Rakhshandehroo T., Wei E.X., et al. Lymphopenia during the COVID-19 infection: what it shows and what can be learned. Immunol. Lett. 2020;225:31–32. doi: 10.1016/j.imlet.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouyang Y., Yin J., Wang W., et al. Downregulated gene expression spectrum and immune responses changed during the disease progression in patients with COVID-19. Clin. Infect. Dis. 2020;71(16):2052–2060. doi: 10.1093/cid/ciaa462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pepys M.B., Hirschfield G.M. C-reactive protein: a critical update. J. Clin. Invest. 2003;111(12):1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrido I., Liberal R., Macedo G. Review article: COVID-19 and liver disease-what we know on 1st May 2020 , Aliment Pharmacol. Ther. 2020;52(2):267–275. doi: 10.1111/apt.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagana S.M., Kudose S., Iuga A.C., et al. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod. Pathol. 2020;33(11):2147–2155. doi: 10.1038/s41379-020-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun J., Aghemo A., Forner A., et al. COVID-19 and liver disease. Liver Int. 2020;40(6):1278–1281. doi: 10.1111/liv.14470. [DOI] [PubMed] [Google Scholar]
- 23.Guler E., Unal N.G., Cinkooglu A., et al. Correlation of liver-to-spleen ratio, lung CT scores, clinical, and laboratory findings of COVID-19 patients with two consecutive CT scans. Abdom. Radiol. (NY) 2021;46(4):1543–1551. doi: 10.1007/s00261-020-02805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh S., Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology. 2020;159(2):768–771.e3. doi: 10.1053/j.gastro.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang Y., Jeon J., Song T.J., et al. Association between the fatty liver index and the risk of severe complications in COVID-19 patients: a nationwide retrospective cohort study. BMC Infect. Dis. 2022;22(1):384. doi: 10.1186/s12879-022-07370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meijnikman A.S., Bruin S., Groen A.K., et al. Increased expression of key SARS-CoV-2 entry points in multiple tissues in individuals with NAFLD. J. Hepatol. 2021;74(3):748–749. doi: 10.1016/j.jhep.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussain A., Mahawar K., Xia Z., et al. Obesity and mortality of COVID-19. Meta-analysis, Obes. Res. Clin. Pract. 2020;14(4):295–300. doi: 10.1016/j.orcp.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Yang J., Hu J., Zhu C. Obesity aggravates COVID-19: a systematic review and meta-analysis. J. Med. Virol. 2021;93(1):257–261. doi: 10.1002/jmv.26237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe M., Caruso D., Tuccinardi D., et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. 2020;111 doi: 10.1016/j.metabol.2020.154319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y., Ding L., Zou X., et al. Visceral adiposity and high intramuscular fat deposition independently predict critical illness in patients with SARS-CoV-2. Obesity (Silver Spring) 2020;28(11):2040–2048. doi: 10.1002/oby.22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen A., Bressem K., Albrecht J., et al. The role of visceral adiposity in the severity of COVID-19: highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;110 doi: 10.1016/j.metabol.2020.154317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pranata R., Lim M.A., Huang I., et al. Visceral adiposity, subcutaneous adiposity, and severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. Clin. Nutr. ESPEN. 2021;43:163–168. doi: 10.1016/j.clnesp.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiernan K., MacIver N.J. The role of the adipokine leptin in immune cell function in health and disease. Front. Immunol. 2020;11:3656. doi: 10.3389/fimmu.2020.622468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guglielmi V., Colangeli L., D’Adamo M., et al. Susceptibility and severity of viral infections in obesity: lessons from influenza to COVID-19. Does leptin play a role? Int. J. Mol. Sci. 2021;22(6):3183. doi: 10.3390/ijms22063183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohashi K., Shibata R., Murohara T., et al. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol. Metab. 2014;25(7):348–355. doi: 10.1016/j.tem.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Tonon F., Di Bella S., Giudici F., et al. Discriminatory value of adiponectin to leptin ratio for COVID-19 pneumonia. Int. J. Endocrinol. 2022;2022 doi: 10.1155/2022/9908450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuk J.L., Church T.S., Blair S.N., et al. Does measurement site for visceral and abdominal subcutaneous adipose tissue alter associations with the metabolic syndrome? Diabetes Care. 2006;29(3):679–684. doi: 10.2337/diacare.29.03.06.dc05-1500. [DOI] [PubMed] [Google Scholar]