Abstract
The genetics of intracranial aneurysms is complex. Much work has been done looking at the extracellular matrix surrounding cerebral vasculature as well as the role of matrix metalloproteinases. This comprehensive review summarizes what is known to date about the important genetic components that predispose to aneurysm formation and critically discusses the published findings. We discuss promising pre-clinical models of aneurysm formation and subarachnoid hemorrhage, and highlight avenues for future discovery, while considering limitations in the research to date. This review will further serve as a comprehensive reference guide to understand the genetic underpinnings for aneurysm pathophysiology and act as a primer for further investigation.
Keywords: Extracellular matrix, Matrix metalloproteinases, Aneurysm genetics, Preclinical models
1. Introduction
Intracranial aneurysms (IA) occur in ~3–6% of the population [1]. The incidence of aneurysmal subarachnoid hemorrhage (aSAH) is between 10 and 15 per 100,000 people per year. For those individuals harboring an IA that results in aSAH, about one-third will die; of the survivors, half will recover sufficiently to lead a functional life, and the remainder will need support in order to continue their normal daily activities [2]. Due to the devastation caused by aSAH, research has been devoted to elucidating the pathogenesis underlying IA formation, with the goal that an increased understanding of the disease process will allow for prevention, early identification, and treatment, for those at risk.
Prior to the rise of genetic sequencing and other genetic tools, epidemiological studies demonstrated several correlations between patterns of inheritance with IA formation and rupture. First and second degree relatives of patients who harbor an IA, or who have suffered aSAH, have a 2–4 fold increased risk of developing an IA themselves [3]. Further, there is also an approximately three times greater risk of aSAH among Japanese and Finnish populations compared to other nationalities [4]. Well-known genetic disorders including autosomal dominant polycystic kidney disease, Ehlers-Danlos syndrome, Loeys-Dietz syndrome, Marfan syndrome, tuberous sclerosis, and fibromuscular dysplasia are all associated with increased risk of IA development [2]. Each of these diseases is characterized by genetic mutations at unique loci, supporting the notion that the pathogenesis of IA may be mediated by a diverse group of gene products and regulatory proteins.
Investigation into the genetics of IAs has since been predicated upon either elucidating the culprit genes behind these recognized associations or discovering novel loci associated with IA risk. Genome-wide association studies (GWAS) have been pivotal to these efforts. The most robust GWAS to date have utilized single nucleotide polymorphism (SNP) data from thousands of cases and controls to reveal several susceptibility loci including the SOX17 and CDKN2A genes [5,6]. The proteins encoded by these genes regulate endothelial function and blood vessel formation, supporting the hypothesis that genetic variation affecting the extracellular matrix or related factors would most impact IA risk.
Histologically, IA aneurysms are characterized by a disruption of the inner elastic lamina (IEL), thinning of the tunica media with a reduction in the number of vascular smooth muscle cells (VSMC), and a disorganization of the extracellular matrix (ECM) [7]. These observed morphological changes have led researchers to focus on the expression of genes responsible for maintaining vascular wall integrity and preventing vascular remodeling following vessel wall injury. Research into the genetic basis of IA may yield the identification of specific biomarkers and provide for novel genetic therapies to alter clinical management of IA. This paper provides a critical evaluation of the current literature implicating specific genes underlying IA pathogenesis and avenues for future research. We specifically look at the supportive vascular matrix, matrix metalloproteinases, nitric oxide, and inflammatory mediators.
2. Pathways: supportive vascular matrix
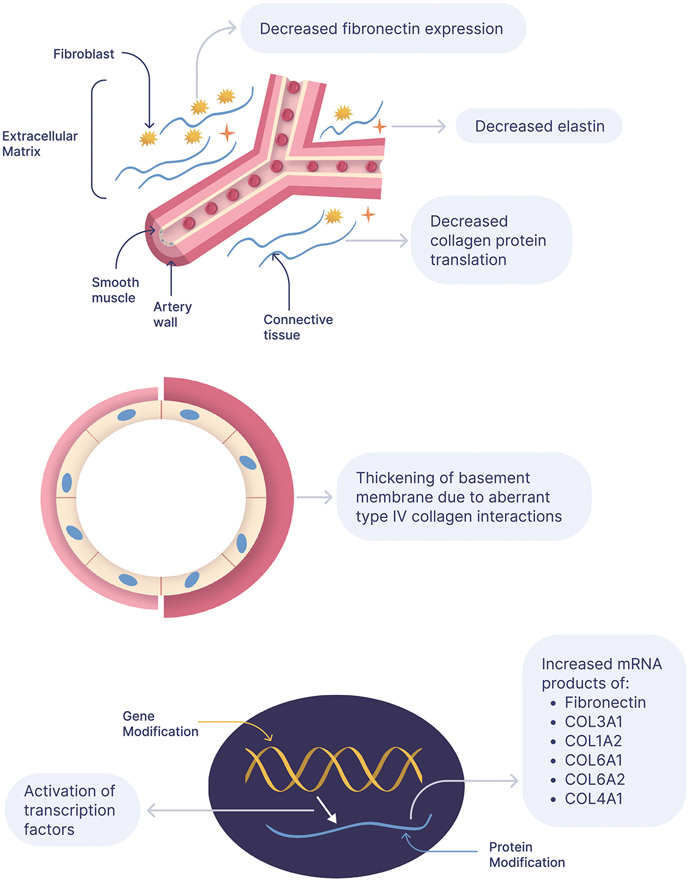
A complex interplay between factors influencing the synthesis, deposition, and proteolysis of ECM proteins is thought to contribute to aneurysmal development (Fig. 1). In order to accurately quantify protein levels, one must understand the balance between synthesis and degradation. An elevation in protein levels may be attributed to an increase in its synthesis, or a decrease in its degradation. Conversely, a reduction in protein levels may be attributed to a decrease in its synthesis, or an increase in its degradation.
Fig. 1.
Overview of the extracellular matrix regulation associated with aneurysm formation.
2.1. Upregulation of fibronectin
Peters and colleagues, reported a significant upregulation of mRNA encoding the ECM constituent fibronectin in the middle cerebral artery (MCA) of a three-year old child harboring multiple saccular aneurysms [8]. Utilizing tissue samples obtained from the same patient’s superior temporal artery (STA) as a control, they found fibronectin expression to be upregulated about 100-fold in intracranial aneurysms. Fibronectin is a glycoprotein dimer, consisting of two ~250 kDA subunits covalently linked near the C-terminus via a pair of disulfide bonds. The most abundant glycoprotein found in connective tissue, fibronectin contains multiple binding domains for various ECM molecules (ie. integrins, collagen/gelatin, heparin, and fibrin). The presence of these multifunctional binding domains permits fibronectin to mediate a myriad of cellular interactions within the ECM, including cell adhesion, migration, growth, and differentiation [9]. It is synthesized by endothelial cells, VSMC, and fibroblasts, and promotes vascular remodeling following a noxious insult [10].
It is thought that aneurysms develop as a result of an inability to maintain vascular homeostasis—that is, a faulty compensatory response to maintain vessel wall integrity after an environmental insult, such as smoking, age related damage, or sustained hypertension [11]. The elevated fibronectin mRNA, suggests a pattern of vascular proliferative repair in aneurysmal tissue [8].
Using a rat animal model of IA, Futami et al., performed immunohistochemical analysis to compare fibronectin levels in cerebral aneurysmal lesions and normal controls. In control rats, they observed continuous fibronectin staining in the arterial subendothelial layer. In contrast, early aneurysmal lesions in experimental rats were found to have an absence of fibronectin within the subendothelial layer. A third cohort, in which a proliferative repair response was induced following aneurysmal formation, was found to be immune-positive for fibronectin within the subendothelial layer [10].
The elevated fibronectin mRNA observed in human IAs suggests the presence of an ongoing reparative response. Yet, without immunohistochemical tissue analysis in human specimens, it is unclear whether these mRNA transcripts yield a functional protein product via translation. Furthermore, the ubiquity of potential protein products, owing to fibronectin mRNA alternative splicing [9], raises the possibility that this elevated population of fibronectin mRNA transcripts is absent in normal tissue. As such, specific nucleotide sequencing is indicated to detail the specific mRNA transcripts that are found to be elevated in IAs. This additional data will indicate whether the problem is at the transcriptional level, resulting in inappropriate mRNA products, or if downstream processes (ie. increased degradation) facilitate fibronectin turnover.
2.2. Altered expression of collagen subtypes
In addition to observing elevated fibronectin mRNA in IA tissue samples, Peters et al. noted an elevation of certain subtypes of the ECM component of collagen. Specifically, they noted that compared to the STA control, the IA tissue was characterized as having an elevation in mRNA transcripts for collagen type III alpha-1 (34:7), collagen type I alpha-2 (20:1), collagen type VI alpha-1 (13:1), collagen type VI alpha-2 (9:1), collagen type IV alpha-1 (5:0), and elastin (5:0) [8].
In tissue isolate from 15 patients with IA, Mimata et al. also reported an elevation of type III collagen mRNA in fibroblastic and VSMC [12]. Collagen type III alpha-1 is a product of the COL3A1 gene, and is the mutation site of individuals suffering from Ehlers-Danlos syndrome type IV [13]. This mutation negatively affects the metabolism of type III collagen, producing a nonfunctional pro-alpha-1 type III collagen chain, a major constituent of vessel walls, thereby predisposing these individuals to arterial rupture and aneurysm formation. In a study encompassing 220 patients with Ehlers Danlos type IV, Pepin et al., reported the incidence of cerebrovascular events to be 2.1% [14].
In a 1983 study published by Nicholls and colleagues, researchers obtained skin and STA biopsies from 17 patients undergoing surgery to repair an IA, and from 6 control patients undergoing surgical resection of either a glioma (3) or a meningioma (3). 11/17 of the aneurysmal patients demonstrated a deficiency of type III collagen, whereas all control patients had normal levels of type III collagen [15]. This finding suggested an association between type III collagen deficiency and aneurysmal development in persons not affected by Ehlers-Danlos type IV.
In an effort to identify any COL3A1 genetic polymorphisms correlated with IAs, Brega and colleagues isolated DNA from peripheral leukocytes extracted from whole blood in 19 patients who had been found to have cerebral aneurysms. DNA from patients with cystic fibrosis and Duchenne muscular dystrophy was used for a control, as these diseases are not known to have a correlation with connective tissue disease or cerebral aneurysms. Using AvaII endonuclease, Brega et al. produced variable COL3A1 gene fragments of sizes 5.7 kilobase (allele A) and 4.3 kilobase (Allele B), and a constant fragment of 2.6 kb. Of note, 58% of aneurysm patients harbored at least one type B allele; whereas, only 13% of control patients were found to have the type B allele—a statistically significant difference [16]. This finding suggests an association of the type B allele with aneurysmal development. However, van den Berg et al. note that this polymorphism is located in a non-coding intron, and suggest that such a variance is unlikely to have any biologically relevant consequence. Furthermore, Van den Berg et al. found no observable link between COL3A1 gene mutation and IA among 41 patients with confirmed berry aneurysms [17].
Currently, one cannot definitively apply causation to a specific COL3A1 gene polymorphism to IA development. The elevated COL3A1 mRNA transcript observed by Peters et al., would not conflict with findings of reduced protein levels in the event that these transcripts do not yield a functional product. This could be attributed to premature degradation of mRNA or proteins due to innate instability, thereby resulting in reduced protein levels [8].
The second collagen transcript Peters et al. found to be elevated, collagen type I alpha-2, is encoded by the COL1A2 gene, is located on chromosome 7q22.1 [18]. This gene partially encodes for type I collagen, which is present in blood vessels, and is the most abundant form of collagen found in the human body. Comprised of two alpha-1, and one alpha-2 chains, type I collagen, when deficient, has been associated with IAs [19]. Mutations in COL1A2 also result in osteogenesis imperfecta, a disease characterized by an autosomal dominant inheritance pattern resulting in bone fragility and connective tissue defects [20]. In a study comprising 260 Japanese patients with IA (115 of the familial variety), and 293 age-matched controls, Yoneyama et al., observed a significant association between three specific COL1A2 gene polymorphisms and IAs. Yoneyama and colleagues, observed correlative small nucleotide polymorphisms (SNPs) at position 32—a silent mutation—and a polymoprhism of intron 46. The greatest allelic association was observed with polymorphism rs42524, a mutation occurring at exon 28, resulting in an A→ P subsitution of the amino acid residue at position 459 [20]. They synthesized 2 collagen-related peptides, one with the normal amino acid sequence, and one with proline substitution at position 459. In solution, both protein structures formed triple helical aggregates, with thermal stability testing demonstrating increased stability following the proline substitution. From this finding, Yonyeama et al., postulate that the Pro-459 variant affects vascular wall stability by altering its rigidity or elasticity [18]. Extending the findings of Yonyeyama, and his colleagues, Zhu et al., analyzed the same polymoprhism using DNA samples taken from 225 IA patients and 326 age-matched controls. Consistent with previous findings, Zhu et al., observed a significant association between IA and the polymorphism rs42524 of COL1A2 for sporadic IA in the Chinese population [21]. Glasker et al., also identified a significant association between rs42524 and IA in a German population [22]. Because these findings are consistent among multiple ethnic groups, the association between rs42524 polymorphism of COL1A2 and IA is likely not unique to a specific demographic.
Peters et al. also observed an elevation in the transcript encoding type IV collagen alpha-1, a product of the COL4A1 gene. The alpha 1 collagen is ubiquitous in basement membrane of tissues located throughout the body [23]. In some individuals, COL4A1 mutations produce a unique phenotype termed HANAC syndrome, whereby these patients present with hereditary angiopathy, nephropathy, aneurysms, and muscle cramps [24]. The gene consists of 52 exons located at chromosome 13q34, and mutations of this gene have been found to cause sporadic, as well as recurrent intracerebral hemorrhages. The increased frequency of intracerebral hemorrhage in these patients is thought to be secondary to small vessel disease. Furthermore, mutations of this gene have been associated with the development of IAs, suggesting a significant role for type IV collagen-alpha 1 DNA mutations resulting in cerebrovascular disease [25].
In animal studies, Futami et al., noted a pattern of type I and IV collagen disintegration similar to that observed in fibronectin. In normal control rats, Futami et al. observed continuous staining of both types of collagen in the subendothelial layer, in the area surrounding VSMC, and a diffuse staining in the tunica adventitia. As with fibronectin, aneurysmal lesions were void of both types of collagen in the subendothelial layer. In addition, for those rats with aneurysmal lesions in which a proliferative repair response was induced, Futami et al., failed to observe a reconstitution of type I and type IV collagen staining [10]. Despite the elevated mRNA transcripts observed in IA tissue, animal studies do not demonstrate an increase in collagen protein in aneurysmal tissue. As with fibronectin, identification of the specific nucleotide sequence increased in human aneurysmal tissue is necessary to completely characterize the pattern of collagen gene expression observed.
The last two collagen subtypes Peters et al., found elevated in IA—collagen type VI-alpha 1 and collagen type VI-alpha 2—together partially encode type VI collagen [8]. The COL6A1 and COL6A2 genes are present on chromosome 21. Mutations in the genes encoding type VI collagen (COL6A1 and COL6A2) result in Bethlem myopathy—a condition characterized by contracture of multiple joints, generalized muscle weakness, and wasting [26]. Type VI collagen is found in all tissues containing type I/III collagen fibers, and is thought to serve as an attachment site to anchor blood vessels to the surrounding ECM. In vitro studies have demonstrated the ability of type VI collagen to interact with various ECM components, including proteoglycans, collagens, hyaluronan, heparin, and integrins. In a study published by Kuo et al., type VI collagen was found to have significant binding capacity for type IV collagen, fibronectin, and type VI collagen—with the strongest binding observed between type VI and type IV collagen. Using immunofluorescent labeling, Kuo and colleagues observed co-localization of type VI and type IV collagen, which suggests a vital role for type VI collagen in maintaining endothelial basement membrane integrity. The interaction between type VI and IV collagen is further substantiated by experiments demonstrating that, following incubation, one can co-immunoprecipitate both forms of collagen using antibodies targeting either type IV or type VI collagen [25]. Interestingly, Armstrong et al., report a downregulation of collagen type VI-alpha-1 mRNA in human abdominal aortic aneurysms [27]. Studies have shown that the specificity of collagen type IV/VI binding is dependent on an intact VI-alpha1 subunit [25]. It stands to reason that a reduction in type VI-alpha-1 protein may lead to arterial structural instability by de-stabilizing basement membranes. In a 1988 study, Roggendorf et al., found type VI collagen to be absent from normal, human cortical vasculature; however, it was found to be present in the intima and media in cerebral blood vessels isolated from hypertensive patients via a surgical biopsy [28]. Mimata et al. were able to confirm these findings. In control samples, the researchers found type VI collagen only present in the adventitia; whereas, in human aneurysmal tissue, type VI collagen was found present in the luminal wall and abluminal layer [12]. Furthermore, type VI collagen was found to be co-distributed with type III collagen. From these findings, Roggendorf et al., surmised that a local elevation in cerebral blood pressure results in an altered pattern of vascular development causing aberrant type VI collagen deposition, and through its interaction/stabilization with other ECM proteins, a thickening of vascular basement membranes [28]. Of note, arterial hypertension has been identified as one of the most significant risk factors associated with the development of IA amongst the general population [29]. Based on the work of Mimata et al. and Roggendorf et al., it appears that aneurysms demonstrate a pattern of inappropriate type VI collagen deposition, and this process begins in hypertensive patients prior to the development of an IA. This theory would coincide with the elevation of type VI alpha-1 and type VI alpha-2 observed in IA by Peters et al. Because the patient in Peters report was only three years old, it seems likely that alterations in type VI collagen gene expression may result in aneurysmal development independent of hemodynamic stressors.
2.3. Altered elastin expression
In addition to collagen and fibronectin, Peters et al. also observed an upregulation of mRNA encoding the protein elastin [8]. Located on chromosome 7q11.2, the 45 kb elastin (ELN) gene contains 34 exons, and its corresponding protein contributes to the elastic characteristic of blood vessels [30]. Synthesized by VSMCs, elastin is released as the monomer tropoelastin, and its final assembly is mediated by the cross-linking enzyme lysyl oxidase [31]. Marfan syndrome, a connective tissue disorder caused by mutation of the FBN1 gene, has been shown to be associated with IAs. It is thought that through its interactions with elastin, fibrillin-1, a product of the FBN1 gene, provides structural stability to connective tissues [32]. Studies have shown that patients with Marfans syndrome have altered elastin metabolism in the vessel wall of the ascending aorta [33].
In a study of familial IA in the Japanese population comprising 104 affected sibling pairs, Onda et al., observed 14 unique polymorphisms of the ELN gene. Moreover, linkage analysis revealed a significant pattern of co-inheritance between the D7S2472 marker and ELN—separated from each other by 400 kb. Furthermore, Onda et al. found the frequency of the Mm haplotype (INT20/INT23) to be significantly higher in the IA Japanese population than in the controls. From this finding, the researchers propose the presence of this haplotype predisposes individuals to developing an IA [34]. Extending upon these results, Farnham et al. also reported a significant linkage between the D7S2421 and the ELN gene in patients from Utah harboring an IA [35]. This finding indicates that ELN linkage association with IA is not restricted to the Japanese population, and may be involved in familial IA development in other demographics.
Further implicating the ELN gene for IA development, Akagawa et al., identified a SNP of the 3’-untranslated region of ELN to be associated with IAs in Japanese patients [31]. Using umbilical artery smooth muscle cells (UASMCs), ex vivo studies demonstrate a reduction of ELN transcription in cells with an adenine insertion SNP at position 502. Furthermore, Akagawa et al. performed in vitro transfection studies of HEK293 cells with the aforementioned ELN 3’-UTR SNP, and showed a reduction in luciferase activity—as compared to the control vector without insertion. Because ELN mRNA levels are regulated by mRNA/protein interactions, the researchers synthesized two 53 nucleotide digoxigenin-labeled RNA transcripts to test for the presence of an A +502 SNP allele specific RNA-protein interaction. One transcript contained the ELN 3’-UTR nucleotides +477 to +529, and the other contained an adenine insertion at +502. When DIG-labeled riboprobes were incubated with HEK293 cytoplasmic extracts, an allele-specific interaction was observed in the A +502 SNP allele—an interaction that was absent in the major allele. From this, they concluded that the A +502 SNP results in mRNA instability that may be attributed to accelerated degradation caused by an unidentified cytoplasmic factor. Due to the inherent instability of this mRNA, the authors suggested that ELN 3’-UTR (+502) produces less elastin, thereby creating a scenario of elastin deficiency in the vascular wall and increased susceptibility to aneurysmal development [36].
Association of the ELN locus with IA has not been without controversy; Yamada et al. and Mineharu et al., found no evidence of linkage for the ELN locus and familial intracranial aneurysms in the Japanese population [37,38]. Berthelemy-Okazaki et al., were not able to replicate the results of Farnham et al., and found no association between ELN polymorphism and familial IA in the Utah population [39]. Furthermore, in a population of Central European patients with IAs, Hofer et al. report no linkage association of the ELN gene with IAs [40]. These findings were corroborated by McColgan et al., in a genetic meta-analysis that revealed no significant association between ELN and IAs [41]. In a post-mortem study, Chyatte et al. compared the elastin content of MCAs harvested at autopsy from five patients who died from aSAH to three control patients whose death was unrelated to cerebrovascular disease. Using Van Gieson stain, no differences in elastin content was observed between the two groups; however, the region of MCA harvested in those patients who died from aSAH was void of aneurysmal lesions [42]. Therefore, the results of this study may not accurately depict the cellular changes that occur at the site of an aneurysmal lesion. In fact, Abruzzo et al. obtained five samples of recently ruptured human IAs, and found the aneurysmal orifice to be demarcated from the parent artery by an absence of the internal elastic lamina [43].
2.4. Abnormal ECM assembly
Another marker, versican, a member of the hylauranon-binding proteoglycan family, is a major ECM component of soft tissues. Found on chromosome 5q12-14, the versican gene consists of 16 exons encompassing more than 90 kb of DNA. It is expressed by VSMC, and immunohistochemical analysis has shown versican to lie in close association with blood vessels [44] Versican interacts with the elastin fiber-associated protein fibrillin, which, in turn binds fibulin 2. Fibulin 2 binds to elastin, and through this indirect action, it is though that versican serves to mediate the organization, and assembly of elastic fibers [45].
Because of its role in ECM assembly, Ruigrok et al. sought to ascertain the presence of a link between the versican gene and IAs in the Dutch population. Of 16 SNPs, Ruigrok and colleagues, observed two markers—rs251124 and rs173686—yielding the highest association with IAs. Rs251124 was found to be present in 18.1% of IA patients, and in only 12.8% of control patients. Furthermore, rs173686 was present in 39.3% of persons with IAs; whereas, 32.2% of control patients were found positive for this allele. From these findings, Ruigrok et al. assert that variation in or near the versican gene plays a role in susceptibility to IAs [45]. Further implicating the versican gene in IA susceptibility, Onda et al. also found evidence of linkage at 5q22-31 in the Japanese population [34]. In addition, the 5q13-14 locus has been previously reported to increase susceptibility to thoracic aortic aneurysms, suggesting a common genetic etiology between IAs and aortic aneurysms [46].
Owing to alternative splicing, the versican gene produces multiple protein isoforms with varying consequences for ECM assembly. Moreover, the two SNPs Ruigrok et al., report to be associated with IAs occur within two exons (6 and 7) where alternative splicing takes place [45]. These exons encode binding domains for the glycosaminoglycan chondroitin sulfate. The largest splice variant (V0) contains both exons 6 and 7; whereas, in the smallest variant (V3) these domains are absent. When overexpressed, V3 promotes cell adhesion, reduces growth and migration, and induces tropoelastin synthesis [47]. Ruigrok et al. suggest that patients with IAs may be characterized by an alternative splicing mechanism that favors production of the largest splice variant, thereby resulting in a reduction in ECM assembly [45]. When comparing IA tissue to an STA control, neither Peters et al. nor Changbin et al., observed differences in the pattern of versican gene expression [48]. However, because of alternative splicing, quantitative analysis of versican gene expression is inefficient. Because the various isoforms mediate distinct functions—owing to the presence, or lack thereof, of specific GAG binding domains—direct nucleotide sequence accurately characterizes the distinct species of versican mRNA present in IA tissue.
2.4.1. Matrix metalloproteinases
In addition to the aforementioned ECM structural components, factors regulating ECM turnover may also be implicated in IA development and pathogenesis. More specifically, abnormalities in ECM metabolism resulting in accelerated degradation could predispose individuals to IA development, and subsequent rupture. Matrix metalloproteinases (MMP) are a family of ECM proteases whose catalytic activity depends on the trace element zinc [49].
2.5. Upregulation of gelatinase A
A specific MMP, MMP2 is produced by fibroblasts, macrophages, leukocyte, smooth muscle cells, and endothelial cells [50,51]. Also referred to as gelatinase A, MMP2 mediates the proteolytic cleavage of type IV collagen found in basement membrane [52]. MMP2 is thought to play a role in both wound healing and angiogenesis [53]. The MMP2 gene is located on chromosome 16q13-q21 and mutations in this gene result in “vanishing bone” syndrome, an osteolytic bone disease [54].
In a study of 31 patients with cerebral aneurysms and 14 control patients—defined as persons undergoing craniotomy with no evidence of an aneurysm as determined by MRI or angiography—Chyatte et al. analyzed serum MMP2 enzymatic activity in samples of venous blood prior to surgical intervention [55]. Of the aneurysmal group, 52% of patients had suffered a previous aSAH. As compared to control patients, there was a three-fold increase in MMP2 enzymatic activity and a concordant reduction in serum procollagen peptide in the aneurysm group. However, in 15 patients with cerebral aneurysms, an increase in serum MMP2 activity was not observed. From these findings, it appears that increased serum MMP2 activity may be associated with the development and/or rupture of cerebral aneurysms. Because 15 patients did not demonstrate an increase in MMP2 enzymatic activity, this finding is best considered correlative, in that it does not reflect a sine qua non [42].
For those patients with an observed increase in serum MMP2, specific nucleotide sequence could indicate a link between MMP2 gene polymorphisms and the observed elevation in serum MMP2. Price et al., report the presence of fifteen SNPs in the MMP2 gene, with six variants in the promoter, one in the 5’UTR, one in a noncoding intron, and one in the 3’UTR. A cytosine to thymine transition mutation at position −1306 has been found to reduce MMP2 promoter activity by destroying the binding site for the transcriptional regulator protein Sp-1 [56].
2.6. MMP2 enzyme kinetics
In addition to alterations in gene expression, the accelerated rate of substrate degradation observed in MMP2 serum derived from aneurysmal patients may be attributed to altered MMP2 enzyme kinetics. As such, three-dimensional characterization of the enzyme of interest, in addition to assays measuring MMP2 enzyme kinetics, are needed to adequately differentiate any potential differences in MMP2 function between these two cohorts.
Li et al. furthered our understanding of MMP2 activity in IA patients by investigating enzyme characteristics of MMP2 specifically localized in aneurysmal lesions. They performed immunohistochemistry and western blot analysis on IA tissue obtained from 31 patients undergoing surgical clipping, and observed a significant increase in IA tissue MMP-2 protein levels—as compared to circle of Willis tissue samples obtained at autopsy from patients who died from causes other than cerebrovascular hemorrhage [57].
In a similar study, Caird et al. compared ten IA tissue samples obtained at autopsy from patients whose death was attributed to aSAH to nine from circle of Willis autopsy specimens obtained from patients whose death was due to reasons other than aSAH [58]. Consistent with the findings submitted by Li et al., Caird and colleagues observed little MMP2 immunostaining in control samples; however, both atherosclerotic and non-atherosclerotic aneurysmal lesions demonstrated moderate to extensive MMP2 immunostaining [58]. Using two IA tissue samples, Caird et al. sought to expand this finding by analyzing the MMP2 enzymatic activity in non-atherosclerotic aneurysmal tissue. Interestingly, they observed an absence of MMP2 enzymatic activity, suggesting an elevation of nonfunctional MMP2 in cerebral aneurysms [58]. Furthermore, Lovett et al. previously demonstrated that certain disease states are characterized by the expression of unique MMP-2 isoforms [59].
None of the aforementioned studies present data detailing the specific peptide sequence of the MMP2 protein in aneurysmal or control patients. As such, it is possible that unique MMP2 proteins exist within each of these groups. Without exhaustive efforts to characterize the functional activity of MMP2, and its 3D conformation in its active state, it is impossible to objectively compare MMP2 activity between aneurysmal and non-aneurysmal vascular tissue. The divergence in observed MMP2 activity as reported by Chyatte et al. and Caird et al. support the notion that serum MMP2 and aneurysmal MMP2 have unique functional properties, and may mediate aneurysmal development via unique pathways [55,58]. As such, complete characterization of the individual MMP2 polypeptide of interest will allow for more accurate comparison between patient groups within a given study, and between experiments seemingly comparing identical isoforms of MMP2—as determined by molecular weight.
2.7. Upregulation of MMP9
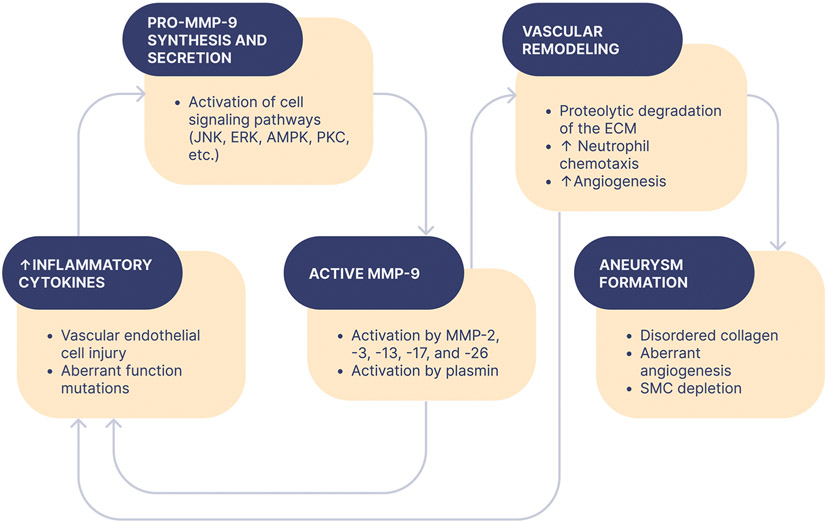
In addition to MMP-2, there is also evidence that MMP-9 plays a role in the development of IAs (Fig. 2) [58]. Transcribed from a gene located on chromosome 20q11.2-a13.1, MMP-9, also known as the collagenase type IV B and gelatinase-B, has been shown to be elevated in plasma obtained from patients with aortic root dilatations [60]. In addition, ten polymorphisms of the MMP9 gene have previously been reported by Zhang and colleagues [61]. In one SNP, a cytosine to thymine transition mutation at position −1562 of the MMP-9 promoter has been shown to result in a loss of a protein nuclear binding site and increased transcriptional activity of this gene [62]. Moreover, individuals harboring this SNP have been showed to be at an increased risk for developing severe coronary arteriosclerosis and IAs [63]. In addition, Shimajiri and colleagues observed an association between the length of a cytosine/adenine dinucleotide microsatellite repeat in the MMP-9 promoter and the transcriptional activity of this gene. More specifically, as compared to a 21 C/A dincucleotide repeat, luciferase assay of the MMP-9 promoter revealed that dinucleotide repeats of 18, 14, and 0 have 50%, 50%, and 5% of the transcriptional activity of a 21 C/A dinucleotide repeat, respectively [64].
Fig. 2.
The role of matrix metalloproteinase-9 (MMP) in aneurysm formation.
Similar to MMP2, Kim and colleagues observed a significant elevation in MMP-9, as determined by western blot analysis, in IAs. Kim et al. obtained aneurysmal tissue from six patients undergoing surgical clipping for comparison with intracranial arterial tissue from six age-matched patients undergoing craniotomy for nonvascular diseases [65]. A portion of the STA from all patients was also obtained for further comparison. Kim and colleagues report an increase in MMP9 levels via nonimmunoprecipitated western blot analysis in aneurysmal samples, as compared to STA from patients in both the control and experimental group. Furthermore, in a separate set of patients—6 of whom had IAs, and 6 of whom did not—Kim, and his colleagues, found zymographic analysis to reveal no apparent differences in plasma MMP-9.
Consistent with these findings, Caird et al. observed significant immunohistochemical staining of MMP-9 in atherosclerotic human aneurysmal lesions, and an absence of staining in control Circle of Willis arteries. In contrast to MMP2, Caird and colleagues report an absence of MMP-9 immunohistochemical staining in non-atherosclerotic aneurysmal lesions in all but one sample—of which, staining was minimal. Furthermore, this patient was known to have diffuse atherosclerosis and coronary artery disease. Zymographic analysis of two non-atherosclerotic aneurysmal lesions revealed weak MMP-9 enzymatic activity, although not absent—as was seen for MMP-2. Of note, the authors do not indicate which of the six non-atherosclerotic aneurysmal tissue specimens was used for zymographic study [58]. If the authors included the unique patient in which immunohistochemical analysis revealed positive staining for MMP-9, the results may skew the data toward higher levels of MMP-9 activity than can be expected to extrapolate to larger populations. As such, the presence of MMP-9 activity in non-atherosclerotic aneurysmal lesions cannot be conclusively determined from this paper alone. Despite this confounding variable, the differential expression of MMP-9 and -2 protein levels in atherosclerotic and non-atherosclerotic aneurysmal lesions suggests disparate mechanisms of pathogenesis. More specifically, MMP-9 and -2 may serve distinct roles in IA pathogenesis depending on whether the IA is superimposed at the site of an atherosclerotic lesion or elsewhere. An analysis of MMP-9 and -2 enzymatic activity for both atherosclerotic and non-atherosclerotic aneurysmal lesions would provide for more complete comparison of the role of these proteases in these two patterns of aneurysmal development.
Aoki et al., found support for MMP-9 in aneurysmal pathogenesis in their study. As with MMP-2, immunohistochemistry revealed MMP-9 expression in aneurysmal lesions after three months of IA induction. Furthermore, control arteries were observed to be absent in MMP-9 expression. In contrast to MMP-2, RT-PCR revealed did not show MMP-9 mRNA expression following one month of aneurysm induction, but mRNA was found to be upregulated after three months. MMP-9 mRNA was absent in the arterial wall of controls [66]. Although, it is not yet currently clear how MMP-2 and -9 are involved in aneurysmal pathogenesis, both human and animal models indicate a clear role for these proteins. Unfortunately, there is significant, unexplained discrepancy between the observed elevation of MMP-2 and -9 in aneurysmal tissue and the low enzymatic activity. Because of documented SNPs in these genes, it seems logical that a study in which the specific nucleotide sequences of MMP-2 and -9 genes is mapped merits warrant in order to further understand differential MMP expression in aneurysmal tissue. However, Zhang and colleagues report no association in promoter SNPs and a predisposition for developing IAs [63].
An increased understanding of MMP-2 and -9 enzyme kinetics may reveal novel alterations in protein confirmation that could explain the low MMP-2 activity that Caird reported in human non-atherosclerotic IAs. Because Caird et al. did not identify the patients selected for MMP-9 zymographic analysis of non-atherosclerotic aneurysmal lesions, uncertainty remains regarding MMP-9 activity in these specimens [58].
2.7.1. Tissue inhibitors of metalloproteinases
In order to fully understand the role of MMP-2 and -9 in aneurysmal pathogenesis, the role of post-translational regulation in modifying matrix metalloproteinase activity has to be considered. Tissue inhibitors of metalloproteinases (TIMPs) mediate a myriad of physiological functions including inhibition of active MMPs and pro-MMP formation. There are four currently identified TIMPs, so called TIMP-1, TIMP-2, TIMP-3, and TIMP-4 [67]. MMP-2 is secreted as an inactive zymogen and its activation requires TIMP-2. Furthermore, activated MMP-2 is inhibited via interaction with the C-terminal domain of TIMP-2. TIMP-1 serves to inhibit MMP-3 by inserting itself into the catalytic site and substrate-binding groove of MMP-3. TIMP-3 is also the only known TIMP to bind with high affinity to various ECM glycosaminoglycans [68].
In an attempt to characterize TIMP levels in IA tissue, Kim et al. obtained aneurysmal tissue from six patients undergoing surgical clipping with intracranial arterial tissue from six age-matched patients undergoing craniotomy for nonvascular diseases for controls [65]. A portion of the STA from all patients was also obtained for further comparison. Of note, western blot analysis revealed a significant increase in TIMP levels in aneurysmal tissue as compared to vessels taken from control patients and non-diseased STA segments taken from both the IA and control group. In addition, they report an increase in MMP9 levels for non-immunoprecipitated western blot analysis in aneurysmal samples as compared to STA from patients in the control group. From these findings, they propose the observed elevation in TIMP found in IA may serve to negatively regulate MMP-9, which was also found to be elevated in IAs [65].
2.8. TIMP genetics
To look for a genetic link between TIMP SNPs and IA pathogenesis, Krex et al. conducted a study including 44 patients with IA confirmed via cerebral angiography, and did not observe an association between SNPs in TIMP-1, -2, or -3 and intracranial aneurysms [69]. Similarly, Hinterseher et al. did not demonstrate an association with TIMP-1 variants and abdominal aortic aneurysms [70].
Jin et al. demonstrated that alterations in MMP may be correlated with the natural history of IAs. Of thirty patients undergoing aneurysmal clipping, patients were divided into groups of 15: those with ruptured aneurysms, and those with unruptured aneurysms. They performed RT-PCR, and report a significant upregulation for both MMP-2 and MMP-9 in tissue samples derived from ruptured IAs in comparison to unruptured IAs. Because ECM turnover is determined by the relationship between ECM synthesis and degradation, Jin et al., sought to compare the ratio between MMPs and TIMPs. They report an increased ratio of mRNA expression in MMP-2/-9 to TIMP-1, -2, and -3, in ruptured IAs in relation to unruptured IAs. Furthermore, they found serum MMP-2 and MMP-9 to be significantly greater in those patients who had suffered aSAH. In line with other studies, Jin et al., report positive immunohistochemical findings for both MMP-2 and MMP-9 in aneurysmal tissue [71].
From this study, it appears that elevations in IA MMP correlate with the probability of IA rupture. Analysis of MMP activity would serve as a useful adjunct to health care providers managing IA patients. Previous studies reporting a correlation between aneurysm size and the incidence of rupture would have found increased accuracy in their results if they had controlled for MMP activity in the patients [72]. Our understanding of MMP is still in its infancy. If Caird et al. are correct in their report of reduced MMP activity in IA tissue, alternate mechanisms in which MMPs serve as pathologic entities must be explored [58]. On the contrary, if the elevation in plasma MMPs provides an indication for the likelihood of IA rupture, blood analysis could prove useful for both patient prognosis and management.
Longitudinal studies monitoring IA size progression and plasma MMP, and IA tissue MMP levels/activity would enhance our understanding of the role of MMPs in the natural history of cerebral aneurysms. Because Jin et al., compared unruptured IA and ruptured IA, it is unclear whether any observed differences are a product of cellular processes occurring subsequent to the rupture event [71].
2.8.1. Nitric oxide
Nitric Oxide (NO) is of particular interest in the natural history of IAs, both in its development and the sequelae following aSAH [73]. NO is formed via the enzyme Nitric Oxide Synthase (NOS), which converts the amino acid L-arginine to NO and citrulline using nicotinamide adenine dinucleotide phosphate (NADPH) and O2 as cofactors in the reaction. NOS exists in three isoforms: endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS), with both eNOS and nNOS characterized as having constitutive calcium dependent enzymatic activity [74]. In addition to inducing arteriolar vasodilation, eNOS serves to inhibit platelet aggregation and adhesion, leukocyte adhesion, and proliferation of VSMCs [75]. eNOS is the primary source of NO within the vasculature, and its dysfunction is thought to contribute to various pathologies including septic shock, hypertension, vasospasm, toxemia, and atherosclerosis [74]. Encoded on chromosome 7q35-36, the eNOS gene (NOS3) is comprised of 26 exons [76].
2.9. NOS SNPs
Khurana et al., report a T→C SNP at position −786 of the eNOS promoter to influence the progression of IA to aSAH. Of 52 patients admitted for aSAH, heterozygosity (T/C −786) was shown to be associated with rupture of larger diameter IAs. More specifically, 100% of aneurysms (n = 13) with a diameter greater than 10 mm were heterozygote patients. In addition, the mean diameter of ruptured IAs was 8.5 mm in heterozygotes; whereas, the mean diameter for the T/T and C/C homozygotes was 4.7 mm and 6.0 mm, respectively. From this finding, Khuarana and colleagues suggest a potential genetic etiology may explain the discrepancy reported in the International Study of Unruptured Intracranial Aneurysms (ISUIA)—that is, the association between aneurysmal size and likelihood of rupture fails to explain the fact that the majority of aSAH observed in clinical practice are of less than 10 mm in diameter [77].
In a study consisting of 336 Japanese and 191 Korean patients, Akagawa et al. sought to reproduce the findings of Khuarana et al. Their findings were inconsistent with previous results, with the authors reporting no association between aneurysmal size and the eNOS (T/C−786) genotype, for either ruptured, or unruptured IAs. From this, the authors conclude that eNOS T-786C SNP genotype does not influence the size of aneurysms [36]. However, a subsequent study produced by Khurana offers some qualification to the discrepant data offered by these two groups [78]. The genetic cause of IAs is not one of Mendelian inheritance; rather, it appears to be the accumulation of multiple genetic variants of little penetrance that, together, predispose individuals to IA development. In other words, IAs are likely the result of multifactorial inheritance. As such, the assertion by Akagawa et al. of a lack of association between the eNOS T-786C SNP and IAs is misleading. Extending on their previous results, and thereby refuting the statement offered by Akagawa et al., Khurana conducted a genotypic comparison similar to their 2003 study. Comparing 49 patients with intact IAs and 58 individuals who had suffered aSAH, Khurana and colleagues, analyzed the association between three genetic variants of the eNOS gene and IA pathogenesis. In addition, this study also included a 27 variable nucleotide tandem repeat (27 VNTR) of intron 4 and a G→T SNP (eNOS G894T) of exon 7. Of note, all three genetic variants were observed to be present in significantly greater numbers in patients having suffered aSAH in comparison to intact, unruptured IA patients. Furthermore, the 4a-C-T haplotype, consisting of all three variant alleles, demonstrated a 11.4-fold increased likelihood of presenting in the patients with aSAH. The 4a-C-G haplotype, consisting of the 27 VNTR variant and T-786C SNP, demonstrated an 8.6-fold increased likelihood of presenting in the patients with aSAH. Lastly, the 4b-C-T haplotype, consisting of T-786C SNP and G894T SNP, demonstrated a 9.3-fold increased likelihood of presenting in the patients with aSAH [78].
It is possible that co-inheritance of multiple alleles results in a predilection for aneurysmal development, and the individual allelic variation in those patients studied by Akagawa et al. was insufficient to result in a significant association between IAs and the eNOS (T/C −786) genotype. However, Krischek et al. observed no significant allelic effect in the three gene variants reported by Khurana’s team [31]. In this study, the experimental cohort consisted of 297 Japanese patients having suffered a previous aSAH and 108 patients harboring at least one UA. 176 volunteers with no evidence of IA served as experimental controls. Furthermore, Song et al., investigated the role for the eNOS (T/C −786) genotype in predisposition for IA in the Korean population. As observed by Krischek, Song et al. also report no association of the eNOS (T/C −786) genotype and aSAH [79]. Furthermore, Song and colleagues, found no relationship between NOS3 allelic variation and aneurysmal diameter at the time of rupture. In addition, the three genetic variants Khurana et al., identified did not demonstrate a significant relation between IA development in the Korean population [80].
The differences in data presented by Krischek et al., Akagawa et al., and Kim et al. could be rooted in differences in the genetic regulation of NOS3 in the Asian population, as compared to the Caucasian population—which comprised the entire patient population in the study by Khurana et al. As such, these three variants in NOS3 may be limited to Caucasians, and may not be applied to other demographics. However, the significant role of eNOS in maintaining cerebral vascular homeostasis undermines the necessity for future research investigating the role of NOS3 genetic variants in IAs in humans.
2.10. The role of INOS
In addition to eNOS, iNOS has been implicated in many human pathologies, including: Alzheimer’s disease, Parkinsons disease, myocardial infarction, certain malignancies, transplant rejection, obliterative bronchiolitis, prostheses failure, and inclusion-body myositis [81]. iNOS is encoded by the NOS2A gene, and is localized to chromosome 17q11.2-q12 [82]. Its transcription is upregulated in response to hemodynamic stresses, and alternative splicing results in mRNA diversity consisting of unique isoforms. In addition, further protein heterogeneity is generated due to multiple AUG codons in the 5’-UTR allowing for eight unique translational initiation sites [81]. Its expression is predominantly mediated via the janus kinase/signal transducer (JAK/-STAT) and the nuclear factor kappa B (NFkB) signal transduction pathways culminating in alterations in nuclear transcription [83].
In addition to the aforementioned pathologies, iNOS has also been implicated in the pathogenesis of AAAs. As compared to normal abdominal aortic tissue, AAA tissue demonstrates both increased mRNA and protein expression [84]. It, therefore, raises the possibility that alterations in iNOS expression patterns may mediate the vascular pathology observed in IAs, similar to its reported role in AAAs.
However, in a genome-wide association study consisting of 29 Japanese families with a high incidence (at least three diseased family members) of familial aneurysms, Yamada and colleagues, report no association between a NOS2A promoter variant at nucleotide −2453 and IAs [38]. Furthermore, Mineharu et al. did not observe a predisposition for aneurysmal development in 362 Japanese patients harboring an IA and NOS2A genetic variants [37]. Despite these observations, immunohistochemistry of an aneurysm neck isolated from the right MCA of a 69 year old woman revealed positive staining for both iNOS and nitrotyrosine—a marker for increased production of reactive nitric oxide, which in the presence of superoxide anion, forms peroxynitrite, a potent oxidizing agent [85]. Because of the transience of iNOS activity, the elevated expression patterns observed in this patient suggest a role for iNOS in human IA pathology. Therefore, altered expression patterns of NOS2A may serve as either a protective, or potentiating, factor for aneurysmal development.
Furthermore, studies using the murine model support a role for iNOS in IA pathogenesis. In 147 male Sprauge-Dawley rats, aged 6 to 8 weeks, Fukuda et al., ligated the right common carotid artery and posterior branches of both renal arteries to induce IA formation. To examine the effects of early aneurysmal formation, rats were administered intraperitoneal (IP) aminoguanidine (+/− L-arginine), an NO antagonist, one day following surgery, and tissues from the bifurcation of the left anterior cerebral artery and olfactory artery were isolated two weeks post-surgery for immunohistochemical analysis. To examine the effects of NO inhibition on mature aneurysm formation, aminoguanidine was administered intraperitoneal (IP) in rats for 90 consecutive days following surgery, and tissues were isolated three months post-surgery for immunohistochemical analysis. In addition to untreated rats—that is, rats in which surgical cerebral induction was not performed—rats administered IP saline served as comparative controls.
In contrast to the arterial bifurcations of untreated rats, immunoreactivity to mouse monoclonal iNOS antibody was observed in VSMCs of aneurysmal tissue in surgically treated rats. Furthermore, endothelial cell and VSMC damage was apparent in early aneurysmal tissue obtained from surgically treated rats—an effect that was attenuated in those animals treated with aminoguanidine. However, this effect was abolished in surgically treated rats that were co-administered aminoguanidine and L-arginine. In addition, the incidence of IA formation was significantly reduced in the aminoguanidine cohort, as compared to those rats dosed with only IP saline. Interestingly, the progression of disease was found to be positively correlated with iNOS expression, and showed an inverse relation to eNOS expression. Also, the elevation in peroxynitrite observed in the IA MCA tissue of the 69-year-old woman was absent in the murine model [85].
To further elucidate the role of iNOS in IA formation, Samadasa and colleagues induced IAs in mice using a procedure analogous to that performed by Fukududa et al. For this experiment, tissue harvested from the bifurcation of the anterior cerebral artery and the olfactory artery was compared between wild type and iNOS knockout mice. Four months after surgery, wild type mice demonstrated iNOS immunoreactivity in the media and adventitia, and expectedly, iNOS was immunonegative in knockout mice. Although Samadasa and colleagues found no significant difference in the incidence of aneurysms between these two groups, IAs in wild type mice were significantly larger than in knockout mice. Furthermore, the number of VSMCs immunopositive for anti-ssDNA antibodies, an apoptosis marker, was significantly higher in the iNOS knockout mice [86].
The data submitted by Fukudua et al. and Samadasa et al., both support a role for iNOS in IA pathogenesis. The reductions in IA incidence, and VSMC/ endothelial cell damage, in surgically treated rats with concomitant aminoguanidine administration suggests iNOS antagonism positively alters the natural history of IA pathogenesis. Because this phenomenon was abolished when these animals were also given L-arginine (an iNOS substrate and aminoguanidine iNOS competitor), the contribution of iNOS in IA pathogenesis garners even more support. However, aminoguanidine also serves other functions, including inhibition of both the enzyme diamine oxidase and non-enzymatic glycosylation, lending faith to the idea that mechanisms distinct from iNOS inhibition lead to the observed effect on IAs [87]. Yet, studies in iNOS knockout mice remove this confounding variable, and show that the absence of iNOS gene expression in IAs is associated with smaller aneurysmal size, and a reduction in the number of apoptotic VSMCs. This suggests that iNOS is intimately associated with IA disease progression, as quantitative measures have demonstrated a direct correlation between an increased number of apoptotic cells and the likelihood of aneurysmal rupture [88]. However, the findings of Fuduka et al. and Samadasa et al., are inconsistent in regards to the role of iNOS and IA incidence, causing uncertainty as to whether iNOS is an inciting agent, or strictly an accelerator of disease progression.
Although Yamada et al. and Mineharu et al., failed to demonstrate an association between NOS2A polymorphisms and IAs, alternate mechanisms of increased NOS2A transcription are possible. More specifically, NF-kB, a positive regulator of iNOS gene expression, has been shown to be elevated in cerebral aneurysms [89].
2.11. NF-kB Inflammation
NF-kB exists as a dimer, complexed to the protein Rel. It is normally sequestered in the cytoplasm by the protein inhibitor of kappa B (ikB), and in response to various stimuli, it is free to translocate into the nucleus to act as a transcription factor for many DNA response elements—predominantly genes involved in immune response regulation. The stimuli capable of invoking NF-kB activation are numerous, such that specific discussion of these factors would warrant a separate paper in and of itself. The same is true for those genes in which NF-kB induces an upregulation in gene expression [90]. Therefore, this paper only addresses those genes/stimuli that have been demonstrated to play a role in IA pathogenesis.
In addition to iNOS, NF-kB causes upregulation of various MMPs, including the aforementioned MMP-2 and -9. A role for NF-kB pathogenesis in AAAs has been demonstrated in the use of a chimeric decoy (ODN) against NF-kB to preserve vascular wall integrity in an animal model of AAA [91]. In an attempt to identify a role for NF-kB pathogenesis in IAs, Aoki et al., obtained IA tissue samples from seven patients for immunohistochemical analysis using a mouse monocolonal antibody specific for the DNA binding form of the NF-kB p65 subunit. For comparison, unaffected MCA tissue taken from four patients was used as a comparison for NF-kB expression. As compared to control specimen, NF-kB protein was found to be significantly upregulated in aneurysm tissue—with a predominance of protein expression observed within the intimal layer [66].
3. Genetics of inflammatory mediators
Consistent with the central role of inflammation in IA pathogenesis, polymorphisms affecting the expression and/or function of innate immune system and inflammatory signaling pathways can predispose affected individuals to develop cerebral aneurysms and/or suffer aSAH. Various polymorphisms in the classical pro-inflammatory cytokines TNFα, IL-1β, and IL-6 are all more prevalent in those with IAs than those without while others offer a protective effect against IAs. Homozygosity of the −511C > T polymorphism in the promoter region of the IL-1β gene is associated with increased risk of aSAH [92]. Other studies have failed to find an association between IL-1β genotype and aneurysmal disease [93]. There is more substantial evidence supporting TNFα and IL-6 genotype as a driver of IA progression. Three unique SNPs in the TNFα gene are associated with increased IA susceptibility in Chinese and Japanese populations [94–96] while the G > A allele of SNP rs1800629 confers a lower risk of IA development in Caucasians [93]. There is strong evidence for two particular SNPs (−572G > C & −174 G > C) in the IL-6 gene promoter region mediating IA risk [97–99]. These SNPs are relatively more common in Asian populations, although the effect is consistent across ethnic groups [100].
While the precise physiological impact of these mutations is unclear, the clinical consequences of are potentially severe. There is clear evidence that TNFα, IL-1β, and IL-6 all drive aneurysm formation and rupture through modulating the phenotypes of vascular smooth muscle cells and infiltrating macrophages [101–104]; thus, mutations which affect the expression [105] and/or function of these cytokines can drastically change IA risk. Further, there are other inflammatory mediators of aneurysmal disease (e.g. PPARγ, IL-17, PGE2) of which there have been no genetic association studies [106,107]. Investigation into polymorphisms of these factors could elucidate novel IA risk factors.
4. Preclinical models
Expanding on this finding, Aoki and colleagues, also examined a murine model of cerebral aneurysms, and reported a role for NF-kB in initiation of IA formation [66]. Following cerebral aneurysm induction, but prior to overt aneurysm formation, NF-kB was observed in both endothelial cells and CD68 positive cells—a cluster of differentiation marker specific for macrohpages [108]. Using p50 knockout mice, they demonstrate a clear role for functional NF-kB involvement in IA formation. The p50 subunit, when complexed with p65 as a heterodimer (p50/p65), serves as the DNA binding domain of NF-kB [109]. Five months post-surgery for IA induction, the number of KO mice demonstrating aneurysmal change was significantly less than wild type. Of those KO mice in which aneurysm formation occurred, the mean diameter of the aneurysm was significantly less than that of wild type. Furthermore, mRNA expression levels in IA tissue of MCP-1, VCAM-1, MMP-2, MMP-9, IL-1B, and iNOS was upregulated in wild type mice, but not in p50 KOs. Similar to the reports of an NF-kB decoy in AAAs, ODN injection at the time of aneurysmal induction via surgery resulted in a significant reduction in the incidence of IEL disruption, a maker of early aneurysm change [66]. In addition, IA diameter was significantly smaller in the ODN administered cohort. This effect persisted if ODN was injected one week following surgery, but the effect was abolished when administration occurred two weeks after surgery. Furthermore, decoy administration resulted in a significant reduction of macrophage infiltration of the cerebral vasculature.
Murine models have also demonstrated an apparent role for MMP2 in the development of IAs. By asymmetrically ligating the left carotid artery and posterior branches of the bilateral renal arteries, in conjunction with a diet containing 0.12% B-aminopropionitrile—an inhibitor of lysyl oxidase—Aoki et al., induced IA formation in Sprague-Dawley rats [66]. At time intervals of one month and three months, the researchers isolated tissue samples from the anterior cerebral artery/olfactory artery bifurcation for immunohistochemical analysis. Three months following aneurysmal induction, they observed immunohistochemical evidence of MMP-2 expression in experimental rats; whereas, MMP-2 was found to be absent in the arterial walls derived from controls. Moreover, RT-PCR indicated an upregulation of MMP-2 mRNA as soon as one month after aneurysmal induction, with a significant increase in MMP-2 expression at three months. Of note, there was no apparent increase in MMP-2 mRNA expression in control arteries. To analyze the functional activity of the MMP-2 observed in aneurysmal tissue, they performed zymography and observed an increase in MMP-2 activity in the aneurysmal neck four months after IA induction. Furthermore, in vitro pretreatment with tosylam—an MMP-2, -9, and -12 competitive inhibitor—abolished gelatin degradation, thereby supporting the belief that matrix metalloproteinases are the key mediators of gelatin digestion in aneurysmal lesions. Because tosylam also serves to inhibits MMP-9 and -12, one cannot delineate the contribution of MMP-2 in ECM turnover from these MMPs in aneurysmal lesions.
Aoki et al., also observed the effects of MMP inhibition on aneurysmal development by feeding experimental animals a diet containing 50 mg/kg/day of tosylam. Three months following aneuysmal inductions, rats were euthanized and those animals fed tosylam were found to have a significant reduction in the rate of advanced aneurysms—defined as an obvious outward bulging of the arterial wall with disappearance of the internal elastic lamina. Furthermore, aneurysmal tissue derived from tosylam fed rats was observed to be absent of MMP activity; whereas, aneurysmal tissue obtained from animals not fed tosylam demonstrated evidence of MMP activity, as per zymography.
The blotchy mouse has a mutated ATP7A gene, which encodes an ATP mediated copper permease and serves as an experimental model to investigate the relationship between elastin and aneurysmal development. As such, these mice are copper deficient, thereby resulting in reduced activity in lysyl oxidase—a copper dependent enzyme responsible for cross linking collagen and elastin fibers [110]. Coutard and colleagues, reported the incidence of small cerebral aneurysm development to be 19% and 17% in hypertensive and normotensive blotchy mice, respectively. Comparatively, no normotensive control mice developed small cerebral aneurysms while 23% of hypertensive control mice were found to have aneurysms. Large cerebral aneurysms were found in 19% of hypertensive blotchy mice, but did not develop in any other group. Furthermore, within the aneurysm wall Coutard observed an absence of the internal elastic lamina. Because elastin maturation is dependent on the enzyme lysyl oxidase, this mouse model demonstrates that alterations in elastin metabolism may perturb the integrity of the IEL, thereby increasing the likelihood of IA formation.
5. Polymorphisms of unknown significance
The main focus of this review is a critical review of genetic variants for those genes in which the primary function has already been, at a minimum, partially elucidated. However, genetic polymorphisms in regions of unknown significance have also been associated with a predisposition for individuals to acquire certain disease states. For example, a locus on chromosome 9p21 has been associated with a phenotype of coronary artery disease, carotid artery disease, stroke, peripheral artery disease, AAAs, and of significance for this manuscript, intracranial aneurysms [111]. In 2008, Helgadottir et al., reported a novel chromosome 9p21 sequence variant, rs10757278-G or allele G, to be significantly associated with IAs [112]. While the G allele is associated with an increased incidence of IAs, its presence also seems to retard the rate of aneurysmal growth development. Furthermore, the sample population included individuals harboring IAs from Iceland, The Netherlands, and Finland, thereby reducing the likelihood that this observed effect is limited to one specific demographic.
Nakaoka et al., designed a study in which they attempted to correlate chromosome 9p21 SNPs to three specific aneurysmal characteristics, or “subphenotypes” [113]. These characteristics included: a prior history of rupture, the number of IAs, and location of IAs. Enrollment consisted of 981 IA patients, of which 659 were sporadic and 242 were familial. 699 individuals with no evidence of IAs, as evidenced by MRI, served as controls. In addition to the rs10757278 polymorphism reported by Helgadottir et al., Nakaoka and colleagues, investigated the association of IAs with three other 9p21 SNPs (rs1333040, rs2891168, and rs2383207). All four genotypes were associated with an increased incidence of IAs, but only rs1333040 (T allele) served as an independent predictor, with the increased association of the other three polymorphisms likely attributed to linkage disequlibrium with the rs1333040 SNP.
In regards to specific aneurysm characteristics, rs1333040 showed greater association with multiple aneurysms than a single aneurysm, and demonstrated no difference between ruptured and unruptured IAs. The greatest association between rs1333040 and IA location was in the posterior communicating artery (Pcomm), followed by the MCA, and with IAs of the anterior communicating artery (Acomm) demonstrating no significant association. Furthermore, the rs1333040 gene variant resulted in a 1.69 fold increased risk of developing a Pcomm aneurysm. This data suggests that those individuals harboring the rs1333040 T allele are at an increased risk of developing an IA in the posterior circulation, which carries a rather poor prognostic outcome. More specifically, Wiebers et al., identified three IA locations as predictors of aSAH: basilar tip, cavernous artery, and Pcomm [72]. In addition, IAs located in the posterior circulation are reported to be associated with an increased incidence of negative outcomes following either endovascular or surgical intervention.
When the findings of Wiebers et al. and Nakaoka et al., are taken together, it would appear that the rs1333040 T allele is associated with an increased incidence of those aneurysms carrying the most perilous natural history—that is, IAs most likely progressing to aSAH. In addition, aneurysms of the posterior circulation are also characteristically the most challenging to cure via surgical, and endovascular, interventions. It seems that genetic analysis of chromosome 9p21 genetic variants would have prognostic implications, allowing for greater predictive value for neurosurgeons managing these patients. For individuals with close relatives harboring IAs, genetic determination of rs1333040 genotype may inform physicians of an increased likelihood of future IA development within the posterior circulation. As such, this would represent a population that may stand to benefit from periodic follow up imaging in order to diagnose the presence of IAs prior to asymptomatic onset, and size progression—which itself, is a prognostic indicator of aSAH.
6. Conclusion
The main focus of this review is a critical review of genetic variants for those genes in which the primary function has already been, at a minimum, partially elucidated. However, as elucidated above, genetic polymorphisms in regions of unknown significance have also been associated with a predisposition for individuals to acquire certain disease states.
Ongoing studies are warranted as emerging genetic polymorphisms continue to be discovered in relation to aneurysmal formation. This primer has highlighted the current knowledge state and serves as a basis for easy reference. The available evidence stems largely from aneurysmal tissue collected and analyzed from around the world. Pre-clinical studies have also allowed focused investigation that have had clear clinical correlation. Stratifying patients by genetic phenotype is likely to warrant clinical utility for determining which patients are more predisposed to aneurysmal rupture. Future investigations must be multiinstitutional in order to garner data from different demographics and ensure validity amongst various cohorts.
Acknowledgements
Brandon Lucke-Wold is Supported by NIH R25 NS108939.
References
- [1].Nakagawa T, Hashi K, The incidence and treatment of asymptomatic, unruptured cerebral aneurysms, J. Neurosurg 80 (2) (1994) 217–223, 10.3171/jns.1994.80.2.0217. [DOI] [PubMed] [Google Scholar]
- [2].Ferreira M, Nahed BV, Babu MA, Walcott BP, Ellenbogen RG, Sekhar LN, Trapped fourth ventricle phenomenon following aneurysm rupture of the posterior circulation: case reports, Neurosurgery 70 (1) (2012) E253–E258, 10.1227/NEU.0b013e31822abf95. [DOI] [PubMed] [Google Scholar]
- [3].Brown BM, Soldevilla F, MR angiography and surgery for unruptured familial intracranial aneurysms in persons with a family history of cerebral aneurysms, AJR Am. J. Roentgenol 173 (1) (1999) 133–138, 10.2214/ajr.173.1.10397113. [DOI] [PubMed] [Google Scholar]
- [4].Wermer MJ, van der Schaaf IC, Algra A, Rinkel GJ, Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: an updated meta-analysis, Stroke 38 (4) (2007) 1404–1410, 10.1161/01.STR.0000260955.51401.cd. [DOI] [PubMed] [Google Scholar]
- [5].Bilguvar K, Yasuno K, Niemelä M, et al. , Susceptibility loci for intracranial aneurysm in European and Japanese populations, Nat. Genet 40 (12) (2008) 1472–1477, 10.1038/ng.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yasuno K, Bilguvar K, Bijlenga P, et al. , Genome-wide association study of intracranial aneurysm identifies three new risk loci, Nat. Genet 42 (5) (2010) 420–425, 10.1038/ng.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Meng H, Wang Z, Hoi Y, et al. , Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation, Stroke 38 (6) (2007) 1924–1931, 10.1161/STROKEAHA.106.481234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Peters DG, Kassam AB, Feingold E, et al. , Molecular anatomy of an intracranial aneurysm: coordinated expression of genes involved in wound healing and tissue remodeling, Stroke 32 (4) (2001) 1036–1042, 10.1161/01.str.32.4.1036. [DOI] [PubMed] [Google Scholar]
- [9].Pankov R, Yamada KM, Fibronectin at a glance, J. Cell Sci 115 (Pt 20) (2002) 3861–3863, 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- [10].Futami K, Yamashita J, Tachibana O, Higashi S, Ikeda K, Yamashima T, Immunohistochemical alterations of fibronectin during the formation and proliferative repair of experimental cerebral aneurysms in rats, Stroke 26 (9) (1995) 1659–1664, 10.1161/01.str.26.9.1659. [DOI] [PubMed] [Google Scholar]
- [11].Kassam AB, Horowitz M, Chang YF, Peters D, Altered arterial homeostasis and cerebral aneurysms: a molecular epidemiology study, Neurosurgery 54 (6) (2004) 1450–1460, 10.1227/01.neu.0000125005.67850.f8. [DOI] [PubMed] [Google Scholar]
- [12].Mimata C, Kitaoka M, Nagahiro S, et al. , Differential distribution and expressions of collagens in the cerebral aneurysmal wall, Acta Neuropathol. 94 (3) (1997) 197–206, 10.1007/s004010050694. [DOI] [PubMed] [Google Scholar]
- [13].Kato T, Hattori H, Yorifuji T, Tashiro Y, Nakahata T, Intracranial aneurysms in Ehlers-Danlos syndrome type IV in early childhood, Pediatr. Neurol 25 (4) (2001) 336–339, 10.1016/s0887-8994(01)00315-0. [DOI] [PubMed] [Google Scholar]
- [14].Pepin M, Schwarze U, Superti-Furga A, Byers PH, Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type, N. Engl. J. Med 342 (10) (2000) 673–680, 10.1056/NEJM200003093421001. [DOI] [PubMed] [Google Scholar]
- [15].Neil-Dwyer G, Bartlett JR, Nicholls AC, Narcisi P, Pope FM, Collagen deficiency and ruptured cerebral aneurysms. A clinical and biochemical study, J. Neurosurg 59 (1) (1983) 16–20, 10.3171/jns.1983.59.1.0016. [DOI] [PubMed] [Google Scholar]
- [16].Petrovic D, Bregar D, Guzic-Salobir B, et al. , Sex difference in the effect of ACE-DD genotype on the risk of premature myocardial infarction, Angiology 55 (2) (2004) 155–158, 10.1177/000331970405500207. [DOI] [PubMed] [Google Scholar]
- [17].van den Berg JS, Pals G, Arwert F, et al. , Type III collagen deficiency in saccular intracranial aneurysms. Defect in gene regulation? Stroke 30 (8) (1999) 1628–1631, 10.1161/01.str.30.8.1628. [DOI] [PubMed] [Google Scholar]
- [18].Yoneyama T, Kasuya H, Onda H, et al. , Collagen type I alpha2 (COL1A2) is the susceptible gene for intracranial aneurysms, Stroke 35 (2) (2004) 443–448, 10.1161/01.STR.0000110788.45858.DC. [DOI] [PubMed] [Google Scholar]
- [19].van Dijk FS, Huizer M, Kariminejad A, et al. , Complete COL1A1 allele deletions in osteogenesis imperfecta, Genet. Med 12 (11) (2010) 736–741, 10.1097/GIM.0b013e3181f01617. [DOI] [PubMed] [Google Scholar]
- [20].Gajko-Galicka A, Mutations in type I collagen genes resulting in osteogenesis imperfecta in humans, Acta Biochim. Pol 49 (2) (2002) 433–441. [PubMed] [Google Scholar]
- [21].Zhu Y, Li W, Ge M, et al. , Polymorphism rs42524 of COL1A2 and sporadic intracranial aneurysms in the Chinese population, J. Neurosurg 109 (6) (2008) 1060–1064, 10.3171/JNS.2008.109.12.1060. [DOI] [PubMed] [Google Scholar]
- [22].Glasker S, Schatlo B, Klingler JH, et al. , Associations of collagen type I alpha2 polymorphisms with the presence of intracranial aneurysms in patients from Germany, J. Stroke Cerebrovasc. Dis 23 (2) (2014) 356–360, 10.1016/j.jstrokecerebrovasdis.2013.04.038. [DOI] [PubMed] [Google Scholar]
- [23].Plaisier E, Chen Z, Gekeler F, et al. , Novel COL4A1 mutations associated with HANAC syndrome: a role for the triple helical CB3[IV] domain, Am. J. Med. Genet. A 152A (10) (2010) 2550–2555, 10.1002/ajmg.a.33659. [DOI] [PubMed] [Google Scholar]
- [24].Jeanne M, Labelle-Dumais C, Jorgensen J, et al. , COL4A2 mutations impair COL4A1 and COL4A2 secretion and cause hemorrhagic stroke, Am. J. Hum. Genet 90 (1) (2012) 91–101, 10.1016/j.ajhg.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kuo DS, Labelle-Dumais C, Gould DB, COL4A1 and COL4A2 mutations and disease: insights into pathogenic mechanisms and potential therapeutic targets, Hum. Mol. Genet 21 (R1) (2012) R97–R110, 10.1093/hmg/dds346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jobsis GJ, Keizers H, Vreijling JP, et al. , Type VI collagen mutations in Bethlem myopathy, an autosomal dominant myopathy with contractures, Nat. Genet 14 (1) (1996) 113–115, 10.1038/ng0996-113. [DOI] [PubMed] [Google Scholar]
- [27].Armstrong PJ, Johanning JM, Calton WC Jr., et al. , Differential gene expression in human abdominal aorta: aneurysmal versus occlusive disease, J. Vasc. Surg 35 (2) (2002) 346–355, 10.1067/mva.2002.121071. [DOI] [PubMed] [Google Scholar]
- [28].Roggendorf W, Opitz H, Schuppan D, Altered expression of collagen type VI in brain vessels of patients with chronic hypertension. A comparison with the distribution of collagen IV and procollagen III, Acta Neuropathol. 77 (1) (1988) 55–60, 10.1007/BF00688243. [DOI] [PubMed] [Google Scholar]
- [29].Pirson Y, Chauveau D, Torres V, Management of cerebral aneurysms in autosomal dominant polycystic kidney disease, J. Am. Soc. Nephrol 13 (1) (2002) 269–276, 10.1681/ASN.V131269. [DOI] [PubMed] [Google Scholar]
- [30].Tassabehji M, Metcalfe K, Hurst J, et al. , An elastin gene mutation producing abnormal tropoelastin and abnormal elastic fibres in a patient with autosomal dominant cutis laxa, Hum. Mol. Genet. 7 (6) (1998) 1021–1028, 10.1093/hmg/7.6.1021. [DOI] [PubMed] [Google Scholar]
- [31].Krischek B, Kasuya H, Akagawa H, et al. , Using endothelial nitric oxide synthase gene polymorphisms to identify intracranial aneurysms more prone to rupture in Japanese patients, J. Neurosurg 105 (5) (2006) 717–722, 10.3171/jns.2006.105.5.717. [DOI] [PubMed] [Google Scholar]
- [32].Schievink WI, Parisi JE, Piepgras DG, Familial intracranial aneurysms: an autopsy study, Neurosurgery 41 (6) (1997) 1247–1251, 10.1097/00006123-199712000-00003. [DOI] [PubMed] [Google Scholar]
- [33].Halme T, Savunen T, Aho H, Vihersaari T, Penttinen R, Elastin and collagen in the aortic wall: changes in the Marfan syndrome and annuloaortic ectasia, Exp. Mol. Pathol 43 (1) (1985) 1–12, 10.1016/0014-4800(85)90050-4. [DOI] [PubMed] [Google Scholar]
- [34].Onda H, Kasuya H, Yoneyama T, et al. , Genomewide-linkage and haplotype-association studies map intracranial aneurysm to chromosome 7q11, Am. J. Hum. Genet 69 (4) (2001) 804–819, 10.1086/323614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Farnham JM, Camp NJ, Neuhausen SL, et al. , Confirmation of chromosome 7q11 locus for predisposition to intracranial aneurysm, Hum. Genet 114 (3) (2004) 250–255, 10.1007/s00439-003-1044-z. [DOI] [PubMed] [Google Scholar]
- [36].Akagawa H, Kasuya H, Onda H, et al. , Influence of endothelial nitric oxide synthase T-786C single nucleotide polymorphism on aneurysm size, J. Neurosurg 102 (1) (2005) 68–71, 10.3171/jns.2005.102.1.0068. [DOI] [PubMed] [Google Scholar]
- [37].Mineharu Y, Inoue K, Inoue S, et al. , Association analysis of common variants of ELN, NOS2A, APOE and ACE2 to intracranial aneurysm, Stroke 37 (5) (2006) 1189–1194, 10.1161/01.STR.0000217408.91389.4d. [DOI] [PubMed] [Google Scholar]
- [38].Yamada S, Utsunomiya M, Inoue K, et al. , Absence of linkage of familial intracranial aneurysms to 7q11 in highly aggregated Japanese families, Stroke 34(4) (2003) 892–900, 10.1161/01.STR.0000062887.71400.B4. [DOI] [PubMed] [Google Scholar]
- [39].Berthelemy-Okazaki N, Zhao Y, Yang Z, et al. , Examination of ELN as a candidate gene in the Utah intracranial aneurysm pedigrees, Stroke 36 (6) (2005) 1283–1284, 10.1161/01.STR.0000166198.05439.f8. [DOI] [PubMed] [Google Scholar]
- [40].Hofer A, Hermans M, Kubassek N, et al. , Elastin polymorphism haplotype and intracranial aneurysms are not associated in Central Europe, Stroke 34 (5) (2003) 1207–1211, 10.1161/01.STR.0000069013.83336.1C. [DOI] [PubMed] [Google Scholar]
- [41].McColgan P, Thant KZ, Sharma P, The genetics of sporadic ruptured and unruptured intracranial aneurysms: a genetic meta-analysis of 8 genes and 13 polymorphisms in approximately 20,000 individuals, J. Neurosurg 112 (4) (2010) 714–721, 10.3171/2009.8.JNS092. [DOI] [PubMed] [Google Scholar]
- [42].Chyatte D, Reilly J, Tilson MD, Morphometric analysis of reticular and elastin fibers in the cerebral arteries of patients with intracranial aneurysms, Neurosurgery 26 (6) (1990) 939–943, 10.1097/00006123-199006000-00003. [DOI] [PubMed] [Google Scholar]
- [43].Abruzzo T, Shengelaia GG, Dawson RC 3rd, Owens DS, Cawley CM, Gravanis MB, Histologic and morphologic comparison of experimental aneurysms with human intracranial aneurysms, AJNR Am. J. Neuroradiol 19 (7) (1998) 1309–1314. [PMC free article] [PubMed] [Google Scholar]
- [44].Naso MF, Zimmermann DR, Iozzo RV, Characterization of the complete genomic structure of the human versican gene and functional analysis of its promoter, J. Biol. Chem 269 (52) (1994) 32999–33008. [PubMed] [Google Scholar]
- [45].Ruigrok YM, Rinkel GJ, Wijmenga C, The versican gene and the risk of intracranial aneurysms, Stroke 37 (9) (2006) 2372–2374, 10.1161/01.STR.0000236499.55301.09. [DOI] [PubMed] [Google Scholar]
- [46].Guo D, Hasham S, Kuang SQ, et al. , Familial thoracic aortic aneurysms and dissections: genetic heterogeneity with a major locus mapping to 5q13-14, Circulation 103 (20) (2001) 2461–2468, 10.1161/01.cir.103.20.2461. [DOI] [PubMed] [Google Scholar]
- [47].Wight TN, Versican: a versatile extracellular matrix proteoglycan in cell biology, Curr. Opin. Cell Biol 14 (5) (2002) 617–623, 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- [48].Shi C, Awad IA, Jafari N, et al. , Genomics of human intracranial aneurysm wall, Stroke 40 (4) (2009) 1252–1261, 10.1161/STROKEAHA.108.532036. [DOI] [PubMed] [Google Scholar]
- [49].Birkedal-Hansen H, Role of matrix metalloproteinases in human periodontal diseases, J. Periodontol 64 (Suppl 5S) (1993) 474–484, 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- [50].Galis ZS, Kranzhofer R, Fenton JW 2nd, Libby P, Thrombin promotes activation of matrix metalloproteinase-2 produced by cultured vascular smooth muscle cells, Arterioscler. Thromb. Vasc. Biol 17 (3) (1997) 483–489, 10.1161/01.atv.17.3.483. [DOI] [PubMed] [Google Scholar]
- [51].Romanic AM, Madri JA, Extracellular matrix-degrading proteinases in the nervous system, Brain Pathol. 4 (2) (1994) 145–156, 10.1111/j.1750-3639.1994.tb00825.x. [DOI] [PubMed] [Google Scholar]
- [52].Forsyth PA, Wong H, Laing TD, et al. , Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas, Br. J. Cancer 79 (11–12) (1999) 1828–1835, 10.1038/sj.bjc.6690291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hsu JY, McKeon R, Goussev S, et al. , Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury, J. Neurosci 26 (39) (2006) 9841–9850, 10.1523/JNEUROSCI.1993-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Martignetti JA, Aqeel AA, Sewairi WA, et al. , Mutation of the matrix metalloproteinase 2 gene (MMP2) causes a multicentric osteolysis and arthritis syndrome, Nat. Genet 28 (3) (2001) 261–265, 10.1038/90100. [DOI] [PubMed] [Google Scholar]
- [55].Chyatte D, Lewis I, Gelatinase activity and the occurrence of cerebral aneurysms, Stroke 28 (4) (1997) 799–804, 10.1161/01.str.28.4.799. [DOI] [PubMed] [Google Scholar]
- [56].Price SJ, Greaves DR, Watkins H, Identification of novel, functional genetic variants in the human matrix metalloproteinase-2 gene: role of Sp1 in allele-specific transcriptional regulation, J. Biol. Chem 276 (10) (2001) 7549–7558, 10.1074/jbc.M010242200. [DOI] [PubMed] [Google Scholar]
- [57].Li B, Li F, Chi L, Zhang L, Zhu S, The expression of SPARC in human intracranial aneurysms and its relationship with MMP-2/-9, PLoS One 8 (3) (2013), e58490, 10.1371/journal.pone.0058490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Caird J, Napoli C, Taggart C, Farrell M, Bouchier-Hayes D, Matrix metalloproteinases 2 and 9 in human atherosclerotic and non-atherosclerotic cerebral aneurysms, Eur. J. Neurol 13 (10) (2006) 1098–1105, 10.1111/j.1468-1331.2006.01469.x. [DOI] [PubMed] [Google Scholar]
- [59].Lovett DH, Mahimkar R, Raffai RL, et al. , A novel intracellular isoform of matrix metalloproteinase-2 induced by oxidative stress activates innate immunity, PLoS One 7 (4) (2012), e34177, 10.1371/journal.pone.0034177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Karakaya O, Barutcu I, Esen AM, et al. , Relationship between circulating plasma matrix metalloproteinase-9 (gelatinase-B) concentration and aortic root dilatation, Am. J. Hypertens 19 (4) (2006) 361–365, 10.1016/j.amjhyper.2005.08.013. [DOI] [PubMed] [Google Scholar]
- [61].Zhang B, Henney A, Eriksson P, Hamsten A, Watkins H, Ye S, Genetic variation at the matrix metalloproteinase-9 locus on chromosome 20q12.2-13.1, Hum Genet. 105 (5) (1999) 418–423, 10.1007/s004390051124. [DOI] [PubMed] [Google Scholar]
- [62].Cotignola J, Reva B, Mitra N, et al. , Matrix Metalloproteinase-9 (MMP-9) polymorphisms in patients with cutaneous malignant melanoma, BMC Med. Genet 8 (2007) 10, 10.1186/1471-2350-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhang B, Ye S, Herrmann SM, et al. , Functional polymorphism in the regulatory region of gelatinase B gene in relation to severity of coronary atherosclerosis, Circulation 99 (14) (1999) 1788–1794, 10.1161/01.cir.99.14.1788. [DOI] [PubMed] [Google Scholar]
- [64].Shimajiri S, Arima N, Tanimoto A, et al. , Shortened microsatellite d(CA)21 sequence down-regulates promoter activity of matrix metalloproteinase 9 gene, FEBS Lett. 455 (1–2) (1999) 70–74, 10.1016/s0014-5793(99)00863-7. [DOI] [PubMed] [Google Scholar]
- [65].Kim SC, Singh M, Huang J, et al. , Matrix metalloproteinase-9 in cerebral aneurysms, Neurosurgery. 41 (3) (1997) 642–666, 10.1097/00006123-199709000-00027. [DOI] [PubMed] [Google Scholar]
- [66].Aoki T, Kataoka H, Morimoto M, Nozaki K, Hashimoto N, Macrophage-derived matrix metalloproteinase-2 and -9 promote the progression of cerebral aneurysms in rats, Stroke 38 (1) (2007) 162–169, 10.1161/01.STR.0000252129.18605.c8. [DOI] [PubMed] [Google Scholar]
- [67].Brew K, Dinakarpandian D, Nagase H, Tissue inhibitors of metalloproteinases: evolution, structure and function, Biochim. Biophys. Acta 1477 (1–2) (2000) 267–283, 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- [68].Yu WH, Yu S, Meng Q, Brew K, Woessner JF Jr., TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix, J. Biol. Chem 275 (40) (2000) 31226–31232, 10.1074/jbc.M000907200. [DOI] [PubMed] [Google Scholar]
- [69].Krex D, Rohl H, Konig IR, Ziegler A, Schackert HK, Schackert G, Tissue inhibitor of metalloproteinases-1, -2, and -3 polymorphisms in a white population with intracranial aneurysms, Stroke 34 (12) (2003) 2817–2821, 10.1161/01.STR.0000099966.51485.5F. [DOI] [PubMed] [Google Scholar]
- [70].Hinterseher I, Krex D, Kuhlisch E, et al. , Analysis of tissue inhibitor of metalloproteinase-2 gene polymorphisms in a caucasian population with abdominal aortic aneurysms, Zentralbl. Chir 133 (4) (2008) 332–337, 10.1055/s-2008-1076862. [DOI] [PubMed] [Google Scholar]
- [71].Jin D, Sheng J, Yang X, Gao B, Matrix metalloproteinases and tissue inhibitors of metalloproteinases expression in human cerebral ruptured and unruptured aneurysm, Surg. Neurol 68 (Suppl 2) (2007) S11–S16, 10.1016/j.surneu.2007.02.060. [DOI] [PubMed] [Google Scholar]
- [72].Wiebers DO, Whisnant JP, Huston J 3rd, et al. , Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment, Lancet 362 (9378) (2003) 103–110, 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- [73].Pluta RM, Delayed cerebral vasospasm and nitric oxide: review, new hypothesis, and proposed treatment, Pharmacol. Ther 105 (1) (2005) 23–56, 10.1016/j.pharmthera.2004.10.002. [DOI] [PubMed] [Google Scholar]
- [74].Cooke JP, Dzau VJ, Derangements of the nitric oxide synthase pathway, L-arginine, and cardiovascular diseases, Circulation 96 (2) (1997) 379–382. [PubMed] [Google Scholar]
- [75].Forstermann U, Munzel T, Endothelial nitric oxide synthase in vascular disease: from marvel to menace, Circulation 113 (13) (2006) 1708–1714, 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- [76].Marsden PA, Heng HH, Scherer SW, et al. , Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene, J. Biol. Chem 268 (23) (1993) 17478–17488. [PubMed] [Google Scholar]
- [77].Khurana VG, Sohni YR, Mangrum WI, et al. , Endothelial nitric oxide synthase T-786C single nucleotide polymorphism: a putative genetic marker differentiating small versus large ruptured intracranial aneurysms, Stroke 34 (11) (2003) 2555–2559, 10.1161/01.STR.0000096994.53810.59. [DOI] [PubMed] [Google Scholar]
- [78].Khurana VG, Meissner I, Sohni YR, et al. , The presence of tandem endothelial nitric oxide synthase gene polymorphisms identifying brain aneurysms more prone to rupture, J. Neurosurg 102 (3) (2005) 526–531, 10.3171/jns.2005.102.3.0526. [DOI] [PubMed] [Google Scholar]
- [79].Song MK, Kim MK, Kim TS, et al. , Endothelial nitric oxide gene T-786C polymorphism and subarachnoid hemorrhage in Korean population, J. Korean Med. Sci 21 (5) (2006) 922–926, 10.3346/jkms.2006.21.5.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kim TG, Kim NK, Baek MJ, et al. , The relationships between endothelial nitric oxide synthase polymorphisms and the formation of intracranial aneurysms in the Korean population, Neurosurg. Focus 30 (6) (2011), E23, 10.3171/2011.2.FOCUS10227. [DOI] [PubMed] [Google Scholar]
- [81].Kroncke KD, Fehsel K, Kolb-Bachofen V, Inducible nitric oxide synthase in human diseases, Clin. Exp. Immunol 113 (2) (1998) 147–156, 10.1046/j.1365-2249.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Barcellos LF, Begovich AB, Reynolds RL, et al. , Linkage and association with the NOS2A locus on chromosome 17q11 in multiple sclerosis, Ann. Neurol 55 (6) (2004) 793–800, 10.1002/ana.20092. [DOI] [PubMed] [Google Scholar]
- [83].Kleinert H, Pautz A, Linker K, Schwarz PM, Regulation of the expression of inducible nitric oxide synthase, Eur. J. Pharmacol 500 (1–3) (2004) 255–266, 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- [84].Zhang J, Schmidt J, Ryschich E, Mueller-Schilling M, Schumacher H, Allenberg JR, Inducible nitric oxide synthase is present in human abdominal aortic aneurysm and promotes oxidative vascular injury, J. Vasc. Surg 38 (2) (2003) 360–367, 10.1016/s0741-5214(03)00148-4. [DOI] [PubMed] [Google Scholar]
- [85].Fukuda S, Hashimoto N, Naritomi H, et al. , Prevention of rat cerebral aneurysm formation by inhibition of nitric oxide synthase, Circulation 101 (21) (2000) 2532–2538, 10.1161/01.cir.101.21.2532. [DOI] [PubMed] [Google Scholar]
- [86].Sadamasa N, Nozaki K, Hashimoto N, Disruption of gene for inducible nitric oxide synthase reduces progression of cerebral aneurysms, Stroke 34 (12) (2003) 2980–2984, 10.1161/01.STR.0000102556.55600.3B. [DOI] [PubMed] [Google Scholar]
- [87].Griffiths MJ, Messent M, MacAllister RJ, Evans TW, Aminoguanidine selectively inhibits inducible nitric oxide synthase, Br. J. Pharmacol 110 (3) (1993) 963–968, 10.1111/j.1476-5381.1993.tb13907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hara A, Yoshimi N, Mori H, Evidence for apoptosis in human intracranial aneurysms, Neurol. Res 20 (2) (1998) 127–130, 10.1080/01616412.1998.11740494. [DOI] [PubMed] [Google Scholar]
- [89].Pahan K, Sheikh FG, Liu X, Hilger S, McKinney M, Petro TM, Induction of nitric-oxide synthase and activation of NF-kappaB by interleukin-12 p40 in microglial cells, J. Biol. Chem 276 (11) (2001) 7899–7905, 10.1074/jbc.M008262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Pahl HL, Activators and target genes of Rel/NF-kappaB transcription factors, Oncogene 18 (49) (1999) 6853–6866, 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- [91].Nakashima H, Aoki M, Miyake T, et al. , Inhibition of experimental abdominal aortic aneurysm in the rat by use of decoy oligodeoxynucleotides suppressing activity of nuclear factor kappaB and ets transcription factors, Circulation 109 (1) (2004) 132–138, 10.1161/01.CIR.0000105725.61763.A2. [DOI] [PubMed] [Google Scholar]
- [92].Slowik A, Borratynska A, Turaj W, et al. , Interleukin 1beta-511 C/T polymorphism and risk of aneurysmal subarachnoid haemorrhage, J. Neurol. Neurosurg. Psychiatry 77 (2) (2006) 279–280, 10.1136/jnnp.2005.075457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Hu L, Li B, Liao X, Yan J, Polymorphisms of inflammatory cytokine genes and risk for intracranial aneurysm: a systematic review and meta-analysis, Yonsei Med. J 61 (5) (2020) 391–399, 10.3349/ymj.2020.61.5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hu J, Luo J, Wang H, et al. , Association of TNF-α-3959T/C gene polymorphisms in the Chinese population with intracranial aneurysms, J. Mol. Neurosci 63 (3–4) (2017) 349–354, 10.1007/s12031-017-0985-y. [DOI] [PubMed] [Google Scholar]
- [95].Low SK, Zembutsu H, Takahashi A, et al. , Impact of LIMK1, MMP2 and TNF-α variations for intracranial aneurysm in Japanese population, J. Hum. Genet 56 (3) (2011) 211–216, 10.1038/jhg.2010.169. [DOI] [PubMed] [Google Scholar]
- [96].Xu L, Hu L, Hu C, et al. , Associations between inflammatory cytokine gene polymorphisms and susceptibilities to intracranial aneurysm in Chinese population, Biomed. Res. Int 2021 (2021), 8865601, 10.1155/2021/8865601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Liu Y, Sun J, Wu C, Cao X, He M, You C, The interleukin-6-572G/C gene polymorphism and the risk of intracranial aneurysms in a Chinese population, Genet. Test. Mol. Biomarkers 16 (7) (2012) 822–826, 10.1089/gtmb.2012.0004. [DOI] [PubMed] [Google Scholar]
- [98].Morgan L, Cooper J, Montgomery H, Kitchen N, Humphries SE, The interleukin-6 gene −174G>C and −572G>C promoter polymorphisms are related to cerebral aneurysms, J. Neurol. Neurosurg. Psychiatry 77 (8) (2006) 915–917, 10.1136/jnnp.2005.081976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Sun H, Zhang D, Zhao J, The interleukin-6 gene −572G>C promoter polymorphism is related to intracranial aneurysms in Chinese Han nationality, Neurosci. Lett 440 (1) (2008) 1–3, 10.1016/j.neulet.2008.04.077. [DOI] [PubMed] [Google Scholar]
- [100].Zheng S, Su A, Sun H, You C, The association between interleukin-6 gene polymorphisms and intracranial aneurysms: a meta-analysis, Hum. Immunol 74 (12) (2013) 1679–1683, 10.1016/j.humimm.2013.08.274. [DOI] [PubMed] [Google Scholar]
- [101].Starke RM, Raper DM, Ding D, et al. , Tumor necrosis factor-α modulates cerebral aneurysm formation and rupture, Transl. Stroke Res 5 (2) (2014) 269–277, 10.1007/s12975-013-0287-9. [DOI] [PubMed] [Google Scholar]
- [102].Aoki T, Fukuda M, Nishimura M, Nozaki K, Narumiya S, Critical role of TNF-alpha-TNFR1 signaling in intracranial aneurysm formation, Acta Neuropathol. Commun 2 (2014) 34, 10.1186/2051-5960-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Moriwaki T, Takagi Y, Sadamasa N, Aoki T, Nozaki K, Hashimoto N, Impaired progression of cerebral aneurysms in interleukin-1beta-deficient mice, Stroke 37 (3) (2006) 900–905, 10.1161/01.STR.0000204028.39783.d9. [DOI] [PubMed] [Google Scholar]
- [104].Wajima D, Hourani S, Dodd W, et al. , Interleukin-6 promotes murine estrogen deficiency-associated cerebral aneurysm rupture, Neurosurgery 86 (4) (2020) 583–592, 10.1093/neuros/nyz220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Aoki T, Frȍsen J, Fukuda M, et al. , Prostaglandin E2-EP2-NF-κB signaling in macrophages as a potential therapeutic target for intracranial aneurysms, Sci. Signal (465) (2017) 10, 10.1126/scisignal.aah6037. [DOI] [PubMed] [Google Scholar]
- [106].Shimada K, Furukawa H, Wada K, et al. , Protective role of peroxisome proliferator-activated receptor-γ in the development of intracranial aneurysm rupture, Stroke 46 (6) (2015) 1664–1672, 10.1161/STROKEAHA.114.007722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hoh BL, Rojas K, Lin L, et al. , Estrogen deficiency promotes cerebral aneurysm rupture by upregulation of Th17 cells and interleukin-17A which downregulates E-cadherin, J. Am. Heart Assoc (8) (2018) 7, 10.1161/JAHA.118.008863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Di Gregorio GB, Yao-Borengasser A, Rasouli N, et al. , Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone, Diabetes 54 (8) (2005) 2305–2313, 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- [109].Hou S, Guan H, Ricciardi RP, Phosphorylation of serine 337 of NF-kappaB p50 is critical for DNA binding, J. Biol. Chem 278 (46) (2003) 45994–45998, 10.1074/jbc.M307971200. [DOI] [PubMed] [Google Scholar]
- [110].Coutard M, Experimental cerebral aneurysms in the female heterozygous Blotchy mouse, Int. J. Exp. Pathol 80 (6) (1999) 357–367, 10.1046/j.1365-2613.1999.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Holdt LM, Teupser D, Recent studies of the human chromosome 9p21 locus, which is associated with atherosclerosis in human populations, Arterioscler. Thromb. Vasc. Biol 32 (2) (2012) 196–206, 10.1161/ATVBAHA.111.232678. [DOI] [PubMed] [Google Scholar]
- [112].Helgadottir A, Thorleifsson G, Magnusson KP, et al. , The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm, Nat. Genet 40 (2) (2008) 217–224, 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]
- [113].Nakaoka H, Takahashi T, Akiyama K, et al. , Differential effects of chromosome 9p21 variation on subphenotypes of intracranial aneurysm: site distribution, Stroke 41 (8) (2010) 1593–1598, 10.1161/STROKEAHA.110.586529. [DOI] [PubMed] [Google Scholar]