Abstract
Aqueous film-forming foams historically were used during fire-training activities on Joint Base Cape Cod, Massachusetts, and created an extensive per- and polyfluoroalkyl substances (PFAS) groundwater contamination plume. Potential for PFAS bioconcentration from exposure to the contaminated groundwater, which discharges to surface-water bodies, was assessed with mobile laboratory experiments using groundwater from the contamination plume and a nearby reference location. The on-site continuous flow 21-day exposures used male and female fathead minnows, freshwater mussels, polar organic chemical integrative samplers (POCIS), and polyethylene tube samplers (PETS) to evaluate biotic and abiotic uptake. Composition of the PFAS-contaminated groundwater was complex and 9 PFAS were detected in the reference groundwater and 17 PFAS were detected in the contaminated groundwater. The summed PFAS concentrations ranged from 115 to 137 ng L−1 in reference groundwater and 6100 to 14,600 ng L−1 in contaminated groundwater. Biotic concentration factors () for individual PFAS were species, sex, source, and compound specific, and ranged from 2.9 to 1000 L kg−1 in whole body male fish exposed to contaminated groundwater for 21 days. The fish and mussel generally increased with increasing fluorocarbon chain length and were greater for sulfonates than for carboxylates. The exception was perfluorohexane sulfonate, which deviated from the linear trend and had a 10-fold difference in between sites, possibly because of biotransformation of precursors such as perfluorohexane sulfonamide. Uptake for most PFAS in male fish was linear over time whereas female fish had bilinear uptake indicated by an initial increase in tissue concentrations followed by a decrease. Uptake of PFAS was less for mussels (maximum ) than for fish, and mussel uptake of most PFAS also was bilinear. Although abiotic concentration factors were greater than , and values for POCIS were greater than for PETS, passive samplers were useful for assessing PFAS that potentially bioconcentrate in fish but are present at concentrations below minimum quantitation limits in water. Passive samplers also accumulate short-chain PFAS that are not bioconcentrated.
Keywords: PFAS, groundwater, surface water, bioconcentration, fathead minnow, freshwater mussel, polar organic chemical integrative sampler, polyethylene tube sampler, aqueous film-forming foams, mobile laboratory
Graphical Abstract
INTRODUCTION
Per- and polyfluoroalkyl substances (PFAS) consist of thousands of individual compounds with diverse chemical structures, uses, environmental fates, and biological effects.1–3 Because of their multiple sources, high mobility, and persistence, PFAS have contaminated aquatic environments and biota on a global scale.4–7 Widespread occurrence of PFAS has been reported in groundwater, surface water, drinking water, and wastewater, and of the thousands of compounds in use, relatively few have been studied in detail.8–15 Although not currently regulated at the federal level in the U.S., perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) have national drinking water health advisories that were recently decreased from 70 ng L−1 (individually or combined) to <0.1 ng L−1.16–18 At the state level, additional PFAS have been targeted for drinking-water regulations.19 For example, Massachusetts regulates six PFAS (perfluoroheptanoate, PFHpA; PFOA; perfluorononanoate, PFNA; perfluorodecanoate, PFDA; perfluorohexane sulfonate, PFHxS; and PFOS) with a combined limit of 20 ng L−1.20 While considerable effort has focused on developing human health guidelines for drinking water, there has been less effort on developing benchmarks for effects on aquatic organisms.21,22 Although there are no established ecotoxicological regulations, draft aquatic life ambient water quality criteria have been recommended for PFOA and PFOS, with acute criterion maximum concentrations in water of 49 and 3.0 mg L−1, respectively, and chronic criterion continuous concentrations of 0.094 and 0.0084 mg L−1, respectively.23
Typically, PFAS occur as complex mixtures that vary widely in composition depending on manufacturing processes, uses, and formulations.24,25 For example, aqueous film-forming foams (AFFF) used to fight aviation fuel fires contain many classes of PFAS with differing chemical structures that have been shown to contaminate groundwater.26 The specific composition of PFAS mixtures in AFFF has changed over time and reflects historical usage of specific formulations.13,27 Likewise, PFAS composition can evolve along a flow path as the result of geochemical fractionation due to sorption, biotransformation of precursor compounds, treatment processes, and contributions from additional sources.14,28
Assessing the occurrence and concentration of PFAS in aquatic environments using biotic and abiotic sampling is complicated by the many processes influencing their fate and transport, including sorption at physical interfaces and biodegradation.29–33 Of particular importance for assessing environmental exposure and potential biological effects on aquatic organisms is the partitioning of PFAS between water and tissue.34–36 Although studies of bioconcentration and bioaccumulation of PFAS in fish typically focus on kinetics and partitioning into internal organ tissue, whole-body and muscle tissue are preferred for assessing human consumption exposure and ecosystem risk.35,37 Despite the extensive and rapidly growing literature on PFAS occurrence, fate, and effects, there are relatively few controlled studies (laboratory or field) reporting on bioconcentration factors (BCFs) for PFAS in freshwater fish and mussels.
Studies on aquatic organisms indicate that complex biological processes such as bioconcentration and toxicity are mediated by the physicochemical properties of the individual PFAS. One of the earliest reports on PFAS bioconcentration by freshwater fish38 assessed the uptake of perfluoroalkyl carboxylates (PFCA) and perfluoroalkyl sulfonates (PFSA) from water by rainbow trout (Oncorhynchus mykiss) and partitioning into various tissue compartments (plasma and liver had the highest uptake and muscle had the lowest). The PFCA and PFSA with less than 7 and 6 fluorocarbons, respectively, were not detected in fish tissue. Bioconcentration factors for carcass tissue (whole organism minus blood and internal organs) increased with increasing fluorocarbon chain length and ranged from 4 L kg−1 for PFOA to 23,000 L kg−1 for perfluorotetradecanoic acid (PFTeDA). For an equivalent fluorocarbon chain length, BCFs were greater for PFSA than PFCA. Bioconcentration of PFAS in common carp (Cyprinus carpio L.)39 also increased with increasing fluorocarbon chain length and BCFs were greater for PFSA than PFCA (PFOS = 720 to 1300 L kg−1; PFOA = 5.1 to 9.4 L kg−1). Similarly, bioconcentration of PFAS in zebrafish (Danio rerio)40 showed a strong relation between uptake and fluorocarbon chain length for various tissue compartments: BCFs ranged from 0.12 L kg−1 for perfluorobutanoic acid (PFBA) to 19,000 L kg−1 for perfluorododecanoic acid (PFDoA).
Although there are limited studies of PFAS uptake by mussels, as with fish, uptake increases with increasing fluorocarbon chain length.41,42 In addition, PFAS uptake in mussels appears to be concentration dependent with lower BCFs at higher water concentrations. The biological mechanisms underlying this effect are ambiguous but may involve concentration-dependent efflux or site-specific adsorption.
In addition to the hydrophobicity-based fluorocarbon chain-length effect,39,40,43,44 another important physicochemical factor controlling aqueous behavior of PFAS is the acid dissociation constant (log pKa), which is <4 for PFCA and PFSA, resulting in the ionic species being predominant at environmental pHs.45,46 In contrast to neutral hydrophobic organic contaminants, which predominantly partition into lipid tissue (and also show a carbon chain length effect),47 bioconcentration of ionic PFAS is predominantly associated with binding to proteins and phospholipids.38,48,49
Passive water samplers are widely used to assess organic contaminant occurrence and have been proposed to mimic uptake in biota.50 However, few studies have evaluated concurrent uptake of PFAS in passive samplers and biota.51 As with biotic media, abiotic partitioning of PFAS between water and passive samplers increases with increasing perfluorocarbon chain length and also depends upon the physicochemical characteristics of the individual compound, attributes of the materials used to construct the passive sampler, background water chemistry, and environmental conditions.50,52
The limited literature on experimental determination of PFAS bioconcentration typically focuses on a single aquatic species and a limited number of compounds at relatively high concentrations. Although controlled laboratory studies can be effective at determining steady state BCFs for individual PFAS, they do not accurately reflect exposure to complex environmental mixtures. Field-based PFAS bioaccumulation studies often include a range of compounds present in environmental mixtures but are limited by lack of hydrological and chemical controls, uncertainty in exposure history, multiple exposure routes (dietary, water, and sediment), and effects of co-occurring contaminants. Many studies on PFAS uptake focus on partitioning into specific tissue rather than whole organisms, which gives a potentially skewed perspective of actual bioconcentration/bioaccumulation.34–36
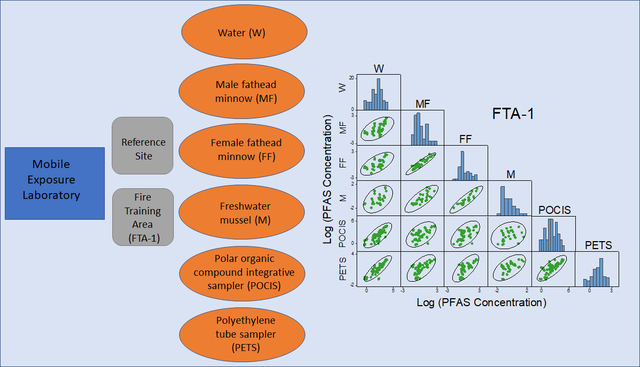
This paper describes controlled field-based multi-media exposure experiments conducted to evaluate PFAS bioconcentration by aquatic organisms. The experiments were conducted at an AFFF-derived PFAS groundwater contamination plume from a legacy fire-training area (FTA) and simultaneously evaluated biotic and abiotic uptake of PFAS from two groundwater sources with contrasting low and high concentrations. The groundwater contamination provided a natural field laboratory to evaluate uptake of PFAS from environmental mixtures under ambient conditions. Groundwater from the reference source had PFAS concentrations representative of low-level contamination in the regional aquifer and were similar to levels in a nearby lake. Groundwater from the FTA-contaminated source contains high concentrations of a complex AFFF-derived PFAS mixture. Biotic and abiotic media (fish, mussels, and passive samplers) were deployed to determine whether (1) PFAS uptake from mixtures varies as a function of concentration and composition, (2) uptake varies between sex and species, and (3) uptake by passive samplers provides a surrogate for uptake by aquatic organisms.
METHODS
Study Site
This investigation was conducted at the U.S. Geological Survey (USGS) groundwater research site at Joint Base Cape Cod (JBCC), Massachusetts, USA (Figure 1). The site is located on the Cape Cod aquifer53 (a sole source drinking-water supply) and has been the focus of investigations on groundwater contamination from multiple sources, including wastewater treatment plant effluent disposal54–58 and FTA activities.14,15 The PFAS groundwater contamination plume originates from a historical FTA where AFFF were used during training from 1970 to 1985.59 The plume is located in a hydrologically well characterized unconfined sand-and-gravel aquifer.60–63 Because groundwater contamination plumes in this area discharge to surface waters,15,64–67 these experiments provide insight into potential PFAS exposure of biota in the receiving ecosystem. There also is the potential for human exposure if drinking-water supplies are obtained from PFAS-contaminated regions of the aquifer.68
Figure 1.
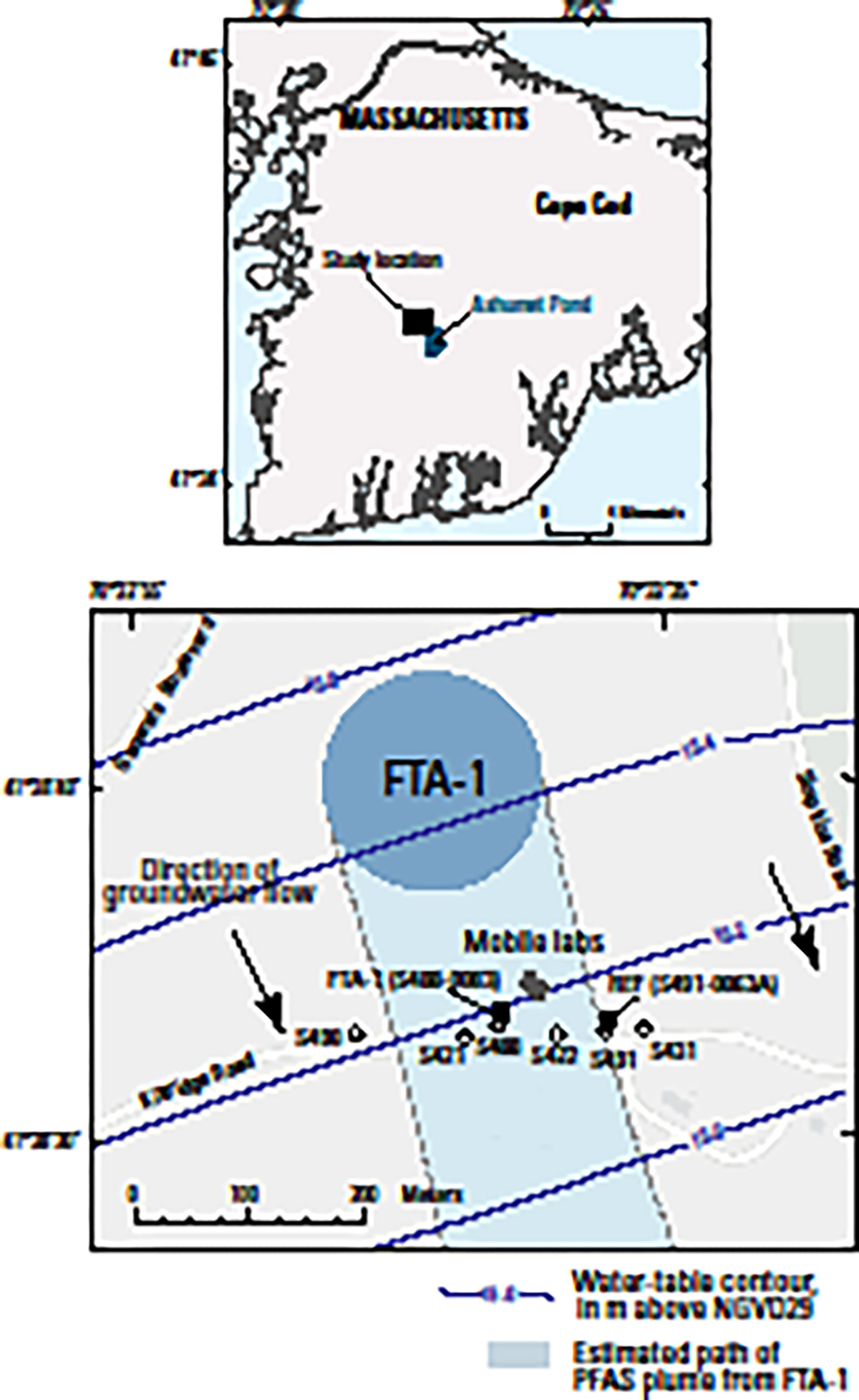
Site map showing locations of (1) the per- and polyfluoroalkyl substance (PFAS) groundwater contamination plume originating from a historical fire-training area (FTA) on Cape Cod, Massachusetts, (2) monitoring wells used to define the plume boundary, (3) water-table contours and inferred groundwater-flow direction, and (4) reference (REF; MA-SDW 491–0063A) and FTA contaminated (FTA-1; MA-SDW 488–0083) groundwater wells used for the mobile laboratory exposure experiments. The REF well was located on the complex “fringe” of the plume and does not represent pristine uncontaminated groundwater but rather provided an order of magnitude concentration gradient from FTA-1. [Water-table contours adapted from reference 63; see Table S1 for well information; dashed lines indicate inferred boundary.]
The glacial deposits that form the aquifer include many kettle lakes in which groundwater seepage is the major input of water (no surface-water inflows), resulting in minimal differences between the non-reactive bulk chemical compositions of the groundwater and surface water.15,69,70 The JBCC PFAS plume discharges to Ashumet Pond, a kettle lake about 1 km downgradient from the FTA The PFAS-contaminated lake water subsequently recharges the downgradient aquifer.15
Mobile-laboratory exposure experiments were conducted near the eastern edge of the PFAS plume at a location approximately 200 m downgradient from the FTA source (Figure 1). Transverse vertical and horizontal macrodispersion are limited (or low) in the Cape Cod aquifer61,62 and the PFAS plume has relatively sharp lateral boundaries.14 This feature was used to advantage in the experimental design to provide reference and contaminated groundwater sources near one another (<100 m apart) to maximize the PFAS concentration gradient for the exposure treatments while minimizing differences in bulk groundwater geochemistry. Supply wells (see Supporting Information, SI; Table S1) were installed in (1) relatively uncontaminated reference groundwater (REF; MA-SDW 491–0063A) and (2) the FTA groundwater contamination plume (FTA-1; MA-SDW 488–0083). Stainless steel submersible pumps fitted with high density polyethylene tubing were installed in each well, and during the experiments groundwater was pumped at a continuous rate of 10 L min−1 to provide flow to the mobile laboratories.
Mobile Laboratory Experiments
Exposure conditions.
Two mobile laboratories were set up adjacent to the supply wells to expose biotic and abiotic media to the PFAS mixtures present in the REF (low concentrations) and FTA-1 (high concentrations) groundwater under environmentally relevant scenarios. The 21-day continuous-flow mobile laboratory fish and passive sampler exposures were conducted from August 29 to September 21, 2018 using protocols (see SI) that maintained controlled conditions, including consistent flow (~200 mL min−1), aeration (oxygen saturation), diet (fed daily), temperature (20 ± 1 °C), and photoperiod (14 h:10 h light:dark).71–73 During the experiments water samples were collected almost daily for PFAS analysis. Temperature, pH, dissolved oxygen, and specific conductance were measured at the time of sample collection.74
Model Organisms.
Adult male and female fathead minnows (Pimephales promelas) were randomly assigned to 10 L glass aquaria and exposed to REF and FTA-1 groundwater for up to 21 days. Each aquaria contained either 5 male or 5 female fish, and during each sampling fish were collected from two aquaria per treatment. Male and female fish carcasses (whole body minus blood, liver, gonad, brain, and gastrointestinal tissue) were sampled at REF and FTA-1 on d0 (, both sexes), d4 (, male only), d7 (, both sexes), d14 (, both sexes), and d21 (, both sexes). Whole-body fish were sampled on d21 (, both sexes). Individual carcass and whole-body samples were wrapped in foil and frozen at −80 °C.
Adult freshwater unionid mussels (Ligumia subrostrata) were used to assess PFAS bioconcentration in an invertebrate species. Each mussel exposure treatment consisted of 10 L glass aquaria containing fine-grained silica sand (see SI). At the start of the 14-day exposures (beginning on d7 of the fish exposures), mussels were randomly transferred into 5 aquaria maintained under the same conditions as fish. Initial control samples were processed on d7 (), and 1 mussel was sampled from each of the individual aquarium () on d11, d14, and d21. Soft tissue was collected, wrapped in foil, and frozen at −80 °C.
Passive samplers.
Polar organic chemical integrative samplers (POCIS) were constructed (see SI) in the standard configuration50,75 using hydrophilic-lipophilic-balance sorbent (HLB) contained between two polyethersulfone membranes and had an exposed sampling surface area of 41 cm2. Polyethylene tube samplers (PETS)76,77 were constructed (see SI) by filling microporous polyethylene tubing with HLB sorbent and had an exposed sampling surface area of 18.8 cm2. The POCIS and PETS were deployed in separate aquaria maintained under the same conditions as fish and mussels, and initial controls consisted of fabrication and field blanks. Exposed samplers () were collected on d4, d7, d14, and d21.
Chemical analysis.
Groundwater samples were analyzed at Harvard University for 25 PFAS using liquid chromatography-tandem mass spectrometry (LC-MS/MS) in negative ion multiple reaction monitoring (MRM) mode (see SI).14,15 Abbreviations for the PFAS measured and the MRM transition ions monitored are presented in Table S2. The targeted PFAS cover a wide range of physicochemical characteristics (Table S3). Quality assurance (QA) procedures for LC-MS/MS analysis of all media included instrument and extraction blanks, sample extraction and field duplicates, extraction spikes, and sample matrix spikes, and the results are summarized in the SI and elsewhere.74 Fish carcass, fish whole body, and mussel soft tissue were analyzed at Harvard University by LC-MS/MS for 27 compounds (see SI; Table S2; addition of FBSA and FHxSA). Tissue concentrations were determined as wet weight. In addition, fish and mussel feed were analyzed following the same procedures.
Following exposure, the POCIS HLB sorbent was removed and extracted with methanol (see SI). The POCIS extracts were analyzed at Harvard University by LC-MS/MS for the same 27 compounds as fish and mussel tissue (Table S2). The intact PETS were extracted with methanol (see SI) and analyzed at the University of Rhode Island by LC-MS/MS for the same 27 PFAS measured in water, tissue, and POCIS.
Concentration Factors, Uptake Rates, and Statistical Analysis
Concentration factors and uptake rates were calculated assuming “quasi” steady state conditions following the 21-day fish and passive sampler exposures and the 14-day mussel exposures. The limited duration of the experiments and dynamic environmental conditions can result in uncertainty regarding achieving steady state. Because this paper reports on PFAS uptake by organisms and passive samplers, the terms biotic concentration factor and abiotic concentration factor were used to describe the partitioning between water and biotic and abiotic media.
Values for (L kg−1) were calculated for each PFAS using mean groundwater concentrations for d3 to d21 and mean fish and mussel tissue concentrations at d21. The passive sampler values (L kg−1) were determined using mean d3 to d21 groundwater concentrations, POCIS concentrations at d21 based on mass of HLB sorbent, and PETS concentrations at d21 based on total PETS mass. Uptake of PFAS by biotic and abiotic media was evaluated using “pseudo” first-order rates and associated half-lives determined from the slope of the curve for the natural logarithm transformed concentration versus time (see SI).
Descriptive and multivariate statistics78 for individual PFAS were calculated for water, fish, mussel, and passive samplers (see SI). Pearson correlation analysis of log transformed concentration data was used to explore relations between PFAS uptake in biotic and abiotic media, and between individual PFAS in all samples and media types ().
RESULTS AND DISCUSSION
Water Exposure Conditions
The PFAS results for groundwater samples collected from d3 to d21 are presented in Table S4. The QA results for all media are summarized in the SI, and the complete dataset is presented elsewhere.74 A unique characteristic of the FTA contaminated groundwater is the low dissolved organic carbon content (<1.0 mg L−1) and minimal co-occurring contaminants.74 Of the 25 PFAS analyzed in all media, 9 were detected in the REF groundwater (Figures 2 and S1). The most abundant PFAS at REF was total-PFHxS (T-PFHxS; combined linear, L, and branched, B, isomers) with a mean concentration (± 1 standard deviation) of 83 ± 5.9 ng L−1 (). The summed concentrations for all detected PFAS (∑PFAS) ranged from 120 to 140 ng L−1 (mean = 130 ± 7.5 ng L−1). The combined mean PFOA and T-PFOS concentrations at REF were <20 ng L−1 (below the 70 ng L−1 USEPA health advisory)16,17 but the combined mean concentrations of the 6 PFAS regulated by the Commonwealth of Massachusetts20 was >100 ng L−1 (5 times the 20 ng L−1 limit). Reported concentrations of ∑PFAS in Ashumet Pond (200 to 230 ng L−1) were similar to REF groundwater.15 Seventeen PFAS were detected at FTA-1 (Figures 2 and S1) with the most abundant being T-PFOS (mean concentration = 4000 ± 960 ng L−1). At FTA-1, the combined PFOA and T-PFOS mean concentration was 5200 ng L−1 and the combined Massachusetts six was 7700 ng L−1.
Figure 2.
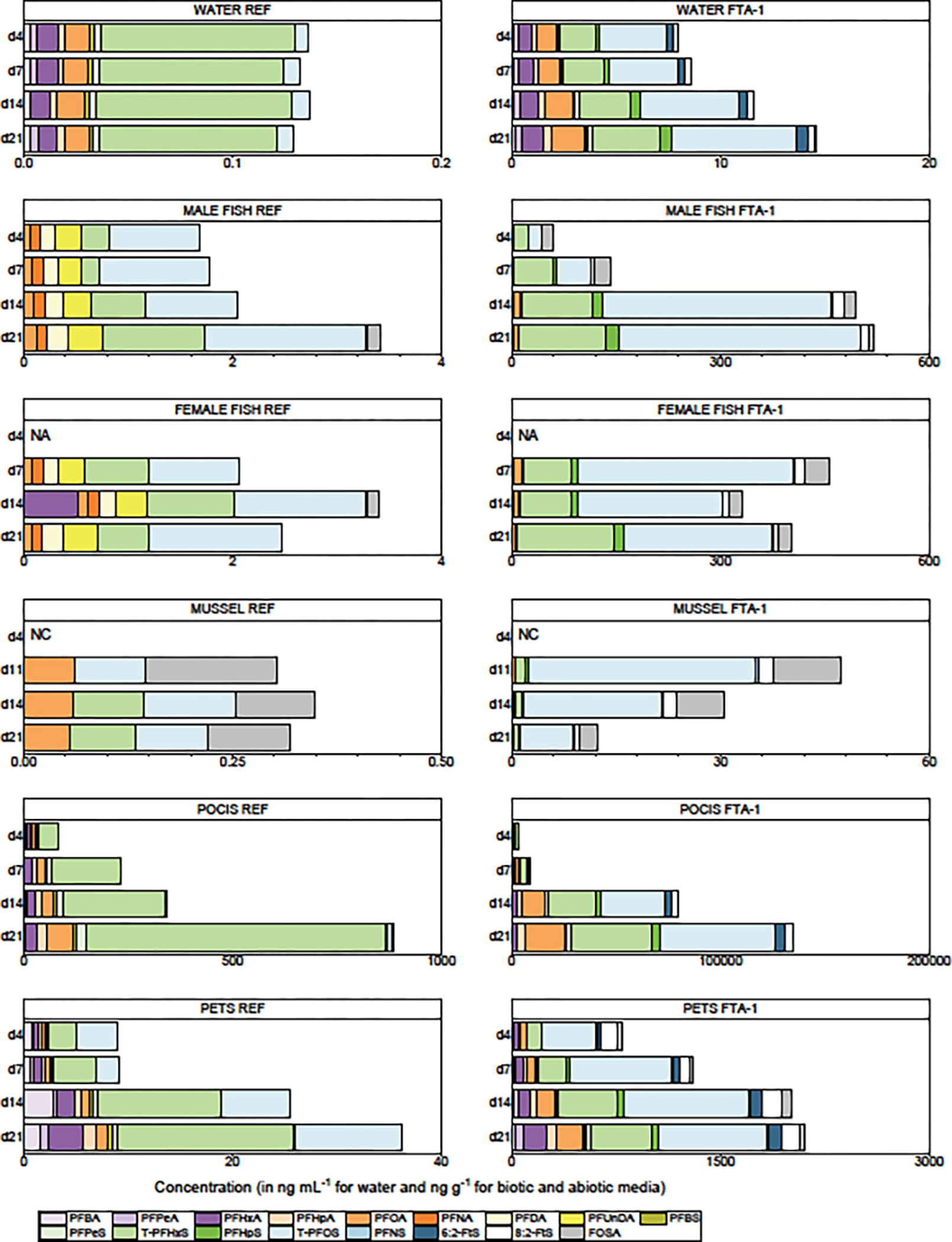
Concentrations of 17 per- and polyfluoroalkyl substances (PFAS) detected in water, male and female fish tissue, mussel tissue, polar organic chemical integrative sampler (POCIS), and polyethylene tube sampler (PETS) media exposed to groundwater from the reference (REF; MA-SDW 491–0063A) and fire-training area contaminated (FTA-1; MA-SDW 488–0083) wells during the 2018 mobile-laboratory experiments conducted on Cape Cod, Massachusetts. [Fish and passive samplers collected on d4, d7, d14, and d21; 14 day mussel exposure began on d7 of the fish and passive sampler exposures and samples were collected on d11, d14, and d21 (after 4, 7, and 14 days of deployment); concentrations for groundwater, POCIS, and PETS (); male and female fathead minnow carcass tissue mean concentrations (d4, d7, d14 ; d21 ); mussel tissue (); see Table S2 for compound abbreviations; see Tables S4 to S8 for individual PFAS concentration measurements and descriptive statistics for each media; NA; day 4 female fish samples were not collected and analyzed; NC, mussel exposures were not conducted on d4; POCIS concentrations in ng g−1 of sorbent; PETS concentrations in ng g−1 of sampler.]
The hydrological and chemical dynamics of groundwater contamination plumes are spatially and temporally heterogeneous, and this study captured the inherent variability that occurs under environmental conditions. Based on a mean groundwater flow velocity of ~0.3 m d−1 in the Cape Cod aquifer,61 it was expected that PFAS concentrations would remain relatively stable during the 21-day experiments (the parcel of water sampled would only be ~7 m long). Stable PFAS concentrations were observed at REF but not at FTA-1 (Figures 2 and S1; Table S4). Over the course of the experiment, ∑PFAS at FTA-1 increased from 6100 ng L−1 at d3 to 15,000 ng L−1 at d21 (mean = 10,000 ± 2400 ng L−1). Although concentrations increased, the composition remained relatively constant. The PFAS contamination plume has strong vertical and lateral concentration gradients and temporal variations in PFAS concentrations were observed previously (Table S1).14 Although the ∑PFAS at FTA-1 increased 2.5-fold over the course of the experiment, it was not possible to determine if the increase resulted from intrinsic variability within the contamination plume as the water naturally flows past the well screen, or if the change was induced by pumping water from a larger zone of influence.
Concentrations in Biotic Tissue
Concentrations of PFAS in male and female fathead minnow carcass tissue from REF and FTA-1 are shown in Figure 2 and data for individual fish are presented in Table S5. The short fluorocarbon chain compounds (PFBA, PFPeA, and PFBS) were detected in REF and FTA-1 groundwater but not in fish exposed to the groundwater, which is consistent with their high water solubility and low partitioning potential (Table S3).45,46 Five PFAS (PFHpS, PFNS, 6:2-FtS, 8:2-FtS, and FOSA) were detected in most FTA-1 fish and groundwater samples but were not detected (or were minimally detected) in REF fish or groundwater. Two additional perfluoroalkyl sulfonamide precursors (FBSA and FHxSA) were analyzed in biota but not water and were detected in FTA-1 fish but not REF fish. Four long-chain PFAS (PFDoDA, PFTrDA, PFTeDA, and PFDS) were detected in fish feed and fish tissue (Table S5) but not in groundwater (Table S4). Groundwater concentrations <MQLs for these compounds could reflect their low water solubility, sorption to sediments, or other physicochemical interactions,45,46 and their detection in fish tissue could be due to bioconcentration. However, their presence in fish feed suggests that diet also is a potential source of these PFAS (see further discussion in passive sampler section). Two PFAS precursors (N-Me-FOSAA and N-Et-FOSAA) were sporadically detected in groundwater and fish at concentrations near their MQLs but were excluded from further analysis.
The PFAS with the maximum d21 fish whole-body concentration at REF was T-PFOS (male = 1.4 ± 0.22 ng g−1; female = 1.8 ± 0.49 ng g−1) with L-PFOS comprising 96% and 89% of T-PFOS in male and female fish, respectively (in contrast to groundwater where L-PFOS comprised 65% of the T-PFOS). The PFAS with the maximum d21 male fish whole-body concentration at FTA-1 also was T-PFOS (707 ± 517 ng g−1; 82% L-PFOS), whereas the maximum d21 female fish whole-body concentration was FHxSA (349 ± 84 ng g−1) followed by T-PFOS (294 ± 147 ng g−1; 74% L-PFOS). Note that FHxSA was not analyzed in groundwater because no analytical standard was available at the time.
Differences in PFAS concentrations between carcass and whole-body tissue varied by compound, but concentrations generally were lower in carcass compared to whole body due to removal of blood, liver, and other organs. At REF, there was little difference in d21 male carcass and whole-body ∑PFAS concentrations (3.6 and 3.4 ng g−1, respectively), whereas female carcass ∑PFAS concentrations were only 58% of whole-body values. At FTA-1, d21 male carcasses comprised 85% of the whole-body weight and carcass ∑PFAS concentrations were 49% of whole-body values. Female carcasses comprised 82% of the whole-body weight and carcass ∑PFAS concentrations were 58% of whole-body values.
The PFAS detected in mussel tissue at REF and FTA-1 (Figure 2; Table S6) also were detected in groundwater and fish. However, mussel concentrations were much lower than fish and the PFAS composition was substantially different. The predominant PFAS detected in REF mussels was FHxSA (0.76 ± 0.36 ng g−1), although PFOA, T-PFHxS, T-PFOS, and FOSA were sporadically detected near the MQL. The predominant PFAS detected in FTA-1 mussels was FHxSA (107 ± 45 ng g−1) followed by T-PFOS (33 ± 38 ng g−1) as was observed for female fish. Mussels had greater uptake of T-PFHxS, T-PFOS, and FOSA relative to PFCA. Mussel feed contained trace levels of PFAS (∑PFAS = 9.7 ng g−1; Table S6) but the predominant compounds in the feed (PFBA and PFHxA) were not detected in tissue.
Biotic Concentration Factors
Male and female fish values were calculated for PFAS that were detected in both water and tissue (Table 1). Variability in PFAS concentrations at FTA-1 prevented steady-state conditions, but it is difficult to quantify additional uncertainty associated with determining . Consequently, mean groundwater concentrations from d3 to d21 were used for the calculations, assuming that concentration variation effects were limited. Although water and fish tissue concentrations were lower at REF than FTA-1 (Figure 2; Tables S4 and S5), male and female fish d21 whole body values generally were similar between sources indicating concentration independence for most compounds. However, T-PFHxS values at FTA-1 were nearly an order of magnitude greater than REF. While T-PFOS values were similar between REF and FTA-1 for female fish they were greater at REF for male fish. It was not possible to calculate values for longer-chain PFAS at REF because their groundwater concentrations were <MQL. The assumption of limited effects on resulting from non-steady state conditions is supported by the similarity of values between FTA-1 and REF (which did not have increasing concentrations).
Table 1.
Biotic concentration factors () for male and female fathead minnow carcass tissue (MFC and FFC; each sex), male and female fathead minnow whole-body tissue (MFW and FFW; each sex), mussel soft tissue (MU; ), and abiotic concentration factors () for polar organic chemical integrative samplers (POCIS; ) and polyethylene tube samplers (PETS; ) exposed to groundwater from the reference (REF; MA-SDW 491-0063A) and fire-training area contaminated (FTA-1; MA-SDW 488-0083) wells located on Cape Cod, Massachusetts. [ values for MFC, MFW, FFC, FFW were calculated from mean d3 to d21 water concentration and mean tissue concentration after 21 day exposures; values for MU were calculated using mean d3 to d21 water concentration and mean tissue concentration after 14 day exposures; for POCIS calculated from mean d3 to d21 water concentration and sorbent media concentration after 21 day exposures; for PETS calculated from mean d3 to d21 water concentration and whole sampler concentration after 21 day exposures; see Table S2 for PFAS compound abbreviations; ND-W, not detected in water; ND-T, not detected in tissue; ND-WT, not detected in water or tissue; ND-P, not detected in passive sampler; ND-WP, not detected in water or passive sampler; NM-P, not measured in PETS.]
| REF | REF | REF | REF | FTA-1 | FTA-1 | FTA-1 | FTA-1 | REF | FTA-1 | REF | FTA-1 | REF | FTA-1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MFC | MFW | FFC | FFW | MFC | MFW | FFC | FFW | MU | MU | POCIS | POCIS | PETS | PETS | |
| Compound | L kg−1 | L kg−1 | L kg−1 | L kg−1 | L kg−1 | L kg−1 | L kg−1 | L kg−1 | L kg−1 | L kg−1 | L kg−1 | L kg−1 | L kg−1 | L kg−1 |
|
| ||||||||||||||
| PFBA | ND-T | ND-T | ND-T | ND-T | ND-T | ND-T | ND-T | ND-T | ND-T | ND-T | 390 | 27 | 350 | 230 |
| PFPeA | ND-T | ND-T | ND-T | ND-T | ND-T | 3.0 | ND-T | ND-T | ND-T | ND-T | ND-P | 280 | 260 | 230 |
| PFHxA | ND-T | ND-T | ND-T | ND-T | 1.3 | 2.9 | 0.9 | 1.2 | ND-T | ND-T | 2800 | 3200 | 330 | 220 |
| PFHpA | ND-T | ND-T | ND-T | ND-T | 2.4 | 4.9 | 1.1 | 1.8 | ND-T | 0.2 | 7000 | 13,000 | 350 | 270 |
| PFOA | 10 | 8.6 | 6.2 | 9.6 | 6.2 | 14 | 3.9 | 8.0 | 5.1 | 0.2 | 5600 | 16,000 | 110 | 160 |
| PFNA | ND-W | ND-W | ND-W | ND-W | 29 | 55 | 21 | 33 | ND-T | 3.5 | ND-W | 14,000 | ND-WP | 300 |
| PFDA | ND-W | ND-W | ND-W | ND-W | 220 | 330 | 240 | 290 | ND-WT | ND-T | ND-WP | 17,000 | ND-WP | 380 |
| PFUnDA | ND-W | ND-W | ND-W | ND-W | 700 | 1000 | 760 | 900 | ND-WT | 120 | ND-W | 14,000 | ND-WP | 730 |
| PFBS | ND-T | ND-T | ND-T | ND-T | ND-T | ND-T | ND-T | ND-T | ND-T | ND-T | 3500 | 4500 | 180 | 290 |
| PFPeS | ND-T | 63 | ND-T | ND-T | 4.4 | 12 | 8.9 | 23 | ND-T | ND-T | 8000 | 14,000 | 200 | 230 |
| L-PFHxS | 13 | 11 | 6.7 | 18 | 59 | 110 | 64 | 100 | 1.1 | 0.4 | 9100 | 18,000 | NM-P | NM-P |
| B-PFHxS | ND-T | ND-T | ND-T | 13 | 45 | 92 | 69 | 140 | 8.1 | 0.4 | 4500 | 18,000 | NM-P | NM-P |
| T-PFHxS | 12 | 9.8 | 5.9 | 16 | 58 | 110 | 64 | 110 | 1.0 | 0.4 | 8600 | 18,000 | 200 | 200 |
| PFHpS | ND-WT | ND-WT | ND-WT | ND-WT | 57 | 120 | 46 | 92 | ND-WT | 1.3 | ND-W | 12,000 | ND-W | 150 |
| L-PFOS | 280 | 280 | 220 | 350 | 110 | 220 | 59 | 84 | 20 | 6.8 | 1800 | 15,000 | NM-P | NM-P |
| B-PFOS | 100 | 88 | 110 | 110 | 53 | 91 | 43 | 53 | ND-T | 1.6 | 2000 | 12,000 | NM-P | NM-P |
| T-PFOS | 200 | 190 | 150 | 260 | 86 | 180 | 53 | 73 | 13 | 5.0 | 1900 | 14,000 | 1400 | 190 |
| PFNS | ND-WT | ND-WT | ND-WT | ND-WT | 280 | 750 | 190 | 360 | ND-WT | 44 | ND-WP | 16,000 | ND-WP | 1200 |
| 6:2-FtS | ND-W | ND-WT | ND-WT | ND-W | 0.2 | 0.2 | 0.4 | 0.7 | ND-WT | 0.2 | ND-W | 13,000 | ND-WP | 260 |
| 8:2-FtS | ND-WT | ND-WT | ND-WT | ND-WT | 44 | 84 | 30 | 50 | ND-WT | 5.8 | ND-WP | 15,000 | ND-WP | 510 |
| FOSA | ND-W | ND-W | ND-W | ND-W | 170 | 560 | 570 | 990 | ND-W | 200 | ND-WP | 9000 | ND-WP | 960 |
At FTA-1, fish values increased with increasing fluorocarbon chain length, ranging from 2.9 L kg−1 for PFHxA to 1000 L kg−1 for PFUnDA in male fish. For a given fluorocarbon chain-length equivalent, PFSA had higher values than PFCA (d21 FTA-1 male whole-body T-PFOS ; PFNA ). The fluorocarbon chain-length effect was less pronounced for female fish and the d21 FTA-1 female whole-body T-PFOS was 73 L kg−1 compared to 33 L kg−1 for PFNA. For male fish, the L-PFOS values were slightly greater than for B-PFOS, whereas for female fish the L-PFHxS values were less than for B-PFHxS. The values for male and female fathead minnows in this study agree with reported whole-body log BCFs for multiple fish species (ranging from 1.36 ± 0.61 for PFOA to 3.01 ± 0.66 for PFOS).35 The FTA-1 male fish whole-body log for PFOA was 1.15 and for T-PFOS was 2.26. The observed carcass to whole-body ratios for d21 FTA-1 male fish (Table 1) were consistent with the strong association of PFAS uptake with protein and phospholipid rich tissue such as blood and liver.38,40,48,49 Removal of blood, liver, and other organs resulted in carcass to whole-body ratios of 0.44 to 0.70 for PFCA and 0.37 to 0.53 for PFSA.
The values for mussels exposed to groundwater from REF and FTA-1 for 14 days (REF, PFOA = 5.1 L kg−1 and T-PFOS = 20 L kg−1; FTA-1, PFOA = 0.2 L kg−1 and T-PFOS = 6.8 L kg−1) were lower than for fish exposed for 21 days (Table 1), partially due to the shorter exposure time. The values measured here fall within the range reported in other studies.35 Log BAFs in green mussels (Perna viridis) exposed to 1000 and 10,000 ng L−1 of PFOS (2.60 and 2.30, respectively)41 were greater than reported here, as were log BAFs for Zebra mussel (Dreissena polymorpha) exposed to 1000 ng L−1 of PFOA and T-PFOS (2.70 and 3.00, respectively).42 Although groundwater and mussel tissue PFAS concentrations were lower at REF than FTA-1, mussel T-PFOS values were greater at REF (13 L kg−1) than FTA-1 (5.0 L kg−1). These results are consistent with the concentration effect noted for green mussels41 where lower BAFs were observed at higher concentrations (attributed to nonlinear adsorption to specific binding sites. Mussel values at FTA-1 (Table 1) ranged from 0.20 to 200 L kg−1 and increased with increasing chain length. The PFSA had slightly greater values than PFCA, and L-PFOS had greater values than B-PFOS.
Concentrations and Concentration Factors in Passive Samplers
Passive samplers provided an assessment of abiotic PFAS uptake. Concentrations of PFAS in POCIS are presented as ng g−1 of HLB sorbent (Figure 2; Table S7). During the exposure experiments, ∑PFAS in the POCIS increased 11-fold at REF (83 ng g−1 at d4; 880 ng g−1 at d21) and 40-fold at FTA-1 (3800 ng g−1 at d4; 150,000 ng g−1 at d21). The predominant PFAS detected in POCIS at REF was T-PFHxS (d4 to d21 mean = 290 ± 290 ng g−1; 92% L-PFHxS). The predominate PFAS detected in POCIS at FTA-1 was T-PFOS (d4 to d21 mean = 22,000 ± 27,000 ng g−1; 69% L-PFOS) followed by T-PFHxS.
Concentrations of PFAS in PETS also increased over time (Figure 2; Table S8) but to a lesser extent than POCIS. At REF, ∑PFAS increased 4.0-fold over the course of the experiment (9.0 ng g−1 at d4; 36 ng g−1 at d21) and at FTA-1 ∑PFAS increased 2.6-fold (800 ng g−1 at d4; 2100 ng g−1 at d21). The predominant PFAS detected in PETS at REF was T-PFHxS (d4 to d21 mean = 8.9 ± 6.7 ng g−1) and at FTA-1 was T-PFOS (d4 to d21 mean = 700 ± 220 ng g−1). Linear- and branched-isomers of PFHxS and PFOS were not quantified separately in PETS. The long-chain PFAS (PFDoDA, PFTrDA, PFTeDA, and PFDS) were not detected in either the POCIS or PETS, supporting the idea that the source of these compounds in fish is food rather than water.
The PFAS values for POCIS at REF (Table 1) ranged from 390 L kg−1 (PFBA) to 9100 L kg−1 (L-PFHxS), and at FTA-1 ranged from 27 L kg−1 (PFBA) to 18,000 L kg−1 (L-PFHxS). Sampling rates for POCIS at FTA-1 (Table S9) ranged from 0.037 to 0.18 L d−1 (mean = 0.090 ± 0.032 L d−1), consistent with reported values.79–81 The PFAS values for PETS were lower than for POCIS (Table 1) and at REF ranged from 110 L kg−1 (PFOA) to 1400 L kg−1 (T-PFOS). At FTA-1, PFAS values for PETS ranged from 150 L kg−1 (PFHpS) to 1200 L kg−1 (PFNS). Sampling rates for PETS (Table S9) ranged from 0.009 to 0.16 L d−1 (mean = 0.042 ± 0.047 L d−1), consistent with reported values.77
Cross-Media Correlation
All PFAS detected in groundwater (REF and FTA-1) also were detected in either biotic or abiotic media (Figure 3). However, several PFAS were detected only in fish tissue and passive samplers and not in groundwater. Of the 15 PFAS detected at REF, only 3 were shared across all media types: 9 were detected in POCIS and PETS, 2 were detected only in POCIS, 1 was detected only in PETS, 4 were detected in water, POCIS, and PETS but not biota, 1 was detected only in water and PETS, and 2 were detected only in fish. At FTA-1, 17 PFAS were detected, with 10 shared between water, biota, and passive samplers. Fish carcass contained all PFAS detected in mussel as well as additional compounds. Whole fish contained 1 additional PFAS not detected in carcass. The POCIS and PETS profiles had complete overlap with water and biota and included several PFAS not detected in biota. Biota and passive samplers included one PFAS not detected in water.
Figure 3.
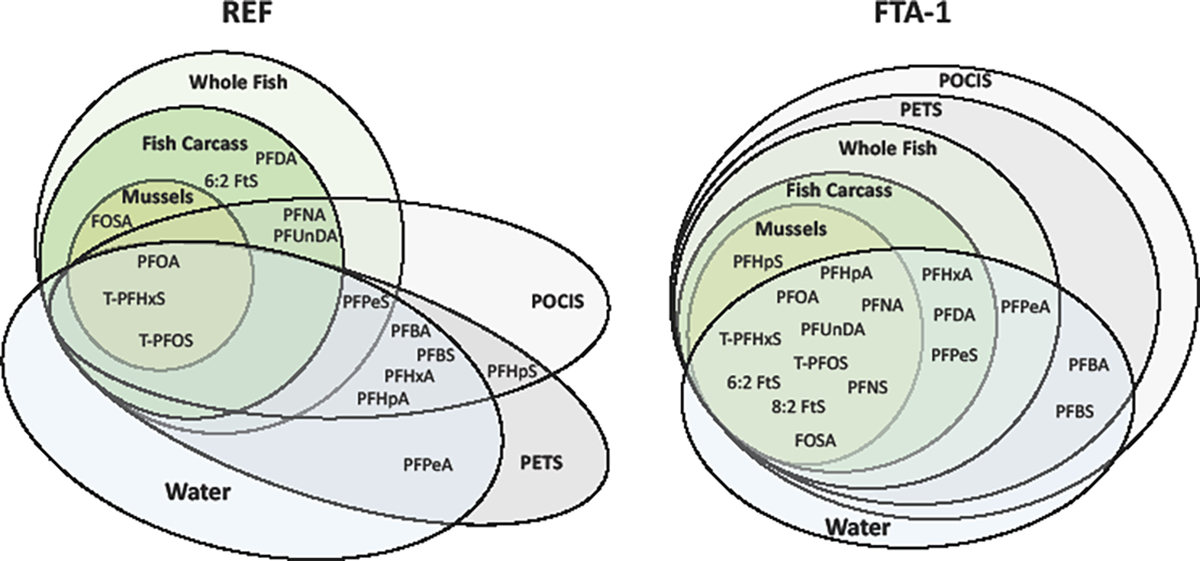
Diagrams showing overlap in detection of individual per- and polyfluoroalkyl substances (PFAS) in biotic and abiotic media exposed to groundwater from the reference (REF; MA-SDW 491–0063A) and fire-training area contaminated (FTA-1; MA-SDW 488–0083) wells during the 2018 mobile-laboratory experiments conducted on Cape Cod, Massachusetts. [See Table S2 for compound abbreviations; see Tables S4 to S8 for individual PFAS concentration data; individual PFAS detected within water, fish carcass, whole fish, mussels, polar organic chemical integrative sampler (POCIS), or polyethylene tube sampler (PETS) are indicated by different color backgrounds; PFAS occurring in multiple compartments are indicated by overlapping ovals; smaller ovals correspond to fewer detects; larger ovals correspond to more detects; oval shapes drawn for efficient distinction of unique versus overlapping profiles.]
Relations between PFAS in water, biota, and passive samplers generally were better at FTA-1 (which had greater concentrations) than REF (Figures S2 and S3). Pearson correlation analysis of log transformed PFAS concentrations for individual media at REF (Figure S2) showed strong correlations between male and female fish, water and passive samplers were moderately correlated, and PETS were correlated with fish and mussels. At FTA-1 (Figure S3), male and female fish were highly correlated, male fish and mussel were moderately correlated, and female fish and mussel were highly correlated. Water concentrations at FTA-1 were highly correlated with POCIS and PETS, moderately correlated with male and female fish, and poorly correlated with mussels. Log-transformed concentration data for individual PFAS in all media (Table S10) generally were correlated but varied by compound. Of the PFAS detected in ≥50% of samples, PFHpA, PFOA, PFNA, and 6:2-FtS had the strongest correlation with other PFAS. In contrast, PFBA, PFPeA, PFBS, T-PFHxS, and T-PFOS were weakly correlated to other PFAS.
Factors Influencing Uptake of PFAS by Biota
The REF and FTA-1 groundwater contained complex mixtures of PFAS, with each compound having unique physicochemical properties (Table S3).45,46 Fluorocarbon chain length is the most environmentally relevant structural feature of PFAS that influences water solubility, bioconcentration, and toxicity, although the anionic head group also plays an important role.38–40,43–46 In male and female fish, increased with increasing fluorocarbon chain length (Figure 4). A similar relation was observed for mussels although values were an order of magnitude lower than in fish. Perfluorobutanoic acid and PFBS were detected in REF and FTA-1 groundwater but not biotic media (Figure 2) and could not be calculated. The co-occurrence of longer chain PFAS has been shown to inhibit uptake of shorter chain PFAS82 and may contribute to the absence of PFBA and PFBS in fish.
Figure 4.
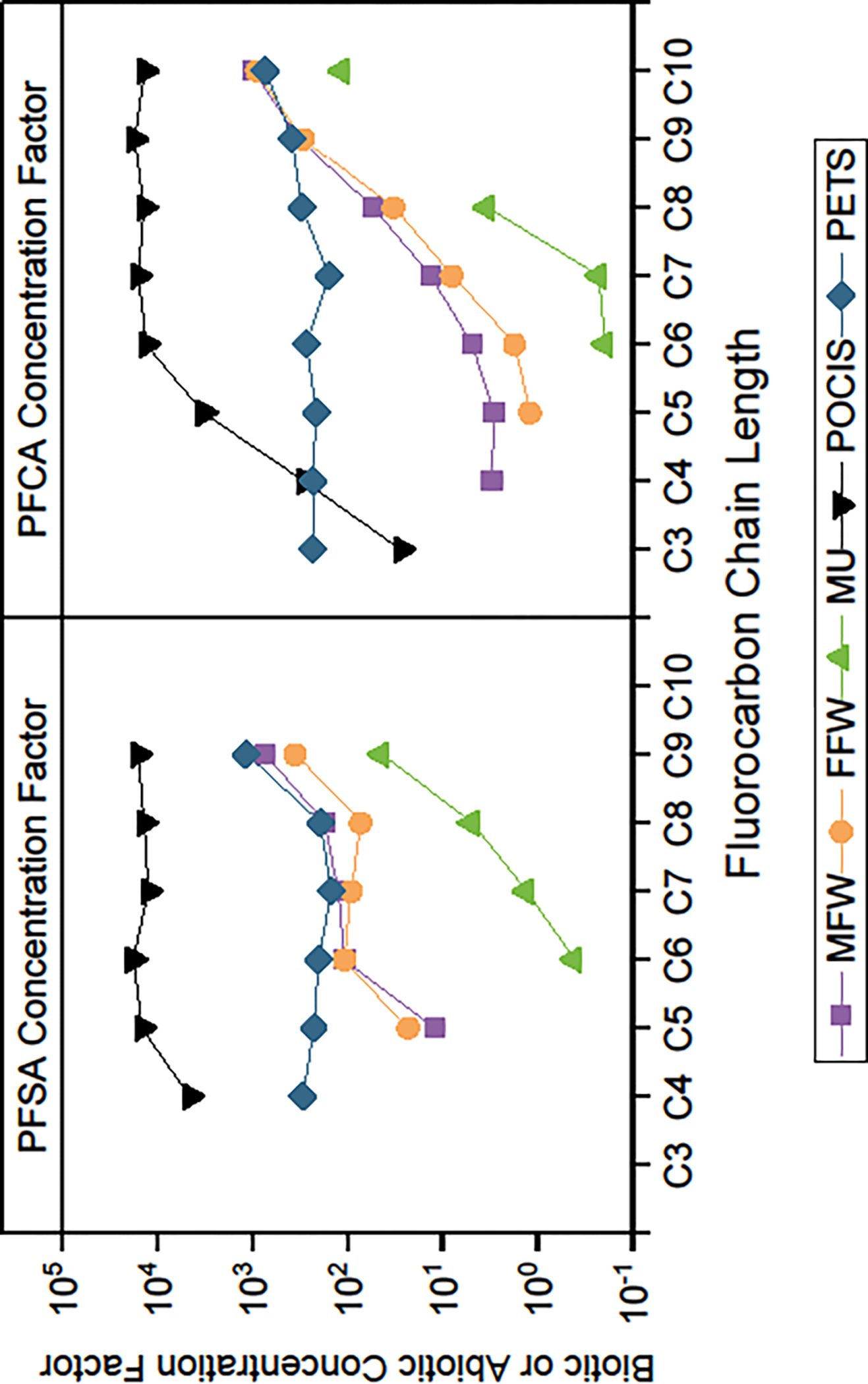
Biotic and abiotic perfluoroalkyl sulfonate (PFSA) and perfluorocarboxylate (PFCA) concentration factors as a function of fluorocarbon chain length for fish, mussels, polar organic chemical integrative samplers (POCIS), and polyethylene tube samplers (PETS) exposed to groundwater from the fire-training area contaminated (FTA-1; MA-SDW 488–0083) well during the 2018 mobile-laboratory experiments conducted on Cape Cod, Massachusetts. [MFW, d21 male fish whole-body tissue; FFW, d21 female fish whole-body tissue; MU, d21 mussel soft tissue; POCIS, d21 sorption media; PETS, d21 sorption media; see Table 1 for biotic and abiotic concentration factors.]
The values for PFHxS in whole body male and female fish from FTA-1 deviated from the trend observed for the equivalent fluorocarbon chain length PFCA (Figure 4). One possible explanation for this anomaly is formation of PFHxS by biotransformation of co-occurring precursor compounds such as FHxSA. Although FHxSA was detected in fish and mussel tissue, could not be determined because it was not measured in groundwater. There is limited availability of fish metabolic transformation pathway data for PFAS,83 and uncertainties in the assumptions that microbial degradation84 or abiotic processes85 are adequate surrogates for fish metabolism. It generally is thought that biotransformation of precursors ultimately results in terminal PFCA and PFSA products.1 Analysis of fish metabolism pathways indicates that a diverse set of precursors can produce PFOS as the end transformation product.83 Although similar biotransformation analysis has not been conducted with PFHxS and the precursor FHxSA, it is reasonable to assume that the structural homologs would follow similar transformation pathway.
The presence of linear- and branched-PFOS isomers and sulfonamide precursors in FTA-1 groundwater indicates that the AFFF-derived PFAS were produced by electrochemical fluorination,14 a process that also produces the 6 fluorocarbon homologs.1 Both PFHxS and PFOS were present in the REF and FTA-1 groundwater (REF T-PFHxS/T-PFOS = 12; FTA-1 T-PFHxS/T-PFOS = 0.54). The REF groundwater T-PFOS/FOSA ratio could not be calculated (FOSA not detected) and the T-PFHxS/FHxSA ratio could not be determined because FHxSA was not measured in the groundwater. The T-PFHxS/FHxSA ratio for d21 FTA-1 male and female fish whole-body tissue was 0.95 and 0.67, respectively. This indicates substantial uptake of FHxSA relative to PFHxS and a pool of precursor that could undergo biotransformation into PFHxS. In contrast, the T-PFOS/FOSA ratios for d21 FTA-1 male and female fish whole-body tissue were 39 and 9.2, respectively, indicating much less uptake of FOSA relative to T-PFOS (or much faster transformation).
Although the relations between fluorocarbon chain-length and for PFCA and PFSA (Figure 4) were similar for male and female fish, there were differences between sexes (Table 1). Uptake rates at REF and FTA-1 varied between male and female fish and by PFAS (Figures S4 and S5; Table S11). Male fish generally had positive uptake rates whereas female fish often had bilinear uptake (initial increase followed by decrease). Ankley et al.86 reported differences in PFOS uptake between male and female fathead minnows after 21-day water exposures, with females having 2- to 3-fold greater concentrations than males, suggesting males had faster elimination rates. In contrast, Lee and Schultz87 reported female fish had a 10-fold faster elimination of PFOA than male fish.
The PFAS composition in mussel differed from fish with respect to enrichment of precursors relative to acids. It is not clear if this is due to higher uptake or lower biotransformation of precursors. Enrichment of PFHxS was not observed in mussel tissue, which also accumulated the precursor compound FHxSA, reflecting different uptake and metabolic mechanisms than fish. Mussels also showed a concentration effect with higher values observed at lower exposure concentrations (Table 1). As with female fish, mussel uptake rates were bilinear with an initial increase followed by a decrease or plateau (Figures S4 and S5; Table S11). Maximum ∑PFAS concentrations for mussels occurred after 4 days of exposure (3.1 ng g−1 at REF; 200 ng g−1 at FTA-1). Inverse concentration dependency and bilinear uptake in mussels have been reported,41 and the decrease in uptake at FTA-1 over time could be influenced by the concomitant increase in concentrations that occurred over the course of the exposures.
Predictability of PFAS Biotic Uptake from Passive Samplers
The potential for passive samplers to predict bioconcentration was explored by comparing to . Passive samplers provided a reasonable approximation of PFAS mixture composition following bioconcentration in fish, particularly for PFAS with ≥6 fluorocarbons. However, passive samplers were poor predictors of PFAS mixture composition following bioconcentration for mussels. Both passive sampler types over-predicted in mussels (factor of 100 for PETS; factor of 1000 for POCIS) because the samplers have one-way uptake with no elimination. Predicted fish bioconcentration based on PETS was within a factor of 3 for PFDA, PFUnDA, PFHxS, PFHpS, PFOS, PFNS, and FOSA. Because POCIS accumulated far more PFAS mass than PETS, the ratios of to were greater, but displayed results consistent with fish for PFHxS, PFHpS, PFOS, and PFNS. Both types of passive samplers displayed greater uptake for PFHxA to PFNA and 6:2 FTS than biota.
The POCIS had the highest concentration factors of any media with more compounds detected and greater values at FTA-1 than REF. Concentrations of PFAS in POCIS (Figures S4 and S5) generally increased linearly with time, except PFBA and PFPeA, which had declining or steady uptake. Likewise, concentrations of PFAS in PETS generally increased linearly with time. Uptake rates at FTA-1 for POCIS ranged from 0.001 to 0.425 d−1 and for PETS ranged from 0.006 to 1.8 d−1 (Table S11).
There was a fluorocarbon chain-length dependency for PFAS uptake by POCIS with an increase in from PFBS to PFPeS but little additional increase for longer-chain lengths (Figure 4). The chain-length effect was more pronounced for PFCA than PFSA with an approximately one log-unit increase in with each additional fluorocarbon unit between PFBA and PFHpA, after which there was no additional increase. The greater for POCIS relative to PETS reflects the larger surface area as well as greater mass transfer through the porous membranes retaining the HLB sorbent relative to diffusion through the polyethylene tube. The fluorocarbon chain-length effect likely reflects the high affinity of long-chain PFAS for the HLB sorbent. The PETS did not exhibit a strong concentration effect (REF ≈ FTA-1) or fluorocarbon chain-length effect, likely reflecting rate-limiting diffusion through the polyethylene tubes.
The passive samplers provided time-weighted average water concentrations for PFAS. Using independent sampling rates for POCIS and PETS reported in the literature (consistent with those measured in this study),77,81 integrated water concentrations were estimated for FTA-1 to evaluate the effect of variable water concentrations on uptake. Using estimated water concentrations from d21 POCIS concentrations and d21 male whole-body concentrations for PFOA and PFOS yielded values of 6.2 and 124 L kg−1, respectively (within factors of 2.5 and 1.4 of values determined from measured water concentration). Using similarly estimated water concentrations from d21 PETS concentrations yielded values of 36 and 368 L kg−1, respectively (factor of 2.6 and 2.0 of values determined from measured water concentration). A field comparison of PFAS uptake by POCIS and liver tissue from wild-caught fish reported poor agreement for individual PFAS between the two media.51 The present study showed good agreement in PFAS uptake and composition between fish and POCIS, likely due to the stable groundwater source relative to more variable surface water conditions.
IMPLICATIONS
This investigation provides an integrated assessment of PFAS uptake by biotic and abiotic media from complex environmental mixtures. Each media had a different PFAS composition and concentration, and biotic uptake was substantially different than abiotic uptake. The results from these “real world” exposures using AFFF-derived PFAS mixtures in contaminated groundwater have transferability to other FTA-impacted sites with respect to field-derived values. The use of passive samplers in addition to model organisms provided data to evaluate the appropriateness of using abiotic media to mimic biotic processes. Although a strategy using discrete samples or passive samplers would be sufficient for characterizing PFAS concentrations in water, it would not adequately characterize biotic uptake dynamics or address specific sex and species differences. Despite differences between male and female fish (carcass and whole body), PFAS uptake by passive samplers largely mimicked uptake by fish but not by mussels. The data suggest that passive samplers are useful screening tools for PFAS that bioconcentrate in fish but are below MQLs in water. In addition, passive samplers can assess exposure to short-chain PFAS not detected in biota.
This unique field experiment allowed sensitive determination of in-situ values for mixtures of PFAS present at environmental concentrations and compositions. The results were consistent with predictions based on compound physicochemical properties and reported field observations for wild fish.34–36 Depending on the individual PFAS chemical structure, values ranged from <10 to 1000 L kg−1. However, the results clearly show that precursor compounds are important components of environmental PFAS mixtures that can bioconcentrate to a greater extent than PFCA and PFSA, and subsequently undergo biotransformation within the organism, which can influence values. Even with low values, body burdens can be substantial with potential for biological effects at the organismal level. Understanding the complexities of PFAS bioconcentration in contaminated groundwater sources provides a foundation for assessing potential impacts on aquatic organisms in surface-water ecosystems receiving contaminated groundwater discharges.
Fish tissue concentrations for the REF exposure experiment provide a reasonable approximation of potential exposure for surface-water organisms, as illustrated by the PFAS contamination plume, which discharges into Ashumet Pond15 resulting in T-PFOS concentrations of 50 ± 4.1 ng L−1 (greater than T-PFOS concentrations of 7.2 ± 1.2 ng L−1at REF). The REF male fish whole-body T-PFOS concentrations after 21-day exposures (1.4 ± 0.22 ng g−1) and the female fish whole-body T-PFOS concentrations (1.8 ± 0.49 ng g−1) were consistent with results for recreational fish collected from lakes in New Hampshire37 (mean = 6.8 ± 8.6 ng g−1; ). The d21 REF fish mean T-PFOS concentration exceeded the New Hampshire reference dose value limit88 of ≤1.1 ng g−1 for daily consumption by adults.
The mobile laboratory experimental approach incorporates the dynamic conditions intrinsic in natural systems. Accounting for this inherent variability is an explicit acknowledgement that bioconcentration factors determined under environmental conditions rarely attain steady state. Studies such as this are an important step in extrapolating results from highly controlled “pseudo steady state” laboratory experiments to highly variable aquatic environments.
Supplementary Material
Synopsis:
Mobile laboratory experiments were used to assess uptake of per- and polyfluoroalkyl substances (PFAS) by biotic and abiotic media from reference and fire-training area contaminated groundwater.
ACKNOWLEDGEMENTS
This research was supported by the U.S. Geological Survey (USGS) Environmental Health Program and Water Quality Processes Program with additional funding from the National Institute for Environmental Health award number P42ES027706. Animal care and handling followed University of Colorado Denver Institutional Animal Care and Use Committee Protocol number 00698. We thank Andrea Tokranov, Robert Hull, and Timothy McCobb (USGS New England Water Science Center), Jeramy Jasmann (USGS Strategic Laboratory Science Branch), and David Bertolatus (University of Colorado Denver) for their contributions. We also thank Rose Forbes (Air Force Civil Engineer Center) for providing site access and logistical support. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Footnotes
SUPPORTING INFORMATION
Eleven tables present site information, analytical methods, analytical results for the various media, passive sampler sampling rates, statistical correlations, and biotic and abiotic uptake rates. Five figures show water concentrations, Pearson correlation analysis, and biotic and abiotic uptake rates.
REFERENCES
- 1.Buck RC; Franklin J; Berger U; Conder JM; Cousins IT; de Voogt P; Jensen AA; Kannan K; Mabury SA; van Leeuwen SP Perfluoroalkyl and polyfluoroalkyl substances in the environment; terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chelcea IC; Ahrens L; Orn S; Mucs D; Andersson PL Investigating the OECD database of per-and polyfluoroalkyl substances – chemical variation and applicability of current fate models. Environ. Chem. 2020. [ 10.1071/EN19296]. [DOI] [Google Scholar]
- 3.Environmental Protection Agency US. PFAS master list of PFAS substances. V2. Accessed July 31, 2022 at [https://comptox.epa.gov/dashboard/chemical_lists/pfasmaster]. [Google Scholar]
- 4.Houde M; Martin JW; Letcher RJ; Solomon KR; Muir DCG Biological monitoring of perfluroalkyl substances: A review. Environ. Sci. Technol. 2006, 40, 3463–3476. [DOI] [PubMed] [Google Scholar]
- 5.Houde M; De Silva AO; Muir DCG; Letcher RJ Monitoring of perfluorinated compounds in aquatic biota: an updated review. Environ. Sci. Technol. 2011, 45, 7962–7973. [DOI] [PubMed] [Google Scholar]
- 6.Lau C; Anitole K; Hodes C; Lai D; Pfahles-Hutchens A; Seed J Perfluoroalkyl acids: A review on monitoring and toxicological findings. Toxicol. Sci. 2007, 99, 366–394. [DOI] [PubMed] [Google Scholar]
- 7.Nakayama SF; Yoshikane M; Onoda Y; Nishihama Y; Iwai-Shimada M; Takagi M; Kobayashi Y; Isobe T Worldwide trends in tracing poly- and perfluoroalkyl substances (PFAS) in the environment. Trends Anal. Chem. 2019, 121, 115410. [Google Scholar]
- 8.Hoffman K; Webster TF; Bartell SM; Weisskopf MG; Fletcher T; Vieira VM Private drinking water wells as a source of exposure to perfluorooctanoic acid (PFOA) in communities surrounding a fluoropolymer production facility. Environ. Health Perspect. 2011, 119, 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houtz EF; Higgins CP; Field JA; Sedlak DL Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil. Environ. Sci. Technol. 2013, 47, 8187–8195. [DOI] [PubMed] [Google Scholar]
- 10.Filipovic M; Woldegiorgis A; Norström K; Bibi M; Lindberg M; Österås A-H Historical usage of aqueous film forming foam: A case study of the widespread distribution of perfluoroalkyl acids from a military airport to groundwater, lakes, soils and fish. Chemosphere 2015, 129, 39–45. [DOI] [PubMed] [Google Scholar]
- 11.Anderson RH; Long GC; Porter RC; Anderson JK Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere 2016, 150, 678–685. [DOI] [PubMed] [Google Scholar]
- 12.Hu XC; Andrews DQ; Lindstrom AB; Bruton TA; Schaider LA; Grandjean P; Lohmann R; Carignan CC; Blum A; Balan SA; Higgins CP; Sunderland EM Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ. Sci. Technol. Lett. 2016, 3, 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houtz EF; Sutton R; Park J-S; Sedlak M Poly- and perfluoroalkyl substances in wastewater: Significance of unknown precursors, manufacturing shifts, and likely AFFF impacts. Water Res. 2016, 95, 142–149. [DOI] [PubMed] [Google Scholar]
- 14.Weber AK; Barber LB; LeBlanc DR; Sunderland EM; Vecitis CD Geochemical and hydrologic factors controlling subsurface transport of poly- and perfluoroalkyl substances, Cape Cod, Massachusetts. Environ. Sci. Technol. 2017, 51, 4269–4279. [DOI] [PubMed] [Google Scholar]
- 15.Tokranov AK; LeBlanc DR; Pickard HM; Ruyle BJ; Barber LB; Hull RB; Sunderland EM; Vecitis CD Surface-water/groundwater boundary effects on seasonal PFAS concentrations and PFAA precursor transformations. Environ. Sci. Process. Impact. 2021, 23, 1893–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Environmental Protection Agency. Drinking water health advisory for perfluorooctanoic acid (PFOA). U.S. Environmental Protection Agency 822-R-16–005; U.S. Environmental Protection Agency: Washington, DC, 2016, 103 p. [Google Scholar]
- 17.Environmental Protection Agency US. Drinking water health advisory for perfluorooctane sulfonate (PFOS). U.S. Environmental Protection Agency 822-R-16–004; U.S. Environmental Protection Agency: Washington, DC, 2016, 88 p. [Google Scholar]
- 18.Environmental Protection Agency US. Drinking water health advisories for PFOA and PFOS: 2022 interim updated PFOA and PFOS health advisories. Accessed August 2022 at [https://www.epa.gov/sdwa/drinking-water-health-advisories-pfoa-and-pfos].
- 19.Post GB Recent US state and federal drinking water guidelines for per- and polyfluoroalkyl substances. Environ. Toxicol. Chem. 2021, 40, 550–563. [DOI] [PubMed] [Google Scholar]
- 20.Massachusetts Department of Environmental Protection. Final PFAS-related amendments, 310 CMR 40.0000, Dec. 27, 2019. Accessed November 2022 at [https://www.mass.gov/doc/final-pfas-related-changes-to-the-mcp-2019-12-13/download].
- 21.Giesy JP; Naile JE; Khim JS; Jones KC; Newsted JL Aquatic toxicology of perfluorinated chemicals. Rev. Environ. Contam. Toxicol. 2010, 202, 1–52. [DOI] [PubMed] [Google Scholar]
- 22.Hoke RA; Bouchelle LD; Ferrell BD; Buck RC Comparative acute freshwater hazard assessment and preliminary PNEC development for eight fluorinated acids. Chemosphere 2012, 87, 725–733. [DOI] [PubMed] [Google Scholar]
- 23.Environmental Protection Agency US. Draft recommended aquatic life ambient water quality criteria for perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS). [EPA–HQ–OW–2022–0365 and EPA–HQ– OW–2022–0366; FRL 8310–01–OW]. Fed. Reg. 2022, 87, 26199–26201. [Google Scholar]
- 24.Wang Z; DeWitt JC; Higgins CP; Cousins IT A never-ending story of per- and polyfluoroalkyl substances (PFAS)? Environ. Sci. Technol. 2017, 51, 2508–2518. [DOI] [PubMed] [Google Scholar]
- 25.Guelfo JL; Korzeniowski S; Mills MA; Anderson J; Anderson RH; Arblaster JA; Conder JM; Cousins IT; Dasu K; Lee LS; Liu J; McKenzie ER; Willey J Environmental sources, chemistry, fate, and transport of per- and polyfluoroalkyl substances: State of the science, key knowledge gaps, and recommendations presented at the August 2019 SETAC focus topic meeting. Environ. Toxicol. Chem. 2021, 40, 3234–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barzen-Hanson KA; Roberts SC; Choyke S; Oetjen K; McAlees A; Riddell N; McCrindle R; Ferguson PL; Higgins CP; Field JA Discovery of 40 classes of per- and polyfluoroalkyl substances in historical aqueous film-forming foams (AFFFs) and AFFF-impacted groundwater. Environ. Sci. Technol. 2017, 51, 2047–2057. [DOI] [PubMed] [Google Scholar]
- 27.Leeson A; Thompson T; Stroo HF; Anderson RH; Speicher J; Mills MA; Willey J; Coyle C; Ghosh R; Lebrón C; Patton C Identifying and managing aqueous film-forming foam-derived per-and polyfluoroalkyl substances in the environment. Environ. Toxicol. Chem. 2021, 40, 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGuire ME; Schaefer C; Richards T; Backe WJ; Field JA; Houtz E; Sedlak DL; Guelfo JL; Wunsch A; Higgins CP Evidence of remediation-induced alteration of subsurface poly- and perfluoroalkyl substance distribution at a former firefighter training area. Environ. Sci. Technol. 2014, 48, 6644–6652. [DOI] [PubMed] [Google Scholar]
- 29.Ahrens L; Bundschuh M Fate and effects of poly- and perfluoroalkyl substances in the aquatic environment: A review. Environ. Toxicol. Chem. 2014, 33, 1921–1929. [DOI] [PubMed] [Google Scholar]
- 30.Guelfo JL; Higgins CP Subsurface transport potential of perfluoroalkyl acids at aqueous film-forming foam (AFFF)-impacted sites. Environ. Sci. Technol. 2013, 47, 4164–4171. [DOI] [PubMed] [Google Scholar]
- 31.Brusseau ML Assessing the potential contributions of additional retention processes to PFAS retardation in the subsurface. Sci. Tot. Environ. 2018, 613–614, 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fitzgerald NJM; Wargenau A; Sorenson C; Pedersen J; Tufenkji N; Novak PJ; Simcik MF Partitioning and accumulation of perfluoroalkyl substances in model lipid bilayers and bacteria. Environ. Sci. Technol. 2018, 52, 10433–10440. [DOI] [PubMed] [Google Scholar]
- 33.Liu J; Mejia Avendaño S Microbial degradation of polyfluoroalkyl chemicals in the environment: A review. Environ. Int. 2013, 61, 98–114. [DOI] [PubMed] [Google Scholar]
- 34.Conder JM; Hoke RA; De Wolf W; Russell MH; Buck RC Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ. Sci. Technol. 2008, 42, 995–1003. [DOI] [PubMed] [Google Scholar]
- 35.Burkhard LP Evaluation of published bioconcentration factor (BCF) and bioaccumulation factor (BAF) data for per- and polyfluoroalkyl substances across aquatic species. Environ. Toxicol. Chem. 2021, 40, 1530–1543. [DOI] [PubMed] [Google Scholar]
- 36.Lewis AJ; Yun X; Spooner DE; Kurz MJ; McKenzie ER; Sales CM Exposure pathways and bioaccumulation of per- and polyfluoroalkyl substances in freshwater aquatic ecosystems: Key considerations. Sci. Tot. Environ. 2022, 822, 153561. [DOI] [PubMed] [Google Scholar]
- 37.Pickard HM; Ruyle BJ; Thackray CP; Chovancova A; Dassuncao C; Becanova J; Voita S; Lohmann R; Sunderland EM PFAS and precursor bioaccumulation in freshwater recreational fish: Implications for fish advisories. Environ. Sci. Technol. 2022, 56, 15573–15583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin JW; Mabury SA; Solomon KR; Muir DCG Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 2003, 22, 196–204. [PubMed] [Google Scholar]
- 39.Inoue Y; Hashizume N; Takata N; Murakami H; Suzuki Y; Kikushima E; Otsuka M Unique physicochemical properties of perfluorinated compounds and their bioconcentration in common carp Cyprinus carpio L. Arch. Environ. Contam. Toxicol. 2012, 62, 672–680. [DOI] [PubMed] [Google Scholar]
- 40.Wen W; Xia X; Zhou D; Wang H; Zhai Y; Lin H; Chen J; Hu D Bioconcentration and tissue distribution of shorter and longer chain perfluoroalkyl acids (PFAAs) in zebrafish (Danio rerio): effects of perfluorinated carbon chain length and zebrafish protein content. Environ. Poll. 2019, 249, 277–285. [DOI] [PubMed] [Google Scholar]
- 41.Liu C; Gin KYH; Chang VWC; Goh BPL; Reinhard M Novel perspectives on the bioaccumulation of PFCs – the concentration dependency. Environ. Sci. Technol. 2011, 45, 9758–9764. [DOI] [PubMed] [Google Scholar]
- 42.Fernández-Sanjuan M; Faria M; Lacorte S; Barata C Bioaccumulation and effects of perfluorinated compounds (PFCs) in zebra mussels (Dreissena polymorpha). Environ. Sci. Pollut. Res. 2013, 20, 2661–2669. [DOI] [PubMed] [Google Scholar]
- 43.Ohmori K; Kudo N; Katayama K; Kawashima Y Comparison of the toxicokinetics between perfluorocarboxylic acids with different carbon chain lengths. Toxicol. 2003, 184, 135–140. [DOI] [PubMed] [Google Scholar]
- 44.Wang T; Lin Z; Yin D; Tian D; Zhang Y; Kong D Hydrophobicity-dependent QSARs to predict the toxicity of perfluorinated carboxylic acids and their mixtures. Environ. Toxicol. Pharmacol. 2011, 32, 259–265. [DOI] [PubMed] [Google Scholar]
- 45.Rayne S; Forest K Perfluoroalkyl sulfonic and carboxylic acids: A critical review of physicochemical properties, levels and patterns in waters and wastewaters, and treatment methods. J. Environ. Sci. Health Part A 2009, 42, 1145–1199. [DOI] [PubMed] [Google Scholar]
- 46.Ding G; Peijnenburg WJGM Physicochemical properties and aquatic toxicity of poly- and perfluorinated compounds. Crit. Rev. Environ. Sci. Technol. 2013, 43, 598–678. [Google Scholar]
- 47.Chiou CT Partition and Adsorption of Organic Contaminants in Environmental Systems. Wiley Interscience, Hoboken, NJ, 2002, 257 p. [Google Scholar]
- 48.Ng CA; Hungerbühler K Bioconcentration of perfluorinated alkyl acids: how important is specific binding. Environ. Sci. Technol. 2013, 47, 7214–7223. [DOI] [PubMed] [Google Scholar]
- 49.Ng CA; Hungerbühler K Bioaccumulation of perfluorinated alkyl acids: Observations and models. Environ. Sci. Technol. 2014, 48, 4637–4648. [DOI] [PubMed] [Google Scholar]
- 50.Alvarez DA Development of semipermeable membrane devices (SPMDs) and polar organic chemical integrative samplers (POCIS) for environmental monitoring. Environ. Toxicol. Chem. 2013, 32, 2179–2181. [DOI] [PubMed] [Google Scholar]
- 51.Cerveny D; Grabic R; Fedorova G; Grabicova K; Turek J; Kodes V; Golovko O; Zlabek V; Randak T Perfluoroalkyl substances in aquatic environment - comparison of fish and passive sampling approaches. Environ. Res. 2016, 144, 92–98. [DOI] [PubMed] [Google Scholar]
- 52.Fauvelle V; Kaserzon SL; Montero N; Lissalde S; Allan IJ; Mills G; Mazzella N; Mueller JF; Booij K Dealing with flow effects on the uptake of polar compounds by passive samplers. Environ. Sci. Technol. 2017, 51, 2536–2537. [DOI] [PubMed] [Google Scholar]
- 53.Ryan BJ Cape Cod Aquifer, Cape Cod, Massachusetts. U.S. Geological Survey Water-Res. Invest. 80–571: U.S. Geological Survey; Washington, DC, 1980, 23 p. [Google Scholar]
- 54.LeBlanc DR Sewage plume in a sand and gravel aquifer, Cape Cod, Massachusetts. U.S. Geological Survey Water-Supply Paper 2218: U.S. Geological Survey; Washington, DC, 1984, 28 p. [Google Scholar]
- 55.Barber LB II; Thurman EM; Schroeder MP; LeBlanc DR Long-term fate of organic micropollutants in sewage-contaminated ground water. Environ. Sci. Technol. 1988, 22, 205–211. [DOI] [PubMed] [Google Scholar]
- 56.Field JA; Barber LB II; Thurman EM; Moore BL; Lawrence DL; Peake DA Fate of alkylbenzenesulfonates and dialkyltetralinsulfonates in sewage contaminated ground water. Environ. Sci. Technol. 1992, 26, 1140–1148. [Google Scholar]
- 57.Repert DA; Barber LB; Hess KM; Keefe SH; Kent DB; LeBlanc DR; Smith RL Long-term natural attenuation of carbon and nitrogen within a groundwater plume after removal of the treated wastewater source. Environ. Sci. Technol. 2006, 40, 1154–1162. [DOI] [PubMed] [Google Scholar]
- 58.Barber LB; Keefe SH; LeBlanc DR; Bradley PM; Chapelle FH; Meyer MT; Loftin KA; Kolpin DW; Rubio F Fate of sulfamethoxazole, 4-nonylphenol, and 17β-estradiol in groundwater contaminated by wastewater treatment plant effluent. Environ. Sci. Technol. 2009, 43, 4843–4850. [DOI] [PubMed] [Google Scholar]
- 59.Air Force Civil Engineer Center. Installation restoration project at Joint Base Cape Cod. Accessed March 2022 at [https://www.massnationalguard.org/JBCC/afcec-documents/FINAL%20PLUME%20BOOKLET%20High%20Quality%201SEP21-1.pdf].
- 60.LeBlanc DR; Garabedian SP; Hess KM; Gelhar LW; Quadri RD; Stollenwerk KG; Wood WW Large-scale natural gradient tracer test in sand and gravel, Cape Cod, Massachusetts: 1. Experimental design and observed tracer movement. Water Resour. Res. 1991, 27, 895–910. [Google Scholar]
- 61.Garabedian SP; LeBlanc DR; Gelhar LW; Celia MA Large-scale natural gradient tracer test in sand and gravel, Cape Cod, Massachusetts: 2. Analysis of spatial moments for a nonreactive tracer. Water Resour. Res. 1991, 27, 911–924. [Google Scholar]
- 62.Hess KM; Wolf SH; Celia MA Large-scale natural gradient tracer test in sand and gravel, Cape Cod, Massachusetts: 3. Hydraulic conductivity variability and calculated macrodispersivities. Water. Resource. Res. 1992, 28, 2011–2027. [Google Scholar]
- 63.Walter DA; McCobb TD; Fienen MN Use of a numerical model to simulate the hydrologic system and transport of contaminants near Joint Base Cape Cod, western Cape Cod, Massachusetts. U.S. Geological Survey Sci. Invest. Report 2018–5139: U.S. Geological Survey; Washington, DC, 2019, 98 p. [Google Scholar]
- 64.McCobb TD; LeBlanc DR; Walter DA; Hess KM; Kent DB; Smith RL Phosphorous in a ground-water contaminant plume discharging to Ashumet Pond, Cape Cod, Massachusetts, 1999. U. S. Geological Survey Water Res. Invest. Report 02–4306: U. S. Geological Survey; Washington, DC, 2003, 69 p. [Google Scholar]
- 65.McCobb TD; Briggs MA; LeBlanc DR; Day-Lewis FD; Johnson CD Evaluating long-term patterns of decreasing groundwater discharge through a lake-bottom permeable reactive barrier. J. Environ, Manag. 2018, 220, 233–245. [DOI] [PubMed] [Google Scholar]
- 66.Briggs MA; Tokranov AK; Hull RB; Haynes AB; Lane JW Hillslope groundwater discharges provide localized stream ecosystem buffers from regional per- and polyfluoroalkyl substances contamination. Hydrol. Process. 2020, 34, 2281–2291. [Google Scholar]
- 67.Ruyle BJ; Pickard HM; LeBlanc DR; Tokranov AK; Thackray CP; Hu XC; Vecitis CD; Sunderland EM Isolating the AFFF signature in coastal watersheds using oxidizable PFAS precursors and unexplained organofluorine. Environ. Sci. Technol. 2021, 55, 3686–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bradley PM; LeBlanc DR; Romanok KM; Smalling KL; Focazio MJ; Cardon MC; Clark JM; Conley JM; Evans N; Givens CE; Gray JL; Gray LE; Hartig PC; Higgins CP; Hladik ML; Iwanowicz LR; Loftin KA; McCleskey RB; McDonough CA; Medlock-Kakaley EK; Weis CP; Wilson VS Public and private tapwater: Comparative analysis of contaminant exposure and potential risk, Cape Cod, Massachusetts, USA. Environ. Inter. 2021, 152, 106487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stoliker DL; Repert DA; Smith RL; Song B; LeBlanc DR; McCobb TD; Conaway CH; Hyun SP; Koh D-C; Moon. H. S.; Kent, D. B. Hydrologic controls on nitrogen cycling processes and functional gene abundance in sediments of a groundwater flow-through lake. Environ. Sci. Technol. 2016, 50, 3649–3657. [DOI] [PubMed] [Google Scholar]
- 70.Smith RL; Repert DA; Stoliker DL; Kent DB; Song B; LeBlanc DR McCobb T.; Bohlke JK.; Hyun SP.; Moon HS. Seasonal and spatial variation in the location and reactivity of a nitrate-contaminated groundwater discharge zone in a lakebed. J. Geophys. Res. Biogeosci. 2019, 124, 2186–2207. [Google Scholar]
- 71.Barber LB; Lee KE; Swackhamer DL; Schoenfuss HL Reproductive responses of male fathead minnows exposed to wastewater treatment plant effluent, effluent treated with XAD8 resin, and an environmentally relevant mixture of alkylphenol compounds. Aquat. Toxicol. 2007, 82, 36–46. [DOI] [PubMed] [Google Scholar]
- 72.Vajda AM; Barber LB; Gray JL; Lopez EM; Bolden AM; Schoenfuss HL; Norris DO Demasculinization of male fish by wastewater treatment plant effluent. Aquat. Toxicol. 2011, 103, 213–221. [DOI] [PubMed] [Google Scholar]
- 73.Barber LB; Rapp JL; Kandel C; Keefe SH; Rice J; Westerhoff P; Bertolatus DW; Vajda AM Integrated assessment of wastewater reuse, exposure risk, and fish endocrine disruption in the Shenandoah River Watershed. Environ. Sci. Technol. 2019, 53, 3429–3440. [DOI] [PubMed] [Google Scholar]
- 74.Vajda AM; Barber LB; Alvarez DA; Becanova J; Jastrow A; Keefe SH; LeBlanc DR; Lohmann R; Pickard HM; Roth DA; Steevens JA Uptake of per- and polyfluoroalkyl substances by fish, mussel, and passive samplers in mobile laboratory exposures using groundwater from a contamination plume at a historical fire training area, Cape Cod, Massachusetts - Chemical and biological data from August to September 2018. U.S. Geological Survey Data Release: U.S. Geological Survey; Washington, DC, 2023. Accessed February 2023 at [ 10.5066/P9LCN0EF]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alvarez DA Guidelines for the use of the semipermeable membrane device (SPMD) and the polar organic chemical integrative sampler (POCIS) in environmental monitoring studies. U.S. Geological Survey Techniques and Methods 1–D4: U.S. Geological Survey; Washington, DC, 2010, 28 p. [Google Scholar]
- 76.Kaserzon SL; Vijayasarathy S; Bräunig J; Mueller L; Hawker DW; Thomas KV; Mueller JF Calibration and validation of a novel passive sampling device for the time integrative monitoring of per- and polyfluoroalkyl substances (PFASs) and precursors in contaminated groundwater. J. Haz. Mat. 2019, 366, 423–432. [DOI] [PubMed] [Google Scholar]
- 77.Gardiner C; Robuck A; Becanova J; Cantwell M; Kaserzon S; Katz D; Mueller I; Lohmann R Field validation of a novel passive sampler for dissolved PFAS in surface waters. Environ. Toxicol. Chem. 2022, 41, 2375–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.OriginLab Corporation. OriginPro, Version 2022. Northampton, MA. [Google Scholar]
- 79.Erhunse A An assessment of perfluoro alkyl substances bioavailability and policy implication for water quality and biota in the Lower Apalachicola River and estuary. Ph.D. Dissertation, Florida A&M University, Environmental Sciences Institute, Tallahassee, FL, 2010, 272 p. [Google Scholar]
- 80.Gobelius L; Persson C; Wiberg K; Ahrens L Calibration and application of passive sampling for per- and polyfluoroalkyl substances in a drinking water treatment plant. J. Haz. Mat. 2019, 362, 230–237. [DOI] [PubMed] [Google Scholar]
- 81.Fedorova G; Golovko O; Randak T; Grabic R Passive sampling of perfluorinated acids and sulfonates using polar organic chemical integrative samplers. Environ. Sci. Pollut. Res. 2013, 20, 1344–1351. [DOI] [PubMed] [Google Scholar]
- 82.Wen W; Xinghui X; Hu D; Zhou D; Wang H; Zhai Y; Lin H Long chain perfluoroalkyl acids (PFAAs) affect the bioconcentration and tissue distribution of short chain PFAAs in zebrafish (Danio rerio). Environ. Sci. Technol. 2017, 51, 12358–12368. [DOI] [PubMed] [Google Scholar]
- 83.Kolanczyk RC; Saley MR; Serrano JA; Daley SM; Tapper MA PFAS biotransformation pathways: A species comparison study. Toxics 2023, 11, 74, 24 p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cook EK; Olivares CI; Antell EH; Yi S; Nickerson A; Choi YJ; Higgins CP; Sedlak DL; Alvarez-Cohen L Biological and chemical transformation of the six-carbon polyfluoroalkyl substance N-dimethyl ammonio propyl perfluorohexane sulfonamide (AmPr-FHxSA). Environ. Sci. Technol. 2022, 56, 15478–15488. [DOI] [PubMed] [Google Scholar]
- 85.Zhong H; Liu W; Li N; Ma D; Zhao C; Li J; Wang Y; Jiang G Assessment of perfluorohexane sulfonic acid (PFHxS)-related compounds degradation potential: Computational and experimental approaches. J. Haz. Mat. 2022, 436, 129240. [DOI] [PubMed] [Google Scholar]
- 86.Ankley GT; Kuehl DW; Kahl MD; Jensen KM; Linnum A; Leino RL; Villeneuve DA Reproductive and developmental toxicity and bioconcentration of perfluorooctanesulfonate in a partial life-cycle test with the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2005, 24, 2316–2324. [DOI] [PubMed] [Google Scholar]
- 87.Lee JJ; Schultz IR Sex differences in the uptake and deposition of perfluorooctanoic acid in fathead minnows after oral dosing. Environ. Sci. Technol. 2010, 44, 491–496. [DOI] [PubMed] [Google Scholar]
- 88.New Hampshire Department of Environmental Services. Technical background report for proposed maximum contaminant levels (MCLs) and ambient groundwater quality standards (AGQSs) for perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), and perfluorohexane sulfonic acid (PFHxS), 2019. [Accessed November 2022 at https://www.des.nh.gov/sites/g/files/ehbemt341/files/documents/r-wd-19-29.pdf]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


