This randomized clinical trial evaluates the efficacy and safety of interleukin-13–targeted treatment with tralokinumab monotherapy in adolescents with atopic dermatitis.
Key Points
Question
What is the efficacy and safety of targeted interleukin-13 treatment with tralokinumab monotherapy in adolescents with moderate to severe atopic dermatitis?
Findings
In this randomized clinical trial of 289 patients aged 12 to 17 years, a statistically significant higher number of patients treated with tralokinumab vs placebo achieved an Investigator’s Global Assessment score of 0 (clear) or 1 (almost clear) and 75% or more improvement in the Eczema Area and Severity Index at week 16. No new safety signals were identified, and there was a low frequency of conjunctivitis through 52 weeks.
Meaning
This trial showed that interleukin-13–targeted treatment with tralokinumab was efficacious and well tolerated in adolescents with moderate to severe atopic dermatitis, suggesting that it is a valuable treatment option in this age group.
Abstract
Importance
Safe and effective long-term treatments for adolescents with moderate to severe atopic dermatitis (AD) are limited.
Objective
To evaluate the efficacy and safety of interleukin-13–targeted treatment with tralokinumab monotherapy in adolescents with AD.
Design, Setting, and Participants
The 52-week, randomized, double-blinded, placebo-controlled, phase 3 ECZTRA 6 trial was conducted from July 17, 2018, through March 16, 2021, at 72 centers across 10 countries in North America, Europe, Asia, and Australia. Enrolled patients were 12 to 17 years old with moderate to severe AD (Investigator’s Global Assessment [IGA] score ≥3; Eczema Area and Severity Index [EASI] ≥16).
Interventions
Patients were randomized (1:1:1) to tralokinumab (150 or 300 mg) or placebo every 2 weeks for 16 weeks. Patients with an IGA score of 0 (clear) or 1 (almost clear) and/or 75% or higher improvement in EASI (EASI 75) at week 16 without rescue medication received maintenance treatment; other patients switched to open-label tralokinumab, 300 mg, every 2 weeks.
Main Outcomes and Measures
Primary end points at week 16 were an IGA score of 0 or 1 and/or achieving EASI 75. Key secondary end points were a reduction of Adolescent Worst Pruritus Numeric Rating Scale of 4 or more, change in SCORing AD, and change in Children’s Dermatology Life Quality Index from baseline to week 16. Safety end points were the number of adverse events and serious adverse events.
Results
Of 301 patients randomized, 289 comprised the full analysis set (median [IQR] age, 15.0 [13.0-16.0] years; 149 [51.6%] male). More patients receiving tralokinumab, 150 mg, (n = 98), and tralokinumab, 300 mg (n = 97), achieved an IGA score of 0 or 1 without rescue medication at week 16 (21 [21.4%] and 17 [17.5%], respectively) vs placebo (n = 94; 4 [4.3%]) (adjusted difference, 17.5% [95% CI, 8.4%-26.6%]; P < .001 and 13.8% [95% CI, 5.3%-22.3%]; P = .002, respectively). More patients receiving tralokinumab, 150 mg (28 [28.6%]), and tralokinumab, 300 mg, (27 [27.8%]) vs placebo (6 [6.4%]) achieved EASI 75 without rescue at week 16 (adjusted difference, 22.5% [95% CI, 12.4%-32.6%]; P < .001 and 22.0% [95% CI, 12.0%-32.0%]; P < .001, respectively). Proportions of patients with Adolescent Worst Pruritus Numeric Rating Scale reduction of 4 or more from baseline were greater with tralokinumab, 150 mg (23.2%), and tralokinumab, 300 (25.0%), vs placebo (3.3%), and adjusted mean changes were greater in SCORing AD with tralokinumab, 150 mg (–27.5), and tralokinumab, 300 mg (–29.1), vs placebo (–9.5) and in Children’s Dermatology Life Quality Index with tralokinumab, 150 mg (–6.1), and tralokinumab, 300 mg (–6.7), vs placebo (–4.1) at week 16. At week 52, tralokinumab efficacy was maintained without rescue in more than 50% of patients meeting primary end point(s) at week 16. In the open-label phase, IGA score of 0 or 1 and EASI 75 were achieved in 33.3% and 57.8%, respectively, at week 52. Tralokinumab was well tolerated, without frequency of conjunctivitis increasing through week 52.
Conclusions and Relevance
In this randomized clinical trial, tralokinumab was efficacious and well tolerated, supporting its value for treating adolescents with moderate to severe AD.
Trial Registration
ClinicalTrials.gov Identifier: NCT03526861
Introduction
Atopic dermatitis (AD) commonly develops in early childhood and affects up to 20% of children.1,2 It is characterized by recurrent eczematous skin lesions and intense pruritus and can lead to anxiety, depression, and reduced quality of life.3 In adolescents, AD is associated with additional burdens, including psychologic effect on family and social life, as well as school performance.4
Interleukin (IL)-13 contributes to immune dysregulation, skin barrier dysfunction, and microbiome dysbiosis and is thought to be the dominant cytokine driving AD disease progression.5,6,7,8 Tralokinumab is a fully human IgG4 monoclonal antibody that binds with high affinity to IL-13.9,10 In contrast, dupilumab, also currently approved for treating adolescents with AD, inhibits both IL-4 and IL-13 signaling by blocking the IL-4R.11 Herein, we report results from ECZTRA 6, a phase 3, randomized, double-blinded, placebo-controlled study that examined efficacy and safety of tralokinumab monotherapy vs placebo in adolescents with moderate to severe AD.
Methods
Study Design
The randomized, double-blinded, placebo-controlled ECZTRA 6 trial evaluated the efficacy, safety, and tolerability of tralokinumab monotherapy in adolescents aged 12 to 17 years with moderate to severe AD who were candidates for systemic therapy (eFigure 11 in Supplement 1). The trial was conducted at 72 centers across 10 countries in North America, Europe, Asia, and Australia (eAppendix 1 in Supplement 1). Patients were randomized (1:1:1) using a central interactive response system to receive subcutaneous tralokinumab, 150 or 300 mg, or placebo every 2 weeks for 16 weeks after a loading dose at week 0 (twice the subsequent dose). Rescue medication was allowed at any time, and potency was classified according to the Anatomical Therapeutic Chemical Classification System. Patients receiving tralokinumab and meeting either primary end point at week 16 without use of rescue medication after week 2 (any topical calcineurin inhibitors [TCIs], topical corticosteroids [TCSs], or systemic AD treatment) were considered responders and were rerandomized (1:1) to receive blinded maintenance treatment until week 52 for either every 2 weeks or every 4 weeks at their original dose (150 or 300 mg). Patients receiving placebo who met the primary end point(s) at week 16 without use of rescue medication continued to receive blinded placebo every 2 weeks until week 52. All other patients, and those who lost response or received rescue medication during maintenance, were transferred to open-label treatment with tralokinumab, 300 mg, every 2 weeks with optional use of weak to moderate potency TCS or TCI. Patients were transferred from maintenance to open-label treatment at any visit from week 16 if they met specific criteria and transfer was considered appropriate by the investigator (eFigure 11 in Supplement 1).
The study protocol (Supplement 2) was approved by all relevant ethics committees and institutional review boards. The trial was conducted according to the principles of the Declaration of Helsinki and the International Council on Harmonization Guidelines for Good Clinical Practice, and conformed with all relevant country-specific laws and regulations. Written informed consent was provided by patients enrolled in the study or their legal representatives. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patients
Eligible patients had body weight of at least 30 kg, history of AD for at least 1 year, history of TCS/TCI treatment failure, AD over 10% or more body surface area (also at screening), an Eczema Area and Severity Index12 (EASI) of 16 or higher (≥12 at screening), an Investigator’s Global Assessment13 (IGA) score of 3 or higher (also at screening), and an Adolescent Worst Pruritus Numeric Rating Scale (NRS) average of 4 or higher during the week prior to baseline.
End Points
The primary end points were proportions of patients achieving IGA13 score of 0 (clear) or 1 (almost clear) at week 16 and/or 75% or higher improvement in EASI12 (EASI 75) at week 16. Key secondary end points were proportion of patients achieving a reduction of Adolescent Worst Pruritus NRS (weekly average) of 4 or higher, change in SCORing AD (SCORAD),14 and change in Children’s Dermatology Life Quality Index (CDLQI)15 from baseline to week 16. Worst Pruritus NRS was adjusted for adolescent understanding and validated in agreement with regulatory authorities. Additional secondary end points included EASI 50 and EASI 90 at week 16 and EASI percentage change from baseline to week 16. Additional patient-reported outcomes included eczema-related sleep NRS, Patient-Oriented Eczema Measure, and Hospital Anxiety and Depression Scale. Maintenance end points assessed at week 52 among patients receiving tralokinumab in the initial treatment period were IGA score of 0 or 1 for patients who had IGA score of 0 or 1 at week 16 and EASI 75 for patients who achieved EASI 75 at week 16. Open-label end points at week 52 were IGA score of 0 or 1 and EASI 50, 75, and 90. Safety end points were number of adverse events (AEs) and serious AEs.
Statistical Analysis
The sample size of 294 patients (98 per arm) was chosen to provide sufficient statistical power to demonstrate the efficacy of tralokinumab vs placebo for the primary end points. The power to detect a 20% difference in IGA score of 0 or 1 at week 16 (assuming response rates for tralokinumab, 300 mg, every 2 weeks vs placebo of 30% vs 10%, respectively) was approximately 94% at a 2-sided 5.0% significance level. For EASI 75, assuming week 16 rates of 40% and 15% for tralokinumab, 300 mg, and placebo, respectively, the power to detect a difference at the 2-sided 5.0% significance level was approximately 98%. The combined power to detect a difference was 92% or higher. For the tralokinumab, 150 mg, every-2-weeks dose, the accumulated power for rejecting the 2 hypotheses for both primary end points at 2.5% significance was 84% (IGA score of 0 or 1) and 80% (EASI 75) under the same assumptions as above. To control the overall type I error rate, the primary analyses of the primary estimands for the primary and key secondary end points followed a hierarchical testing procedure (eFigure 1 in Supplement 1). The full analysis set was defined as all patients randomized to initial treatment who were exposed to investigational medicinal product (excluding those enrolled at 2 sites with good clinical practice noncompliance [eTable 8 in Supplement 1]) and was used in all week 16 efficacy analyses (primary and secondary end points).
Several prespecified statistical analyses were conducted within the estimand framework, incorporating 2 major events that could influence the treatment effect: (1) initiation of rescue medication and (2) permanent discontinuation of treatment. Binary end points used a composite estimand for the primary approach, which assessed differences in response rates at week 16 without rescue medication, regardless of treatment discontinuation. Patients who received rescue medication were considered nonresponders, and patients with missing data were imputed as nonresponders. The difference between treatment groups was analyzed using the Cochran–Mantel–Haenszel test stratified by region and baseline IGA score. Continuous end points used a hypothetical estimand, which assessed treatment differences in change from baseline to week 16, assuming all patients adhered to the treatment regimen and no rescue medication was available. Data collected after permanent discontinuation or initiation of rescue medication were excluded from the analysis, and end points were analyzed using a linear mixed-effect model for repeated measurements. The IGA scores of 0 or 1 and EASI 75 at week 52 (among patients with IGA scores of 0 or 1 at week 16 and those achieving EASI 75 at week 16, respectively) were summarized descriptively by treatment group using the same response definition as previously described. Maintenance end points were assessed by pooled and individual treatment arms. Atopic dermatitis rescue medication/concomitant treatment was summarized for each treatment period. Safety analyses were performed and reported separately for 3 treatment periods. SAS, version 9.4 (SAS Institute) was used for the statistical analyses. All significance tests were 2-sided using 5% significance. Additional methods are provided in eAppendix 2 in Supplement 1 and in Supplement 3.
Results
Baseline Characteristics
Of 347 patients screened, 301 were randomized, and 289 comprised the full analysis set receiving tralokinumab, 150 mg, every 2 weeks (n = 98); tralokinumab, 300 mg, every 2 weeks (n = 97); or placebo (n = 94) (Figure 1 and eFigure 2 in Supplement 1). Baseline characteristics were comparable among treatment groups (Table 1).
Figure 1. CONSORT Diagram.
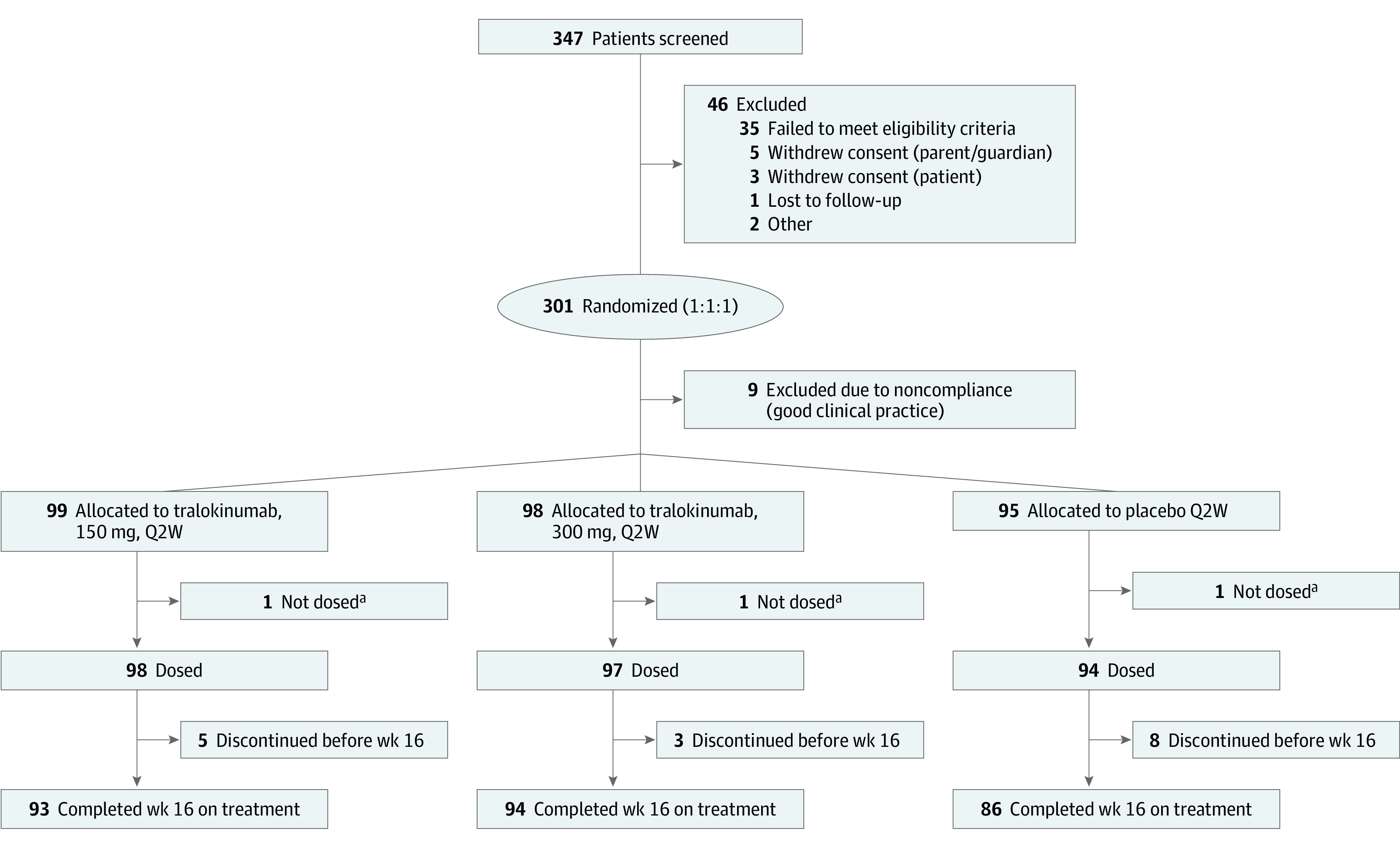
Q2W indicates every 2 weeks.
aWithdrew from the trial prior to first dosing.
Table 1. Demographics and Baseline Characteristics, Full Analysis Set.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| Total (N = 289) | Placebo (n = 94) | Tralokinumab every 2 wk | ||
| 150 mg (n = 98) | 300 mg (n = 97) | |||
| Age, median (IQR), y | 15.0 (13.0-16.0) | 14.0 (13.0-16.0) | 15.0 (13.0-16.0) | 15.0 (13.0-16.0) |
| Age group, y | ||||
| 12-14 | 131 (45.3) | 49 (52.1) | 37 (37.8) | 45 (46.4) |
| 15-17 | 158 (54.7) | 45 (47.9) | 61 (62.2) | 52 (53.6) |
| Weight, median (IQR), kg | 59.0 (49.0-70.0) | 59.0 (50.0-74.0) | 59.5 (50.0-68.0) | 58.0 (48.0-71.0) |
| Sex | ||||
| Female | 140 (48.4) | 43 (45.7) | 47 (48.0) | 50 (51.5) |
| Male | 149 (51.6) | 51 (54.3) | 51 (52.0) | 47 (48.5) |
| Region | ||||
| North America | 145 (50.2) | 47 (50.0) | 50 (51.0) | 48 (49.5) |
| Europe | 98 (33.9) | 32 (34.0) | 33 (33.7) | 33 (34.0) |
| Australia | 14 (4.8) | 4 (4.3) | 5 (5.1) | 5 (5.2) |
| Asia | 32 (11.1) | 11 (11.7) | 10 (10.2) | 11 (11.3) |
| Duration of AD, median (IQR), y | 13.0 (11.0-15.0) | 13.0 (11.0-15.0) | 13.0 (11.0-16.0) | 13.0 (11.0-15.0) |
| Affected BSA, median (IQR), % | 49.0 (32.0-67.0) | 52.0 (31.0-68.0) | 49.0 (37.0-65.0) | 44.0 (30.0-66.0) |
| IGA score of 4 | 135 (46.7) | 43 (45.7) | 44 (44.9) | 48 (49.5) |
| EASI, median (IQR) | 28.0 (21.1-38.1) | 27.2 (19.7-35.8) | 28.9 (21.4-39.4) | 28.0 (21.1-37.8) |
| SCORAD, median (IQR) | 66.9 (58.1-76.7) | 66.7 (57.8-76.7) | 65.0 (57.8-77.6) | 68.3 (59.4-75.6) |
| CDLQI, median (IQR) | 13.0 (8.0-18.0) | 13.0 (9.0-17.0) | 13.0 (8.0-18.0) | 13.0 (6.0-19.0) |
| Adolescent Worst Pruritus NRS, median weekly average (IQR) | 7.7 (6.6-8.8) | 7.6 (6.4-8.7) | 7.5 (6.6-8.7) | 8.1 (6.7-8.9) |
| Any previous treatment | 289 (100) | 94 (100) | 98 (100) | 97 (100) |
| Topical corticosteroid | 289 (100) | 94 (100) | 98 (100) | 97 (100) |
| Topical calcineurin inhibitor | 169 (58.5) | 56 (59.6) | 53 (54.1) | 60 (61.9) |
| Systemic corticosteroids | 130 (45.0) | 49 (52.1) | 48 (49.0) | 33 (34.0) |
| Systemic immunosuppressantsa | 61 (21.1) | 20 (21.3) | 22 (22.4) | 19 (19.6) |
| Monoclonal antibodies (type not specified)b | 7 (2.4) | 3 (3.2) | 2 (2.0) | 2 (2.1) |
| Other immunosuppressants | 1 (0.3) | 1 (1.1) | 0 | 0 |
| Wet wraps | 77 (26.2) | 29 (30.9) | 27 (27.6) | 21 (21.6) |
| Phototherapy | 74 (25.6) | 29 (30.9) | 29 (29.6) | 16 (16.5) |
| Comorbidities | ||||
| None (AD only)c | 38 (13.1) | 10 (10.6) | 10 (10.2) | 18 (18.6) |
| ≥1 Comorbid atopic diseases | 251 (86.9) | 84 (89.4) | 88 (89.8) | 79 (81.4) |
| ≥2 Comorbid atopic diseases | 194 (67.1) | 67 (71.3) | 71 (72.4) | 56 (57.7) |
| ≥3 Comorbid atopic diseases | 122 (42.2) | 42 (44.7) | 45 (45.9) | 35 (36.1) |
| ≥4 Comorbid atopic diseases | 40 (13.8) | 14 (14.9) | 13 (13.3) | 13 (13.4) |
Abbreviations: AD, atopic dermatitis; BSA, body surface area; CDLQI, Children’s Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; IGA, Investigator’s Global Assessment; NRS, Numeric Rating Scale; SCORAD, SCORing Atopic Dermatitis.
Mycophenolate, cyclosporine, or methotrexate (no patients previously used azathioprine).
No reason was given for discontinuation.
Asthma, food allergy, seasonal allergy, or allergic conjunctivitis.
Primary End Points and Related Outcomes
There were statistically significant higher proportions of patients achieving IGA score of 0 or 1 at week 16 without rescue medication in the tralokinumab, 150 mg (21.4%), and tralokinumab, 300 mg (17.5%), arms vs the placebo (4.3%) arm (adjusted difference, 17.5% [95% CI, 8.4%-26.6%]; P < .001 and 13.8% [95% CI, 5.3%-22.3%]; P = .002, respectively; Figure 2A and eFigure 3 and eTables 1 and 2 in Supplement 1). Similarly, a statistically significant higher number of patients achieved EASI 75 without rescue medication in the tralokinumab, 150 mg (28.6%), and tralokinumab, 300 mg (27.8%), arms vs the placebo (6.4%) arm at week 16 (adjusted difference, 22.5% [95% CI, 12.4%-32.6%]; P < .001 and 22.0% [95% CI, 12.0%-32.0%]; P < .001, respectively; Figure 2B and eFigure 3 and eTables 1 and 3 in Supplement 1); EASI 50 and EASI 90 responses were achieved by more patients in both tralokinumab arms vs the placebo arm at week 16 (eTable 1 and eFigure 4 in Supplement 1). Rescue medications were used in the initial treatment phase by 33.7% and 29.9% of patients receiving tralokinumab, 150 mg, and tralokinumab, 300 mg, respectively, compared with 56.4% in the placebo arm (predominantly TCS of any strength; eTable 4 in Supplement 1). Rescue use was initiated more rapidly in patients receiving tralokinumab, 150, vs tralokinumab, 300 mg (eFigure 5 in Supplement 1).
Figure 2. Tralokinumab Efficacy vs Placebo Across Primary and Key Secondary End Points up to Week 16 (Initial Treatment Period), Full Analysis Set.
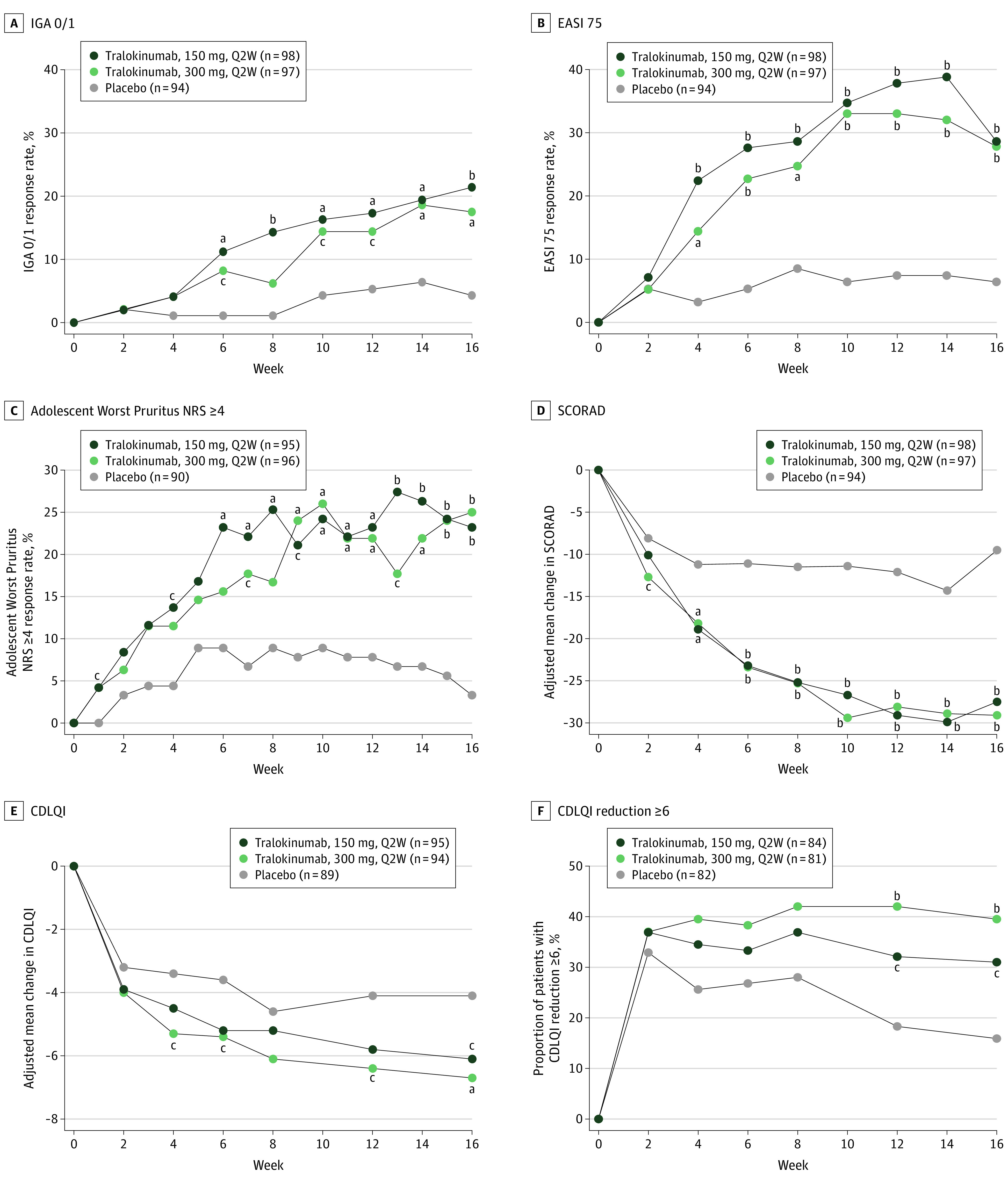
Primary end points were Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) (A) and 75% or more improvement in the Eczema Area and Severity Index (EASI 75) (B) by visit up to week 16 (initial treatment period) in the full analysis set. Secondary end points included the proportion of patients with a reduction in weekly average Adolescent Worst Pruritus Numeric Rating Scale (NRS) of 4 or more from baseline by visit (C), change from baseline in SCORing Atopic Dermatitis (SCORAD) by visit (D), change from baseline in Children’s Dermatology Life Quality Index (CDLQI) by visit in the initial treatment period up to week 16 (E), and proportion of patients with a CDLQI reduction of 6 or more from baseline by visit (F). For binary end points, patients who received rescue medication after week 2 were considered nonresponders, and those with missing values at week 16 were imputed as nonresponders. For continuous end points, data collected after permanent discontinuation of tralokinumab or initiation of rescue medication after week 2 were not included. Q2W indicates every 2 weeks.
aP < .01 vs placebo.
bP < .001 vs placebo.
cP < .05 vs placebo.
Key Secondary and Related End Points
Tralokinumab treatment resulted in statistically significant improvements in all key secondary end points compared with placebo (eTable 1 and eFigure 6 in Supplement 1). Proportions of patients with a 4-point or higher reduction in Adolescent Worst Pruritus NRS from baseline to week 16 were significantly greater in the tralokinumab, 150 mg (23.2%), and tralokinumab, 300 mg (25.0%), arms vs the placebo (3.3%) arm (difference, 19.9% [95% CI, 10.6%-29.2%]; P < .001 and 21.7% [95% CI, 12.3%-31.1%]; P < .001, respectively; Figure 2C and eTable 1 in Supplement 1). Adjusted mean (SE) changes in SCORAD from baseline to week 16 were significantly greater in the tralokinumab, 150 mg (–27.5 [2.4]), and tralokinumab, 300 mg (–29.1 [2.4]), arms vs the placebo (–9.5 [3.0]) arm (difference, –18.0 [95% CI, −25.6 to −10.4]; P < .001 and –19.7 [95% CI, −27.1 to −12.2]; P < .001), respectively; Figure 2D and eTable 1 in Supplement 1). Adjusted mean (SE) changes in CDLQI were significantly greater in the tralokinumab, 150 mg (–6.1 [0.6]), and tralokinumab, 300 mg (–6.7 [0.6]), arms vs the placebo arm (–4.1 [0.7]) (difference, –2.0 [95% CI, −3.9 to −0.1]; P = .04 and –2.6 [95% CI, −4.5 to −0.7]; P = .007), respectively; Figure 2E and eTable 1 in Supplement 1), and more patients in the tralokinumab vs placebo arms had CDLQI reductions of 6 or more (minimal important difference in adolescents16; 31.0% in the 150-mg arm and 39.5% in the 300-mg arm vs 15.9% with placebo at week 16; Figure 2F). The sensitivity, secondary, and tertiary analyses all supported the primary analysis results for both primary and key secondary end points (eTable 5 in Supplement 1).
Additional Patient-Reported Outcomes (Initial Period)
Both tralokinumab doses resulted in improvements in eczema-related sleep NRS vs placebo at week 16, with adjusted mean changes of –2.9 in the 150-mg arm and –3.1 in the 300-mg arm vs –1.8 with placebo (difference, –1.1 [95% CI, –2.0 to –0.2]; P = .01 and –1.3 [95% CI, –2.2 to –0.4]; P = .005, respectively; eFigure 7 in Supplement 1). Similarly, proportions of patients experiencing clinically meaningful reductions in the Patient-Oriented Eczema Measure (≥6-point improvement) were higher at week 16 in the tralokinumab, 150 mg (36 of 93 [38.7%]), and tralokinumab, 300 mg (44 of 94 [46.8%]), arms vs the placebo arm (9 of 86 [10.5%]; difference, 28.4% [95% CI, 16.4%-40.3%] and 36.5% [95% CI, 24.7%-48.3%], respectively; P < .001 for both comparisons; eFigure 7 in Supplement 1). Patients receiving tralokinumab, 300 mg, every 2 weeks experienced a greater decrease in Hospital Anxiety and Depression Scale from baseline vs placebo at week 16 (–4.4 vs –2.1; difference, –2.3 [95% CI, –4.3 to –0.3]; P = .02), but a decrease was not observed in the tralokinumab, 150 mg, arm (–1.8; difference vs placebo, 0.3 [95% CI, –1.8 to 2.3]; P = .81; eFigure 7 in Supplement 1).
Maintenance Phase, Open-label Phase, and Additional Outcomes
At week 16, 50 patients receiving tralokinumab and meeting either primary end point at week 16 without rescue medication were rerandomized (1:1) to maintenance phase treatment of either every 2 weeks or every 4 weeks at their original dose of 150 or 300 mg (eFigures 2 and 11 in Supplement 1). Patients who required rescue or met specific criteria were transferred to open label (eFigure 11 in Supplement 1). At week 52, responses of IGA score of 0 or 1 were maintained without rescue medication in 22 of 35 (62.9%) patients across all tralokinumab maintenance arms (ie, 150 or 300 mg, every 2 weeks or every 4 weeks) (eFigure 8 in Supplement 1). The EASI 75 responses were maintained without use of rescue medication by 25 of 47 (53.2%) patients across all tralokinumab maintenance arms (eFigure 8 in Supplement 1). Rescue medication use in the maintenance phase is summarized in eTable 4 in Supplement 1.
Overall, 214 patients were transferred to open-label tralokinumab, 300 mg, every 2 weeks (with optional use of weak to moderate potency TCS or TCI) at week 16 (eFigures 2 and 11 in Supplement 1). At week 52, IGA score of 0 or 1 was achieved by 23 of 65 (35.4%) patients transferring from the tralokinumab, 150 mg, every 2 weeks group; 22 of 70 (31.4%) from the tralokinumab, 300 mg, every 2 weeks group; and 22 of 79 (27.8%) from the placebo group, all without use of high-potency TCS, crisaborole, or systemic AD treatment (Figure 3A). In addition, EASI 75 was achieved by 41 of 65 (63.1%) patients transferring from the tralokinumab, 150 mg, every 2 weeks group; 37 of 70 (52.9%) from the tralokinumab, 300 mg, every 2 weeks group; and 52 of 79 (65.8%) from the placebo group, all without use of high-potency TCS, crisaborole, or additional systemic AD treatment (Figure 3B). In the open-label phase, 52.1% of patients used concomitant AD medication (mainly topical), and only 5.6% of patients used high-potency TCS, which classified them as nonresponders (eTable 4 in Supplement 1). Concomitant AD medication in the open-label phase is listed in eTable 4 in Supplement 1). A high proportion of patients not meeting EASI 75 response criteria at week 16 reached clinically meaningful EASI 50 responses within 4 to 8 weeks after transferring to the open-label phase; the proportion of patients achieving EASI 90 response continued to increase over the duration of the open-label phase (eFigure 9 in Supplement 1). An additional analysis at the individual patient level showed that an increasing number of patients initiated on tralokinumab (n = 195) achieved EASI 50, 75, and 90 responses from week 4 to week 52 (eFigure 10 in Supplement 1).
Figure 3. Tralokinumab Efficacy During Weeks 16-52 of the Open-label Phase.
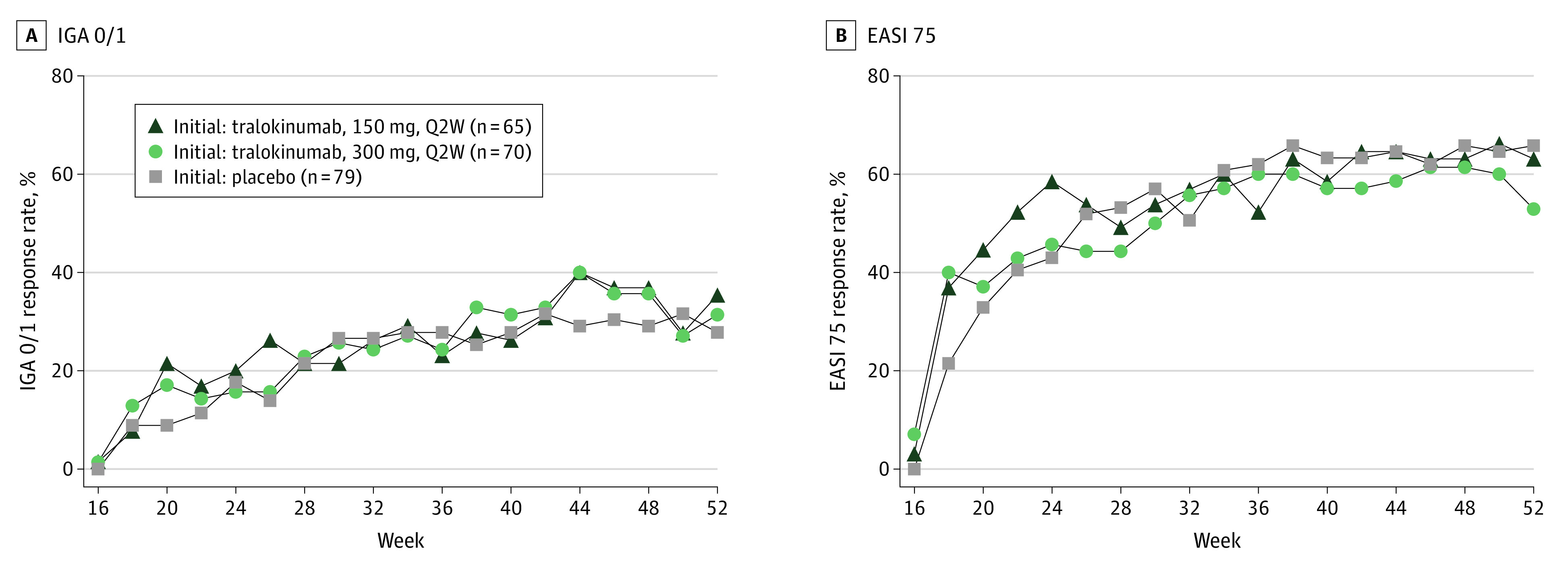
The response rates for Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) (A) and 75% or more improvement in the Eczema Area and Severity Index (EASI 75) (B) by visit up to week 52 in the open-label treatment period (open-label analysis set) included all patients from the initial treatment period who did not meet the primary end point without rescue medication at week 16. All patients received open-label tralokinumab, 300 mg, every 2 weeks (Q2W) plus optional topical corticosteroids. Patients could use weak to moderate potency topical corticosteroids and/or topical calcineurin inhibitors as needed on lesional skin at the investigator’s discretion. Patients who received high-potency topical calcineurin inhibitor or systemic atopic dermatitis treatment during open-label treatment were considered nonresponders, and patients with missing data were imputed as nonresponders.
Safety Outcomes
Tralokinumab was well tolerated, with a majority of AEs being mild or moderate in severity (Table 2). In the initial treatment period, proportions of patients with 1 or more AE were similar among those receiving tralokinumab, 150 mg, every 2 weeks (67.3%; rate [events per patient-years of exposure × 100], 596.6); tralokinumab, 300 mg, every 2 weeks (64.9%; rate, 441.0); and placebo (61.7%; rate, 479.7), with few serious AEs and only 1 treatment-emergent event leading to treatment withdrawal (not considered related to treatment); the most frequent AEs were upper respiratory tract infection, dermatitis atopic (disease exacerbation), injection-site reaction, asthma, and headache (Table 2). Proportions of patients with conjunctivitis as an AE of special interest—including the 4 preferred terms conjunctivitis, conjunctivitis bacterial, conjunctivitis viral, and conjunctivitis allergic—were low and similar between the 2 tralokinumab arms vs the placebo arm (Table 2). Only 2 events were reported with the preferred term conjunctivitis in the tralokinumab, 150 mg, arm and none in the placebo or tralokinumab, 300 mg, arms. Frequencies of other AEs of special interest, including eczema herpeticum and skin infections requiring systemic treatment, were low across all treatment arms. There was no increase in acne with tralokinumab (pooled doses, 1.5%) vs placebo (4.3%), while the proportions of patients with viral and bacterial skin infections (1.5% vs 0%), nausea (2.1% vs 0%), and herpes simplex infections (1.0% vs 2.1%) were low in the initial period; the types and frequencies of AEs observed in the maintenance and open-label phases were similar to the initial phase (eTables 6 and 7 in Supplement 1). At baseline, mean levels of eosinophils were above the normal reference range in all treatment groups. In the initial phase, laboratory measurements showed transient increases in eosinophils in a few patients receiving tralokinumab. Continued treatment in the open-label phase did not indicate further increases over time, and no clinical consequences related to eosinophilia were reported. There were no AEs of eosinophilia reported throughout the trial.
Table 2. Safety Outcomes in the Initial Treatment Phase, Safety Analysis Set (N = 289).
| Outcome | No. (%) | ||
|---|---|---|---|
| Placebo (n = 94) | Tralokinumab every 2 wk | ||
| 150 mg (n = 98) | 300 mg (n = 97) | ||
| Adverse events (patients with ≥1) | 58 (61.7) | 66 (67.3) | 63 (64.9) |
| Serious adverse events (patients with ≥1)a | 5 (5.3) | 3 (3.1) | 1 (1.0) |
| Severity of adverse events | |||
| Mild | 40 (42.6) | 48 (49.0) | 47 (48.5) |
| Moderate | 31 (33.0) | 33 (33.7) | 32 (33.0) |
| Severe | 7 (7.4) | 5 (5.1) | 3 (3.1) |
| Adverse event related to investigational medicinal product | 20 (21.3) | 26 (26.5) | 25 (25.8) |
| Adverse event leading to withdrawal | 0 | 1 (1.0)b | 0 |
| Frequent adverse events (≥5% in any group) | |||
| Viral upper respiratory tract infection | 8 (8.5) | 19 (19.4) | 12 (12.4) |
| Upper respiratory tract infection | 4 (4.3) | 8 (8.2) | 11 (11.3) |
| Dermatitis atopic | 12 (12.8) | 13 (13.3) | 7 (7.2) |
| Injection-site reaction | 0 | 6 (6.1) | 2 (2.1) |
| Asthma | 5 (5.3) | 0 | 3 (3.1) |
| Headache | 3 (3.2) | 5 (5.1) | 6 (6.2) |
| Adverse events of special interest | |||
| Eye disorders | 2 (2.1) | 4 (4.1) | 4 (4.1) |
| Conjunctivitis | 2 (2.1) | 4 (4.1) | 3 (3.1) |
| Conjunctivitis (preferred term) | 0 | 2 (2.0) | 0 |
| Conjunctivitis bacterial (preferred term) | 0 | 0 | 1 (1.0) |
| Conjunctivitis allergic (preferred term) | 2 (2.1) | 2 (2.0) | 2 (2.1) |
| Conjunctivitis viral (preferred term) | 0 | 0 | 0 |
| Keratitis | 0 | 0 | 1 (1.0) |
| Eczema herpeticum | 1 (1.1) | 1 (1.0) | 0 |
| Malignant neoplasms | 0 | 0 | 0 |
| Skin infections requiring systemic treatment | 2 (2.1) | 5 (5.1) | 2 (2.1) |
| Injection-site reactionsc | 1 (1.1) | 9 (9.2) | 7 (7.2) |
Serious adverse events were cellulitis (n = 1), dermatitis atopic (n = 1), and cerebrovascular accident (n = 1) in the tralokinumab, 150 mg, every 2 weeks arm; radius fracture (n = 1) in the tralokinumab, 300 mg, every 2 weeks arm; and infectious mononucleosis (n = 1), dermatitis atopic (n = 1), acute respiratory failure (n = 1), asthma (n = 1), and anaphylactic reaction (n = 1) in the placebo arm. Only treatment-emergent adverse events were included.
Due to cerebrovascular accident and not considered related to treatment by the investigator or sponsor.
Includes injection-site pain, swelling, and other injection-site reactions.
Discussion
This phase 3 trial examined targeting IL-13 with tralokinumab monotherapy in pediatric patients aged 12 to 17 years with moderate to severe AD. Tralokinumab had superior efficacy to placebo for all primary and key secondary end points, and key psychosocial and symptomatic effects of AD were also improved at week 16. The full clinical benefit of tralokinumab was not achieved for all patients at week 16, consistent with results in the adult phase 3 ECZTRA 1 and 2 monotherapy studies.10 In the majority of those who achieved clinical responses without any rescue medication at week 16, responses were maintained through week 52, without use of any rescue medication, including TCS. Of patients who were initially treated with tralokinumab and did not meet either primary end point at week 16 or who used rescue treatment, around one-third achieved an IGA score of 0 or 1, and more than half achieved EASI 75 at week 52 after receiving open-label tralokinumab, 300 mg, every 2 weeks without use of high-potency TCS or systemic AD treatment. Importantly, similar to the adult studies, the number of patients reaching EASI 90 also continued to increase over time toward week 52.
The current study is, to our knowledge, the first to show that targeting IL-13 alone improved AD signs and symptoms along with improvements in multiple high-effect disease domains in a pediatric population. Despite the heterogeneity of AD, the patient-level data shown herein from week 4 to 52 suggest that most patients are likely to respond to tralokinumab, consistent with the predominance of cutaneous IL-13 expression in AD phenotypes, including in pediatric patients.17
Tralokinumab was well tolerated over 52 weeks with low rates of treatment discontinuation (eFigure 2 in Supplement 1). The majority of AEs were nonserious and mild or moderate in severity, and the frequency and type of most AEs in adolescent patients receiving tralokinumab were similar to or, in some cases, lower (eg, conjunctivitis, disease flares) than those observed in phase 3 adult studies.10 Biologic treatments targeting the type 2 inflammatory pathway have been shown to increase the incidence of conjunctivitis in adult and adolescent patients.10,11,18,19 Conjunctivitis incidence among adolescents was low and similar between tralokinumab and placebo arms at week 16, with no increases seen for up to 52 weeks of treatment. Moreover, the incidence of acne did not increase with tralokinumab treatment; herpes simplex infections and nausea were infrequent across tralokinumab and placebo arms; and we did not see any signals of head and neck dermatitis among the AEs reported. No clinical consequences related to eosinophilia were reported, and the laboratory parameters did not indicate a need for additional laboratory monitoring. Both the tralokinumab, 150 mg, and tralokinumab, 300 mg, every-2-weeks doses showed efficacy on the primary and key secondary end points, with numerically greater improvement for tralokinumab, 300 mg, in some patient-reported outcomes and also later initiation of rescue medication. The safety profile of both doses was favorable, and no dose relationship with the incidence of adverse events was observed. In the tralokinumab, 300 mg, arm there were no conjunctivitis (preferred term) events, and fewer disease exacerbation events than in the tralokinumab, 150 mg, arm. Importantly, the safety and tolerability profile of tralokinumab in adolescents was consistent with the established profile of tralokinumab in adults.
While clinical signs are the mainstay for evaluating treatments for AD, patient-reported outcomes are extremely important when assessing treatments due to the psychosocial effect of the disease, particularly in adolescents.3,4,16,20 Tralokinumab was associated with improvements in several patient-reported outcomes, including scores assessing burden of sleep, pruritus, quality of life, and anxiety/depression.
The results presented herein show substantial tralokinumab efficacy at week 16, with continued improvement up to week 52. Other approved treatments in this age group, including dupilumab, have also shown substantial efficacy.18 Interpretation of comparisons between trial results is challenging due to differences in study design, analysis methods, and populations,21 and any comparison only on primary end points at week 16 would ignore both the chronic nature of the disease and the changes reported by the patient. It is notable that the safety profile of tralokinumab is favorable; although no statistical comparisons have been made, the frequency of acne is numerically lower than that reported with Janus kinase inhibitors,22,23 and the frequency of conjunctivitis in adolescents is numerically lower than that reported with dupilumab.18 Recent data suggest that IL-4 plays a similar role as IL-13 in goblet cell homeostasis,24 which may explain why blocking only IL-13 (and not both IL-4 and IL-13) could result in lower frequency of conjunctivitis with tralokinumab compared with dupilumab.
Limitations
Limitations include low sample size and lack of a placebo group in the maintenance phase, as well as lack of an active comparator arm in general, meaning direct comparison of tralokinumab relative to other targeted treatments or immunosuppressants could not be evaluated. Additionally, the open-label treatment phase was not blinded and hence may have introduced bias by influencing reporting/measurement of outcomes.
Conclusions
In this randomized clinical trial, tralokinumab monotherapy was effective and had a favorable benefit-to-risk profile in adolescents with moderate to severe AD over 52 weeks. Importantly, tralokinumab improved symptomatic and psychosocial aspects of AD in adolescents, including itch, sleep, anxiety/depression, and overall quality of life, with few adverse effects and low treatment discontinuation rates. These results are consistent with tralokinumab data reported in adults with AD,9,10 suggesting that specific targeting of IL-13 with tralokinumab is an effective and well-tolerated long-term treatment option for uncontrolled AD in adolescents.
eAppendix 1. Study investigators/institutions
eAppendix 2. Additional methods
eTable 1. Primary and secondary efficacy outcomes for the initial treatment period, full analysis set
eTable 2. IGA 0/1 by visit (initial treatment period; observed data, full analysis set)
eTable 3. Percentage change from baseline in EASI by visit (initial treatment period; observed data, full analysis set)
eTable 4. Rescue medications used in the (A) initial and (B) maintenance treatment periods, and concomitant AD medications in the (C) open-label treatment period, by type
eTable 5. Primary efficacy analyses for confirmatory endpoints using different estimand approaches
eTable 6. Safety outcomes in the maintenance treatment phase
eTable 7. Safety outcomes in the open-label treatment phase
eTable 8. Primary endpoint at Week 16 in all dosed patients, including nine patients from two sites with GCP non-compliance
eFigure 1. Testing hierarchy used for the primary and secondary endpoints
eFigure 2. Patient disposition
eFigure 3. IGA0/1 and EASI 75 up to Week 16 by individual tralokinumab arm (150 or 300 mg Q2W) vs placebo
eFigure 4. Achievement of EASI 50 or EASI 90 up to Week 16 (initial treatment period), full analysis set
eFigure 5. Initiation of rescue medication and permanent discontinuation of IMP before or at Week 16, full analysis set
eFigure 6. Reduction in Adolescent Worst Pruritus NRS ≥4, change in SCORAD, and change in CDLQI from baseline to Week 16 by individual tralokinumab arm (150 or 300 mg Q2W) vs placebo
eFigure 7. Tralokinumab efficacy vs placebo across additional patient-reported outcomes up to Week 16 (initial treatment period), full analysis set
eFigure 8. Tralokinumab efficacy at Week 52 of the maintenance phase
eFigure 9. Achievement of EASI 50 or EASI 90 by Week 52 of the open-label treatment period, open-label analysis set
eFigure 10. Individual patient change from baseline in EASI over time (Week 4, 16, and 52)
eFigure 11. Study design
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement
References
- 1.Peters AS, Kellberger J, Vogelberg C, et al. Prediction of the incidence, recurrence, and persistence of atopic dermatitis in adolescence: a prospective cohort study. J Allergy Clin Immunol. 2010;126(3):590-595. doi: 10.1016/j.jaci.2010.06.020 [DOI] [PubMed] [Google Scholar]
- 2.Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1. doi: 10.1038/s41572-018-0001-z [DOI] [PubMed] [Google Scholar]
- 3.Ghio D, Greenwell K, Muller I, Roberts A, McNiven A, Santer M. Psychosocial needs of adolescents and young adults with eczema: a secondary analysis of qualitative data to inform a behaviour change intervention. Br J Health Psychol. 2021;26(1):214-231. doi: 10.1111/bjhp.12467 [DOI] [PubMed] [Google Scholar]
- 4.Ricci G, Bellini F, Dondi A, Patrizi A, Pession A. Atopic dermatitis in adolescence. Dermatol Reports. 2011;4(1):e1. doi: 10.4081/dr.2012.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bieber T. Interleukin-13: targeting an underestimated cytokine in atopic dermatitis. Allergy. 2020;75(1):54-62. doi: 10.1111/all.13954 [DOI] [PubMed] [Google Scholar]
- 6.Furue K, Ito T, Tsuji G, et al. The IL-13-OVOL1-FLG axis in atopic dermatitis. Immunology. 2019;158(4):281-286. doi: 10.1111/imm.13120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nomura I, Goleva E, Howell MD, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171(6):3262-3269. doi: 10.4049/jimmunol.171.6.3262 [DOI] [PubMed] [Google Scholar]
- 8.Berdyshev E, Goleva E, Bronova I, et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight. 2018;3(4):e98006. doi: 10.1172/jci.insight.98006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverberg JI, Toth D, Bieber T, et al. ; ECZTRA 3 study investigators . Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184(3):450-463. doi: 10.1111/bjd.19573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wollenberg A, Blauvelt A, Guttman-Yassky E, et al. ; ECZTRA 1 and ECZTRA 2 study investigators . Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437-449. doi: 10.1111/bjd.19574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson EL, Bieber T, Guttman-Yassky E, et al. ; SOLO 1 and SOLO 2 Investigators . Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335-2348. doi: 10.1056/NEJMoa1610020 [DOI] [PubMed] [Google Scholar]
- 12.Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M; EASI Evaluator Group . The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. Exp Dermatol. 2001;10(1):11-18. doi: 10.1034/j.1600-0625.2001.100102.x [DOI] [PubMed] [Google Scholar]
- 13.Futamura M, Leshem YA, Thomas KS, Nankervis H, Williams HC, Simpson EL. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol. 2016;74(2):288-294. doi: 10.1016/j.jaad.2015.09.062 [DOI] [PubMed] [Google Scholar]
- 14.Kunz B, Oranje AP, Labrèze L, Stalder JF, Ring J, Taïeb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195(1):10-19. doi: 10.1159/000245677 [DOI] [PubMed] [Google Scholar]
- 15.Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132(6):942-949. doi: 10.1111/j.1365-2133.1995.tb16953.x [DOI] [PubMed] [Google Scholar]
- 16.Simpson EL, de Bruin-Weller M, Eckert L, et al. Responder threshold for Patient-Oriented Eczema Measure (POEM) and Children’s Dermatology Life Quality Index (CDLQI) in adolescents with atopic dermatitis. Dermatol Ther (Heidelb). 2019;9(4):799-805. doi: 10.1007/s13555-019-00333-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renert-Yuval Y, Pavel AB, Bose S, et al. Tape strips capture atopic dermatitis-related changes in nonlesional skin throughout maturation. Allergy. 2022;77(11):3445-3447. doi: 10.1111/all.15423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(1):44-56. doi: 10.1001/jamadermatol.2019.3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019;181(3):459-473. doi: 10.1111/bjd.17869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitt J, Spuls P, Boers M, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy. 2012;67(9):1111-1117. doi: 10.1111/j.1398-9995.2012.02874.x [DOI] [PubMed] [Google Scholar]
- 21.Silverberg JI, Simpson EL, Armstrong AW, de Bruin-Weller MS, Irvine AD, Reich K. Expert perspectives on key parameters that impact interpretation of randomized clinical trials in moderate-to-severe atopic dermatitis. Am J Clin Dermatol. 2022;23(1):1-11. doi: 10.1007/s40257-021-00639-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151-2168. doi: 10.1016/S0140-6736(21)00588-2 [DOI] [PubMed] [Google Scholar]
- 23.Eichenfield LF, Flohr C, Sidbury R, et al. Efficacy and safety of abrocitinib in combination with topical therapy in adolescents with moderate-to-severe atopic dermatitis: the JADE TEEN randomized clinical trial. JAMA Dermatol. 2021;157(10):1165-1173. doi: 10.1001/jamadermatol.2021.2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen PM, Tollenaere MAX, Hedengran A, et al. IL-4 and IL-13 both contribute to the homeostasis of human conjunctival goblet cells in vitro. Allergy. 2022;77(8):2555-2558. doi: 10.1111/all.15326 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Study investigators/institutions
eAppendix 2. Additional methods
eTable 1. Primary and secondary efficacy outcomes for the initial treatment period, full analysis set
eTable 2. IGA 0/1 by visit (initial treatment period; observed data, full analysis set)
eTable 3. Percentage change from baseline in EASI by visit (initial treatment period; observed data, full analysis set)
eTable 4. Rescue medications used in the (A) initial and (B) maintenance treatment periods, and concomitant AD medications in the (C) open-label treatment period, by type
eTable 5. Primary efficacy analyses for confirmatory endpoints using different estimand approaches
eTable 6. Safety outcomes in the maintenance treatment phase
eTable 7. Safety outcomes in the open-label treatment phase
eTable 8. Primary endpoint at Week 16 in all dosed patients, including nine patients from two sites with GCP non-compliance
eFigure 1. Testing hierarchy used for the primary and secondary endpoints
eFigure 2. Patient disposition
eFigure 3. IGA0/1 and EASI 75 up to Week 16 by individual tralokinumab arm (150 or 300 mg Q2W) vs placebo
eFigure 4. Achievement of EASI 50 or EASI 90 up to Week 16 (initial treatment period), full analysis set
eFigure 5. Initiation of rescue medication and permanent discontinuation of IMP before or at Week 16, full analysis set
eFigure 6. Reduction in Adolescent Worst Pruritus NRS ≥4, change in SCORAD, and change in CDLQI from baseline to Week 16 by individual tralokinumab arm (150 or 300 mg Q2W) vs placebo
eFigure 7. Tralokinumab efficacy vs placebo across additional patient-reported outcomes up to Week 16 (initial treatment period), full analysis set
eFigure 8. Tralokinumab efficacy at Week 52 of the maintenance phase
eFigure 9. Achievement of EASI 50 or EASI 90 by Week 52 of the open-label treatment period, open-label analysis set
eFigure 10. Individual patient change from baseline in EASI over time (Week 4, 16, and 52)
eFigure 11. Study design
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement


