Abstract
On 8 July 2021, EFSA published a Conclusion on the peer review of the pesticide risk assessment for the active substance acibenzolar‐S‐methyl in light of confirmatory data submitted. EFSA concluded that based on the confirmatory information submitted by the applicant, the assessment of endocrine‐disrupting properties could not be finalised for humans and non‐target organisms and identified further data deemed necessary to finalise the assessment. Consequently, during the decision‐making stage it could not be concluded by risk managers that acibenzolar‐S‐methyl still meets the approval criteria laid down in Article 4 of Regulation (EC) No 1107/2009 and therefore the European Commission decided to launch a review of the existing approval in accordance with Article 21 of that Regulation and on 6 July 2022 invited the applicant to submit comments on the findings in the EFSA Conclusion including any relevant information. On 14 December 2022, the European Commission requested EFSA to consider the proposal as submitted by the applicant in light of the EFSA Conclusion and to confirm whether the proposed studies are considered sufficient to complete the assessment of the endocrine disrupting properties of the substance in line with Commission Regulation (EU) 2018/605. The current statement contains EFSA's considerations as regards the testing strategy and associated timelines for additional data generation proposed by the applicant to complete the assessment of the endocrine disrupting properties of acibenzolar‐S‐methyl in line with Commission Regulation (EU) 2018/605.
Keywords: acibenzolar‐S‐methyl, peer review, risk assessment, pesticide, plant activator, endocrine disrupting properties
Summary
The approval of acibenzolar‐S‐methyl was renewed under Regulation (EC) No 1107/2009 on 1 April 2016 by Commission Implementing Regulation (EU) No 2016/389. It was a specific provision of the approval that the applicant was required to submit to the European Commission confirmatory information by 1 June 2017 as regards the relevance and reproducibility of the morphometric changes observed in the cerebellum of fetuses linked to exposure to acibenzolar‐S‐methyl and whether these changes may be produced via an endocrine mode of action. The confirmatory information was requested to include a systematic review of the available evidence assessed on the basis of available guidance (e.g. EFSA guidance on Systematic Review methodology, 2010). In accordance with the specific provision, the applicant, Syngenta, submitted an updated dossier in May 2017 as well as additional information in February 2019 in line with the EFSA/ECHA guidance for the identification of endocrine disruptors (2018), which was evaluated by the designated rapporteur Member State (RMS), France and then reviewed by the Member States and EFSA. As an outcome of the procedure of the assessment of the submitted confirmatory information, in compliance with the guidance document SANCO 5634/2009‐rev.6.1 (European Commission, 2013), EFSA issued a Technical Report on 15 April 2020 (EFSA, 2020a).
Following consideration of the conclusions of the Technical Report, in October 2020, the European Commission requested EFSA to organise a peer review, to further assess the relevance of the endocrine disrupting properties of acibenzolar‐S‐methyl and to deliver its conclusions, and if applicable, to establish which additional tests are needed to conclude on the endocrine disrupting properties both for humans and non‐target organisms under consideration of the new scientific criteria set out under points 3.6.5. and 3.8.2 of Regulation (EC) No 1107/2009 as amended by Commission Regulation 2018/605. Following the specific request from the Commission, EFSA delivered its Conclusion on the peer review of the pesticide risk assessment of the active substance acibenzolar‐S‐methyl on 14 June 2021 (EFSA, 2021). Overall, EFSA concluded that based on the confirmatory information submitted by the applicant, the assessment of endocrine disrupting properties could not be finalised for humans and non‐target organisms and identified further data deemed necessary to finalise the assessment.
Consequently, during the decision‐making stage it could not be established by risk managers that acibenzolar‐S‐methyl still meets the approval criteria laid down in Article 4 of Regulation (EC) No 1107/2009 and therefore the European Commission decided to launch a review of the existing approval in accordance with Article 21 of that Regulation and on 6 July 2022 invited the applicant to submit comments on the findings in the EFSA Conclusion including any relevant information by 6 October 2022.
Subsequently, on 14 December 2022 the European Commission requested EFSA to consider the proposal as submitted by the applicant in light of the EFSA Conclusion and to confirm whether the proposed studies are considered sufficient to complete the assessment of the endocrine disrupting properties of the substance in line with Commission Regulation (EU) 2018/605.
The current statement contains EFSA's considerations as regards the testing strategy and associated timelines for additional data generation proposed by the applicant to complete the assessment of the endocrine disrupting properties of acibenzolar‐S‐methyl in line with Commission Regulation (EU) 2018/605.
As regards the testing strategy to complete the assessment for the endocrine disrupting properties of acibenzolar‐S‐methyl for the oestrogen, androgen and steroidogenesis (EAS)‐modalities for mammals, EFSA agrees with the assays and associated timelines proposed by the applicant; though, the testing strategy and timelines should include the possibility that a level 5 OECD test guideline (TG) 443 study may be needed if any proposed level 2 and/or level 3 study would result to be positive. The proposed testing strategy, with the inclusion of the OECD TG 443 study, is in line with the EFSA/ECHA (2018) guidance for the identification of endocrine disruptors and previous EFSA recommendations (EFSA, 2021). Regarding the thyroid (T)‐modality, EFSA agrees with the assays and timelines proposed by the applicant but the testing strategy should include the execution of a Comparative Thyroid Assay (CTA), as proposed by EFSA in the confirmatory data Conclusion (EFSA, 2021) and in agreement with the RMS France. This is in line with the Appendix A of the EFSA/ECHA guidance and previous EFSA recommendations. The level 5 OECD TG 443 study should be conducted only if triggered by positive outcome in the level 2 and/or 3 studies as proposed by EFSA; differently, the CTA study should be performed as recommended by EFSA in the confirmatory data Conclusion (EFSA, 2021), and the proposed in vitro assays for the T‐modality should be conducted to complement the mode of action (MoA) analysis. The full package of studies should be conducted in the timeframe proposed by the applicant. Therefore, the timelines proposed by the applicant are considered acceptable also for the execution of the CTA study and of the in vitro mechanistic studies.
The testing strategy to complete the assessment for the endocrine disrupting properties through the T‐modality for non‐mammalian species deviates from the recommendations of the ECHA/EFSA guidance on endocrine disruptors (ED). Comparison of the different available protocols on amphibians has been presented by EFSA aiming at providing an overview of the advantages and disadvantages of the available test methods. The extended Amphibian Metamorphosis Assay (extended AMA) may present some added values when compared to a standard AMA, such as the inclusion of a time‐to‐event parameter, however, it is not clear how it contributes to a MoA, when an AMA is already available and positive. Although it is acknowledged that the validation of the Larval Amphibian Growth and Development Assay (LAGDA) was limited to few chemicals (only one substance known to act through multiple ED MoA including a T‐MoA), it is noted that the extended AMA suggested by Ortego et al. (2021) is a non‐standardised protocol as well, when comparing it to the existing AMA. In both cases the reproducibility of the results by different laboratories is a source of uncertainty. Therefore, overall, based on the above consideration and the test design, a LAGDA is considered more fit for purpose in this case. For the EAS‐modalities, the proposed testing strategy is considered adequate. However, in the case the performed Fish Short‐Term Reproduction Assay (FSTRA) would not be negative, a MoA should be postulated and additional testing might be needed, i.e. a test in line with OECD TG 240 (Medaka Extended One‐Generation Reproduction Test (MEOGRT)) to further investigate adversity. The timelines are, in general, considered appropriate. However, if a LAGDA or a MEOGRT is conducted, a 27/28‐month time period, respectively would be necessary.
1. Introduction
The approval of acibenzolar‐S‐methyl was renewed under Regulation (EC) No 1107/2009 1 on 1 April 2016 by Commission Implementing Regulation (EU) No 2016/389 2 . EFSA previously finalised a Conclusion on this active substance on 12 August 2014 in the EFSA Journal (EFSA, 2014).
It was a specific provision of the approval that the applicant was required to submit to the European Commission confirmatory information by 1 June 2017 as regards the relevance and reproducibility of the morphometric changes observed in the cerebellum of fetuses linked to exposure to acibenzolar‐S‐methyl and whether these changes may be produced via an endocrine mode of action. The confirmatory information was requested to include a systematic review of the available evidence assessed on the basis of available guidance (e.g. EFSA guidance on Systematic Review methodology, 2010). In accordance with the specific provision, the applicant, Syngenta, submitted an updated dossier in May 2017 as well as additional information in February 2019 in line with the EFSA/ECHA guidance for the identification of endocrine disruptors (2018), which was evaluated by the designated rapporteur Member State (RMS), France and then reviewed by the Member States and EFSA. As an outcome of the procedure of the assessment of the submitted confirmatory information, in compliance with the guidance document SANCO 5634/2009‐rev.6.1 (European Commission, 2013), EFSA issued a Technical Report on 15 April 2020 (EFSA, 2020a). Following consideration of the conclusions of the Technical Report, in October 2020, the European Commission requested EFSA to organise a peer review, to further assess the relevance of the endocrine disrupting (ED) properties of acibenzolar‐S‐methyl and to deliver its conclusions on the ED properties, and if applicable, to establish which additional tests are needed to conclude on the ED properties both for humans and non‐target organisms under consideration of the new scientific criteria set out under points 3.6.5. and 3.8.2 of Regulation (EC) No 1107/2009 as amended by Commission Regulation 2018/605.
Following the specific request from the Commission, EFSA delivered its Conclusion on the peer review of the pesticide risk assessment of the active substance acibenzolar‐S‐methyl on 14 June 2021 (EFSA, 2021). Overall, EFSA concluded that based on the confirmatory information submitted by the applicant, the assessment of endocrine‐disrupting properties according to points 3.6.5 and 3.8.2 of Regulation (EC) No 1107/2009, as amended by Commission Regulation 2018/605 3 , could not be finalised and identified further data deemed necessary to finalise the assessment for both humans and non‐target organisms.
Consequently, during the decision‐making stage, it could not be established by risk managers that acibenzolar‐S‐methyl still meets the approval criteria laid down in Article 4 of Regulation (EC) No 1107/2009 and therefore the European Commission decided to launch a review of the existing approval in accordance with Article 21 of that Regulation. As a result of this process, following a specific mandate received from the European Commission on 14 December 2022, EFSA prepared a draft statement in February 2023 which was circulated to the rapporteur Member State, France, and subsequently to all Member States for commenting via a written procedure before finalisation. A key supporting document to this statement is the peer review report (EFSA, 2023), which contains the comments received on the draft statement, in which all views including minority views, where applicable, can be found.
Given the importance of the peer review report, this document is considered as background document to this statement and thus is made publicly available.
1.1. Background and terms of reference as provided by the requestor
On 6 July 2022, in accordance with Article 21(1) of Regulation (EC) No 1107/2009, the European Commission invited the applicant to submit comments on the findings of the EFSA Conclusion on the assessment of the endocrine disrupting properties for acibenzolar‐S‐methyl in light of confirmatory data published on 8 July 2021 (EFSA, 2021), including any relevant information.
On 6 October 2022, the applicant submitted to the European Commission their response including a proposal for additional tests with estimated timelines for data availability and submission of the full data package. The applicant's response document including their testing strategy and estimated timing for data generation has been forwarded to EFSA by the European Commission and is included in Appendix A of this statement.
On 14 December 2022 the European Commission requested EFSA to consider – in consultation with the Rapporteur Member State France – the proposal as submitted by the applicant and to confirm within 3 months from the date of the request from the Commission whether the proposed studies are considered sufficient to complete the assessment of the endocrine disrupting properties of the substance in line with Commission Regulation (EU) 2018/605. If relevant, EFSA was requested to also identify which additional studies would be required and to specify the period of time needed for conducting each of the planned (or additional) studies.
For this purpose, a statement has been produced containing EFSA's considerations as regards the testing strategy and associated timelines for additional data generation proposed by the applicant with a view to complete the assessment of the endocrine disrupting properties of acibenzolar‐S‐methyl in line with Commission Regulation (EU) 2018/605.
2. Assessment
As part of the confirmatory data process, the ED assessment for acibenzolar‐S‐methyl was thoroughly discussed at the Pesticides Peer Review Experts' Teleconference 48 on Mammalian Toxicology and Ecotoxicology joint session on endocrine disruption in April 2021. Advice from the EFSA Endocrine Disruption (ED) Working Group was also provided and taken into account in the conclusions reached in the finalised EFSA Conclusion (EFSA, 2021).
In particular, EFSA identified the need for the following studies to complete the data package for humans and non‐target organisms:
For the thyroid (T)‐modality:
A thyroid assessment assay with measurement of thyroid hormones (THs) and thyroid‐stimulating hormone (TSH) in fetus and pups in line with the: US EPA, Office of Pesticide Programs, Health Effects Division, Washington (DC) 4 , 5 ;
A test according to OECD test guideline (TG) 231 (AMA). If the test is negative, the active substance will not meet the ED criteria for the T‐modality for non‐target organisms. However, in case of positive results, additional testing is needed, i.e. a test according to OECD TG 241 (LAGDA).
For the oestrogen, androgen and steroidogenesis (EAS) 6 ‐modalities:
A study in line with OECD TG 458 (Stably Transfected Human Androgen Receptor Activation Assay (AR STTA) assay);
Aromatase assay (human recombinant) OPPTS 890.1200 (US EPA 2009 In: Endocrine Disruptor Screening Program Test Guidelines. Office of Prevention, Pesticides and Toxic Substances (OPPTS), US EPA, Washington (DC));
A study in line with OECD TG 456 (H295R Steroidogenesis assay);
A study in line with OECD TG 441 (Hershberger Assay) in case OECD TG 456, OPPTS 890.1200 and OECD TG 458 are negative;
For non‐target organisms, a test according to OECD TG 229 (FSTRA).
If the above tests are negative, the active substance will not meet the ED criteria for the EAS‐modalities for humans and/or non‐target organisms. However, in case of positive result/s for one of the above tests for at least one modality, additional testing is needed:
A test according to OECD TG 443 (with the inclusion of cohort 1B) or OECD TG 416 (including additional endpoints in accordance with the EFSA, 2020b) technical report: ‘Outcome of the pesticides peer review meeting on general recurring issues in mammalian toxicology’. 7
A test according to OECD TG 240 (Medaka Extended One‐Generation Reproduction Test (MEOGRT)) for further investigating adversity in fish.
A summary of the proposal for ED data generation plan, as submitted by the applicant on 6 October 2022 following the request from the European Commission in the context of the review of the approval of the substance under Article 21 of Regulation (EC) No 1107/2009, is provided in Table 1 below. Full details of the available data and anticipated final reporting dates of ongoing studies are provided in Appendix A.
Table 1.
Summary of data generation plan as provided by the applicant
| Study | Objective | Expected reporting date |
|---|---|---|
| EAS‐modalities | ||
| Stably Transfected Human Androgen Receptor Activation Assay (OECD TG 458) | Evaluate the potential for acibenzolar‐S‐methyl to exhibit an (anti)‐Androgenic (A) endocrine activity | Report Finalised |
| Hershberger Assay (OECD TG 441) | December 2022 | |
| H295R Steroidogenesis Assay (OECD TG 456) | Evaluate the potential for acibenzolar‐S‐methyl to exhibit steroidogenic (S) activity in vitro | Report Finalised |
| Aromatase Assay (human recombinant) (OPPTS 890.1200) | Report Finalised | |
| Fish Short‐Term Reproduction Assay (OECD TG 229) | Investigate the ED potential on non‐target organisms | December 2022 |
| T‐modality | ||
| In vitro comparative study of hepatic Phase II metabolism in multiple species (human, rat, mouse and dog) | Measure the expressions of CYPs isoforms and the T4‐UGT enzymatic activity (Phase II) and will be conducted on multiple species in order to assess human relevance of the effect of acibenzolar‐S‐methyl on hepatic metabolism. | Report Finalised |
| In vitro assessment of inhibition of thyroid peroxidase activity (TPO) | Investigate the potential molecular initiating events (MIEs) for thyroid‐disrupting modes of action. | Q2/Q3 2023* |
| In vitro assessment of inhibition of sodium‐iodide symporter activity (NIS) | Q2/Q3 2023* | |
| In vitro assessment of inhibition of the deiodinase enzyme (DIO 1,2,3) | Q4 2024/Q1 2025* | |
| Amphibian Metamorphosis Assay (OECD TG 231) | Investigate the ED potential on non‐target organisms | December 2022 |
| Extended Amphibian Metamorphosis Assay | Investigate findings from existing AMA to understand potential population relevance | Q1 2024 |
Best estimate based on validation work completed by Syngenta.
2.1. EFSA considerations on the testing strategy and timelines for data generation for the ED assessment for humans
2.1.1. Testing strategy for the T‐modality
As regards to the testing strategy to complete the assessment for the endocrine disrupting properties of acibenzolar‐S‐methyl for the T‐modality, EFSA notes that the in vitro assays 8 proposed by the applicant are indeed intended to measure molecular initiating events (MIEs) and/or key events (KEs) relevant for screening potential thyroid disruptive chemicals. Although validation efforts are ongoing for these assays at international level, the current lack of validated methods to be used for regulatory decisions is acknowledged and this uncertainty is also included in the weight of evidence (WoE) proposed in the Appendix A of the EFSA/ECHA ED guidance (ECHA/EFSA, 2018). The current dataset for the T‐modality indicates that the developmental neurotoxicity (DNT) study is the main reason of concern for the T‐modality because of the observed effects in the available in vivo study and that there is insufficient evidence to conclude on a pattern of T‐mediated adversity based on the histopathological assessment of the thyroid.
EFSA noted that the in vivo DNT endpoints are ranked as ‘T‐sensitive’ in the OECD GD 150 (meaning that the observed effect/s can also be consequent to non‐endocrine MoA) and, as such, accordingly reported in the EFSA/ECHA ED guidance. In addition, acibenzolar‐S‐methyl is positive (i.e. is a ‘hit’) in several endpoints measured in the in vitro DNT testing battery (Blum et al., 2023), and therefore the distinction between an endocrine mediated DNT effect and non‐endocrine mediated DNT effects is uncertain. This is because there are limitations in the DNT‐in vitro testing battery (IVB) to identify DNT effects consequent to endocrine mechanisms. Therefore, no conclusion on the ED properties of the active substance can be drawn based on the in vivo and in vitro DNT studies though, DNT data (in vivo and in vitro) should be included in the WoE analysis.
The current concern is additionally substantiated by evidence that in the NCC_TPO_AUR 9 assay (protein single format assay using rat thyroid gland cell line) reported in ToxCast, acibenzolar‐S‐methyl is positive (AC50 of 0.27 μΜ). 10 However, EFSA noted that there is uncertainty as acibenzolar‐S‐methyl is a substance where the AUR‐TPO result and the GUA‐TPO 11 result do not agree. EFSA is aware that AUR‐TPO AC50/potency tends to be left shifted (less) than GUA‐TPO potency, but GUA‐TPO is negative at > 100 μM and no other thiadiazoles are in the ToxCast database, so the hypothesis that the chemistry of acibenzolar‐S‐methyl is possibly related to a non‐specific reaction with the AUR chemistry could not be assessed.
The Comparative Thyroid Assay (CTA), which is currently not included in the applicant's data generation plan, was therefore proposed by EFSA to conclude on the T‐modality by measuring T‐mediated adversity in the most sensitive population in vivo for a MoA known to include changes in THs and TSH as intermediate KEs for which the rat is recognised as sensitive model. This is in line with the EFSA/ECHA ED guidance.
However, EFSA acknowledges that the level of uncertainties can be reduced by providing additional investigations on thyroid MIEs/KEs as proposed by the applicant. In particular, the TPO assay proposed by the applicant is considered appropriate to resolve the uncertainty as to the concern of TPO inhibition and well complements the available assays/data reported in ToxCast. The methodology proposed by the applicant would likely resolve the divergence observed in the outcomes between GUA‐TPO and AUR‐TPO by providing direct evidence via analytical detection of monoiodotyrosine (MIT). The proposed control/reference chemicals to evaluate the success of the assay are considered appropriate. EFSA also agrees on the remaining in vitro assays proposed by the applicant 12 to explore the additional MIEs/KEs.
Before concluding on the applicant's proposal on how to further investigate the T‐modality, EFSA, in line with the mandate, consulted the RMS France, and the following is a summary of the feedback received by EFSA:
In the database available for acibenzolar‐S‐methyl, adverse outcome was reported in the developmental neurotoxicity study (morphometric changes in the cerebellum, increased auditory startle amplitude) and could be attributed to an endocrine mode of action made of Thyroid Hormones variations and impaired thyroid homeostasis. In vitro, positive outcomes were observed in in vitro neurotoxicity data package and a strong positive result was noted for TPO inhibition in one of the two assays available in the ToxCast database. Based on these considerations, it was agreed during the Pesticide Peer Review Expert meeting 13 to request a CTA, in agreement with the advice of the EFSA ED Working Group in April 2021.
The proposal of the applicant to investigate only a selection of several MIE and/or KE associated with thyroid‐disrupting mode of actions is acknowledged and would give insights to the MoA associated with DNT effects but may also be regarded as insufficient. If these in vitro assays were negative, the RMS would consider that the level of uncertainty would remain too high to conclude that acibenzolar‐S‐methyl is not an endocrine disruptor for T‐modality. Investigating only some MIEs is not comprehensive enough with regard to the entire AOP published by Noyes et al., 2019 . This AOP acknowledges the existence of other important KEs. Moreover, interpretation of such in vitro data, which are not internationally validated for the time being, may be a regulatory limitation. None of the proposed assays are validated and focusing only on upstream parts of the AOP introduces a bias and would result in an overall dataset that does not consider the most important KE. Indeed, this KE of decreased thyroid hormone levels is the common and major key event, which triggers the need for investigation. It is therefore deemed that investigation of hormone levels in sensitive population using the CTA developed by US EPA would lead to lesser uncertainties than investigation of each individual MIE as proposed. The resulting data would be more comprehensive and fit much better to the available AOP. Furthermore, even if each MIE taken individually were not able to induce TH level changes and subsequent adverse outcome, the sum of the activities of all the MIEs could lead to THs interference. Based on thyroid physiology, the proposed investigations are not likely to capture feedback loops, which are well known to occur in all mammalian species. Without this investigation, RMS France considers that the level of biological plausibility would be hindered by the absence of suitable supporting data. The available AOP is not linear, THs variation is one of the common KEs and therefore ignoring this KE appears not to be justified. Overall, the RMS considers that the CTA assay remains the most appropriate test to reduce the level of uncertainties. This is also in line with the EFSA/ECHA ED Guidance.
Overall, EFSA agrees with the proposal to conduct additional in vitro assays exploring MIEs/KEs relevant for the assessment of the T‐modality and EFSA acknowledges that currently there is not sufficient experience for testing for further MIEs, not included in the applicant's proposal, of interest for the T‐modality. The proposed testing battery is indeed expected to be a critical step for understanding the MoA linking the endocrine activity to the adverse outcome, if any. EFSA is however considering the feedback from the RMS France and the regulatory implication thereof, i.e. to allow unequivocally concluding on the T‐modality and is therefore recommending the execution of the CTA study as in the confirmatory data Conclusion (EFSA, 2021). EFSA considers the timeframe proposed by the applicant as sufficient to complete the study package also with the inclusion of the CTA study.
2.1.2. Testing strategy for the EAS‐modalities
EFSA notes that the assays proposed by the applicant (OECD TG 458, 441, 456 and OPPTS 890.1200) are in line with the testing strategy recommended by EFSA in 2021 (EFSA, 2021) to conclude on the potential ED properties of acibenzolar‐S‐methyl for the EAS‐modalities. However, the strategy proposed by the applicant does not include the triggering of a level 5 study, i.e. preferably the OECD TG 443 study with the inclusion of the cohort 1b, if any of the level 2 and/or 3 assays would result to be positive. Because the execution of a level 5 study is time demanding and is likely that an additional DRF study in pregnant rat is necessary, the additional time needed for the execution of these studies should eventually be considered in the testing strategy and associated timelines. The timeframe proposed by the applicant is considered sufficient to also cover the execution of the OECD TG 443 study if the outcome of the level 2 and/or 3 studies performed to evaluate the EAS‐modalities would result to be positive.
2.2. EFSA considerations on the testing strategy and timelines for data generation for the ED assessment for non‐target organisms
2.2.1. Testing strategy for the T‐modality
As reported in Table 1, in order to sufficiently investigate the endocrine activity for non‐mammalian species through the T‐modality, the applicant conducted a test in line with OECD TG 231 (AMA). This test was in line with the testing strategy recommended by EFSA in 2021 (EFSA, 2021) and in accordance with the ECHA/EFSA ED guidance (ECHA/EFSA, 2018). Based on preliminary results, in the AMA, some changes in T‐mediated parameters (according to the applicant's preliminary summary decreased normalised hind‐limb length at the 2 highest tested concentrations and some changes in the thyroid histopathology at the top concentration) were observed. According to the ECHA/EFSA ED guidance and the flowchart reported in Figure 1 of the ED guidance, if level 3 tests are positive, a MoA should be postulated and the need for further data to investigate adversity should be considered, i.e. a LAGDA according to OECD TG 241 should be performed. In the case of acibenzolar‐S‐methyl and based on the preliminary results of the AMA, the applicant suggested an anti‐thyroidal MoA. In order to understand whether the effects observed in the AMA may be relevant at the level of the population, the applicant proposed to further perform an extended AMA according to a non‐standardised protocol proposed by Ortego et al. (2021). This latter proposed to extend the AMA until animals reach NF (Nieuwkoop and Faber, 1994) stage 62, instead of stopping after 21 days of exposure (see Figure 1 below). The proposed protocol, therefore, recommends a fixed stage protocol instead of fixed time one and, according to the applicant, would allow to better understand whether the changes observed in the available AMA are relevant for amphibian populations.
Figure 1.
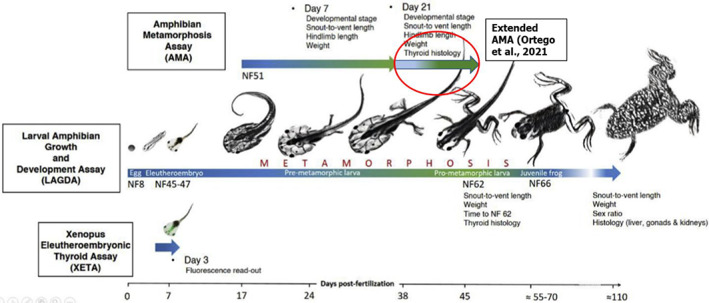
Duration of exposure and life stages covered in the available protocol with Xenopus spp. (Adapted from Couderq et al., 2020)
Table 2 below gives an overview of all the biological endpoints measured in the three available protocols with amphibians (i.e. AMA, extended AMA and LAGDA). In terms of biological endpoints measured, the extended AMA differs from the standard AMA as the animals are exposed until they reach stage NF 62 and not for a fixed number of days, i.e. 21 days. As a consequence, the extended AMA foresees the investigation of time to reach NF stage 62, while in the standard AMA, the developmental stage after 21 days is measured. This measured parameter in the extended AMA may allow employing alternative statistical tools (USEPA, 2013a,b; Haselman et al., 2016; Ortego et al., 2021) which might be increasingly informative or powerful. The animals are exposed from stage NF 51 to 57 (21 days) in the case of the AMA, and from stage NF 51 to stage NF 62 (until 95% of the surviving tadpoles reached NF stage 62 or up to 45 days post‐fertilisation, whichever occurred first) in the extended AMA. Therefore, the difference in the exposure duration between an AMA and an extended one is unlikely to lead to different results. Moreover, in both cases, three concentrations are used making the two tests not suitable for endpoint derivation and use in risk assessment. The LAGDA also foresees the measurement of a time to event parameter, i.e. time to reach NF 62 and therefore to this respect there is no valid justification on the choice of one test (extended AMA) over the other (LAGDA). In both protocols, thyroid histopathology is assessed in animals reaching stage NF 62. The LAGDA foresees the use of at least 4 concentrations and the animals are exposed for 16 weeks overall, although the assessment of T‐mediated parameters is only performed at the interim sampling, i.e. when animals reach stage NF 62. Moreover, in the LAGDA, after subsamples reaching NF stage 62 are collected, the remaining animals are exposed continuously until all frogs reached NF stage 66, i.e. completion of metamorphosis. These three aspects, i.e. time‐to‐event endpoint, duration of exposure and experimental design make the test more suitable for confirming whether an effect may be relevant at the level of population. Additionally, the LAGDA also allows to better understand and contextualise potential change in T‐mediated parameters in the weight of evidence as, due to the inclusion of parameters like liver and kidney histopathology and liver somatic index, systemic toxicity and target organ toxicity may be better characterised.
Table 2.
Biological endpoints measured in the different tests with amphibians. The X marked with a red circle indicates when the most relevant T‐mediated parameters (developmental stage/time to reach NF 62 and thyroid histology) are measured in the different protocols

In conclusion, although the extended AMA may present some added values when compared to a standard AMA, such as the inclusion of a time‐to‐event parameter, it is not clear how it contributes to a MoA, when an AMA is already available and positive. For example, based on its protocol and the length of exposure, it is questionable whether an extended AMA may be considered a more relevant test to assess potential adversity at the level of the population.
Although it is acknowledged that the validation of the LAGDA was limited to few chemicals (USEPA, 2013c) (only one substance known to act through multiple ED MoA including a T‐MoA), it is noted that the extended AMA suggested by Ortego et al. (2021) is a non‐standardised protocol as well, when comparing it to the existing AMA. In both cases the reproducibility of the results by different laboratories is a source of uncertainty.
Overall, based on the available evidence and in order to confirm whether the effects observed are relevant at the population level, a LAGDA is considered more fit for purpose.
The timelines as proposed (see Table 1) are considered to fit with the testing strategy proposed. However, if a LAGDA is performed instead of an extended AMA, 27 months are considered to be needed.
2.2.2. Testing strategy for the EAS‐modalities
With regard to EAS‐modalities, the applicant performed a FSTRA (OECD, 2012) which is in line with the testing strategy suggested in EFSA (2021). Interim results were already provided, and the final report was reported to be ready in December 2022 (see Table 1). Based on interim results, the applicant considered the FSTRA overall negative for endocrine activity as the effects observed were attributed to systemic toxicity. However, since the full report was not available and therefore full details could not be assessed, if positive evidence for EAS‐mediated endocrine activity is found in the level 3 study, a MoA should be postulated and additional testing might be needed, i.e. a test in line with OECD TG 240 (MEOGRT) (OECD, 2015) to further investigate adversity. In such case, the timeline proposed shall account for the additional testing and be extended up to 28 months.
3. Conclusion
As regards the testing strategy to complete the assessment for the ED properties of acibenzolar‐S‐methyl for the EAS‐modalities for mammals, EFSA agrees with the assays and associated timelines proposed by the applicant; though, the testing strategy and timelines should include the possibility that a level 5 OECD TG 443 study may be needed if any proposed level 2 and/or level 3 study would result to be positive. EFSA noted that the applicant's proposal, with the inclusion of the OECD TG 443 study, is in line with the EFSA/ECHA ED guidance and with the previous EFSA recommendations (EFSA, 2021). Regarding the T‐modality, EFSA agrees with the assays and timelines proposed by the applicant but the testing strategy should include the execution of a CTA study as proposed by EFSA (2021). This is in line with the previous EFSA recommendations and with the Appendix A of the EFSA/ECHA ED guidance. The level 5 study, i.e. OECD TG 443 study will be not necessary if the studies proposed by the applicant assessing in vitro and in vivo endocrine activity for the EAS‐modalities will result to be unequivocally negative. This is not applicable for the T‐modality and the CTA study is considered necessary to unequivocally conclude on the T‐modality. The timelines proposed by the applicant are considered acceptable to also cover the execution of the CTA study and of the in vitro mechanistic studies.
The proposal to deviate from the testing strategy for non‐mammalian species for the T‐modality (i.e. extended AMA according to Ortego et al., 2021 instead of a LAGDA according to OECD TG 241) as proposed in the ECHA/EFSA ED guidance, in case of positive evidence for endocrine activity, as indicated in the interim results of the available AMA, does not seem fully justified. A comparison of the available test methods in terms of, e.g. duration of exposure, parameters measured has been presented by EFSA. Although the extended AMA may present some added values when compared to a standard AMA, such as the inclusion of a time‐to‐event parameter, it is not clear how it contributes to a MoA, when an AMA is already available and positive. For example, based on its protocol and the length of exposure, it is questionable whether an extended AMA may be considered a more relevant test to assess potential adversity at the level of the population. Therefore, overall, a LAGDA is considered more fit for purpose. The timelines as indicated may be suitable if an extended AMA is conducted however, if a LAGDA is performed, up to 27 months are needed. For the EAS‐modalities, the testing strategy and proposed timeline are considered adequate. However, if positive evidence for EAS‐mediated endocrine activity is found in the level 3 study, a MoA should be postulated and additional testing might be needed, i.e. a test in line with OECD TG 240 (MEOGRT) to further investigate adversity. In such case, the timeline proposed shall account for the additional testing and be extended up to 28 months.
Abbreviations
- AMA
amphibian metamorphosis assay
- AOP
adverse outcome pathway
- CTA
Comparative Thyroid Assay
- DIO
iodothyronine deiodinases
- DNT
developmental neurotoxicity
- EAS
oestrogen, androgen and steroidogenesis modalities
- ECHA
European Chemicals Agency
- ED
endocrine disrupting
- EEC
European Economic Community
- ERSTTA
stably transfected human oestrogen receptor‐alpha transcriptional activation assay
- FSTRA
fish short‐term reproduction assay
- GD
guidance
- KE
key events
- LAGDA
larval amphibian growth and development assay
- MEOGRT
Medaka Extended One‐Generation Reproduction Test
- MIE
molecular initiating events
- MIT
monoiodotyrosine
- MOA
mode of action
- OECD
Organisation for Economic Co‐operation and Development
- S
Svedberg, S (10−13 s)
- TC
technical material
- TG
test guideline
- TH
thyroid hormone
- TPO
thyroid peroxidase
- TSH
thyroid‐stimulating hormone (thyrotropin)
- WHO
World Health Organization
Appendix A – Acibenzolar‐S‐methyl: Applicant comments to Article 21 review of approval in accordance with Regulation (EC) No 1107/2009 – Submission date: 6 October 2022
1.
Appendix A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2023.7968.
Supporting information
Acibenzolar‐S‐methyl: Applicant comments to Article 21 review of approval in accordance with Regulation (EC) No 1107/2009 –Submission date: 6 October 2022
Suggested citation: EFSA (European Food Safety Authority) , 2023. Statement concerning the testing strategy and timelines proposed by the applicant for the assessment of the endocrine disruption properties of acibenzolar‐S‐methyl in the context of the review of the approval of the active substance. EFSA Journal 2023;21(4):7968, 16 pp. 10.2903/j.efsa.2023.7968
Requestor: European Commission
Question number: EFSA‐Q‐2023‐00031
Declarations of interest: If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Adopted: 22 March 2023
Notes
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, pp. 1–50.
Commission Implementing Regulation (EU) 2016/389 of 17 March 2016 renewing the approval of the active substance acibenzolar‐S‐methyl in accordance with regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011. OJ L 73, 18.3.2016, pp. 77–80.
Commission Regulation (EU) 2018/605 of 19 April 2018 amending Annex II to Regulation (EC) No 1107/2009 by setting out scientific criteria for the determination of endocrine disrupting properties. OJ L 101, 20.4.2018, pp. 33–36.
Guidance for Thyroid Assays in Pregnant Animals, Fetuses and Postnatal Animals, and Adult Animals. October 24, 2005. Available online: https://www.epa.gov/sites/production/files/2015-06/documents/thyroid_guidance_assay.pdf.
With regard to the iodine content in rodent diets for use in the comparative thyroid assay (CTA), the following corrigendum is made: the correct expression is 5 μg of Iodine as daily intake. This is based on the assumption that the average food consumption is of ~20 g/day, and that 225 μg of iodine/kg of chow is sufficient. This corresponds to 4.15 μg iodine/day based on daily food intake. The 1,000 μg/g (1 mg/kg or 1 ppm) is the typical rat chow.
For humans, the endocrine activity for the E modality was sufficiently investigated and negative. The ER ToxCast model was available. Therefore, the E modality needs only to be further investigated for non‐target organisms other than mammals. For wild mammals as non‐target organisms, the same conclusion for the E modalities as the one for humans applies.
It is noted that with evolving experience EFSA now strongly recommends the OECD TG 443 study.
i.e. in vitro assessment of inhibition of thyroid peroxidase activity (TPO), in vitro assessment of inhibition of sodium‐iodide symporter activity (NIS), in vitro assessment of inhibition of the deiodinase enzyme (DIO 1,2,3), in vitro comparative liver metabolism induction.
Sodium‐chloride cotransporter Amplex UltraRed‐thyroperoxidase assay.
AC50: Active Concentration 50 (concentration at which 50% of maximum activity is observed)
Guaiacol oxidation thyroperoxidase assay.
i.e. in vitro assessment of inhibition of thyroid peroxidase activity (TPO), in vitro assessment of inhibition of sodium‐iodide symporter activity (NIS), in vitro assessment of inhibition of the deiodinase enzyme (DIO 1,2,3), in vitro comparative liver metabolism induction.
Pesticide Peer Review TC 48 (13–16 April 2021).
References
- Blum J, Masjosthusmann S, Bartmann K, Bendt F, Dolde X, Dönmez A, Förster N, Holzer AK, Hübenthal U, Keßel HE, Kilic S, Klose J, Pahl M, Stürzl L‐C, Mangas I, Terron A, Crofton KM, Scholze M, Mosig A, Leist M and Fritsche E, 2023. Establishment of a human cell‐based in vitro battery to assess developmental neurotoxicity hazard of chemicals, Chemosphere, 311(Part 2), 137035. 10.1016/j.chemosphere.2022.137035 [DOI] [PubMed] [Google Scholar]
- Couderq S, Leemans M and Fini JB, 2020. Testing for thyroid hormone disruptors, a review of non‐mammalian in vivo models. Molecular and Cellular Endocrinology, 508, 110779. [DOI] [PubMed] [Google Scholar]
- ECHA (European Chemicals Agency) and EFSA (European Food Safety Authority) with the technical support of the Joint Research Centre (JRC) , Andersson N, Arena M, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Parra Morte JM, Pellizzato F, Tarazona J, Terron A and Van der Linden S, 2018. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal 2018;16(6):5311,135 pp. 10.2903/j.efsa.2018.5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2010. Application of systematic review methodology to food and feed safety assessments to support decision making. EFSA Journal 2010;8(6):1637, 90 pp. 10.2903/j.efsa.2010.1637 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2014. Conclusion on the peer review of the pesticide risk assessment of the active substance acibenzolar‐S‐methyl. EFSA Journal 2014;12(8):3691, 73 pp. 10.2903/j.efsa.2014.3691 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2020a. Technical report on the outcome of the consultation with Member States, the applicant and EFSA on the pesticide risk assessment for acibenzolar‐S‐methyl in light of confirmatory data. EFSA supporting publication 2020:EN‐1846, 29 pp. 10.2903/sp.efsa.2020.EN-1846 [DOI]
- EFSA (European Food Safety Authority) , 2020b. Technical report on the outcome of the pesticides peer review meeting on general recurring issues in mammalian toxicology. EFSA supporting publication 2020:EN‐1837, 26 pp. 10.2903/sp.efsa.2020.EN-1837 [DOI]
- EFSA (European Food Safety Authority) , Alvarez F, Arena M, Auteri D, Borroto J, Castoldi AF, Chiusolo A, Colagiorgi A, Colas M, Crivellente F, De Lentdecker C, Ferilli F, Ippolito A, IstaceF, Kardassi D , Kienzler A, Linguadoca A, Mangas I, Molnar T, Parra Morte JM, Sharp R, Szentes C, Terron A, Tiramani M and Villamar‐Bouza L, 2021. Conclusion on the peer review of the pesticide risk assessment for the active substance acibenzolar‐S‐methyl in light of confirmatory data submitted. EFSA Journal 2021;19(7):6687, 12 pp. 10.2903/j.efsa.2021.6687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2023. Peer Review Report to the EFSA Statement concerning the testing strategy and timelines proposed by the applicant for the assessment of the endocrine disruption properties of acibenzolar‐S‐methyl in the context of the review of the approval of the active substance. Available online: www.efsa.europa.eu [DOI] [PMC free article] [PubMed]
- European Commission , 2013. Guidance document on the procedures for submission and assessment of confirmatory information following approval of an active substance in accordance with Regulation (EC) No 1107/2009. SANCO 5634/2009‐rev. 6.1. www.efsa.europa.eu/publications
- Haselman JT, Kosian PA, Korte JJ, Olmstead AW, Iguchi T, Johnson RD and Degitz SJ, 2016. Development of the Larval Amphibian Growth and Development Assay: effects of chronic 4‐tert‐octylphenol or 17β‐trenbolone exposure in Xenopus laevis from embryo to juvenile. Journal of Applied Toxicology, 36(12), 1639–1650. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD and Faber J, 1994. Normal Table of Xenopus laevis. Normal Table of Xenopus laevis (Daudin): A Systematical & Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis. 1st edn. Garland Science. 10.1201/9781003064565 [DOI] [Google Scholar]
- Noyes PD, Friedman KP, Browne P, Haselman JT, Gilbert ME, Hornung MW, Barone S Jr, Crofton KM, Laws SC, Stoker TE, Simmons SO, Tietge JE and Degitz SJ, 2019. Evaluating chemicals for thyroid disruption: opportunities and challenges with in vitro testing and adverse outcome pathway approaches. Environmental Health Perspectives, 127(9), 95001. 10.1289/EHP5297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) , 2012. Test no. 229: fish short term reproduction assay, OECD guidelines for the testing of chemicals, section 2. OECD Publishing, Paris. 10.1787/9789264185265-en [DOI]
- OECD (Organisation for Economic Co‐operation and Development) , 2015. Test no. 240: Medaka Extended One Generation Reproduction Test (MEOGRT), OECD guidelines for the testing of chemicals, section 2. OECD Publishing, Paris 10.1787/9789264242258-en [DOI]
- Ortego LS, Olmstead AW, Weltje L, Wheeler JR, Bone AJ, Coady KK, Banman CS, Burden N and Lagadic L, 2021. The extended amphibian metamorphosis assay: a thyroid‐specific and less animal‐intensive alternative to the larval amphibian growth and development assay. Environmental Toxicology and Chemistry, 40(8), 2135–2144. 10.1002/etc.5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA (US Environmental Protection Agency) , 2013a. Scientific review of the Endocrine Disruptor Screening Program (EDSP); tier 1 screening assay and battery performance. EPA‐HQ‐OPP‐2013‐0075‐0023. Washington, DC.
- USEPA (US Environmental Protection Agency) , 2013b. Endocrine Disruptor Screening Program SAP review of EDSP tier 1 screening: Assay and battery performance. EPA‐HQ‐OPP‐2013‐0075‐0003. Washington, DC.
- USEPA (US Environmental Protection Agency) , 2013c. Validation of the larval amphibian growth and development assay: integrated summary report.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Acibenzolar‐S‐methyl: Applicant comments to Article 21 review of approval in accordance with Regulation (EC) No 1107/2009 –Submission date: 6 October 2022


