Abstract
Sepsis is defined as infection with organ dysfunction due to a dysregulated immune response. The lung is one of the most vulnerable organs at the onset of sepsis. Interleukin (IL)-33 can be released by injured epithelial and endothelial cells in the lung and regulate immune activation and infiltration. Therefore, we postulated that IL-33 would contribute to the immune response in the lung during sepsis. Using the cecal ligation and puncture (CLP) sepsis model, we show here that IL-33 contributes significantly to both sepsis-induced inflammation in the lung and systemic inflammatory response in the early phase of sepsis. Despite the higher intra-peritoneal bacterial burden, the absence of IL-33 resulted in less infiltration of neutrophils and monocytes into the lungs in association with lower circulating, lung and liver cytokine levels as well as reduced lung injury at 6 h after sepsis. IL-33 was required for the upregulation of IL-5 in type 2 Innate Lymphoid Cells (ILC2), while IL-5 neutralization suppressed neutrophil and monocyte infiltration in the lungs during CLP sepsis. This reduction in leukocyte infiltration in IL-33-deficient mice was reversed by administration of recombinant IL-5. These results indicate that IL-33 plays a major role in the local inflammatory changes in the lung, in part, by regulating IL-5 and this axis contributes to lung injury early after the onset of sepsis.
Keywords: interleukin-33, lung, sepsis
INTRODUCTION
Sepsis is defined as infection with organ dysfunction1 and is a leading cause of mortality in Intensive Care Units worldwide.1,2 The ultimate cause of death is due to secondary development of life-threatening organ dysfunction, which is thought to result, in part, from a dysregulated inflammatory response.3 The lung is one of the most vulnerable organs during sepsis.3,4 and acute lung injury develops early during the course of sepsis and is associated with high morbidity and increased mortality.4 The infiltration of neutrophils and production of proinflammatory and chemotactic molecules, in particular tumor necrosis factor-α (TNF-α) and interleukin (IL)-1β, play central roles in the process of sepsis-induced acute lung inflammation and acute lung injury.5–7 The release of TNF-α and IL-1β initiates and also amplifies inflammatory cascades via myeloid cell activation and the upregulation of a network of proinflammatory cytokines, lipid mediators, and reactive oxygen species, leading to vascular leak and tissue injury.8 Simultaneously, anti-inflammatory responses, in particular IL-10 production, are engaged to counteract the actions of proinflammatory cytokines and to restore immunological balance.8–10 Although these pathophysiological events are well documented, the events that initiate the inflammatory cascades are not clearly understood.
IL-33 is a member of the IL-1 family, and has recently emerged as a cytokine with diverse functions in infectious and inflammatory diseases.11–13 IL-33 is mainly expressed in barrier cells, including the epithelial and endothelial cells.13–15 Upon cell damage or tissue injury, IL-33 expression increases and is released into the extracellular space, to subsequently alarm the neighboring tissues by activating various target cells, including T cells, mast cells, eosinophils, type 2 Innate Lymphoid Cells (ILC2), dendritic cells, monocytes, macrophages, NKT cells and NK cells.14,16,17 These cells express the IL-33 receptor ST2 (also known as IL-1RL1), and respond to IL-33/ST2 signaling by producing cytokines and chemokines.14,18 However, IL-33 can activate multiple arms of the immune response. For example, IL-33 can promote both Th1 and Th2 immune responses depending on type of cells activated, the cytokine microenvironment and host immune context.18–20 In cecal ligation and puncture (CLP) sepsis, IL-33 is important for immune cell activation and bacterial clearance21 but its role in systemic and pulmonary inflammatory responses is not known.
IL-33 has been shown to be a potent activator of ILC2, resulting in enhanced production of the key effector cytokines IL-5 and IL-13, which are responsible for the development of allergic airway inflammation.22–24 ILC2 are a recently described subset of linage-/ST2+, GATA3-dependent lymphocytes identified in multiple barrier tissues,24 and are known to have important functions in allergic diseases,25 influenza virus infection26 and dermatitis.27 IL-33 can also contribute to lung protection through restoring airway epithelial integrity by stimulating ILC2 to produce amphiregulin.26 However, the roles of IL-33 in regulating early inflammatory responses in the lung in sepsis remain to be determined.
In this study, we tested the hypothesis that IL-33 is a regulator of lung inflammation in the early phases of sepsis. Using a model of polymicrobial intra-abdominal sepsis, we showed that IL-33 plays a central role in inducing lung injury within hours after the onset of severe sepsis. IL-33 was found to drive systemic inflammation, ILC2 IL-5 expression as well as neutrophil and monocyte infiltration and cytokine production in the lungs. Exogenous rIL-5 partially reversed the IL-33-deficient state in the lung response during sepsis. These findings identify IL-33 as a driver of systemic and pulmonary inflammation during intra-abdominal sepsis.
RESULTS
IL-33 levels increase rapidly in the plasma and lungs during CLP sepsis
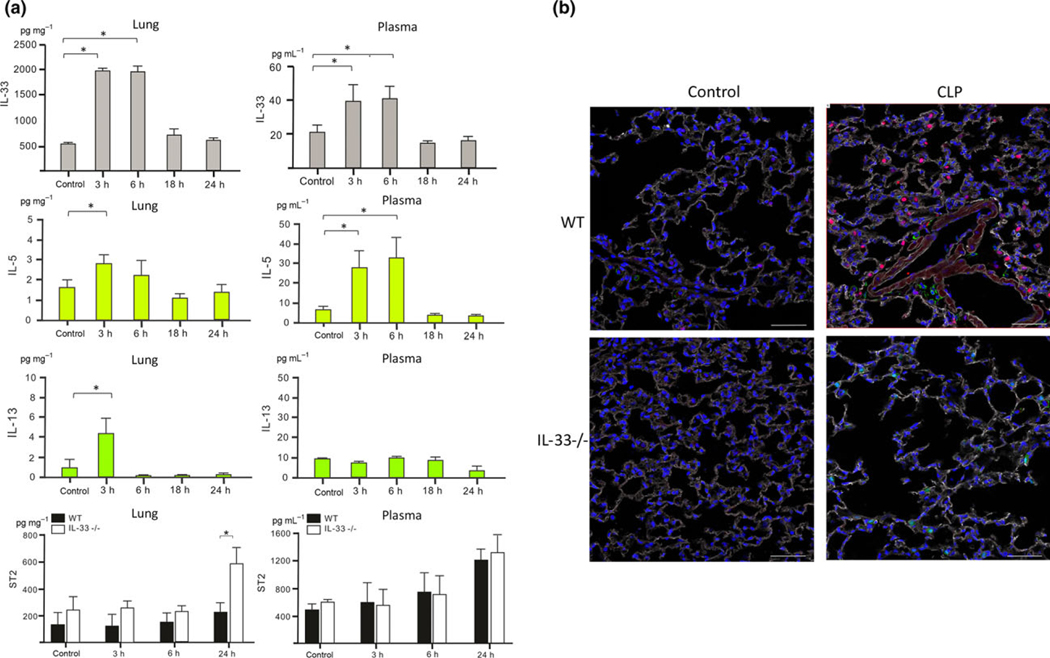
To determine the roles of IL-33 in local lung and systemic inflammatory responses during sepsis wild-type C57BL/6 (WT) and IL-33-deficient (IL-33−/−) mice were subjected to CLP sepsis. As shown in Figure 1a, IL-33 levels were markedly increased in the plasma and lungs as early as 3–6 h and returned to baseline levels by 18 h in WT mice after CLP. IL-33 levels corresponded with IL-5 levels in the plasma and lungs in a 0–24 h time frame after CLP, but not IL-13 levels (Figure 1a). Immunohistochemistry of the lung localized IL-33 to epithelial cells at 6 h after CLP (Figure 1b).
Figure 1.
IL-33 increased in the lung and in the circulation during sepsis. (a) IL-33, IL-5, IL-13 and ST2 protein levels in the plasma or in the lung at indicated time points after CLP. Results shown are mean ± s.d. of n = 5–8 mice per group. Duplicates were measured for each sample. Significance is defined as *P < 0.05. (b) Immunofluorescence staining for IL-33 in the lung tissue. Red: IL-33, White: Collagen-I, Green: CD45, Blue: DAPI. Scale bar = 50 μm. Unmanipulated mice serve as controls. Images shown are representative of n = 4 (WT), n = 4 (IL-33−/−) mice.
IL-33 promotes systemic inflammation
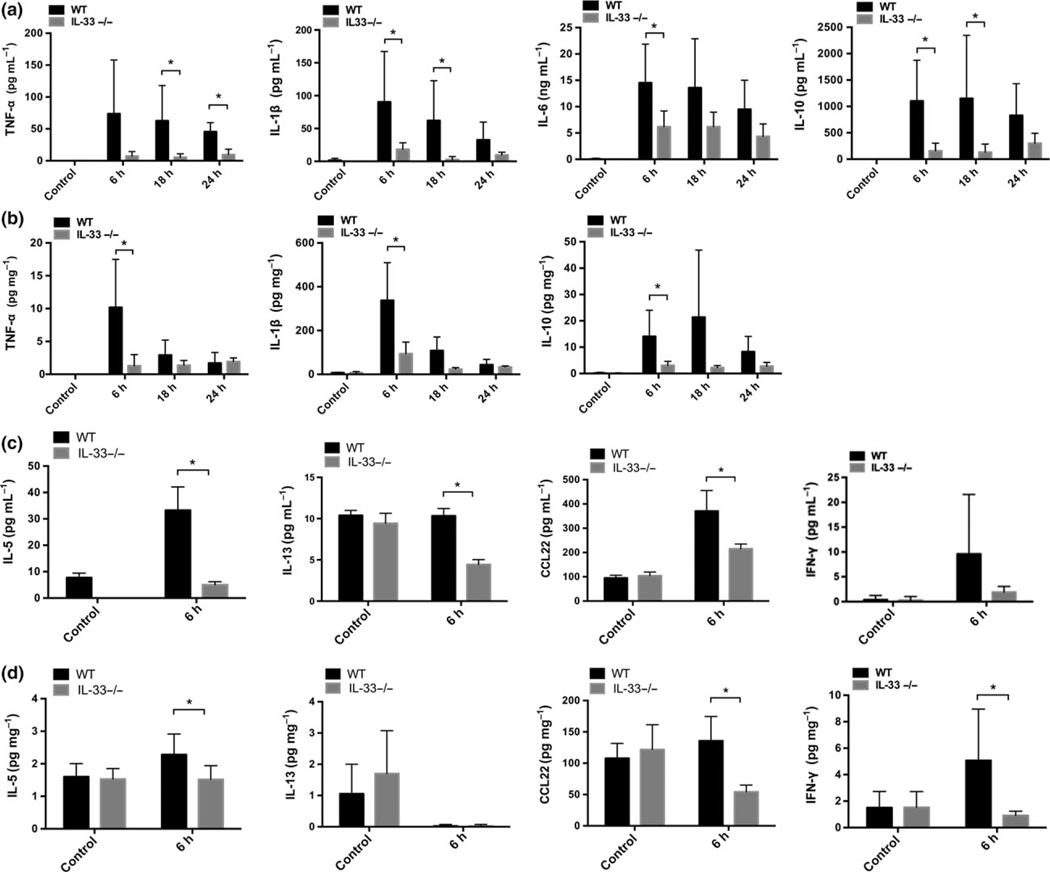
As expected, CLP lead to marked increases in TNF-α, IL-1β, IL-6 and IL-10 in the plasma (Figure 2a) and TNF-α, IL-1β and IL-10 in lung homogenates (Figure 2b) between 6 and 24 h. Levels of these cytokines were significantly lower in IL-33−/−mice across the first 24 h following CLP. IL-33 also promoted a Th2 response as we show that IL-33 deletion resulted in significantly reduced levels of IL-5, IL-13 and CCL-22 in the plasma (Figure 2c) and in lung homogenates (Figure 2d) at 6 h after CLP. Deletion of IL-33 also prevented CLP-induced increases in liver IL-1β, IL-10, IL-5, and CCL22 levels measured at 6 h (Supplementary figure 1). No increases in soluble ST2 (sST2) levels were detected in the lung in the first 24 h of -sepsis and plasma ST2 levels did not increase until 24 h following CLP in WT mice (Figure 1a). Interestingly, IL-33−/− mice subjected to CLP exhibited an increase in lung sST2 levels suggesting a negative IL-33-mediated feedback for sST2 expression or release. These time course dynamics implicate IL-33 in the early inflammatory pathways activated by CLP.
Figure 2.
IL-33 deletion resulted in reduced levels of circulating and pulmonary cytokines. TNF-α, IL-1β, IL-6 and IL-10 levels in the plasma (a) or in lung homogenates (b) of WT mice or IL-33−/− mice in a 0–24 h time frame after CLP. IL-5, IL-13, CCL-22 and IFN-γ levels in the plasma (c) or in lung homogenates (d) of WT mice or IL-33−/− mice at 6 h after CLP. Unmanipulated mice serve as controls. Data shown are mean ± s.d. of n = 5–8 mice per group. Duplicates were measured for each sample. *P < 0.05.
To address the role of IL-33 in the setting of controlled infection, we treated mice with antibiotics which results in efficient clearance of bacteria and markedly improved survival in this model.28 IL-33−/− mice showed significantly lower circulating levels of TNF-α and IL-10 as compared to WT mice at 18 h after CLP with antibiotics (Supplementary figure 2), indicating that even when the infection is controlled by antibiotics, IL-33 contributes to systemic inflammatory response during intra-abdominal infection.
IL-33 contributed to CLP sepsis-induced infiltration of neutrophils and monocytes into the lung
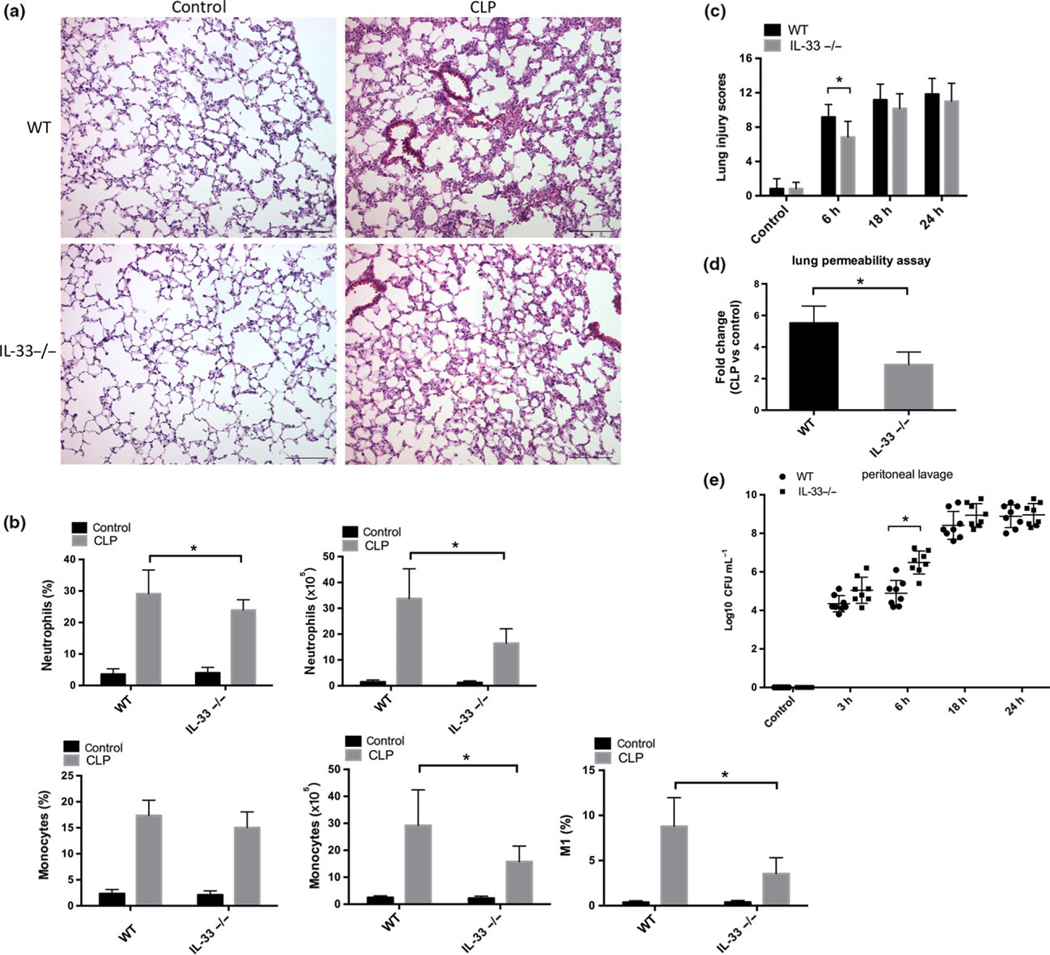
H&E staining revealed a robust influx of leukocytes into the interstitium and broncheoalveolar space in the lung of mice subjected to CLP (6 h) (Figure 3a). IL-33−/− mice displayed reduced leukocyte infiltration (Figure 3a). The average numbers of CD45+ cells per lung was higher in WT mice (2.86 × 107) than IL-33−/− mice (1.70 × 107) at 6 h after CLP. Leukocytes were isolated from the lungs as described in the methods and flow cytometry performed to identify leukocyte subsets (Supplementary figure 3) as described by Zaynagetdinov et al.29 Abdominal sepsis induced a sharp increase in both the percentages and numbers of the neutrophils and pulmonary monocytes (Figure 3b) in the lungs at 6 h. In comparison to the WT mice, IL-33−/− mice had significantly reduced percentages and numbers of neutrophils (P < 0.05) in the lung after CLP. Pulmonary monocytes (P < 0.05) were also lower in number without a significant effect on the percentage (Figure 3b) in IL-33−/− mice.
Figure 3.
IL-33 deletion ameliorated sepsis-induced lung injury and infiltration of neutrophil and monocyte into the lung. (a) Representative HE staining images of lung histopathology in unmanipulated mice (control) and septic mice at 6 h after CLP. Images are representative of n = 8 (WT), n = 8 (IL-33−/−) mice. (b) Percentages and numbers of neutrophils and pulmonary monocytes, and of M1 percentages of monocytes in the lung were assessed using flow cytometry. Data are presented as mean ± s.d. of n = 8–12 mice per group, *P < 0.05. (c) Scoring of lung histopathology in unmanipulated mice (control) and septic mice in a 0–24 h time frame after CLP. Data are presented as mean ± s.d. of n = 6 per group. Three different views were scored for each sample. *P < 0.05. (d) The changes in vascular permeability in lung were assessed by EBD leakage from blood into lung airways at 6 h after CLP. Data shown are mean ± s.d. of n = 7 mice per group, *P < 0.05. (e) Bacteria load in the peritoneum of WT mice or IL-33−/− mice at indicated time points after CLP. Data shown are mean ± s.d. Symbols represent individual mice, *P < 0.05.
To assess the macrophage subsets, we analyzed the expression of polarization markers within the F4/80+/CD11b+ cells, including intracellular inducible nitric oxide synthase (iNOS) for classical (M1) macrophages and CD206 (mannose receptor) surface expression for alternatively activated (M2) macrophages. We assessed the expression of Gr1 and CD11b within the CD206+ (or iNOS+) gate, and found that interstitial macrophages (IMs) represented the major source of CD206+ cells, while iNOS was mainly detected in pulmonary monocytes (Supplementary figure 3b). IL-33 deficiency resulted in a significantly reduced frequency of iNOS+ monocytes in the lung (Figure 3b) but did not affect the percentage of CD206+ IMs (data not shown). These results indicated that IL-33 promoted the recruitment of neutrophils and monocytes into the lung within hours of the onset of sepsis and contributed to a M1 polarizaiton in the pulmonary monocytes.
IL-33 deletion resulted in less lung injury in the presence of a greater bacterial burden at 6 h after the onset of sepsis
Lung injury was assessed over the first 24 h by calculating lung injury scores (Figure 3c). As expected CLP lead to an increase in lung injury scores at 6, 18 and 24 h that was significantly lower in the IL-33−/− mice at 6 h. To determine whether IL-33 also contributed to vascular leak, another hallmark of lung injury in sepsis, endothelial permeability was measured using Evans Blue Dye leakage. As shown in Figure 3d, IL-33 deletion prevented the sepsis-induced increase in pulmonary vascular permeability measured at 6 h. During polymicrobial sepsis, the efficiency of bacterial clearance determines the magnitude of inflammatory response and end organ injury.28 Others have shown that IL-33 is important for promoting bacterial clearance in the peritoneal cavity in CLP sepsis.21 We confirmed that IL-33−/− mice had higher peritoneal levels of bacteria and a trend toward higher counts in the blood and lung than their WT counterparts at 6 h (Figure 3e and Supplementary figure 4a). In line with bacterial clearance, IL-33 deletion resulted in a slight increase in mortality after CLP (Supplementary figure 4b). Thus, the reduction in systemic inflammatory cytokine levels (Figure 2) and pulmonary leukocyte infiltration and lung injury (Figure 3a–d) observed in IL-33−/− mice occurred in the presence of a higher bacterial burden (Figure 3e) in the early phase of CLP.
IL-33 contributed to sepsis-induced TNF-α, IL-1β and IFN-γ production from pulmonary neutrophils and monocytes
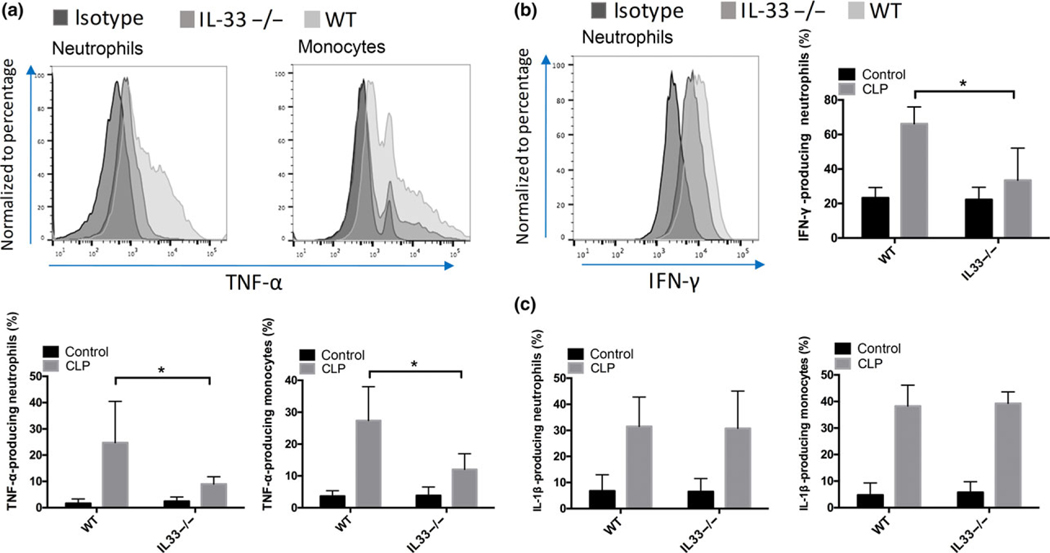
As described above, cytokine levels in lung homogenates were found to correspond to the circulating levels (Figure 2). IL-33−/− mice displayed significantly lower (P < 0.05) levels of TNF-α, IL-1β (Figure 2b) and IFN-γ (Figure 2d) in the lung after CLP than the WT mice at 6 h. To identify the cellular sources of proinflammatory and anti-inflammatory cytokines in the lungs, we assessed the expression of Gr1, CD11b and F4/80 within the TNF-α+ gate and found that TNF-α in the lung was produced by several cell subsets, including neutrophils, monocytes, IMs and alveolar macrophages (AMs) (data not shown). These four cell subsets were also found to express the pro-form of IL-1β. We found that Gr1high/CD11b+ neutrophils were the major cellular source of IFN-γ. In comparison to the WT mice, IL-33−/− mice showed lower expression of TNF-α (Figure 4a) and IFN-γ (Figure 4b) within pulmonary neutrophils, and reduced levels of TNF-α in pulmonary monocytes (Figure 4a). Deletion of IL-33 did not impact the expression of TNF-α within IMs and AMs at 6 h after CLP (data not shown).
Figure 4.
IL-33 contributes to sepsis-induced cytokine production in pulmonary neutrophils and monocytes at 6 h after CLP. Expressions of TNF-α (a), IFN-γ (b) and IL-1γ (c) in neutrophils or in pulmonary monocytes were detected by flow cytometry at 6 h after CLP. Representative histograms show the comparison of TNF-a expression between WT and IL-33−/− mice. Plots shown are representative of n = 6 (WT), n = 6 (IL-33−/−) mice. Percentages of TNF-α-producing neutrophils and monocytes in the lung were calculated by flow cytometry. Data are mean ± s.d. of n = 6 mice per group. *P < 0.05.
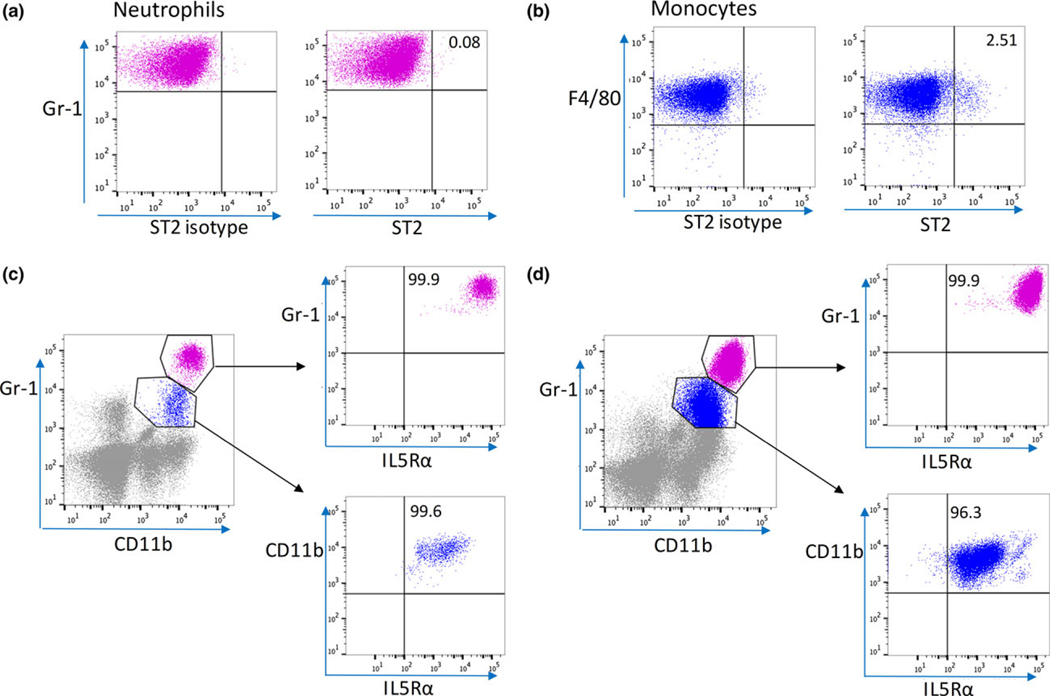
Pulmonary neutrophils were ST2-negative but highly expressed IL-5Ra
To investigate the mechanism by which IL-33 regulated the inflammatory response in the lungs, leukocytes isolated from lungs were analyzed by flow cytometry for ST2 (IL-33 receptor) expression. We also assessed the expression of the IL-5 receptor, IL-5α, since it is known that IL-33 can induce IL-5 production by ILC2.22–24 We found that the majority of pulmonary neutrophils were ST2-negative (Figure 5a), and only 2.5% of pulmonary monocytes were ST2-positive (Figure 5b). In contrast, 99.9% of the pulmonary neutrophils highly expressed IL-5Rα, and over 90% of pulmonary monocytes were intermediate positive for IL-5Rα, in both control mice (Figure 5c) and septic mice (Figure 5d). Other cell types that are known to express ST2 include eosinophils, mast cells and regulatory T cells.11,12 Using flow cytometry, we failed to find mast cells in the lungs, and observed that IL-33 deficiency had no impact on low percentages of eosinophils or Tregs in the lung tissue at 6 h after CLP (Supplementary figure 4c).
Figure 5.
Neutrophils and pulmonary monocytes express IL-5Ra in the lung at 6 h after CLP. Representative flow cytometry plots showing ST2 expressions (a, b) and IL-5Ra expressions (c, d) on neutrophils and pulmonary monocytes in the lung from WT mice at 6 h after CLP. Numbers in quadrants indicate percentages of cells positive for ST2 or IL-5Rα. Plots shown are representative of n = 5 (WT), n = 5 (IL-33−/−) mice.
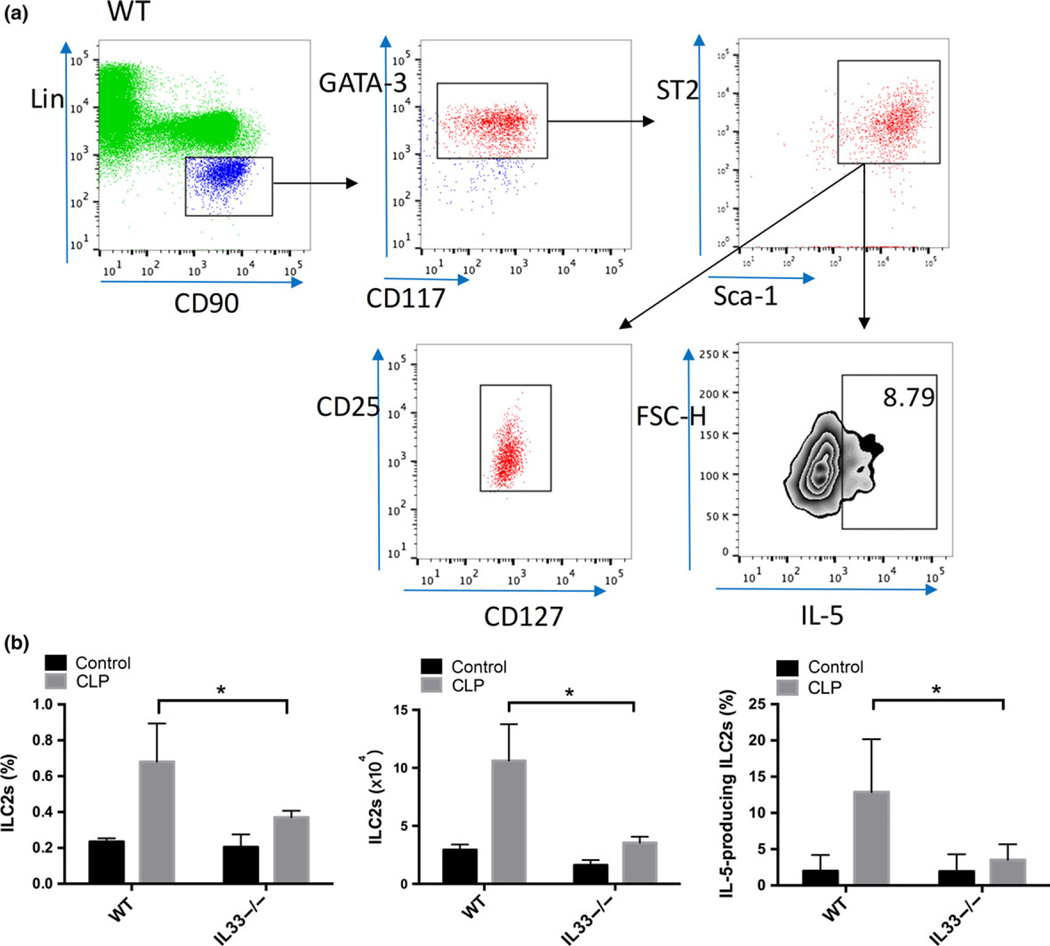
Sepsis-induced increases in lung ILC2 numbers and IL-5 production were IL-33-dependent
Using a combination of markers including CD45, lineage markers (Lin, including CD11b, CD3e, CD45R, NK1.1), CD90.2, ST2, sca-1, GATA-3, CD117(c-Kit), CD127 and CD25,24,25 we identified a population of CD45+/Lin−/CD90.2+/ST2+/sca-1+/GATA-3+/CD117+/CD127+/CD25+ cells as ILC2 (Figure 6a). These cells were strongly positive for ST2 and represented about 0.2% of the total leukocytes in the lung at baseline (Figure 6b). Sepsis induced a rapid increase in the prevalence of ILC2 (P < 0.05) in lungs of WT mice at 6 h after CLP (Figure 6b). These ILC2 had enhanced expression of IL-5 (P < 0.05) (Figure 6a, b) and this was accompanied by a significant increase in circulating IL-5 levels (P < 0.05) in the plasma (Figure 2c) and in lung (Figure 2d). Both the increase in ILC2 frequency in the lung and upregulation of IL-5 in the ILC2 population were significantly lower in IL-33−/− mice (P < 0.05) than that observed in WT mice at 6 h after CLP (Figure 6b). IL-33 deficiency also prevented the increase in IL-5 levels in the plasma (Figure 2c) and in lung (Figure 2d) in this early phase of sepsis.
Figure 6.
Sepsis-induced increases in lung ILC2 numbers and IL-5 production are IL-33-dependent. Flow cytometry plots showing populations of ILC2 in the lungs of WT mice (a) at 6 h after CLP. After gating on the viable CD45+/Lin−/CD90.2+/CD117+/GATA-3+/sca-1+/ST2+ leukocyte subset, it revealed that this cell population was also positive for CD127 and CD25, and thus can be identified as ILC2. Intracellular IL-5 was detected within the ILC2 subsets. Numbers in the gate indicates percentage of IL-5-producing ILC2. Plots shown are representative of n = 8 (WT), n = 8 (IL-33−/−) mice. (b) Percentages and numbers of ILC2s and IL-5-producing ILC2s in the lung at 6 h after CLP are shown. Data are mean ± s.d. of n = 6 mice per group, *P < 0.05.
We also measured the plasma levels of IL-13, which is known to be produced by ILC2,23,30 but found no significant difference (P > 0.05) between the control and septic WT mice at 6 h after CLP (Figure 2c). The pulmonary levels of IL-13 were very low at 6 h following CLP in both strains (Figure 2d). At 6 h after CLP, IL-33−/− mice had plasma IL-13 levels even lower (P < 0.05) than the baseline levels, indicating that IL-33 may play a role in maintaining IL-13 expression during sepsis (Figure 2c).
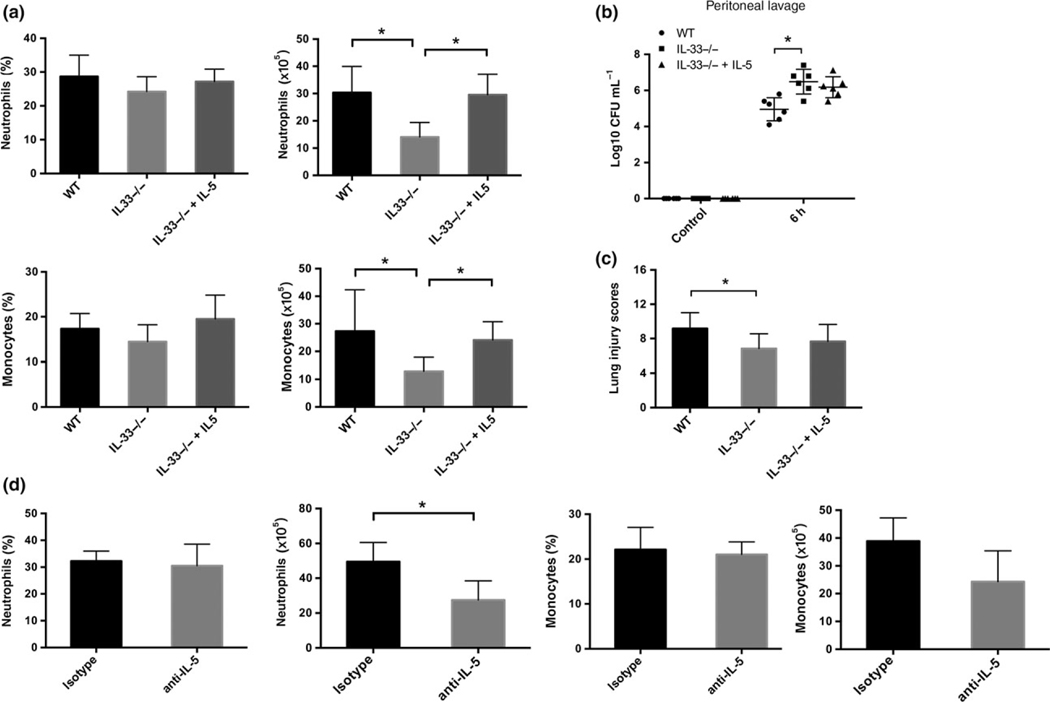
IL-5, but not ILC2, plays a role in IL-33-induced neutrophil and monocyte influx into the lungs in sepsis
Our observation that IL-5 expression in the lung is IL-33-dependent during CLP suggests that the effects of IL-33 on lung inflammation could be mediated partially through IL-5. Treatment of IL-33−/− mice with intravenous rIL-5 (50 μg kg−1) at the time of CLP increased recruitment of neutrophils and monocytes into the lung to levels similar to that observed in WT mice (Figure 7a). Administration of rIL-5 did not impact the peritoneal bacterial burden (Figure 7b) or the lung injury score (Figure 7c) in IL33−/− mice at 6 h after CLP. Pretreatment of WT mice with anti-IL-5 neutralizing antibodies (8 mg kg−1) resulted in reduced numbers of pulmonary neutrophils and monocytes (Figure 7d). Taken together, these data raise the possibility that IL-33 stimulates ILC2 to produce IL-5 that then participates in leukocyte recruitment and activation in the lung within hours of the onset of intra-abdominal sepsis. To test whether ILC2 deletion prevents lung injury following CLP sepsis, mice lacking ILC2 were generated through adoptive transfer of Rora+/+ or Rora−/− bone marrow into lethally irradiated WT mice as described in the methods. We have previously shown that this approach leads to complete irradication of ILC2 in the lungs.31 There were no significant differences in the plasma or lung levels of TNF-α or IL-1β, the frequency of neutrophils or monocytes in lung leukocytes, or lung injury scores in ILC2-deficient mice as compared with the bone marrow transplant control WT (Rora+/+) mice at 6 h after CLP (Supplementary figure 5). Thus, ILC2 alone do not account for the IL-33-dependent early lung injury and inflammation during abdominal sepsis.
Figure 7.
Exogenous rIL-5 partially reverses the suppressed inflammatory cell infiltration in the lung of IL-33/ mice in sepsis. (a) IL-33−/− mice were given rIL-5 or PBS i.v. at the onset of CLP. Experiments were terminated at 6 h. Total cell numbers were calculated and percentages of pulmonary neutrophils and monocytes were analyzed by flow cytometry. Bacteria load (b) in the peritoneum was examined and scoring of lung histopathology (c) was performed at 6 h after CLP. (d) WT mice were given anti-mouse IL-5 or isotype control i.v. at the onset of CLP. Lung samples were harvested at 6 h for flow cytometry. WT: wild-type mice, IL-33−/−: PBS-treated IL-33−/− mice, IL-33−/−+ IL-5: rIL-5-treated IL-33−/− mice, anti-IL-5: anti-IL-5-treated WT mice, isotype: isotype-treated WT mice. n = 6 mice per group. *P < 0.05.
DISCUSSION
The activation and recruitment of inflammatory cells such as neutrophils and monocytes into the lungs is a feature of pulmonary inflammatory processes associated with acute lung injury.7,8 We assessed the role of IL-33 in these early events in a clinically relevant model of polymicrobial intra-abdominal sepsis. While we confirmed that IL-33 participated in promoting bacterial clearance in the peritoneal cavity, our results established that the early upregulation of IL-33 has the adverse consequence of increasing inflammation-associated lung injury in the early phase of sepsis. These events occurred prior to increases in sST2, which would be expected to limit the extracellular actions of IL-33 and may involve the early upregulation of IL-5. These results established a dominant role for IL-33 in the initial lung and systemic inflammation response in sepsis and double-edged sword actions of IL-33, that is more efficient bacterial clearance at the cost of worse lung injury.
Previous studies have linked IL-33 signaling with LPS-induced lung injury.32,33 For example, overexpression of sST2 (a decoy receptor for IL-33) resulted in local inhibition of IL-33 signaling and reduced IL-1β and IFN-γ levels in the lungs of LPS-treated mice, leading to reduced inflammatory cell infiltration and vascular leakage.32 sST2 gene transfer has been shown to provide a protective effect on LPS-induced acute lung injury by repressing inflammatory cell infiltration, alveolar hemorrhage and proinflammatory cytokines.33 Recent studies, using administration of recombinant protein28 or overexpression of the IL-33 gene,34,35 demonstrated that IL-33 could induce neutrophil migration and activation. Here, we show that IL-33 drives systemic and pulmonary inflammatory responses during intra-abdominal, polymicrobial sepsis. Our findings highlighted the central role of IL-33 in these conditions by showing that the deletion of IL-33 resulted in less inflammation in the lungs and less lung injury even in the face of higher bacterial counts in the peritoneal cavity at 6 h after sepsis. No effect of the absence of IL-33 is found with regards to the bacterial load and lung injury at later time points during sepsis.
The observation that IL-33 deletion resulted in lower systemic and liver levels of IL-1β, IL-10 and IL-5 at 6 h after CLP supports the role for IL-33 in driving systemic inflammatory responses during CLP sepsis. We speculate that the impact of extracellular IL-33 could be especially prominent in the early hours of the response when IL-33 levels peak and levels of the endogenous inhibitor of IL-33, sST2, remain low.
During polymicrobial sepsis, the efficiency of bacterial clearance determines the magnitude of inflammatory response and end organ injury.28 Previous studies have shown that genetically knockout IL-33 impairs bacterial clearance21 as well as increases mortality36 after CLP. In line with these studies, IL-33 deletion resulted in significantly higher bacterial loads in peritoneal cavities as well as a slight increase in mortality in our sublethal CLP model. Furthermore, our results indicated that IL-33 contributed to systemic inflammatory response during intra-abdominal infection even when the infection is controlled by antibiotics. Therefore, it seems unlikely that prevention of lung inflammation and injury was due to a change in local bacterial counts in the lungs.
IL-33 levels in the lungs increased rapidly after the onset of sepsis and triggered robust inflammatory responses characterized by upregulation of both proinflammatory (TNF-α, IL-1β) and anti-inflammatory (IL-10) cytokines as well as an influx of neutrophils and monocytes. We provide evidence that these cytokines were mainly produced by neutrophils and monocytes. Previous work has shown that IL-33 has a direct impact on macrophages and can augment LPS-induced expression of proinflammatory mediators (iNOS, IL-6 and TNF-α) in macrophages.37 We showed that up to 2.5% of lung monocytes were positive for ST2. Therefore, a small population of monocytes might respond directly to IL-33 for increased TNF-α production in the presence of other stimulators, such as LPS. However, we also showed that pulmonary neutrophils and monocytes were positive for IL-5Ra. As is also shown, IL-33 activates ST2-expressing ILC2 to produce IL-5, and IL-5 drives recruitment of neutrophils and monocytes into the lung. Therefore, IL-33-driven cytokine upregulation may partially result from IL-5-mediated neutrophil and monocyte recruitment and activation. Other proinflammatory mediators in the lung microenvironment may also drive cytokine expressions. Our studies focused on the sources of cytokines in the CD45+ population of cells in the lungs. It is also possible that other cell types, including the epithelium and endothelium could contribute to the local production of some of these cytokines during sepsis.
Membrane-bound ST2 is recognized to be responsible for IL-33/ST2 signaling.12,18 However, recent studies suggested that IL-33 can also function independently of ST2.19,38 Blocking IL-33 signaling by sST2 administration significantly reduced LPS-mediated mortality and systemic levels of IL-6, IL-12, and TNF-α. Conversely, inhibition of endogenous ST2 through anti-ST2 antibodies aggravated the toxic effects of LPS.39 These conflicting results in studies using IL33−/−, ST2−/− mice and application of anti-ST2 antibodies in the same model of disease indicate that some functions of IL-33 and ST2 signaling are distinct.38–40 In this study, we observed increased neutrophil recruitment and inflammatory cytokine production in response to IL-33. However, since ST2, the only known IL-33 receptor, was shown to be minimally expressed in these cells, we concluded that the effects of IL-33 on neutrophils and monocytes required an intermediate signal. The observation that both neutrophils and pulmonary monocytes were positive for IL-5Rα identified these cells as targets for IL-5 in sepsis-induced lung inflammation. Endogenous IL-33 has been shown to induce the expansion of ILC2 and their production of IL-5 in allergic diseases22–24 and parasite infections.41 Using models of nematode infection induced acute lung inflammation, Jackson-Jones et al. reported crucial role of IL-33 in pleural B1-cell activation and local IgM secretion via IL-33-ILC2-IL-5 pathway, in which B1 cells are not the direct target of IL-33 but require IL-5 for activation.42 We recently reported that IL-33 can drive neutrophil IL-5 production through ILC2 activation in a model of sterile injury.31 In the current study, IL-33−/− septic mice had less IL-5-producing ILC2 in the lungs and lower IL-5 levels. The suppressed inflammatory cell infiltration in the lung of IL-33−/− mice was partially reversed by administration of rIL-5. Thus, some of the proinflammatory actions of IL-33 in the lung during sepsis appear to be mediated by IL-5. However, deletion of ILC2 had no effect on lung cytokine levels, leukocyte infiltration, or injury. The lethal irradiation and adoptive transfer of bone marrow could have impacted other aspects of the immune response to CLP. Nonetheless, the findings with ILC2 deletion raise the possibility that other sources of IL-5 or mediators other than IL-5 may be important to the IL-33-mediated inflammatory changes.
IL-5 is recognized to promote eosinophil recruitment and maturation.43,44 However, the protective role of IL-5 in sepsis has been shown to be eosinophil-independent.45 Increased recruitment of monocytes and neutrophils has been described in IL-5-overexpressing mice and in mice with exogenous IL-5 administration46 and a chemotactic effect of IL-5 for monocytes and neutrophils has been reported.46,47 In addition, neutralization of IL-5 inhibited neutrophil accumulation in a mouse model of filariasis,48 while anti-IL-5Rα treatment resulted in reduced numbers of neutrophils and monocytes in a clinical study on asthma.49 Consistent with the results from Linch et al.,45 we found in this study that neutrophils were highly positive for IL-5Rα and pulmonary monocytes were moderately positive. Therefore, one of the major effects of IL-33 induced IL-5 upregulation in the lung during sepsis could be the recruitment of IL-5α positive neutrophils and monocytes to the lungs.
In summary, our studies demonstrate a major role for IL-33 not only in lung inflammation and injury in the early phases of sepsis but also in the systemic inflammatory response to bacterial infection. We propose that within hours of onset of sepsis, IL-33 is released from cells and increases the expression of other mediators including IL-5. This is associated with the recruitment of neutrophils and monocytes into the lung which likely exacerbates sepsis-induced lung injury. These findings open new perspectives for the roles of IL-33 in the pathogenesis of sepsis-induced lung injury and identify IL-33 as one of the major drivers of early systemic inflammation in polymicrobial sepsis.
METHODS
Reagents
For drug administration, recombinant Mouse IL-5, rat anti-mouse IL-5 (TRFK5) and Rat IgG1 κ isotype control (R3–34) were purchased from BD Biosciences (San Diego, CA). The panel of antibodies used in flow cytometry are listed in Supplementary table 1. For immunofluorescence staining, the following primary antibodies were used: anti-IL33 (5 μg mL−1 + 0.01% Triton X-100 in PBB, goat IgG; R&D Systems, Minneapolis, MN), anti-collagen I (2 μg mL−1 in PBB, rabbit IgG; Abcam, Cambridge, MA); anti-CD45 polyclonal antibody (2 μg mL−1 + 0.01% Triton X-100 in PBB, rat IgG; Abcam). Alexa Cy5-conjugated donkey anti-goat IgG (Jackson ImmunoResearch, West Grove, PA), Alexa 488-conjugated donkey anti-rat (Jackson ImmunoResearch) and Alexa 488-conjugated donkey anti-rabbit (Molecular Probes, San Jose, CA) were used as secondary antibodies. Nuclear staining was performed with Bisbenzimide H 33258 (Sigma-Aldrich, St Louis, MO).
Mice
Male C57BL/6 WT mice were purchased from Jackson Laboratories (Bar Harbor, ME). IL-33−/− mice were originally from Dr S Nakae50 and are now bred in our facility. All mice were on a C57BL/6 genetic background and were used at the age of 8–12 weeks. Animals were handled according to the protocols approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Experiments were performed in compliance with the National Institutes of Health Guidelines for the Use of Laboratory Animals.
For ILC2 deletion, B6.SJL-Ptprc Pepc mice (n = 4) were lethally irradiated at 1000 Rads and then received 107 bone marrow cells from 2- to 3-week-old WT (Rora+/+) or Rorasg/sg (ILC2 deficient) mice. After 12 weeks, the reconstituted mice were subjected to CLP sepsis.
Cecal ligation and puncture
Sepsis was induced in mice by a CLP model. Mice were anesthetized with isoflurane (Piramal Critical Care, Bethlehem, PA) with oxygen. The cecum was 50% ligated and punctured twice with a 22-gauge needle. Mice received a subcutaneous injection of saline (0.9% w/v, 1 mL) immediately after surgery for fluid resuscitation. In some experiments for setting of controlled infection, mice received antibiotics (Primaxin 25 mg kg−1; Fresenius Kabi USA, LLC, Lake Zurich, IL, USA) subcutaneously every 12 h, starting 2 h after CLP. At the end time point, mice were anesthetized, peritoneal lavage fluid (PLF) was collected under sterile conditions and blood was withdrawn by cardiac puncture. In a subset of mice, survival was monitored for 7 consecutive days after CLP.
Bacterial counts
Peritoneal cavity was washed with 1 mL sterile PBS for 1 min. Peritoneal lavage fluid was collected. The left lung was homogenated with 1 mL sterile PBS. Peritoneal lavage fluid, lung homogenates and blood were subjected to serial 10-fold dilutions and cultured overnight in 5% sheep blood agar (Teknova, Hollister, CA) at 37°C. Colony-forming units were quantified by manual counting.
Immunofluorescence
For immunofluorescence staining, the right upper lobe of the lung was inflated followed by perfusion-fixation with PBS and then 2% paraformaldehyde. The tissue was slowly frozen in 2-methylbutane according to a standardized protocol for cryopreservation. Tissue sections (5 μm) were incubated with 2% bovine serum albumin (BSA) in PBS for 1 h, then were incubated overnight with primary antibodies. The primaries were removed followed by five washes with PBB followed by addition of the corresponding secondary antibodies for one hour at room temperature, and then nuclear staining was performed. Imaging conditions were maintained at identical settings within each antibody-labeling experiment with original gating performed using the negative control. Imaging was performed using a Nikon A1 confocal microscope (Nikon, Melville, NY). Quantification was performed using NIS Elements (Nikon).
Hematoxylin and eosin staining
Lung samples were fixed in 2% paraformaldehyde overnight at 4°C, and then dehydrated and embedded in paraffin. The sections were stained with Hematoxylin and eosin and evaluated for histological changes. The histological alterations were quantitatively scored on a scale from 0 to 4 (0, absent and appears normal; 1, mild; 2, moderate; 3, strong; and 4, intense) for four parameters: infiltration or aggregation of neutrophils in airspaces or vessel walls, hemorrhage, alveolar congestion, and thickening of alveolar wall membrane. The sum of all the scores for the four parameters was calculated as the total scores, ranging between 0 (least severe) and 16 (most severe).
Lung permeability assay
The changes in vascular permeability in lung were assessed by Evans Blue Dye (EBD) leakage from blood into lung airways. EBD (20 mg kg−1 bodyweight; Sigma-Aldrich) in 200 mL PBS was injected intravenously by penial vein 30 min before the end of observation time point (6 h). Lungs were perfused with PBS to remove intravascular dye. Lungs were collected and dried at 60°C for 48 h. The dry weights of lungs were documented. EBD were extracted from lungs by incubated in formamide (Sigma-Aldrich) at 37°C for 24 h and centrifuged at 5000 g for 30 min. The EBD in the supernatant was quantitated by a dual wavelength (620 and 740 nm) spectrophotometric method, correcting for contaminating heme pigments using formula Corrected OD620 = Observed × OD620–1.2641 9 Observed OD740–0.0087. The EBD concentration in lung was calculated against a standard curve and expressed as the dye normalized by dry lung weight. The fold change of EBD in lung between CLP and normal control was calculated.
Enzyme-linked immunosorbent assay
Lung tissue samples were lysed, and protein concentration for each was determined using BCA. Plasma and lung homogenates were analyzed for cytokine protein levels using Enzyme-linked immunosorbent assay kits from R&D Systems (Minneapolis, MN). Assays were performed according to the manufacturer’s protocols.
Flow cytometry
The right lung lobes were perfused with sterile PBS via heart puncture before excising, then removed into gentle MACS C tubes (Miltenyi Biotec Inc, Auburn, CA) and digested using a lung dissociation kit and an automated tissue dissociator (Miltenyi Biotec). Single-cell suspensions were obtained after being filtered and washed. Cells numbers were calculated using a Hemacytometer. For intracelluar staining, cells were stimulated with PMA (10 ng mL−1), protein transport inhibitor (1 μL mL−1) and ionomycin (500 ng mL−1) for 3 h at 37°C. After having been stained with Fixable Viability Dye, cells were incubated with anti-mouse CD16/CD32 (BD Biosciences, San Diego, CA) at 4°C for 10 min. Afterwards, cells were incubated with different sets of fluorochrome-conjugated antibodies for 30 min at 4°C in the dark. Intracelluar staining was performed using the intracellular fixation and permeabilization buffer set (eBioscience, San Diego, CA), positive signals were identified by comparison of staining with antibodies to the markers or isotype-matched control antibodies. Data were acquired using BD LSR II and BD FACS LSR Fortessa flow cytometer (BD Bioscience) and were analyzed with FlowJo software (TreeStar, Ashland, OR). Leukocyte subsets were identified based on the following markers: neutrophils (CD45+/Gr1high/CD11b+/F4/80−), alveolar macrophages (AMs: CD45+/F4/80+/CD11b−/CD11chigh/Gr1−), pulmonary monocytes (CD45+/F4/80+/CD11b+/CD11c−/Gr1low) and interstitial macrophages (IMs: CD45+/F4/80+/CD11b+/CD11clow/Gr1−).
Statistical analysis
All data were analyzed using GraphPad Prism software (GraphPad Software Inc., San Diego, CA). Comparisons were performed using one-way ANOVA and a two-tailed t-test. For measurements of bacterial Colony-forming units, groups were compared using nonparametric Mann–Whitney U-tests. Survival data were analyzed using the log-rank test. A value of P < 0.05 was considered statistically significant. All values are presented as mean values ± s.d.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grant RO-1 GM-50441.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Lakshmikanth CL, Jacob SP, Chaithra VH, et al. Sepsis: in search of cure. Inflamm Res 2016; 65: 587–602. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J, Vincent JL, Adhikari NK, et al. Sepsis: a roadmap for future research. Lancet Infect Dis 2015; 15: 581–614. [DOI] [PubMed] [Google Scholar]
- 3.Tavares E, Maldonado R, Miñano FJ. Immunoneutralization of endogenous aminoprocalcitonin attenuates sepsis-induced acute lung injury and mortality in rats. Am J Pathol 2014; 184: 3069–3083. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J. The immunopathogenesis of sepsis. Nature 2002; 420: 885–891. [DOI] [PubMed] [Google Scholar]
- 5.Grommes J, Soehnlein O. Contribution of neutrophils toacute lung injury. Mol Med 2011; 17: 293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ware LB. Pathophysiology of acute lung injury and theacute respiratory distress syndrome. Semin Respir Crit Care Med 2006; 27: 337–349. [DOI] [PubMed] [Google Scholar]
- 7.Parsey MV, Tuder RM, Abraham E. Neutrophils are major contributors to intraparenchymal lung IL-1 beta expression after hemorrhage and endotoxemia. J Immunol 1998; 160: 1007–1013. [PubMed] [Google Scholar]
- 8.Bhatia M, Moochhala S. Role of inflammatory mediatorsin the pathophysiology of acute respiratory distress syndrome. J Pathol 2004; 202: 145–156. [DOI] [PubMed] [Google Scholar]
- 9.Fumeaux T, Pugin J. Role of interleukin-10 in the intracellular sequestration of human leukocyte antigen-DR in moncocytes during septic shock. Am J Respir Crit Care Med 2002; 166: 1475–1482. [DOI] [PubMed] [Google Scholar]
- 10.Latifi SQ, O’Riordan MA, Levine AD. Levine. Interleukin-10 controls the onset of irreversible septic shock. Infect Immun 2002; 70: 4441–4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol 2010; 10: 103–110. [DOI] [PubMed] [Google Scholar]
- 12.Rostan O, Arshad MI, Piquet-Pellorce C, et al. Crucial and diverse role of the interleukin-33/ST2 axis in infectious diseases. Infect Immun 2015; 83: 1738–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paris G, Pozharskaya T, Asempa T, et al. Damage-associated molecular patterns stimulate interleukin-33 expression in nasal polyp epithelial cells. Int Forum Allergy Rhinol 2014; 4: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cayrol C, Girard JP. IL-33: an alarmin cytokine withcrucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol 2014; 31: 31–37. [DOI] [PubMed] [Google Scholar]
- 15.Zeyda M, Wernly B, Demyanets S, et al. Severe obesity increases adipose tissue expression of interleukin-33 and its receptor ST2, both predominantly detectable in endothelial cells of human adipose tissue. Int J Obes (Lond) 2013; 37: 658–665. [DOI] [PubMed] [Google Scholar]
- 16.Mirchandani AS, Salmond RJ, Liew FY. Interleukin-33 andthe function of innate lymphoid cells. Trends Immunol 2012; 33: 389–396. [DOI] [PubMed] [Google Scholar]
- 17.Oboki K, Nakae S, Matsumoto K, et al. IL-33 and airway inflammation. Allergy Asthma Immunol Res 2011; 3: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu H, Turnquist HR, Hoffman R, et al. Role of the IL-33-ST2 axis in sepsis. Mil Med Res 2017; 4: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villarreal DO, Weiner DB. Interleukin 33: a switch-hittingcytokine. Curr Opin Immunol 2014; 28: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurowska-Stolarska M, Hueber A, Stolarski B, et al. Interleukin-33: a novel mediator with a role in distinct disease pathologies. J Intern Med 2011; 269: 29–35. [DOI] [PubMed] [Google Scholar]
- 21.Alves-Filho JC, Sonego F, Souto FO, et al. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med 2010; 16: 708–712. [DOI] [PubMed] [Google Scholar]
- 22.Van Dyken SJ, Mohapatra A, Nussbaum JC, et al. Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and γδ T cells. Immunity 2014; 40: 414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein Wolterink RG, Kleinjan A, van Nimwegen M, et al. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol 2012; 42: 1106–1116. [DOI] [PubMed] [Google Scholar]
- 24.Sonnenberg GF, Artis D. Innate lymphoid cells in theinitiation, regulation and resolution of inflammation. Nat Med 2015; 21: 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Gonzalez I, Steer CA, Takei F. Lung ILC2 linkinnate and adaptive responses in allergic inflammation. Trends Immunol 2015; 36: 189–195. [DOI] [PubMed] [Google Scholar]
- 26.Monticelli Laurel A, Sonnenberg Gregory F, Abt MichaelC, et al. Innate lymphoid cells promote lung tissue homeostasis following acute influenza virus infection. Nat Immunol 2011; 12: 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salimi M, Barlow JL, Saunders SP, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med 2013; 210: 2939–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng M, Scott MJ, Loughran P, et al. Lipopolysaccharide clearance, bacterial clearance, and systemic inflammatory responses are regulated by cell type-specific functions of TLR4 during sepsis. J Immunol 2013; 190: 5152–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaynagetdinov R, Sherrill TP, Kendall PL, et al. Identification of myeloid cell subsets in murine lungs using flow cytometry. Am J Resp Cell Mol 2013; 49: 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hung LY, Lewkowich IP, Dawson LA, et al. IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proc Natl Acad Sci USA 2013; 110: 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, Guardado J, Hoffman R, et al. IL-33 mediated ILC2 activation and neutrophil IL-5 production in the lung response after severe trauma: a reverse translation study from a human cohort to a mouse trauma model. PLoS Med 2017; 14: e1002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez-González I, Roca O, Masclans JR, et al. Human mesenchymal stem cells overexpressing the IL-33 antagonist soluble IL-1 receptor-like-1 attenuate endotoxin-induced acute lung injury. Am J Respir Cell Mol Biol 2013; 49: 552–562. [DOI] [PubMed] [Google Scholar]
- 33.Yin H, Li XY, Yuan BH, et al. Adenovirus-mediated overexpression of soluble ST2 provides a protective effect on lipopolysaccharide-induced acute lung injury in mice. Clin Exp Immunol 2011; 164: 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talabot-Ayer D, Martin P, Vesin C, et al. Severe neutrophil-dominated inflammation and enhanced myelopoiesis in IL-33-overexpressing CMV/IL33 mice. J Immunol 2015; 194: 750–760. [DOI] [PubMed] [Google Scholar]
- 35.Zhiguang X, Wei C, Steven R, et al. Over-expression of IL-33 leads to spontaneous pulmonary inflammation in mIL-33 transgenic mice. Immunol Lett 2010; 131: 159–165. [DOI] [PubMed] [Google Scholar]
- 36.Lv R, Zhao J, Lei M, et al. IL-33 attenuates sepsis by inhibiting IL-17 receptor signaling through upregulation of SOCS3. Cell Physiol Biochem 2017; 42: 1961–1972. [DOI] [PubMed] [Google Scholar]
- 37.Xiang Y, Eyers F, Herbert C, et al. MicroRNA-487b is a negative regulator of macrophage activation by targeting IL-33 production. J Immunol 2016; 196: 3421–3428. [DOI] [PubMed] [Google Scholar]
- 38.Luzina IG, Pickering EM, Kopach P, et al. Full-length IL-33 promotes inflammation but not Th2 response in vivo in an ST2-independent fashion. J Immunol 2012; 189: 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sweet MJ, Leung BP, Kang D, et al. A novel pathway regulating lipopolysaccharide-induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression. J Immunol 2001; 166: 6633–6639. [DOI] [PubMed] [Google Scholar]
- 40.Ohto-Ozaki H, Kuroiwa K, Mato N, et al. Characterization of ST2 transgenic mice with resistance to IL-33. Eur J Immunol 2010; 40: 2632–2642. [DOI] [PubMed] [Google Scholar]
- 41.Yasuda K, Muto T, Kawagoe T, et al. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode- infected mice. Proc Natl Acad Sci USA 2012; 109: 3451–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson-Jones LH, Duncan SM, Magalhaes MS, et al. Fat-associated lymphoid clusters control local IgM secretion during pleural infection and lung inflammation. Nat Commun 2016; 7: 12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takatsu K, Nakajima H. IL-5 and eosinophilia. Curr Opin Immunol 2008; 20: 288–294. [DOI] [PubMed] [Google Scholar]
- 44.Fulkerson PC, Schollaert KL, Bouffi C, et al. IL-5 triggers a cooperative cytokine network that promotes eosinophil precursor maturation. J Immunol 2014; 193: 4043–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linch SN, Danielson ET, Kelly AM, et al. Interleukin 5 is protective during sepsis in an eosinophil-independent manner. Am J Respir Crit Care Med 2012; 186: 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hakansson L, Venge P. Priming of eosinophil and neutrophil migratory responses by interleukin 3 and interleukin 5. APMIS 1994; 102: 308–316. [DOI] [PubMed] [Google Scholar]
- 47.Ringheim GE. Mitogenic effects of interleukin-5 on microglia. Neurosci Lett 1995; 201: 131–134. [DOI] [PubMed] [Google Scholar]
- 48.Al-Qaoud KM, Pearlman E, Hartung T, et al. A new mechanism for IL-5-dependent helminth control: neutrophil accumulation and neutrophil-mediated worm encapsulation in murine filariasis are abolished in the absence of IL-5. Int Immunol 2000; 12: 899–908. [DOI] [PubMed] [Google Scholar]
- 49.Busse WW, Katial R, Gossage D, et al. Safety profile, pharmaco- kinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. J Allergy Clin Immunol 2010; 125: 1237–1244, e1232 [DOI] [PubMed] [Google Scholar]
- 50.Oboki K, Ohno T, Kajiwara N, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci USA 2010; 107: 18581–18586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.