Abstract
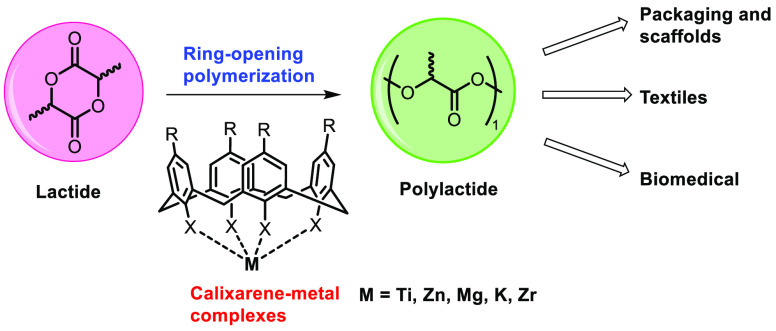
Polylactide synthetic procedures have lately gained attention, possibly due to their biocompatibility and the environmental problems associated with fossil-fuel-based polymers. Polylactides can be obtained from natural sources such as cassava, corn, and sugar beet, and polylactides can be manufactured in a laboratory using a variety of processes that begin with lactic acid or lactide. One of the most effective synthetic pathways is through a Lewis acid catalyzed ring-opening polymerization of lactides to obtain a well-defined polymer. In this regard, calixarenes, because of their easy functionalization and tunable properties, have been widely considered to be a suitable 3D molecular scaffold for new metal complexes that can be used for lactide polymerization. This review summarizes the progress made in applying some metal-calixarene complexes in the ring-opening polymerization of lactide.
1. Introduction
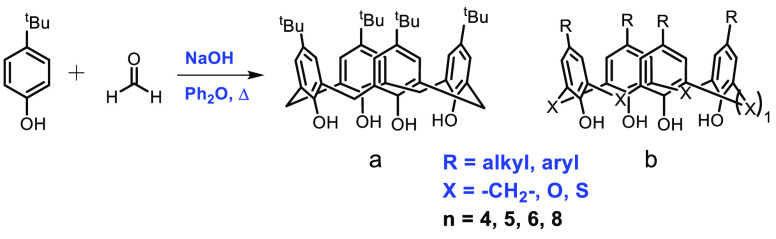
The history of calixarenes dates back to 1872, when Adolf von Baeyer reported the reaction between phenol and formaldehyde.1 After almost 70 years, the compound was termed “calixarene” and its structure was assigned by C. D. Gutsche in 1978, derived from the Latin “calix” meaning vase and describing the cone-shaped conformation which the macromolecule often adopts (Figure 1a).2,3 Calix[n]arenes possess a common structure in which n represents the number of phenolic units linked together through a methylene group or heteroatom (Figure 1b). The existence of a hydrophobic cavity and the possibility of incorporating a wide variety of analytes create extensive opportunities for their use as catalysts and chemosensors.4−6 Many catalytically valuable ligands, such as the ubiquitous multidentate salen and porphyrin molecules, act as electron donors in their metal complexes. Like salens and porphyrins, a similar tetradentate dianionic motif is also found in calixarene, where the metal center is coordinated to the lower rim oxygen atoms. An increase in studies on the use of calix[n]arenes as auxiliary ligands in metal catalysts for various transformations is a result of the development of calixarene coordination chemistry. Synthetic protocols toward selectively modified calixarene ligands are straightforward, making these ligands highly attractive for inorganic and analytical chemists. The conformational flexibility, presence of cavities, and ability to concurrently coordinate numerous metal centers are essential characteristics of the higher calixarene systems. Most calix[4]arene systems tend to retain the cone conformation upon metalation, binding to metal centers in both exo and endo conformations. Nonetheless, calixarene ligands have yet to compete with other multidentate ligands in catalytic applications. One possible explanation for this disparity is that the calixarene ligand exclusively contains hard oxygen donors. Thus, calixarene-based compounds have been utilized as ligands for complexation with a large number of oxophilic metals.7,8 The presence of only hard O-donors also limits the use of calixarene in biomimetic studies.8 Nonetheless, calixarenes are easily and selectively modified, and high-yield synthetic protocols toward various mono-, di-, or tri-O-substituted ethers and esters are available.9 Moreover, despite calixarene not exactly taking part in the catalysis, it may have a positive role in many chemical transformations. Calixarenes are frequently used in transition-metal bond activation and catalysis because they give a metal more stability and control over its coordination environment than monodentate ligands.7−9
Figure 1.
(a) Synthesis and (b) structure of calix[n]arene.
The world’s reliance on petroleum-based polymers has grown significantly over time. Polyolefins like polyethylene, polypropylene, polystyrenes, and polyvinyl chloride, polyesters like polyethylene terephthalate, polyamides like nylon, and epoxy (commonly known as plastic) are synthetic polymers made from petroleum hydrocarbons.10,11 Because of the omnipresent qualities of petroleum-based polymers, they are finding utility in a wide range of applications. These applications include consumer products like appliances, toys, packaging materials, electronics, coatings, and automobile components to name a few.12,13 These polymers have also gained significant traction in the biomedical field, where they are used in the design and development of implants, monitoring, diagnostic, or therapeutic devices, and drug delivery techniques, among others.14,15 Petrochemical-based polymers have been extensively used owing to numerous advantages like ease of preparation and low cost. However, the utilization of these polymers poses significant hurdles, which include a paucity of organic compounds as a result of dwindling oil and gas supplies and rising oil and gas prices. Additional effects include toxicity, unaffordable pricing, cross-contamination in recycling, and environmental issues caused by their disintegration. These concerns inspired the search for materials capable of fixing these limitations while retaining the needed qualities for diverse applications. Biocomposites and biopolymers are two materials which have gained recent traction because of their easily adjustable properties.16−18 Biocomposites from cheap and renewable resources offer significant sustainability and have found use in building materials, the aerospace industry, circuit boards, automotive applications, and the energy sector.19−21
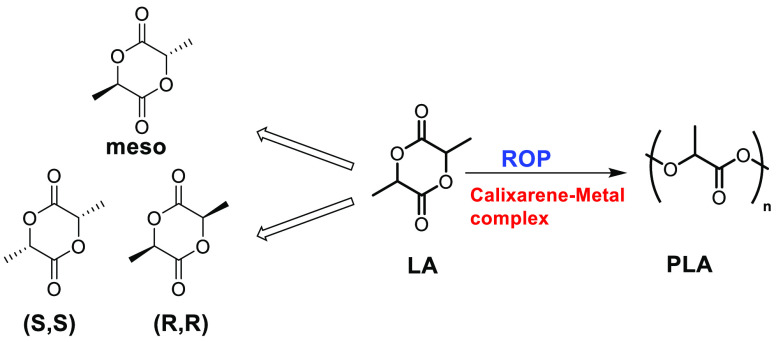
Biopolymers have been around for a while now but have generally been held back due to their higher cost and issues with durability. However, the development of improved catalytic systems coupled with rising environmental concerns accompanying the use of petroleum-based polymers has created tremendous interest in alternatives to plastic. One such alternative is a polylactide (PLA) (Figure 2), a polymer of lactic acid which is synthesized either through polycondensation of lactic acid or by ring-opening polymerization (ROP) of lactide (LA).22−24 The pathway of polycondensation has been found to be less influential, as water is liberated constantly, which is difficult to remove, necessitating harsh conditions to obtain the polymer with a high molecular weight.25 Hence, the ring-opening polymerization (ROP) of LA has been emphasized, as it allows good control over the molecular parameters like molecular weight, polydispersity index, etc. under mild reaction conditions compared to the polycondensation pathway.26 PLA and its modified products have received great attention as functional materials due to their biocompatibility, nontoxicity, mechanical stability, durability, and applications.27,28 Lactide copolymerization with monomers like malic acid or polymers such as polyethylene glycol (PEG) or polyglycolic acid (PGA) has also been explored.29,30 Although calixarene-metal complexes have found use in many functional group transformations, initially, only a few papers on their application in polymerization were reported.31−42 An exciting work by Frediani and co-workers in 2008 reported the use of a Ti-calixarene complex in lactide polymerization.43 After that, several articles have been published in this field by different research groups worldwide.43−64 Despite some progress in this area and the importance of PLA, no such review has been published to cover the developments (made in this field) critically and also entirely. Thus, this review proposes to not only fill the gap but also provide new insights and analyses of the progress and limitations of different aspects. In this review, we will discuss a few of the protocols adopted to synthesize calixarene-metal complexes, touch upon the structure of a few interesting complexes, and deliberate upon their activity as catalysts for LA ring-opening polymerization.
Figure 2.
Structures of LA and PLA.
2. Calixarene-Metal Complexes in ROP
Metal complexes of calixarenes have received immense attention in the field of catalysis.31−41 The utilization of a properly designed ligand plays a very important role in the polymerization of LA.42 Much work has been carried out by using transition-metal complexes of calixarenes, which have proved to be very effective toward the LA polymerization.43−64 Here we will discuss the synthesis and application of some calixarene metal complexes in the ROP of LA.
2.1. Titanium-Calixarene Complexes in ROP
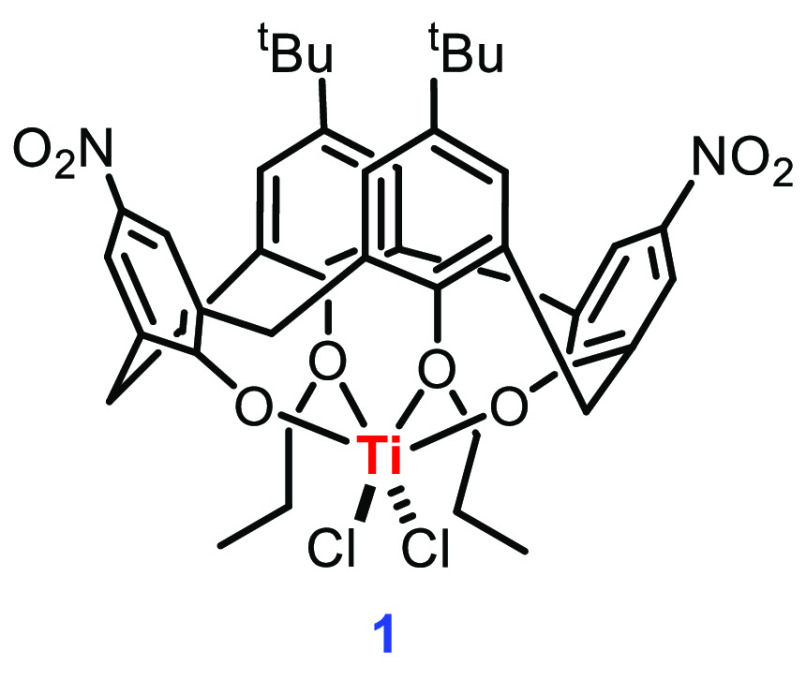
Frediani’s group described the synthesis of the first titanium complex of modified calixarene 1 in lactide polymerization.43 The dicholorotitanium complex 1 was obtained in 88% yield by treating 5,17-bis-tert-butyl-25,27-dihydroxy-11,23-dinitro-26,28-dipropyloxycalix[4]arene with TiCl4 in hot toluene. The NMR spectra of 1 revealed a C2v-symmetrical structure, confirming the cone conformation (Figure 3). According to single-crystal X-ray diffraction studies, the titanium atom is found in an octahedral ligand environment composed of four oxygen and two chlorine atoms, with the two propylated oxygen atoms occupying trans positions. The polymerizations with catalyst 1 were carried out under solvent-free conditions, and it was found to be more active than other reported chlorotitanium catalysts. One possible reason given was the presence of two strongly electron withdrawing nitro functional groups, which enhanced the Lewis acidic properties of complex 1. Another important observation reported by Frediani’s group was the role of an external alcohol in reactivity and polymerization. Despite the fact that Ti complex 1 was successful in the polymerization of LA in the absence of any external alcohol activator, the presence of 75 equiv of n-BuOH further improved the activity. Under these conditions, PLA was obtained with 98.3% monomer conversion and an excellent polydispersity index (PDI 1.16). One reason for increased activity would be the in situ formation of a more labile Ti-dialkoxide complex. The presence of a unique quartet in the methine region (at 5.16 ppm) is indicative of isotactic polylactide formation. The authors report a living polymerization mechanism with catalyst 1, signifying that the active Ti-metal center is present at the end of the polymerization cycle.
Figure 3.
Structure of Cl2Ti-calixarene complex 1.
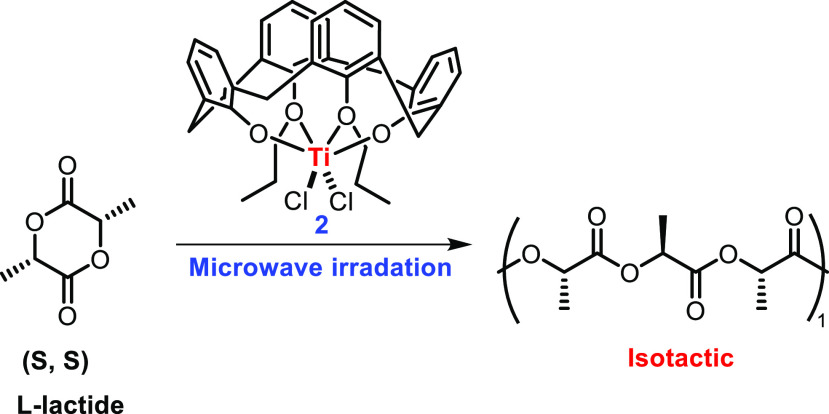
In a continuation of their work on the application of Ti-calixarene complexes in PLA synthesis, Frediani and co-workers in 2010 reported the synthesis of a Cl2Ti-calixarene complex 2 (Scheme 1) and described its use in ROP of rac-LA or l,d-LA.44 To initiate the polymerization reaction, his group opted for two techniques: i.e., microwave irradiation and conventional thermal treatment with complex 2. In comparison with the other experiments carried out, it was found that microwave energy enhances the polymerization rate. It was also observed that the activity of complex 2 was somewhat higher in the case of rac-LA than for l-LA.45 The remarkable feature of the polymer formed was the partial isotactic stereoblock microstructure in spite of the C2v symmetry with a conversion of 88% and PDI of 1.23 with conventional heating. The spectrum shows two well-resolved peaks, of equal normalized intensity, at 5.21 and 5.22 ppm representing hexad stereosequences indicating random polymerization. Under microwave-assisted conditions a polymer with a PDI of 1.30 (88% conversion of monomer) was obtained within 80 min. Although lower molecular weights were observed with microwave irradiation (Mw = 27100 and Mn = 20900) compared to those with the conventional method (Mw = 27600 and Mn = 22400), better stereocontrol (isotactic) could be witnessed with microwave heating.
Scheme 1. Microwave-Assisted ROP.
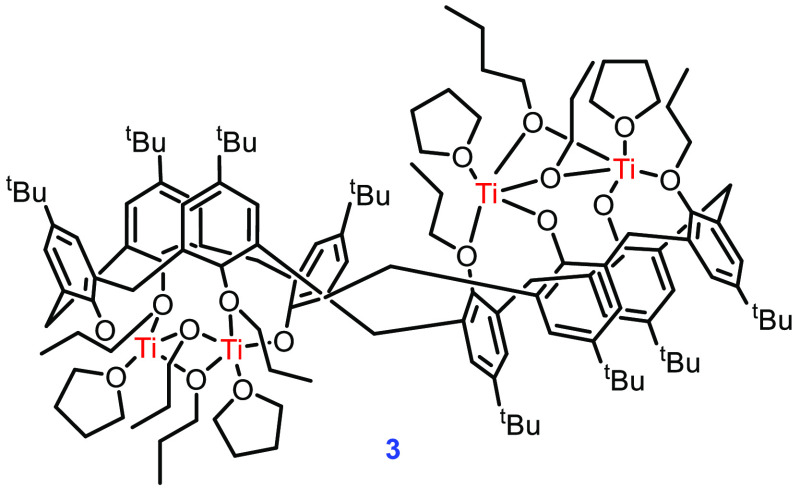
Jason et al. have prepared several structurally diverse metal clusters stabilized by tert-butylcalix[8]arene. They noted that the usual deprotection–metalation strategy is ineffective in preparing Ti-calix[8]arene complex 3 (Figure 4). Complex 3 was prepared by directly treating the corresponding calixarene with an excess of Ti(OiPr)4 in THF. The single crystal of complex 3 shows that the two Ti(IV) centers are 3.2876 Å apart within half of the molecule. The Ti centers are also supported by two bridging propoxides. The complex was only moderately successful in the polymerization of rac-LA, with the maximum m/z observed at 1956. The absence of higher molecular weight polymer units was attributed to the competing intramolecular transesterification process.46 The decreased activity of complex 3 may be due to the higher flexibility of the calix[8]arene scaffold, which allows for unusual binding and less access to the catalytically active core.
Figure 4.
Structure of complex 3.
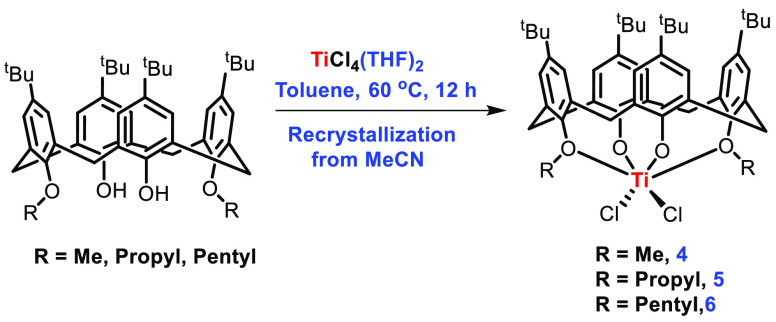
Redshaw et al. have synthesized the titanium complexes of a series of functionalized calix[4]arene molecules of the type TiCl2L(O)2(OR)2 (R = Me (4), n-Pr (5), and n-pentyl (6)), the dimeric compound TiL(O)3(OR)]2(Cl)2 (R = n-decyl (7)) and Ti(MeCN)ClL(O)3(OMe) (8) for the ROP of cyclic esters. For the preparation of complexes 4–6, the method reported by Taoufik and Bonnamour was followed.47 In this procedure 1,3-alkoxy calix[4]arene was treated with a THF complex of TiCl4 in toluene and the reaction mixture was stirred at 60 °C for 12 h. Crystallization was done by using CH3CN during workup (Scheme 2). The crystal structure of these complexes indicates that the Ti-metal center resides in a distorted-octahedral geometry with a pinched cone conformation.
Scheme 2. Synthesis of Titanocalixarene Complexes 4–6.
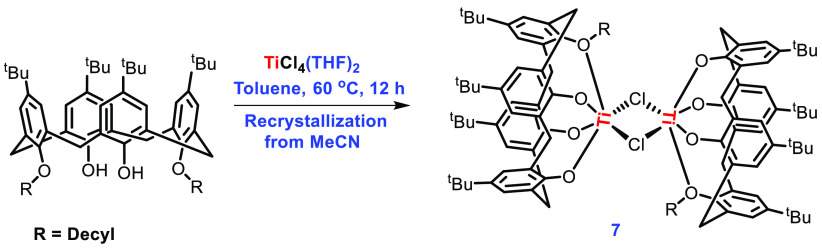
Interestingly, in the case of 1,3-n-decyl-functionalized calixarene, a choloro-bridged dimer was obtained, in which each Ti center is best described to be present in a distorted-octahedral geometry (Scheme 3).
Scheme 3. Synthesis of Titanocalixarene Complex 7.
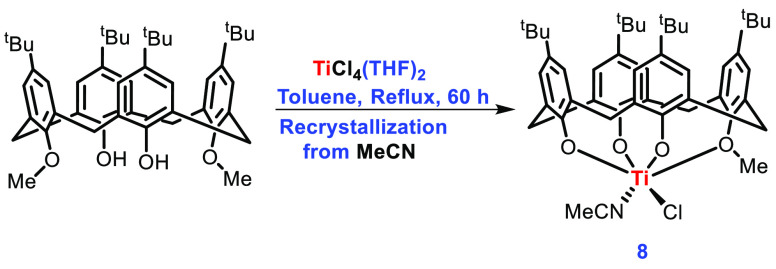
Redshaw’s group also prepared many other titanocalixarene complexes for their catalytic study of ROP in cyclic esters using a modified Floriani procedure.48 Consequently, the treatment of 1,3-dimethoxy calix[4]arene with TiCl4(THF)2 in hot toluene (60 h) followed by refluxing with acetonitrile (24 h) afforded orange-red crystals of complex 8 on cooling (Scheme 4).
Scheme 4. Synthesis of Titanocalixarene Complex 8.
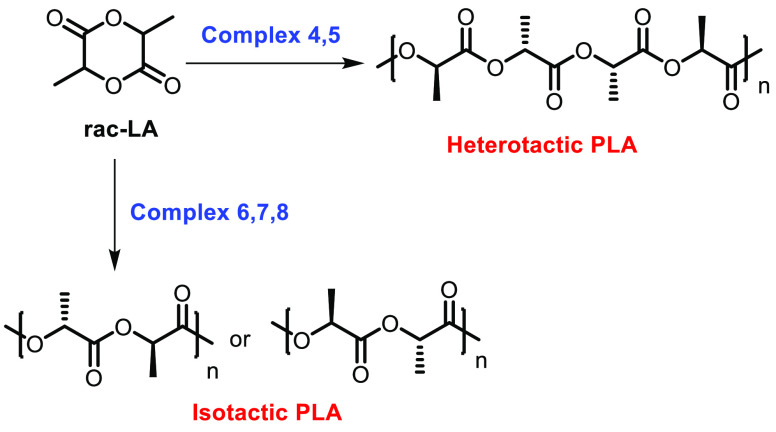
All of the complexes were investigated for their ability to catalyze the ROP of rac-LA. It was reported that the catalyst systems 4–8 were inactive at 80 °C even when the reaction mixture was stirred for 24 h. At an elevated temperature (130 °C), almost quantitative conversion was observed with complexes 4–6 and 8 (95%, 97%, 95%, and 95%, conversions, respectively), while only moderate conversion (65%) could be realized with complex 7. Ring-opening polymerization was carried out with complexes 4 and 5, which yielded the heterotactic polymers (Pr 0.46 and 0.42, respectively), and with complexes 6–8 isotactic materials were obtained (Scheme 5). Interestingly, catalyst 8 was also found to be active even in air, with complete conversion being observed.
Scheme 5. Microstructures of Heterotactic and Isotactic PLA.
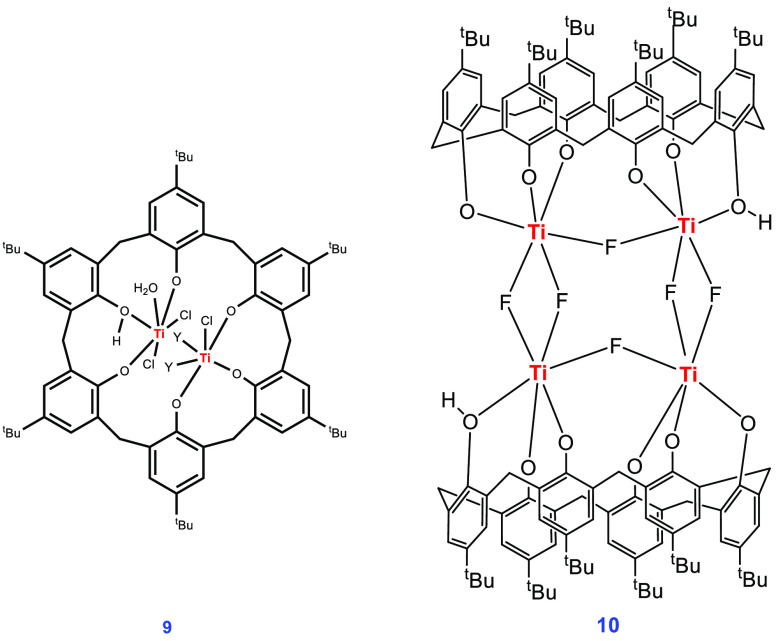
Very recently, Redshaw and co-workers synthesized Ti complexes of calix[6]arene and calix[8]arene (9–13) and screened them in the ROP of cyclic esters both in air and under an inert atmosphere. The catalyst 9 was prepared by direct treatment of calix[6]arene with 2 equiv of TiCl4 in hot toluene followed by workup with acetonitrile. In each case, the two pseudo-octahedral Ti(IV) ions are bound to a calix[6]arene ligand via three phenolate oxygens, while the Cl, CH3CN and H2O molecules are facial (Figure 5, complex 9).
Figure 5.
Structures of complexes 9 (Y = CH3CN) and 10.
Treatment of calix[6]arene with TiF4 (3 equiv) and extraction into MeCN provided complex 10. The pseudo-octahedral Ti centers of the two macrocyclic units in complex 10 are connected to each other through an F bridge. The central core consists of two Ti2F2 diamonds that connect the calixarenes, while the two fluoride ions connect the Ti centers (Figure 5, complex 10).
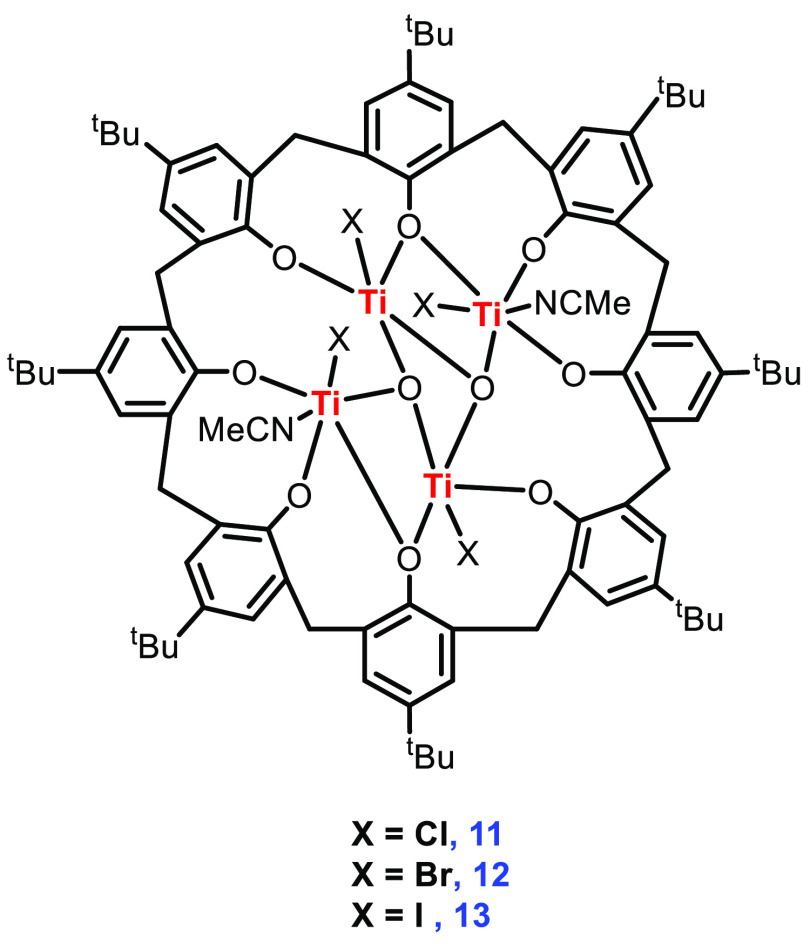
The reaction of p-tert-butylcalix[8]arene with 4 equiv of TiCl4 in refluxing toluene and workup with MeCN furnished small red crystals of 11 in moderate yield. The X-ray diffraction studies show a complex where Ti is occupying a pseudo-octahedral position with trans Cl and MeCN groups, while the other Ti centers are square-pyramidal with apical Cl ligands. Treatment of p-tert-butylcalix[8]arene with 4 equiv of TiI4 followed by workup in dichloromethane afforded the dark red complex 13. Similarly, the analogous complex 12 could be realized with TiBr4 (Figure 6). Interestingly, the molecular structure of the prepared compounds revealed a ladder-like complex similar to that observed for the chloride and iodide systems.
Figure 6.
Structures of complexes 11–13.
All of the catalysts were screened under different reaction conditions (for a brief overview, see Table 1). Among all the catalysts prepared, only complex 9 was found to be highly active toward the polymerization of rac-LA with a conversion of 87%, and the syndiotactic bias (Pr) of 0.51 suggested atactic polylactide formation.49,50 Regardless of the reaction conditions investigated, no reaction was detected while using 10 and 11 and the bromide complex 12. In contrast, a 11% conversion was observed when catalyst 13 was used even after 24 h.
Table 1. Catalyst Screening for ROP of rac-LA.
| entry | complex no. | rac-LA:Ti:BnOH | T (°C) | time (h) | conversion (%) |
|---|---|---|---|---|---|
| 1 | 9 | 500:1:3 | 130 | 24 | 87 |
| 2 | 9 | 500:1:3 | 130 | 1 | 33 |
| 3 | 10 | 250:1:4 | 130 | 24 | |
| 4 | 11 | 500:1:2 | 130 | 24 | |
| 5 | 12 | 250:1:1 | 130 | 24 | |
| 6 | 13 | 250:1:1 | 130 | 24 | 11 |
2.2. Zinc-Calixarene Complexes in ROP
From enzymatic transformations to asymmetric organic synthesis, zinc complexes are at the heart of a wide range of catalytic processes.51,52 Both bi- and monometallic Zn complexes have been found to be active in LA polymerization. In 1996 Raston and co-workers reported the formation of an unusual bimetallic Zn complex when calixarene 1,3-dimethyl ether was treated with 2 equiv of Et2Zn.53,54
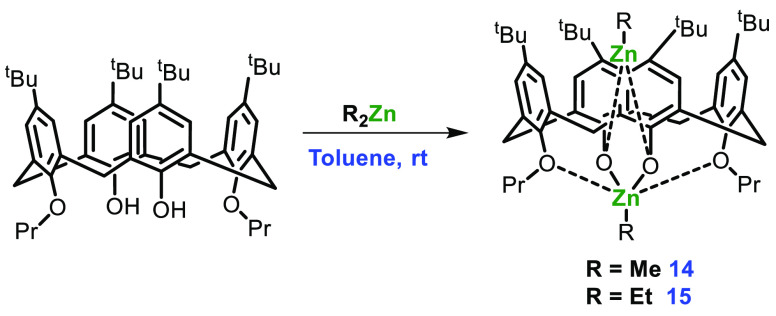
Vigalok et al. in 2004 synthesized and studied a variety of bimetallic alkyl zinc complexes of 1,3-alkylated calix[4]arenes.55 They envisaged that the small methyl substituent might favor flipping in the calixarene scaffold, and thus much of their work was on larger 1,3-substituted calix[4]arenes. A series of bimetallic Zn complexes were prepared by treating appropriately substituted calix[4]arene with 2 equiv of Et2Zn in toluene at room temperature (Scheme 6).
Scheme 6. Bimetallic Zn-Calixarene Complexes 14 and 15.
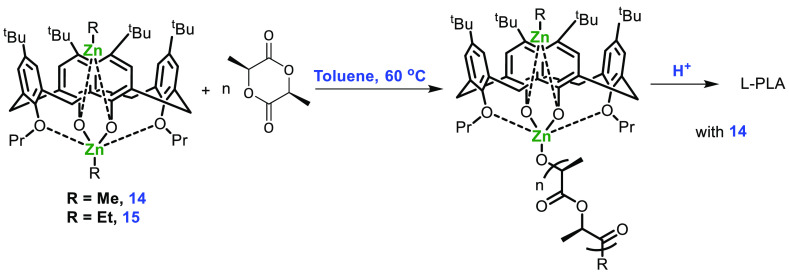
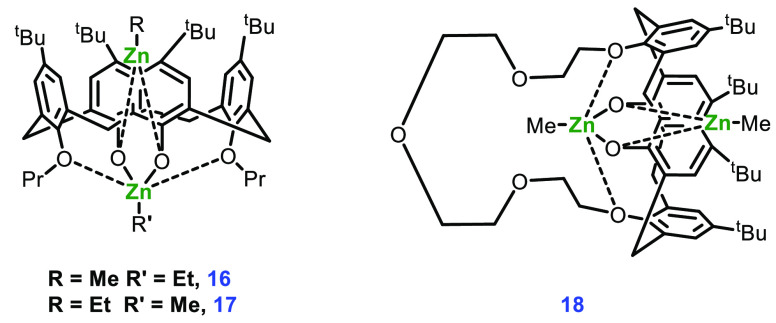
The alkyl-incorporated complexes 14 and 15 containing two Zn atoms share the same aryl oxide framework; calixarene trapping protects one of the alkyl zinc fragments from an attack of the organic substrate. It has been observed that the ethyl zinc group is a comparatively poor nucleophile and less reactive than Zn-alkoxide, therefore moving from an ethyl zinc to a methyl zinc initiating group drastically improved the performance of the catalyst.56 Ring polymerization was carried out in toluene at a temperature above 60 °C to produce PLA with low polydispersity and a conversion rate of 98% (Scheme 7). To ensure that conformational changes in calixarene did not play a significant role in catalysis, they also prepared complexes 16–18 (Figure 7) and checked the efficiency in the ROP of l-lactide. The results indicated that the catalyst activity depended only on the external alkyl group (behaving as an initiator for reaction) and was independent of the alkyl zinc group inside the calixarene cavity.
Scheme 7. Polymerization of l-Lactide with Complexes 14 and 15.
Figure 7.
Complexes 16–18.
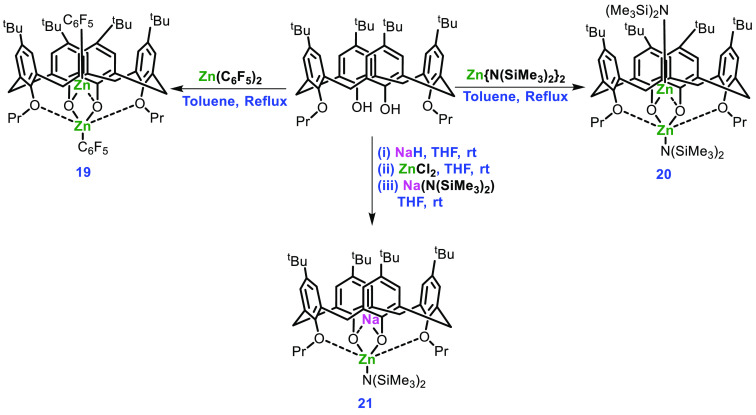
Redshaw and co-workers explored the use of the calix[4]arene and the oxacalix[3]arene ligand in the preparation of the corresponding Zn complexes and reported their use in ROP of cyclic esters.57 The authors identified the effect of additional chain transfer agents and the tactility of the resulting polymers. The synthesis of several new zinc-containing calix[4]arene complexes (19–21) is outlined in Scheme 8.
Scheme 8. Synthesis of Complexes 19–21.
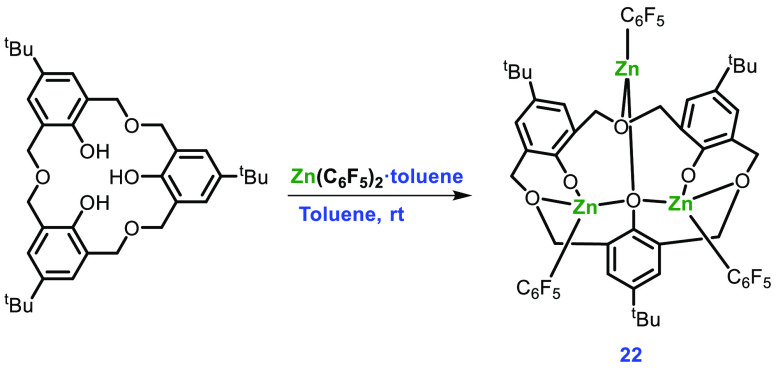
Among the synthesized complexes, only 19 was found to be more active for the ROP of rac-LA at high temperature (100 °C) with 90% conversion and Pr = 0.62, but the molecular weight of the polymer was found to be lower than expected. One of the possible reasons for this could be the unwanted trans-esterification reaction occurring at that temperature. Replacing the pentafluorophenyl group with an amide group (N(SiMe3)2) in 19 afforded complex 20, which was found to be more effective with 64% conversion and Pr = 0.54 at room temperature and the polymer molecular weight was near the expected value. While complex 21 differs from complex 20 by substitution of Zn-N(SiMe3)2 with a sodium cation, it was not found to be active under the same reaction conditions. The group also synthesized an oxacalix[3]arene complex for the first time, the synthetic scheme of which is given in Scheme 9. After removing the volatiles from a reaction between 3 equiv of Zn(C6F5)2·toluene and oxacalix[3]arene at room temperature, compound 22 was formed. However, while attempting crystallization from hot acetonitrile, ring opening and rearrangement of the parent oxacalix[3]arene were observed. Compound 22 was found to be active in ROP at high temperature, leading to the possibility of ring opening of the parent oxacalix[3]arene and rearrangement.
Scheme 9. Synthesis of Complex 22.
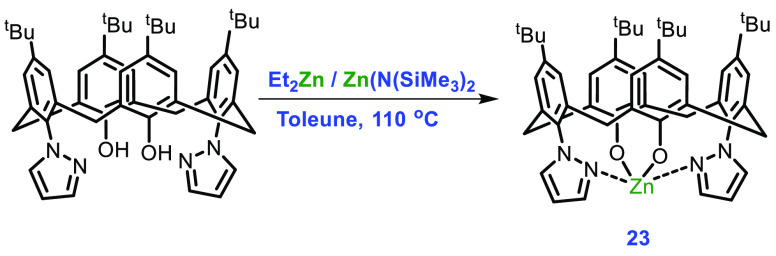
Rawat et al. in 2019 reported for the first time a monometallic Zn complex of an oxygen-depleted p-tert-butylcalix[4]arene ligand in the ROP of rac-LA.58 Complex 23 was synthesized in quantitative yield by treating 1,3-bis(pyrazole)-p-tert-butylcalix[4]arene with ZnEt2/Zn(HMDS)2 in toluene at 110 °C. According to a qualitative study, the oxygen of the 1,3-phenols has substituted the two ethyl groups of the reagent, indicating the development of a monometallic Zn complex. Diffusion NMR studies further confirmed the structure of monometallic Zn complex 23 with diffusion constants for the complex and ligand of 0.4926 × 10–5 and 0.4979 × 10–5 cm2 s–1, respectively (Scheme 10). The coordination mode in complex 23 was verified by preparing the 15N-labeled equivalent of 23 and studying its 15N NMR. Complex 23 was found to be effective when ROP was carried out in toluene at 110 °C, which afforded the polymer with a molecular weight of 29 kDa and a PDI of 1.78.
Scheme 10. Synthesis of Complex 23.
2.3. Magnesium-Calixarene Complexes in ROP
Magnesium compounds show exceptionally high activity for the ROP of rac-LA compared to their zinc counterparts.59−61 However, the use of calix[4]arene-Mg complexes in this area remains scant. Monoanionic ligands are preferred for reactions with Mg precursors. They invariably result in a metal with a viable nucleophilic group for ROP, which may explain why calix[4]arenes are seldom used.
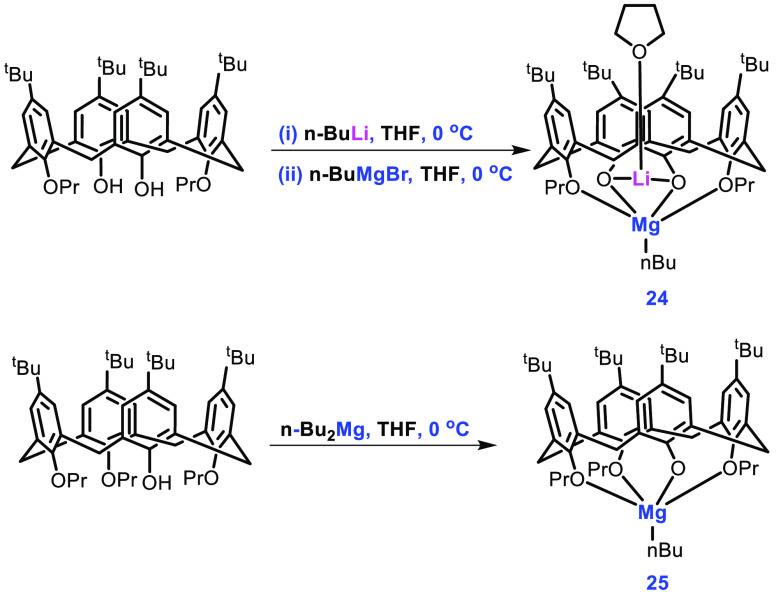
Redshaw in 2014 reported the synthesis of heterobimetallic complex 24 and monometallic Mg complex 25.62 For the synthesis of complex 24, lithiation of 1,3-dipropoxy-p-tert-butylcalix[4]arene was carried out with nBuMgBr in THF and, for complex 25, tripropoxy-p-tert-butylcalix[4]arene was treated with n-Bu2Mg (Scheme 11). By using a single-crystal XRD technique, it was reported that both complexes exist in a cone conformation with the Li atom (in 24) residing in the cavity of the calixarene. Both of the catalysts were proven to be effective for the ROP of rac-LA, with complex 24 showing a conversion of 94%, while complex 25 displayed a conversion of 99%.
Scheme 11. Synthesis of Complexes 24 and 25.
Initially, the polymerization of rac-LA was attempted by using benzyl alcohol as an activator. However, this led to the formation of methyl-(R,S)-lactate instead of the desired PLA product. The author reported that using 1 equiv of MeOH in combination with catalyst 24 resulted in greater activity for the ROP of rac-LA in CH2Cl2 than any other solvent. Other alcohols as activators were also found to be ineffective. In contrast to 24, compound 25 showed increased activities in THF and toluene, rather than CH2Cl2, with conversions of 97%, 99%, and 55%, respectively. For catalyst 25, the addition of alcohols (i-PrOH, t-BuOH, or BnOH) other than MeOH led to increased activities, particularly when BnOH was used, which gave a 92% conversion of rac-LA in 3 min. PLA obtained from both catalysts had a respectable PDI of 1.19 with catalyst 24 and 1.25 with catalyst 25.
2.4. Potassium-Calixarene Complexes in ROP
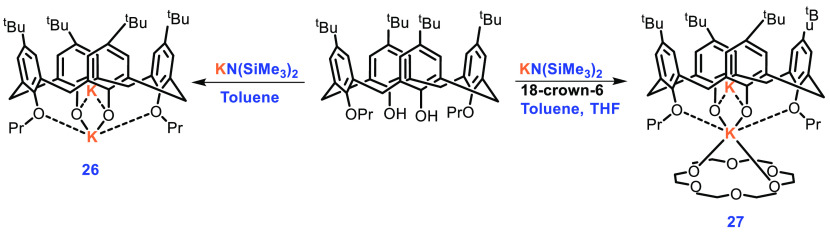
Wu et al. based their synthesis of K-calixarene complexes on the fact that potassium is nontoxic and calix[4]arenes show a good inclusion effect. They were able to synthesize four calix[4]arene potassium complexes and reported their use in the ROP of rac-LA.63 Complex 26 was prepared in 50.7% yield by the treatment of distal functionalized 1,3-dipropoxy tert-butyl calix[4]arene and KN(SiMe3)2. Complex 27 could be obtained from 26 in moderate yield (34.5%) by reacting with 18-crown-6 in a mixed solvent of toluene and THF at room temperature. On the other hand, direct treatment of the ligand with KN(SiMe3)2 and 18-crown-6 also afforded the desired complex 27 (Scheme 12).
Scheme 12. Synthesis of Complexes 26 and 27.
Unsurprisingly, complex 26 showed a cone conformation with one K atom at the exo position with the second K atom occupying the endo position. Complex 26 also shows aggregation, due to which several active sites were possible, and as expected 26 was active in ROP. The oxygen atoms of crown ether caps the potassium ion in an exo position in complex 27. The other K atom, as in complex 26, is occupying the cavity of the calix[4]arene. Four oxygen atoms of the calix[4]arene ligand and two oxygen atoms of 18-crown-6 are sandwiched around the possible catalytic center. A quantitative conversion in ROP of rac-LA was observed within 3 min when a 200:1:2 monomer:catalyst:activator ratio was used with a complex concentration of 2.0 mM in 5.0 mL of toluene at room temperature, while the same reaction consumed 5.5 h in CH2Cl2 to reach a 97% yield, and only 95% lactide could be converted to polymer in THF within 10 h. It was discovered that, when 1.0 equiv of BnOH was used as an activator, the molecular weights of the final polymers were lower than predicted, which may be due to a cyclization side reaction caused by the phenoxy groups. To suppress the side reactions, polymerization was conducted with 2, 5, and 10 equiv of BnOH to good effect with a conversion as high as 98%, but with an increasing concentration of activator the polymer was obtained as a wider PDI of 1.48.
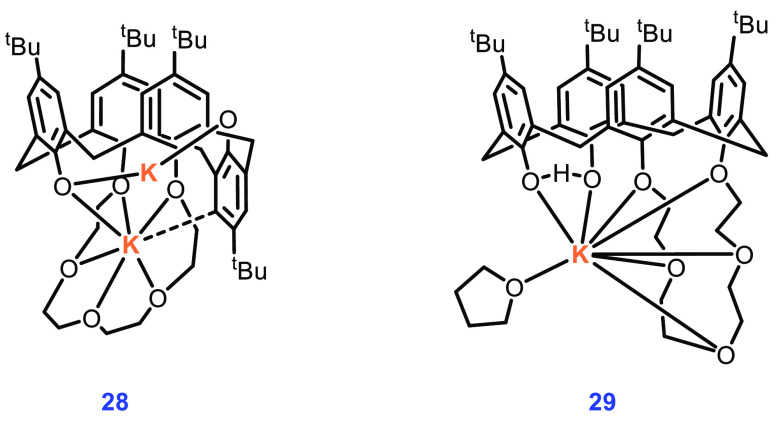
Compounds 28 and 29 were prepared from tert-butyl calix[4]arene with the aim to synthesize other bimetallic complexes bearing a crown ether as a second auxiliary ligand. Structural analysis with NMR and single-crystal XRD indicated the formation of the dinuclear complex 28. The rotation of two phenoxy groups leads to a quick transformation equilibrium reaction in solution (Figure 8). On the other hand, 29 is a stable mononuclear complex, possibly due to intramolecular hydrogen bonding; consequently, it shows substantially lower activity in ROP. Complex 28, which contains a 1,3-substituent crown moiety, is also a highly active catalyst for the ROP of rac-LA with 2 equiv of BnOH as a coinitiator, but final polymer molecular weights were smaller than expected (Mn.cal 12900 g/mol; Mn,obs 6200 g/mol), possibly due to some side reactions, like transesterification and/or cyclization. It was observed that a 10-fold increase in the amount of BnOH led to faster polymerization, partially suppressing the side reactions due to phenoxides. The molecular weights of polymers were found to be closer to the calculated Mn now (Mn,cal 2900 g/mol; Mn,obs 2700 g/mol); the dispersity values of the polymers were also low.
Figure 8.
Structures of complexes 28 and 29.
2.5. Zirconium Complexes
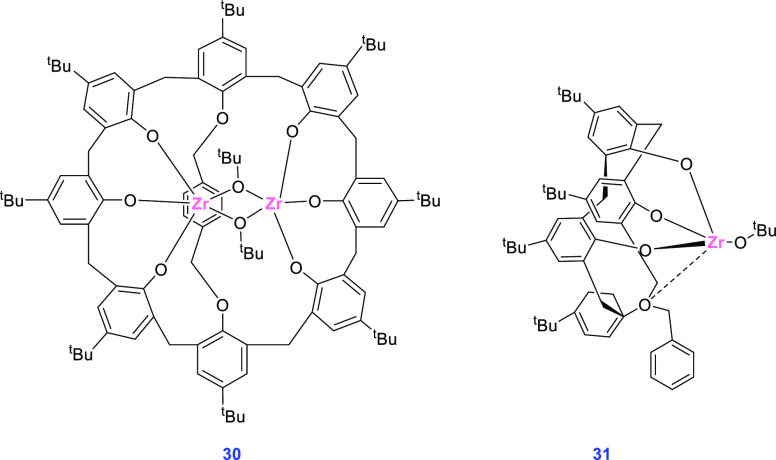
Zr complexes are of particular interest in the ROP of cyclic esters due to their low toxicity and high reactivity in contrast to the corresponding Ti complexes in the polymerization process. Researchers have used a bridge between the different phenolic groups of the calixarene scaffold to minimize the number of conformers that the ligand could attain and to keep the metal atoms in an open catalytic pocket. As a result, Neri and colleagues developed the di-Zr 1,5-bridged calix[8]arene complex 30 with a xylene functionality, which they used in the ROP of cyclic esters. The polymerization results were then compared with those obtained using a mono-Zr complex featuring a calix[4]arene ligand.
The dinuclear heteroleptic complex 30 was obtained by protonolysis of 1,5-bridged calix[8]arene with 2 equiv of Zr(IV) tert-butoxide in toluene (or THF) at room temperature (Figure 9). The mononuclear Zr complex 31, with a chemical environment as similar as possible to that of the dinuclear complex 30, was also prepared and tested for polymerization of rac-LA to verify the importance of two close Zr atoms. The derived complex (31) was synthesized by metathesis with 1 equiv of Zr(IV) tert-butoxide.
Figure 9.
Structures of Zr complexes 30 and 31.
Using a monomer/initiator ratio of 100:1, complex 30 was screened in the ROP of rac-LA at various temperatures. It was observed that, with increasing temperature, the activity improved rapidly, and at 100 °C polymerization was completed in 30 min, with a turnover frequency of 194 h–1. Complex 30, which possesses C2v symmetry, having Zr atoms lying in the vicinity, was highly active in the ROP of rac-lactide. One of the reasons for this could be the backbiting of the growing polymer chain that promotes a chain reaction. DFT calculations proved that chain folding in complex 30 was exergonic, while that of complex 31 was slightly endergonic. Hence, all polymerization reactions carried out by complex 31 led to polymers with monomodal and narrow molecular weight distributions (PDI = 1.10–1.30).64
3. Mechanistic Aspects of ROP by Calixarene-Metal Complexes
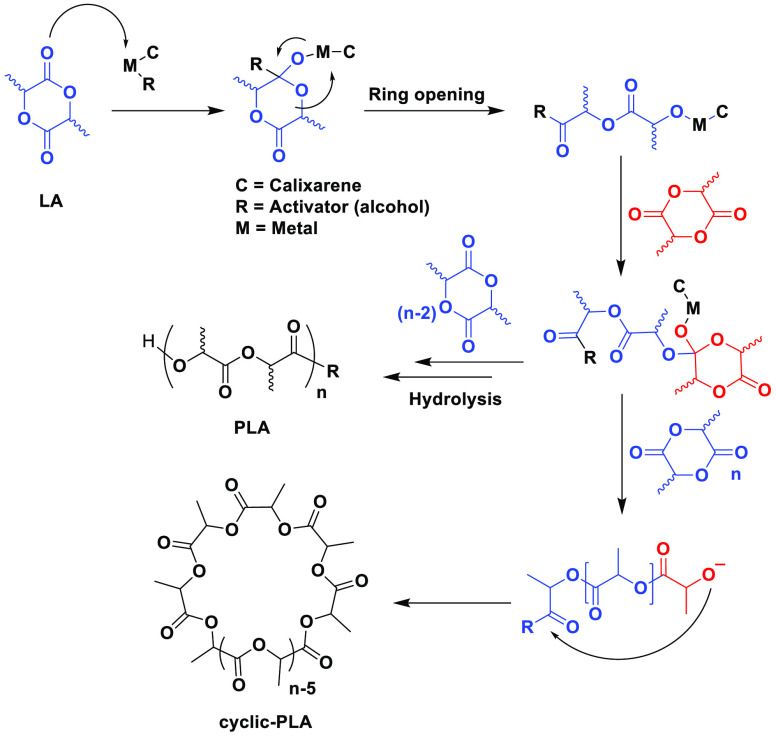
As previously mentioned, the ROP of LA is the best way to achieve the synthesis of a high-molecular-weight PLA. In comparison to polycondensation, ROP of LA can generate a polymer with a wide molecular weight range by regulating monomer purity and synthesis conditions without the use of a chain coupling agent or an azeotropic method. Various ions initiate ring-opening polymerization, which is a type of chain-growth mechanism for the polymerization of cyclic monomers. The terminal end of a polymer, as the reactive center of propagation, divides the system into anionic ROP, cationic ROP, and radical ROP. For PLA, the cyclic monomer (lactide, which is the cyclic dimer of lactic acid) is attacked by the terminus of the growing polymer chain leading to propagation (Figure 10). It should also be mentioned that alcohols as activators are likely to react with the metal–halogen bonds to result in a metal-alkoxide. Such species are known to be faster catalysts in the ROP of cyclic esters. In the case of Zn complexes 14, 15, and 23, an external alcohol (or activator) was not required to initiate ROP.53−58 Talatta et al. reported that the bimetallic or multimetallic calixarene complexes show higher activity than the corresponding monometallic analogues, clearly indicating that a cooperative interaction between two metals is a determinant for controlling the performance of ROP.64 Generally, alcohol is needed as an initiator in the lactide polymerization process that is facilitated by metal complexes. Through ligand exchange, the alcohol first reacts with metal centers to generate metal-alkoxides. The catalyst’s metal atom then briefly coordinates with the exocyclic carbonyl oxygen of the lactide, increasing both the carbonyl group’s electrophilicity and the nucleophilicity of the initiator’s alkoxide group. Then, the lactide’s acyl-oxygen bond breaks, permitting insertion into the catalyst’s metal–oxygen bond (alkoxide). The polymer is created by repeatedly introducing new lactide molecules into the metal–oxygen link.
Figure 10.
General mechanism for the ROP of LA.
Table 2 describes the efficiency of various calixarene-based catalysts in lactide polymerization presented in this review: the catalyst number, PDI, and molecular weight (Mw) of the polymer formed and corresponding references are provided.
Table 2. Summary of Calixarene-Based Catalysts in Lactide Polymerization.
| entry | catalyst | PDI | Mw (g/mol) | ref |
|---|---|---|---|---|
| 1 | 1 | 1.16 | 42000 | (43) |
| 2 | 2 | 1.23 | 22400 | (44) |
| 3 | 3 | not reported | not reported | (46) |
| 4 | 4 | 1.68 | 13520 | (49) |
| 5 | 5 | 1.94 | 11170 | (49) |
| 6 | 6 | 2.09 | 15770 | (49) |
| 7 | 7 | 1.04 | 22040 | (49) |
| 8 | 8 | 1.94 | 69950 | (49) |
| 9 | 9 | 1.29 | 8190 | (50) |
| 10 | 14 | 1.06 | 143000 | (56) |
| 11 | 15 | 1.45 | 72000 | (56) |
| 12 | 19 | 1.26 | 1440 | (57) |
| 13 | 20 | 1.13 | 8970 | (57) |
| 14 | 23 | 1.78 | 29000 | (58) |
| 15 | 24 | 1.19 | 1790 | (62) |
| 16 | 25 | 1.25 | 14800 | (62) |
| 17 | 26 | 1.42 | 13400 | (63) |
| 18 | 27 | 1.21 | 13400 | (63) |
| 19 | 28 | 1.08 | 2700 | (63) |
| 20 | 29 | 1.06 | 2800 | (63) |
| 21 | 30 | 1.23 | 705 | (64) |
| 22 | 31 | 1.14 | 2363 | (64) |
4. Some Recent Examples of Catalysts Used in ROP of LA
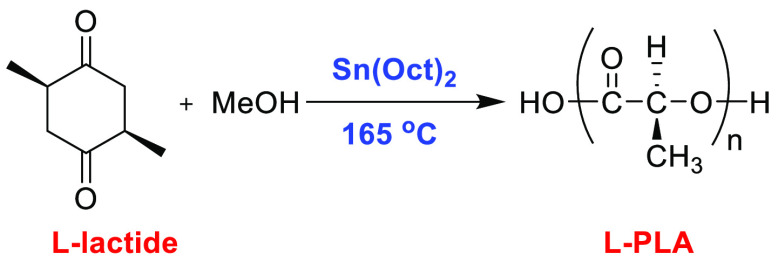
Stannous octoate (Sn(Oct)2) has been extensively used in industry for the ROP of LA due to its solubility in a monomer melt, high reaction rate, and potential to generate higher molecular weight polymers.65 Morris et al. were the first to study the role of alcohols as initiators in the ROP of LA. His group has utilized different alcoholic systems (1-dodecanol, 1-octanol, methanol) and studied the effect on yield, thermal properties, and stability in the presence of Sn(Oct)2 as a catalyst. It was observed that methanol was found to be an effective initiator, which afforded the polymer in an 89% yield, with an Mn value of 11800 g/mol and a PDI of 1.22 (Scheme 13).
Scheme 13. Synthesis of PLA Using Stannous Octoate.
Guanidines are neutral ligands of high donor strength capable of forming metal complexes with a wide variety of coordinations. Guanidine-based metal complexes have proven to be superior catalysts in lactide polymerization sustainability and decreased toxicity. They are stable at high temperatures in the presence of residual protic impurities, show promising activity, and lead to a high-molecular-mass polymer.66 Many Zn- and Fe-guanidine complexes have been synthesized and examined toward the ROP of LA, but complex 32 was found to be the most effective with a PDI of 1.4 and Mn of 71000 g/mol (Figure 11).
Figure 11.
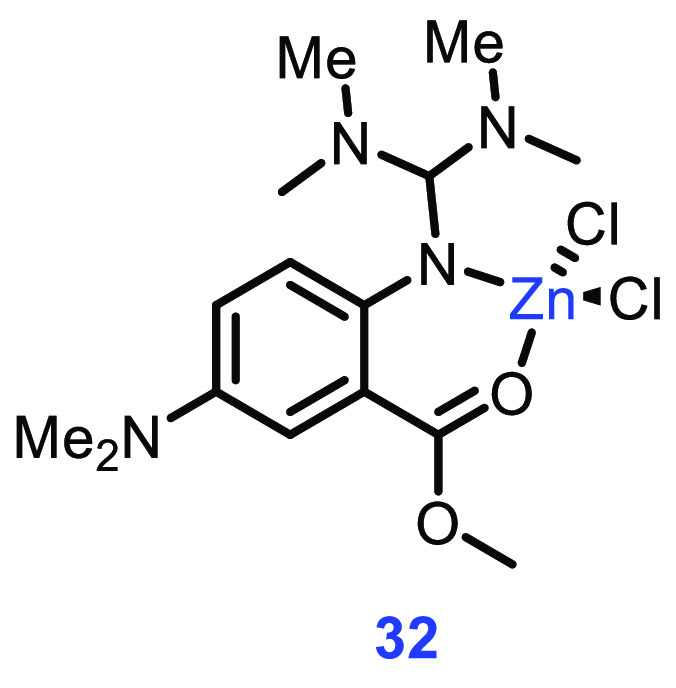
Structure of complex 32.
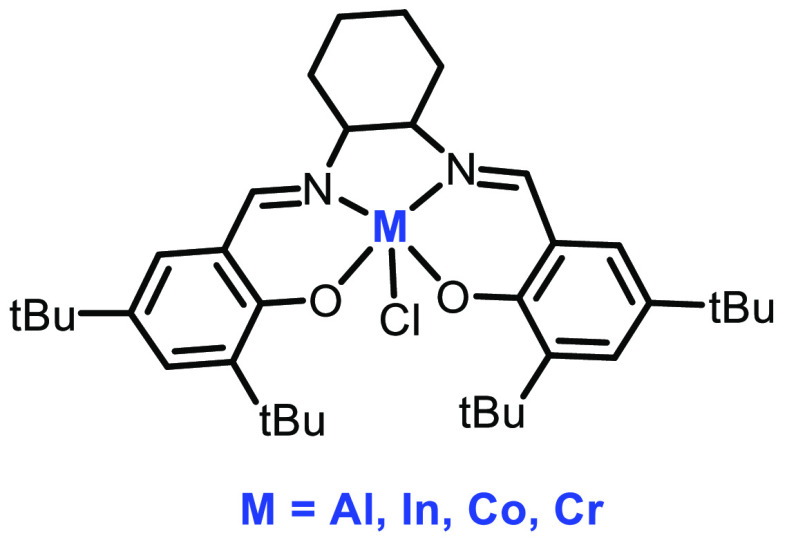
Phomphrai and co-workers explored the use of cyclohexylsalen-ligated metal complex 33 (M = Al(III), In(III), Cr(III), and Co(III)) in ROP of LA. The catalytic system is comprised of a metal complex, initiator, and epoxide as solvent. All the complexes were examined for ROP, but the (Cy-salen)InCl complex showed the highest activity and afforded a polymer with a PDI of 1.23 and a molecular weight of 4300 Da (Figure 12). One possible reason for this could be the larger ionic radius of In; hence, the coordination environment of the ligand affects the rate of polymerization.67
Figure 12.
Structure of complex 33.
Phomphrai et al. also reported the synthesis and application of several metal complexes of tetraphenylporphyrin (TPP) in the ROP of LA (Figure 13). The (TPP)AlCl catalyst is one of the few reported complexes to be active in polymerizing rac-LA at room temperature, leading to a isotactic polylactide. The ionic initiator PPN+Cl– was proven to have higher catalytic activity compared to a neutral initiator such as pyridine or DMAP, and among the many metal complexes, Cr-TPP 34 was found to be the most active, producing polymers with a good PDI (1.08) and molecular weight (5150 Da).67
Figure 13.
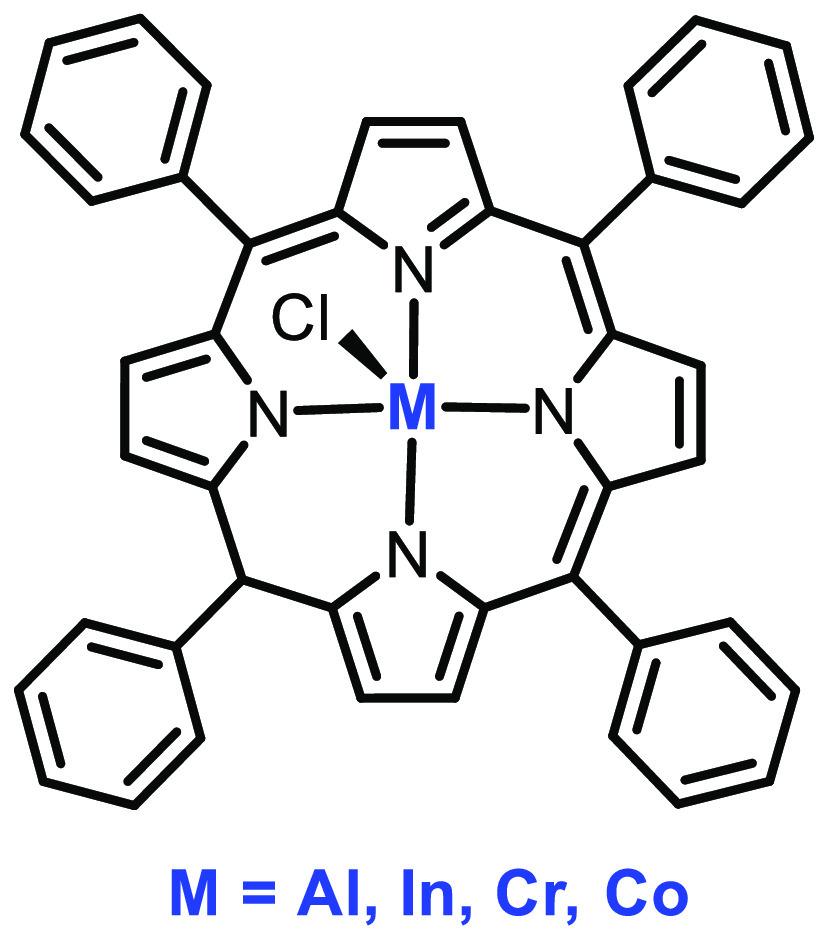
Structure of complex 34.
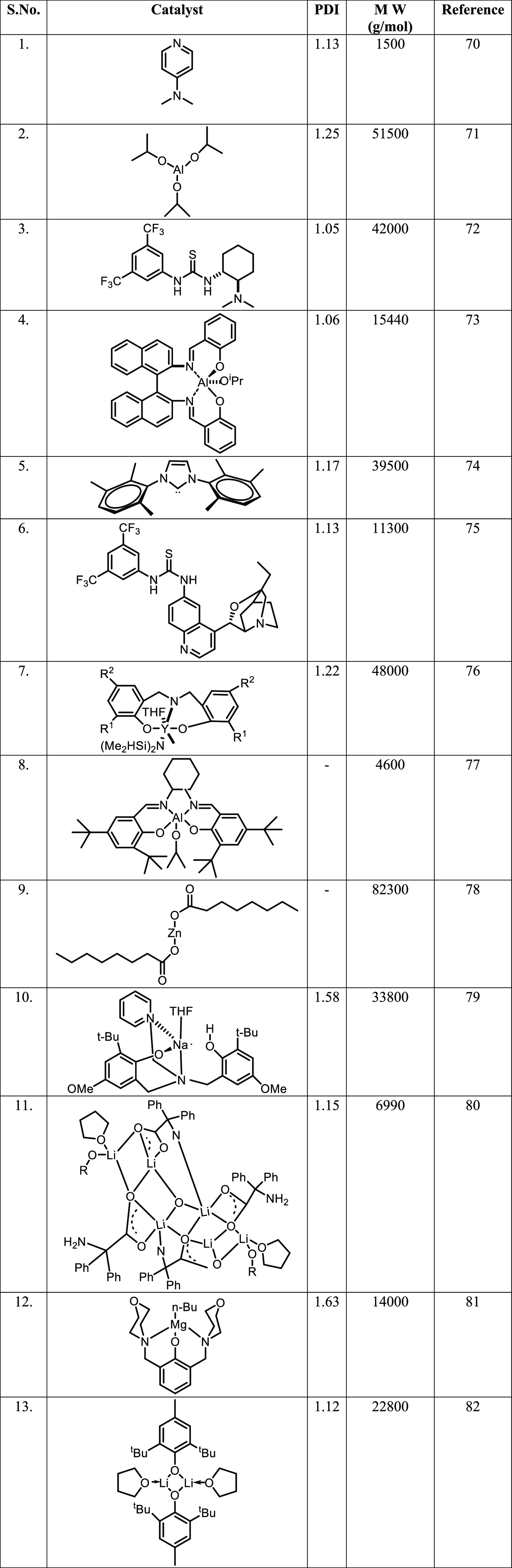
Although ROP of LA is the method of choice, Yamaguchi in 1995 reported high-molecular-weight synthesis (300000 Da) of PLA by polycondensation of l-lactic acid with Sn powder at 130 °C.68 Kimura et al. also reported a polycondensation route to high-molecular-weight poly(l-lactic acid) from l-lactic acid.69 In their protocol, a lactic acid melt was created by heat treatment at around 105 °C in the presence of a binary system of Sn(II) chloride hydrate/p-toluenesulfonic acid and further heating at 150 °C (10–30 h) for polycondensation. This route gave a polycondensate with a high molecular weight (2 × 104) in a relatively short reaction time. The polymer formed by this procedure was comparable to the poly(l-lactic acid) obtained by ring-opening polymerization of l-lactide.69 Although the FDA has approved Sn and its salt as a food additive, the toxicity of most tin compounds is a significant disadvantage in biomedical applications. Table 3(70−82) gives a few examples of notable catalysts used for polylactide preparation.
Table 3. Some Notable Catalysts Used in Lactide Polymerization.
Conclusion
The production of PLA using different metal-based calixarene complexes, which have been known in the past few decades, are reported in this review. Due to the unique properties possessed by PLA, like biodegradability, biocompatibility, and mechanical stability, it offers a wide range of applications in packaging units, textile industries, and medicinal fields. Ring-opening polymerization (ROP) is an efficient and convenient method for the synthesis of high-molecular-weight polylactide. These calix[n]arene-metal complexes offered advantages such as a high yield and PDI and a shorter reaction time. Comparing the different catalyst systems also show that complexes having more than one metal center may not always be more efficient in ROP. For instance, monometallic complexes 25, 29, and 31 are more efficient (better PDI and higher molecular weight) when compared to their multimetallic counterparts. There is a huge scope for the extension of the applications of calixarene-metal complexes in polymerization, since only a limited number of metals such as Zn, Ti, Mg, K, and Zr incorporated with a calixarene scaffold have been reported. Others metals like Li, Fe(III), Cr, and Co may also be employed for lactide polymerization, but no previous reports are available. Predominantly, the controlled ROP could lead to a polymer with specific and needed properties (e.g., refractive index, molecular weight), making it a very important catalyst in polymer synthesis nowadays.
Acknowledgments
G.S., Y.B., and A.L. thank Prof. A. K. Yadav (Director) and Prof. S. R. Pathak (HOD), ASAS, for their constant encouragement and support.
Author Contributions
G.S. and Y.B. contributed equally.
Author Contributions
The manuscript was written through the contributions of all authors.
The authors declare no competing financial interest.
References
- Bayer A. Ueber die Verbindungen der Aldehyde mit den Phenolen. Ber. Dtsch. Chem. Ges. 1872, 5, 280–282. 10.1002/cber.18720050186. [DOI] [Google Scholar]
- Gutsche C. D.; Muthukrishnan R. Calixarenes. 1. Analysis of the product mixtures produced by the base-catalyzed condensation of formaldehyde with para-substituted phenols. J. Org. Chem. 1978, 43 (25), 4905–4906. 10.1021/jo00419a052. [DOI] [Google Scholar]
- Gutsche C. D.Calixarenes revisited, monographs in supramolecular chemistry; Stoddart J. F., Ed.; The Royal Society of Chemistry: 1998. [Google Scholar]
- Ludwig R. Calixarenes in analytical and separation chemistry. Fresenius’ J. Anal. Chem. 2000, 367 (2), 103–128. 10.1007/s002160051611. [DOI] [PubMed] [Google Scholar]
- Ludwig R.; Dzung N. T. K. Calixarene-based molecules for cation recognition. Sens. 2002, 2 (10), 397–416. 10.3390/s21000397. [DOI] [Google Scholar]
- Kim S. K.; Sessler J. L. Ion pair receptors. Chem. Soc. Rev. 2010, 39 (10), 3784–3809. 10.1039/c002694h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzen N.; Goldberg I.; Lipstman S.; Vigalok A. Unexpected Pt(II) migration between the calixarene oxygen atoms. Inorg. Chem. 2006, 45 (14), 5266–5268. 10.1021/ic0606126. [DOI] [PubMed] [Google Scholar]
- Keck M.; Hoof S.; Rawat V.; Vigalok A.; Limberg C. The Coordination Behavior of Oxygen-depleted Calixarenes towards d10 Noble Metal Ions. Zeitschrift für anorganische und allgemeine Chemie 2020, 646, 904–908. 10.1002/zaac.202000138. [DOI] [Google Scholar]
- Arduini A.; Casnati A.. Calixarenes, in macrocycle synthesis: a practical approach, Parker D., Ed.; Oxford University Press: 1996; pp 145–173. [Google Scholar]
- Albano C.; Cataño L.; Figuera L.; Perera R.; Karam A.; González G.; Noris K. Evaluation of a composite based on high-density polyethylene filled with surface-treated hydroxyapatite. Polym. Bull. 2009, 62, 45–55. 10.1007/s00289-008-1011-x. [DOI] [Google Scholar]
- Nagalakshmaiah M.; Afrin S.; Malladi R. P.; Elkoun S.; Robert M.; Ansari M. A.; Svedberg A.; Karim Z.. Biocomposites: Present trends and challenges for the future. Green Composites for Automotive Applications; Woodhead Publishing: 2019; pp 197–215. [Google Scholar]
- Nagalakshmaiah M.; Nechyporchuk O.; El-Kissi N.; Dufresne A. Melt extrusion of polystyrene reinforced with cellulose nanocrystals modified using poly [(styrene)-co-(2-ethylhexyl acrylate)] latex particles. Eur. Polym. J. 2017, 91, 297–306. 10.1016/j.eurpolymj.2017.04.020. [DOI] [Google Scholar]
- Luzi F.; Torre L.; Kenny J. M.; Puglia D. Bio- and fossil-based polymeric blends and nanocomposites for packaging: structure–property relationship. Mater. 2019, 12 (3), 471–520. 10.3390/ma12030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce A. K.; O’ Reilly R. K. Polymers for Biomedical Applications: The Importance of Hydrophobicity in Directing Biological Interactions and Application Efficacy. Biomacromolecules 2021, 22 (11), 4459–4469. 10.1021/acs.biomac.1c00434. [DOI] [PubMed] [Google Scholar]
- Bolívar-Monsalve E. J.; Alvarez M. M.; Hosseini S.; Espinosa-Hernandez M. A.; Ceballos-González C. F.; Sanchez-Dominguez M.; Shin S. R.; Cecen B.; Hassan S.; Maiog E. D.; Santiago G. T.-de Engineering bioactive synthetic polymers for biomedical applications: a review with emphasis on tissue engineering and controlled release. Mater. Adv. 2021, 2, 4447–4478. 10.1039/D1MA00092F. [DOI] [Google Scholar]
- Bharath K. N.; Basavarajappa S. Applications of biocomposite materials based on natural fibers from renewable resources: a review. Sci. Eng. Compos. Mater. 2016, 23 (2), 123–133. 10.1515/secm-2014-0088. [DOI] [Google Scholar]
- Amici J.; Torchio C.; Versaci D.; Dessantis D.; Marchisio A.; Caldera F.; Bella F.; Francia C.; Bodoardo S. Nanosponge-based composite gel polymer electrolyte for safer Li-O2 batteries. Polymers 2021, 13 (10), 1625–1639. 10.3390/polym13101625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomietto P.; Russo F.; Galiano F.; Loulergue P.; Salerno S.; Paugam L.; Audic J. L.; Bartolo L. D.; Figoli A. Sustainable fabrication and pervaporation application of bio-based membranes: Combining a polyhydroxyalkanoate (PHA) as biopolymer and Cyrene as green solvent. J. Membr. Sci. 2022, 643, 120061–120077. 10.1016/j.memsci.2021.120061. [DOI] [Google Scholar]
- Ramesh M.; Palanikumar K.; Reddy K. H. Plant fibre based bio-composites: Sustainable and renewable green materials. Renew. Sust. Energy Rev. 2017, 79, 558–584. 10.1016/j.rser.2017.05.094. [DOI] [Google Scholar]
- Zhang Z.; Lu J.; Zhang Z.; Yang J.; Xin K.; Zhao Z.; An L.; Kong D. Effect of potassium ferrate treatment on adhesive gelatinous biopolymer structure and erosion resistance of sewer sediments: Promotion or inhibition?. Chem. Eng. J. 2022, 431 (1), 134025–134038. 10.1016/j.cej.2021.134025. [DOI] [Google Scholar]
- Abdah M. A. A. M.; Mokhtar M.; Khoon L. T.; Sopian K.; Dzulkurnain N. A.; Ahmad A.; Sulaiman Y.; Bella F.; Su’ait M. S. Synthesis and electrochemical characterizations of poly(3,4-ethylenedioxythiophene/manganese oxide coated on porous carbon nanofibers as a potential anode for lithium-ion batteries. Energy Rep. 2021, 7, 8677–8687. 10.1016/j.egyr.2021.10.110. [DOI] [Google Scholar]
- Bian X.; Zhang B.; Sun Z.; Xiang S.; Li G.; Chen X. Synthesis of multi-arm poly(L-lactide) and its modification on linear polylactide. Polym. Bull. 2017, 74, 245–262. 10.1007/s00289-016-1713-4. [DOI] [Google Scholar]
- Taib N. A. A. B.; Rahman M. R.; Huda D.; Kuok K. K.; Hamdan S.; Bakri M. K. B.; Julaihi M. R. M. B.; Khan A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179. 10.1007/s00289-022-04160-y. [DOI] [Google Scholar]
- Cheng Y.; Deng S.; Chen P.; Ruan R. Polylactic acid (PLA) synthesis and modifications: a review. Front. Chem. China 2009, 4 (3), 259–264. 10.1007/s11458-009-0092-x. [DOI] [Google Scholar]
- Hu Y.; Daoud W. A.; Cheuk K. K. L.; Lin C. S. K. Newly developed techniques on polycondensation, ring-opening polymerization and polymer modification: Focus on poly (lactic acid). Mater. 2016, 9 (3), 133. 10.3390/ma9030133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford M. J.; Dove A. P. Stereocontrolled ring-opening polymerisation of lactide. Chem. Soc. Rev. 2010, 39 (2), 486–494. 10.1039/B815104K. [DOI] [PubMed] [Google Scholar]
- Subramanian B.; Sanjeeviraja C.; Jayachandran M. Review on materials properties of Sn (S, Se) compound semiconductors useful for photoelectrochemical solar cells. Bull. Electrochem. 2002, 18 (8), 349–366. [Google Scholar]
- Mostovaya O. A.; Gorbachuk V. V.; Padnya P. L.; Vavilova A. A.; Evtugyn G. A.; Stoikov I. I. Modification of oligo-and polylactides with macrocyclic fragments: synthesis and properties. Front. Chem. 2019, 7, 554. 10.3389/fchem.2019.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taşkin E.; Hazer B.; Beşirli N.; Çavuş G. Synthesis of some novel blends of polylactide with polylactide-b-poly (ethylene glycol) block copolymers. J. Macromol. Sci. A 2012, 49 (2), 164–170. 10.1080/10601325.2012.642222. [DOI] [Google Scholar]
- Hazer B.; Baysal B. M.; Köseoğlu A. G.; Beşirli N.; Taşkın E. Synthesis of polylactide-b-poly (Dimethyl Siloxane) block copolymers and their blends with pure Polylactide. J. Polym. Environ. 2012, 20 (2), 477–484. 10.1007/s10924-011-0406-1. [DOI] [Google Scholar]
- Soriente A.; De Rosa M.; Fruilo M.; Lepore L.; Gaeta C.; Neri P. Study on an aldol reaction catalyzed by Ti (IV)/calix [n] arene complexes. Adv. Synth. Catal. 2005, 347 (6), 816–824. 10.1002/adsc.200505023. [DOI] [Google Scholar]
- Ladipo F. T.; Sarveswaran V.; Kingston J. V.; Huyck R. A.; Bylikin S. Y.; Carr S. D.; Watts R.; Parkin S. Synthesis, characterization, and alkyne cyclotrimerization chemistry of titanium complexes supported by calixarene-derived bis (aryloxide) ligation. J. Organomet. Chem. 2004, 689 (3), 502–514. 10.1016/j.jorganchem.2003.10.039. [DOI] [Google Scholar]
- Ozerov O. V.; Patrick B. O.; Ladipo F. T. Highly Regioselective [2+ 2+ 2] Cycloaddition of terminal alkynes catalyzed by η6-arene complexes of titanium supported by dimethylsilyl-bridged p-tert-butyl calix [4] arene ligand. J. Am. Chem. Soc. 2000, 122 (27), 6423–6431. 10.1021/ja994543o. [DOI] [Google Scholar]
- Massa A.; D’Ambrosi A.; Proto A.; Scettri A. Critical importance of molecular sieves in titanium (IV)–calix[4]arene catalyzed epoxidation of allylic alcohols. Tetrahedron Lett. 2001, 42 (10), 1995–1998. 10.1016/S0040-4039(01)00059-4. [DOI] [Google Scholar]
- Lee J.; Kim Y.; Do Y. Novel chlorotitanium complexes containing chiral tridentate Schiff base ligands for ring-opening polymerization of lactide. Inorg. Chem. 2007, 46 (19), 7701–7703. 10.1021/ic700493p. [DOI] [PubMed] [Google Scholar]
- Chmura A. J.; Davidson M. G.; Jones M. D.; Lunn M. D.; Mahon M. F.; Johnson A. F.; Khunkamchoo P.; Roberts S. L.; Wong S. S. Group 4 complexes with aminebisphenolate ligands and their application for the ring opening polymerization of cyclic esters. Macromolecules 2006, 39 (21), 7250–7257. 10.1021/ma061028j. [DOI] [Google Scholar]
- Dechy-Cabaret O.; Martin-Vaca B.; Bourissou D. Controlled ring-opening polymerization of lactide and glycolide. Chem. Rev. 2004, 104 (12), 6147–6176. 10.1021/cr040002s. [DOI] [PubMed] [Google Scholar]
- Redshaw C.; Rowan M. A.; Warford L.; Homden D. M.; Arbaoui A.; Elsegood M. R.; Dale S. H.; Yamato T.; Casas C. P.; Matsui S.; Matsuura S. Oxo-and imidovanadium complexes incorporating methylene-and dimethyleneoxo-bridged calix [3]- and -[4]arenes: synthesis, structures and ethylene polymerisation catalysis. Chem. - Eur. J. 2007, 13 (4), 1090–1107. 10.1002/chem.200600679. [DOI] [PubMed] [Google Scholar]
- Capacchione C.; Neri P.; Proto A. Polymerization of ethylene in the presence of 1, 3-dimethoxy-p-But-calix [4] arene titanium dichloride. NMR evidence of the cationic titanium compound generated by methylalumoxane. Inorg. Chem. Commun. 2003, 6 (4), 339–342. 10.1016/S1387-7003(02)00772-4. [DOI] [Google Scholar]
- Kuran W.; Listos T.; Abramczyk M.; Dawidek A. Epoxide Polymerization and Copolymerization with Carbon Dioxide Using Diethylaluminum. J. Macromol. Sci. - Pure Appl. Chem. A 1998, 35, 427–437. 10.1080/10601329808001987. [DOI] [Google Scholar]
- Arbaoui A.; Redshaw C.; Elsegood M. R.; Wright V. E.; Yoshizawa A.; Yamato T. Iron (III) and Zinc (II) calixarene complexes: synthesis, structural studies, and use as procatalysts for ε-caprolactone Polymerization. Chem. - Asian J. 2010, 5 (3), 621–633. 10.1002/asia.200900514. [DOI] [PubMed] [Google Scholar]
- Roy S. S.; Sarkar S.; Chakraborty D. Macrocycles in dual role: ancillary ligands in metal complexes and organocatalysts for the ring-opening polymerization of lactide. J. Incl. Phenom. Macrocycl. Chem. 2021, 100, 1–36. 10.1007/s10847-021-01045-x. [DOI] [Google Scholar]
- Frediani M.; Sémeril D.; Mariotti A.; Rosi L.; Frediani P.; Rosi L.; Matt D.; Toupet L. Ring opening polymerization of lactide under solvent-free conditions catalyzed by a chlorotitanium calix[4]arene complex. Macromol. Rapid Commun. 2008, 29 (18), 1554–1560. 10.1002/marc.200800383. [DOI] [Google Scholar]
- Frediani M.; Sémeril D.; Matt D.; Rosi L.; Frediani P.; Rizzolo F.; Papini A. M. Ring-opening polymerisation of rac-lactide using a calix [4] arene-based titanium (IV) complex. Int. J. Polym. Sci. 2010, 2010, 1–6. 10.1155/2010/490724. [DOI] [Google Scholar]
- Frediani M.; Sémeril D.; Matt D.; Rizzolo F.; Papini A. M.; Frediani P.; Rosi L.; Santella M.; Giachi G. L-Lactide polymerization by calix [4] arene-titanium (IV) complex using conventional heating and microwave irradiation. e-Polym. 2010, 10 (1), 1. 10.1515/epoly.2010.10.1.177. [DOI] [Google Scholar]
- Ryan J. D.; Gagnon K. J.; Teat S. J.; McIntosh R. D. Flexible macrocycles as versatile supports for catalytically active metal clusters. Chem. Commun. 2016, 52 (58), 9071–9073. 10.1039/C6CC00478D. [DOI] [PubMed] [Google Scholar]
- Espinas J.; Darbost U.; Pelletier J.; Jeanneau E.; Duchamp C.; Bayard F.; Boyron O.; Broyer J. P.; Thivolle-Cazat J.; Basset J. M.; Taoufik M.; et al. Titanacalixarenes in homogeneous catalysis: synthesis, conformation and catalytic activity in ethylene polymerisation. Eur. J. Inorg. Chem. 2010, 2010, 1349–1359. 10.1002/ejic.200901185. [DOI] [Google Scholar]
- Zanotti-Gerosa A.; Solari E.; Giannini L.; Floriani C.; Re N.; Chiesi-Villa A.; Rizzoli C. Titanium-carbon functionalities on an oxo surface defined by a calix [4] arene moiety and its redox chemistry. Inorg. Chim. Acta 1998, 270 (1–2), 298–311. 10.1016/S0020-1693(97)05863-5. [DOI] [Google Scholar]
- Sun Z.; Zhao Y.; Santoro O.; Elsegood M. R.; Bedwell E. V.; Zahra K.; Walton A.; Redshaw C. Use of titanocalix [4] arenes in the ring opening polymerization of cyclic esters. Catal. Sci. Technol. 2020, 10 (6), 1619–1639. 10.1039/C9CY02571E. [DOI] [Google Scholar]
- Santoro O.; Elsegood M. R.; Bedwell E. V.; Pryce J. A.; Redshaw C. Insights into the structures adopted by titanocalix [6 and 8] arenes and their use in the ring opening polymerization of cyclic esters. Dalton Trans. 2020, 49 (34), 11978–11996. 10.1039/D0DT02130J. [DOI] [PubMed] [Google Scholar]
- Jedrzejas M. J.; Setlow P. Comparison of the binuclear metalloenzymes diphosphoglycerate-independent phosphoglycerate mutase and alkaline phosphatase: their mechanism of catalysis via a phosphoserine intermediate. Chem. Rev. 2001, 101 (3), 607–618. 10.1021/cr000253a. [DOI] [PubMed] [Google Scholar]
- Pu L.; Yu H. B. Catalytic asymmetric organozinc additions to carbonyl compounds. Chem. Rev. 2001, 101 (3), 757–824. 10.1021/cr000411y. [DOI] [PubMed] [Google Scholar]
- Gardiner M. G.; Lawrence S. M.; Raston C. L.; Skelton B. W.; White A. H. Multinuclear metallocalix [4] arenes incorporating ethylzinc groups. Chem. Commun. 1996, 21, 2491–2492. 10.1039/cc9960002491. [DOI] [Google Scholar]
- Atwood J. L.; Junk P. C.; Lawrence S. M.; Raston C. L. Zinc dimerization of p-tert-butylcalix[4] arene. Supramol Chem. 1996, 7 (1), 15–17. 10.1080/10610279608054990. [DOI] [Google Scholar]
- Bukhaltsev E.; Goldberg I.; Vigalok A. Substituent-dependent formation of organotransition-metal bimetallic calix [4] arene inclusion complexes. Organometallics 2004, 23 (20), 4540–4543. 10.1021/om049568x. [DOI] [Google Scholar]
- Bukhaltsev E.; Frish L.; Cohen Y.; Vigalok A. Single-site catalysis by bimetallic zinc calixarene inclusion complexes. Org. Lett. 2005, 7 (23), 5123–5126. 10.1021/ol051741d. [DOI] [PubMed] [Google Scholar]
- Walton M. J.; Lancaster S. J.; Wright J. A.; Elsegood M. R.; Redshaw C. Zinc calixarene complexes for the ring opening polymerization of cyclic esters. Dalton trans. 2014, 43 (48), 18001–18009. 10.1039/C4DT02226B. [DOI] [PubMed] [Google Scholar]
- Sinha A. K.; Vigalok A.; Rawat V. Catalytic application of zinc complex of oxygen depleted 1, 3-bis (pyrazole)-p-tert-butylcalix [4] arene. Tetrahedron Lett. 2019, 60 (11), 796–799. 10.1016/j.tetlet.2019.02.017. [DOI] [Google Scholar]
- Chamberlain B. M.; Cheng M.; Moore D. R.; Ovitt T. M.; Lobkovsky E. B.; Coates G. W. Polymerization of lactide with Zinc and Magnesium β-diiminate complexes: stereocontrol and mechanism. J. Am. Chem. Soc. 2001, 123 (14), 3229–3238. 10.1021/ja003851f. [DOI] [PubMed] [Google Scholar]
- Chisholm M. H.; Choojun K.; Gallucci J. C.; Wambua P. M. Chemistry of magnesium alkyls supported by 1, 5, 9-trimesityldipyrromethene and 2-[(2, 6-diisopropylphenyl) amino]-4-[(2, 6-diisopropylphenyl) imino] pent-2-ene. A comparative study. Chem. Sci. 2012, 3 (12), 3445–3457. 10.1039/c2sc21017g. [DOI] [Google Scholar]
- Wang Y.; Zhao W.; Liu D.; Li S.; Liu X.; Cui D.; Chen X. Magnesium and zinc complexes supported by N, O-bidentate pyridyl functionalized alkoxy ligands: synthesis and immortal ROP of ε-CL and L-LA. Organometallics 2012, 31 (11), 4182–4190. 10.1021/om300113p. [DOI] [Google Scholar]
- Walton M. J.; Lancaster S. J.; Redshaw C. Highly Selective and Immortal Magnesium Calixarene Complexes for the Ring-Opening Polymerization of rac-Lactide. ChemCatChem. 2014, 6 (7), 1892–1898. 10.1002/cctc.201402041. [DOI] [Google Scholar]
- Li Y.; Zhao H.; Mao X.; Pan X.; Wu J. Structures of potassium calix [4] arene crown ether inclusion complexes and application in polymerization of rac-lactide. Dalton trans. 2016, 45 (23), 9636–9645. 10.1039/C6DT01417H. [DOI] [PubMed] [Google Scholar]
- Lapenta R.; De Simone N. A.; Buonerba A.; Talotta C.; Gaeta C.; Neri P.; Grassi A.; Milione S. Dinuclear zirconium complex bearing a 1, 5-bridged-calix [8] arene ligand as an effective catalyst for the synthesis of macrolactones. Catal. Sci. Technol. 2018, 8 (10), 2716–2727. 10.1039/C7CY02537H. [DOI] [Google Scholar]
- Pholharn D.; Srithep Y.; Morris J. Effect of initiators on synthesis of poly(L-lactide) by ring opening polymerization. Mater. Sci. Eng. 2017, 213, 012022. 10.1088/1757-899X/213/1/012022. [DOI] [Google Scholar]
- Schäfer P. M.; Herres-Pawlis S. Robust Guanidine Metal Catalysts for the Ring-Opening Polymerization of Lactide under Industrially Relevant Conditions. ChemPlusChem. 2020, 85 (5), 1044–1052. 10.1002/cplu.202000252. [DOI] [PubMed] [Google Scholar]
- Praban S.; Piromjitpong P.; Balasanthiran V.; Jayaraj S.; Chisholm M. H.; Tantirungrotechai J.; Phomphrai K. Highly efficient metal(III) porphyrin and salen complexes for the polymerization of rac-lactide under ambient conditions. Dalton trans. 2019, 48, 3223–3230. 10.1039/C8DT04699A. [DOI] [PubMed] [Google Scholar]
- Ajioka M.; Enomoto K.; Suzuki K.; Yamaguchi A. Basic Properties of Polylactic Acid Produced by the Direct Condensation Polymerization of Lactic Acid. Bull. Chem. Soc. Jpn. 1995, 68 (8), 2125–2131. 10.1246/bcsj.68.2125. [DOI] [Google Scholar]
- Moon S. I.; Lee C. W.; Taniguchi I.; Miyamoto M.; Kimura Y. Melt/solid polycondensation of L-lactic acid: an alternative route to poly(l-lactic acid) with high molecular weight. Polymer 2001, 42, 5059–5062. 10.1016/S0032-3861(00)00889-2. [DOI] [Google Scholar]
- Nederberg F.; Connor E. F.; Möller M.; Glauser T.; Hedrick J. L. New paradigms for organic catalysts: the first organocatalytic living polymerization. Angew. Chem., Int. Ed. 2001, 40 (14), 2712–2715. . [DOI] [PubMed] [Google Scholar]
- Dubois P.; Jacobs C.; Jerome R.; Teyssie P. Macromolecular engineering of polylactones and polylactides. 4. Mechanism and kinetics of lactide homopolymerization by aluminum isopropoxide. Macromolecules 1991, 24 (9), 2266–2270. 10.1021/ma00009a022. [DOI] [Google Scholar]
- Dove A. P.; Pratt R. C.; Lohmeijer B. G. G.; Waymouth R. M.; Hedrick J. L. Thiourea-based bifunctional organocatalysis: supramolecular recognition for living polymerization. J. Am. Chem. Soc. 2005, 127 (40), 13798–13799. 10.1021/ja0543346. [DOI] [PubMed] [Google Scholar]
- Ovitt T. M.; Coates G. W. Stereochemistry of lactide polymerization with chiral catalysts: new opportunities for stereocontrol using polymer exchange mechanisms. J. Am. Chem. Soc. 2002, 124 (7), 1316–1326. 10.1021/ja012052+. [DOI] [PubMed] [Google Scholar]
- Connor E. F.; Nyce G. W.; Myers M.; Möck A.; Hedrick J. L. First example of n-heterocyclic carbenes as catalysts for living polymerization: organocatalytic ring-opening polymerization of cyclic esters. J. Am. Chem. Soc. 2002, 124 (6), 914–915. 10.1021/ja0173324. [DOI] [PubMed] [Google Scholar]
- Zhu J.-Bo.; Chen E. Y.-X. From meso-lactide to isotactic polylactide: epimerization by b/n Lewis pairs and kinetic resolution by organic catalysts. J. Am. Chem. Soc. 2015, 137 (39), 12506–12509. 10.1021/jacs.5b08658. [DOI] [PubMed] [Google Scholar]
- Amgoune A.; Thomas C. M.; Roisnel T.; Carpentier J.-F. Ring-opening polymerization of lactide with group 3 metal complexes supported by dianionic alkoxy-amino-bisphenolate ligands: combining high activity, productivity, and selectivity. Chem. - Eur. J. 2006, 12 (1), 169–179. 10.1002/chem.200500856. [DOI] [PubMed] [Google Scholar]
- Zhong Z.; Dijkstra P. J.; Feijen J. Controlled and stereoselective polymerization of lactide: kinetics, selectivity, and microstructures. J. Am. Chem. Soc. 2003, 125 (37), 11291–11298. 10.1021/ja0347585. [DOI] [PubMed] [Google Scholar]
- Gadomska-Gajadhur A.; Ruskowski P.ł Biocompatible catalysts for lactide polymerization-catalyst activity, racemization effect and optimization of the polymerization based-on design of experiments. Org. Process. Res. Dev. 2020, 24 (8), 1435–1442. 10.1021/acs.oprd.0c00149. [DOI] [Google Scholar]
- Devaine-Pressing K.; Oldenburg F. J.; Menzel J. P.; Springer M.; Dawe L. N.; Kozak C. M. Lithium, sodium, potassium and calcium amine-bis(phenolate) complexes in the ring-opening polymerization of rac-lactide. Dalton trans. 2020, 49 (5), 1531–1544. 10.1039/C9DT04561A. [DOI] [PubMed] [Google Scholar]
- Al-Khafaji Y. F.; Prior T. J.; Horsburgh L.; Elsegood M. R. J.; Redshaw C. Multimetallic lithium complexes derived from the acids Ph2C(X)CO2H (X = OH, NH2): synthesis, structure and ring opening polymerization of lactides and lactones. ChemistrySelect 2017, 2 (2), 759–768. 10.1002/slct.201602029. [DOI] [Google Scholar]
- Poirier V.; Roisnel T.; Carpentier J.-F.; Sarazin Y. Versatile catalytic systems based on complexes of zinc, magnesium and calcium supported by a bulky bis(morpholinomethyl)phenoxy ligand for the large-scale immortal ring-opening polymerisation of cyclic esters. Dalton trans. 2009, 44, 9820–9827. 10.1039/b917799j. [DOI] [PubMed] [Google Scholar]
- Chen H.-Y.; Mialon L.; Abboud K. A.; Miller S. A. Comparative study of lactide polymerization with lithium, sodium, magnesium, and calcium complexes of BHT. Organometallics 2012, 31 (15), 5252–5261. 10.1021/om300121c. [DOI] [Google Scholar]