Abstract
Background
Giardia has been associated with reduced risk of diarrhea in children in low-resource settings, but the mechanism underlying this association is unknown. To assess whether Giardia may shape colonization or infection with other enteric pathogens and impact associations with diarrhea, we examined Giardia and enteric pathogen codetection among children <5 years old in Kenya, The Gambia, and Mali as part of the Vaccine Impact on Diarrhea in Africa study.
Methods
We tested for Giardia and other enteric pathogens using enzyme-linked immunosorbent assays and real-time polymerase chain reaction (PCR) on stool, respectively. We evaluated associations between Giardia and enteric pathogen detection using multivariable logistic regression models separately for children with moderate-to-severe diarrhea (MSD, cases) and free of diarrhea (controls).
Results
Among 11 039 enrolled children, Giardia detection was more common among controls (35%) than cases (28%, P < .001). Campylobacter coli/jejuni detection was associated with Giardia in controls in The Gambia (adjusted odds ratio [aOR] [95% confidence interval {CI}]: 1.51 [1.22‒1.86]) and cases across all sites (1.16 [1.00‒1.33]). Among controls, the odds of astrovirus (1.43 [1.05‒1.93]) and Cryptosporidium spp. (1.24 [1.06‒1.46]) detection were higher among children with Giardia. Among cases, the odds of rotavirus detection were lower in children with Giardia in Mali (.45 [.30‒.66]) and Kenya (.31 [.17‒.56]).
Conclusions
Giardia was prevalent in children <5 years old and was associated with detection of other enteric pathogens, with differing associations in cases versus controls and by site. Giardia may affect colonization or infection by certain enteric pathogens associated with MSD, suggesting an indirect mechanism of clinical impact.
Keywords: Giardia; pediatric diarrhea, rotavirus, enteric pathogens
Giardia was prevalent in children <5 years old and was associated with detection of other enteric pathogens, including rotavirus. Giardia may affect colonization or infection by certain enteric pathogens associated with MSD, suggesting an indirect mechanism of clinical impact.
The protozoan parasite Giardia duodenalis, also referred to as Giardia lamblia and Giardia intestinalis (hereafter Giardia), is one of the most common enteric pathogens among children and adults worldwide, with >200 million persons infected annually [1]. This high prevalence is particularly pronounced among children in low-resource settings; several studies have found overall prevalence rates in children of 14‒53% [2–4]. A multisite birth-cohort study (the Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project [MAL-ED]) found that for two-thirds of children, Giardia was detected at least once in their first 2 years of life [2].
Acute Giardia infection is treatable but can cause diarrhea and other gastrointestinal symptoms, particularly among adults and children in high-income countries [5]. However, where Giardia prevalence is high, as among children in low-resource settings, the relationship between Giardia and diarrhea is more complex. The Global Enteric Multicenter Study (GEMS)—a 2007–2011 prospective case-control study of the incidence, etiology, and adverse consequences of medically attended acute moderate-to-severe diarrhea (MSD, ≥3 loose stools within a 24-hour period and dehydration, dysentery, or hospitalization) among children <5 years old—found that Giardia was more frequently detected in the stool of children free of diarrhea compared to children with MSD [6], with similar results among children with milder diarrhea enrolled in MAL-ED [7]. A meta-analysis of 12 studies conducted in low-resource settings found children with Giardia had lower odds of developing acute diarrhea (≥3 loose stools within a 24-hour period lasting <14 days), although paradoxically, Giardia detection was positively associated with persistent diarrhea (uninterrupted diarrhea for ≥14 days) [8].
The mechanisms underlying the different clinical impacts of pediatric Giardia in low-resource settings are still an area of active investigation and are likely due to a combination of differences in the host, parasite, and host-parasite interactions [8]. Giardia is known to alter intestinal villi, epithelial cells, mucous barrier, and permeability [9–12], and it can promote the production of host antimicrobial peptides [13] and reduce inflammation [14, 15]. In GEMS, Giardia was found to be associated with changes to the microbiome of children with MSD and free of diarrhea [16]. Giardia-induced changes in children could impact the ability of other enteric pathogens to invade the intestinal mucosa and epithelium, in turn promoting or preventing their colonization/infection [17]. Giardia could then have no association with MSD or be indirectly protective against MSD in children in low-resource settings by preventing colonization/infection with other enteric pathogens associated with MSD.
From 2015 to 2018, the Vaccine Impact on Diarrhea in Africa (VIDA) study collected data to evaluate the etiology and burden of MSD among children <5 years old [Kotloff et al VIDA]. This prospective case-control study was designed as a follow-on to GEMS in 3 countries—The Gambia, Kenya, and Mali—to investigate the pathogen-specific burden of pediatric diarrhea following rotavirus vaccine introduction. We described Giardia prevalence in the VIDA study and assessed whether Giardia is associated with detection of other enteric pathogens among children with MSD and children free of diarrhea. Our goal was to investigate whether pathogens known to cause MSD were less prevalent in children with Giardia compared to those without Giardia in order to understand whether this parasite may be associated with lower risk of MSD via indirect routes, such as inhibition of pathogen colonization/infection.
METHODS
VIDA Study Design and Enrollment
The VIDA study design has been described elsewhere [Kotloff et al VIDA; Powell et al VIDA]. Briefly, the study was conducted from May 2015‒July 2018 in 5 censused populations with ongoing demographic surveillance systems in 3 countries (study sites): a rural population in Basse and Bansang, The Gambia; 2 urban neighborhoods in Bamako, Mali; and a rural location in Siaya County, Kenya. Children <5 years old were enrolled as cases if they presented to healthcare facilities with MSD, defined as: ≥3 loose stools within a 24-hour period with ≥1 clinical indication of moderate-to-severe dehydration (the need for intravenous fluids, loss of skin turgor, and/or sunken eyes), dysentery, or admission to the healthcare facility. MSD onset had to be within the past 7 days after ≥7 days without diarrhea. Also, 1 to 3 controls per case, matched by age group and sex, were randomly selected within 14 days of the index MSD case from near the case-home using site-specific demographic surveillance system records. Control children had to have been diarrhea-free for ≥7 days before enrollment.
Data Collection
Giardia was detected in stool collected at enrollment using an enzyme-linked immunosorbent assay (ELISA; TechLab, Inc., Blacksburg, Virginia, USA). All other enteric pathogens were detected using TaqMan Array Card (TAC) assays on extracted nucleic acids from stool specimens [18], applying a cycle threshold (Cq) of 35 to determine specimens positive (Cq <35) or negative (Cq ≥35) for a pathogen. We used ELISA as the Giardia detection method because its detection probe was excluded from a subset of array cards due to experimental and logistical constraints, which left 34% of specimens untested by TAC assay.
Sociodemographic information was collected at enrollment at healthcare facilities for cases and households for controls. History of breastfeeding among cases was assessed for whether they had exclusively, partially, or not breastfed during the week before the onset of their diarrhea, whereas controls were assessed by the same criteria as part of their normal diet. Water, sanitation, and animal-associated risk factors for exposure to Giardia in each household have been described elsewhere [Berendes et al. VIDA] and were not assessed in these analyses.
Statistical Analysis
All analyses were performed in R (version 4.0.3) [19]. Cases and controls were analyzed separately because associations between Giardia and detection of other pathogens may differ between children with and without diarrhea. Unadjusted associations between Giardia detection and sociodemographic factors were examined using χ2 tests. Cut points for fuel source, caregiver's education, household assets, flooring type, and crowding were selected in order to be consistent with other published analyses [20, 21] [Berendes et al VIDA, Nasrin et al VIDA].
To understand how Giardia might impact colonization/infection with pathogens associated with MSD, the 9 enteric pathogens with the largest attributable fractions for MSD in the VIDA study were included in these analyses after excluding pathogens whose codetection with Giardia was rare (<5%: Aeromonas, Salmonella): Campylobacter coli or Campylobacter jejuni, Shigella spp., Helicobacter pylori, enterotoxigenic Escherichia coli bearing genes for heat-stable toxin (ST-ETEC), astrovirus, norovirus genotype II (GII), rotavirus, sapovirus, and Cryptosporidium spp. [Kotloff et al VIDA]. The association between Giardia and enteric pathogen detection was estimated using multivariable logistic regression, treating Giardia as the primary exposure and detection of an enteric pathogen (positive/negative) as the outcome variable. Models were adjusted for site, child's age group, sex, caregiver's education, household assets, and flooring type; breastfeeding was not included in these models due to its high collinearity with age group (Supplementary Table 1). We did not adjust by environmental risk factors as we wanted to include possible codetections due to shared exposure pathways. To consider whether study site or age group acted as potential effect modifiers, regression models were run for each enteric pathogen to include an interaction term between Giardia and these variables. Results were presented by strata that showed a significant interaction.
Ethical Considerations
This study was approved by the ethical review committees at the University of Maryland, Baltimore (HP-00062472), the US Centers for Disease Control and Prevention (CDC) (reliance agreement 6729), The Gambia Government/Medical Research Council/Gambia at the London School of Hygiene & Tropical Medicine (1409), the Comité d'Ethique de la Faculté de Médecine, de Pharmacie, et d'Odonto-Stomatologie, Bamako, Mali (no number), and the Kenya Medical Research Institute Scientific & Ethics Review Unit in Siaya County, Kenya (SSE 2996). Informed, written consent was obtained from all participants prior to initiation of study procedures.
RESULTS
Giardia Detection and Sociodemographic Factors
A total of 11 039 children were enrolled: 4840 children with MSD (cases) and 6213 matched children free of diarrhea (controls). Giardia was detected among 3556 (32%) of the enrolled children and was lower among cases (28%) than controls (35%) (P < .001) (Table 1). Among controls, prevalence was highest in The Gambia (43%) and Mali (41%), with lower detection rates in Kenya (21%, P < .001), and it was higher among older children, with 50% prevalence in 24- to 59-month-old children as compared to 18% in children 0–11 months old (P < .001). Detection was more common in female controls compared to male controls (37% vs 34%, P = .007) and was lower among controls whose caregivers completed at least primary school (25%) as compared to controls whose caregivers did not (40%, P < .001). Detection was higher among controls in households with >3 assets (television, telephone, radio, bicycle/rickshaw, car/truck, motorcycle/scooter, refrigerator, motorized boat, animal-drawn cart) than controls living in households with ≤3 assets (40% vs 29%, P < .001). Controls living in households with finished floors had higher rates of Giardia detection (38%) as compared to children living in households with rudimentary or natural floors (28%, P < .001). Finally, Giardia detection was highest among controls who were not breastfed: 47% of controls who were not breastfed, 28% who were partially breastfed, and 8% who were exclusively breastfed had Giardia detected in their stool (P < .001).
Table 1.
Sociodemographic Indicators Among Children <5 Years With and Without Giardia Detection Among Controls and Cases at Enrollment in the VIDA Study, 2015–2018
| Controls | Cases | |||||
|---|---|---|---|---|---|---|
| No Giardia |
Giardia
n (%) |
P valuea | No Giardia n (%) |
Giardia
n (%) |
P valuea | |
| All | 4021 (65) | 2183 (35) | … | 3462 (72) | 1373 (28) | … |
| Site | <.001 | <.001 | ||||
| ȃThe Gambia | 1212 (57) | 926 (43) | 1087 (65) | 590 (35) | ||
| ȃMali | 1164 (59) | 816 (41) | 1100 (68) | 508 (32) | ||
| ȃKenya | 1645 (79) | 441 (21) | 1275 (82) | 275 (18) | ||
| Age group | <.001 | <.001 | ||||
| ȃ0–11 mo | 1727 (82) | 380 (18) | 1487 (87) | 232 (13) | ||
| ȃ12–23 mo | 1299 (61) | 827 (39) | 1158 (68) | 538 (32) | ||
| ȃ24–59 mo | 995 (50) | 976 (50) | 817 (58) | 603 (42) | ||
| Sex | .007 | .042 | ||||
| ȃMale | 2210 (66) | 1122 (34) | 1887 (73) | 704 (27) | ||
| ȃFemale | 1811 (63) | 1061 (37) | 1575 (70) | 669 (30) | ||
| Caregiver education | <.001 | <.001 | ||||
| ȃ<Primary school | 2390 (60) | 1626 (40) | 2198 (69) | 1008 (31) | ||
| ȃ≥Primary school | 1631 (75) | 557(25) | 1261 (78) | 365 (22) | ||
| Household assetsb | <.001 | <.001 | ||||
| ȃ>3 | 1965 (60) | 1332 (40) | 1757 (67) | 852 (33) | ||
| ȃ≤3 | 2054 (71) | 848 (29) | 1703 (77) | 520 (23) | ||
| Fuel source | .804 | .678 | ||||
| ȃClean fuelc | 227 (65) | 120 (35) | 197 (73) | 74 (27) | ||
| ȃOther | 3784 (65) | 2059 (35) | 3260 (72) | 1298 (28) | ||
| Crowding | .359 | .722 | ||||
| ȃ≤2 persons sleeping/room | 1795 (64) | 1001 (36) | 1571 (71) | 631 (29) | ||
| ȃ>2 persons sleeping/room | 2226 (65) | 1182 (35) | 1890 (72) | 742 (28) | ||
| Flooring | <.001 | <.001 | ||||
| ȃFinished floord | 2807 (62) | 1722 (38) | 2446 (70) | 1068 (30) | ||
| ȃNatural or rudimentary floor | 1203 (72) | 457 (28) | 1012 (77) | 305 (23) | ||
| Breastfeeding | <.001 | <.001 | ||||
| ȃExclusive | 133 (92) | 12 (8) | 137 (90) | 15 (10) | ||
| ȃPartial | 2558 (72) | 994 (28) | 2177 (78) | 604 (22) | ||
| ȃNone | 1330 (53) | 1177 (47) | 1148 (60) | 754 (40) | ||
The bold values are P values < 0.05.
Abbreviation: VIDA, Vaccine Impact on Diarrhea in Africa.
Tests the difference in Giardia detection rate across all subgroups.
Includes television, telephone, radio, bicycle/rickshaw, car/truck, motorcycle/scooter, refrigerator, motorized boat, animal-drawn cart.
Includes electric, propane, butane, natural gas.
Includes parquet/polished wood, vinyl/asphalt strips, ceramic tile, cement, or carpet.
Giardia detection by sociodemographic indicators among cases followed similar trends to controls, with the highest prevalence among cases in The Gambia (35%) and Mali (32%) followed by Kenya (18%, P < .001). Detection was more common among 24- to 59-month-old cases (42%) compared to younger cases aged 0–11 months old (13%, P < .001) and among female (30%) compared to male cases (27%, P = .042). Giardia prevalence was lower among cases whose caregivers completed at least primary school compared to those who did not (22% vs 31%, P < .001), but it was higher among cases living in households with >3 assets compared to households with ≤3 assets (33% vs 23%, P < .001) and among cases living in households with finished floors compared to households with rudimentary or natural floors (30% vs 23%, P < .001). As with controls, detection was most common among children who were not breastfed, with 40% of non-breastfed cases having Giardia detected in their stool, as compared to 22% of partially breastfed and 10% of exclusively breastfed cases (P < .001). For both controls and cases, breastfeeding data were highly co-linear with age, whereby most older children did not breastfeed (Supplementary Table 1), and both groups had the highest Giardia prevalence (Table 1). Giardia prevalence was not significantly different among children living in households using clean versus other fuel sources or experiencing crowding for both controls and cases.
Adjusted Associations Between Giardia and Enteric Pathogen Detection
We tested whether enteric pathogen detection differed between children with versus without Giardia, considering whether differences were unique to either cases or controls. Of the 9 enteric pathogens considered in the study (Table 2), detection of C. coli/jejuni, Shigella spp., H. pylori, and Cryptosporidium spp. was more common among children with Giardia compared to children without Giardia for both controls (C. coli/jejuni: 32% vs 26%, P < .001; Shigella spp.: 28% vs 20%, P < .001; H. pylori: 14% vs 11%, P = .001; Cryptosporidium spp.: 21% vs 17%, P < .001) and cases (C. coli/jejuni: 35% vs 31%, P = .014; Shigella spp.: 41% vs 32%, P < .001; H. pylori: 23% vs 17%, P = .014; Cryptosporidium spp.: 26% vs 22%, P = .011). Among controls only, the rate of astrovirus and sapovirus detection was higher among children with Giardia relative to children without Giardia (astrovirus: 5% vs 4%, P = .028; sapovirus: 12% vs 10%, P = .042). Among cases only, detection of norovirus GII and rotavirus was lower among children with Giardia relative to children without Giardia (norovirus GII: 9% vs 12%, P = .002; rotavirus: 9% vs 15%, P < .001).
Table 2.
Detection of Enteric Pathogens Overall and Codetection With Giardia Among Controls and Cases in the VIDA Study
| Controls | Cases | |||||||
|---|---|---|---|---|---|---|---|---|
| Detection n (%)a |
Codetection With Giardia n (%)b |
Detection Without Giardia n (%)c |
P valued | Detection n (%)a |
Codetection With Giardia n (%)b |
Detection Without Giardia n (%)c |
P valued | |
| Bacteria | ||||||||
| ȃCampylobacter coli/jejuni | 1359 (28) | 530 (32) | 829 (26) | <.001 | 1545 (32) | 475 (35) | 1070 (31) | .014 |
| ȃShigella spp. | 1084 (23) | 468 (28) | 616 (20) | <.001 | 1641 (34) | 553 (41) | 1088 (32) | <.001 |
| ȃHelicobacter pylori | 558 (12) | 226 (14) | 332 (11) | .001 | 879 (18) | 311 (23) | 568 (17) | .014 |
| ȃST-ETEC | 944 (20) | 340 (21) | 604 (19) | .225 | 1084 (23) | 321 (24) | 763 (22) | .336 |
| Viruses | ||||||||
| ȃAstrovirus | 210 (4) | 87 (5) | 123 (4) | .028 | 304 (6) | 91 (7) | 213 (6) | .509 |
| ȃNorovirus GII | 513 (11) | 167 (10) | 346 (11) | .343 | 552 (11) | 125 (9) | 427 (12) | .002 |
| ȃRotavirus | 208 (4) | 74 (5) | 134 (4) | .717 | 642 (13) | 122 (9) | 520 (15) | <.001 |
| ȃSapovirus | 529 (11) | 203 (12) | 326 (10) | .042 | 668 (14) | 194 (14) | 474 (14) | .649 |
| Parasites | ||||||||
| ȃCryptosporidium spp. | 873 (18) | 349 (21) | 524 (17) | <.001 | 1106 (23) | 348 (26) | 758 (22) | .011 |
The bold values are P values < 0.05.
Abbreviations: ST-ETEC, heat-stable enterotoxigenic E. coli; VIDA, Vaccine Impact on Diarrhea in Africa.
Percent is representative of detection among controls and cases in the study overall.
Percent is representative of detection among controls and cases with Giardia.
Percent is representative of detection among controls and cases without Giardia.
Tests the difference between enteric pathogen detection in children with versus without Giardia.
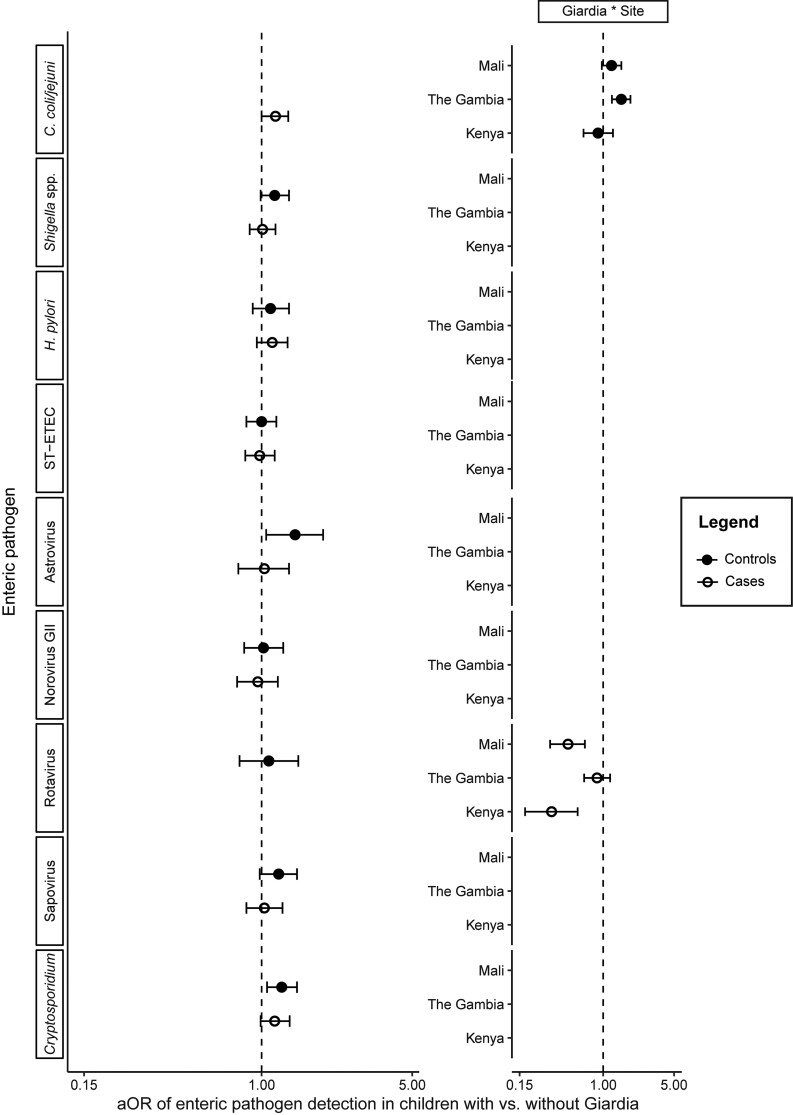
When examining the association between Giardia and detection of other enteric pathogens, no significant associations were found in both cases and controls across all sites. There was also no significant effect of age on Giardia-enteric pathogen codetection in our models despite the high prevalence of Giardia among older children. Only C. coli/jejuni detection was found to be positively associated with Giardia detection in both controls and cases; however, although in controls this association was site-specific to The Gambia (adjusted odds ratio [aOR] [95% confidence interval {CI}]: 1.51 [1.22‒1.86]), in cases it was across all 3 sites (1.16 [1.00‒1.33]) (Figure 1, Supplementary Table 2). There were positive associations between Giardia and astrovirus (1.43 [1.05‒1.93]) and Cryptosporidium spp. detection (1.24 [1.06‒1.46]) among controls across all 3 sites, but not among cases (Figure 1, Supplementary Tables 3 and 4). There was a site-specific significant association between Giardia and rotavirus detection unique to cases; the odds of rotavirus detection were lower among cases with Giardia in Mali (0.45 [.30‒.66]) and Kenya (0.31 [.17‒.56]) but not in The Gambia (0.87 [.65‒1.17]) (Figure 1, Supplementary Table 5). No other enteric pathogens associated with MSD in the VIDA study were associated with Giardia detection in our analyses (Figure 1, Supplementary Tables 6–10).
Figure 1.
The aORs (circles) and 95% confidence intervals (whiskers) of enteric pathogen detection for children with versus without Giardia. Each pathogen row represents 2 models: 1 model for the association between Giardia and codetection of that pathogen in controls (filled circles), and 1 model for that same association in cases (open circles). The vertical dotted line at aOR = 1.0 represents no association between Giardia detection and enteric pathogen codetection; aOR <1.0 represent a negative association, while aOR > 1.0 represent a positive association. If significant country-specific interactions with Giardia were detected (Giardia * Site), country-level estimates are presented in lieu of single pan-site estimates. Abbreviation: aOR, adjusted odds ratio.
DISCUSSION
We investigated whether detection of Giardia in children free of diarrhea and children with MSD was associated with detection of other enteric pathogens to better understand the relationship between Giardia and diarrhea among children in low-resource settings. In a population of children where Giardia detection was more common in children free of diarrhea compared to children with MSD, we found significant positive associations between detection of Giardia and C. coli/jejuni, Cryptosporidium spp., and astrovirus and a significant negative association between Giardia and rotavirus, but none of these associations were found among both clinical groups across all 3 sites. These findings suggest that interactions between Giardia and other pathogens may impact clinical outcomes normally associated with either pathogen, but that the outcome of this interaction could vary by context or other risk factors.
We observed a negative association between Giardia and rotavirus detection among children with MSD in Mali and Kenya. This finding that a pathogen known to cause MSD [6] [Kotloff et al VIDA] was less prevalent in children with Giardia compared to those without Giardia suggests that one possible mechanism behind Giardia's protective effect against diarrhea could be that it decreases the pathogenicity of specific co-infecting organisms. A study in Bedouin infants found that infants coinfected with Giardia and rotavirus experienced reduced diarrheal disease severity compared to children with rotavirus alone [22]. Although another case-control study contradicts these results, showing greater risk of diarrhea in individuals with rotavirus and Giardia as compared to single-pathogen infections, the study population included children and adults, and controls were not matched to cases on age [23]. Alternatively, colonization/infection with one of these enteric pathogens could promote a gut environment not conducive to colonization/infection with the other. Indeed, Giardia has been shown to promote secretion of antimicrobial peptides, nitric oxide, reactive oxygen species, mucins, and other immune factors [24] which could in turn inhibit the pathogenesis of diarrhea due to other pathogens, including rotavirus. Understanding the possible molecular interactions between Giardia and rotavirus might be relevant to understanding vaccine performance given high Giardia prevalence and routine infant rotavirus immunization over the last decade in Mali and Kenya, where studies have found lower efficacy of these vaccines [25].
We found a positive association between Giardia and C. coli/jejuni detection in both children with MSD and free of diarrhea; only in the case of children free of diarrhea was this effect site-specific in The Gambia. This positive link is compatible with results from the MAL-ED study showing a positive correlation between Giardia and Campylobacter carriage [2]. Codetection could be common because Giardia and Campylobacter have shared pathways of exposure through untreated water and contact with dogs and cats [26, 27]; however, no shared pathways were found when examining environmental risk factors in the VIDA study [Berendes et al VIDA]. Alternatively, one pathogen could foster an environment conducive to colonization/infection with the other. Giardia attaches to the intestinal epithelium, a site critical for production of pro-inflammatory chemokines in response to microbial infection. In in vitro and ex vivo experiments, Giardia attenuated secretion and directly degraded a pro-inflammatory chemokine, CXCL8, produced by human intestinal epithelial cells following exposure to Salmonella Typhimurium [14]. By dampening the immune response to other microbes, Giardia could create an environment conducive to Campylobacter colonization.
Similarly, Giardia was found to be positively associated with both Cryptosporidium spp. and astrovirus detection; however, this relationship was only present across all sites in children free of diarrhea at enrollment, despite both these pathogens being associated with MSD [6, 7] [Kotloff et al VIDA]. As with C. coli/jejuni, one enteric pathogen could promote a gut environment conducive to the other, but because the Cryptosporidium spp. and astrovirus link with Giardia was unique to children free of diarrhea, co-carriage could reduce inflammation that would otherwise cause MSD. Indeed, previous work found that children in Tanzania with Giardia had reduced levels of C-reactive protein in their serum, a marker for inflammation, whereas Giardia detection was associated with lower rates of subsequent diarrheal episodes in those children [28].
This study is subject to several limitations. First, although cases and controls are analyzed independently in our analyses, the selection of controls was based on matching to cases, so data from controls may not be generalizable to all children without diarrhea in these sites. Second, these analyses only consider single instances of codetection between Giardia and one enteric pathogen; as a result, multiple combinations of enteric pathogens in association with Giardia not captured in our analyses may have biased estimated effects. Third, some codetections may have been missed because we used assays with differing sensitivities to detect Giardia (ELISA) versus other enteric pathogens (TAC) in stool. However, the use of TAC for Giardia would have excluded more than 2000 specimens from these analyses. Finally, our analyses are cross-sectional and therefore cannot determine the causal relationship between Giardia and other enteric pathogens.
Our analyses demonstrate that Giardia is prevalent in young children living in low-resource settings and is associated with detection of other enteric pathogens associated with MSD, although there were no consistent associations across all sites and children with MSD or free of diarrhea. Given our findings, although Giardia on its own may not cause diarrhea in high prevalence settings, assessment of the risk of MSD from other enteric pathogens should include this pathogen in future models. Possible associations and underlying mechanisms between Giardia and enteric pathogen codetection and altered clinical outcomes, including diarrhea or malnutrition, should be analyzed in future studies.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Perrine Marcenac, Division of Foodborne, Waterborne, and Environmental Diseases, US Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Awa Traoré, Centre pour le Développement des Vaccins du Mali (CVD-Mali), Bamako, Mali.
Sunkyung Kim, Division of Foodborne, Waterborne, and Environmental Diseases, US Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Graeme Prentice-Mott, Division of Foodborne, Waterborne, and Environmental Diseases, US Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
David M Berendes, Division of Foodborne, Waterborne, and Environmental Diseases, US Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Helen Powell, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA; Department of Pediatrics, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Irene N Kasumba, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA; Department of Medicine, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Dilruba Nasrin, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA; Department of Medicine, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Joquina Chiquita M Jones, Medical Research Council (UK) Unit, The Gambia at the London School of Hygiene & Tropical Medicine, Banjul, The Gambia.
Syed M A Zaman, Medical Research Council (UK) Unit, The Gambia at the London School of Hygiene & Tropical Medicine, Banjul, The Gambia.
John B Ochieng, Kenya Medical Research Institute, Center for Global Health Research (KEMRI-CGHR), Kisumu, Kenya.
Jane Juma, Kenya Medical Research Institute, Center for Global Health Research (KEMRI-CGHR), Kisumu, Kenya.
Doh Sanogo, Centre pour le Développement des Vaccins du Mali (CVD-Mali), Bamako, Mali.
Marc-Alain Widdowson, Division of Global Health Protection, Center for Global Health, Nairobi, Kenya.
Jennifer R Verani, Division of Global Health Protection, Center for Global Health, Nairobi, Kenya.
Jie Liu, Division of Infectious Diseases and International Health, Department of Medicine, University of Virginia, Charlottesville, Virginia, USA; School of Public Health, Qingdao University, Qingdao, China.
Eric R Houpt, Division of Infectious Diseases and International Health, Department of Medicine, University of Virginia, Charlottesville, Virginia, USA.
M Jahangir Hossain, Medical Research Council (UK) Unit, The Gambia at the London School of Hygiene & Tropical Medicine, Banjul, The Gambia.
Samba O Sow, Centre pour le Développement des Vaccins du Mali (CVD-Mali), Bamako, Mali.
Richard Omore, Kenya Medical Research Institute, Center for Global Health Research (KEMRI-CGHR), Kisumu, Kenya.
Sharon M Tennant, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA; Department of Medicine, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Eric D Mintz, Division of Foodborne, Waterborne, and Environmental Diseases, US Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Karen L Kotloff, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA; Department of Pediatrics, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Notes
Acknowledgments. The authors wish to express their deep gratitude to the families who participated in these studies, the clinical and field staff for their exceptional hard work and dedication, and to the physicians, administration, and health officials at every site who generously provided facilities and support for the conduct of the study. They also wish to thank members of the Waterborne Disease Prevention Branch at CDC for helpful conversations and support. They are grateful to Catherine Johnson, Chris Focht, and Nora Watson at the Emmes Company, LLC, for expert data management and reporting. Special thanks go to Carl Kirkwood, Duncan Steele, and Anita Zaidi at the Bill & Melinda Gates Foundation for helpful oversight, Kathy Neuzil for thoughtful suggestions, and to the following members of our International Scientific Advisory Committee for providing insightful comments and guidance: Janet Wittes (Chair), George Armah, John Clemens, Christopher Duggan, Stephane Helleringer, Ali Mokdad, James Nataro, and Halvor Sommerfelt.
Supplement sponsorship. This supplement was sponsored by the Bill & Melinda Gates Foundation. This study is based on research funded in part by grants from the Bill & Melinda Gates Foundation (OPP1111236/OPP1116751).
Disclaimer. The funding organizations had no role in the design, collection, analysis, or interpretation of data, or in the writing of the article. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Kenya Medical Research Institute, University of Maryland, US Centers for Disease Control and Prevention, nor any of the collaborating partners in this project.
Financial support. This study was funded by the Bill & Melinda Gates Foundation (grant numbers OPP1111236, OPP1129479, and OPP1116751).
References
- 1. Karanis P, Kourenti C, Smith H. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J Water Health 2007; 5:1–38. [DOI] [PubMed] [Google Scholar]
- 2. Rogawski ET, Bartelt LA, Platts-Mills JA, et al. Determinants and impact of Giardia infection in the first 2 years of life in the MAL-ED birth cohort. J Pediatric Infect Dis Soc 2017; 6:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McQuade ETR, Platts-Mills JA, Gratz J, et al. Impact of water quality, sanitation, handwashing, and nutritional interventions on enteric infections in rural Zimbabwe: the Sanitation Hygiene Infant Nutrition Efficacy (SHINE) trial. J Infect Dis 2020; 221:1379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin A, Ercumen A, Benjamin-Chung J, et al. Effects of water, sanitation, handwashing, and nutritional interventions on child enteric protozoan infections in rural Bangladesh: a cluster-randomized controlled trial. Clin Infect Dis 2018; 67:1515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Conners EE, Miller AD, Balachandran N, Robinson BM, Benedict KM. Giardiasis outbreaks—United States, 2012–2017. MMWR Morb Mortal Wkly Rep 2021; 70: 304–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 7. Platts-Mills JA, Babji S, Bodhidatta L, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 2015; 3:e564–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muhsen K, Levine MM. A systematic review and meta-analysis of the association between giardia lamblia and endemic pediatric diarrhea in developing countries. Clin Infect Dis 2012; 55(Suppl 4):S271–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amat CB, Motta JP, Fekete E, Moreau F, Chadee K, Buret AG. Cysteine protease-dependent mucous disruptions and differential mucin gene expression in Giardia duodenalis infection. Am J Pathol 2017; 187:2486–98. [DOI] [PubMed] [Google Scholar]
- 10. Halliez MC, Motta JP, Feener TD, et al. Giardia duodenalis induces paracellular bacterial translocation and causes postinfectious visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 2016; 310:G574–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beatty JK, Akierman SV, Motta JP, et al. Giardia duodenalis induces pathogenic dysbiosis of human intestinal microbiota biofilms. Int J Parasitol 2017; 47:311–26. [DOI] [PubMed] [Google Scholar]
- 12. Ortega-Pierres G, Argüello-García R, Laredo-Cisneros MS, et al. Giardipain-1, a protease secreted by Giardia duodenalis trophozoites, causes junctional, barrier and apoptotic damage in epithelial cell monolayers. Int J Parasitol 2018; 48:621–39. [DOI] [PubMed] [Google Scholar]
- 13. Manko-Prykhoda A, Allain T, Motta JP, et al. Giardia spp. promote the production of antimicrobial peptides and attenuate disease severity induced by attaching and effacing enteropathogens via the induction of the NLRP3 inflammasome. Int J Parasitol 2020; 50:263–75. [DOI] [PubMed] [Google Scholar]
- 14. Cotton JA, Bhargava A, Ferraz JG, Yates RM, Beck PL, Buret AG. Giardia duodenalis Cathepsin B proteases degrade intestinal epithelial interleukin-8 and attenuate interleukin-8-induced neutrophil chemotaxis. Infect Immun 2014; 82:2772–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cotton JA, Motta JP, Schenck LP, Hirota SA, Beck PL, Buret AG. Giardia duodenalis infection reduces granulocyte infiltration in an in vivo model of bacterial toxin-induced colitis and attenuates inflammation in human intestinal tissue. PLoS One 2014; 9:e109087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berry ASF, Johnson K, Martins R, et al. Natural infection with Giardia is associated with altered community structure of the human and canine gut microbiome. mSphere 2020; 5:e00670–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fekete E, Allain T, Siddiq A, Sosnowski O, Buret AG. Giardia spp. and the gut microbiota: dangerous liaisons. Front Microbiol 2021; 11:618106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu J, Gratz J, Amour C, et al. A laboratory-developed TaqMan array card for simultaneous detection of 19 enteropathogens. J Clin Microbiol 2013; 51:472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. R Core Team . R: A language and environment for statistical computing. Available at: https://www.R-project.org/.
- 20. Benjamin-Chung J, Crider YS, Mertens A, et al. Household finished flooring and soil-transmitted helminth and Giardia infections among children in rural Bangladesh and Kenya: a prospective cohort study. Lancet Glob Health 2021; 9:e301–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mohan VR, Karthikeyan R, Babji S, et al. Rotavirus infection and disease in a multisite birth cohort: results from the MAL-ED study. J Infect Dis 2017; 216:305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bilenko N, Levy A, Dagan R, Deckelbaum RJ, El-On Y, Fraser D. Does co-infection with Giardia lamblia modulate the clinical characteristics of enteric infections in young children? Eur J Epidemiol 2004; 19:877–83. [DOI] [PubMed] [Google Scholar]
- 23. Bhavnani D, Goldstick JE, Cevallos W, Trueba G, Eisenberg JN. Synergistic effects between rotavirus and coinfecting pathogens on diarrheal disease: evidence from a community-based study in northwestern Ecuador. Am J Epidemiol 2012; 176:387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fink MY, Singer SM. The intersection of immune responses, microbiota, and pathogenesis in giardiasis. Trends Parasitol 2017; 33:901–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clark A, van Zandvoort K, Flasche S, et al. Efficacy of live oral rotavirus vaccines by duration of follow-up: a meta-regression of randomised controlled trials. Lancet Infect Dis 2019; 19:717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Division of Foodborne, Waterborne, and Environmental Diseases . Campylobacter (Campylobacteriosis): questions and answers. Available at: https://www.cdc.gov/campylobacter/faq.html. Accessed 29 June 2021.
- 27. Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Division of Foodborne, Waterborne, and Environmental Diseases . Parasites—Giardia: general information. Available at: https://www.cdc.gov/parasites/giardia/general-info.html. Accessed 29 June 2021.
- 28. Veenemans J, Mank T, Ottenhof M, et al. Protection against diarrhea associated with Giardia intestinalis is lost with multi-nutrient supplementation: a study in Tanzanian children. PLoS Negl Trop Dis 2011; 5:e1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.