Abstract
Background
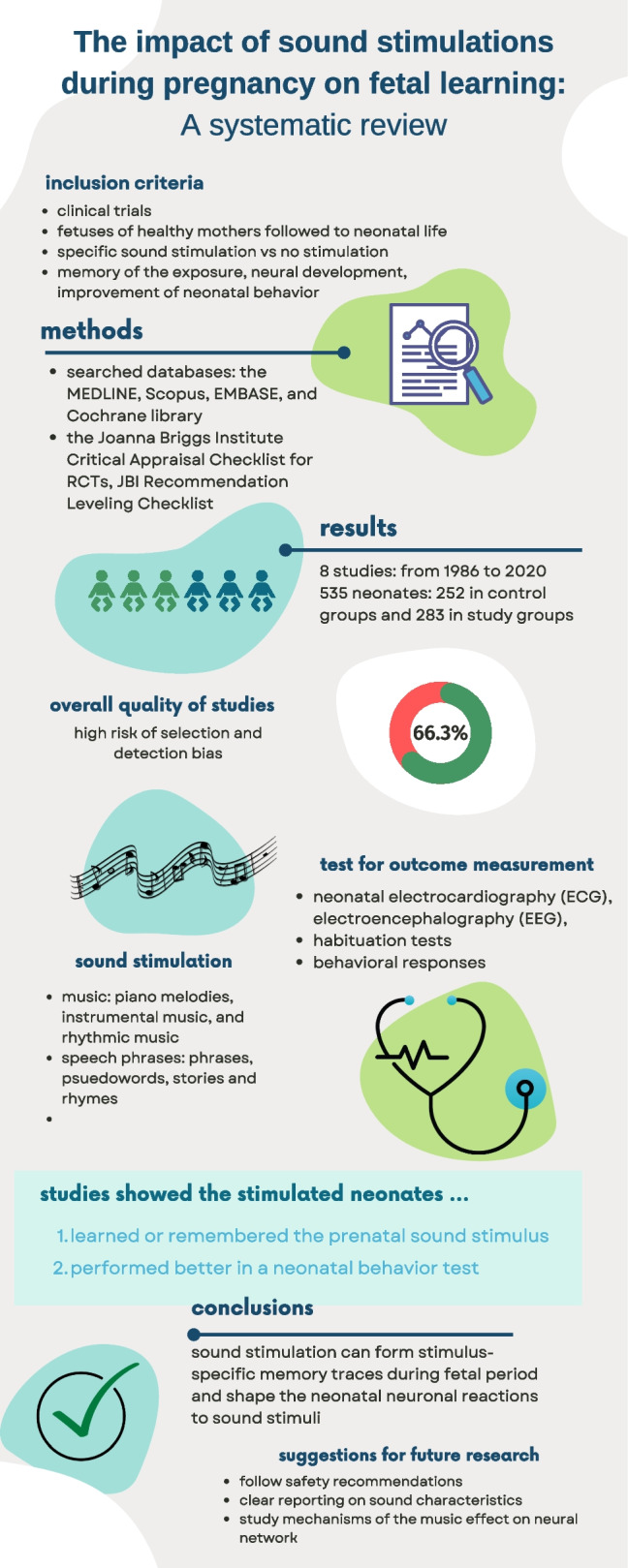
The developing nervous system in utero is exposed to various stimuli with effects that may be carried forward to the neonatal period. This study aims to investigate the effects of sound stimulation (music and speech) on fetal memory and learning, which was assessed later in neonatal period.
Methods
The MEDLINE (pubmed), Scopus, EMBASE, and Cochrane Library were searched. Two reviewers selected the studies and extracted the data independently. The quality of eligible studies was assessed using The Joanna Briggs Institute Critical Appraisal Checklist for Randomized Controlled Trials (RCTs).
Results
Overall 3930 articles were retrieved and eight studies met the inclusion criteria. All of the included studies had good general quality; however, high risk of selection and detection bias was detected in most of them. Fetal learning was examined through neonatal electrocardiography (ECG), electroencephalography (EEG), habituation tests, and behavioral responses. Seven studies showed that the infants had learned the fetal sound stimulus and one study indicated that the prenatally stimulated infants performed significantly better on a neonatal behavior test. There was considerable diversity among studies in terms of sound stimulation type, characteristics (intensity and frequency), and duration, as well as outcome assessment methods.
Conclusions
Prenatal sound stimulation including music and speech can form stimulus-specific memory traces during fetal period and effect neonatal neural system. Further studies with precisely designed methodologies that follow safety recommendations, are needed.
Graphical Abstract
Keywords: Sound stimulation, Pregnancy, Fetal learning, Neonatal behavior, Fetal memory, Neural development
Introduction
The developing nervous system in utero is exposed to various stimuli with effects that may be carried forward to the newborn period. The human brain is influenced by music experience, beginning in utero and lasting across the lifetime [1]. The Neural processing of music encompasses an extremely complex and extensive network of cortical and subcortical structures which integrate auditory, sensory motor and cognitive functions as well as emotional changes [2, 3]. The wide effects of music on brain function, including auditory perception, language processing, attention and memory, emotion, and motor skills have proposed the use of music as a noninvasive intervention for patients [4]. Since the 1980s, several experimental studies have been performed on fetal sensory competencies in relation to different forms of sound stimuli [5–11]. The first empirical studies were designed to explore the fetus’ hearing ability [12]. Initial responsiveness to different frequencies of sound starts from around 23 weeks of pregnancy. This is consistent with the development of neurosensory part of the auditory system which was indicated by startle response to vibroacoustic stimulation in 24 weeks of GA. Consistent responses, from all fetuses, observed between 28–30 weeks [13, 14]. The primary studies focused on the immediate response of fetus to sound stimulations and later studies followed up the effects into neonatal life. The concerning effects were memory persistence and improvements in general behavior of exposed infants. The retention of the effects of prenatal sound exposure, for instance fetal learning, suggests that fetal neurodevelopment may be positively influenced and enhanced. The repetition of stimuli shortens the time of fetal habituation, thus memory formation might happen gradually [15]. This article considers memory as a behavioral change caused by an experience, and learning as a process for acquiring memory [16]. Fetal learning has been measured using various outcome assessments including habituation testing, classical conditioning, exposure learning, heart rate (HR), and brain event-related potentials (ERPs) [17, 18]. Different forms of acoustic stimulations have been used to influence the fetus. Furthermore, there are great methodological differences in the music exposure protocols such as sound frequency and volume, means of music administration, and exposure region (directly to the maternal abdomen or in the environment). All these variables can impact the amount and quality of the sound reaching the fetus and therefore its effects [19, 20]. A previous systematic review of the effects of visual and auditory stimuli in functional fetal brain imaging also showed great variation in methodology of similar studies. This study showed that differences in the measurement strategies and study designs can lead to variable results [21].
There have been reviews concerning the therapeutic role of music during pregnancy; however, no review focused on the effects of music or speech on neurodevelopment, memory, and learning in term infants of healthy mothers. Considering the wide variation of studies and the lack of a systematic review about this concept, this study was conducted to determine the effects of sound stimulations (music and speech) on fetal memory and learning, which was assessed later in neonatal period.
Method
Registration and protocol
This systematic review was done in accordance with The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) [22] and registered at Pazhoohan Research Information System (registration code: 66065).
Data sources
Four databases including the MEDLINE, Scopus, EMBASE, and Cochrane library were thoroughly searched. Also, The International Prospective Register of Systematic Reviews (PROSPERO) was searched to identify ongoing systematic reviews in the same topic. The references of recent reviews and other eligible articles were manually searched for additional studies not identified through the electronic search.
We ran our initial search strategy in May 2019 and updated it in November 2021. Controlled trials with accessible English titles, abstracts, and full-texts were included without time limitation.
Search strategy
The search strategy was designed using the following database-specific vocabularies (MeSH, EMTREE) and additional free-text terms: (((((("Infant, Newborn"[Mesh]) OR infant) OR newborn) OR neonate)) AND (((((((((((((((((((((((("brain function") OR "brain development") OR "fetal sensory competencies ") OR "development of fetal hearing") OR "fetal development") OR "infant behavior") OR "neonatal behavior") OR "Infant Behavior"[Mesh]) OR "auditory brain development") OR "visual brain development") OR "thinking") OR "recognize ability") OR "memory") OR "neuropsycological behavior") OR "auditory attention") OR "visual attention") OR "mental function") OR "fetal learning") OR "endocrine effect") OR "metabolism effect") OR "Prenatal Exposure Delayed Effects"[Mesh])) OR ("Embryonic and Fetal Development"[Mesh] OR "Fetal Development"[Mesh])) OR "Language Development"[Mesh])) AND ((((((((((((((((("mother voice") OR "transnatal auditory learning") OR "auditory stimulation") OR "prenatal music education") OR "maternal voice") OR "maternal music") OR "antenatal training with music") OR "music effect") OR "effect of music") OR "fetal exposure to music") OR "fetal music exposure") OR "fetal exposure to mother voice") OR "fetal exposure to maternal music") OR "antenatal music education") OR "prenatal sound stimulation") OR "music during pregnancy") OR "auditory habituation").
Inclusion and exclusion criteria
Types of studies
Only intervention studies (RCTs or quasi-RCTs) were considered. Publications were included without time limitation with available English titles, abstracts, and full-texts.
Participants
Participants comprised fetuses followed up to neonatal life and later. Non-singleton pregnancies, mothers with coexisting medical diseases, and high-risk pregnancies including diabetic patients were excluded. Studies on premature or unhealthy infants, newborns after birth, and neonates with existing congenital disease were not included.
Interventions
The selected studies had conducted sound stimulation including music and speech phrases.
Outcomes
The findings of included studies focused on the memory of the exposure, development of brain as well as improvement of neonatal behavior. The neonate’s memory of sound stimulation can be assessed by ECG, EEG, habituation tests, and behavior change. The trials that did not follow up the fetus into neonatal period were excluded.
Data extraction
Two reviewers screened all titles and abstracts of retrieved papers independently. Additionally, full-texts of relevant papers were screened for eligibility by two independent reviewers and the reasons for exclusion were documented for the excluded full-texts. Two researchers extracted the data separately and disagreements were discussed and resolved. The following information was extracted from the articles: the first author, publishing year, country, study design, sample size, intervention type, enrolment time, intervention duration, content of intervention, exposure region, age, state of neonate at time of test, and the type of test.
Quality assessment
The quality of studies was assessed according to the Joanna Briggs Institute Critical Appraisal Checklist for RCTs.
Results
Search results
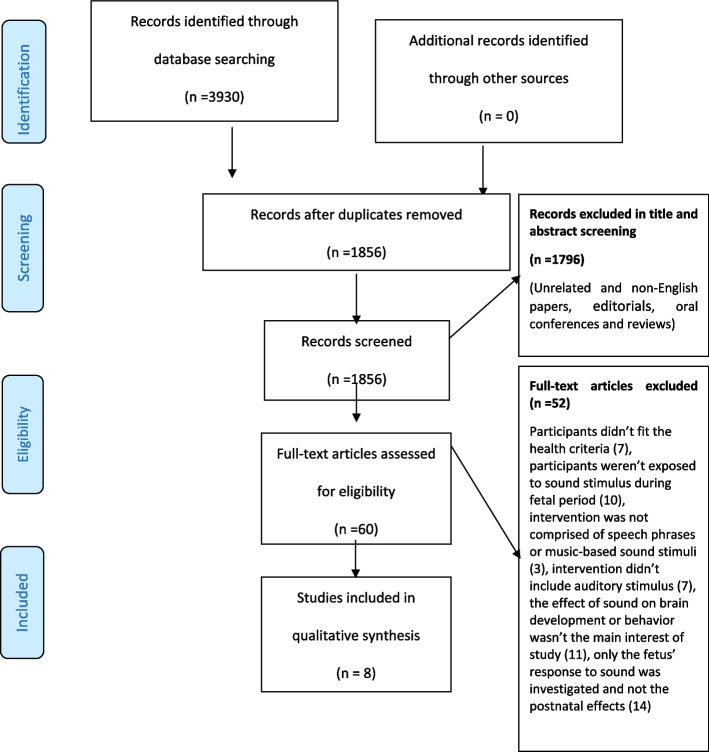
Overall, 3930 titles were found in the searching process. After removal of duplicated articles using EndNote X9.3.3 literature manager software, 1856 articles remained. Initial evaluation of the titles and abstracts decreased the number of papers to 60 after elimination of unrelated and non-English papers, editorials, oral conferences, and reviews and further reduced to 8 after selecting studies which their full text met the inclusion and exclusion criteria (Fig. 1). Fifty two studies were excluded during full text assessment process, due to the following reasons: in 7 studies, participants did not fit the health criteria; in 10 studies exposure to sound stimulus was not during fetal period; in 7 studies intervention did not encompass any types of auditory stimulus; and in 3 studies the sound intervention was not comprised of speech phrases or music-based sound stimuli. Moreover, the main focus of 11 studies was not the sound’s effects on brain development and behavior. In 14 studies, only the fetus’ response to sound, and not the postnatal effect, was investigated.
Fig. 1.
PRISMA flow diagram
Study characteristics
The basic characteristics of the 8 included studies are summarized (Table 1). The 8 RCTs were published from 1986 to 2020. The sample size varied from 18 to 260, with a total population of 535 neonates (252 in control groups and 283 in study groups).
Table 1.
Characteristics of the included studies
| Number | Study number | Author | Country | Method | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study design | Sample size | Intervention type | Enrolment time | Intervention duration | Content of intervention | Exposure region | Neonatal state at time of test | Age at time of test | Type of test | ||||||
| Control group | Experiment group | Final total | |||||||||||||
| 1 | Lang A et al. (2020) [23] | Austria | RCT | 11 | 23 | 34, 11excluded | Rhymes with mother voice | 34 GA weeks | mean:84 times of 5 min(420 min) | recorded mother and unfamiliar female voice with calm or lively rhythm | listening loudly | Preferably sleep | 14.78 days (SD = 4.29) in the first recording and a mean age of 36.65 days (SD = 4.49) in the second recording | sleep-awake states based on polysomnography | |
| 2 | Speech–brain coupling | Lang et al. (2020) [24] | Austria | RCT | 12 | 22 | 34, 21 excluded | nursery rhymes with mother voice and non-maternal voice | < 34 GA weeks | At least 420 min | recorded mother and unfamiliar female voice with calm or lively rhythm | listening loudly | Preferably sleep | 14.35 days (SD = 2.67) in the first recording and 36.48 days (SD = 3.43) in the second recording | hdEEG |
| Heart rate analyses | 13 | 24 | 37, 18 excluded | ECG | |||||||||||
| 3 | 1 | Partanen E et al.(2013) [17] | Finland | RCT | 11, 1excluded | 10, 2 excluded | 21 | music melody, speech | 29 + 0 GA weeks | mean:57 times of 15 min ( 855 min) | musical melodies | listening loudly | sleep | EG:16 d CG: 13 d | ERP, MMR |
| 2 | RCT | 8, 4excluded | 10, 2excluded | 18 | music melody, speech | 29 + 0 GA weeks | mean:57 times of 15 min ( 855 min) | changed musical melodies | listening loudly | EG:144 d CG:133 d | ERP, MMR | ||||
| 4 | Partanen E et al.(2013) [25] | Finland | RCT | 16 | 17,11 excluded | 33 | nonvocal music, speech | 29 + 0 GA weeks | mean: 60 times of CD (two 4-min sequences)(480 min) | three variants of pseudowords, free choice of nonvocal | listening via headphones | sleep | EG:5.5 d CG: 4 d | EEG, ERP, MMR | |
| 5 | Arya R et al.(2012) [26] | India | RCT | 134, 36excluded | 126, 43excluded | 260 | music | EG:13.1 ± 2.4, CG:12.7 ± 2.9 GA weeks | 173.3 (± 18.9) hours | instrumental music, natural sounds, chants from religious | listening via headphones | awake | day 2 or 3 of life | BNBAS | |
| 6 | Experimental melody | Granier-Deferre C et al.(2011) [27] | France | RCT | 15 | 13 | 50, 75excluded | music | 35th,36th, and 37th weeks | mean: 39.24, SD = 2.05 sessions of 12 min (470 .88 min) | piano melody | listening loudly | sleep | EG:30.44 d, SD = 6.36 CG: Mean = 31.48 d, SD = 6.7 | HR,HRV |
| Control melody | RCT | 10 | 12 | music | 35th,36th, and 37th weeks | mean: 39.24, SD = 2.05 sessions of 12 min (470 .88 min) | piano melody | listening loudly | sleep | EG:30.44 d, SD = 6.36 CG: Mean = 31.48 d, SD = 6.7 | HR,HRV | ||||
| 7 | James D et al.(2002) [28] | UK | RCT | 10 | 10 | 20 | music | 72 h prior to elective delivery (M:38 GA weeks) | 240 min | a rhythmical music (little Brown Jug) | earphone on the maternal abdomen | days 3–5 | neonatal behavioral states | ||
| 8 | Mother voice | Decasper A et al. (1986) [29] | USA | RCT | 5 | 9 | 28, 17excluded | stories with mother voice | 7.5 months pregnant | 67 times of story ( about 210 min) | live mother voice of stories, recorded mother voice for testing | listening loudly | awake | 55.8 h postnatal | IBI of nonnutritive sucking |
| Others voice | RCT | 7 | 7 | stories with mother voice | 7.5 months pregnant | 67 times of story ( about 210 min) | live mother voice of stories, recorded mother voice for testing | listening loudly | 55.8 h postnatal | IBI of nonnutritive sucking | |||||
The participants in intervention groups underwent different kinds of sound stimulations including music (piano melodies, instrumental music, and rhythmic music) [17, 25–28] and speech phrases (speech phrases, psuedowords, stories, and rhymes) [17, 23–25, 29].
Mean enrolment time of pregnant mothers ranged from 13 to 40 weeks (gestational age). The duration of interventions was extremely different between studies, with 173.3 h of music in one study with larger population and intervention duration [26], and 210 to 855 min of music or speech in the other studies [17, 23–25, 28, 29].
Pregnant mothers and their fetuses were exposed to stimulus by listening loudly in four studies [17, 23, 24, 27, 29], via headphones in two studies [25, 26], and by devices on maternal abdomen in one studies [28].
The infant’s age at the time of test was different across studies; neonates were tested at first week of life in four studies [25, 26, 28, 29], and at the end of first month and through fifth months of life in two studies [17, 27]. Two studies evaluated the memory of infants two times, during second and fifth weeks of life [23, 24]. Five studies examined the infants during sleep periods [17, 23–25, 27] and two studies tested them while they were awake [26, 29].
The type of test used to measure outcomes was EEG in three studies [17, 24, 25], ECG and HR in two studies [24, 27], neonatal behavioral assessment in two studies [26, 28], habituation test in one study [29], and sleep-awake states based on polysomnography in one study [23].
The eight included studies reported different, and in some cases controversial, results of the sound stimulation on two comparing groups.
Findings
The specific findings of the included studies are summarized in Table 2. Partanen et al. has reported long-term plastic effects, lasting for several months, on the developing brain in addition to boosted neural responsiveness to the music used in the fetal training [17]. Similarly, in another study by Partanen et al., the results indicated modulation of neural responsiveness and enhancement of neural commitment in neonates that were exposed to selected speech stimuli during the prenatal period [25]. In the study conducted by Arya et al., the results of behavior assessment in tested neonates demonstrated that prenatal music exposure to mother had significant favorable effects on neonatal behavior [25]. Other studies has referenced the positive effects of prenatal sound intervention in forms of habituation, learning and memory formation. Decasper et al. found that the neonates had learned and remembered some features of the acoustic cues that helped them prefer their mother voice to others’ [29]. Also, in a randomized study by James et al., examination of neonatal behavioral states, suggested that a simple form of fetal programming or learning has happened for prenatally stimulated fetuses [28]. Moreover, Granier et al. showed that repetitive prenatal exposure to a specific melody impacted neonate’s auditory perception and formed a memory of the sound stream with a retention interval from 3–4 days to six weeks [27].
Table 2.
Specific findings
| Number | Author | Specific finding | Effect size |
|---|---|---|---|
| 1 | Lang A et al. (2020) [23] | Effect of Auditory Stimulation on Sleep–Wake-States | The time spent in the three behavioral states: F(1.53, 49.03) = 14.71, p < 0.001 |
| Effect of Voice Familiarity on Sleep–Wake-States | STATE*VOICE: F(1.58, 51.30) = 1.71, p = 0.196 | ||
| Effect of Rhyme Familiarity on Sleep–Wake-States | STATE*RHYME: F(1.44, 31.70) = 0.21, p = 0.752 | ||
| Effect of Auditory Stimulation on Physiology (HR) | GROUP (F(1, 29) = 8.99, p = 0.006 | ||
| 2 | Lang et al. (2020) [24] | Effect of Rhyme Familiarity on Infant’s Heart Rate | OR; F(1, 21) = 0.01, p = 0.972, |
| Effect of Voice Familiarity on Infant’s Heart Rate | VOICE F(1, 32) = 0.10, p = 0.750 | ||
| Effect of Rhyme Familiarity on Infant’s Brain Physiology | RHYME _ FREQ (F(2, 46) = 6.16, p = 0.004, after correcting for multiple comparisons:p > 0.0166 | ||
| Effect of Voice Familiarity on Infant’s Brain Physiology | VOICE (F(1, 36) = 9.39, p = 0.004 | ||
| 3 | Partanen E et al.(2013) [17] | MMRs for vowel identity changes of the syllable |
Learning group: t(16) = 2.536, P < 0.022, control group: t(15) = 2.577,P < 0.021 |
| MMRs for pitch changes of the syllable |
Learning group: t(16) = 3.640, P < 0.002 Control group: t(31) = 2.122, P < 0.042, d = 0.763 |
||
| 4 | Partanen E et al.(2013) [25] | responses to the unchanged sounds |
Learning group at birth: (t(19) = 2.11, p,0.049, d = 0.97) Learning group at the age of four months (t(16) = 3.33, p,0.004, d = 1.68) |
| Amplitudes of response at birth | unchanged (r = 0.74, p,0.015, R2 = 0.54) and changed sounds (r = 0.68, p,0.032, R2 = 0.46) | ||
| 5 | Arya R et al.(2012) [26] | BNBAS |
Habituation (ES 1.05, 95% CI 0.53–1.57, P = 0.0001), orientation (ES 1.13, 95% CI 0.82–1.44, P < 0.0001), motor performance (ES 0.25, 95% CI 0.0–0.5, P = 0.0479), range of State (ES 0.31 95% CI 0.17- 0.45 P < 0.0001), regulation of state (ES 0.54 95% CI 0.28, 0.80 P < 0.0001), and autonomic stability (ES 0.26 95% CI 0.06, 0.46 P = 0.0102) |
| 6 | Granier-Deferre C et al.(2011) [27] | Heart Rate analysis of the subjects with a cardiac deceleration |
mixed ANOVA on the z-scores: Group, F (1, 28) = .30, p = .59, Melody, F (1,28) = 1.35, p = .26, Group x Melody interaction, F (1, 28) = 1.22, p = .28 Time, F (149, 4172) = 13.02, p,.00001, Time x Group, F (149, 4172) = 1.38, p,.0017, Time x Melody, F (149, 4172) = 1.79, p,.00001, |
| 7 | James D et al.(2002) [28] | Neonatal behavioral states |
median time to a state change (P = 0.01) median time to S1 (P = 0.06) median time to exhibited awake states (S4 and S5) (P = 0.05) transitions during exposure to music compared to the baseline period (P = 0.05 and P = 0.04) |
| 8 | Decasper A et al. (1986) [29] | IBI of nonnutritive sucking |
baseline conditional probabilities of target and novel stories: t(11) < 0.1 reinforcement ratios of matched-subject pairs target-story ratios: t(11) = 2.68, p < .05, novel-story ratios: t(11) < 0.1 |
RCT Randomized Control Trial, EG Experiment Group, CG Control Group, GA Gestational Age, EEG Electroencephalography, hdEEG high-density Electroencephalography, ECG Electrocardiography, MMR Mismatch Response, ERP Event-related Potentials, BNBAS Neonatal Behavioral Assessment, HR Heart Rate, HRV Heart Rate Variability, IBI Interburst intervals, ES Effect size
* interaction
In contrast, Lang et al. found no stimulus-specific effect of sound, rhyme or voice familiarity on the newborns’ behavioral states in the prenatally exposed group. Nevertheless, a general calming effect of the experienced stimulus was found, which indicated fetal learning [23]. Another clinical trial by Lang et al. showed that newborns react distinctly to the maternal voice at second and fifth weeks of birth, identifiable with ECG and EEG. Therefore, it appears that basic memory traces are formed in fetal period and effect the neonate’s autonomic and neuronal reactions to sound stimuli [24].
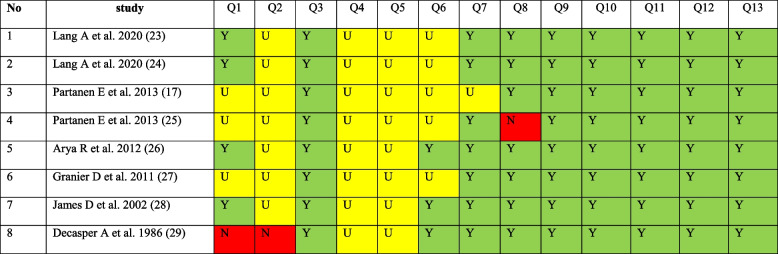
Quality assessment of studies
The risk of bias assessment of included studies is summarized (Table 3). All studies had a good general quality based on JBI critical appraisal checklist for RCTs. Four trials had a high risk of selection bias [17, 25, 27, 29]; in all studies, treatment groups were similar at the baseline characteristics. Blinding of participants and those delivering the treatment was not applicable in the studies since mothers listened to the music directly or they could be aware of vibrations produced by the device (earphones, artificial larynx) on their abdomen. In five trials, blinding of outcome assessor was not reported, so there was a high risk of detection bias [17, 23–25, 27]. In one study, the reasons of loss to follow up were not discussed, thus there was a risk of attrition bias [25]. Low risk of information bias was detected for all studies since outcomes were measured in a reliable and the same way for the compared groups. Participants were analyzed in the groups to which they were randomized in all trials. Appropriate statistical analysis was used in all studies. The designs of all trials were appropriate for the topic.
Table 3.
(Y Yes, N No, U Unclear; JBI critical appraisal checklist for randomized controlled trials: Q1 Was true randomization used for assignment of participants to treatment groups?; Q2 = Was allocation to treatment groups concealed?; Q3 = Were treatment groups similar at baseline?; Q4 = Were participants blind to treatment assignment?; Q5 = Were those delivering treatment blind to treatment assignment?; Q6 = Were outcome assessors blind to treatment assignment?; Q7 = Were treatment groups treated identically other than the intervention of interest?; Q8 = Was follow-up complete, and if not, were strategies to address incomplete follow-up utilized?; Q9 = Were participants analyzed in the groups to which they were randomized?; Q10 = Were outcomes measured in the same way for treatment groups?; Q11 = Were outcomes measured in a reliable way?; Q12 = Was appropriate statistical analysis used?; Q13 = Was the trial design appropriate, and any deviations from the standard RCT design (individual randomization, parallel groups) accounted for in the conduct and analysis of the trial?)
Level of recommendations
The JBI Recommendation Leveling Checklist was used to evaluate the results of included Studies, the results of which are presented in the Table 4.
Table 4.
Grading of evidence based on JBI grading
| Number | Author | Recommendations | Grade |
|---|---|---|---|
| 1 | Lang A et al. (2020) [23] | Prenatal experience or “fetal programming” may have an effect on behavioral (sleep/wake states) and physiological (heartrate) reactions just weeks after birth as evident in less waking periods and HR habituation to stimuli heard already in the last trimester before birth. Parents and societies should be aware of such effects and may consider this in their parenting methods even before birth. This is not to say that we are in favor of overambitious stimulation of the fetus in order to maximize learning even before birth. Rather we call into attention that much of what a fetus is exposed to before birth—whether it is parental movements, touch, music, or speech—may shape the infants’ physiology and later perception of the world | A |
| 2 | Lang et al. (2020) [24] | Our results indicate that newborns show distinct reactions to the maternal voice already at birth (two and five weeks) even on a physiological level and identifiable with ECG and EEG. Furthermore, it appears that basic memory traces are formed in utero and shape the newborn’s autonomic and neuronal reactions to speech and voice stimuli, namely, in such a way that newborns familiarized to nursery rhymes prenatally show distinctly different reactions than newborns being naïve in this respect. This again emphasizes the importance of the prenatal environment and calls into attention that already at these times the brain is tuned or “programmed” for the postnatal environment predicted and most likely experienced | A |
| 3 | Partanen E et al.(2013) [17] | These results indicate that auditory experiences during the fetal period can induce changes in neural processing and therefore have several important practical implications. First, these results indicate that the shaping of the central auditory system begins before birth. Repeated exposure to certain types of sounds leads to the development of neural memory traces for these sounds, as suggested by the strengthening of the activation in the MMR time range to changes in the learned material in the learning group. Thus, it appears likely that hearing a great deal of speech before birth may have positive effects, preparing the neural apparatus for the accurate analysis and discrimination of the fine acoustic features of speech. These early experiences may, then, affect the individual’s later abilities of speech perception and language acquisition | B |
| 4 | Partanen E et al.(2013) [25] | Our results show that prenatal exposure to music can have long-term plastic effects on the developing brain and enhance neural responsiveness to the sounds used in the prenatal training. Furthermore, we found that these plastic changes are long lasting. These findings have several practical implications. First, since the prenatal auditory environment modulates the neural responsiveness of fetuses, it seems plausible that the adverse prenatal sound environment may also have long-lasting detrimental effects. Such environments may be, for example, noisy workplaces and, in case of preterm infants, neonatal intensive care units. Furthermore, as prenatal exposure still affected the ERP responses months after birth, additional fetal exposure to structured sound environments might be beneficial for supporting the auditory processing of, for example, infants at risk for dyslexia in whom basic auditory processing was shown to be impaired | B |
| 5 | Arya R et al.(2012) [26] | In conclusion, this study provides preliminary evidence that maternal music exposure beneficially affects neonatal behaviour. A trained clinician can utilize the behavioural organization of the newborn infant to gain insights into the intrauterine experience and the perinatal events which may have influenced the neonate’s CNS organization | A |
| 6 | Granier-Deferre C et al.(2011) [27] | 3-weeks of prenatal exposure to a specific melodic contour affects infants ‘auditory processing’ or perception, i.e., impacts the autonomic nervous system at least six weeks later, when infants are 1-month old. The long-term memory for the descending melody is interpreted in terms of enduring neurophysiological tuning and its significance for the developmental psychobiology of attention and perception, including early speech perception, is discussed | B |
| 7 | James D et al.(2002) [28] | The first prospective randomized controlled study to demonstrate that fetal exposure to a complex sound stimulus results in the development of altered behavior in the fetus and the occurrence of altered behavior in the newborn period compared to unexposed controls. We have not examined whether this effect is specific to this stimulus or sound exposure in general. Furthermore, there is no information that such effects are either long lasting or beneficial | A |
| 8 | Decasper A et al. (1986) [29] | The study provides the first direct evidence that prenatal auditory experience with a particular maternally generated speech stimulus influences the reinforcing value of that stimulus after birth. The present study suggests noninvasive, ethically acceptable methods to further study the effects of prenatal auditory stimulation on postnatal auditory function and development, especially the development of speech perception | B |
Discussion
Overall seven studies showed that the tested neonates had learned or remembered the prenatal sound stimulus [17, 23–25, 27–29] and one study showed that the prenatally stimulated infants performed significantly better on orientation and habituation in a neonatal behavior test [26]. These findings suggest that stimulus-specific memory traces are formed during fetal period and shape the neonate’s autonomic and neuronal reactions to sound stimuli. Moreover, music exposure during pregnancy might have beneficial influence on neonatal behavior responses, which is an indicator of the integrity of nervous system at several levels. However, our findings also imply that because the fetal neural system is malleable to the surrounding sounds, it is also vulnerable to potential harmful environmental acoustic stimuli. Nonstandard, unstructured, and unusual acoustic stimulation, which the fetus could perceive as noise, cannot be recommended until further researches on such stimulation have been thoroughly conducted [30].
There have been multiple clinical trials regarding the impact of prenatal sound-based interventions on different aspects of fetal development. Systematic reviews and meta-analyses has been conducted to evaluate the effects of sound stimulations during pregnancy. Two of these studies indicated that music therapy as a non-pharmacological approach, can reduce anxiety levels during pregnancy and labor [31, 32]. Music interventions may decrease stress levels and physiological anxiety related indexes such as HR, systolic and diastolic pressures. Two other systematic reviews showed positive influence of music therapy on well-being and quality of life after birth in premature neonates in the neonatal intensive care unit (NICU). Music therapy can significantly improve preterm infant's HR, respiratory rate, anxiety level, and oral feeding volume [33, 34]. A systematic review by Carvalho et al. explored the immediate responses of the fetus to mother voice [35]. But this review did not investigate the memory of neonates of mother voice experienced in fetal period. Another study conducted by He et al. systematically reviewed the effect of prenatal music therapy on fetal and neonatal status; however, the neonatal effects weren’t discussed properly [36]. Moreover, studies with unhealthy participants such as pre-eclamptic and diabetic mothers, and pregnant women during labor were included. This study didn’t investigate fetal learning and the memory formation of prenatal music exposure. Therefore, this is the first systematic review to determine the effect of sound stimulations including music and speech, on fetal learning, memory and neonatal behavior, which was assessed later in neonatal period. This article could provide useful information for family-centered maternity care, pregnant women and neurodevelopment researchers.
Of the eight studies included, all had good general quality based on having more low-risk domains than high-risk ones. In four studies, application of randomization had a high risk of bias [17, 25, 27, 29]. There was high risk of bias in terms of allocation concealment in all of the studies. In five studies, the blinding of outcome assessor was not reported [17, 23–25, 27]. There was a high risk of bias concerning the reasons of loss to follow up in one study [25]. The final results might have been influenced by the high risk of selection and detection bias. Hence, there is a need for rigorously designed RCTs to provide reliable information on this concept.
There was considerable variability in the existing literature. Dissimilarities were found in the intervention process and outcome measurement. The sample size of trials varied from 18 to 260. The fetus’ age in the start of exposure ranged from before 20 weeks of gestation to 72 h prior to elective delivery. Considering the fact that the onset of fetal hearing occurs at about 23 weeks of gestation and completes at 31 weeks, sound exposure in most of the studies took place at third trimester of pregnancy [13]. However, it is not clearly known when the beneficial effect of fetal sound stimulation starts and thus optimal timing for such intervention in clinical practice cannot be determined. There is a lack of evidence concerning the role of timing of prenatal sound simulation [26].
The types of sound stimulation applied in the studies could be divided into music and speech by maternal or non-maternal voice. There have been other studies evaluating the effects of VAS on fetal learning and habituation [37]. Although, it’s important to note that using VAS is not recommended due to the potential adverse effects on fetal auditory system and the lack of reliable evidence on this matter [38].
Studies have used recorded as well as live maternal speech to stimulate the fetus, though the recorded music was not often described in details. In a research performed by Krueger et al. the fetal response to maternal live voicing was compared to a recorded format. Although the outcomes were difficult to interpret, the different response was easily detected [39]. All studies attempted to use novel stimuli with no possibility of exposure before and after birth, other than the controlled time. There were different ways of comparison between types of sound stimulation among included articles. The content of stimulation was altered in postnatal testing in the study by Decasper et al. in order to investigate the specificity of the formed memory [29]. Partanen et al. changed a number of notes in the original melody and showed that the responses to the unchanged music were greater in the study than control group, both at birth and at the age of four months [17]. Granier et al. used two ascending and descending piano melodies which had inverse contours, but similar spectra, same duration, tempo, rhythm, and amplitude. The results showed significant change in HR to the familiar melody compared to unfamiliar one in the study group [27]. Two studies by Lang et al. compared the effect of rhythm and familiarity of the voice of nursery rhymes in newborns’ response. Interestingly, the results revealed that familiarity of rhyme and voice (mother vs. other female) had no significant difference on infant’s HR and sleep-awake state. Though, the brain-level data demonstrated a distinct response of the neonate’s brain to the familiarity of voice [23, 24].
Standardization of the intensity of sound stimulation appeared to be an important challenge in the studies. Most of the studies used sounds with more than 80 dB of intensity, since diminishing of about 20 dB is expected through maternal abdomen [40]. Also, the maternal voice undergoes no or little attenuation when transmitted to the uterus [41–44]. Sound levels between 80 and 110 dB adequate to reach the fetal cochlea [13]. It is important to control the sound volume, due to the fact that intensity of sound influences the habituation time for fetus [45]. In the study conducted by Arya et al., mothers were allowed to decide about the volume of music, since the stimulus was not directly applied to maternal abdomen [26].
Frequency is another characteristic of acoustic stimulations that can impact the quantity and quality of the sound that reaches the fetus, and consequently its effects. Fetal detection of frequency changes happens in womb early in fetal development, at 28 weeks of pregnancy [46, 47]. Fetuses first respond to low frequency 250 or 500 Hz tones at about 25–27 weeks of gestational age, and afterwards to the 1000 or 3000 Hz tones by 29–31 weeks of gestational age [13]. In two studies, the range of frequency used for stimulation was about 100–1000 Hz [25, 27]. The frequency of sound was not reported in the other six studies. Further studies are required to determine the optimal frequency of music to affect the fetus in utero. Studies should provide clear reporting of the sound frequency and consider the possible adverse effects of uncontrolled sound stimuli. Future researches are recommended to avoid prolonged fetal exposure to low-frequency sound levels (< 250 Hz) higher than 65 dB [48, 49].
The duration of intervention was decided based on each study’s objectives. It ranged from 210 to 855 min for studies designed to investigate learning and memory [17, 23–25, 27–29], and about 173 h for a study designed to investigate changes in neonatal behavior [26]. Partanen et al. reported that longer exposure to speech material, such as psuedowords, may generally improve speech discrimination [25]. Nevertheless, none of the included articles focused on the influence of stimulation duration on the outcomes. Another study conducted on premature infants examined the effect of sound intervention until the infants reached term using cranial ultrasonography. The results showed that the degree of the right and left auditory cortex development was not significantly correlated with the duration of music exposure [50].
The age of neonates at time of test varied between studies; four studies tested the neonates at first week of life so as to evaluate the hypothesis of learning during prenatal period and transferring the memory into neonatal life [25, 26, 28, 29]. The other studies tested the infants at 2 and 5 weeks of life, the end of first month, and through 5 months of age in order to assess memory retention [17, 23, 24, 27]. No studies were found to investigate the retention of memory in the exposed infants after five months of age.
Most of the studies tested the infants during sleep periods, which might diminish the observable effects of the stimulation [17, 23–25, 27]. Although, recognition of familiar stimuli can occur during sleep in adult brain [51].
There were different kinds of outcome assessment methods within studies as follows:
1- EEG: in 3 studies EEG, mismatch response (MMR), and ERPs were used for detecting memory traces of sounds experienced in the womb [17, 24, 25].
2- ECG and HR: in 2 studies the cardiac response elicited by prenatally exposed melodies was used for testing the neonates [24, 27].
3- sleep-awake states based on polysomnography: in one study changes in behavioral states (quiet sleep, active sleep, transitional sleep, awake) to auditory stimulation was examined with polysomnography. This included monitoring infants with electromyogram, electrooculogram, ECG and videography [23].
4- Neonatal Behavioral Assessment: in one study Brazelton Neonatal Behavioral Assessment Scale (BNBAS) was employed for outcome evaluation [52]. One other study used conventional neonatal behavioral analysis criteria [53]. These tests examine the integrity of neonatal nervous system at different levels.
5- Nonnutritive sucking: alteration of sucking behavior in newborns when exposed to a familiar and unfamiliar recorded voice was utilized as a measurement tool in one study [29].
The region of exposure was an indicator factor of the underlying mechanism of the resulted effects. Stimulation was either direct (using devices on the maternal abdomen) [28] or indirect (mothers listened to music with headphones) [25, 26]. It’s important to note that earphones or other sound producing devices are not recommended to be used directly attached to the pregnant woman's abdomen [48, 49]. There was a better practical implication when the mother used conventional headphones over her ears due to its easier adaptability to the routine lifestyle [26]. In the studies which the sound stimulation was transmitted via environment (listening loudly to sound, reading stories out loud by mother), both direct and indirect mechanisms could be involved [17, 23, 24, 27, 29].
There were two probable mechanisms for the effects of prenatal music exposure on neonatal behavior, one of which was the indirect mechanism. The indirect effects are likely to be mediated by endocrine changes in mother [26]. Music is known to have several endocrine effects including increased growth hormone, which modifies the production of certain cytokines, increased levels of ovarian steroid secretion, alterations in the biorhythms, cortisol, testosterone, and estrogen levels [43, 44]. Corticosteroids [1] regulate growth of neuroblasts, myelination, and metabolism in developing brain in different ways [33]. Thus, the indirect mechanism was likely to be mediated via endocrine changes of stress reduction in mothers that caused enhancement of fetal neural network [25].
The direct mechanism included neural mechanisms, like music processing. Music perception along with auditory signal transduction triggers a sequence of motor, cognitive, and emotional processes that evokes a number of brain areas, unilaterally and bilaterally [46]. Another direct mechanism was neuro-physiological adaptive process. This process is mediated by the autonomic nervous system and aims at tuning the auditory system. The neuro-physiological adaptive mechanism might form a better neuro-functional organization of fetal auditory system by structuring cellular and synaptic plasticity and improving receptive field selectivity [27]. As a result, fetal sound stimulation might develop a more effective neural network for detection of the changes in sounds. However, further researches are needed to shed light on the precise mechanisms of the discussed effects.
Limitation
The main limitation of this systematic review was the heterogeneity of intervention and outcome assessment methods across the studies that made it difficult to reach a consistent conclusion. The search strategy and study selection processes were restricted to English-language publications and limited databases. Furthermore, it should be noted that most of the studies had high risk of selection and detection bias that might influence the final conclusion.
Suggestion and implication
There is a need for methodologically strong RCTs about this concept with rigorously designed interventions, consistent outcomes, and standardized reporting measures. Future studies may include comparisons of different intervention durations and sound stimulation types. Specific effects of different types of music including instrumental music compared to vocal one and maternal voice versus other voices should be considered in future studies. Recommended areas for further research are the mechanisms of the effect of music on fetus, the association between behavioral development of newborn and intrauterine central nervous system (CNS) organization, and more long-term follow-up studies. Using music during pregnancy may have positive implications for maternal-neonatal bonding after birth [35]. This field of study could be useful for family-centered maternity care to provide a novel and pleasant care for pregnant mothers and neuro-researchers to extend their measures for understanding neurodevelopment process.
The findings of this study emphasizes the importance of the prenatal environment on fetal development, therefore, the influence of adverse prenatal sound environment on fetus is an essential field to be studied in future researches. Studies should follow safety recommendations strictly and provide clearer reporting on sound stimulation characteristics [48, 49]. It’s important to note that although this review shows evidence that the fetus can learn, it does not mean that they need to be taught anything. Misinterpretation of the data has resulted in the development of commercial products that promote use of headphones applied to the pregnant abdomen that play certain types of music and sounds to help "improve" prenatal brain development. This might be a potentially harmful practice due to the currently unsufficient knowledge on the matter.
Conclusion
The findings of this study suggest that sound stimulation (music and speech) can form stimulus-specific memory traces during fetal period and shape the neonatal autonomic and neuronal reactions to sound stimuli. Also, fetal music exposure might have beneficial effects on neonatal behavior responses, which is an indicator of the integrity of nervous system. However, these outcomes also imply that the fetal neural system is vulnerable to potential harmful surrounding sounds. There is a need for methodologically strong RCTs that follow safety recommendations strictly and provide clearer reporting on sound stimulation characteristics. Mechanisms of the effect of music on fetus, the influence of adverse prenatal sound environment on fetus, the association between behavioral development of newborn and intrauterine CNS organization, and more long-term follow-up studies are the recommended areas for further research.
Acknowledgements
The research protocol was approved and supported by Student Research Committee, Tabriz University of Medical Sciences (grant number: 66065).
Abbreviations
- VAS
Vibroacoustic stimulation
- ECG
Electrocardiography
- EEG
Electroencephalography
- HR
Heart rate
- ERPs
Brain event-related potentials
- PRISMA
The Preferred Reporting Items for Systematic reviews and Meta-Analyses
- NICU
Neonatal intensive care unit
- MMR
Mismatch response
- BNBAS
Brazelton Neonatal Behavioral Assessment Scale
- CNS
Central nervous system
Authors’ contributions
Study concept and design: AS, KM, MG and LN, Acquisition of data: AS, KM, MG and LN, Drafting of the Manuscript: AS, KM, MG and LN, Critical revision of the manuscript: AS, KM, MG and LN. The authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki of the World Medical Association and approval was obtained from the local ethics committees (Research Ethics Committees of Vice-Chancellor in Research Affairs—Tabriz University of Medical Sciences, approval ID: IR.TBZMED.VCR.REC.1399.378). Consent to participate is not applicable in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Trehub SE. Musical predispositions in infancy. Ann N Y Acad Sci. 2001;930(1):1–16. doi: 10.1111/j.1749-6632.2001.tb05721.x. [DOI] [PubMed] [Google Scholar]
- 2.Koelsch S. Toward a neural basis of music perception–a review and updated model. Front Psychol. 2011;2:110. doi: 10.3389/fpsyg.2011.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel AD. Language, music, syntax and the brain. Nat Neurosci. 2003;6(7):674–681. doi: 10.1038/nn1082. [DOI] [PubMed] [Google Scholar]
- 4.Chorna O, Filippa M, De Almeida JS, Lordier L, Monaci M, Hüppi P, et al. Neuroprocessing mechanisms of music during fetal and neonatal development: A role in neuroplasticity and neurodevelopment. Neural Plast. 2019;2019:3972918. doi: 10.1155/2019/3972918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lecanuet J-P, Graniere-Deferre C, Jacquet AY, DeCasper A. Fetal discrimination of low-pitched musical notes. Dev Psychobiol. 2000;36(1):29–39. doi: 10.1002/(SICI)1098-2302(200001)36:1<29::AID-DEV4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 6.Groome LJ, Mooney DM, Holland SB, Smith LA, Atterbury JL, Dykman RA. Behavioral state affects heart rate response to low-intensity sound in human fetuses. Early Human Dev. 1999;54(1):39–54. doi: 10.1016/S0378-3782(98)00083-8. [DOI] [PubMed] [Google Scholar]
- 7.Lecanuet J-P, Granier-Deferre C, DeCasper A, Maugeais R, Andrieu A-JJ. Perception et discrimination foetales de stimuli langagiers; mise en évidence à partir de la réactivité cardiaque; résultats préliminaires. Comptes rendus de l'Académie des sciences Série 3, Sciences de la vie 1987;305(5):161–4. [PubMed]
- 8.Lecanuet J, Granier-Deferre C, Busnel M, editors. Differential fetal auditory reactiveness as a function of stimulus characteristics and state. Seminars in Perinatology; 1989. [PubMed]
- 9.Hepper PG, Shahidullah BS. Development of fetal hearing. Arch Dis Child Fetal Neonatal Ed. 1994;71(2):F81–F87. doi: 10.1136/fn.71.2.F81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Qahtani NH. Foetal response to music and voice. Aust N Z J Obstet Gynaecol. 2005;45(5):414–7. doi: 10.1111/j.1479-828X.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- 11.Granier-Deferre CRA, Jacquet AY, Bassereau S. Near-term fetuses process temporal features of speech. Dev Sci. 2011;14(2):336–352. doi: 10.1111/j.1467-7687.2010.00978.x. [DOI] [PubMed] [Google Scholar]
- 12.Preyer WT. Specielle Physiologie des Embryo: Untersuchungen ueber die Lebenserscheinungen vor der Geburt: Grieben; 1885.
- 13.Hepper PG, Shahidullah BS. The development of fetal hearing. Fetal Matern Med Rev. 1994;6(3):167–179. doi: 10.1017/S0965539500001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herschkowitz N. Brain development in the fetus, neonate and infant. Biol Neonate. 1988;54(1):1–19. doi: 10.1159/000242818. [DOI] [PubMed] [Google Scholar]
- 15.Kisilevsky BS, Hains SM. Exploring the relationship between fetal heart rate and cognition. Infant Child Dev. 2010;19(1):60–75. doi: 10.1002/icd.655. [DOI] [Google Scholar]
- 16.Okano H, Hirano T, Balaban E. Learning and memory. Proc Natl Acad Sci. 2000;97(23):12403–12404. doi: 10.1073/pnas.210381897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Partanen E, Kujala T, Tervaniemi M, Huotilainen M. Prenatal music exposure induces long-term neural effects. PLoS ONE. 2013;8(10):e78946. doi: 10.1371/journal.pone.0078946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hepper PG. Memory in utero? Dev Med Child Neurol. 1997;39(5):343–346. doi: 10.1111/j.1469-8749.1997.tb07442.x. [DOI] [PubMed] [Google Scholar]
- 19.Lecanuet JP, Gautheron B, Locatelli A, Schaal B, Jacquet AY, Busnel MC. What sounds reach fetuses: biological and nonbiological modeling of the transmission of pure tones. Dev Psychobiol. 1998;33(3):203–219. doi: 10.1002/(SICI)1098-2302(199811)33:3<203::AID-DEV2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 20.Nyman M, Arulkumaran S, Hsu T, Ratnam S, Till O, Westgren M. Vibroacoustic stimulation and intrauterine sound pressure levels. Obstet Gynecol. 1991;78(5 Pt 1):803–806. [PubMed] [Google Scholar]
- 21.Dunn K, Reissland N, Reid VM. The functional foetal brain: A systematic preview of methodological factors in reporting foetal visual and auditory capacity. Dev Cogn Neurosci. 2015;13:43–52. doi: 10.1016/j.dcn.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. 2021;372. [DOI] [PMC free article] [PubMed]
- 23.Lang A, Del Giudice R, Schabus MJBS. Sleep, Little Baby: The Calming Effects of Prenatal Speech Exposure on Newborns’ Sleep and Heartrate. Brain Sci. 2020;10(8):511. doi: 10.3390/brainsci10080511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang A, Ott P, Del Giudice R, Schabus MJBS. Memory Traces Formed in Utero—Newborns’ Autonomic and Neuronal Responses to Prenatal Stimuli and the Maternal Voice. Brain Sci. 2020;10(11):837. doi: 10.3390/brainsci10110837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Partanen E, Kujala T, Näätänen R, Liitola A, Sambeth A, Huotilainen M. Learning-induced neural plasticity of speech processing before birth. Proc Natl Acad Sci USA. 2013;110(37):15145–15150. doi: 10.1073/pnas.1302159110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arya R, Chansoria M, Konanki R, Tiwari DK. Maternal music exposure during pregnancy influences neonatal behaviour: An open-label randomized controlled trial. Int J Pediatr. 2012;2012:901812. doi: 10.1155/2012/901812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granier-Deferre C, Bassereau S, Ribeiro A, Jacquet AY, Decasper AJ. A melodic contour repeatedly experienced by human near-term fetuses elicits a profound cardiac reaction one month after birth. PLoS ONE. 2011;6(2):e17304. doi: 10.1371/journal.pone.0017304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.James DK, Spencer CJ, Stepsis BW. Fetal learning: a prospective randomized controlled study. Ultrasound Obstet Gynecol. 2002;20(5):431–438. doi: 10.1046/j.1469-0705.2002.00845.x. [DOI] [PubMed] [Google Scholar]
- 29.DeCasper AJ, Spence MJ. Prenatal maternal speech influences newborns' perception of speech sounds. Infant Behav Dev. 1986;9(2):133–150. doi: 10.1016/0163-6383(86)90025-1. [DOI] [Google Scholar]
- 30.Kim H, Lee MH, Chang HK, Lee TH, Lee HH, Shin MC, et al. Influence of prenatal noise and music on the spatial memory and neurogenesis in the hippocampus of developing rats. Brain Develop. 2006;28(2):109–114. doi: 10.1016/j.braindev.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Lin H-H, Chang Y-C, Chou H-H, Chang C-P, Huang M-Y, Liu S-J, et al. Effect of music interventions on anxiety during labor: a systematic review and meta-analysis of randomized controlled trials. PeerJ. 2019;7:e6945. doi: 10.7717/peerj.6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Willenswaard KC, Lynn F, McNeill J, McQueen K, Dennis C-L, Lobel M, et al. Music interventions to reduce stress and anxiety in pregnancy: a systematic review and meta-analysis. BMC Psychiatry. 2017;17(1):1–9. doi: 10.1186/s12888-017-1432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bieleninik Ł, Ghetti C, Gold CJP. Music therapy for preterm infants and their parents: A meta-analysis. Pediatrics. 2016;138(3):e20160971. doi: 10.1542/peds.2016-0971. [DOI] [PubMed] [Google Scholar]
- 34.Yue W, Han X, Luo J, Zeng Z, Yang M. Effect of music therapy on preterm infants in neonatal intensive care unit: Systematic review and meta-analysis of randomized controlled trials. J Adv Nurs. 2021;77(2):635–52. doi: 10.1111/jan.14630. [DOI] [PubMed] [Google Scholar]
- 35.Carvalhoa MES, De Miranda Justo JMR, Gratier M, Da Silva HMFR. The impact of maternal voice on the fetus: A systematic review. Curr Women's Health Revi. 2019;15(3):196–206. doi: 10.2174/1573404814666181026094419. [DOI] [Google Scholar]
- 36.He H, Huang J, Zhao X, Li Z. The Effect of Prenatal Music Therapy on Fetal and Neonatal Status: A Systematic Review and Meta-Analysis. Complement Ther Med. 2021;60:102756. doi: 10.1016/j.ctim.2021.102756. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez-Gonzalez NL, Suarez MN, Perez-Piñero B, Armas H, Domenech E, Bartha JL. Persistence of fetal memory into neonatal life. Acta Obstet Gynecol Scand. 2006;85(10):1160–1164. doi: 10.1080/00016340600855854. [DOI] [PubMed] [Google Scholar]
- 38.Gagnon R, Hunse C, Carmichael L, Fellows F, Patrick J. Effects of vibratory acoustic stimulation on human fetal breathing and gross fetal body movements near term. Am J Obstet Gynecol. 1986;155(6):1227–1230. doi: 10.1016/0002-9378(86)90149-3. [DOI] [PubMed] [Google Scholar]
- 39.Krueger CA, Cave EC, Garvan C. Fetal response to live and recorded maternal speech. Biol Res Nurs. 2015;17(1):112–120. doi: 10.1177/1099800414532308. [DOI] [PubMed] [Google Scholar]
- 40.Gerhardt KJ, Abrams RM, editors. Fetal hearing: characterization of the stimulus and response. Seminars in perinatology; 1996: Elsevier. [DOI] [PubMed]
- 41.Querleu D, Renard X, Versyp F, Paris-Delrue L, Vervoort P. La transmission intra-amniotique des voix humaines. Rev Fr Gynecol Obstet. 1988;83(1):43–50. [PubMed] [Google Scholar]
- 42.Querleu D, Renard X, Boutteville C, Crepin G, editors. Hearing by the human fetus? Seminars in perinatology; 1989. [PubMed]
- 43.Richards DS, Frentzen B, Gerhardt KJ, McCANN ME, Abrams RM. Sound levels in the human uterus. Obstet Gynecol. 1992;80(2):186–190. [PubMed] [Google Scholar]
- 44.Benzaquen S, Gagnon R, Hunse C, Foreman J. The intrauterine sound environment of the human fetus during labor. Am J Obstet Gynecol. 1990;163(2):484–490. doi: 10.1016/0002-9378(90)91180-K. [DOI] [PubMed] [Google Scholar]
- 45.Lecanuet J, Granier-Deferre C, Busnel M. Fetal cardiac and motor responses to octave-band noises as a function of central frequency, intensity and heart rate variability. Early Human Dev. 1988;18(2–3):81–93. doi: 10.1016/0378-3782(88)90045-X. [DOI] [PubMed] [Google Scholar]
- 46.Holst M, Eswaran H, Lowery C, Murphy P, Norton J, Preissl H. Development of auditory evoked fields in human fetuses and newborns: a longitudinal MEG study. Clin Neurophysiol. 2005;116(8):1949–1955. doi: 10.1016/j.clinph.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Draganova R, Eswaran H, Murphy P, Lowery C, Preissl H. Serial magnetoencephalographic study of fetal and newborn auditory discriminative evoked responses. Early Human Dev. 2007;83(3):199–207. doi: 10.1016/j.earlhumdev.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 48.Graven SN. Sound and the Developing Infant in the NICU: Conclusions and Recommendations for Care. J Perinatol. 2000;20(1):S88–S93. doi: 10.1038/sj.jp.7200444. [DOI] [PubMed] [Google Scholar]
- 49.Krueger C, Horesh E, Crossland BA. Safe sound exposure in the fetus and preterm infant. J Obstet Gynecol Neonatal Nurs: JOGNN. 2012;41(2):166–170. doi: 10.1111/j.1552-6909.2012.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Webb AR, Heller HT, Benson CB, Lahav AJ. Mother’s voice and heartbeat sounds elicit auditory plasticity in the human brain before full gestation. Proc Natl Acad Sci USA. 2015;112(10):3152–3157. doi: 10.1073/pnas.1414924112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Legendre G, Andrillon T, Koroma M, Kouider S. Sleepers track informative speech in a multitalker environment. Nat Hum Behav. 2019;3(3):274–283. doi: 10.1038/s41562-018-0502-5. [DOI] [PubMed] [Google Scholar]
- 52.Brazelton TB, Nugent JK. Neonatal behavioral assessment scale: Cambridge University Press; 1995.
- 53.Prechtl HF. The behavioural states of the newborn infant (a review) Brain Res. 1974;76(2):185–212. doi: 10.1016/0006-8993(74)90454-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.