Abstract
Aims:
To estimate incidence of type 1 diabetes (T1D) and to develop a T1D prediction model among young adults.
Methods:
Adults 20–45 years newly-diagnosed with diabetes in 2017 were identified within Kaiser Permanente’s healthcare systems in California and invited for diabetes autoantibody (DAA) testing. Multiple imputation was conducted to assign missing DAA status. The primary outcome for incidence rates (IR) and the prediction model was T1D defined by ≥1 positive DAA.
Results:
Among 2,347,989 persons at risk, 7862 developed diabetes, 2063 had DAA measured, and 166 (8.0%) had ≥1 positive DAA. T1D IR (95% CI) per 100,000 person-years was 15.2 (10.2–20.1) for ages 20–29 and 38.2 (28.6–47.8) for ages 30–44 years. The age-standardized IRs were 32.5 (22.2–42.8) for men and 27.2 (21.0–34.5) for women. The age/sex-standardized IRs were 30.1 (23.5–36.8) overall; 41.4 (25.3–57.5) for Hispanics, 37.0 (11.6–62.4) for Blacks, 21.4 (14.3–28.6) for non-Hispanic Whites, and 19.4 (8.5–30.2) for Asians. Predictors of T1D among cases included female sex, younger age, lower BMI, insulin use and having T1D based on diagnostic codes.
Conclusions:
T1D may account for up to 8% of incident diabetes cases among young adults. Follow-up is needed to establish the clinical course of patients with one DAA at diagnosis.
Keywords: Type 1 diabetes, Young adults, Incidence rates, Prediction model, Autoimmunity
Preliminary results of this study were described in two published abstracts from the American Diabetes Association Annual Scientific Sessions in June 2019 in Chicago.
1. Introduction
In the United States, three in 1000 young adults are diagnosed with diabetes annually [1]. Although the overall incidence of diabetes has not increased in recent years [2], the number of new cases that have type 1 diabetes (T1D) versus type 2 diabetes (T2D) is not known. T1D is due to the autoimmune cell-mediated destruction of the pancreatic β-cells resulting in the loss of insulin secretion [3]. While classification of diabetes type is important for determining the best therapeutic approach, the type of diabetes is not always evident around the time of diagnosis. The onset of T1D in adults may be more variable then in children; adults may not present with the classic symptoms observed in children, may have a slower progressing glucose homeostasis decompensation, and may not require insulin treatment for months after diagnosis leading to possible misdiagnosis of T2D [4–7].
The continuum in the presentation and progression has made it difficult to conduct surveillance of incident adult-onset T1D [8]. Although European registries suggest that most T1D cases are diagnosed after age 20 years [8–10], there are not similar registries in the US and estimates of prevalence of T1D among young adults in the U.S are often based on self-report of diabetes type and treatment status [11].
To fill the gap in our knowledge of T1D in young adults, the Diabetes in Young Adults (DiYA) study had two objectives: to (1) estimate the incidence of T1D among 20–45 year olds and (2) develop prediction models to identify individuals who may have T1D from among all incident diabetes cases for possible use in clinical settings and national surveillance.
2. Subjects, materials and methods
2.1. Population and data sources
This study was conducted in the Kaiser Permanente (KP) Southern California (KPSC) and Northern California (KPNC) regions which combined had over 9 million members in 2017. KP is a prepaid, integrated health care system that uses an Epic electronic health record (EHR). Members predominantly receive their health care in KP facilities, with claims for outside services included in the EHR. This study was reviewed and approved by the Institutional Review Boards for KPSC and KPNC.
2.2. Population at risk
As shown in Figure 1, we identified all individuals who were KP members on 1/1/2017 that would be 20 years or older and less than46 years by 12/31/2017 and had 12 consecutive months of health plan enrollment in 2016. We then excluded members that had evidence of prevalent diabetes using the validated KPNC Diabetes Registry case definition that includes diagnosis code, laboratory test results and dispensed prescriptions indicative of diabetes [12]. The remaining members were the population without recognized diabetes and thus at risk for a diabetes diagnosis in 2017.
Fig. 1 –
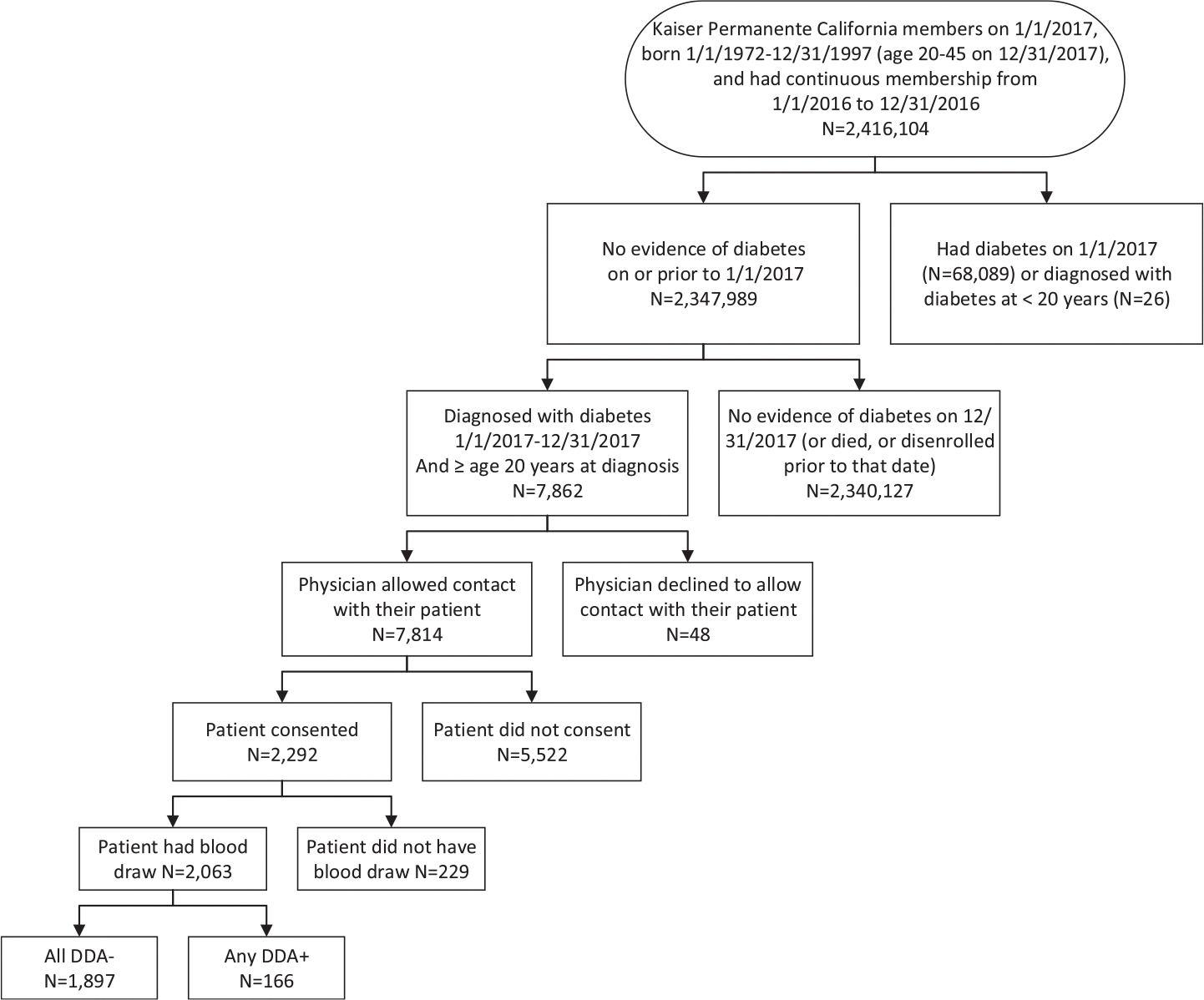
Flow chart for study sample selection, case identification, recruitment, diabetes autoantibody testing and associated results: The DiYA study.
2.3. Ascertainment and recruitment of incident cases
We conducted monthly ascertainment for newly-diagnosed (incident) diabetes cases through review of EHRs of more than 2 million individuals at risk of diabetes from 1 January through 31 December 2017. Based on information in the EHR, we considered the individual to have incident diabetes if they met at least one of the criteria shown in Table 1. We used the date of the second diabetes indicator as the diagnosis date. Women with diabetes indicators detected exclusively during pregnancy were not classified as incident cases.
Table 1 -.
Criteria used to identify Kaiser Permanente members in California ages 20–45 years as having incident (newly-diagnosed) diabetes: the DiYA study.
| Criterion | Value | Details |
|---|---|---|
|
| ||
| Outpatient diabetes diagnosis | ≥ 2 ICD-10-CM codes for diabetes. | Any position (primary, secondary, etc.). Codes must be on separate days. Included E10.xxx, E11.xxx, and E13. xxx (does not include gestational diabetes) |
| OR | ||
| Outpatient diabetes diagnosis AND outpatient prescription for a diabetes medication | ≥ 1 ICD-10-CM code for diabetes and ≥ 1 diabetes medication | See details above for ICD-10-CM codes |
| OR | ||
| Outpatient diabetes diagnosis AND Outpatient abnormal laboratory result indicative of diabetes | ≥ 2 abnormal laboratory result including plasma glucose fasting ≥ 126 mg/dL, 2 h after oral glucose load ≥ 200 mg/dL, or random ≥ 200 mg/dL or HbA1c ≥ 6.5% (≥48 mmol/mol) | Test must be on separate days if the same test type. Tests can occur on the same day if they are different test types. |
| OR | ||
| Inpatient diabetes diagnosis AND any outpatient diabetes indicator | ≥ 1 inpatient diagnosis and ≥1 outpatient diabetes indicator (e.g., diagnosis, prescription for a diabetes medication or abnormal laboratory result indicative of diabetes) | Primary discharge code only |
ICD-10-CM = International Classification of Diseases, Tenth Revision, Clinical Modification
After incident cases were identified, their primary health care providers were given the opportunity to exclude them from study outreach for any reason (n = 48, 0.6%). After these exclusions, outreach was conducted in English and Spanish by electronic mail and/or postal mail with telephone follow-up to invite these individuals to participate in the DiYA Study by providing informed consent, completing a survey, and having blood drawn at any KPSC or KPNC clinical laboratory for DAA testing. Recruitment was conducted from June 2017 through December 2018.
2.4. Diabetes autoantibody testing
Serum samples were stored at −80 °C prior to shipment on dry ice to the Biochemical Autoantibody Laboratory at the University of Colorado Barbara Davis Center for Diabetes. The laboratory is a National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) North America Core Laboratory. All blood samples were analyzed for four DAAs with standard radio-binding assay (RBA) including antibodies to GAD65 (GADA), insulinoma-associated protein 2 (IA-2A), zinc transporter-8 (ZnT8A) and insulin (IAA). Briefly, GADA and IA-2A assays were performed with NIDDK harmonized standard RBA [13] with results expressed in DK units. IAA and ZnT8A assays were assessed using the RBA developed at the Barbara Davis Center for Diabetes as previously published [14,15] and the results are expressed as an index. The sensitivity and specificity were 82% and 99% for GADA, 62% and 100% for IA-2A, 66% and 99% for IAA, 68% and 100% for ZnT8A in the 2018 Islet Autoantibody Standardization Program workshop [16]. Primary care providers were sent their patients’ results if at least one DAA was positive and the participant consented to share their results.
2.5. Definition of diabetes type
Our a priori primary outcome was T1D defined as having diabetes (see Table 1) and the presence of at least one of the four measured DAAs as suggested by the 2019 ADA criteria [5]. A positive IAA result did not count toward the case definition if there was evidence that insulin had been dispense for at least two weeks prior to the blood draw (n = 102). Individuals that did not meet the criteria for T1D were categorized as having T2D or other forms of non-gestational diabetes.
2.6. Other variable definitions
Date of birth, sex, race, ethnicity, height, weight, census block of residence and payer for insurance were extracted from the EHR. Age on 1/1/2017 and on the date of diabetes diagnosis were calculated from the date of birth. Individuals whose race/ethnicity was missing (6.6% of overall sample, 3.3% of cases) and those categorized as Native American, Pacific Islander, or multiple or other race, were combined as other/unknown. BMI was calculated as kg/m2 using measured height and weight closest to 1/1/2017, and median household income was based on the median for their residence’s census block on 1/1/2017. For individual with diabetes, we selected BMI and HbA1c levels measured closest to the diagnosis date (median: on date of diagnosis for weight; 4 days before diagnosis for HbA1c), diabetes treatment regimen based on all diabetes medications dispensed from 4 months before diagnosis date (to capture medication dispensed prior to the second diabetes indicator) until one year after diagnosis, and presence of diabetic ketoacidosis (DKA) based on diagnosis codes from 90 days before the first indicator of diabetes until 180 days after the diagnosis date. To approximate diabetes type assigned by their physician (“clinician type”), individuals were considered to have T1D if ≥50% of their diabetes diagnosis codes were for T1D (E10.xxx, Supplemental Table 1) out of all diabetes diagnosis codes from the first diabetes indicator until 365 days after diagnosis [17].
2.7. Statistical analysis
All of the analyses were conducted using SAS Enterprise Guide version 7.1 (SAS Institute, Inc., Cary, NC, USA).
2.7.1. Multiple imputation
Multiple imputation was used to handle missing DAA status (positive vs negative) for individuals who did not provide a blood sample for the study and for missing covariates of interest tested for inclusion in the prediction models (BMI, change in BMI in the year before diagnosis and the 6 months after diagnosis, and clinical laboratory values). For the final analytic data set, 50 multiple imputed datasets with complete and imputed data were used to calculate incidence rates and to construct the prediction models. Details of the methods for imputation of missing variables (including DAA results for individuals whose antibodies were not tested by the study) are in the Supplemental Material; Supplemental Table 2 includes variables used for the imputation.
2.7.2. Diabetes incidence rates
Person-time (months) for all persons at risk for diabetes was used to account for censoring due to disenrollment or death prior to 12/31/2017. We estimated diabetes incidence rates per 100,000 person years (PY) and 95% CIs overall and by type (T1D vs T2D/other) standardized to the age and sex of the 2010 US Census population, by sex standardized by age, and by race/ethnicity standardized by age and sex using the direct standardization method. Once rates were calculated, Poisson regression was used to test for differences in rates by sex and race/ethnicity.
2.7.3. T1D prediction models
The purpose of the prediction models was to identify individuals who may have T1D from among the total sample of individuals with diabetes. Briefly, the dataset with all incident diabetes cases was randomly split into training (66.3%) and validation (33.7%) samples. Modelling techniques including stepwise analysis, SAS rapid predictive modeler and recursive partitioning (trees) were applied to the training dataset to identify potential splits in continuous factors, as well as the strongest predictors of T1D and determine the models with the greatest predictive ability. Variable selection was primarily based on improvement in model fit measured by Bayesian Information Criterion rather than p-value. Final models were then tested in the validation data. The area under the receiver operating characteristic curve (AUC) with its 95% CI was used to assess the overall fit of the predictive model relative to the prediction of T1D. Additional information including the variables tested for inclusion in the prediction models which were selected from an array of clinical information in the EHR collected as part of routine clinical care are in Supplemental Table 2.
3. Results
Of the 2,347,989 individuals at risk for diabetes, 7862 incident cases were identified in 2017 (Table 2; Figure). Of these, 2063 (26.2%) consented and had their blood drawn for DAA testing. A comparison of the characteristics of tested versus untested individuals is shown in Supplemental Table 3. The median time from diagnosis to blood draw was 299 days (interquartile range: 166 days). Eight percent of the 2063 participants tested were DAA positive; 115 (5.6%) had one and 51 (2.4%) had two or more positive DAAs. Frequency of positivity by DAA were 6.1%, 2.2%, 2.0%, and 1.7% for GADA, IAA, IA-2A and ZnT8A, respectively (Table 3). Characteristics of individuals by measured DAA status are shown in Supplemental Table 4. Over three-quarters of participants who were DAA+ had a clinical diagnosis of T2D. The final datasets which categorized all incident cases as antibody positive or negative using multiple imputations for missing DAA results, resulted in a mean DAA positive rate of 8.0%, ranging from 7.0% to 9.6% across the 50 imputed datasets.
Table 2 -.
Characteristics of individuals in the population at risk, overall and by incident diabetes status in 2017: The DiYA study.
| Population at Risk on 1/1/2017 | Diabetes Status at end of 2017 |
||
|---|---|---|---|
| Diabetes | No Diabetes | ||
|
| |||
|
N
Sex, n (%) |
2,347,989 | 7,862 | 2,340,127 |
| Male | 1,133,031 (48.3) | 4313 (54.9) | 1,128,718 (48.2) |
| Female | 1,214,847 (51.7) | 3549 (45.1) | 1,211,298 (51.8) |
| Other/Unknown | 111 (0.0) | 0 (0.0) | 111 (0.0) |
|
Age, years, mean (SD)
Age Categories, years, n (%) |
31.5 (7.5) | 36.8 (6.0) | 31.5 (7.5) |
| Age 20–24 | 564,390 (24.0) | 400 (5.1) | 563,990 (24.1) |
| Age 25–29 | 412,323 (17.6) | 611 (7.8) | 411,712 (17.6) |
| Age 30–34 | 462,358 (19.7) | 1397 (17.8) | 460,961 (19.7) |
| Age 35–39 | 467,922 (19.9) | 2234 (28.4) | 465,688 (19.9) |
| Age 40–45 | 440,996 (18.8) | 3220 (41.0) | 437,776 (18.7) |
| Race/Ethnicity, n (%) * | |||
| Hispanic (regardless of race) | 812,462 (34.6) | 3882 (49.4) | 808,580 (34.6) |
| White (non-Hispanic) | 780,012 (33.2) | 1381 (17.6) | 778,631 (33.3) |
| Asian | 360,861 (15.4) | 1380 (17.6) | 359,481 (15.4) |
| Black (non-Hispanic) | 164,205 (7.0) | 640 (8.1) | 163,565 (7.0) |
| Other race/ethnicity/missing | 212,341 (9.0) | 579 (7.4) | 236,170 (10.1) |
|
BMI nearest to 1/1/17, mean ± SD
BMI, kg/m2, n (%) |
28.3 (6.72) | 37.0 (8.72) | 28.3 (6.69) |
| BMI < 18.5 | 32,622 (1.4) | 7 (0.1) | 32,615 (1.4) |
| BMI 18.5–24.9 | 648,209 (27.6) | 368 (4.7) | 647,841 (27.7) |
| BMI 25–29.9 | 608,465 (25.9) | 1270 (16.2) | 607,195 (25.9) |
| BMI 30–35.9 | 346,943 (14.8) | 1952 (24.8) | 344,991 (14.7) |
| BMI 35+ | 277,797 (11.8) | 4233 (53.8) | 273,564 (11.7) |
| Missing | 433,953 (18.5) | 32 (0.4) | 433,921 (18.5) |
| Insurance type, n (%) | |||
| Commercial | 2,055,397 (87.5) | 6961 (88.5) | 2,048,436 (87.5) |
| Medicaid, Medicare or state-subsidized | 168,782 (7.2) | 647 (8.2) | 168,135 (7.2) |
| Private pay or Self-funded | 74,984 (3.2) | 165 (2.1) | 74,819 (3.2) |
| Other/Missing | 48,826 (2.1) | 89 (1.2) | 48737(2.1) |
|
Median household Income in census block, US$ mean ± SD
Median household income, US$, n (%) |
75,268 (31324) | 68,681 (28369) | 75,291 (31331) |
| <$30,000 | 67,646 (2.9) | 280 (3.6) | 67,366 (2.9) |
| $30–<50 K | 453,957 (19.3) | 2030 (25.8) | 451,927 (19.3) |
| $50–<75 K | 768,813 (32.7) | 2756 (35.1) | 766,057 (32.7) |
| $75 < 100 K | 567,747 (24.2) | 1666 (21.2) | 566,081 (24.2) |
| $100 < 150 K | 423,688 (18.0) | 1006 (12.8) | 422,682 (18.1) |
| $150,000+ | 51,197 (2.2) | 92 (1.2) | 51,105 (2.2) |
| Missing | 14,941 (0.6) | 32 (0.4) | 14,909 (0.6) |
| Diabetes Medication † | |||
| No diabetes medications | − | 1936 (24.6) | − |
| Insulin +/− other diabetes medication | − | 728 (9.3) | − |
| Other non-insulin diabetes medication(s) | − | 5198 (66.1) | − |
| Diabetes type based on majority of ICD-10-CM codes † | |||
| Type 1 | − | 126 (1.6) | − |
| Type 2 | − | 7710 (98.1) | − |
| Other type or unknown‡ | − | 26 (0.3) | − |
Other race/ethnicity (% of total population) includes Multiple Race (1.4%), Pacific Islander (0.9%), “Other Race” (0.8%), and Native American (0.3%).
In the 365 days after diabetes diagnosis.
Of the 26 individuals categorized as other diabetes type or unknow, 9 individuals had >50% of their codes for diabetes types other than type 1 diabetes or type 2 diabetes, 12 had equal numbers of codes for type 1 diabetes and type 2 diabetes or type 2 diabetes and other diabetes type, and 5 had codes for three types of diabetes, none of which exceeded 50% of the ICD-10 codes.
Table 3 -.
Distribution of Antibody Test Results for 2063 individuals whose antibodies were tested by the DiYA Study, for Each Antibody.
| Diabetes Autoantibody | Test Results |
|---|---|
|
| |
| GAD65 | |
| Positive, n/N (%) | 126/2,063 (6.1) |
| Median value, DK units (all positives) | 168.0 |
| Mean (SD), DK units | 330.0 (326.0) |
| Range of values, DK units | 20.5–1086.3 |
| Threshold for positive, DK units | 20.0 |
| mIAA * | |
| Positive, n/N (%) | 43/1961 (2.2) |
| Median value (all positives) | 0.035 |
| Mean (SD) | 0.259 (0.590) |
| Range of values | 0.011–3.024 |
| Threshold for positive | 0.010 |
| IA2 | |
| Positive, n/N (%) | 42/2063 (2.0) |
| Median value (all positives) | 155.3 |
| Mean (SD) | 172.1 (116.1) |
| Range | 12.2–378.9 |
| Threshold for positive | 5.0 |
| ZnT8 | |
| Positive, n/N (%) | 36/2063 (1.7) |
| Median value (all positives) | 0.112 |
| Mean (SD) | 0.218 (0.262) |
| Range of values | 0.024–1.138 |
| Threshold for positive | 0.020 |
| Number of DAA+, N (%) | |
| 0 | 1897 (92.0) |
| 1 | 115 (5.6) |
| 2 | 24 (1.2) |
| 3 | 24 (1.2) |
| 4 | 3 (0.1) |
Excludes participants with at least 2 weeks of insulin use prior to blood draw for DAA measurement.
3.1. Diabetes incidence rates
The age- and sex-standardized diabetes incidence rates per 100,000 person years (PY) and 95% CI were 30.1 (23.5–36.8) for T1D and 349.2 (339.1–359.3) for T2D/other diabetes types (Table 3). The age-standardized T1D incidence rates were 32.5 (22.2–42.8) for males and 27.2 (21.0–34.5) for females (p < 0.05). The race/ethnic-specific age- and sex-standardized incidence rates per 100,000 PY for T1D were 41.4 (25.3–57.5) for Hispanics, 37.0 (11.6–62.4) for non-Hispanic Blacks (NHB), 21.4 (14.3–28.6) for NHWs and 19.4 (8.5–30.2) for Asians (p < 0.001 for all race/ethnic comparison except NHW vs NHB (p = 0.536) and Hispanic vs. NHB (p = 0.475)).
The age-standardized T2D/other diabetes types incidence rate per 100,000 PY were 401.0 (385.4–416.6) for males and 297.9 (285.7–310.2) for females (p < 0.001). The race/ethnic-specific age- and sex-standardized rates for T2D/other diabetes types were 528.6 (506.3–550.9) for Hispanic, 449.4 (405.1–493.8) for NHB, 380.5 (356.6–404.4) for Asian, and 174.9 (162.9–186.8) for NHW individuals (p < 0.001 for all race/ethnic group comparisons).
3.2. Prediction models for T1D
Of the >50 variables tested for inclusion in the prediction models (Supplemental Table 2), eight variables contributed to the prediction of T1D among all individuals with incident diabetes (Table 5). In the training dataset, younger age, female sex, and non-Hispanic white race/ethnicity (demographics); higher HbA1c levels, lower BMI, and lower total serum cholesterol levels around the time of diagnosis, insulin treatment within 6 months of diagnosis and percent of T1D diagnosis codes were predictors of T1D. The AUC (95% CI) was 0.706 (0.700–0.711) for the training dataset (Fig. 1). When insulin treatment and T1D diagnosis codes were excluded, the same 6 demographic and clinical variables were identified as predictors of T1D, and the AUC was 0.678 (0.674–0.682) for the training dataset, a reduction of only 0.028.
Table 5 -.
Demographic and Clinical Variables associated with Autoimmune Type 1 Diabetes in the Training and Validation Cohorts: the DiYA Study.
| Variables | Individuals in the Training Cohort (66.7%) |
Individuals in the Validation Cohort (33.3%) |
||||||
|---|---|---|---|---|---|---|---|---|
| DAA negative | DAA positive | Adjusted OR (95% CI) Model 1 |
Adjusted OR (95% CI) Model 2 |
DAA negative | DAA positive | Adjusted OR (95% CI) Model 1* |
Adjusted OR (95% CI) Model 2† |
|
| 92.3% | 7.7% | 91.6% | 8.4% | |||||
|
| ||||||||
|
DEMOGRAPHICS
Age at Dx (years) | ||||||||
| 20–24 | 82.2% | 17.8% | Reference | Reference | 82.3% | 17.7% | Reference | Reference |
| 25–29 | 90.7% | 9.3% | 0.58 (0.28,1.18) | 0.68 (0.31,1.47) | 88.2% | 11.8% | 0.75 (0.30,1.86) | 0.76 (0.27,2.13) |
| 30–39 | 92.2% | 7.8% | 0.51 (0.28,0.92) | 0.69 (0.35,1.35) | 91.6% | 8.4% | 0.60 (0.27,1.33) | 0.81 (0.33,2.00) |
| 40–45 | 93.5% | 6.5% | 0.39 (0.20,0.78) | 0.57 (0.26,1.25) | 93.2% | 6.8% | 0.46 (0.19,1.14) | 0.67 (0.24,1.86) |
| Sex | ||||||||
| Male | 92.7% | 7.3% | 0.70 (0.47,1.05) | 0.70 (0.47,1.06) | 92.1% | 7.9% | 0.71 (0.44,1.16) | 0.71 (0.43,1.18) |
| Female | 91.8% | 8.2% | Reference | Reference | 91.0% | 9.0% | Reference | Reference |
| Race/ethnicity | ||||||||
| Hispanic | 92.7% | 7.3% | 0.70 (0.41,1.18) | 0.79 (0.46,1.37) | 92.3% | 7.7% | 0.67 (0.39,1.17) | 0.82 (0.46,1.48) |
| Non-Hispanic White | 89.8% | 10.2% | Reference | Reference | 87.7% | 12.3% | Reference | Reference |
| Asian | 95.1% | 4.9% | 0.35 (0.16,0.75) | 0.43 (0.2,0.95) | 94.8% | 5.2% | 0.33 (0.14,0.80) | 0.43 (0.17,1.06) |
| Non-Hispanic Black | 92.2% | 7.8% | 0.66 (0.30,1.45) | 0.7 (0.31,1.59) | 93.0% | 7.0% | 0.52 (0.20,1.38) | 0.66 (0.24,1.78) |
| Other/missing race | 87.8% | 12.2% | 1.19 (0.52,2.77) | 1.38 (0.59,3.21) | 86.4% | 13.6% | 1.17 (0.39,3.51) | 1.51 (0.49,4.65) |
|
CLINICAL
BMI Category at Dx (kg/m2) | ||||||||
| <20 | 50.1% | 49.9% | 2.66 (0.92,7.64) | 2.15 (0.63,7.3) | 41.6% | 58.4% | 2.38 (0.42,13.47) | 1.19 (0.16,8.67) |
| 20–24 | 80.4% | 19.6% | Reference | Reference | 71.1% | 28.9% | Reference | Reference |
| 25–29 | 89.7% | 10.3% | 0.51 (0.31,0.84) | 0.67 (0.39,1.16) | 88.3% | 11.7% | 0.35 (0.18,0.70) | 0.48 (0.22,1.04) |
| 30–39 | 93.5% | 6.5% | 0.27 (0.16,0.45) | 0.38 (0.21,0.70) | 93.8% | 6.2% | 0.16 (0.08,0.33) | 0.25 (0.11,0.58) |
| ≥40 | 94.4% | 5.6% | 0.19 (0.11,0.35) | 0.29 (0.15,0.56) | 94.5% | 5.5% | 0.12 (0.05,0.29) | 0.20 (0.08,0.52) |
| HbA1c % mean (SD) | 8.4 (2.3) | 9.2 (2.7) | 1.11 (1.02,1.22) | 1.08 (0.98,1.18) | 8.5 (2.3) | 9.5 (2.7) | 1.15 (1.04,1.27) | 1.11 (0.99,1.23) |
| HbA1c (mmol/mol), mean, (SD) | 68 (2) | 77 (6) | 69 (2) | 80 (6) | ||||
| Total Cholesterol | 194.3 (54.1) | 185.9 (48.6) | 0.995 (0.991,1.000) | 0.995 (0.991,1.000) | 196.3 (55.7) | 189.9 (50.4) | 0.995(0.990,1.000) | 0.995(0.990,1.000) |
| Any Insulin use (≤6 mos.) | 77.5% | 22.5% | 1.86 (1.06,3.27) | 73.1% | 26.9% | 1.90 (0.98,3.68) | ||
| % type 1 diabetes ICD10 codes ‡ | 0.6 (6.8) | 8.7 (25.9) | 1.02 (1.01,1.03) | 0.9 (8.51) | 14.6 (32.45) | 1.02 (1.01,1.03) | ||
| AUC (95% CI)§ | 0.678 (0.674–0.682) | 0.706 (0.700–0.711) | 0.686 (0.682–0.690) | 0.719 (0.714–0.724) | ||||
Note: All of the variables shown in these models improved the AUC, even if they do not have significant CIs.
Model 1 includes: demographics (younger age, female sex, NHW race/ethnicity) and clinical characteristics at time of diagnosis (higher hemoglobin HbA1c, lower BMI, and lower total cholesterol).
Model 2 includes: variables in model 1 plus insulin use within 6 months of diabetes diagnosis and percent of ICD-10 codes that were for type 1 diabetes out of all diabetes diagnosis codes in the first 6 months after diagnosis.
Out of all diabetes codes in the 6 months after diagnosis.
AUC: Area under the curve. AUC > 0.5 indicates that the model predicts diabetes autoantibody status greater than chance alone.
4. Discussion
Using one or more positive DAA to define T1D among individuals diagnosed with diabetes, we estimated that the age- and sex-standardized incidence of T1D was 30.1 per 100,000 PY in adults ages 20–45 years, with T1D accounting for up to 8% of all incident diabetes cases. Incidence rates were higher among those aged 30–45 than among 20–30 years old, among men than women, and among Hispanics and NHBs than among Asian and NHW. Incidence of T2D and other types in DiYA had similar findings for age (increases with age) and race-ethnicity categories (higher rates in race/ethnic minorities) but rates were the same for men and women.
Globally, few studies [18–20] reported on the incidence of T1D in adults 20–45 years of age, and, among those, most did not define T1D based on autoimmunity exclusively. Other studies have used a clinical phenotype consistent with T1D (for example, insulin use from diagnosis or within 6 months of diagnosis, age <30 years, lower fasting C-peptide, low BMI, or DKA) with some including diagnosis codes and GADA (collected in a small number of individuals during clinical care) and a genetic risk score for T1D to construct the phenotype [7,21–25]. The US National Health Interview Survey uses self-reports of insulin use and diabetes type [11] and the National Health and Nutrition Examination Survey uses self-reported diabetes diagnosis, current insulin use and age <30 years and <40 years at diagnosis [26].
Our results on higher T1D incidence in the older group and in men, are consistent with previous studies. Bruno et al assessed DAA (islet cell autoantibodies and GADA) in 617 of 1135 (54.4%) incident cases in 30–49 year olds in Italy at a mean of one year after diagnosis and reported increasing T1D incidence with age (6.1, 6.7, and 7.9/100,000 for ages 30–34, 35–39, and 40–44 years, respectively) [24]. In all age groups, the incidence was higher in males than in females. In a cohort from Sweden, an area known to have very high T1D incidence rates, Thunander et al used DAA results and/or C-peptide values to classify individuals as having T1D. They reported T1D incidence rates of 19.7, 11.7, and 20.0/100,000 adults ages 20–29, 30–39, and 40–49 years, respectively, although incidence rates rose in subsequent age groups (for example, 36.1/100,000 for ages 50–59 years) [9]. Incidence was higher for males than for females among most age groups. However, in a study conducted using a large national US commercial health insurance provider database that used two or more diagnosis codes for T1D to categorize individuals as having T1D [27], incidence rates were 18.0/100,000 among the 20–24 year olds and 16.0/100,000 among the 40–44 year olds.
Only one previous study has estimated the incidence of T1D in young adults across racial/ethnic groups. This study was based on U.S. military personnel aged 18–44 years and defined incidence of T1D, based on hospital discharge diagnosis or multiple outpatient diagnoses of insulin-requiring diabetes. The age-adjusted incidence of T1D from 1990 to 2005 was 17.5/100,000 PY for men and 13.6/100,000 PY for women [28], almost half of the incidence estimates reported in DiYA. However, similarly to our results, among both men and women, incidence rates of T1D were almost twice has high for NHB as they were for NHW and increased with age. A possible explanation of these findings by race/ethnicity are that the lifetime risk of T1D for Hispanics and NHB individuals may be similar to NHW individuals, but age of onset is older in individuals from these race/ethnic groups. Similarly, in youth <20 years at diagnosis from the SEARCH study, steeper increases in age- and sex adjusted incidence of T1D were observed among NHBs (2.7% per year), Hispanics (4.0% per year) and Asians and Pacific Islanders (4.4% per year) than among NHWs (0.7% per year) from 2002 to 2015 [29].
Although the presence of a single DAA may have lower specificity compared with the presence of two or more DAA, it may assist in the diagnosis of T1D and understanding of the clinical course of individuals with symptomatic hyperglycemia at the time of diagnosis in the real word clinical settings. We included all 20–45 year olds with any positive DAA in our case definition of T1D, of which approximately one-third were prescribed insulin in the 12 months year after diagnosis. In a recent analysis of individuals in the UKPDS longitudinal cohort of adults with T2D, 84% with positive GADA and 94% of with ICA required insulin treatment by 6 years of diabetes duration [30]. Similar results were reported for a Swedish cohort of young adults initially considered to have T2D or unclassifiable diabetes [9]. These findings suggest that the presence of only one DAA may identify individuals more likely to require insulin and to have T1D. There is debate as to whether slowly progressive autoimmune diabetes with onset during adulthood should be categorized as latent autoimmune diabetes in adults (LADA) or whether the priority is to focus awareness on the potential for slower autoimmune beta cell destruction in adults with inclusion of all forms of diabetes mediated by autoimmune b-cell destruction under the rubric of T1D [30]. Therefore, there is the need to further investigate whether the presence of only one DAA is associated with a clinical course that is typical of T1D among racially and ethnically diverse populations of young adults.
Our predictive model identified demographic and clinical characteristics that predicted autoimmunity among 7862 individuals with incident diabetes in 2017 with AUCs of 0.678 and 0.706 for models without and with diabetes treatment and diagnosis codes. Although the discrimination of this predictive model is marginally acceptable [31], it is not high enough to be used for the diagnosis of T1D, but it may assist clinicians in identifying individuals who may benefit from DAA testing as part of clinical care. The use of DAA results to identify T1D cases among young adults with incident diabetes is rare since DAAs are not commonly tested as part of routine clinical care, however DAA results may help clinicians in the management of their patients with diabetes. Many of the characteristics that we identified in the prediction models such as younger age, NHW race/ethnicity, and lower BMI are associated with the characteristics we consider typical for T1D. However, we found that female sex, which usually has a lower risk than males for T1D in childhood, was a predictor of T1D among young adults with diabetes, suggesting the possibility of sex differences in age of onset. The model only improved marginally when we included insulin use and clinical diabetes type based on the majority of ICD-10 codes. Therefore, our model may be useful in clinical settings to determine which patients may benefit from DAA testing and for surveillance to identify individuals who may be more likely to have T1D from among individuals with newly-diagnosed diabetes. Previous studies that developed prediction models for T1D have not systematically assessed DAA.
Our study has several limitations and strengths. While multiple efforts were made to contact individuals to invite them to participate in the study and the study’s blood draw was convenient (non-fasting, a KP laboratory of their choice), only 26% consented and had their blood drawn. As with other studies, men and individuals from race/ethnic minority groups were less likely to participate. However, using a wealth of clinical and demographic information from the EHRs, we imputed the DAA status (positive or negative) for the untested cases to estimate incidence rates by diabetes type and to develop T1D prediction models. Individuals in the study population were insured, however this is unlikely to have impacted DAA status and likely led to more accurate and complete identification of cases and estimated date of diagnosis as insured individuals have access to diabetes screening and treatment. Additionally, incidence rates were standardized to the to the age and sex of the 2010 US Census population. While we used NIDDK harmonized standard RBA for measurement of DAAs and the four assays had very high specificity ranging from 99 to 100%, the sensitivity of the four assays range from 62% for IA-2A to 82% for GADA [16] and therefore we cannot exclude the possibility of false positives.
The strengths of the study are the large population size of over 2.3 million individuals 20–45 at risk for diabetes, the race/ethnic diversity of the population, and our ability to have accurate estimates of the population at risk and of incident cases using the rich, robust longitudinal clinical and demographic data from KP’s EHR. Additionally, samples were tested for all four DAA in a central laboratory at the Barbara Davis Center for Diabetes, an internationally recognized NIDDK North America Core Laboratory. These DAA results could be linked back to clinical and demographic data in the EHR to facilitate these analyses.
Approximately 8% of adults in this age group newly diagnosed with diabetes may have T1D based on the presence of at least one DAA. Higher T1D incidence rate were observed for men than for women, among the 30–45 year age group than the 20–29 year olds, and among Hispanic, Black and Asian individuals compared with NHW individuals. Demographic and clinical factors available in the EHR such as race/ethnicity, age, sex, BMI, and total cholesterol may help predict incident T1D from among individuals with incident diabetes. In turn, this may assist clinicians in identifying individuals who may benefit from DAA testing as part of clinical care. This method could also be used to identify individuals who are more likely to have T1D compared to T2D and thus to estimate T1D incidence when DAA results are not available. Future studies assessing DAA in incident diabetes cases among racial and ethnic diverse young adults and with long-term follow-up may elucidate whether the clinical evolution of diabetes in young adults may vary according to presence, number and type of DAA and will contribute to our knowledge on autoimmune T1D.
Supplementary Material
Table 4 -.
Crude and Age- and Sex-Standardized Diabetes Incidence Rates (IR) and 95% CI for Total Diabetes, Type 1 Diabetes and Type 2 Diabetes per 100,000 person-years at Risk among Members of Kaiser Permanente California aged 20–45 years in 2017: The DiYA Study.
| All (total) Diabetes |
DAA + Type 1 Diabetes |
DAA − Type 2 diabetes/other |
|||||
|---|---|---|---|---|---|---|---|
| PERSON- YEARS AT RISK | IR | 95% CI | IR | 95% CI | IR | 95% CI | |
|
| |||||||
| CRUDE RATES | |||||||
| Overall | 2,121,318 | 370.6 | (362.4,378.8) | 29.5 | (23.1, 35.9) | 341.1 | (331.5, 350.7) |
| By Sex | |||||||
| Male | 1,016,285 | 424.4 | (411.7, 437.1) | 32.0 | (21.7, 42.2) | 392.4 | (377.5, 407.4) |
| Female | 1,105,034 | 321.2 | (310.6, 331.8) | 27.3 | (21.0,33.5) | 293.9 | (282.3, 305.5) |
| By Race/ethnicity | |||||||
| Non-Hispanic White | 710,304 | 195.4 | (185.1, 205.7) | 21.2 | (14.5, 28.0) | 174.2 | (162.8, 185.6) |
| Hispanic | 731,157 | 533.5 | (516.8, 550.2) | 39.6 | (24.9, 54.4) | 493.9 | (473.7, 514.1) |
| Asian | 332,898 | 419.3 | (397.3, 441.3) | 20.8 | (9.3,32.4) | 398.5 | (374.4, 422.6) |
| Non-Hispanic Black | 147,357 | 435.7 | (402.0,469.4) | 32.8 | (10.6, 55.0) | 402.9 | (364.8, 441.0) |
| By Age at Diagnosis | |||||||
| 20.0–29.9 yrs. | 801,056 | 117.0 | (109.5, 124.5) | 15.2 | (10.2, 20.1) | 101.8 | (93.6, 110.0) |
| 30.0–44.9 yrs. | 1,320,263 | 524.5 | (512.2, 536.9) | 38.2 | (28.6, 47.8) | 486.3 | (471.8, 500.7) |
| STANDARDIZED RATES | |||||||
| Overall* | 2,121,318 | 379.3 | (370.6, 388.0) | 30.1 | (23.5, 36.8) | 349.2 | (339.1, 359.3) |
| By Sex | |||||||
| Male | 1,016,285 | 433.5 | (420.1,446.9) | 32.5 | (22.2,42.8) | 401.0 | (385.4, 416.6) |
| Female | 1,105,034 | 325.7 | (314.6, 336.7) | 27.7 | (21.0, 34.5) | 297.9 | (285.7, 310.2) |
| By Race/ethnicitya | |||||||
| Non-Hispanic White | 710,304 | 196.3 | (185.6, 207.0) | 21.4 | (14.3, 28.6) | 174.9 | (162.9, 186.8) |
| Hispanic | 731,157 | 570.0 | (551.4,588.6) | 41.4 | (25.3, 57.5) | 528.6 | (506.3, 550.9) |
| Asian | 332,898 | 399.9 | (378.0, 421.8) | 19.4 | (8.5,30.2) | 380.5 | (356.6,404.4) |
| Non-Hispanic Black | 147,357 | 486.4 | (446.9, 525.9) | 37.0 | (11.6,62.4) | 449.4 | (405.1, 493.8) |
Race/ethnicity specific rates are shown for the four race/ethnic groups with a sufficient number of individuals to show meaningful rates. All individuals are included in the overall rates and in the sex, and age specific rates.
Age- and sex-standardized rates using the US 2010 populations ages 20 to 45 years old.
Acknowledgements
We appreciate Kaiser Permanente members for their participation in this study and their contribution of their electronic health record information to this study and the Kaiser Permanente clinicians that collaborated with us on this study.
Funding
This study was funded by the Centers for Disease Control and Prevention (1U18DP006289, PIs JM Lawrence and A Ferrara). Testing for insulin autoantibodies was funded by JDRF International (2-SRA-2018–533-S-B, PI: L Yu).
Role of the funder
The funding for this study was provided by the Centers for Disease Control and Prevention. Scientists from the CDC (G. I., S.S., A.A., D.R.) participated in the interpretation of data and writing of this manuscript.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institute of Diabetes and Digestive and Kidney Diseases.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2020.108624.
REFERENCES
- [1].Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017. [Google Scholar]
- [2].Benoit SR, Hora I, Albright AL, Gregg EW. New directions in incidence and prevalence of diagnosed diabetes in the USA. BMJ Open Diabetes Res Care 2019;7(1):e000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 2017;66(2):241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Buzzetti R, Zampetti S, Maddaloni E. Adult-onset autoimmune diabetes: current knowledge and implications for management. Nat Rev Endocrinol 2017;13(11):674–86. [DOI] [PubMed] [Google Scholar]
- [5].American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019;42(Suppl 1):S13–S28. [DOI] [PubMed] [Google Scholar]
- [6].American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43(Suppl 1):S14–S31. [DOI] [PubMed] [Google Scholar]
- [7].Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol 2018;6(2):122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chiang JL, Kirkman MS, Laffel LM, Peters AL. Type 1 diabetes sourcebook A. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 2014;37(7):2034–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Thunander M, Petersson C, Jonzon K, et al. Incidence of type 1 and type 2 diabetes in adults and children in Kronoberg, Sweden. Diabetes Res Clin Pract 2008;82(2):247–55. [DOI] [PubMed] [Google Scholar]
- [10].Vandewalle CL, Falorni A, Lernmark A, Goubert P, Dorchy H, Coucke W, Semakula C, et al. Associations of GAD65- and IA-2- autoantibodies with genetic risk markers in new-onset IDDM patients and their siblings. The Belgian Diabetes Registry. Diabetes Care 1997;20(10):1547–52. [DOI] [PubMed] [Google Scholar]
- [11].Bullard KM, Cowie CC, Lessem SE, et al. Prevalence of diagnosed diabetes in adults by diabetes type - United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67(12):359–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Selby JV, Ray GT, Zhang D, Colby CJ. Excess costs of medical care for patients with diabetes in a managed care population. Diabetes Care 1997;20(9):1396–402. [DOI] [PubMed] [Google Scholar]
- [13].Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab 2010;95(7):3360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA 2007;104(43):17040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yu L, Robles DT, Abiru N, et al. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci USA 2000;97(4):1701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].University of Florida. Islet Autoantibody Standardization Program (IASP) Certification of 2018 Workshop Participation November/29/2018. [Google Scholar]
- [17].Chi GC, Li X, Tartof SY, Slezak JM, Koebnick C, Lawrence JM. Validity of ICD-10-CM codes for determination of diabetes type for persons with youth-onset type 1 and type 2 diabetes. BMJ Open Diabetes Res Care 2019;7(1):e000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Diaz-Valencia PA, Bougneres P, Valleron AJ. Global epidemiology of type 1 diabetes in young adults and adults: a systematic review. BMC Public Health 2015;15:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Saydah S, Imperatore G. Emerging approaches in surveillance of type 1 diabetes. Curr Diab Rep 2018;18(9):61. [DOI] [PubMed] [Google Scholar]
- [20].Imperatore G, Mayer-Davis EJ, Orchard T, Zhong VW. Prevalence and Incidence of Type 1 Diabetes among Children and Adults in the United States and Comparison with Non-U. S. Countries. In: Diabetes in America, 3rd ed. Vol NIH Pub No. 17–1468. National Institutes of Health; 2017:2.1–2.17. [Google Scholar]
- [21].Bruno G, Novelli G, Panero F, et al. The incidence of type 1 diabetes is increasing in both children and young adults in Northern Italy: 1984–2004 temporal trends. Diabetologia 2009;52(12):2531–5. [DOI] [PubMed] [Google Scholar]
- [22].Klompas M, Eggleston E, McVetta J, Lazarus R, Li L, Platt R. Automated detection and classification of type 1 versus type 2 diabetes using electronic health record data. Diabetes Care 2013;36(4):914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bruno G, Gruden G, Songini M . Incidence of type 1 diabetes in age groups above 15 years: facts, hypothesis and prospects for future epidemiologic research. Acta Diabetol 2016;53(3):339–47. [DOI] [PubMed] [Google Scholar]
- [24].Bruno G, Maule M, Biggeri A, et al. More than 20 years of registration of type 1 diabetes in Sardinian children: temporal variations of incidence with age, period of diagnosis, and year of birth. Diabetes 2013;62(10):3542–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Berhan Y, Waernbaum I, Lind T, Mollsten A, Dahlquist G. Swedish Childhood Diabetes Study G. Thirty years of prospective nationwide incidence of childhood type 1 diabetes: the accelerating increase by time tends to level off in Sweden. Diabetes 2011;60(2):577–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Menke A, Orchard TJ, Imperatore G, Bullard KM, Mayer-Davis E, Cowie CC. The prevalence of type 1 diabetes in the United States. Epidemiology. 2013;24(5):773–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rogers MAM, Kim C, Banerjee T, Lee JM. Fluctuations in the incidence of type 1 diabetes in the United States from 2001 to 2015: a longitudinal study. BMC Medicine 2017;15(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gorham ED, Barrett-Connor E, Highfill-McRoy RM, et al. Incidence of insulin-requiring diabetes in the US military. Diabetologia 2009;52(10):2087–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Divers J, Mayer-Davis EJ, Lawrence JM, et al. Trends in incidence of type 1 and type 2 diabetes among youths - selected counties and Indian reservations, United States, 2002–2015. MMWR Morb Mortal Wkly Rep 2020;69(6):161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Maddaloni E, Coleman RL, Agbaje O, Buzzetti R, Holman RR. Time-varying risk of microvascular complications in latent autoimmune diabetes of adulthood compared with type 2 diabetes in adults: a post-hoc analysis of the UK Prospective Diabetes Study 30-year follow-up data (UKPDS 86). Lancet Diabetes Endocrinol 2020;8(3):206–15. [DOI] [PubMed] [Google Scholar]
- [31].Hosmer DW, Lemeshow S, Sturdivant RX. Chapter 5: Assessing the Fit of the Model in Applied logistic regression, Third Edition. John Wiley & Sons, Inc., Hoboken, New Jersey, 2013. ISBN:9780470582473. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


