Abstract
Aims
the literature regarding the association between multimorbidity and dementia is still unclear. Therefore, we aimed to explore the potential association between multimorbidity at the baseline and the risk of future dementia in the SHARE (Survey of Health, Ageing and Retirement in Europe) study, a large European research survey, with a follow-up of 15 years.
Methods
in this longitudinal study, multimorbidity was defined as the presence of two or more chronic medical conditions, among 14 self-reported at the baseline evaluation. Incident dementia was ascertained using self-reported information. Cox regression analysis, adjusted for potential confounders, was run and hazard ratios (HRs), with their 95% confidence intervals (CIs), that were estimated in the whole sample and by 5 year groups.
Results
among 30,419 participants initially considered in wave 1, the 23,196 included participants had a mean age of 64.3 years. The prevalence of multimorbidity at baseline was 36.1%. Multimorbidity at baseline significantly increased the risk of dementia in the overall sample (HR = 1.14; 95% CI: 1.03–1.27) and in participants younger than 55 years (HR = 2.06; 95% CI: 1.12–3.79), in those between 60 and 65 years (HR = 1.66; 95% CI: 1.16–2.37) and in those between 65 and 70 years (HR = 1.54; 95% CI: 1.19–2.00). In the overall sample, high cholesterol levels, stroke, diabetes and osteoporosis increased the risk of dementia, particularly if present among participants between 60 and 70 years of age.
Conclusions
multimorbidity significantly increases the risk of dementia, particularly in younger people, indicating the need for early detection of multimorbidity for preventing cognitive worsening.
Keywords: multimorbidity, dementia, cohort: Survey of Health, Ageing and Retirement in Europe (SHARE) study, Parkinson, cognitive impairment, risk factor, stroke, diabetes, older people
Key Points
Dementia is one of the most common conditions in older people. The identification of potential risk factors is of importance since most dementias are irreversible.
In the Survey of Health, Ageing and Retirement in Europe study, multimorbidity at baseline significantly increased the risk of dementia in the overall sample and in younger participants.
Considering the single conditions, high cholesterol levels, stroke, diabetes and osteoporosis increased the risk of dementia.
Introduction
Demographic projections suggest that the global population will age rapidly in the next decades, with persons aged 65 years or over rising from 703 million in 2019 to double to 1.5 billion by 2050 [1]. As an effect, the number of people with dementia worldwide, which was estimated at 47.5 million in 2015, is likely to reach 75.6 million in the next 20 years [2]. Future projections indicate that this number will further increase to 135 million in 2050 making dementia a new epidemic [2]. Approximately 7.7 million new cases of dementia are anticipated each year; it is reported that one person in the world will be affected by dementia every 3 seconds [3].
Another consequence of population ageing is the increase in multimorbidity [4], usually defined as the presence of two or more chronic medical diseases [5]. Increasing literature has reported that more than 50% of older adults have multiple chronic conditions [6], but it is important to note that multimorbidity can also affect younger people [6]. Moreover, it has been reported that multimorbidity accounts for 78% of all primary care consultations in high-income countries [7] and that this condition is often associated with other complications including mood disorders [8, 9], sarcopenia [10] and urinary incontinence [11], typically considered geriatric syndromes. Of importance, chronic medical conditions occurring at younger ages may have relevant implications for their management and can increase the risk of premature mortality and healthcare costs [12].
Multimorbidity is extremely common in people already affected by dementia; however, it seems that multimorbidity in people affected by dementia receives less attention than people without dementia in terms of management [13]. Moreover, multimorbidity per se can increase the risk of dementia, as shown in some recent studies. One longitudinal study, conducted among older adults living in Sweden, showed that over 8 years of follow-up, people with multimorbidity had a significantly higher risk of dementia compared to people without this condition [14]; another cohort study confirmed these findings, while it also discovered that age and duration of multimorbidity are important factors in the development of future dementia [15]. It was reported that multimorbidity can increase the risk of dementia through several pathways including low-grade inflammation, faster cognitive decline and an increased frequency of cerebrovascular problems [16].
Despite these studies, advancing our knowledge on the topic of multimorbidity as a potential risk factor for dementia is of importance, and the literature available is still limited. Given this background, we aimed to explore the potential association between multimorbidity at baseline and the risk of future dementia in the SHARE (Survey of Health, Ageing and Retirement in Europe) study, a large European research study with a follow-up of 15 years.
Materials and methods
Standard protocol approvals, registrations and patient consents
The SHARE study is subject to continuous ethics review. During waves 1–4, SHARE was reviewed and approved by the Ethics Committee of the University of Mannheim. Wave 4 and the continuation of the project were reviewed and approved by the Ethics Council of the Max Planck Society. In addition, the country implementations of SHARE were reviewed and approved by the respective ethics committees or institutional review boards whenever this was required. The numerous reviews covered all aspects of the SHARE study, including sub-projects, and confirmed the project to be compliant with the relevant legal norms and that the project and its procedures agree with international ethical standards (http://www.share-project.org/fileadmin/pdf_documentation/SHARE_ethics_approvals.pdf). Informed consent was obtained from all subjects involved in the study in written form.
Study design
SHARE is an ongoing longitudinal study involving the European population and Israel aged 50 years and over (http://www.share-project.org/organisation/share-eric.html) [17]. The SHARE study is a multidisciplinary and cross-national panel database of microdata on health, socio-economic status, and social and family networks.
In this longitudinal cohort study, we used the data of wave 1 (baseline, between 2004 and 2006), wave 2 (2006/2007), wave 3 (2008/2009), wave 4 (2011/2012), wave 5 (2013), wave 6 (2015), wave 7 (2017/2018) and wave 8 (2019/2020) [18]. The final dataset included Austria, Germany, Sweden, The Netherlands, Spain, Italy, France, Denmark, Greece, Switzerland, Belgium, Israel, Czech Republic, Poland, Luxembourg, Hungary, Portugal, Slovenia, Estonia, Croatia, Lithuania, Bulgaria, Cyprus, Finland, Latvia, Malta, Romania and Slovakia.
Using a multistage clustered sampling, nationally representative, the SHARE study explores this cross-country setting as a ‘natural laboratory’ across scientific disciplines and over time in order to turn the challenges of population ageing into opportunities and provide policymakers with reliable information for evidence-based policies. SHARE data collection is based on computer-assisted personal interviewing (CAPI). The interviewers conducted face-to-face interviews using a laptop on which the CAPI instrument is installed in the native language of the participants.
Using the SHARE data, a cohort study was designed to estimate the incidence of dementia among people 65 years old with multimorbidity (exposed) compared to those without multimorbidity (unexposed). All exposed and unexposed were followed up for 15 years using the date of the first interview as index date until dementia incidence, death or last follow-up available, whichever came first (Supplementary Figure 1). Among 30,419 participants initially included in wave 1, we excluded 83 since no sufficient information regarding multimorbidity was available, and 6,870 since there were no information regarding dementia during follow-up, leaving 23,196 participants at the baseline evaluation (Supplementary Figure 1).
Exposure: multimorbidity
According to the most common definition used [5], we defined the presence of two or more chronic medical conditions collected in the SHARE study at wave 1 (baseline evaluation), except for those with dementia who were excluded by study design. For each medical condition, the investigators asked the following question: ‘Has a doctor ever told you that you had/do you currently have any of the conditions on this card?’; a show-card with multiple non-mutually exclusive options was presented to the participants [17]. The help of caregiver or a family member was permitted. The 14 medical conditions analysed were the presence of heart attack, high blood pressure or hypertension, high blood cholesterol, stroke, diabetes or high blood sugar, chronic lung disease, asthma, arthritis, osteoporosis, cancer, stomach or duodenal or peptic (gastrointestinal) ulcer, Parkinson’s disease, cataracts and hip fracture or femoral fracture.
Outcomes: incidence of dementia
The outcome of interest of this research was incident dementia, defined as the presence of dementia during follow-up ascertained using the following question posed to the participants or to their caregivers or family members: ‘Doctor said you had: Alzheimer’s disease, dementia, senility’? Response options were yes, no, refusal and don’t know [19]. The presence of dementia was ascertained at wave 2 (2006/2007), wave 3 (2008/2009), wave 4 (2011/2012), wave 5 (2013), wave 6 (2015), wave 7 (2017/2018) and wave 8 (2019/2020).
Covariates
For assessing associations between multimorbidity and the incidence of dementia, we considered several possible confounding factors at the baseline evaluation including age (as continuous variable), sex, marital status (married and living together with spouse vs. others), educational level (no educational level vs. other degrees), vigorous or moderate level of physical activity more than once a week versus other levels of physical activity, days a week consumed alcohol last 6 months (almost every day), smoke at the present time versus previous/never, and country (categorised as mentioned before).
Statistical analysis
Means and standard deviations (SD) were used to describe continuous measures, while percentages were used for categorical variables. Characteristics of the study participants at baseline (wave 1) were compared according to the presence or absence of dementia during the follow-up period, using chi-squared for categorical variables and independent T-test for continuous variables.
The association between the presence of multimorbidity at wave 1 (as two or more chronic medical conditions) in people without dementia and incident dementia was analysed through several analyses. First, we reported the number of incident cases of dementia cases/total cases (cumulative incidence), the incidence rate (per 1,000 person-years) and the incidence rate ratio (95% CI) by presence or not of multimorbidity. Second, using a Cox regression analysis, we reported the association between multimorbidity and incident dementia, adjusted for the confounders mentioned in the ‘Covariates’ section. All analyses were reported in the sample as whole and stratified by birth cohort (5 year group) (<55, 55–60, 60–65, 65–70, 70–75, >75 years). The results were reported as hazard ratios (HRs) with their 95% confidence intervals (CIs).
All statistical tests were two-tailed, and a P-value <0.05 was statistically significant. All analyses were performed using SPSS version 26.0 software.
Results
The 23,196 included participants had a mean age of 64.3 (SD = 10.1) years (range: 27–103) and they were predominantly females (56.2%). Overall, the prevalence of multimorbidity at wave 1 was 36.1% (95% CI: 35.5–36.7%), ranging from 16.8% in those younger than 55 years to 56.4% in those older than 75 years (Figure 1).
Figure 1.
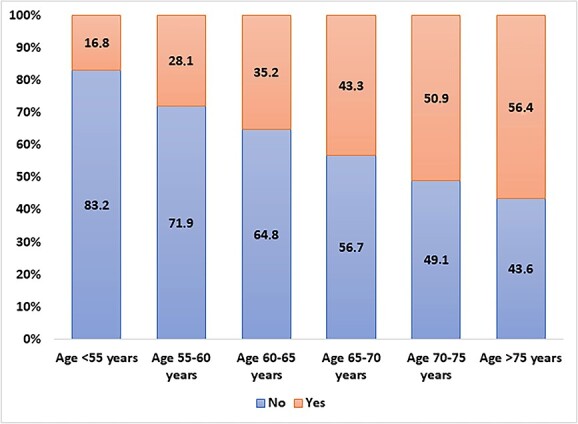
Prevalence of multiple chronic conditions at different ages. Data are reported as percentage based on birth cohort (5 year groups).
During the 15 years of follow-up, the global incidence of dementia was 7.29 (95% CI: 6.92–7.67) per 1,000 person-years [202,461 person-years]. When divided by the presence or absence of multimorbidity at baseline, the incidence rate was almost doubled in the first group compared to the second (10.24 vs. 5.37 cases of dementia per 1,000 person-years) (Supplementary Table 1). The incidence rate ratio was higher in the youngest group (2.18) and lower in the oldest participants (0.95). When the sample was divided into those who did and did not develop dementia during follow-up, the 1,475 participants receiving a diagnosis of dementia were significantly older, more frequently females and with no educational title and less frequently married at wave 1 than the 21,721 counterparts without incident dementia (all comparisons: P < 0.0001) (Table 1). Among health behaviours, incident cases of dementia reported less vigorous and moderate levels of physical activity than their counterparts, but a lower prevalence of smokers (Table 1). Finally, participants who developed dementia during follow-up reported at baseline a higher prevalence of all the chronic medical conditions assessed at the baseline (P < 0.0001) except for asthma (P = 0.09) and cancer (P = 0.67), leading to a significantly higher prevalence of multimorbidity in people with dementia compared to those not developing this condition (51.3 vs. 35.1%, P < 0.0001) (Table 1).
Table 1.
Descriptive baseline characteristics by dementia status during follow-up
| Parameter | Dementia (n = 1,475) |
No dementia (n = 21,721) |
|---|---|---|
| Demographics | ||
| Mean age (SD) | 73.4 (8.9) | 63.7 (9.9) |
| Age <55 years | 3.5 | 23.5 |
| Age 55–60 years | 5.4 | 20.2 |
| Age 60–65 years | 8.7 | 16.8 |
| Age 65–70 years | 16.5 | 14.6 |
| Age 70–75 years | 22.1 | 11.0 |
| Age >75 years | 43.8 | 13.9 |
| Females (n, %) | 915 (62.0) | 12,110 (55.8) |
| Married and living together with spouse (n, %) | 922 (62.5) | 15,826 (72.9) |
| No educational title (n, %) | 438 (29.8) | 4,746 (21.8) |
| Health behaviours | ||
| Vigorous physical activities, more than once a week (n, %) | 359 (24.4) | 8,371 (38.5) |
| Activities requiring a moderate level of energy, more than once a week (n, %) | 873 (59.2) | 15,633 (72.0) |
| Days a week consumed alcohol last 6 months, almost every day (n, %) | 281 (19.1) | 4,693 (21.6) |
| Actual smoke at baseline (n, %) | 171 (11.6) | 4,241 (19.5) |
| Chronic conditions | ||
| Heart attack (n, %) | 253 (17.2) | 2,439 (11.2) |
| High blood pressure (n, %) | 595 (40.3) | 6,715 (30.9) |
| High cholesterol level (n, %) | 383 (26.0) | 4,567 (21.0) |
| Stroke (n, %) | 119 (8.1) | 659 (3.0) |
| Diabetes (n, %) | 219 (14.8) | 2,040 (9.4) |
| Chronic lung disease (n, %) | 96 (6.5) | 979 (4.5) |
| Asthma (n, %) | 76 (5.2) | 918 (4.2) |
| Arthritis (n, %) | 370 (25.1) | 3,993 (18.4) |
| Osteoporosis (n, %) | 197 (13.4) | 1,579 (7.3) |
| Cancer (n, %) | 74 (5.0) | 1,038 (4.8) |
| Gastrointestinal ulcer (n, %) | 118 (8.0) | 1,235 (5.7) |
| Parkinson’s disease (n, %) | 32 (2.2) | 95 (0.4) |
| Cataracts (n, %) | 225 (15.3) | 1,449 (6.7) |
| Hip fracture (n, %) | 57 (3.9) | 371 (1.7) |
| Multimorbidity (≥2 chronic medical conditions) (n, %) |
757 (51.3) | 7,622 (35.1) |
Data are reported as means with SD for continuous variables and numbers and corresponding percentages for categorical factors.
Figure 2 shows the association between multimorbidity at baseline and incident dementia, after adjusting for nine different potential confounders at baseline. In the overall sample, multimorbidity at baseline increased the risk of dementia by about 14% (HR = 1.14; 95% CI: 1.03–1.27; P < 0.0001). The presence of multimorbidity significantly increased the risk of dementia in participants younger than 55 years (HR = 2.06; 95% CI: 1.12–3.79; P < 0.0001), in those between 60 and 65 years (HR = 1.66; 95% CI: 1.16–2.37; P < 0.0001), and in those between 65 and 70 years (HR = 1.54; 95% CI: 1.19–2.00; P < 0.0001), but not in those between 55 and 60 years or in people aged more than 75 years (Figure 2).
Figure 2.
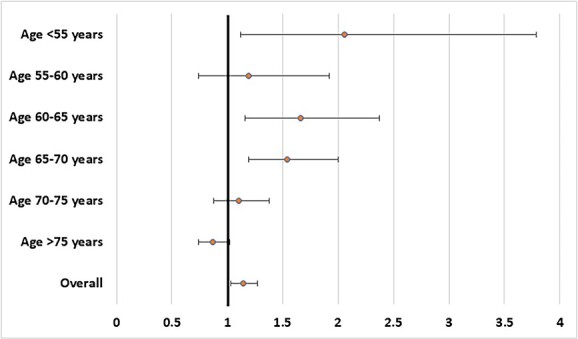
Association between multimorbidity and incident dementia in the overall sample and by age. Data are reported as hazard ratios and their corresponding 95% confidence intervals. Analyses were stratified by birth cohort (5 year groups) and adjusted for age, sex, marital status, educational level, vigorous or moderate level of physical activity, days a week consumed alcohol last 6 months, current smoking and country. Covariate measurement was concurrent with measure of chronic diseases.
When considering single medical conditions increasing the risk of dementia, only Parkinson’s disease was associated with incident dementia in all age groups and in the overall sample (HR = 55.33 in people <55 years to 1.67 in those >75 years) (Table 2). In the overall sample, high cholesterol levels, stroke, diabetes and osteoporosis increased the risk of dementia. Overall, the associations seemed to be stronger and more significant in participants between 60 and 70 years of age (Table 2).
Table 2.
Association between single chronic disease and risk of incident dementia
| Chronic condition | <55 years | 55–60 years | 60–65 years | 65–70 years | 70–75 years | >75 years | Overall |
|---|---|---|---|---|---|---|---|
| Heart attack | 0.76 (0.18–3.20) | 0.84 (0.33–2.11) | 1.60 (0.96–2.68) | 1.45 (1.03–2.03) | 1.06 (0.79–1.43) | 0.87 (0.71–1.06) | 1.03 (0.90–1.19) |
| High blood pressure | 1.19 (0.60–2.35) | 1.25 (0.77–2.03) | 1.16 (0.81–1.67) | 1.40 (1.09–1.81) | 0.93 (0.74–1.17) | 0.88 (0.75–1.04) | 1.05 (0.94–1.16) |
| High cholesterol level | 1.77 (0.89–3.50) | 1.02 (0.59–1.46) | 1.30 (0.89–1.91) | 1.34 (1.02–1.76) | 0.82 (0.63–1.07) | 1.08 (0.90–1.30) | 1.12 (1.00–1.26) |
| Stroke | 6.61 (1.96–22.34) | 2.02 (0.82–4.97) | 3.35 (1.72–6.51) | 2.23 (1.32–3.77) | 1.92 (1.25–2.93) | 1.62 (1.23–2.13) | 1.97 (1.63–2.39) |
| Diabetes | 2.45 (1.07–5.61) | 1.30 (0.66–2.58) | 1.63 (1.01–2.63) | 1.74 (1.27–2.41) | 1.22 (0.89–1.66) | 1.00 (0.79–1.27) | 1.28 (1.11–1.49) |
| Chronic lung disease | 4.84 (1.98–11.83) | 0.85 (0.26–2.82) | 1.19 (0.51–2.74) | 0.62 (0.32–1.22) | 1.35 (0.90–2.02) | 1.22 (0.89–1.67) | 1.22 (0.98–1.50) |
| Asthma | 1.58 (0.48–5.16) | 2.06 (0.82–5.20) | 2.19 (1.16–4.16) | 0.93 (0.51–1.72) | 1.14 (0.69–1.87) | 1.02 (0.70–1.49) | 1.17 (0.93–1.48) |
| Arthritis | 1.82 (0.88–3.75) | 0.62 (0.31–1.24) | 1.00 (0.65–1.54) | 0.85 (0.62–1.17) | 1.30 (1.01–1.67) | 1.77 (1.64–1.93) | 0.91 (0.80–1.03) |
| Osteoporosis | 2.65 (0.89–7.87) | 1.17 (0.52–2.65) | 1.76 (1.03–3.00) | 0.77 (0.49–1.23) | 1.36 (0.98–1.88) | 1.13 (0.90–1.42) | 1.24 (1.06–1.45) |
| Cancer | 0.63 (0.08–4.74) | 0.28 (0.04–2.02) | 1.52 (0.73–3.15) | 1.31 (0.73–2.38) | 0.89 (0.55–1.44) | 0.81 (0.57–1.15) | 0.91 (0.72–1.15) |
| Gastrointestinal ulcer | 2.24 (0.85–5.88) | 1.45 (0.66–3.21) | 1.52 (0.86–2.70) | 1.03 (0.63–1.70) | 1.00 (0.65–1.56) | 1.16 (0.88–1.11) | 1.20 (0.99–1.46) |
| Parkinson’s disease | Not available | 55.33 (11.1–275) | 8.31 (2.50–27.8) | 3.98 (1.39–11.4) | 4.16 (2.08–8.33) | 1.67 (1.07–2.88) | 2.64 (1.84–3.78) |
| Cataracts | 1.31 (0.18–9.76) | 2.54 (0.88–7.33) | 1.54 (0.74–3.18) | 1.25 (0.81–1.92) | 1.12 (0.80–1.56) | 1.00 (0.83–1.21) | 1.10 (0.95–1.28) |
| Hip fracture | Not available | 3.11 (0.74–13.05) | 2.29 (0.83–6.34) | 1.13 (0.42–3.05) | 1.18 (0.64–2.18) | 1.18 (0.84–1.67) | 1.19 (0.91–1.56) |
Data are reported as hazard ratios and their corresponding 95% confidence intervals. Analyses were stratified by birth cohort (5 year groups) and adjusted for age, sex, marital status, educational level, vigorous or moderate level of physical activity, days a week consumed alcohol last 6 months, current smoking and country. Covariate measurement was concurrent with measure of chronic diseases. In bold, statistically significant results as P-value <0.05.
Discussion
In this study, including 23,196 participants followed up for 15 years, we found that multimorbidity at baseline was associated with a higher risk of dementia, after adjusting for several potential important confounders. The association seems to be stronger in younger than in older people. Among the chronic medical conditions investigated, Parkinson’s disease was associated with a higher risk of incident dementia independently of age, while other conditions predicted the onset of dementia, particularly if present below 70 years of age.
One first important epidemiological finding is the high prevalence of multimorbidity in the SHARE study. Multimorbidity affected more than one out of three people; even if the prevalence of multimorbidity was higher in older people (more than half of participants over 75 years reported multimorbidity), it is important to recognise that in people between 50 and 55 years, a considerable proportion of people had two or more chronic medical conditions. We believe that these findings are important since they clearly indicate that multimorbidity is not only a geriatric condition, confirming findings of other studies [20, 21]. Moreover, we have reported that the association between multimorbidity and dementia was stronger in younger than in older age groups. Considering the reported trend of increased burden of several chronic conditions in the adult population in different countries, the strong determinant for this issue raises special concern condition [22]. We can hypothesise that for older people, the follow-up period is too short for reaching the outcome of interest or that participants with multimorbidity could have died before having the diagnosis of dementia. Moreover, we can also propose that a longer exposure to multimorbidity can increase the risk of dementia than a lesser exposure indicating a likely time-dependent association. At the same time, we cannot exclude that the SHARE study prevalently includes older people; therefore, younger participants may be underrepresented.
The evidence regarding the possible association between multimorbidity and dementia is mainly based on several large cross-sectional studies, which have mainly reported that multimorbidity prevalence was extremely high in people with dementia compared to their counterparts [13]. However, cross-sectional studies may suffer from reverse causality, where dementia leads to higher presence for multimorbidity. On the contrary, only limited research of longitudinal design is available on multimorbidity as potential risk factor for dementia. Specifically, one study reported that, among 2,478 older adults living in Sweden with a mean age of 75 years, over a mean follow-up of 8.4 years, multimorbidity (in particular neuropsychiatric, cardiovascular and sensory impairment/cancer) was associated with a higher incidence of dementia [14]. Another large North American study conducted among 2,176 older adults followed up for 4 years showed a significant association between multimorbidity at baseline and the risk of incident mild cognitive impairment/dementia [23]. Finally, another large epidemiological study conducted in the context of the Whitehall II study and involving more than 10,000 participants with a follow-up of 30 years indicated that multimorbidity, particularly when onset was in midlife, had a robust association with the risk of dementia [15].
In the overall sample, some known cardiovascular risk factors (such as high cholesterol levels and diabetes), stroke and osteoporosis increased the risk of dementia The epidemiological evidence provided by our findings is supported by other works. For example, cardiac and metabolic conditions present in midlife, rather than at older ages, may be more likely to increase the risk of dementia [24, 25]. Similarly, depression and other medical conditions may increase the risk of dementia, when considered singularly [26, 27]. Finally, as also reported by other investigations, Parkinson’s disease probably has the strongest association with future dementia [15, 28, 29]. These findings support the idea that this specific condition, among all investigated, raised the risk of dementia, indicating the need of frequently assessing cognitive profile in patients with Parkinson’s disease. Moreover, by promoting education, social networking and support, lifestyle and leisure activities, a cognitive reserve can be constituted to prevent the occurrence of neuropathological changes, as dementia [30].
However, it is likely that multimorbidity may have specific cumulative effects of clustering of chronic diseases, eventually accelerating cognitive decline, leading to dementia, and it has been suggested that the cumulative effect of diseases rather than specific combinations leads to the association with dementia [31]. These findings may indicate that common pathogenetic mechanisms can justify our findings. For example, inflammation is a risk factor for several medical conditions in older people, including dementia [32]. Another important factor could be the number and type of drugs taken in case of multimorbidity that seem to significantly affect cognition in older people [23]. Finally, it was reported that a faster accumulation of chronic conditions was associated with greater cognitive decline [33]. In this sense, multimorbidity may reflect an age-related multisystem failure that is associated with [34] or mediated by cognitive issues [35]. Of importance, one previous study found that several health problems are associated with an increased risk for dementia when combined and not taken singularly [31]. Unfortunately, we were not able to investigate the role of these factors in moderating our findings since data were not available in the SHARE study at the baseline evaluation.
Finally, we believe that our findings are novel and contribute to the debate regarding the importance of multimorbidity as a risk factor for dementia. The SHARE study is representative of the older people living in Europe. Therefore, to report that multimorbidity can increase the risk of dementia, particularly in younger people, is of critical importance from a public health perspective. From a clinical point of view, our study may indicate the necessity of early screening for cognitive issues in people affected by multimorbidity, also in their young age, in order to try to reverse the effect of multimorbidity on cognition.
The major strength of this study is the large cohort included, being representative of Europe and Israel, and the long follow-up period. However, the findings of our investigation must be interpreted within its inherent limitations. First, all the information regarding multimorbidity and dementia are self-reported and not based on medical records or based on a standardised protocol investigating all the possible information regarding diseases. It is known that self-reported information regarding chronic medical conditions can introduce important biases, such as underestimation of the real prevalence of a condition [36]. Moreover, the information on the disease duration of the conditions included in multimorbidity were not available. Similarly, the severity of medical conditions was not considered and, practically, we included participants having different levels of severity of the same condition under the same group. Second, we were not able to clarify the cause of dementia in relation to multimorbidity and this should be investigated in future research. Another limitation is that the time of onset of dementia was when it was first reported in the survey, but this does not represent when the person really received a dementia diagnosis, and its exact timing of onset is not clear especially in those who were not followed in all the surveys. Third, as expected in longitudinal studies involving older people, about a quarter of the participants initially included in our analyses had no information regarding incident dementia. To exclude participants due to missing data can, unfortunately, introduce an important selection bias in our findings. Finally, as also mentioned before, we were not able to adjust our analyses for some potential confounders, including serum markers of inflammation or metabolism or similar that could significantly modify our findings.
In conclusion, our research based on the data of the SHARE showed that multimorbidity significantly increases the risk of dementia over 15 years of follow-up. These findings were stronger in younger people, suggesting that people having multimorbidity at young ages should be routinely and frequently screened for their cognitive profile to attenuate the adverse outcomes of dementia in old age. Future studies assessing the role of a better management of multimorbidity in young and midlife periods could add its importance in preventing dementia.
Supplementary Material
Contributor Information
Nicola Veronese, Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties, University of Palermo, Palermo, Italy.
Ai Koyanagi, Research and Development Unit, Parc Sanitari Sant Joan de Déu, CIBERSAM, ISCIII, 08830 Barcelona, Spain; ICREA, Pg. Lluis Companys 23, 08010 Barcelona, Spain.
Ligia J Dominguez, Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties, University of Palermo, Palermo, Italy; Faculty of Medicine and Surgery, Kore University of Enna, 94100 Enna, Italy.
Stefania Maggi, National Research Council, Neuroscience Institute, Padua, Italy.
Pinar Soysal, Department of Geriatric Medicine, Faculty of Medicine, Bezmialem Vakif University, Istanbul, Turkey.
Francesco Bolzetta, Medical Department, Geriatric Unit, Azienda ULSS (Unità Locale Socio Sanitaria) 3 “Serenissima”, Dolo-Mirano District, Venice, Italy.
Laura Vernuccio, Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties, University of Palermo, Palermo, Italy.
Lee Smith, Centre for Health, Performance, and Wellbeing, Anglia Ruskin University, Cambridge, UK.
Domenica Matranga, Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties, University of Palermo, Palermo, Italy.
Mario Barbagallo, Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties, University of Palermo, Palermo, Italy.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
The SHARE data collection has been funded by the European Commission through FP5 (QLK6-CT-2001-00360), FP6 (SHARE-I3: RII-CT-2006-062193, COMPARE: CIT5-CT-2005-028857, SHARELIFE: CIT4-CT-2006-028812), FP7 (SHARE-PREP: GA N°211909, SHARE-LEAP: GA N°227822, SHARE M4: GA N°261982, DASISH: GA N°283646) and Horizon 2020 (SHARE-DEV3: GA N°676536, SHARE-COHESION: GA N°870628, SERISS: GA N°654221, SSHOC: GA N°823782) and by DG Employment, Social Affairs, and Inclusion. Additional funding from the German Ministry of Education and Research, the Max Planck Society for the Advancement of Science, the U.S. National Institute on Aging (U01_AG09740-13S2, P01_AG005842, P01_AG08291, P30_AG12815, R21_AG025169, Y1-AG-4553-01, IAG_BSR06-11, OGHA_04-064 and HHSN2712013000 71C) and from various national funding sources is gratefully acknowledged (see www.share-project.org). Publication costs were supported by the FFR2021 fund of the University of Palermo assigned to M.B. and N.V.
References
- 1. United Nations . World Population Ageing 2019 (ST/ESA/SER. A/444). New York, USA: Department of Economic and Social Affairs, 2020. [Google Scholar]
- 2. Prince M, Guerchet M, Prina M.. The Global Impact of Dementia 2013-2050. 2013. Website: https://kclpure.kcl.ac.uk/portal/files/11621711/GlobalImpactDementia2013.pdf (1 June 2022, date last accessed).
- 3. Hand MD. Every three seconds: a review of an innovative documentary on research and stigma surrounding dementia across the globe. J Gerontol Soc Work 2019; 62: 369–73. [DOI] [PubMed] [Google Scholar]
- 4. Barnett K, Mercer S, Norbury M, Watt G, Wyke S, Guthrie B. The epidemiology of multimorbidity in a large cross-sectional dataset: implications for health care, research and medical education. Lancet 2012; 380: 37–43. [DOI] [PubMed] [Google Scholar]
- 5. Xu X, Mishra GD, Jones M. Evidence on multimorbidity from definition to intervention: an overview of systematic reviews. Ageing Res Rev 2017; 37: 53–68. [DOI] [PubMed] [Google Scholar]
- 6. Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition—multimorbidity. JAMA 2012; 307: 2493–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adams L. Multimorbidity. Nat Rev Dis Primers 2022; 8: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith L, Shin JI, Jacob Let al. Physical multimorbidity predicts the onset and persistence of anxiety: a prospective analysis of the Irish longitudinal study on ageing. J Affect Disord 2022; 309: 71–6. [DOI] [PubMed] [Google Scholar]
- 9. Smith L, Shin JI, Butler Let al. Physical multimorbidity and depression: a mediation analysis of influential factors among 34,129 adults aged ≥50 years from low- and middle-income countries. Depress Anxiety 2022; 39: 376–86. [DOI] [PubMed] [Google Scholar]
- 10. Veronese N, Smith L, Cereda Eet al. Multimorbidity increases the risk for sarcopenia onset: longitudinal analyses from the English longitudinal study of ageing. Exp Gerontol 2021; 156: 111624. 10.1016/j.exger.2021.111624. [DOI] [PubMed] [Google Scholar]
- 11. Smith L, Shin JI, Ghayda RAet al. Physical multimorbidity and incident urinary incontinence among community-dwelling adults aged ≥50 years: findings from a prospective analysis of the Irish longitudinal study on ageing. Age Ageing 2021; 50: 2038–46. [DOI] [PubMed] [Google Scholar]
- 12. Smith SM, Wallace E, Clyne B, Boland F, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community setting: a systematic review. Syst Rev 2021; 10: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bunn F, Burn A-M, Goodman Cet al. Comorbidity and dementia: a scoping review of the literature. BMC Med 2014; 12: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grande G, Marengoni A, Vetrano DLet al. Multimorbidity burden and dementia risk in older adults: the role of inflammation and genetics. Alzheimers Dement 2021; 17: 768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hassen CB, Fayosse A, Landré Bet al. Association between age at onset of multimorbidity and incidence of dementia: 30 year follow-up in Whitehall II prospective cohort study. BMJ 2022; 2: 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shabir O, Moll TA, Matuszyk MMet al. Preclinical models of disease and multimorbidity with focus upon cardiovascular disease and dementia. Mech Ageing Dev 2020; 192: 111361. 10.1016/j.mad.2020.111361. [DOI] [PubMed] [Google Scholar]
- 17. Börsch-Supan A, Brandt M, Hunkler Cet al. Data resource profile: the Survey of Health, Ageing and Retirement in Europe (SHARE). Int J Epidemiol 2013; 42: 992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scherpenzeel A, Axt K, Bergmann Met al., eds. Collecting survey data among the 50+ population during the COVID-19 outbreak: the Survey of Health, Ageing and Retirement in Europe (SHARE). In: Survey Research Methods, 2020.
- 19. Ferreira RG, Brandão MP, Cardoso MF. An update of the profile of older adults with dementia in Europe: findings from SHARE. Aging Ment Health 2020; 24: 374–81. [DOI] [PubMed] [Google Scholar]
- 20. Veronese N, Noale M, Sinclair Aet al. Risk of progression to diabetes and mortality in older people with prediabetes: the English longitudinal study on ageing. Age Ageing 2022; 51: afab222. 10.1093/ageing/afab222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barbagallo M, Smith L, Koyanagi Aet al. Multimorbidity increased the risk of urinary incontinence in community-dwelling adults: results from the English longitudinal study on ageing. Maturitas 2023; 169: 40–5. [DOI] [PubMed] [Google Scholar]
- 22. Karvonen-Gutierrez CA, Strotmeyer ES. The urgent need for disability studies among midlife adults. Women's midlife health 2020; 6: 1–5. 10.1186/s40695-020-00057-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vassilaki M, Aakre JA, Cha RHet al. Multimorbidity and risk of mild cognitive impairment. J Am Geriatr Soc 2015; 63: 1783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amidei CB, Fayosse A, Dumurgier Jet al. Association between age at diabetes onset and subsequent risk of dementia. JAMA 2021; 325: 1640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abell JG, Kivimäki M, Dugravot Aet al. Association between systolic blood pressure and dementia in the Whitehall II cohort study: role of age, duration, and threshold used to define hypertension. Eur Heart J 2018; 39: 3119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holmquist S, Nordström A, Nordström P. The association of depression with subsequent dementia diagnosis: a Swedish nationwide cohort study from 1964 to 2016. PLoS Med 2020; 17: e1003016. 10.1371/journal.pmed.1003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karthik L, Kumar G, Keswani T, Bhattacharyya A, Chandar SS, Bhaskara RK. Protease inhibitors from marine actinobacteria as a potential source for antimalarial compound. PLoS One 2014; 9: e90972. 10.1371/journal.pone.0090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hobson P, Meara J. Mild cognitive impairment in Parkinson’s disease and its progression onto dementia: a 16-year outcome evaluation of the Denbighshire cohort. Int J Geriatr Psychiatry 2015; 30: 1048–55. [DOI] [PubMed] [Google Scholar]
- 29. Dag A, Lucia B, Halliday GMet al. Parkinson disease-associated cognitive impairment (primer). Nat Rev Dis Primers 2021; 7: 47. 10.1038/s41572-021-00286-x. [DOI] [PubMed] [Google Scholar]
- 30. Miceli S, Maniscalco L, Matranga D. Social networks and social activities promote cognitive functioning in both concurrent and prospective time: evidence from the SHARE survey. Eur J Ageing 2019; 16: 145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Song X, Mitnitski A, Rockwood K. Nontraditional risk factors combine to predict Alzheimer disease and dementia. Neurology 2011; 77: 227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fabbri E, An Y, Zoli Met al. Aging and the burden of multimorbidity: associations with inflammatory and anabolic hormonal biomarkers. J Gerontol A Biol Sci Med Sci 2015; 70: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fabbri E, An Y, Zoli Met al. Association between accelerated multimorbidity and age-related cognitive decline in older Baltimore longitudinal study of aging participants without dementia. J Am Geriatr Soc 2016; 64: 965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koyanagi A, Lara E, Stubbs Bet al. Chronic physical conditions, multimorbidity, and mild cognitive impairment in low- and middle-income countries. J Am Geriatr Soc 2018; 66: 721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maniscalco L, Miceli S, Bono F, Matranga D. Self-perceived health, objective health, and quality of life among people aged 50 and over: interrelationship among health indicators in Italy, Spain, and Greece. Int J Environ Res Public Health 2020; 17: 2414. 10.3390/ijerph17072414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kehoe R, Wu S-Y, Leske MC, Chylack LT Jr. Comparing self-reported and physician-reported medical history. Am J Epidemiol 1994; 139: 813–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


