Abstract
Objective
To assess semicircular canal function in benign paroxysmal positional vertigo (BPPV) using the video head impulse test (vHIT) and caloric test.
Methods
We retrospectively reviewed 39 patients with idiopathic BPPV who underwent both vHIT and the caloric test. Twenty‐one patients had posterior BPPV (p‐BPPV) and eighteen had horizontal BPPV (h‐BPPV). Vestibulo‐ocular reflex (VOR) gain and corrective saccades (CS) were analyzed in vHIT and canal paresis (CP) was calculated in the caloric test.
Results
The mean VOR gain of the posterior canal in p‐BPPV was 0.75 ± 0.28 on the affected side, which was significantly smaller than that on the contralateral side (0.93 ± 0.24, p = .00738). On the other hand, there were no significant differences in the VOR gain of the horizontal canal in h‐BPPV between the affected and the contralateral sides (p = .769). The rates of the presence of CS were not significantly different between the affected canal and the contralateral canal either in p‐BPPV (p = .111) or h‐BPPV (p = .0599). The mean CP value in h‐BPPV patients (43.5 ± 31.3%) was significantly higher than that in p‐BPPV patients (22.2 ± 22.9%; p = .0184).
Conclusion
The VOR gain of vHIT in the affected canal was significantly smaller than that in the contralateral canal in p‐BPPV, but not in h‐BPPV. The caloric responses of the affected canal are reduced to a significantly larger extent in h‐BPPV compared to p‐BPPV. These results suggest that BPPV affects the semicircular canal function differently depending on which semicircular canal is involved.
Keywords: BPPV, caloric test, corrective saccade, vestibulo‐ocular reflex, vHIT
The VOR gain of vHIT in the affected canal was significantly smaller than that in the contralateral canal in p‐BPPV, but not in h‐BPPV. The caloric responses of the affected canal are reduced to a significantly larger extent in h‐BPPV compared to p‐BPPV. These results suggest that BPPV affects the semicircular canal function differently depending on which semicircular canal is involved.

1. INTRODUCTION
Benign paroxysmal positional vertigo (BPPV), the most commonly recognized form of peripheral vestibular vertigo, 1 is characterized by a spinning sensation for periods of a few seconds to 1 min, triggered by an abrupt change in head position relative to gravity. 2 , 3 The pathogenesis of BPPV is considered to be small particles trapped in the semicircular canals. These particles most likely consist of otoconia dislodged from the macula of the utricle. 4 The symptoms of BPPV are caused by mechanical stimulation of the semicircular canal, for example by free‐floating otoconia in the canal (canalolithiasis) or by adherence of otoconia to the cupula of a canal making it sensitive to gravity (cupulolithiasis). 4
Dislodgement of the otoconia from the otolithic membrane and their entrapment in the semicircular canals may cause vestibular dysfunction in patients with BPPV. Abnormal findings in vestibular evoked myogenic potentials (VEMPs), the clinical test of otolithic function, have been reported on the affected side in BPPV. 5 , 6 Another study reported that VEMP abnormalities were more prevalent on the affected side as well as the contralateral side in a BPPV patient group compared to a healthy control group. 7 Regarding semicircular canal function, it has been reported that approximately 30% of BPPV patients showed unilateral caloric hypoexcitability. 8 , 9 Previous studies using the video head impulse test (vHIT), which provides an objective measurement of the vestibulo‐ocular reflex (VOR), revealed varying results in BPPV patients, ranging from normal VOR gain in all three semicircular canals 10 , 11 to reduced gain in the affected canal in comparison to the contralateral healthy canal. 11 , 12 , 13
The vHIT can be used to evaluate the VOR of all semicircular canals in the high‐frequency range, up to 5 Hz, whereas the caloric test can only evaluate the VOR of the horizontal semicircular canals in the low‐frequency range, up to 0.003 Hz. 14 , 15 Combined use of these two tests would enable evaluation of VOR function in both high‐ and low‐frequency ranges.
Since vestibular dysfunction in BPPV can be a cause of persistent dizziness and instability during head movement 16 as well as residual dizziness after successful treatments, 17 , 18 it is important to investigate vestibular function in BPPV in detail. The purpose of the present study was to assess the semicircular canal function in BPPV patients using both vHIT and the caloric test.
2. MATERIALS AND METHODS
This study was approved by the Research Ethics Committee, Graduate School of Medicine, Nagoya City University (#60‐21‐0132), and was conducted according to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants.
2.1. Patients
We retrospectively reviewed the medical records of patients with a confirmed diagnosis of BPPV, who visited the Dizziness Clinic of Nagoya City University Hospital, from December 2019 to April 2021. We included 39 patients (11 men and 28 women; age range 31–90 years, mean ± SD 60.9 ± 12.2 years) who were diagnosed as having idiopathic BPPV and underwent vestibular function tests including vHIT and the caloric test. The diagnosis of BPPV was made according to the Barany Society's diagnostic criteria for BPPV. 4 We included patients who showed nystagmus on the Dix‐Hallpike test or the supine roll test performed in our clinic. Of the 39 patients, 21 were diagnosed as having posterior canal BPPV (p‐BPPV) and 18 as having horizontal canal BPPV (h‐BPPV) (canalolithiasis). We excluded patients’ comorbid with other types of peripheral vestibular vertigo including Meniere's disease, vestibular neuritis and vestibular migraine and those with a known history of other neuro‐otological or neurological diseases. We also excluded patients who failed to complete vHIT in all the stimulation planes.
Patients were treated using the appropriate canalith repositioning procedure according to the semicircular canal involved: Epley's maneuver was performed for p‐BPPV and Lempert's maneuver was performed for h‐BPPV. Patients returned to the clinic for treatment every 1 or 2 weeks until the disappearance of nystagmus on positional testing was confirmed.
Table 1 shows the demographics of the patients. A detailed history was taken and a battery of tests was performed including a physical examination, neurological examination, pure‐tone audiometry, and positional/positioning nystagmus testing under infrared CCD goggles. The neuro‐otological examination included vHIT, electronystagmography, caloric testing, and cervical and ocular VEMPs. Neuroimaging studies such as computed tomography and magnetic resonance imaging of the brain were performed when considered necessary to exclude disorders of the central nervous system such as cerebellar infarction/hemorrhage and neurodegenerative diseases.
TABLE 1.
Patient demographics.
| p‐BPPV | h‐BPPV | |
|---|---|---|
| (N = 21) | (N = 18) | |
| Age (year) | ||
| Mean ± SD | 61.8 ± 13.4 | 59.9 ± 11.0 |
| Range | 36–90 | 31–73 |
| Sex, N (%) | ||
| Male | 7 (33.3%) | 4 (22.2%) |
| Female | 14 (66.7%) | 14 (77.8%) |
| Duration from the onset (months) | 11.3 ± 26.6 | 4.9 ± 10.6 |
| Comorbidities, N | ||
| Hypertension | 6 | 3 |
| Migraine | 3 | 3 |
| Hyperlipidemia | 3 | 1 |
| Cardiovascular disease | 0 | 1 |
2.2. Vestibular function tests
2.2.1. Video head impulse test
The vHIT was performed to assess the vestibulo‐ocular reflex (VOR) in the three semicircular canal planes. Subjects were seated 1 m from a wall with a black fixation dot that served as the visual target. While the subject was asked to stare at the fixation dot, the examiner briefly and unpredictably rotated the subject's head through a 10°‐to‐20°‐angle. The head rotations were made in the horizontal, the left anterior‐right posterior (LARP), and the right anterior left posterior (RALP) planes. The head impulses were repeated at least 15 times in each direction, and the eye and head velocities were recorded and analyzed using an Eye‐See‐Cam system (Interacoustics, Denmark). A mean VOR gain in vHIT of <0.7 for the vertical canals and <0.8 for the horizontal canals was regarded as functionally abnormal. 19 Corrective saccades (CS) were identified by their peak velocity. We only included CS that brought the eye toward the target with a peak velocity >50°/s in our analyses since tiny saccades were frequently observed in healthy subjects. 20 We included both overt and covert saccades.
2.2.2. Caloric test
Caloric testing was carried out using air at 24°C and 50°C for 60 s each. The maximum slow‐phase eye velocity was measured using electronystagmography and canal paresis (CP%) was calculated using Jongkee's index formula. 21 A maximum slow phase eye velocity <10°/s bilaterally or CP% > 20 was regarded as indicating a unilateral or bilateral weakness of responses, respectively. 22
2.2.3. Statistical analysis
The paired t‐test was used to compare the affected side and the contralateral side for the value of VOR gain. Fisher's exact test was used to evaluate the binary data between the two groups. The Mann–Whitney test was used to compare p‐BPPV and h‐BPPV for CP%. A difference of p < .05 was considered significant. To measure the effect size, Cohen's d was calculated for the paired t‐test, r for the Mann–Whitney test, and φ for Fisher's exact test. All statistical analyses were performed using EZR version 1.37 for Windows (Saitama Medical Center, Jichi Medical University, Saitama, Japan). 23
3. RESULTS
We assessed semicircular canal function using vHIT as well as the caloric test in 39 patients with BPPV (21 patients with p‐BPPV and 18 patients with h‐BPPV) (Tables 2 and 3). The mean VOR gain of the posterior semicircular canal on the affected side in p‐BPPV patients was 0.75 ± 0.28 while it was 0.93 ± 0.24 for the posterior semicircular canal on the contralateral side (n = 21). There was a significant difference between them (p = .00738, d = 0.690; Figure 1A). The VOR gains of the horizontal and anterior semicircular canals were normal on both sides in p‐BPPV patients. The rate of the presence of CS on head rotation toward the affected posterior semicircular canal was 52.4% (11 of 21 patients) while it was 23.8% on rotation toward the contralateral posterior semicircular canal (5 of 21 patients). The majority of the type of CS were isolated overt on head rotation toward the affected posterior canal (isolated overt: 10 patients, mixed 1 patient) as well as on rotation toward the contralateral posterior canal (isolated overt: 4 patients, mixed: one patient). There was no significant difference between these values (p = .111, φ = 0.347). Figure 2 shows the results of vHIT in a typical patient with BPPV involving the right posterior semicircular canal. The VOR gain of the right posterior canal was reduced and CS were present while the gains of the other semicircular canals were within normal limits.
TABLE 2.
VOR gain and corrective saccades in vHIT.
| p‐BPPV | h‐BPPV | |||||||
|---|---|---|---|---|---|---|---|---|
| (N = 21) | (N = 18) | |||||||
| Affected a | Contra a | p‐Value | d | Affected a | Contra a | p‐Value | d | |
| Gain, mean ± SD | ||||||||
| Horizontal canal | 1.05 ± 0.20 | 1.07 ± 0.36 | .730 | 0.0690 | 1.13 ± 0.23 | 1.12 ± 0.17 | .769 | 0.0490 |
| Anterior canal | 1.04 ± 0.26 | 0.99 ± 0.27 | .436 | 0.189 | 1.15 ± 0.25 | 1.29 ± 0.27 | .137 | 0.538 |
| Posterior canal | 0.75 ± 0.28 | 0.93 ± 0.24 | .00738 | 0.690 | 0.98 ± 0.44 | 1.08 ± 0.26 | .396 | 0.277 |
| Presence of CS b , N (%) | φ | φ | ||||||
| Horizontal canal | 6 (28.5%) | 7 (33.3%) | 1.000 | 0 | 8 (44.4%) | 2 (11.1%) | .0599 | 0.439 |
| Overt /covert /mixed c | 4/ 0/ 2 | 5/ 1/ 1 | 6/ 2/ 0 | 2/ 0/ 0 | ||||
| Anterior canal | 0 (0.0%) | 1 (4.8%) | 1.000 | 0 | 1 (5.6%) | 0 (0.0%) | 1.000 | 0 |
| Overt /covert /mixed | 0/ 0/ 0 | 0/ 0/1 | 0/ 1/ 0 | 0/ 0/ 0 | ||||
| Posterior canal | 11 (52.4%) | 5 (23.8%) | .111 | 0.347 | 5 (27.8%) | 1 (5.6%) | .177 | 0.316 |
| Overt /covert /mixed | 10/ 0/1 | 4/ 0/ 1 | 3/ 0/ 2 | 0/ 1/ 0 | ||||
Affected, affected side; Contra, contralateral side.
CS, corrective saccades.
Overt/covert/ mixed: isolated overt saccades/isolated covert sacades/both overt and covert saccades.
TABLE 3.
Vestibular function findings with the caloric test.
| p‐BPPV | h‐BPPV | p‐Value | ||
|---|---|---|---|---|
| (N = 18) | (N = 18) | |||
| r | ||||
| CP % value (%), mean ± SD | 43.5 ± 31.3 | 22.2 ± 22.2 | 0.0184 | 0.393 |
| φ | ||||
| Normal function, N (%) | 12 (66.7%) | 6 (33.3%) | 0.0943 | 0.278 |
| Abnormal function, N (%) | 6 (33.3%) | 12 (66.7%) |
FIGURE 1.
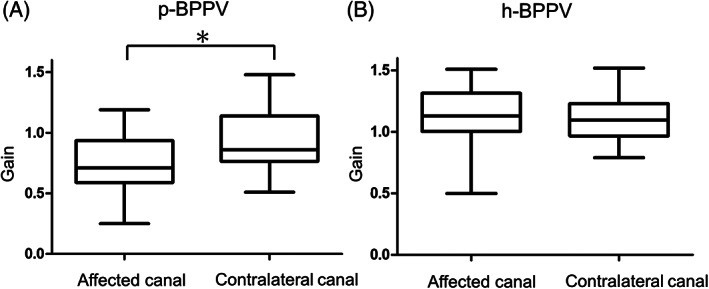
The VOR gain in vHIT of the affected canal and the contralateral canal in BPPV patients. (A) The VOR gain of the affected and contralateral sides of the posterior semicircular canals in patients with p‐BPPV (N = 21). (B) The VOR gain of the affected and contralateral sides of the horizontal semicircular canals in patients with h‐BPPV (N = 18). *p < .05.
FIGURE 2.
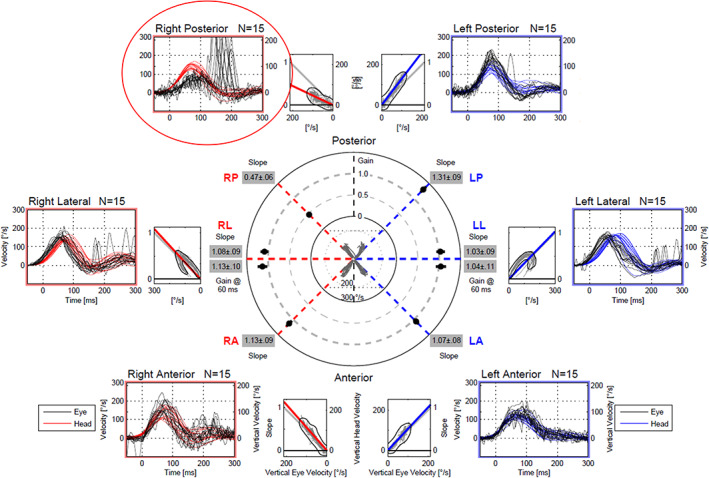
The results of vHIT in a patient with BPPV of the right posterior semicircular canal. vHIT showed reduced VOR gain (0.47) with the presence of corrective saccades during head impulses to the right posterior semicircular canal (red circle). The gains of the other semicircular canals were within normal limits. The caloric testing of this patient gave normal responses on both sides.
On the other hand, in h‐BPPV patients there were no significant differences in the VOR gain of the horizontal canal between the affected side and the contralateral side (p = .769, d = 0.0490; Figure 1B). The rate of the presence of CS on head rotation toward the affected horizontal semicircular canal was 44.4% (7 of 18 patients) while it was 11.1% (2 of 18 patients) on head rotation toward the contralateral horizontal semicircular canal in h‐BPPV patients. There was no significant difference between these values (p = .0599, φ = 0.439).
In the caloric test, the mean CP% value was 43.5 ± 31.3% in h‐BPPV (n = 18) and 22.2 ± 22.9% in p‐BPPV (n = 18). There was a significant difference between these values (p = .0184, r = 0.393). Abnormal caloric responses were found in 66.7% of h‐BPPV patients (12 of 18 patients) and 33.3% of p‐BPPV patients (6 of 18 patients). There was no significant difference between these values (p = .0943, φ = 0.278).
4. DISCUSSION
In the present study, we evaluated semicircular canal function in BPPV patients using vHIT as well as the caloric test, and revealed that the VOR gain in vHIT of the affected semicircular canal was significantly smaller than that of the contralateral canal in p‐BPPV, but not in h‐BPPV and that the value of CP% in h‐BPPV patients was significantly greater than that in p‐BPPV patients. These results suggest that BPPV affects semicircular canal function differently depending on the affected semicircular canal.
The causal mechanism of BPPV is thought to be fragments of otoconia, dislodged from the maculae of the otolith organ, entering the duct of the semicircular canals. 24 This entrapment of the otoconia into the semicircular canals can affect the dynamics of semicircular canal function. Strupp et al. reported that caloric hypo‐excitability in a patient with h‐BPPV resolved after successful liberatory maneuvers, 25 suggesting that ipsilateral caloric hypo‐excitability in this patient was attributed to functional interference of the dynamics of the VOR in the horizontal semicircular canals caused by the entrapped debris. Previous studies reported that about one third of h‐BPPV patients showed reduced caloric responses on the affected side. 8 , 9 In the present study, approximately 60% of patients with h‐BPPV showed unilateral or bilateral abnormalities. The degree of caloric hypo‐excitability in h‐BPPV patients might be dependent on the size and the quantity of the dislodged debris in the affected horizontal semicircular canals.
Abnormal caloric responses have also been reported in p‐BPPV as well as in h‐BPPV. Previous studies reported that approximately 20% of p‐BPPV patients showed canal paresis on the affected side. 26 , 27 In the present study, caloric abnormalities were observed in approximately one third of the patients with p‐BPPV. The reason for caloric weakness in p‐BPPV is unknown. Korres et al. reported that 22% of p‐BPPV patients had canal paresis on the affected side, and speculated that repeated, intense activation of the posterior semicircular canal by movement of otoconia could cause hypesthesia on the side of the lesion. 26 Roberts et al. reported that p‐BPPV patients with a prior history of otologic disease showed a greater prevalence of caloric weakness in comparison with patients with a negative history. 27 They suggested that the involvement of the horizontal semicircular canals in p‐BPPV could be due to previous extensive damage of the vestibular endorgan before the onset of p‐BPPV. 27 While we excluded patients with a prior history of vestibulopathy, it is possible that some patients also had a prior history of otologic diseases.
There are several previous studies of vHIT in patients with BPPV in the literature. 10 , 11 , 13 Saltuk and Yetiser performed vHIT in patients with BPPV and reported there were no significant differences in gain in any canals compared to healthy control groups in either p‐BPPV or h‐BPPV patients. 11 However, gain asymmetry of the posterior semicircular canals was greater than that in controls in p‐BPPV patients whereas there were no significant differences in gain asymmetries in the horizontal semicircular canals between h‐BPPV patients and control groups. Our study has shown that the VOR gain was significantly smaller in the affected canal compared to the contralateral canal in p‐BPPV, but not in h‐BPPV, confirming the results of the aforementioned study. 11 A meta‐analysis of the results of vHIT in BPPV revealed that VOR gain of the posterior semicircular canal was diminished relative to that on the contralateral healthy side, and concluded that vHIT can be valuable as a supporting test in the diagnosis of p‐BPPV. 13
The pathophysiological mechanism of abnormal vHIT findings in BPPV is considered to be either that the otoconia in the affected semicircular canal cause a partial blockage of endolymphatic flow, resulting in a low‐pass filter condition, 10 or that a cupular deviation in either direction due to otoconia adhesion results in a partial mechanical and electrophysiological saturation, decreasing the cupular sensitivity to high acceleration stimuli. 28
What is the cause of the discrepant results in VOR gain in vHIT between p‐BPPV and h‐BPPV? We propose two possible explanations. One is the different distribution of debris caused by the different orientation of each canal relative to gravity. The debris in the posterior semicircular canal would be aggregated in the lowest part of the posterior semicircular canal in the upright position in which vHIT is performed, where it would easily disturb canal flow during rapid head turns along the plane of the canal. In contrast, the debris in the horizontal semicircular canal would be dispersed along the horizontal semicircular canal in the upright position, and the disturbance of canal flow during the head turn should be minimal (Figure 3). The other possible cause is related to the different cross‐sectional area along the semicircular canal. The cross‐sectional area of the semicircular canal is smallest at the middle portion while it is largest at the portion close to ampulla. 29 In p‐BPPV, the head turn toward the affected canal causes canal flow from the wide area to the narrow area, and the debris in the semicircular canal can have more effect on the canal flow. In contrast, in h‐BPPV, although the head turn causes flow from the narrow to the wide area, the effect of the debris on the canal flow would be smaller. To test these hypotheses, experiments using an artificial model of the semicircular canal may be necessary.
FIGURE 3.

Schematic diagram of endolymphatic flow during vHIT in p‐BPPV (A) and h‐BPPV (B). (A) In p‐BPPV, the debris in the posterior semicircular canal is aggregated in the lowest part of the canal in the upright position, disturbing the endolymphatic flow (yellow arrows) during rapid head turns along the plane of the posterior canal (red arrows). (B) In h‐BPPV, the debris in the horizontal semicircular canal is dispersed and the disturbance of the endolymphatic flow (yellow arrows) during horizontal head turns (red arrows) is minimal.
The presence of CS is another important parameter of vHIT. A previous study reported that CS on head rotation toward the affected canal were observed in 25% of p‐BPPV patients and in 11% of h‐BPPV patients. 11 Another study reported that there were no patients who showed CS in p‐BPPV. 12 In the present study, 52.4% of patients with p‐BPPV and 44.4% of patients with h‐BPPV showed CS during head rotation toward the affected canal. These rates of the presence of CS in BPPV patients were higher than reported in the previous studies. 11 The presence of CS was accompanied by decreased VOR gain of the affected canal in most patients with p‐BPPV whereas CS were frequently observed in the presence of normal VOR gain in h‐BPPV patients in the present study. The reason why CS were observed in the presence of normal VOR gain in h‐BPPV patients is unknown. While the origin of CS is not fully understood yet, it is possible that the disturbance of canal flow by debris in the semicircular canal might affect the generation of CS in h‐BPPV.
This study has several limitations. First, this is a retrospective study. There might be a selection bias in the distribution of patients with BPPV. The duration from the onset of disease in the patients included in the present study is relatively long. It is possible that the rate of abnormality in the vestibular function tests is affected by the duration of the disease or previous canalith repositioning procedures. It is also possible that there might be confounding factors between the results of vHIT and the caloric test. Furthermore, this study may not have ruled out bilateral vestibular hypofunction if present as a potential confounder due to lack of rotational studies. Second, we could not evaluate the VOR of the affected canal in the low‐frequency range in p‐BPPV patients since the VOR of the posterior semicircular canal was evaluated by vHIT only. Although a rotational test to evaluate vertical semicircular canal function has been developed, 30 this test is not able to evaluate the low‐frequency VOR of the vertical semicircular canal unilaterally. Third, the number of patients included in this study was small. Conduction of a prospective, multicenter study including many patients is necessary to elucidate the characteristics of semicircular canal function in patients diagnosed with BPPV.
5. CONCLUSION
We evaluated the function of the semicircular canal in patients with BPPV using vHIT as well as the caloric test. We revealed that VOR gain in vHIT of the affected semicircular canal was significantly smaller than that of the contralateral semicircular canal in p‐BPPV while caloric responses were reduced on the affected side compared to the contralateral side in h‐BPPV. These results suggest that BPPV affects the semicircular canal function differently depending on which semicircular canal is involved.
FUNDING INFORMATION
This study was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology (20K11161, 21H03088).
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Kabaya K, Katsumi S, Fukushima A, Esaki S, Minakata T, Iwasaki S. Assessment of semicircular canal function in benign paroxysmal positional vertigo using the video head impulse test and caloric test. Laryngoscope Investigative Otolaryngology. 2023;8(2):525‐531. doi: 10.1002/lio2.1020
REFERENCES
- 1. Bhattacharyya N, Baugh RF, Orvidas L, et al. Clinical practice guideline: benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2008;139(5 Suppl 4):S47‐S81. [DOI] [PubMed] [Google Scholar]
- 2. Kim JS, Zee DS. Clinical practice. Benign paroxysmal positional vertigo. N Engl J Med. 2014;370(12):1138‐1147. [DOI] [PubMed] [Google Scholar]
- 3. You P, Instrum R, Parnes L. Benign paroxysmal positional vertigo. Laryngoscope Investig Otolaryngol. 2019;4(1):116‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. von Brevern M, Bertholon P, Brandt T, et al. Benign paroxysmal positional vertigo: Diagnostic criteria. J Vestib Res. 2015;25(3–4):105‐117. [DOI] [PubMed] [Google Scholar]
- 5. Yang WS, Kim SH, Lee JD, Lee WS. Clinical significance of vestibular evoked myogenic potentials in benign paroxysmal positional vertigo. Otol Neurotol. 2008;29(8):1162‐1166. [DOI] [PubMed] [Google Scholar]
- 6. Singh NK, Apeksha K. Efficacy of cervical and ocular vestibular‐evoked myogenic potentials in evaluation of benign paroxysmal positional vertigo of posterior semicircular canal. Eur Arch Otorhinolaryngol. 2016;273(9):2523‐2532. [DOI] [PubMed] [Google Scholar]
- 7. Kim EJ, Oh SY, Kim JS, Yang TH, Yang SY. Persistent otolith dysfunction even after successful repositioning in benign paroxysmal positional vertigo. J Neurol Sci. 2015;358(1–2):287‐293. [DOI] [PubMed] [Google Scholar]
- 8. Pagnini P, Nuti D, Vannucchi P. Benign paroxysmal vertigo of the horizontal canal. ORL J Otorhinolaryngol Relat Spec. 1989;51(3):161‐170. [DOI] [PubMed] [Google Scholar]
- 9. Baloh RW, Jacobson K, Honrubia V. Horizontal semicircular canal variant of benign positional vertigo. Neurology. 1993;43(12):2542‐2549. [DOI] [PubMed] [Google Scholar]
- 10. Califano L, Iannella R, Mazzone S, Salafia F, Melillo MG. The video head impulse test in the acute stage of posterior canal benign paroxysmal positional vertigo. Acta Otorhinolaryngol Ital. 2021;41(1):69‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salturk Z, Yetiser S. Video head impulse testing in patients with benign paroxysmal positional vertigo. Acta Otolaryngol. 2020;140(12):977‐981. [DOI] [PubMed] [Google Scholar]
- 12. Fallahnezhad T, Adel Ghahraman M, Farahani S, Hoseinabadi R, Jalaie S. Vestibulo‐ocular reflex abnormalities in posterior Semicircular Canal benign paroxysmal positional vertigo: a pilot study. Iran J Otorhinolaryngol. 2017;29(94):269‐274. [PMC free article] [PubMed] [Google Scholar]
- 13. Elsherif M, Eldeeb D, Eldeeb M. Clinical significance of video head impulse test in benign paroxysmal positional vertigo: a meta‐analysis. Eur Arch Otorhinolaryngol. 2021;278(12):4645‐4651. [DOI] [PubMed] [Google Scholar]
- 14. Halmagyi GM, Chen L, MacDougall HG, et al. The video head impulse test. Front Neurol. 2017;8:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahringer A, Rambold HA. Caloric test and video‐head‐impulse: a study of vertigo/dizziness patients in a community hospital. Eur Arch Otorhinolaryngol. 2014;271(3):463‐472. [DOI] [PubMed] [Google Scholar]
- 16. Kitahara T, Ota I, Horinaka A, et al. Idiopathic benign paroxysmal positional vertigo with persistent vertigo/dizziness sensation is associated with latent canal paresis, endolymphatic hydrops, and osteoporosis. Auris Nasus Larynx. 2019;46(1):27‐33. [DOI] [PubMed] [Google Scholar]
- 17. Giommetti G, Lapenna R, Panichi R, et al. Residual dizziness after successful repositioning maneuver for idiopathic benign paroxysmal positional vertigo: a review. Audiol Res. 2017;7(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dispenza F, Mazzucco W, Mazzola S, Martines F. Observational study on risk factors determining residual dizziness after successful benign paroxysmal positional vertigo treatment: the role of subclinical BPPV. Acta Otorhinolaryngol Ital. 2019;39(5):347‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McGarvie LA, MacDougall HG, Halmagyi GM, et al. The video head impulse test (vHIT) of Semicircular Canal function ‐ age‐dependent normative values of VOR gain in healthy subjects. Front Neurol. 2015;6:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weber KP, Aw ST, Todd MJ, McGarvie LA, Curthoys IS, Halmagyi GM. Head impulse test in unilateral vestibular loss: vestibulo‐ocular reflex and catch‐up saccades. Neurology. 2008;70(6):454‐463. [DOI] [PubMed] [Google Scholar]
- 21. Jongkees LB, Maas JP, Philipszoon AJ. Clinical nystagmography. A detailed study of electro‐nystagmography in 341 patients with vertigo. Pract Otorhinolaryngol (Basel). 1962;24:65‐93. [PubMed] [Google Scholar]
- 22. Kabaya K, Tamai H, Okajima A, et al. Presence of exacerbating factors of persistent perceptual‐postural dizziness in patients with vestibular symptoms at initial presentation. Laryngoscope Investig Otolaryngol. 2022;7(2):499‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Epley JM. The canalith repositioning procedure: for treatment of benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 1992;107(3):399‐404. [DOI] [PubMed] [Google Scholar]
- 25. Strupp M, Brandt T, Steddin S. Horizontal canal benign paroxysmal positioning vertigo: reversible ipsilateral caloric hypoexcitability caused by canalolithiasis? Neurology. 1995;45(11):2072‐2076. [DOI] [PubMed] [Google Scholar]
- 26. Korres SG, Balatsouras DG, Ferekidis E. Electronystagmographic findings in benign paroxysmal positional vertigo. Ann Otol Rhinol Laryngol. 2004;113(4):313‐318. [DOI] [PubMed] [Google Scholar]
- 27. Roberts RA, Gans RE, Kastner AH, Lister JJ. Prevalence of vestibulopathy in benign paroxysmal positional vertigo patients with and without prior otologic history. Int J Audiol. 2005;44(4):191‐196. [DOI] [PubMed] [Google Scholar]
- 28. Tamas LT, Obrist D, Avan P, et al. Biasing the semicircular canal cupula in excitatory direction decreases the gain of the vestibuloocular reflex for head impulses. J Vestib Res Equilib Orient. 2019;29(6):281‐286. [DOI] [PubMed] [Google Scholar]
- 29. Curthoys IS, Oman CM. Dimensions of the horizontal semicircular duct, ampulla and utricle in the human. Acta Otolaryngol. 1987;103(5–6):254‐261. [DOI] [PubMed] [Google Scholar]
- 30. Morita M, Imai T, Kazunori S, et al. A new rotational test for vertical semicircular canal function. Auris Nasus Larynx. 2003;30(3):233‐237. [DOI] [PubMed] [Google Scholar]


