Abstract
Objective
To explore whether narrow‐band imaging (NBI) endoscopy is accurate in the diagnosis of malignant transformation of vocal cord leukoplakia.
Methods
The PubMed, Embase, Cochrane Library and Web of Science databases were searched to collect data on studies reporting the use of NBI endoscopy as a diagnostic test for diagnosing vocal cord leukoplakia from January 2015 to December 2021. Study design, analysis method, and extraction results were performed according to the PRISMA guidelines. The sensitivity, specificity, pooled positive likelihood ratio (PLR), negative likelihood ratio (NLR) and area under the curve (AUC) were used to summarize the performance metrics of the meta‐analysis. Risk of bias data and the quality of the included studies was evaluated according to the Quality Assessment of Diagnostic Accuracy Studies‐2 tool (QUADAS‐2).
Results
Nine studies were finally included in the analysis. The results of the meta‐analysis showed that the pooled sensitivity and specificity of NBI endoscopy for diagnosing leukoplakia lesions were 0.76 (95% CI: 0.72–0.8) and 0.93 (95% CI: 0.91–0.95), respectively. The PLR and NLR were 10.09 (95% CI: 6.53–15.59) and 0.22 (95% CI: 0.13–0.38), respectively. The comprehensive diagnostic odds ratio (DOR) was 54.96 (95% CI: 24.32–124.17), and the area under the curve was 0.9584. The eight articles had a low risk of bias risk and one article was unclear.
Conclusion
NBI likely has good accuracy for diagnosing malignant transformation of vocal cord leukoplakia. However, multicenter studies and large samples are still needed.
Keywords: malignant transformation, meta‐analysis, narrow‐band imaging, vocal cord leukoplakia
Narrow‐band imaging (NBI) likely has good accuracy for diagnosing malignant transformation of vocal cord leukoplakia and has a high diagnostic value. But the diagnostic value of NBI for leukoplakia needs to be further verified by multicenter, large sample, and high‐quality clinical trials.
1. INTRODUCTION
Vocal cord leukoplakia refers to the white plaque attached to the surface or edge of the vocal cord mucosa. It develops mainly due to various stimuli such as smoking, alcohol addiction, chronic inflammation of the larynx, and vitamin A and B deficiency. 1 , 2 , 3 The main clinical symptom is hoarseness. 4 Vocal cord leukoplakia tends to undergo malignant transformation, and in some cases, it transforms into laryngeal carcinoma. Therefore, a more precise histopathological diagnosis of early vocal cord leukoplakia is critical for timely prognosis and selection of the appropriate clinical treatment. 5 Presently, according to the basic pathological manifestations, vocal cord leukoplakia is classified into hyperkeratosis, dysplasia (including mild dysplasia, moderate dysplasia, and severe dysplasia), carcinoma in situ (CIS), and invasive carcinoma. 6 The different pathological types of leukoplakia show significant differences in treatment and prognosis.
Narrow‐band imaging (NBI) endoscopy uses a filter to remove the broadband spectrum in the red, blue, and green light emitted by the endoscopic light source, thereby leaving a narrow band spectrum (415 and 540 nm). Comparison of the morphology of the mucosal epithelium and the microvascular morphology of epithelial cells can enable us to better observe and evaluate the morphology of intraepithelial papillary capillary loop (IPCL) and detect some lesions that are difficult to find under ordinary white light endoscopy; this provides a new approach for the early detection of lesions with different properties and different degrees. 7 , 8 In recent years, NBI endoscopy has been gradually used for diagnosing laryngopharyngeal lesions. 9 Therefore, we compared the diagnostic results of vocal cord lesions examined by NBI endoscopy‐based IPCL classification with those of vocal cord lesions examined by the reference standard pathological test. In the present study, the review with meta‐analysis aimed to investigate the accuracy and clinical application value of NBI for diagnosing malignant transformation of vocal cord leukoplakia. The study also provides a basis for selecting clinical treatment options to guide the clinical prognosis.
2. MATERIALS AND METHODS
The review protocol was registered on the International Perspective Register of Systematic Review (PROSPERO; CRD42022356834).
2.1. Search strategy
The PubMed, Embase, Cochrane Library and Web of Science databases were searched for relevant articles on this topic from January 2015 to December 2021. Studies published in the English language on the diagnosis of vocal cord leukoplakia by NBI endoscopy were comprehensively searched, and the references provided in these published studies were searched again to obtain supplementary information. English search terms included (“Vocal Cords” OR “Cord, Vocal” OR “Cords, Vocal” OR “Vocal Cord” OR “Vocal Fold” OR “Fold, Vocal” OR “Folds, Vocal” OR “Vocal Folds” OR “Vocal Ligament” OR “Ligament, Vocal” OR “Ligaments, Vocal” OR “Vocal Ligaments”) AND (“Leukoplakia” OR “Leukoplakias” OR “Leukoplakic Lesions” OR “Lesion, Leukoplakic” OR “Lesions, Leukoplakic” OR “Leukoplakic Lesion” OR “Leukokeratosis” OR “Leukokeratoses”) AND (“Narrow Band Imaging” OR “Band Imaging, Narrow” OR “Band Imagings, Narrow” OR “Imaging, Narrow Band” OR “Imagings, Narrow Band” OR “Narrow Band Imagings” OR “Narrow band Imaging” OR “Imaging, Narrow band” OR “Imagings, Narrow band” OR “Narrow band Imagings”).
2.2. Study inclusion criteria
Studies meeting the following criteria were included: (1) Randomized or nonrandomized studies on the diagnosis of vocal leukoplakia by NBI endoscopy, including prospective and retrospective studies; (2) all the subjects had underwent NBI endoscopy and histopathological diagnosis; (3) the research data included data before histopathological examination (reference standard); and (4) the clinical data included in the study (four‐grid tables) were complete, which could enable to extract true‐positive (TP), false‐positive (FP), true‐negative (TN), and false‐negative (FN).
2.3. Study exclusion criteria
The following types of studies were excluded: (1) reviews, case reports, comments, and animal experiments; (2) studies having errors in data or having incomplete data that prevent designing four‐grid tables; (3) studies lacking discussion on histopathological diagnosis of lesions; and (4) studies lacking full text.
2.4. Study selection and data extraction
Two researchers independently extracted the data from the included studies through the blind method. For any inconsistency in the results of the two researchers, the consensus was reached through discussion between them or through discussion with the third researcher, and the information obtained in the selected study was summarized. The information extracted from the literature included the first author, year of publication, country, research design, number of cases, number of lesions, average age of the patient, course of disease, and numerical data of four‐grid table (TP, FP, TN, and FN) for NBI endoscopic diagnosis of malignant transformation of leukoplakia.
2.5. Statistical analysis
Sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and 95% confidence interval (95% CI) were calculated by MetaDiSc1.4. The summary receiver operating characteristic (SROC) curve was constructed, and the area under the curve (AUC) was calculated. Heterogeneity was tested by the Cochrane Q test (p < .10, indicating heterogeneity among the studies) and by the I 2 test (I 2 > 50%, indicating large heterogeneity among the studies). If the research effect variables were homogeneous, the fixed‐effect models were adopted. If the research effect variables were heterogeneous, the random‐effect models were used. If there was heterogeneity between the studies, the source of heterogeneity was further determined. Spearman's correlation coefficient test was used to determine whether there is a threshold effect, and subgroup analysis was performed.
2.6. Risk of bias assessment
The Quality Assessment of Diagnostic Accuracy Studies (QUADAS) were applied to evaluate the methodological quality and possible bias of the included research studies. Deeks' funnel was used to evaluate the publication bias of the included studies by Stata 12.0. p > .05 was considered to indicate no publication bias.
3. RESULTS
3.1. Study selection
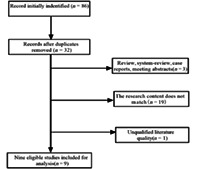
A total of 86 relevant articles were initially retrieved from the search. According to the inclusion and exclusion criteria, 29 articles were initially screened. After full‐text reading, nine articles were finally included in the meta‐analysis. These nine articles included 873 patients and 995 lesions. Figure 1 shows the literature screening process, and Table 1 shows the extracted literature information.
FIGURE 1.

Literature search flow diagram.
TABLE 1.
Characteristics of the selected studies.
| Author | Year | Sample size/sex | Number of lesions | Country | Course of disease | Research type | Age, mean | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rzepakowska et al. | 2018 | 62 (M:46, W:16) | 91 | Poland | NA | Prospective | 61 | 14 | 2 | 0 | 75 |
| Huang et al. | 2017 | 57 (M:56, W:1) | 78 | China | NA | Prospective | 60.54 | 25 | 1 | 4 | 48 |
| Staníková. et al. | 2017 | 63 (M:44, W:19) | 63 | Czech | NA | Prospective | 61 | 22 | 4 | 3 | 34 |
| Lin et al. | 2020 | 112 (M:104, W:8) | 123 | China | 3 m‐1y | Prospective | NA | 72 | 7 | 5 | 39 |
| Guowei et al. | 2021 | 96 (M:87, W:9) | 119 | China | NA | Prospective | 63 | 65 | 3 | 12 | 39 |
| Wioletta et al. | 2021 | 258 (M:212, W:47) | 296 | Poland | ≥3w | Prospective | NA | 88 | 12 | 39 | 157 |
| Fang et al. | 2021 | 90 (M:82, W:8) | 90 | China | NA | Prospective | 61.78 | 50 | 0 | 16 | 24 |
| Zhu et al. | 2019 | 89 (NA) | 89 | China | NA | Prospective | 57.5 | 21 | 4 | 29 | 35 |
| Li et al. | 2020 | 46 (NA) | 46 | China | NA | Prospective | NA | 7 | 1 | 5 | 33 |
Abbreviations: M, men; NA, not available; W, women.
3.2. Study characteristics and quality assessment
All nine articles were prospective studies, and all included subjects had vocal cord leukoplakia. According to the QUADAS‐2 evaluation standard, the selection bias risk of the research objects in eight articles was small, and the clinical applicability was high, whereas the selection bias risk of the research objects in one article was unclear.
3.3. Heterogeneity analysis
The heterogeneity test was performed on the data extracted from the included studies by using the random‐effects model, and the results showed that the sensitivity and specificity of NBI in diagnosing malignant transformation of vocal cord leukoplakia were 0.76 (95% CI: 0.72–0.80) and 0.93 (95% CI: 0.91–0.95), respectively, and the sensitivity (I 2 = 87.3%) and specificity (I 2 = 45.8%) showed significant heterogeneity (Figure 2). The PLR, NLR, and DOR of NBI‐based diagnosis were 10.09 (95% CI: 6.53–15.59), 0.22 (95% CI: 0.13–0.38), and 54.96 (95% CI: 24.32–124.17), respectively (Figure 3). The AUC of the SROC curve was 0.9584 (SE = 0.0170), thus, indicating that NBI endoscopy is a good method to diagnosing malignant transformation of vocal leukoplakia (Figure 4). Significant heterogeneity was found in PLR (I 2 = 33.4%), NLR (I 2 = 88.3%), and DOR (I 2 = 60.4%). Spearman's correlation coefficient was −0.55 (p = .667). Thus, no apparent threshold effect was detected.
FIGURE 2.

Forest plot showing pooled sensitivity and specificity of narrow‐band imaging for diagnosing malignant change of vocal cord leukoplakia. I 2 > 50% was considered significant for heterogeneity. p < .10, indicating heterogeneity among studies.
FIGURE 3.

Forest plot showing the positive likelihood ratio (PLR), negative likelihood ratio (NLR) and diagnostic odds ratio (DLR) of narrow‐band imaging for diagnosing malignant change of vocal cord leukoplakia. I 2 > 50% was considered significant for heterogeneity. p < .10, indicating heterogeneity among studies.
FIGURE 4.

Summary receiver operating characteristic curve (SROC) showing the diagnostic performance of narrow‐band imaging for diagnosing malignant change of vocal cord leukoplakia. AUC, area under the curve.
3.4. Subgroup analysis
To determine the potential sources of heterogeneity, we conducted a subgroup analysis on the diagnostic methods, design types, literature quality, course of the disease, and patient's age for the included studies. The results showed that the age of patients with vocal cord leukoplakia might be a source of heterogeneity. Excluding the articles whose mean age was less than 60 years and whose age was difficult to determine, the remaining articles were analyzed for heterogeneity. The sensitivity and specificity were 0.83 (95% CI: 0.78–0.88) and 0.96 (95% CI: 0.92–0.98), respectively. Sensitivity heterogeneity (I 2 = 51.4%) and specificity heterogeneity (I 2 = 40.9%) were analyzed (Figure 5). The PLR, NLR, and DOR values were 15.59 (95% CI: 8.29–29.32), 0.18 (95% CI: 0.12–0.27), and 108.16 (95% CI: 46.32–252.56), respectively (Figure 6). The AUC of the SROC was 0.9574 (SE = 0.0203), and PLR (I 2 = 12.4%), NLR (I 2 = 36.9%), and DOR (I 2 = 0.0%) (Figure 7). We believe that the five articles included after excluding the age factor showed no significant heterogeneity.
FIGURE 5.

Subgroup analysis. Forest plot showing pooled sensitivity and specificity of narrow‐band imaging for diagnosing malignant change of vocal cord leukoplakia, excluding articles whose mean age was less than 60 years and whose age was difficult to determine.
FIGURE 6.

Forest plot showing the positive likelihood ratio (PLR), negative likelihood ratio (NLR) and diagnostic odds ratio (DLR) of narrow‐band imaging for diagnosing malignant change of vocal cord leukoplakia, excluding articles whose mean age was less than 60 years and whose age was difficult to determine.
FIGURE 7.

Summary receiver operating characteristic curve (SROC) showing the diagnostic performance of narrow‐band imaging for diagnosing malignant change of vocal cord leukoplakia, excluding articles whose mean age was less than 60 years and whose age was difficult to determine.
3.5. Publication bias analysis
The funnel plot of Deeks showed no significant asymmetry and tended to be vertical (p = .21), thus, indicating no significant publication bias in this study (Figure 8).
FIGURE 8.

Deeks' funnel plot for publication bias. p > .05 indicating that there is no publication bias. ESS, effective sample size.
4. DISCUSSION
4.1. Summary of evidence
The present systematic review with meta‐analysis is a distinctive approach to evaluating the accuracy of NBI endoscopy in the diagnosis malignant transformation of vocal cords leukoplakia. Collectively, NBI endoscopy is accurate. Vocal cord leukoplakia is a laryngeal inflammatory lesion caused by abnormal growth or maturation and excessive keratinization of the vocal mucosa epithelium, which occurs due to the accumulation of epithelial surface keratin. It is prevalent in middle‐aged male patients and can be a precancerous lesion; moreover, according to relevant studies, 90% of laryngeal squamous cell carcinomas (SCCs) evolve from precancerous lesions. 10 , 11 The etiology of vocal cord leukoplakia remains unclear, and smoking and drinking are usually considered to be their main risk factors. 12
In 2005, WHO classified vocal cord leukoplakia into squamous epithelial hyperplasia (keratosis, hyperkeratosis, and keratosis insufficiency); mild atypical hyperplasia, moderate atypical hyperplasia, and severe atypical hyperplasia; carcinoma in situ; and invasive carcinoma according to pathological classification. Mild atypical hyperplasia and moderate atypical hyperplasia belong to the category of benign lesions, whereas severe atypical hyperplasia, carcinoma in situ, and invasive carcinoma belong to the category of malignant lesions. 13 The latest WHO revised classification of head and neck tumors classifies pathological types of vocal cord leukoplakia into low‐grade dysplasia and high‐grade dysplasia. 14 , 15 Many treatments are available for vocal cord leukoplakia, including conservative treatment, surgical resection, and even radiotherapy, but the probability of recurrence is high. 2 , 16 Because the treatment and prognosis of leukoplakia significantly differ with the different pathological types, effective examination measures are the key to making informed treatment decisions. 17
In recent years, the use of NBI endoscopy for diagnosing malignant transformation of vocal cord lesions has gradually increased and has a good application prospect as compared to ordinary white light endoscopy. It can highlight pathological blood vessels in precancerous lesions and cancerous lesions by enhancing the comparison of the mucosal epithelium with the submucosal capillary loop and thus improve the detection rate of early cancer. 18 , 19 According to the vascular pattern of NBI described by Ni et al. 20 in 2011, which is based on the dynamic changes of the IPCL pattern on the surface of vocal cord mucosa, vocal cord leukoplakia was classified into the following types: in types I–III IPCL is invisible and observed in the benign lesions of the vocal cord and larynx; in type IV, IPCL is seen in shape, but the arrangement is basically regular, showing small brown spots, without inclination, with tree‐like blood vessels, and with mild to moderate atypical hyperplasia; and type V is classified into three subtypes of a, b and c, which suggests malignant lesions. The diameter of type Va IPCL is thickened, showing solid or hollow brown plaques, which are mostly severe atypical hyperplasia and carcinoma in situ. Type Vb and Vc are invasive carcinoma. 5 Around the white plaque, if more than one NBI type is found in the NBI mode, the highest type found is considered the NBI type of the lesion. 21
In the present study, we used prospective studies to extract data from published studies on vocal cord leukoplakia and NBI for diagnostic meta‐analysis. According to the inclusion and exclusion criteria, nine studies were included in the meta‐analysis. The number of V‐type lesions, which were detected by NBI endoscopy, was compared with the number of malignant transformation of vocal cord leukoplakia (including severe atypical hyperplasia, carcinoma in situ, and invasive SCC) under the reference standard. We used sensitivity and specificity to represent the final results. In addition, we constructed the SROC and calculated the AUC. On the basis of these results, we conducted a more comprehensive analysis to demonstrate the value of NBI for diagnosing vocal cord leukoplakia. The results showed that the sensitivity and specificity of NBI for diagnosing vocal leukoplakia were 0.76 (95% CI: 0.72–0.80) and 0.93 (95% CI: 0.91–0.95), respectively, and the combined AUC was 0.9584. These results indicated that NBI endoscopy might have good accuracy for the diagnosis malignant transformation of vocal cord leukoplakia.
The results of the I 2 test suggested high heterogeneity, and a subgroup analysis was conducted to determine the sources of heterogeneity. By analyzing the diagnostic method, design type, literature quality, lesion morphology type, course of the disease, and age subgroup, it was found that only when age analysis was performed and four studies that did not meet the patient age standard were removed, the obtained heterogeneity was quite different from that before. Therefore, it was considered that the age factor might be the main source of heterogeneity in this study. The summary sensitivity was 0.83 (95% CI: 0.78–0.88), specificity was 0.96 (95% CI: 0.92–0.98), and the combined AUC was 0.9574; these results still indicated that the accuracy of NBI for diagnosing malignant transformation of leukoplakia lesions was good.
4.2. Study limitations
In the present study, studies on vocal cord leukoplakia and NBI were comprehensively collected through various databases, and the inclusion and exclusion criteria were strictly followed to minimize the interference of selection bias on the authenticity and reliability of the study. The present study, however, has some limitations. First, the number of the collected cases from the included studies was relatively small, which prevented the further study of the factors affecting heterogeneity. If heterogeneity is significant, it may affect the reliability and authenticity of the overall results. Secord, the literature research objects were excluded improperly, which leads to incomplete collection of this part of data.
5. CONCLUSION
The study shows that NBI likely has good accuracy and high diagnostic value for diagnosing malignant transformation of vocal cord leukoplakia. However, the diagnostic value of NBI for leukoplakia needs to be further verified by multicenter, large sample, and high‐quality clinical trials.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
We would like to acknowledge all of the staff from Department of Otolaryngology, Head and Neck Surgery and we would also like to thank Shandong Provincial Clinical Research Center for Otorhinolaryngologic Diseases for helpful comments.
Chen J, Li Z, Wu T, Chen X. Accuracy of narrow‐band imaging for diagnosing malignant transformation of vocal cord leukoplakia: A systematic review and meta‐analysis. Laryngoscope Investigative Otolaryngology. 2023;8(2):508‐517. doi: 10.1002/lio2.1049
Jingyu Chen and Zhuojun Li contributed equally to this work.
REFERENCES
- 1. Fang Y, Yang Y, Chen M, et al. Correlating intraepithelial papillary capillary loops of vocal cord leukoplakia with histopathology. Acta Otolaryngol. 2022;142(1):106‐111. [DOI] [PubMed] [Google Scholar]
- 2. Klimza H, Jackowska J, Tokarski M, Piersiala K, Wierzbicka M. Narrow‐band imaging (NBI) for improving the assessment of vocal fold leukoplakia and overcoming the umbrella effect. PLoS One. 2017;12(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tateya I, Tateya T, Surles RL, Kanehira K, Tanumihardjo S, Bless DM. Vitamin A deficiency causes metaplasia in vocal fold epithelium: a rat study. Ann Otol Rhinol Laryngol. 2008;117(2):153‐158. [DOI] [PubMed] [Google Scholar]
- 4. Mau T. Diagnostic evaluation and management of hoarseness. The Medical Clinics of North America. 2010;94(5):945‐960. [DOI] [PubMed] [Google Scholar]
- 5. Lin C, Zhang S, Lu L, Wang M, Qian X. Diagnostic value and pathological correlation of narrow band imaging classification in laryngeal lesions. Ear Nose Throat J. 2021;100(10):737‐741. [DOI] [PubMed] [Google Scholar]
- 6. Chen YL, Bao YY, Zhou SH, Yao HT, Chen Z. Relationship between pepsin expression and dysplasia grade in patients with vocal cord leukoplakia. Otolaryngol Head Neck Surg Off Journal Am Acad Otolaryngol‐Head Neck Surg. 2021;164(1):160‐165. [DOI] [PubMed] [Google Scholar]
- 7. Huang F, Yu J, Zhang F, He C, Li S, Shao J. The usefulness of narrow‐band imaging for the diagnosis and treatment of vocal fold leukoplakia. Acta Otolaryngol. 2017;137(9):1002‐1006. [DOI] [PubMed] [Google Scholar]
- 8. Witkiewicz J, Klimza H, Piersiala KA‐O, Jackowska J, Wierzbicka M. The usefulness of the narrow band imaging (NBI) in decision‐making process regarding second look procedure (SL) in laryngeal cancer follow‐up after transoral laser microsurgery. PloS one. 2020;15(8):e0236623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qi X, Yu D, Zhao X, et al. Clinical experiences of NBI laryngoscope in diagnosis of laryngeal lesions. Int J Clin Exp Med. 2014;7(10):3305‐3312. [PMC free article] [PubMed] [Google Scholar]
- 10. Staníková L, Šatanková J, Kučová H, Walderová R, Zeleník K, Komínek P. The role of narrow‐band imaging (NBI) endoscopy in optical biopsy of vocal cord leukoplakia. Eur Arch Otorhinolaryngol. 2017;274(1):355‐359. [DOI] [PubMed] [Google Scholar]
- 11. Zhukhovitskaya A, Battaglia D, Khosla S, Murry T, Sulica L. Gender and age in benign vocal fold lesions. Laryngoscope. 2015;125(1):191‐196. [DOI] [PubMed] [Google Scholar]
- 12. Singhi AD, Arnold CA, Crowder CD, Lam‐Himlin DM, Voltaggio L, Montgomery EA. Esophageal leukoplakia or epidermoid metaplasia: a clinicopathological study of 18 patients. Mod Pathol. 2014;27(1):38‐43. [DOI] [PubMed] [Google Scholar]
- 13. Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006;85(2):74. [PubMed] [Google Scholar]
- 14. Gale NA‐O, Poljak M, Zidar N. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: what is new in the 2017 WHO blue book for tumours of the hypopharynx, larynx, trachea and parapharyngeal space. Head Neck Pathol. 2017;11(1):23‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zidar N, Gale N. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: hypopharynx, larynx, trachea and parapharyngeal space. Head Neck Pathol. 2022;16(1):31‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rohde M, Grøntved ÅM, Krogdahl A, Godballe C. Aggressive elimination of precancerous lesions of the vocal cords to avoid risk of cancer. Dan Med J. 2012;59(5):A4399. [PubMed] [Google Scholar]
- 17. Young C, Lin W, Lee L, et al. Laryngoscopic characteristics in vocal leukoplakia: inter‐rater reliability and correlation with histology grading. Laryngoscope. 2015;125(2):E62‐E66. [DOI] [PubMed] [Google Scholar]
- 18. Piazza C, Cocco D, De Benedetto L, Bon FD, Nicolai P, Peretti G. Role of narrow‐band imaging and high‐definition television in the surveillance of head and neck squamous cell cancer after chemo‐ and/or radiotherapy. European Archives of Oto‐Rhino‐Laryngology 2010;267(9):1423‐1428. [DOI] [PubMed] [Google Scholar]
- 19. Cosway B, Drinnan M, Paleri V. Narrow band imaging for the diagnosis of head and neck squamous cell carcinoma: a systematic review. Head Neck. 2016;38:E2358‐2367. [DOI] [PubMed] [Google Scholar]
- 20. Ni XG, He S, Xu ZG, et al. Endoscopic diagnosis of laryngeal cancer and precancerous lesions by narrow band imaging. J Laryngol Otol. 2011;125(3):288‐296. [DOI] [PubMed] [Google Scholar]
- 21. Rzepakowska A, Sielska‐Badurek E, Cruz R, Sobol M, Osuch‐Wójcikiewicz E, Niemczyk K. Narrow band imaging versus laryngovideostroboscopy in precancerous and malignant vocal fold lesions. Head Neck. 2018;40(5):927‐936. [DOI] [PubMed] [Google Scholar]


