Abstract
Objectives
To investigate the relationship between auditory pathway function and cochlear size in deaf children with a radiologically normal inner ear or Mondini malformation.
Methods
Thirty‐five deaf children without inner ear malformations (IEMs) and forty cases with Mondini malformation were included in this study. The electrically evoked auditory brainstem responses (EABRs) evoked by electrical stimulation at the round window niche (RWN) and round window membrane (RWM) were recorded during cochlear implantation (CI) surgery. The anatomical parameters of the cochlea were assessed by high‐resolution computed tomography and OTOPLAN 3‐D construction software. Correlations between EABRs and cochlear sizes were analyzed.
Results
The EABR thresholds and/or latencies were negatively correlated with the basal cochlear diameter, cochlear width and/or cochlear duct length in both patients without IEMs and those with Mondini malformation.
Conclusion
The physiological function of the peripheral auditory system depends on the anatomical structure of the cochlea to an extent. A larger cochlear size appears to be associated with better auditory conduction function. Our findings may be beneficial to selection of the proper electrode type and prediction of postoperative auditory rehabilitation.
Level of Evidence
Level 4.
Keywords: auditory pathway function, cochlear implantation, cochlear size, electrically evoked auditory brainstem response, Mondini malformation
Relationships between auditory pathway function and cochlear size were assessed in deaf children. EABR thresholds and latencies were negatively correlated with cochlear sizes. A larger cochlear size appears to be associated with better auditory conduction function.
1. INTRODUCTION
While cochlear implantation (CI) can help deaf patients to reconstruct hearing abilities, 1 CI outcomes still vary greatly. Variations in auditory functions after CI may, at least partially, result from variations in cochlear structures. Evidence has shown that cochlear morphology can affect the insertion of the CI electrode array. 2 Pelliccia et al. found that the degree of deafness is related to the height and basal turn lumen diameter of the cochlea. 3 They emphasized that selecting a proper length of a straight electrode according to the cochlear size may be important for obtaining an ideal insertion depth angle and further preserving most residual hearing. Ketterer et al. also found that the electrode array is more likely to dislocate in the cochlea with a smaller size. 4 Cochlear size appears to be an important factor in determining CI outcomes but not necessarily by affecting the electrode insertion depth or degree. For example, Kuthubutheen et al. showed that the speech performance after CI was correlated with cochlear duct length (CDL) and cochlear diameter, but not with the degree of insertion or the number of channels inserted. 5 Therefore, there may be a relationship between auditory pathway function before CI and cochlear size itself, but relevant evidence is still lacking.
There are large variations in the size and shape of the human cochlea with inner ear malformations (IEMs), 6 which causes a great challenge for implant electrode selection and prediction of CI outcomes. Mondini malformation is one of the most common IEMs and is characterized by a cochlea with a defective apical modiolar and always together with a minimally dilated vestibule and an enlarged vestibular aqueduct. 7 Evidence has shown poorer auditory rehabilitation of deaf patients with Mondini malformation at least at an early stage after CI. 8 Our previous study also demonstrated a lower extraction rate of the electrically evoked auditory brainstem response (EABR) in patients with Mondini malformation than in those without IEMs. 9 Whether the auditory pathway function involved in the cochlea with Mondini malformation is related to cochlear size remains unclear.
Therefore, in this study, we investigated the relationship between cochlear size and auditory conduction function in deaf children with no IEMs and those with Mondini malformation. The cochlear anatomical parameters, including the basal cochlear diameter, cochlear width, cochlear height and CDL, were measured by the OTOPLAN software. The OTOPLAN has been used to select electrodes of proper length, 10 assess cochlear structures for far‐advanced otosclerosis candidates, 11 and improve the image quality of the facial nerve. 12 We also recorded the EABRs evoked by electrical stimulation at the round window niche (RWN) and round window membrane (RWM) during CI surgery. The EABR is an objective neurophysiological measure for assessing the function of the auditory pathway up to the level of the brainstem. 13 , 14 We hypothesized that the threshold or latency of the intraoperative EABR would be correlated with the basal cochlear diameter, cochlear width, cochlear height, or CDL, reflecting the effect of cochlear size on auditory pathway function.
2. MATERIALS AND METHODS
2.1. Participants
Seventy‐five children with severe or profound bilateral sensorineural hearing loss (SNHL) who received their first CI in our hospital were recruited for this study. SNHL was confirmed by preoperative tests, including the auditory brainstem response (ABR), 40‐Hz auditory evoked potential, auditory steady‐state response (ASSR), distortion product otoacoustic emission (DPOAE), and acoustic impedance. The inner ear structure was assessed by high‐resolution computed tomography (HRCT) or magnetic resonance imaging (MRI). Of them, 35 patients had no IEMs and 40 patients had Mondini malformation characterized by a cochlea with a defective apical modiolar, a minimally dilated vestibule, and an enlarged vestibular aqueduct (Table 1). Participants with a mental disability, intracranial lesions, or head trauma were excluded from this study. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The protocols and experimental procedures in the present study were reviewed and approved by the Anhui Provincial Hospital Ethics Committee (No. 2019‐KY‐51). Each child's guardians provided an informed consent.
TABLE 1.
Demographic information of patients with no inner ear malformations (IEMs) and Mondini malformation
| Variable | No IEMs | Mondini malformation |
|---|---|---|
| N | 35 | 40 |
| Sex (M/F, N) | 16/19 | 24/16 |
| Age at test (mean ± SD, years) | 4.84 ± 5.61 | 6.16 ± 5.01 |
| Side of test (L/R, N) | 13/22 | 14/26 |
| Onset of deafness (prelingual/postlingual, N) | 31/4 | 29/11 |
Abbreviations: F, female; L, left; M, male; R, right; SD, standard deviation.
2.2. EABR recording and analysis
The EABR was recorded by Neuro‐Audio NET1.0.103.3. (Neurosoft, Ivanovo, Russia) during CI surgery. The recording, reference, and ground electrodes were body surface button electrodes and were placed in the middle of the forehead, 1 cm in front of the tragus of the operative ear, and between the eyebrows, respectively. Electrical stimulation was generated by an EMG external electric stimulator (Neurosoft, Ivanovo, Russia). A facial nerve stimulation probe (Medtronic, Minneapolis, MN, USA) was used as the stimulation positive electrode. The tail end of the electrode was exposed with a diameter of 500 μm and the rest of the electrode surface was coated with an insulating coating of parylene. The electrical pulse was the alternating wave with a duration of 100‐μs and a delivery rate of 21 Hz. A needle electrode was placed in the coarse protuberance of the occipital bone on the operative side as the reference electrode for stimulation. All electrode impedances were <3 kΩ.
CI surgery was performed through a mastoidectomy. Posterior tympanotomy was performed through the facial recess. After the RWN was exposed, cis‐atracurium (0.5 mg/kg based on the patient's body weight), a muscle relaxant, was administered to reduce the interference of muscle activity on EABR signals. During the first EABR recording, a stimulation probe was placed on the surface of the RWN. Then, a diamond bur was used to remove the RWN and maximally expose the RWM. We performed a second EABR recording by placing the stimulation probe on the surface of the RWM. The stimulation intensity started from 2.0 mA, and increased or decreased in a step of 0.5 mA followed by a smaller step of 0.1 mA until the wave III (eIII) and wave V (eV) appeared or disappeared. The minimum stimulation intensity eliciting eIII and eV was regarded as the EABR threshold. The maximum stimulation intensity was 5.0 mA. Each EABR test for RWN or RWM stimulation lasted 3–5 min. The EABR signal was filtered online between 0.1 and 3 KHz and averaged from 512 sweeps at each stimulus level, with a time window of 15 ms.
2.3. Cochlear parameters
OTOPLAN software (CAScination AG; Bern, Switzerland) was used to reconstruct accurate views of the cochlear cavity based on the HRCT images and provide cochlear anatomical parameters, including the basal cochlear diameter, cochlear width, cochlear height, and CDL (Figure 1). The basal cochlear diameter indicates the distance between the midpoint of the round window passing through the mid‐modiolus axis and the contralateral wall. The cochlear width and height were measured as a straight line perpendicular to the basal cochlear diameter at the mid‐modiolus and from the basal point to the apical point, respectively. The length of the cochlear lateral wall (LW) was obtained: LW = 2.62A × ln (1.00 + θ/235), where A = basal cochlear diameter and θ = cochlear turn expressed in degrees. The CDL was then calculated using LW: CDL = 0.86 × LW.
FIGURE 1.
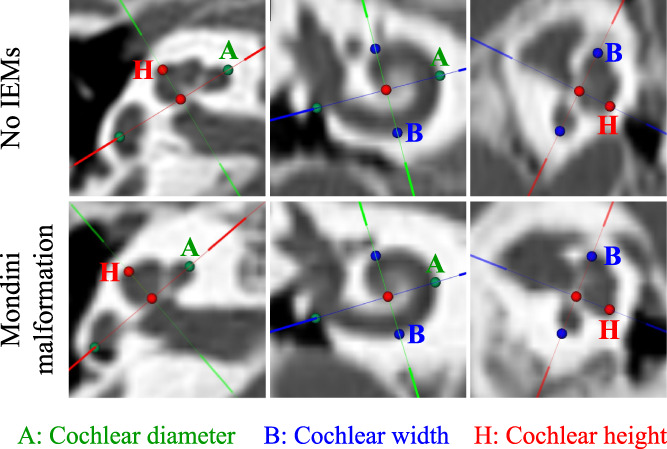
The OTOPLAN software reconstructed cochlear images based on high‐resolution computed tomography from patients with (upper) no inner ear malformations (IEMs) and (lower) Mondini malformation. The A value (green dots) indicates the cochlear diameter, namely the distance between the midpoint of the round window passing through the mid‐modiolus axis and the contralateral wall. The B value (blue dots) indicates the cochlear width measured as a straight line perpendicular to the basal cochlear diameter at the mid‐modiolus. The H value (red dots) indicates the cochlear height measured as a straight line that starting from the basal point to the apical point.
2.4. Statistical analysis
The eIII and eV components were marked by two observers who were experienced in analyzing the EABR waveforms and were blinded to the information of each patient. Markings made by the first observer showed a high correlation with those made by the second observer (r > 0.85). eIII and eV should be repeatable at least at two different stimulation intensities. The statistical analyses were performed by SPSS Statistics V.24 (IBM, Somers, NY, USA). The differences in EABR thresholds, eIII latencies and eV latencies and cochlear sizes between the two groups were analyzed by a nonparametric Mann–Whitney U test because not all data were normally distributed. The correlations between the EABR thresholds and latencies and different cochlear parameters (basal cochlear diameter, cochlear width, cochlear height, and CDL) in each group were further analyzed by a nonparametric Spearman test.
3. RESULTS
3.1. EABRs and Cochlear sizes
Among patients with Mondini malformation, 26 (age range: 0.67–18 years; mean age ± standard deviation [SD]: 6.42 ± 5.24 years) and 27 (age range: 0.67–18 years; mean age ± SD: 6.48 ± 5.12 years) patients showed robust EABRs evoked by electrical stimulation at the RWN and RWM, respectively, and 29 patients (age range: 0.58–18 years; mean age ± SD: 5.53 ± 5.93 years) with no IEMs showed EABRs for both RWN and RWM stimulation. Typical EABR waveforms are shown in Figure 2. No significant difference in EABR thresholds (for RWN: p = .901; for RWM: p = .953), eIII latencies (for RWN: p = .993; for RWM: p = .101), or eV latencies (for RWN: p = .619; for RWM: p = .870) was observed between patients with Mondini malformation and those with no IEMs (Table 2).
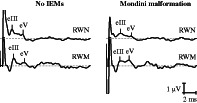
FIGURE 2.
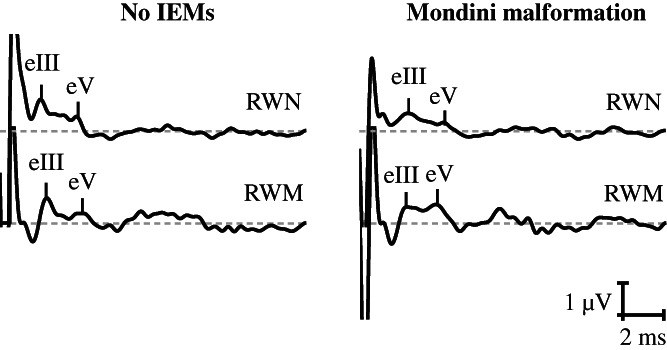
Typical electrically evoked auditory brainstem response (EABR) waveforms of patients (left) with no inner ear malformations (IEMs) and (right) Mondini malformation.
TABLE 2.
Electrically evoked auditory brainstem responses (EABRs) in patients with no inner ear malformations (IEMs) and Mondini malformation
| No IEMs | Mondini Malformation | |
|---|---|---|
| Threshold (μV) | ||
| For RWN | 1.70 ± 0.58 | 1.79 ± 0.65 |
| For RWM | 1.80 ± 0.82 | 1.91 ± 0.81 |
| eIII latency (ms) | ||
| For RWN | 2.11 ± 0.25 | 2.10 ± 0.31 |
| For RWM | 2.11 ± 0.25 | 2.18 ± 0.23 |
| eV latency (ms) | ||
| For RWN | 4.19 ± 0.46 | 4.24 ± 0.48 |
| For RWM | 4.09 ± 0.40 | 4.12 ± 0.49 |
Note: Values are expressed as the mean ± standard deviation.
Abbreviations: eIII, wave III; eV, wave V; RWM, round window membrane; RWN, round window niche.
The cochlear sizes in patients who showed EABRs were also assessed. The cochlear diameters (for RWN: p = .381; for RWM: p = .517) were similar between the two patient groups. However, patients with Mondini malformation showed a smaller cochlear width (for RWN: p = .011; for RWM: p = .007) and CDL (for RWN: p < .001; for RWM: p < .001), but a larger cochlear height (for RWN: p < .001; for RWM: p < .001) than those with no IEMs (Table 3).
TABLE 3.
Cochlear sizes in patients with no inner ear malformations (IEMs) and Mondini malformation
| No IEMs | Mondini Malformation | |
|---|---|---|
| Basal cochlear diameter (mm) | ||
| For RWN | 9.01 ± 0.44 | 8.90 ± 0.42 |
| For RWM | 9.01 ± 0.44 | 8.94 ± 0.47 |
| Cochlear width (mm) | ||
| For RWN | 6.66 ± 0.43 | 6.37 ± 0.43* |
| For RWM | 6.66 ± 0.43 | 6.37 ± 0.41* |
| Cochlear height (mm) | ||
| For RWN | 3.76 ± 0.30 | 4.19 ± 0.30*** |
| For RWM | 3.76 ± 0.30 | 4.21 ± 0.33*** |
| CDL (mm) | ||
| For RWN | 35.1 ± 1.97 | 26.41 ± 1.44*** |
| For RWM | 35.1 ± 1.97 | 26.45 ± 1.44*** |
Note: Values are expressed as the mean ± standard deviation. *p < .05, ***p < .001 versus patients with no IEMs. It should be noted that the cochlear sizes were only assessed for patients with electrically evoked auditory brainstem responses (EABRs). Twenty‐six and 27 patients with Mondini malformation showed EABRs for RWN and RWM stimulation, respectively, and 29 patients with no IEMs showed EABRs for both RWN and RWM stimulation.
Abbreviations: CDL, cochlear duct length; RWM, round window membrane; RWN, round window niche.
3.2. Correlations between EABRs and Cochlear sizes
For patients with no IEMs, the basal cochlear diameter (r = −0.409, p = .028), cochlear width (r = −0.429, p = .020), and CDL (r = −0.501, p = .006) were negatively correlated with the threshold for RWN stimulation (Figure 3). Cochlear width was also negatively correlated with eIII latency for RWM stimulation (r = −0.426, p = .021).
FIGURE 3.
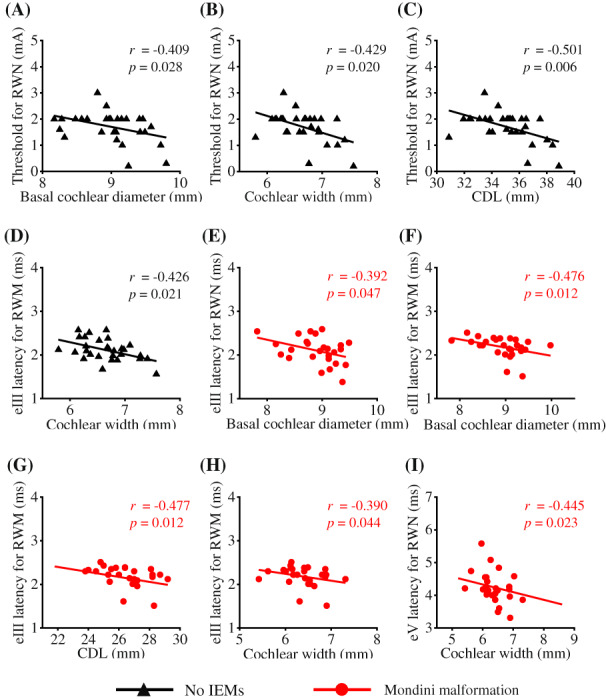
Correlations between electrically evoked auditory brainstem responses (EABRs) and cochlear sizes. (A–C) For patients with no inner ear malformations (IEMs), the basal cochlear diameter, cochlear width, and CDL were negatively correlated with the EABR threshold for RWN stimulation, respectively. (D) The cochlear width was also negatively correlated with the wave III (eIII) latency for RWM stimulation. (E–G) For patients with Mondini malformation, there were negative correlations between the basal cochlear diameter and the eIII latency for RWN and RWM stimulation, and between the CDL and the eIII latency for RWM stimulation. (H–I) The cochlear width was negatively correlated with the eIII latency for RWM stimulation and wave V (eV) latency for RWN stimulation, respectively.
For patients with Mondini malformation, there were negative correlations between the basal cochlear diameter and eIII latency for RWN (r = −0.392, p = .047) and RWM stimulation (r = −0.476, p = .012), and between the CDL and eIII latency for RWM stimulation (r = −0.477, p = .012) (Figure 3). Furthermore, the cochlear width was negatively correlated with the eIII latency for RWM stimulation (r = −0.390, p = .044) and eV latency for RWN stimulation (r = −0.445, p = .023). There was no other significant correlation between the EABR and cochlear parameter.
4. DISCUSSION
In this study, we analyzed the intraoperative EABRs and cochlear sizes of patients with no IEMs and with Mondini malformation and further assessed the relationships between them. The EABR thresholds and/or latencies were negatively correlated with the basal cochlear diameter, cochlear width, and/or CDL in both patient groups, suggesting that a larger cochlear size appears to be associated with better auditory conduction function. Interestingly, compared with patients with no IEMs, those with Mondini malformation showed significantly smaller cochlear width and CDL but similar EABRs. This finding implies that cochlear size is not the only important factor contributing to peripheral auditory system function, especially in patients with IEMs.
The cochlear size (basal cochlear diameter, cochlear width, cochlear height and CDL) in patients with no IEMs varies within certain ranges. 15 , 16 , 17 , 18 Cochlear size may affect electrode insertion (e.g., insertion depth or degree), further resulting in differential auditory outcomes. 2 However, in the present study, auditory conduction function was assessed before CI and was found to be correlated with cochlear size. This finding suggests that a larger cochlear size appears to be associated with better auditory conduction function, but not necessarily by affecting electrode insertion. The number of survival spiral ganglion cells (SGCs) in the cochlea is crucial for the auditory performance. 19 , 20 Furthermore, evidence from deafened animals demonstrates a relationship between the enhanced survival of SGCs and auditory function, as revealed by the higher EABR amplitude. 21 Thus, we infer that the larger cochlea may contain more SGCs, contributing to auditory conduction function in deaf patients.
Correlations between the EABRs and cochlear sizes were also found in deaf children with Mondini malformation, suggesting that larger sizes are beneficial for auditory conduction function not only in the radiologically normal cochlea, but also in the malformed cochlea. The cochlea with Mondini malformation shortens to only one and a half turns because of a deficient interscalar septum or osseous spiral lamina between the middle and apical turns. Interestingly, patients with Mondini malformation have a smaller cochlear width and CDL but similar EABRs compared with those with no IEMs. Previous evidence has shown a great variation of SGC populations (7000–30,000 neurons) in cases with Mondini malformation. 22 Our previous study also suggests that the physiological functions of the peripheral auditory system in patients with Mondini malformation may divide into two opposite extremes, as revealed by either a robust EABR or the absence of the EABR. 9 Therefore, in addition to cochlear size, other factors such as the severity of cochlear malformation may also contribute to auditory conduction function. This study has a limitation. It should be noted that 13 of 40 patients with Mondini malformation had no EABR and were excluded from the correlation analysis. In other words, we mainly assessed the correlations between cochlear sizes and EABRs in patients who had EABRs reflecting less severe deformities. These patients may have similar numbers of SGCs compared to patients with no IEMs, possibly resulting in similar EABRs between the two groups.
5. CONCLUSION
We used the intraoperative EABR to evaluate the physiological function of the auditory pathway up to the level of the brainstem and found negative correlations between the EABR thresholds and latencies and the cochlear sizes in patients with no IEMs and with Mondini malformation. A larger cochlea may contain more SGCs, contributing to better auditory conduction function. Our findings may be beneficial to selection of the proper electrode type and prediction of postoperative auditory rehabilitation.
CONFLICT OF INTEREST STATEMENT
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grants 82201278 and 82271180) and Natural Science Foundation of Anhui Province (Grants 2008085QH429 and 2208085MH231).
Zhu H‐Y, Sun J‐Q, Sun J‐W, Guo X‐T. The effect of cochlear size on electrically evoked auditory brainstem responses in deaf children. Laryngoscope Investigative Otolaryngology. 2023;8(2):532‐537. doi: 10.1002/lio2.1029
Contributor Information
Jia‐Qiang Sun, Email: sunjq0605@126.com.
Jing‐Wu Sun, Email: entsun@ustc.edu.cn.
Xiao‐Tao Guo, Email: gxt2012@mail.ustc.edu.cn.
REFERENCES
- 1. Carlson ML. Cochlear implantation in adults. N Engl J Med. 2020;382:1531‐1542. [DOI] [PubMed] [Google Scholar]
- 2. van der Marel KS, Briaire JJ, Wolterbeek R, Snel‐Bongers J, Verbist BM, Frijns JH. Diversity in cochlear morphology and its influence on cochlear implant electrode position. Ear Hear. 2014;35:e9‐e20. [DOI] [PubMed] [Google Scholar]
- 3. Pelliccia P, Venail F, Bonafe A, et al. Cochlea size variability and implications in clinical practice. Acta Otorhinolaryngol Ital. 2014;34:42‐49. [PMC free article] [PubMed] [Google Scholar]
- 4. Ketterer MC, Aschendorff A, Arndt S, et al. The influence of cochlear morphology on the final electrode array position. Eur Arch Otorhinolaryngol. 2018;275:385‐394. [DOI] [PubMed] [Google Scholar]
- 5. Kuthubutheen J, Grewal A, Symons S, et al. The effect of Cochlear size on Cochlear implantation outcomes. Biomed Res Int. 2019;2019:5849871‐5849878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dhanasingh A. Variations in the size and shape of human Cochlear malformation types. Anat Rec (Hoboken). 2019;302:1792‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sennaroğlu L, Bajin MD. Classification and current Management of Inner ear Malformations. Balkan Med J. 2017;34:397‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen X, Yan F, Liu B, et al. The development of auditory skills in young children with Mondini dysplasia after cochlear implantation. PLoS One. 2014;9:e108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu HY, Chen L, Hou XY, et al. Electrically evoked auditory brainstem responses in deaf patients with Mondini malformation during cochlear implantation. Eur Arch Otorhinolaryngol. 2022;279:4847‐4852. [DOI] [PubMed] [Google Scholar]
- 10. Spiegel JL, Polterauer D, Hempel JM, Canis M, Spiro JE, Muller J. Variation of the cochlear anatomy and cochlea duct length: analysis with a new tablet‐based software. Eur Arch Otorhinolaryngol. 2022;279:1851‐1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lovato A, Marioni G, Gamberini L, Bonora C, Genovese E, de Filippis C. OTOPLAN in Cochlear implantation for far‐advanced Otosclerosis. Otol Neurotol. 2020;41:e1024‐e1028. [DOI] [PubMed] [Google Scholar]
- 12. Ping L, Barazzetti L, Chandran V, et al. Facial nerve image enhancement from CBCT using supervised learning technique. Annu Int Conf IEEE Eng Med Biol Soc. 2015;2015:2964‐2967. [DOI] [PubMed] [Google Scholar]
- 13. Chen L, Zhang JG, Sun JW, Guo XT, Sun JQ. Electrically evoked auditory brainstem responses to electrical stimulation at round window membrane in congenitally deaf children at different ages. Int J Pediatr Otorhinolaryngol. 2021;148:110821. [DOI] [PubMed] [Google Scholar]
- 14. Zhang JG, Chen L, Li P, Sun JW, Guo XT, Sun JQ. Effect of unilateral cochlear implant use on contralateral electrically evoked auditory brainstem responses to round window membrane electrical stimulation. Acta Otolaryngol. 2021;141:588‐593. [DOI] [PubMed] [Google Scholar]
- 15. Sato H, Sando I, Takahashi H. Sexual dimorphism and development of the human cochlea. Computer 3‐D measurement. Acta Otolaryngol. 1991;111:1037‐1040. [DOI] [PubMed] [Google Scholar]
- 16. Meng J, Li S, Zhang F, Li Q, Qin Z. Cochlear size and shape variability and implications in Cochlear implantation surgery. Otol Neurotol. 2016;37:1307‐1313. [DOI] [PubMed] [Google Scholar]
- 17. Martinez‐Monedero R, Niparko JK, Aygun N. Cochlear coiling pattern and orientation differences in cochlear implant candidates. Otol Neurotol. 2011;32:1086‐1093. [DOI] [PubMed] [Google Scholar]
- 18. Wurfel W, Lanfermann H, Lenarz T, Majdani O. Cochlear length determination using cone beam computed tomography in a clinical setting. Hear Res. 2014;316:65‐72. [DOI] [PubMed] [Google Scholar]
- 19. Incesulu A, Nadol JB Jr. Correlation of acoustic threshold measures and spiral ganglion cell survival in severe to profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1998;107:906‐911. [DOI] [PubMed] [Google Scholar]
- 20. Shearer AE, Eppsteiner RW, Frees K, et al. Genetic variants in the peripheral auditory system significantly affect adult cochlear implant performance. Hear Res. 2017;348:138‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agterberg MJ, Versnel H, van Dijk LM, de Groot JC, Klis SF. Enhanced survival of spiral ganglion cells after cessation of treatment with brain‐derived neurotrophic factor in deafened Guinea pigs. J Assoc Res Otolaryngol. 2009;10:355‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmidt JM. Cochlear neuronal populations in developmental defects of the inner ear. Implications for Cochlear Implantation Acta Otolaryngol. 1985;99:14‐20. [DOI] [PubMed] [Google Scholar]


