Abstract
Paraplegia secondary to spinal cord ischemia is a devastating complication in operations on the descending and thoracoabdominal aorta. We hypothesized that the tolerance of the spinal cord to an ischemic insult could be improved by means of regional administration of lidocaine. Thirty-one New Zealand white rabbits were anesthetized and spinal cord ischemia was induced by the placement of clamps both below the left renal vein and above the aortic bifurcation. The animals were divided into 5 groups. Aortic occlusion time was 20 minutes in Group 1 and 30 minutes in all other groups. Groups 1 and 2 functioned as controls. Lidocaine (Group 5) or normal saline solution (Group 3) was infused into the isolated aortic segment after cross-clamping. Group 4 animals received 20% mannitol regionally, before and after reperfusion. Postoperatively, rabbits were classified as either neurologically normal or injured (paralyzed or paretic). Among controls, 20 minutes of aortic occlusion did not produce any neurologic deficit (Group 1: 0/4 injured), while 30 minutes of occlusion resulted in more consistent injury (Group 2: 6/8 injured). Animals that received normal saline (Group 3) or mannitol (Group 4) regionally showed 80% neurologic injury (4/5). Animals treated with the regional lidocaine infusion (Group 5) showed much better neurologic outcomes (7/9 normal: 78%). This superiority of Group 5 over Groups 2, 3, and 4 was significant (P < 0.02). We conclude that regional administration of lidocaine reduced neurologic injury secondary to spinal cord ischemia and reperfusion after aortic occlusion in the rabbit model.
Key words: Animal; aorta, abdominal/surgery; ischemia/prevention & control; lidocaine/therapeutic use; paraplegia/prevention & control; spinal cord injuries/prevention & control; vascular surgical procedures/adverse effects
Spinal cord ischemic injury resulting in paraparesis or paraplegia occurs after all types of procedures on the descending thoracic and thoracoabdominal aorta, but with different frequency. 1 It has been reported that injury rates may range from 10% to 40% in patients with dissections or among those undergoing emergency repairs. 2 Numerous clinical and experimental studies aimed at preventing ischemic spinal cord injury have yielded beneficial techniques that are currently in use in clinical settings. 3–11 Since there is no consensus about spinal cord protection, institutions have adopted widely variant protection techniques, such as reimplantation of major intercostal or lumbar arteries, cerebrospinal fluid drainage, monitoring of the somatosensorial evoked potentials, and the use of shunts, atriofemoral bypass, local or systemic hypothermia, and adjunctive medications.
In common with many other investigators, 7–11 we used a rabbit model for our study of spinal cord ischemia, because of the unique segmental arterial blood supply to the spinal cord from the infrarenal aorta in this animal. We hypothesized that infusing agents with protective properties into the isolated aortic segment that supplies blood to the spinal cord would enable delivery of much higher concentrations of those agents to the cord, than would be feasible via systemic delivery. We chose lidocaine, a widely used local anesthetic and antiarrhythmic agent, as a protective agent because it has a stabilizing effect on neuronal cell membranes and also has some effects on the ischemic brain that resemble those of hypothermia. 12,13 Cocaine-derived anesthetics have been shown to be effective in decreasing the metabolic rate of the neuronal cell. We investigated the effect of regional infusion of lidocaine on the neurologic outcomes of rabbits undergoing spinal cord ischemia secondary to aortic occlusion.
Materials and Methods
Forty New Zealand white rabbits (1.3 to 2.8 kg: mean, 1.7 ± 0.4 kg) were divided into 5 groups. Three animals died before the operation due to anesthesia. Two other animals were given 80 mg/kg regional lidocaine at the outset of the study and died during operation due to severe hypotension and bradycardia. Four additional animals from different groups died of causes unrelated to the specific therapeutic interventions; there were no more deaths in one group than in another. These 9 animals were eliminated from the study.
All 31 animals that remained in the study were anesthetized with intramuscular ketamine (50 mg/kg) and xylazine (5 mg/kg) prior to surgery. One-quarter of this combination was given intravenously as a single dose in the middle of the operation. No animals received blood-pressure or ventilatory support. Hypothermia was not induced and each procedure was performed in the same operating room at ambient temperature. An intravenous catheter (24 gauge) was placed in an ear vein to give preoperative cefazolin (10 mg/kg) and maintenance fluid (0.9% saline solution at a rate of 20 mL/hr). In order to monitor the blood pressure of animals in the lidocaine and control groups, an arterial line was placed in an ear artery through a 24-gauge catheter, and this was connected to a blood-pressure/heart-rate transducer and monitor. Both catheters were removed after completion of the procedure. Each animal underwent laparotomy through a 10-cm midline incision after sterile preparation. Abdominal contents were reflected to the right and the abdominal aorta was isolated and looped distal to the left renal artery and just proximal to the aortic bifurcation. Each rabbit was anticoagulated with heparin 150 U/kg before aortic cross-clamping. Hep-arin was not reversed at the end of the procedure. The aorta was cross-clamped just below the left renal artery and just proximal to the aortic bifurcation by using arterial bulldog clamps, and the aorta was palpated to confirm loss of pulse.
The cross-clamp time was 20 minutes in Group 1 and 30 minutes in the other groups. In control Groups 1 and 2, the aorta was cross-clamped without infusion of any solution. In Groups 3, 4, and 5, a 24-gauge catheter was inserted into each rabbit's aorta distal to the proximal clamp immediately after aortic occlusion. Through this catheter, Group 3 animals received a bolus of 3 mL of normal saline solution in the isolated aortic segment. In Group 4 rabbits, we infused the 1st half of a dose of 0.5 gm/kg mannitol (20% solution) into the aorta before releasing the aortic clamp. After 1 minute of reperfusion, the aorta was reclamped and the 2nd half of the mannitol dose was given as a bolus. We administered the mannitol in 2 stages in order to take advantage of its possible beneficial effects on tissue edema and reperfusion injury. Group 5 animals received a bolus of 40 mg/kg lidocaine (2% Aritmal, 20 mg/mL). Because 2 preliminary animals had not tolerated a bolus of 80 mg/kg lidocaine, we decreased the dose to 40 mg/kg, which was well tolerated by the Group 5 animals.
The aortic catheter was removed and the puncture site was closed with a 7-0 polypropylene suture. The abdomen was closed in 2 layers. Blood pressures and heart rates were recorded at the time of catheter insertion, after cross-clamping, during infusion of saline or lidocaine, at 10, 20, and 30 minutes after cross-clamping, and before removal of the catheter.
At 24 and 48 hours after the operation, the neurologic status of the rabbits was assessed by an independent observer who was blinded to the groups and who assigned grades as follows: 1) normal (able to hop), 2) paretic (unable to hop, but with some lower-extremity movement), and 3) paralyzed (unable to hop, and with no lower-extremity movement). After neurologic evaluation, the animals were administered a lethal injection of pentobarbital. Spinal cords were harvested immediately and stored in 10% formalin solution for 24 hours, before being set in paraffin blocks for sectioning and histologic examination. Four-micron-thick sections of the lower thoracic and lumbar cord were stained with hematoxylin and eosin, Weil (specific for myelin), or Holmes (specific for axon).
This research was performed with the permission of the Department of Experimental Surgery at Ege University Medical School. All animals received humane care in compliance with the animal-welfare regulations of this department and with the “Guide for the Care and Use of Laboratory Animals,” published by the National Institutes of Health.
Statistical Analysis.
Comparisons were made by classifying rabbits as either neurologically normal or injured (paralyzed or paretic). Statistical evaluation was performed using the binomial test. The comparisons of the differences in the blood pressures and heart rates among animals within the same group were performed by the Wilcoxon signed rank test, and among the different groups by the Kruskal-Wallis test. Differences between groups were considered significant at a P value of <0.05.
Results
Experimental results are presented in Table I.
TABLE I. Experimental Results

Controls (Groups 1 and 2).
Twenty minutes of aortic occlusion did not cause any neurologic dysfunction (100% normal). Thirty minutes of occlusion resulted in neurologic injury in 75% of the animals.
Regional Normal Saline (Group 3).
After 30 minutes of occlusion, 80% of the animals in this group had neurologic injury.
Regional Mannitol (Group 4).
After 30 minutes of occlusion, 80% of the animals had neurologic injury.
Regional Lidocaine (Group 5).
Thirty minutes of aortic occlusion resulted in 78% of the animals exhibiting normal neurologic function. This is a statistically significant difference, in comparison with Groups 2, 3, and 4 (P <0.02).
Histologic examination of the lower thoracic and lumbar cords from animals in Groups 2 and 5 was done by a pathologist who did not know from which groups the tissues had been harvested. This examination revealed vacuolization, pyknosis, and dissolution of Nissl bodies, mainly in rabbits with neurologic injury (Fig. 1). However, small areas of vacuolization were seen in the cords of 2 rabbits from the lidocaine group that exhibited no neurologic dysfunction (Fig. 2). Therefore, there was little histologic difference between these 2 groups. This finding has been noted in the rabbit model by other authors. 10
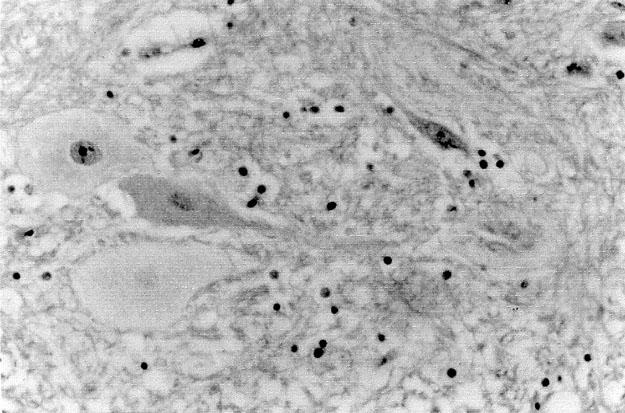
Fig. 1 Dissolution of Nissl bodies and cellular swelling in the lumbar spinal cord of a rabbit that exhibited neurologic dysfunction. (H + E, orig. ×400)

Fig. 2 Small areas of vacuolization in the lumbar spinal cord of a rabbit from the lidocaine group. This rabbit exhibited no neurologic dysfunction. (H + E, orig. ×200)
Both heart rates and systolic blood pressures decreased substantially in Group 5 animals immediately after administration of the lidocaine bolus (P <0.01). This was transient and resolved. Changes in heart rate and systolic blood pressure of Group 5 animals are diagrammed (Fig. 3). Among the groups, the beginning and ending heart rates and systolic blood pressures were not statistically different.

Fig. 3 Changes in heart rate and systolic blood pressures of Group 5 animals (values are expressed as mean).
BPM = beats per minute, Lid = lidocaine infusion; X = cross-clamping
Discussion
Lidocaine, a widely used local anesthetic and antiarrhythmic agent, has a stabilizing effect on neuronal cell membranes due to its action of blocking the sodium/potassium pump and preventing potassium ion fluxes across the cell membrane. 12 Astrup and co-workers 13 have reported that lidocaine has some effects in the ischemic brain that resemble those of hypothermia: additional metabolic inhibition in spite of a flat electroencephalogram, and a delay of the ischemic potassium efflux. It has been reported that lidocaine reversibly inhibits electron transport and uncouples oxidative phosphorylation on porcine brain mitochondria. 14 Continuous infusion of lidocaine has been shown to decrease the infarct size in a focal feline cerebral-ischemia model. 15
To the best of our knowledge, there have been 4 earlier studies on the efficacy of lidocaine and its analogues in preventing ischemic spinal cord injury. Robertson and colleagues 16 administered 160 mg/kg lidocaine through an ear vein in rabbits and occluded the abdominal aorta with a balloon-tip catheter 15 minutes later. In this study, 60% of animals were para-plegic after 25 minutes of ischemia. The authors postulated that this could be secondary to prolonged hypotension after systemic administration. In a por-cine model, Svensson and associates 17 administered, after cross-clamping, a single dose of 120 mL of cold saline containing lidocaine (the concentration is not clear, but according to our estimation it is about 0.5 mg/kg). The authors reported that this solution, which they called a type of “spinoplegia,” reduced the incidence of severe spinal motor-evoked potential amplitude loss, but it apparently did not alter the neurologic deficit rate. Breckwoldt's group 7 showed that intrathecal administration of tetracaine significantly prevented spinal cord injury after 30 minutes of abdominal aortic occlusion in the rabbit model. In contrast to that study, Wakamatsu and co-authors 18 reported that intrathecal tetracaine did not attenuate the glutamate release or the neurologic outcome after 20 minutes of spinal cord ischemia in rabbits.
There are variations among the ischemic threshold periods of aortic occlusion in this rabbit model for spinal ischemia. 7,16,19,20 Consistent paralysis in our study was produced after 30 minutes of infrarenal occlusion. About 20% of control animals in each study, including ours, do tolerate the ischemia and have normal postischemic neurologic function, which could be due to variations in collateral arterial flow to the anterior spinal artery in the rabbit.
In conclusion, regional administration of lidocaine significantly reduced the neurologic injury secondary to spinal cord ischemia and reperfusion after 30 minutes of aortic occlusion in the rabbit model.
Footnotes
Address for reprints: Anil Z. Apaydin, MD, Department of Cardiovascular Surgery, Ege University Medical Faculty Hospital, Bornova – Izmir 35100, Turkey
References
- 1.Mauney MC, Blackbourne LH, Langenburg SE, Buchanan SA, Kron IL, Tribble CG. Prevention of spinal cord injury after repair of the thoracic or thoracoabdominal aorta. Ann Thorac Surg 1995;59:245–52. [DOI] [PubMed]
- 2.Crawford ES, Crawford JL, Safi HJ, Coselli JS, Hess KR, Brooks B, et al. Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate and long-term results of operations in 605 patients. J Vasc Surg 1986;3:389–404. [DOI] [PubMed]
- 3.Acher CW, Wynn M, Hock JR, Popic P, Archibald J, Tur-nipseed WD. Combined use of cerebrospinal fluid drainage and naloxone reduces the risk of paraplegia in thoracoabdominal aneurysm repair. J Vasc Surg 1994;19:236–48. [DOI] [PubMed]
- 4.Cunningham JN Jr, Laschinger JC, Spencer FC. Monitoring of somatosensory evoked potentials during surgical procedures on the thoracoabdominal aorta. IV. Clinical observations and results. J Thorac Cardivasc Surg 1987;94: 275–85. [PubMed]
- 5.Safi HJ, Miller CC 3rd, Carr C, Iliopoulos DC, Dorsay DA, Baldwin JC. Importance of intercostal artery reattachment during thoracoabdominal aortic aneurysm repair. J Vasc Surg 1998;27:58–68. [DOI] [PubMed]
- 6.Svensson LG, Grum DF, Bednarski M, Cosgrove DM 3rd, Loop FD. Appraisal of cerebrospinal fluid alterations during aortic surgery with intrathecal papaverine administration and cerebrospinal fluid drainage. J Vasc Surg 1990;11: 423–9. [DOI] [PubMed]
- 7.Breckwoldt WL, Genco CM, Connolly RJ, Cleveland RJ, Diehl JT. Spinal cord protection during aortic occlusion: efficacy of intrathecal tetracaine. Ann Thorac Surg 1991;51: 959–63. [DOI] [PubMed]
- 8.Moore WM Jr, Hollier LH. The influence of severity of spinal cord ischemia in the etiology of delayed-onset paraplegia. Ann Surg 1991;213:427–32. [DOI] [PMC free article] [PubMed]
- 9.Agee JM, Flanagan T, Blackbourne LH, Kron IL, Tribble CG. Reducing postischemic paraplegia using conjugated superoxide dismutase. Ann Thorac Surg 1991;51:911–5. [DOI] [PubMed]
- 10.Seibel PS, Theodore P, Kron IL, Tribble CG. Regional adenosine attenuates postischemic spinal cord injury. J Vasc Surg 1993;18:153–60. [PubMed]
- 11.Herold JA, Kron IL, Langenburg SE, Blackbourne LH, Tribble CG. Complete prevention of postischemic spinal cord injury by means of regional infusion with hypothermic saline and adenosine. J Thorac Cardiovasc Surg 1994; 107:536–42. [PubMed]
- 12.Astrup J, Sorensen PM, Sorensen HR. Inhibition of cerebral oxygen and glucose consumption in the dog by hypothermia, pentobarbital, and lidocaine. Anesthesiology 1981; 55:263–8. [DOI] [PubMed]
- 13.Astrup J, Skovsted P, Gjerris F, Sorensen HR. Increase in extracellular potassium in the brain during circulatory arrest: effects of hypothermia, lidocaine, and thiopental. Anesthesiology 1981;55:256–62. [DOI] [PubMed]
- 14.Haschke RH, Fink BR. Lidocaine effects on brain mitochondrial metabolism in vitro. Anesthesiology 1975;42:737–40. [DOI] [PubMed]
- 15.Shokunbi MT, Gelb AW, Wu XM, Miller DJ. Continuous lidocaine infusion and focal feline cerebral ischemia. Stroke 1990;21:107–11. [DOI] [PubMed]
- 16.Robertson CS, Foltz R, Grossman RG, Goodman JC. Protection against experimental ischemic spinal cord injury. J Neurosurg 1986;64:633–42. [DOI] [PubMed]
- 17.Svensson LG, Crawford ES, Patel V, McLean TR, Jones JW, DeBakey ME. Spinal oxygenation, blood supply localization, cooling, and function with aortic clamping. Ann Thorac Surg 1992;54:74–9. [DOI] [PubMed]
- 18.Wakamatsu H, Matsumoto M, Nakakimura K, Sakabe T. The effects of moderate hypothermia and intrathecal tetracaine on glutamate concentrations of intrathecal dialysate and neurologic and histopathologic outcome in transient spinal cord ischemia in rabbits. Anesth Analg 1999;88(1): 56–62. [DOI] [PubMed]
- 19.Yum SW, Faden AI. Comparison of the neuroprotective effects of the N-methyl-D-aspartate antagonist MK-801 and the opiate-receptor antagonist nalmefene in experimental spinal cord ischemia. Arch Neurol 1990;47:277–81. [DOI] [PubMed]
- 20.Fowl RJ, Patterson RB, Gewirtz RJ, Anderson DK. Protection against postischemic spinal cord injury using a new 21-aminosteroid. J Surg Res 1990;48:597–600. [DOI] [PubMed]


