Abstract
We conducted this retrospective study to compare methods for measuring atrial septal defects and to identify factors affecting echocardiographic measurement of such defects before transcatheter closure with the CardioSEAL™ Septal Occluder. We reviewed the records of patients considered for device placement at our institution from January 1997 to April 1999. Atrial septal defect size was measured by transthoracic and transesophageal echocardiography; the stretched diameter was measured during catheterization by fluoroscopy and transesophageal echocardiography. The stretched-diameter fluoroscopic measurement was used for device size selection. Analysis of variance was used to calculate the effect of size, age, and size-by-age interaction.
Thirty-one patients (3.3 to 72 years of age) underwent transthoracic and transesophageal echocardiography. One patient was excluded from catheterization because of a 25-mm septal defect as indicated by transesophageal echocardiography (our maximum diameter, 15 mm). Thirty patients underwent transcatheter stretched-diameter sizing; 5 were excluded from device implantation because of defects >20 mm by stretched-diameter fluoroscopy (4) or septal length insufficient for device support (1). Implantation was successful in 23/25 patients; 2/23 had a residual shunt. In patients with available results (26/30), the stretched diameter was the same whether measured by stretched-diameter fluoroscopy or transesophageal echocardiography (P=0.007, R square=0.963). Compared with stretched-diameter fluoroscopy, precatheterization transthoracic and transesophageal echocardiography underestimated defect size by a mean of 22% and 13.2%, respectively. When data from those same tests were compared in defects of ≤10 mm and >10 mm, transthoracic and transesophageal echo-cardiography were reliable predictors (P=0.003 and P=0.05, respectively) of stretched-diameter size in defects ≤10 mm.
Key words: Balloon dilatation; device closure; echocardiography; heart septal defects, atrial
Transcatheter occlusion of secundum atrial septal defects (ASDs) has been performed safely and effectively using several different devices. Patients are selected for attempted device implantation on the basis of noninvasive measurement of ASD size and location by transthoracic echocardiography (TTE). 1 Despite the increasing use of transcatheter devices for ASD closure, precatheterization echocardiographic predictors of successful closure have not been well defined. The purpose of this retrospective study was to compare the various methods of measuring ASDs and to identify factors affecting the echocardiographic measurement of ASD size before transcatheter closure with the CardioSEAL™ Septal Occluder (Nitinol Medical Technologies, Inc.; Boston, Mass).
Methods
We retrospectively reviewed the records of all patients undergoing cardiac cath-eterization for possible transcatheter CardioSEAL device closure of an isolated secundum ASD at our institution from January 1997 to April 1999. The size of the ASD was the primary determining factor for transcatheter ASD closure. Precatheterization TTEs were performed, including standard 2-dimensional imaging, and color and pulsed Doppler studies from the subxiphoid, apical 4-chamber, and left parasternal views. If the ASD was clearly visible on TTE, the maximum diameter of the ASD was determined. If this diameter was ≤15 mm, the patient was eligible to be taken to the catheterization laboratory for attempted device implantation. In the catheterization laboratory, before insertion of the sizing balloon, a comprehensive transesophageal echocardiographic study (TEE) was performed with a sequence of tomographic sections, as previously described, 2 to provide optimal imaging of the atrial septum, confirm the ASD size, determine the total atrial septal length, and measure the superior and inferior rim of the atrial septum.
If TEE showed the ASD size to be suitable for attempted closure, 1 of 2 sizing balloons was used to determine the stretched diameter of the ASD. (The type of balloon was determined by product availability.) In 70% of patients, a spherical sizing balloon catheter (Medi-Tech, Boston Scientific Corporation; Natick, Mass) was used (Fig. 1). Before balloon insertion, the balloon size was measured with a sterile caliper and specific quantities of saline to ensure that it could be inflated to the proper size during insertion. The balloon was introduced over a wire that had been placed through the ASD into a left pulmonary vein. In the left atrium, the balloon was inflated with increasing quantities of dilute contrast medium and was then pulled toward the right atrium until there was no shunting across the ASD, as evidenced by stretched-diameter fluoroscopy and TEE. In the other 30% of patients, a very compliant balloon catheter (NuMed; Hopkinton, NY) was used (Fig. 2). This balloon was advanced over the wire, centered across the defect, and inflated with dilute contrast medium until the balloon had a waist that was visible on fluoroscopy and there was no shunting across the ASD according to fluoroscopy and TEE.
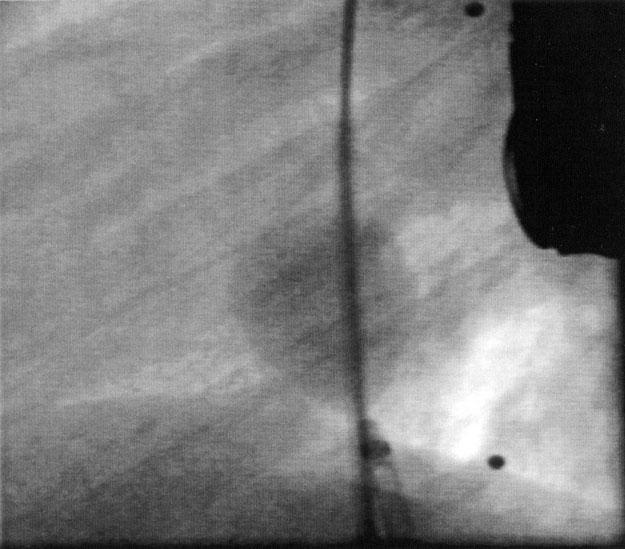
Fig. 1 Angiogram (straight lateral view) shows the atrial septal defect occluded with the Medi-Tech spherical sizing balloon.
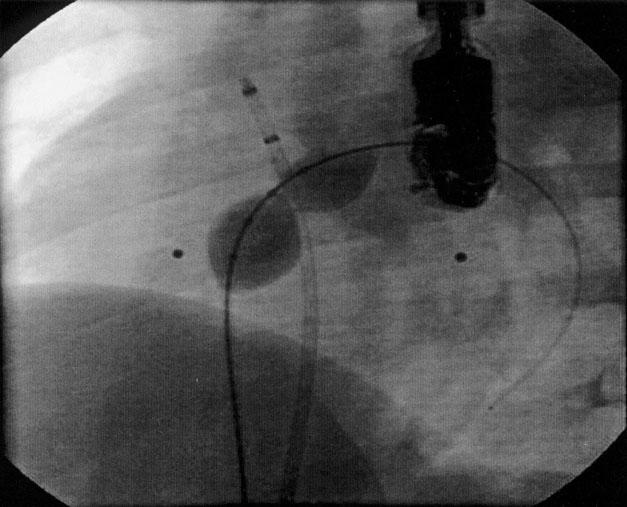
Fig. 2 Angiogram (left anterior oblique view with cranial angulation) shows the atrial septal defect occluded with the NuMed sizing balloon.
When TEE demonstrated no ASD shunting, the stretched diameter of the ASD was defined as the dimension of the balloon at the site of indentation (Fig. 3). The balloon indentation was measured by 2 methods: stretched-diameter fluoroscopy (SD-Fluoro) and stretched-diameter TEE (SD-TEE). The size of the CardioSEAL device was selected on the basis of the SD-Fluoro results: the device size chosen was at least twice the SD-Fluoro measurement (Fig. 4).
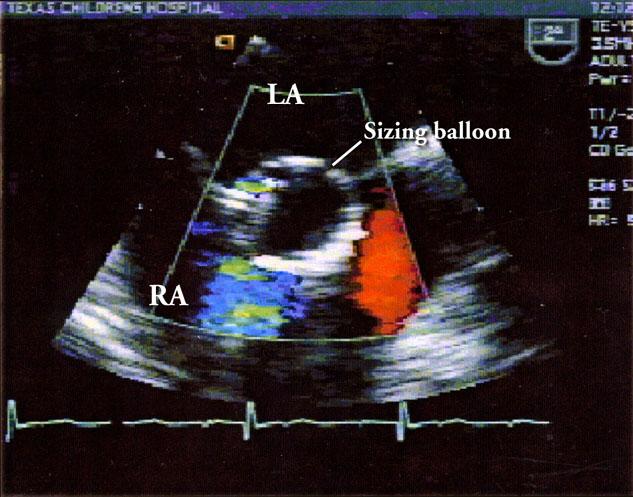
Fig. 3 Transesophageal echocardiogram shows occlusion of the atrial septal defect with the sizing balloon.
LA = left atrium; RA = right atrium
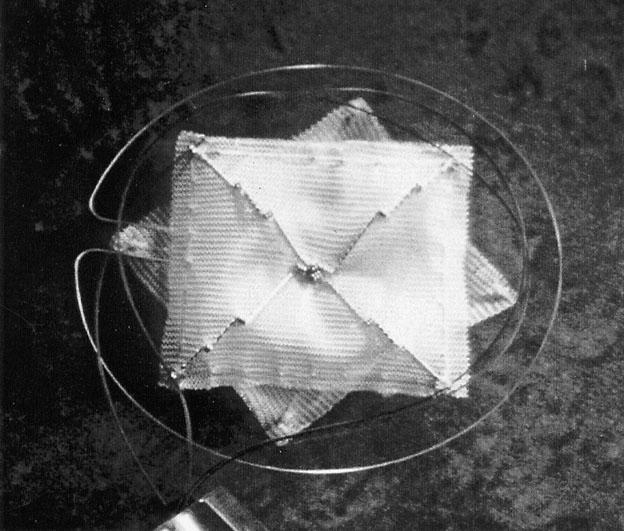
Fig. 4 The CardioSEAL™ Septal Occluder.
Effective ASD closure was defined either by complete defect closure or by a residual Doppler color-flow diameter of <3 mm as determined by TEE in the catheterization laboratory and by TTE the following day. Biplane chest radiographs were reviewed to ascertain the position and integrity of the arms of the device. Arm position was considered acceptable when all of the device arms were on the appropriate side of and in close opposition to the atrial septum. Electrocardiograms were obtained 6 months after ASD closure and were reviewed for the presence of ectopic beats and arrhythmia.
The following formula 3 was used to estimate the stretched diameter of the ASD from the TTE measurement: 1.05 × TTE diameter of ASD in mm + 5.49. The value was compared with the SD-Fluoro measurement obtained in the catheterization laboratory.
Statistical Analysis.
Atrial septal defect size was determined by 4 methods: TTE, TEE, SD-Fluoro, and SD-TEE. The results of these methods were compared for each patient whose results were available. Analysis of variance was used to calculate the effect of size, age, and the interaction between size and age. A P value of <0.05 was considered statistically significant.
Results
Thirty-one patients fulfilled the initial inclusion criteria. Ages ranged from 3.3 to 72 years (median, 12 years). Atrial septal defect sizes ranged from 4.4 to 25 mm (median, 13.5 mm). The patients were divided into 2 groups according to ASD size for the purpose of statistical assessment: 13 had ASDs of ≤10 mm and 18 had ASDs of >10 mm. All 31 patients underwent precatheterization TTE and TEE; however, only 27 of the TTE results were available for review.
Thirty of 31 patients underwent transcatheter stretched diameter (SD) sizing (results were available for only 26 of the SD-TEE patients). The other patient was excluded from catheterization and balloon sizing because the ASD was too large (25 mm) for occlusion with the device available at our institution. Twenty-one patients underwent sizing with the spherical (Medi-Tech) balloon catheter; the NuMed sizing balloon was used in the other 9 patients. Of the 30 patients who underwent balloon sizing, 5 were excluded from device placement: 4 because SD-Fluoro indicated that their ASDs were >20 mm, and 1 because the amount of atrial septum was insufficient for device support as shown by TEE. Device implantation was attempted in 25 patients and was successful in 23 (92%). In 1 of the patients in whom implantation was unsuccessful, the device legs pulled through the septum while the device was still attached to the loader, and the device was withdrawn through the sheath. In the other patient, the device embolized to the left pulmonary artery and was surgically removed. Of the 23 patients in whom the device was implanted successfully, 21 (91%) had effective closure and the other 2 had a residual shunt. All 23 had radiographic evidence of correct arm position. In 1 of those patients, a fractured device arm was discovered at the 12 month follow-up; however, no corrective repair was necessary. There was complete occlusion of the ASD and no embolization in this patient.
Seventeen of the 23 patients had post-implant electrocardiograms available for review; in 16 of these, the results showed no change from the pre-implant evaluation. The other patient developed 2nd-degree atrioventricular block 2 days after device implantation, which resolved spontaneously on the following day.
The formula used to estimate the stretched diameter of ASD from the TTE measurement was accurate in only 6/30 cases (20%).
Comparison of the SD-Fluoro and SD-TEE results showed that these 2 methods yielded equal measurements in all 26 patients for whom records were available (P=0.007, R square=0.963) (Fig. 5). The results of TTE and TEE evaluation were the same in only 13 of the 27 patients (48%) whose records were available. Compared with SD-Fluoro, TTE under-estimated ASD size by an average of 3.8 mm (22%). In defects ≤10 mm, it underestimated the ASD size by an average of 1.8 mm (7.8%), and in defects >10 mm, it underestimated the ASD size by an average of 4.8 mm (17.1%). Compared with SD-Fluoro, TEE underestimated ASD size by an average of 2.2 mm. (13.2%); however, both TTE and TEE measurements were equal to the SD-Fluoro results in the smaller ASDs (≤10 mm; TTE, P=0.003 and TEE, P=0.05) (Figs. 6 and 7). In 21 of 26 patients (81%) for whom SD-TEE results were available, the SD-TEE measurement was larger than the 2-dimensional measurement indicated by TEE before catheterization.
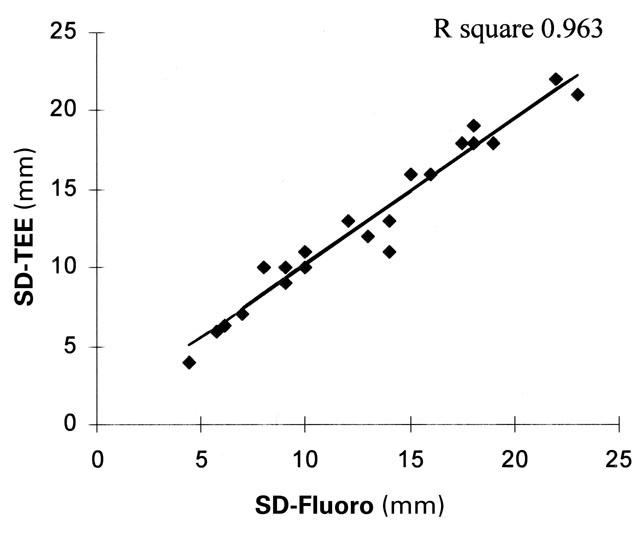
Fig. 5 Linear regression analysis graph, comparing 2 stretched-diameter methods: transesophageal echo-cardiography (SD-TEE) and fluoroscopy (SD-Fluoro).
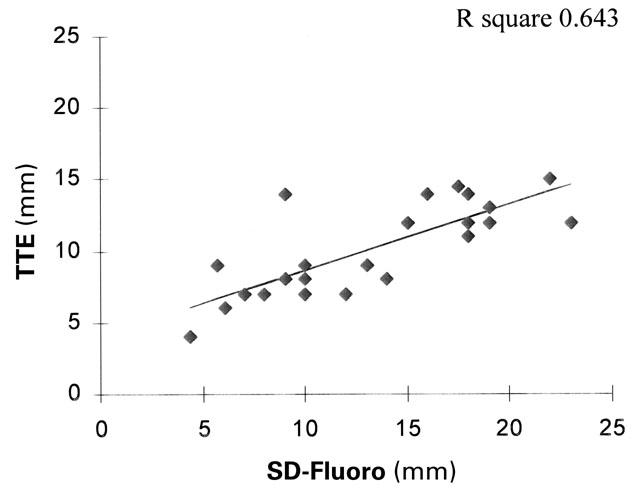
Fig. 6 Linear regression analysis graph, comparing transtho-racic echocardiography (TTE) and stretched-diameter fluoros-copy (SD-Fluoro).
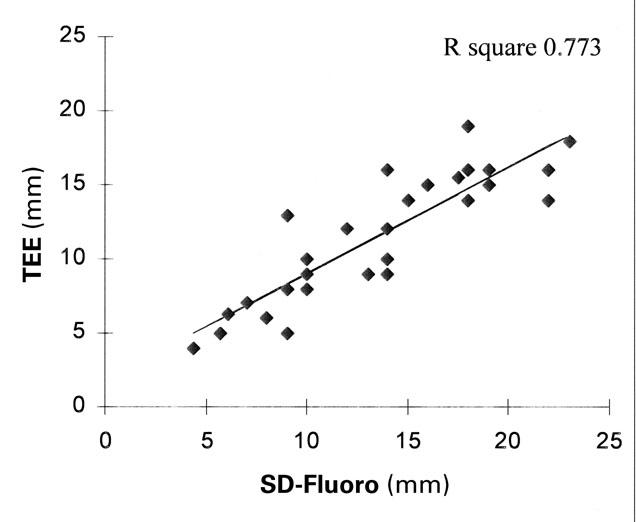
Fig. 7 Linear regression analysis graph, comparing trans-esophageal echocardiography (TEE) and stretched-diameter fluoroscopy (SD-Fluoro).
We found neither age nor age-to-size correlation between the ASD measurements by TTE, TEE, or the stretched-diameter methods.
Discussion
Isolated ASDs constitute approximately 7% of all congenital heart defects. 4 The most common type of ASD is the secundum ASD, which is ideally situated for transcatheter closure. Accumulated experience and improved device design have made transcatheter device closure of secundum ASDs an increasingly viable alternative to surgical repair. 5 Since the 1st description of the use of paired umbrellas to close ASDs by King and colleagues, 6 several devices have been studied. 7,8 Among these are the Clamshell™ double-umbrella device, 9 the “buttoned” double-disk device (Custom Medical Devices; Amarillo, Tex), the Angel Wings™ device (Microvena Corp.; Vadnais, Minn), and the Amplatzer® Septal Occluder (AGA Medical Corp.; Golden Valley, Minn), which have been approved by the FDA for investigational use. 9,10 Approximately 400 Clamshell devices were implanted in a multicenter clinical trial from 1990 to 1991. Despite encouraging results, the device was withdrawn in 1991 because of fractures and embolization of arms of the device. The device has since been redesigned and named the CardioSEAL™ Septal Occluder by Nitinol Medical Technologies, Inc.; clinical trials have been in progress with the CardioSEAL device since 1996.
Surgical closure of secundum ASDs has resulted in low morbidity and mortality rates (<1%). Residual ASD shunting after surgery on long-term follow-up has been reported to be as high as 7.8%, with no difference between primary suture closure and Dac-ron patch closure. 11 In a study evaluating perioperative complications after surgical ASD closure in 232 patients, Galal and coworkers 12 noted severe early complications (pericardial tamponade, renal failure, and sepsis) in 2.5% of children and 8.8% of adults, moderately severe complications (pneumonia and pleural effusion requiring thoracocentesis) in 3.4% of children and 6.1% of adults, and mild complications (atelectasis, gastrointestinal and urinary tract infection, and pleural effusion) in 72.9% of children and 67.5% of adults. Those authors concluded that complications were more common in adults than in children. 12 In our patients who underwent successful CardioSEAL device occlusion, there was a 4% (1/25) incidence of complications (device embolization that required surgical retrieval) and a residual shunt in 8.7% of patients (2/23).
Various atrial arrhythmias, including sinus node dysfunction and atrioventricular conduction defects, have been reported after surgical repair of ASDs. 13,14 In our study, only 1 patient had transient 2nd-degree atrioventricular block, which occurred 2 days after device implantation and resolved spontaneously the next day. All patients in whom a CardioSEAL device was placed were in sinus rhythm at their 6-month follow-up evaluations.
The indications for use of transcatheter devices for ASD device closure, as described in the American Heart Association's 1998 scientific statement, are as follows: 15
-
Conditions for which there is general agreement that ASD devices are appropriate: Patients with secundum ASDs or patients with patent foramen ovale and an associated stroke (or a transient ischemic attack) who meet the following criteria:
ASD diameter less than 20 mm
The presence of sufficient rim of tissue (at least 5 mm) surrounding the defect
Patients with fenestrated Fontan lateral tunnels if temporary balloon occlusion is tolerated 16
Patients with right-to-left atrial shunt and hypoxemia
Conditions for which ASD devices may be indicated: None
-
Conditions for which there is general agreement that ASD devices are inappropriate:
Sinus venosus ASD
Primum ASD
Secundum ASD with significant other forms of congenital heart disease requiring surgical correction
There are 2 main considerations for effective closure of a secundum ASD: the absolute size of the ASD and the amount of supporting rim of the atrial septum. The latter helps determine the stability of the device after implantation. Sizing of the ASD has been attempted with use of angiography, echocar-diography, and balloon inflation. 3,17–20 Prior to trans-catheter ASD occlusion, the size of the ASD is also determined by the stretched-balloon diameter method. 1,3,9,21,22
Successful transcatheter ASD closure depends on accurate selection of the ASD. The best ASD for transcatheter closure is centrally located in the septum with a >5-mm rim of septal tissue and is situated >5 mm from the atrioventricular valves, the coronary sinus, and the pulmonary veins. 23 In a study of pathologic specimens to test the feasibility of ASD occlusion using the Clamshell device, 9 a mean ASD size of 8 × 10 mm was considered favorable and ASDs of >25 mm in diameter with a circumferential rim of <2 mm were judged unsuitable for transcatheter closure. In clinical studies with the Bard Clamshell Occluder 1 (C.R. Bard, USCI Division; Billerica, Mass), patients were selected if they had an ASD diameter <50% of the largest available umbrella size, and supporting atrial septal rims that measured at least 4 mm circumferentially; 32 of 34 patients underwent successful ASD occlusion. In a study of the buttoned ASD device, 24 3 predictive factors were identified for successful ASD occlusion: ASD size, ≤15 mm; ratio of ASD length to atrial septal length, ≤0.35; and ratio of superior and inferior rim length to ASD diameter, ≥0.75. Of the 3 factors, investigators found the ASD size to be the best predictor of successful occlusion. Rosenfeld and co-authors 5 observed that only the ASD diameter predicted effective closure, while other factors (atrial rim size and atrial dimensions) did not. They reported that an ASD diameter of <13 mm on a precatheterization TTE was predictive of effective device closure with the Bard double-umbrella device.
Rao and colleagues 3 used the following formula to estimate the stretched diameter of ASD from the TTE measurement: 1.05 × TTE diameter of ASD in mm + 5.49. In their prospective study, this equation was found to accurately predict the stretched diameter (P <0.001). We used that formula and compared the estimated stretched diameter size with the results obtained by SD-Fluoro in the catheterization laboratory. We found the equation to be accurate in only 20% of cases. However, Rao's group actually pulled the sizing balloon through the septum, rather than just occlude the septum. In the patients whom we studied, the balloons were used for occlusion only.
In our study of 31 patients, 25 patients underwent attempted ASD closure using the CardioSEAL device, and 23 of the 25 closures were successful (92%). Of those 23 patients, 2 had a residual shunt. In 21 of 26 patients for whom results were available (81%), the SD-TEE result was larger than the 2-dimensional measurement indicated by TEE before catheterization. The stretched diameter measured fluoroscopically and the stretched diameter measured by TEE were statistically the same (Fig. 5).
The “true” diameter of the ASD is somewhat enigmatic, especially since the ASD might be oval (from its origin as the fossa ovalis). It is likely that the standard method for sizing—using the stretched diameter—may stretch the ASD, necessitating the use of a larger device or even excluding the patient from transcatheter ASD closure. However, in our experience, the NuMed sizing balloon appears less likely than other such devices to stretch the defect (unpublished observation). Compared with SD-Fluoro, TEE underestimated ASD size by an average of 2.2 mm (13.2%), and TTE underestimated ASD size by an average of 3.8 mm (22%). If the ASD size is ≤10 mm by TTE or TEE, it is highly likely that the results are accurate (P=0.003 and P=0.05, respectively) and, therefore, may be used to select patients for trans-catheter ASD occlusion before catheterization (Figs. 6 and 7). However, if the ASD is >10 mm by TTE, it is likely that TTE will underestimate the size of the defect, necessitating further testing with TEE, which can be performed in the cardiac catheterization laboratory before the catheterization procedure. We found no age or age-to-size correlation between the ASD measurements by TTE, TEE, or the stretched diameter methods.
Conclusions
When determining ASD size for transcatheter device occlusion, the stretched diameter is used for device selection. The stretched diameter of a secundum ASD is equal by SD-TEE and SD-Fluoro. Transthoracic and transesophageal echocardiography are reliable predictors of the stretched diameter (P=0.003 and P=0.05, respectively) if the ASD is ≤10 mm.
Footnotes
Address for reprints: Howaida El-Said, MD, Section of Pediatric Cardiology, Texas Children's Hospital, 6621 Fannin, Houston, TX 77030
References
- 1.Rome JJ, Keane JF, Perry SB, Spevak PJ, Lock JE. Double-umbrella closure of atrial defects. Initial clinical applications. Circulation 1990;82:751–8. [DOI] [PubMed]
- 2.Seward JB, Khandheria BK, Oh JK, Abel MD, Hughes RW Jr, Edwards WD, et al. Transesophageal echocardiography: technique, anatomic correlations, implementation, and clinical applications. Mayo Clin Proc 1988;63:649–80. [DOI] [PubMed]
- 3.Rao PS, Langhough R, Beekman RH, Lloyd TR, Sideris EB. Echocardiographic estimation of balloon-stretched diameter of secundum atrial septal defect for transcatheter occlusion. Am Heart J 1992;124:172–5. [DOI] [PubMed]
- 4.Carlgren L-E. The incidence of congenital heart disease in children born in Gothenburg 1941–1950. Br Heart J 1959; 21:40–50. [DOI] [PMC free article] [PubMed]
- 5.Rosenfeld HM, van der Velde ME, Sanders SP, Colan SD, Parness IA, Lock JE, Spevak PJ. Echocardiographic predictors of candidacy for successful transcatheter atrial septal defect closure. Cathet Cardiovasc Diagn 1995;34:29–34. [DOI] [PubMed]
- 6.King TD, Thompson SL, Steiner C, Mills NL. Secundum atrial septal defects. Nonoperative closure during cardiac catheterization. JAMA 1976;235:2506–9. [PubMed]
- 7.Mills NL, King TD. Nonoperative closure of left-to-right shunts. J Thorac Cardiovasc Surg 1976;72:371–8. [PubMed]
- 8.Sideris EB, Rao PS. Transcatheter closure of atrial septal defects: role of buttoned devices. J Invasive Cardiol 1996;8: 289–96. [PubMed]
- 9.Lock JE, Rome JJ, Davis R, Van Praagh S, Perry SB, Van Praagh R, Keane JF. Transcatheter closure of atrial septal defects. Experimental studies. Circulation 1989;79:1091–9. [DOI] [PubMed]
- 10.Sideris EB, Sideris SE, Fowlkes JP, Ehly RL, Smith JE, Gulde RE. Transvenous atrial septal defect occlusion in piglets with a “buttoned” double-disk device. Circulation 1990;81:312–8. [DOI] [PubMed]
- 11.Pastorek JS, Allen HD, Davis JT. Current outcomes of surgical closure of secundum atrial septal defect. Am J Cardiol 1994;74:75–7. [DOI] [PubMed]
- 12.Galal MO, Wobst A, Halees Z, Hatle L, Schmaltz AA, Khougeer F, et al. Perioperative complications following surgical closure of atrial septal defect type II in 232 patients—a baseline study. Eur Heart J 1994;15:1381–4. [DOI] [PubMed]
- 13.Bolens M, Friedli B. Sinus node function and conduction system before and after surgery for secundum atrial septal defect: an electrophysiologic study. Am J Cardiol 1984;53: 1415–20. [DOI] [PubMed]
- 14.Mycinski C, Fauchier JP, Cosnay P, Chantepie A, Marchand M, Moquet B, et al. Pre- and postoperative study of arrhythmia in atrial septal defects [in French]. Arch Mal Coeur Vaiss 1988;81:685–92. [PubMed]
- 15.Allen HD, Beekman RH 3rd, Garson A Jr, Hijazi ZM, Mullins CE, O'Laughlin MP, Taubert KA. Pediatric therapeutic cardiac catheterization: a statement for healthcare professionals from the Council on Cardiovascular Disease in the Young, American Heart Association [published erratum notice appears in Circulation 1998;97:2375]. Circulation 1998;97:609–25. [DOI] [PubMed]
- 16.Hijazi ZM, Fahey JT, Kleinman CS, Kopf GS, Hellenbrand WE. Hemodynamic evaluation before and after closure of fenestrated Fontan. An acute study of change in oxygen delivery. Circulation 1992;86:196–202. [DOI] [PubMed]
- 17.Forfar JC, Godman MJ. Functional and anatomical correlates in atrial septal defect. An echocardiographic analysis. Br Heart J 1985;54:193–200. [DOI] [PMC free article] [PubMed]
- 18.Rao PS, Langhough R. Relationship of echocardiographic, shunt flow, and angiographic size to the stretched diameter of the atrial septal defect. Am Heart J 1991;122:505–8. [DOI] [PubMed]
- 19.Faletra F, Scarpini S, Moreo A, Ciliberto GR, Austoni P, Donatelli F, Gordini V. Color Doppler echocardiographic assessment of atrial septal defect size: correlation with surgical measurements. J Am Soc Echocardiogr 1991;4:429–34. [DOI] [PubMed]
- 20.Godart F, Rey C, Francart C, Jarrar M, Vaksmann G. Two-dimensional echocardiographic and color Doppler measurements of atrial septal defect, and comparison with balloon- stretched diameter. Am J Cardiol 1993;72:1095–7. [DOI] [PubMed]
- 21.Sideris EB, Sideris SE, Thanopoulos BD, Ehly RL, Fowlkes JP. Transvenous atrial septal defect occlusion by the buttoned device. Am J Cardiol 1990;66:1524–6. [DOI] [PubMed]
- 22.Sideris EB, Rao PS, Lloyd TR, Beekman RH, Worms AM, Hausdorf G, et al. International experience with atrial septal defect occlusion with the buttoned device [abstract]. J Am Coll Cardiol 1993;21(2 Suppl A):260A.8417069
- 23.Hijazi ZM, Marx GR. Transcatheter closure of atrial septal defects and patent foramen ovale: Angel Wings device. In: Beyar R, Keren G, Leon MB, editors. Frontiers in interventional cardiology. London: Martin Dunitz; 1997. p. 443–9.
- 24.Reddy SC, Rao PS, Ewenko J, Koscik R, Wilson AD. Echo-cardiographic predictors of success of catheter closure of atrial septal defect with the buttoned device. Am Heart J 1995;129:76–82. [DOI] [PubMed]


