Abstract
The objective of this study was to determine the prevalence, incidence, and clinical diagnostic accuracy for neuropathologically diagnosed progressive supranuclear palsy (PSP) with data from a longitudinal clinicopathological study using Rainwater criteria to define neuropathological PSP. Of 954 autopsy cases, 101 met Rainwater criteria for the neuropathologic diagnosis of PSP. Of these, 87 were termed clinicopathological PSP as they also had either dementia or parkinsonism or both. The prevalence of clinicopathologically defined PSP subjects in the entire autopsy dataset was 9.1%, while the incidence rate was estimated at 780 per 100 000 persons per year, roughly 50-fold greater than most previous clinically determined PSP incidence estimates. A clinical diagnosis of PSP was 99.6% specific but only 9.2% sensitive based on first examination, and 99.3% specific and 20.7% sensitive based on the final clinical exam. Of the clinicopathologically defined PSP cases, 35/87 (∼40%) had no form of parkinsonism at first assessment, while this decreased to 18/83 (21.7%) at final assessment. Our study confirms a high specificity but low sensitivity for the clinical diagnosis of PSP. The low clinical sensitivity for PSP is likely primarily responsible for previous underestimates of the PSP population incidence rate.
Keywords: Autopsy, Dementia, Diagnosis, Neuropathology, Parkinsonism, Tau, Tufted astrocyte
INTRODUCTION
Progressive supranuclear palsy (PSP) may have been first reported in 1877 by Charcot, but the classic “Steele-Richardson-Olszewski” syndrome was not described until 1964 in a series of 9 cases (1). These patients had gait instability, rigidity, and cognitive changes with classic supranuclear downgaze palsy and neurofibrillary tangles in selective brain regions at autopsy. Hauw et al (2) and Litvan et al (3) developed and subsequently tested the National Institute of Neurological Disorders and Stroke (NINDS) neuropathologic criteria for PSP on 62 postmortem cases. The criteria outlined 3 pathologic distinctions: typical PSP, atypical PSP, and combined PSP, However, there was difficulty in both clinically and pathologically differentiating typical from atypical PSP (3). The NINDS, together with the Society for PSP (NINDS-SPSP) held an International Workshop in 1996 where a new set of clinical research criteria were developed to improve the specificity and sensitivity of the clinical diagnosis of PSP (4). The criteria defined clinically “possible” PSP and clinically “probable” PSP, outlining mandatory exclusion and supportive criteria, while leaving the diagnosis of “definite” PSP to histopathologic confirmation (4). The NINDS-SPSP criteria have subsequently been shown to have high specificity, over 90% but have lacked sensitivity, particularly at first examination, with a median of only 24% from multiple autopsy-validated studies (5–12). Unexplained falls and supranuclear gaze palsy have been the most useful predictive factors for making the diagnosis but may be absent or late clinical findings (13). Thus, making the clinical diagnosis of PSP, particularly early in the clinical course remains problematic if patients lack the cardinal eye findings and early falls or present with a variant PSP syndrome.
To address these diagnostic deficiencies, the International Parkinson and Movement Disorder Society (MDS) formed a PSP Study Group charged with providing an evidence-and consensus-based revision of the NINDS-SPSP criteria. Specific goals were to improve the sensitivity for early and variant PSP presentations while maintaining high specificity versus other forms of parkinsonism. The new criteria were published in 2017 and there has been one autopsy study that has tested the new criteria since then (14). In a study involving 129 subjects, Ali et al (15) calculated sensitivity and specificity of the new MDS-PSP criteria, finding that, as compared to the NINDS-SPSP criteria, the MDS criteria had improved sensitivity, to 47%, but reduced specificity, at 82%.
The poor clinical sensitivity for autopsy-confirmed PSP suggests that published incidence and prevalence rates for PSP, which are based on clinical diagnosis, are likely underestimates. The incidence rate for clinically ascertained PSP is estimated to be <1 to 5 per 100 000 in the United States, with an increased rate of 14.7 per 100 000 in those aged 80–99 (16–18). Our previously published clinicopathological outcomes from a prospective community-based autopsy series revealed an unexpectedly high incidence of PSP; 8 of 119 initially unimpaired subjects (6.7%) progressed to PSP pathology at autopsy (19). Two large, unselected autopsy studies in Japan found 4% of 324 consecutive autopsied cases and 2.9% of 998 cases had PSP pathology, respectively, well above historical prevalence reports from clinical studies (20,21). A similar observation has been reported in a European community-based cohort (22). These large autopsy studies also indicated that typical PSP pathology exists in some clinically normal elderly subjects (12,19).
Despite intervening notable discoveries that completely revolutionized the diagnostic approach, consensus neuropathologic criteria for PSP were not updated since the 1994 NINDS criteria. The use of “enhanced” silver stains such as the Gallyas method (23), as well as the application of immunohistochemical methods for abnormally phosphorylated tau protein (24–27) allowed not only improved detection of PSP neurofibrillary tangles but also of previously unsuspected but characteristic astrocytic morphologies that clearly distinguished PSP from Alzheimer disease (AD) and corticobasal degeneration (CBD) (28–30). Recent sponsorship by the Rainwater Charitable Foundation galvanized a project aimed at providing new PSP neuropathologic criteria; these criteria were found to have high inter-rater reliability for diagnosing PSP in comparison with other tauopathies (31). This development in turn provided an incentive to assess both PSP incidence as well as PSP clinical diagnostic accuracy in our longitudinal clinicopathological study, the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND) and Brain and Body Donation Program (BBDP) (32,33).
MATERIALS AND METHODS
Research subjects
This study was approved by Banner Sun Health Research Institute’s designated Institutional Review Boards. All subjects completed informed consent and were enrolled in the AZSAND/BBDP at Banner Sun Health Research Institute (32,33). Most subjects are recruited directly from the surrounding communities through public speaking events, media reports, and monthly public tours of the Institute. Community neurologists refer additional subjects. Over the history of the program, recruitment has been directed at independently living retired people residing in Maricopa County, Arizona, especially the Sun Cities. As a result of this, and reflective of the general population characteristics as obtained from US census data, the demographics of the population are largely Caucasian, middle- to high-income individuals. In addition to neurological controls, subjects with dementia and parkinsonism are also recruited.
Clinical assessments
Research-dedicated physical, neurological, cognitive, and movement examinations have been previously described (32,33). All subjects completed at least one research visit; most completed annual visits. Standardized neuropsychological test batteries included global testing as well as testing of specific cognitive domains. For each subject, a subspecialist cognitive-behavioral neurologist performed a comprehensive evaluation and, subsequently, a cognitive diagnosis was assigned at a consensus conference attended by neuropsychologists, neurologists, and cognitive neurology subspecialists. Additionally, all subjects received an annual standardized movement disorders examination, including the Unified Parkinson’s Disease Rating Scale ([UPDRS], original version) and a clinical diagnosis by movement disorders fellowship-trained neurologists (E.D.D.-D., C.H.A., S.H.M., and H.A.S.). For this study, we examined clinical diagnoses given at the first and last examination visit as well as the final clinical diagnosis which is made after death at a consensus conference, by review of all prior clinical assessments as well as review of the private medical records, prior to availability of the neuropathological examination results. As the majority of the subjects were clinically evaluated and autopsied before the 2017 MDS-PSP criteria were published (14), to provide continuity over time, clinical PSP was defined according to the NINDS-SPSP criteria (4). Incidental PSP (iPSP) was defined as cases meeting a neuropathological diagnosis for PSP without a clinical diagnosis (without dementia and without parkinsonism). Other definitions for probable Parkinson disease (ProbPD), possible Parkinson disease (PossPD), parkinsonism not otherwise specified (ParkNOS), and multiple system atrophy (MSA) can be found in our previous publication (34). Fall data were assessed from outside medical records in addition to the database UPDRS Part II scores.
Case selection
For the estimation of PSP clinical diagnostic accuracy, as well as an estimation of the prevalence of an autopsy diagnosis of PSP, all subjects who had a complete neuropathological examination between January 1997 and May 2021, as well as at least one complete set of AZSAND/BBDP clinical assessments with both a final clinical diagnosis (considering all clinical data available before autopsy results were available), and a final clinicopathological diagnosis (with autopsy results considered) were included. Because our program has had a recruitment bias favoring AD and Parkinson disease (PD) subjects, we also examined a separate dataset defined as all subjects who were found to be cognitively unimpaired and without parkinsonism or any other defined neurodegenerative disease at their first research assessment, effectively eliminating diagnosis-based selection bias for this subset.
Tissue processing and neuropathologic diagnosis
Autopsies were performed by AZSAND/BBDP. Details regarding the clinical and neuropathological methods have previously been published (32,33). All neuropathological examinations were performed by the same neuropathologist (T.G.B.), blinded to clinical findings. For PSP, the recently published Rainwater neuropathologic criteria were used (31), requiring neurofibrillary tangles or pretangles in 2 of 3 regions (substantia nigra, subthalamic nucleus, globus pallidus) and tufted astrocytes in 1 of 2 regions (peri-Rolandic cortices, putamen). T.G.B. was 1 of 14 neuropathologists participating in the Rainwater criteria evaluation study. The criteria showed high sensitivity (0.97) and specificity (0.91), as well as very high inter-rater reliability for differentiating PSP from other tauopathies (Fleiss kappa 0.826). Semiquantitative average density scores (0–3) were assigned, for tufted astrocytes and neuronal tangles in prefrontal cortex (large-format sections comprising superior, middle, and inferior frontal gyri at the coronal level of the genu of the corpus callosum) and striatum (putamen and/or caudate nucleus), while additional density estimates were obtained for neuronal tangles in the globus pallidus, subthalamic nucleus, and substantia nigra. Subjects received a clinicopathological diagnosis of PD if they had 2 or more of the 3 cardinal clinical signs as well as Lewy bodies and pigmented neuron loss in the substantia nigra (35). Dementia with Lewy bodies (DLB) was distinguished from PD with dementia according to consensus criteria published by the DLB Consortium (36); the diagnosis of DLB was assigned if dementia was diagnosed prior to or within 1 year of the onset of parkinsonism and if the distribution and density of Lewy body-type pathology met “intermediate” or “high” criteria. Other diagnoses followed previously published guidelines (32,33).
Tissue processing methods have been previously described (33). Briefly, the cerebrum was cut in the coronal plane at the time of brain removal into 1 cm thick slices and then divided into left and right halves. The slices from the right half were frozen between slabs of dry ice, while the slices from the left half were fixed by immersion in neutral-buffered 4% formaldehyde for 48 hours at 4°C. Formaldehyde-fixed paraffin-embedded sections were stained with hematoxylin and eosin (H&E) and immunohistochemical stains for phosphorylated α-synuclein and phosphorylated TDP-43, while large-format, 40- to 80-µm-thick formaldehyde-fixed sections were stained for H&E as well as amyloid plaques, neurofibrillary tangles, tufted astrocytes, and other features using Gallyas silver, Thioflavin-S, and Campbell-Switzer silver methods (33,37,38). An initial set of 20 PSP cases were stained for pathological tau protein with both immunohistochemical (AT8 monoclonal antibody, Cat. MN1020, ThermoFisher, Waltham, MA) and Gallyas stains. As the results were qualitatively identical, the Gallyas stain alone was used for subsequent PSP cases and for the semi-quantitative tau pathology assessments. The Gallyas and Campbell-Switzer methods are well suited for differentiating non-AD tauopathies, as they give a sharper image of glial tauopathies than immunohistochemical methods (38). Additionally, the thick sections enable a near 3-dimensional depiction of the microscopic morphologies that is not possible with the conventional, much-thinner paraffin sections (39,40). The Rainwater PSP study found the Gallyas silver stain to be practically equivalent to immunohistochemical stains for phosphorylated tau protein (31). These methods are also well suited to staging AD, as thioflavin-S is 1 of 2 methods recommended and validated for neuritic plaque density grading by the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) and the National Institute on Aging (41,42). The Braak neurofibrillary tangle staging protocol was originally described using the Gallyas stain and the Thal amyloid plaque staging protocol was originally described using the Campbell-Switzer stain (37) on similarly thick sections (43) and previous studies have shown these to be equivalent to immunohistochemical stains for phosphorylated tau and Aβ peptide, respectively (40,43–47). Subjects with Lewy body-related histopathology were classified according to the Unified Staging System for Lewy Body Disorders (48,49).
Statistical analysis
All analyses were conducted with SAS statistical software (Version 9.3 SAS Institute Inc., Cary, NC) and Sigma Plot 12.0 (Systat Software, Inc., San Jose, CA). Group comparisons were done using the Kruskal-Wallis test, Wilcoxon rank sum test, ANOVA test, Chi-square test, or Fisher exact test as appropriate. The diagnostic performance values of predicting clinicopathologically defined PSP, using the clinical diagnosis of PSP or other specific clinical signs and symptoms, were expressed as sensitivity, positive predictive value (PPV), negative predictive value (NPV), and overall accuracy. The 95% confidence intervals (CIs) for sensitivity and specificity were estimated using the binomial distribution method. Youden index was used to choose the optimal cutoff point for receiver-operating characteristic curves. Logistic regression models were further implemented to investigate the association between the clinical diagnosis of PSP, as well as selected clinical signs, with the clinicopathological diagnosis of PSP. For all tests, 0.05 was chosen as the criterion for statistical significance.
RESULTS
Cases meeting rainwater neuropathologic criteria for PSP
There were 954 autopsy cases with at least one complete set of AZSAND/BBDP neuropsychological, behavioral neurology, and movement disorders neurology assessments including a final clinical consensus diagnosis. Of these, 101 cases met Rainwater criteria for the neuropathologic diagnosis of PSP, and 853 cases did not (Table 2; Supplementary Data Table S1). Table 1 shows, separately for iPSP and clinically affected PSP cases (i.e. having either parkinsonism or dementia), semiquantitative density scores for tufted astrocytes and neuronal tangles in 5 brain regions relevant to PSP, including those areas specified by the Rainwater criteria. The density of both lesion types was greater, in every brain region, in the clinically affected group. For neuronal tangles, the highest densities were in the subthalamic nucleus, followed by the substantia nigra, globus pallidus, striatum, and prefrontal cortex. Density scores for tufted astrocytes were only available, in sufficient subject numbers, in the striatum and prefrontal cortex and their densities were greater in the striatum. Additionally, qualitative observations of tau pathology were made in other classically described affected brain regions, including pontine nuclei and cerebellar dentate nucleus. See Figure 1 for representative photomicrographs.
Table 2.
Prevalence of PSP and other major clinicopathological diagnoses
| Clinicopathological diagnosis | Entire dataset (N = 954; except where noted) |
Normal at first assessment dataset (N = 302; except where noted) |
||
|---|---|---|---|---|
| N (%) | Rank | N (%) | Rank | |
| Alzheimer’s disease (AD) | 402 (42.14%) | 1 | 78 (25.83%) | 1 |
| Vascular dementia (VaD) | 102 (10.69%) | 3 | 32 (10.60%) | 2 |
| Progressive supranuclear palsy (PSP) | 87 (9.1%) | 4 | 18 (5.96%) | 3 |
| Dementia with Lewy bodies (DLB) | 76 (7.97%) | 5 | 12 (3.97%) | 4 |
| Parkinson’s disease (PD) | 205 (21.49%) | 2 | 8 (2.65%) | 5 |
| Hippocampal sclerosis (HS) | 33 (3.46%) | 6 | 7 (2.32%) | 6 |
| Frontotemporal lobar degeneration with TDP-43 | 16/815 (1.96%) | 7 | 3/202 (1.48%) | 7 |
| Motor neuron disease | 4 (0.42%) | 10 | 2 (0.66%) | 8 |
| Corticobasal degeneration | 5 (0.52%) | 9 | 1 (0.33%) | 9 |
| Multiple system atrophy | 9 (0.94%) | 8 | 1 (0.33%) | 10 |
| Pick’s disease | 2 (0.21%) | 11 | 0 | 11 |
| Huntington’s disease | 2 (0.21%) | 11 | 0 | 11 |
| Incidental pathology at autopsy in non-demented subjects without parkinsonism | Entire dataset (N = 278) |
Normal at first assessment dataset (N = 192) |
||
|---|---|---|---|---|
| N (%) | Rank | N (%) | Rank | |
| Neurofibrillary tangles | 274/275 (99.64%) | 1 | 192 (100%) | 1 |
| Amyloid plaques | 211/277 (79.03%) | 2 | 138 (71.87) | 2 |
| Cerebral amyloid angiopathy | 152 (54.68%) | 3 | 111 (57.81%) | 3 |
| Cerebral infarcts | 99 (35.6%) | 4 | 64 (33.33%) | 4 |
| Lewy bodies/alpha-synuclein | 48 (17.27%) | 5 | 48 (15.89%) | 5 |
| Tufted astrocytes | 14 (5.04%) | 6 | 12 (6.25%) | 6 |
Disease categories are not mutually exclusive.
Table 1.
Semiquantitative density scores for tufted astrocytes and neuronal tangles
| Brain region | iPSP | ClinPark PSP | ClinDem PSP | ClinMixed PSP | p-Value | |
|---|---|---|---|---|---|---|
| Tufted astrocytes | Prefrontal cortex | 1.1 (0.8), 11 | 1.4 (0.9), 14 | 2.0 (1.0), 12 | 1.6 (0.9), 31 | 0.143 |
| Striatum | 1.1 (0.7), 13 | 1.8 (0.9), 21 | 1.9 (1.1), 17 | 1.9 (0.8), 41 | 0.035 | |
| Neuronal tangles | Prefrontal cortex | 0.9 (1.1), 14 | 0.6 (0.7), 22 | 1.3 (1.0), 17 | 1.2 (0.9), 42 | 0.036 |
| Striatum | 0.8 (0.5), 12 | 1.1 (0.7), 15 | 1.3 (0.7), 14 | 1.3 (0.5), 34 | 0.035 | |
| Globus pallidus | 0.8 (0.5), 12 | 1.8 (0.9), 15 | 1.5 (0.7), 13 | 1.6 (0.8), 38 | 0.007 | |
| Subthalamic nucleus | 1.3 (0.8), 7 | 2.3 (0.8), 13 | 1.8 (0.9), 9 | 2.4 (0.6), 29 | 0.014 | |
| Substantia nigra | 0.8 (0.7), 9 | 1.7 (0.8), 21 | 2.0 (0.5), 11 | 1.6 (0.9), 38 | 0.011 |
Shown are mean score, standard deviation (in parentheses), and number of subjects with data; Kruskal-Wallis test was used to compare the groups; ClinPark PSP is clinicopathologically defined PSP who had parkinsonism but no dementia at their final clinical diagnosis, ClinDem PSP is clinicopathologically defined PSP who had no parkinsonism but had dementia at their final clinical diagnosis, ClinMixed PSP is clinicopathologically defined PSP who had both parkinsonism and dementia at their final clinical diagnosis.
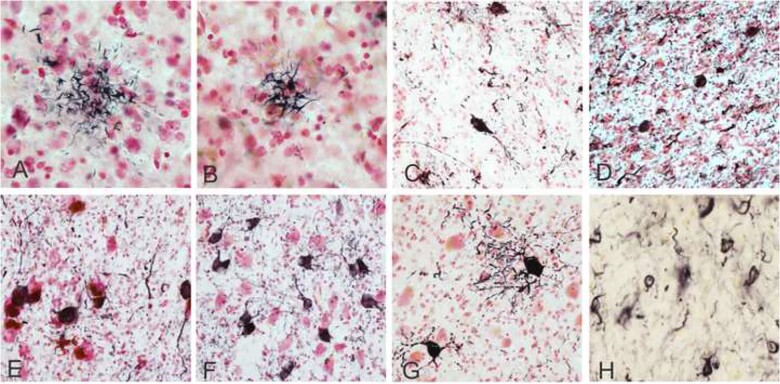
Figure 1.
Photomicrographs depicting PSP pathology. Tufted astrocytes captured on Gallyas silver stain in the middle frontal cortex (A) and in the putamen (B). Immunohistochemical staining for hyperphosphorylated tau (AT8 antibody) showing neuronal tangles in the globus pallidus (C), the subthalamic nucleus (D), the substantia nigra (E), basal pons (F), the dentate nucleus of the cerebellum (G), and coiled bodies in oligodendrocytes in the globus pallidus (H). Positive immunostaining is black and Neutral Red is used as a counterstain. Photomicrographs were taken at 40× magnification (A and B, H) and 20× magnification (C–H).
Prevalence and incidence of PSP pathology
The overall prevalence of PSP pathology was 101/954 (10.6%). Of the neuropathologically defined PSP cases, 87 had been diagnosed with parkinsonism or dementia by the time of their last clinical assessment during life and are here designated as “clinicopathologically defined PSP,” while 14 had neither dementia or parkinsonism and were thereby defined as iPSP. The prevalence of clinicopathologically defined PSP subjects in the entire autopsy dataset was therefore 9.1%, while the prevalence of iPSP was 1.5%. In comparison, of the total 954 autopsied cases, clinicopathological classification identified 402 cases of AD (∼42%), 205 cases of PD (∼21%), 102 cases of vascular dementia (VaD) (∼11%), 76 cases of DLB (∼9%), and lesser percentages of several other neurodegenerative diseases (Table 2). Most cases had more than one major neuropathologic diagnosis (see Supplementary Data Table S1 for complete neuropathologic diagnoses on all cases).
The prevalences of the various clinicopathologically defined conditions were somewhat different in the dataset based on subjects who were initially clinically normal (N = 302; Table 2; Supplementary Data Tables S1 and S2). When the diagnoses were ranked by most common to least common, it is notable that PD ranked as the second most common diagnosis in the whole dataset but only fifth most common in the initially clinically normal dataset, with a prevalence of ∼21% in the entire dataset but only ∼3% in the latter. For PSP, there was not as much difference between datasets, ranking fourth in the entire dataset (9.1%) and third (6.0%) in the initially clinically normal dataset, surpassing PD in the latter. The incidence rate of clinicopathologically defined PSP, for the 302 subjects who were clinically normal at first exam is estimated at 0.0078 per person-year, or 7.8 per 1000 person-years or 780 per 100 000 persons per year.
The percentages of several common neuropathological lesion types present in autopsied subjects who remained non-demented and without parkinsonism are also shown in Table 2, where they are designated as “incidental pathology.” The ranking of these frequencies was identical between the entire dataset and the dataset restricted to subjects who were initially clinically normal. Tufted astrocytes, the pathognomonic lesion of PSP, were present in 5%–7%, while neurofibrillary tangles were present in virtually all subjects, amyloid plaques in 70%–80%, cerebral amyloid angiopathy in 55%–58%, cerebral infarcts in 33%–36%, and Lewy-type α-synucleinopathy in 15%–18%. Age- and gender-specific incidence rates for PSP are shown in Table 3.
Table 3.
Specific incidence of PSP for age and gender
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Age at baseline | No. of patients | Person-years at risk | Incidence (per 100 000) | No. of patients | Person-years at risk | Incidence (per 100 000) |
| 50–79 | 6 | 427.699 | 1402.86 | 2 | 625.517 | 319.74 |
| 80–99 | 3 | 556.454 | 539.13 | 7 | 695.075 | 1007.09 |
Movement and cognitive diagnoses at first and last examinations, comparing PSP and non-PSP groups
Comparison of the PSP, iPSP, and non-PSP groups (Table 4) showed a significant difference in ages at first and final movement examinations and in age at death, with the iPSP group being significantly older. The clinicopathologically defined PSP and non-PSP groups were almost identical in age at all timepoints. The groups were not significantly different in sex distribution.
Table 4.
Demographics and clinical diagnosis at first and last examination by clinicopathologically defined groups with and without PSP pathology
| Non-PSP (N = 853) | ClinPath-PSP (N = 87) | iPSP (N = 14) | Total (N = 954) | p-Value | |
|---|---|---|---|---|---|
| Female, n (%) | 378 (44.3%) | 37 (42.5%) | 8 (57.1%) | 423 (44.3%) | 0.593* |
| Age at first movement exam, mean (SD) | 78.9 (7.8) | 78.7 (8.8) | 83.2 (6.0) | 78.9 (7.9) | 0.100† |
| Age at last movement exam, mean (SD) | 83.6 (7.8) | 83.5 (8.9) | 88.9 (4.5) | 83.7 (7.9) | 0.018 † |
| Death age (years), mean (SD) | 85.5 (8.0) | 85.5 (8.8) | 91.3 (4.5) | 85.6 (8.0) | 0.009 † |
| Time from first movement exam to death (years), mean (SD) | 6.2 (4.9) | 6.3 (5.0) | 7.6 (4.7) | 6.2 (4.9) | 0.370† |
| Time from last movement exam to death (years), mean (SD) | 1.5 (1.5) | 1.5 (1.4) | 1.9 (1.4) | 1.5 (1.5) | 0.202† |
| Number of movement exam, mean (SD) | 4.3 (3.5) | 4.3 (3.4) | 5.4 (4.1) | 4.3 (3.5) | 0.576† |
| Parkinsonism, n (%) | First exam | Last exam | First exam | Last exam | First exam | Last exam | First exam | Last exam | <0.001*,‡ |
|---|---|---|---|---|---|---|---|---|---|
| No | 477 (55.9%) | 440 (51.6%) | 35 (40.2%) | 18 (21.7%) | 14 (100.0%) | 10 (90.9%) | 526 (55.1%) | 468 (49.4%) | <0.001*,§ |
| SuPD-B | 24 (2.8%) | 8 (0.9%) | 1 (1.1%) | 1 (1.2%) | 0 (0.0%) | 0 (0.0%) | 25 (2.6%) | 9 (1.0%) | |
| SuPD-T | 18 (2.1%) | 8 (0.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 18 (1.9%) | 8 (0.8%) | |
| PoPD | 53 (6.2%) | 17 (2.0%) | 10 (11.5%) | 2 (2.4%) | 0 (0.0%) | 0 (0.0%) | 63 (6.6%) | 19 (2.0%) | |
| PrPD | 162 (19.0%) | 187 (21.9%) | 19 (21.8%) | 20 (24.1%) | 0 (0.0%) | 0 (0.0%) | 181 (19.0%) | 207 (21.9%) | |
| ParkNOS | 98 (11.5%) | 158 (18.5%) | 13 (14.9%) | 23 (27.7%) | 0 (0.0%) | 1 (9.1%) | 111 (11.6%) | 182 (19.2%) | |
| PSP | 3 (0.4%) | 6 (0.7%) | 8 (9.2%) | 18 (21.7%) | 0 (0.0%) | 0 (0.0%) | 11 (1.2%) | 24 (2.5%) | |
| DLB | 12 (1.4%) | 24 (2.8%) | 1 (1.1%) | 1 (1.2%) | 0 (0.0%) | 0 (0.0%) | 13 (1.4%) | 25 (2.6%) | |
| MSA-P | 3 (0.4%) | 3 (0.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 3 (0.3%) | 3 (0.3%) | |
| MSA-C | 2 (0.2%) | 1 (0.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.2%) | 1 (0.1%) | |
| MSA-A | 0 (0.0%) | 1 (0.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.1%) | |
| CBD | 1 (0.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.1%) | 0 (0.0%) |
ClinPath PSP, subjects diagnosed with either parkinsonism or dementia as well as meeting Rainwater neuropathology criteria for PSP. iPSP, incidental PSP, without either dementia or parkinsonism.
Chi-square p-value.
Kruskal-Wallis p-value.
p-Value for first parkinsonism exam.
p-Value for last parkinsonism exam.
The elapsed time between first movement examination and death, as well as the total number of movement examinations, were not significantly different between groups. A clinical diagnosis of PSP was made, at the first movement assessment, in only 8/87 (9.2%) of the clinicopathologically defined PSP cases but this increased to 18/87 (20.7%) at the final movement assessment. Only 3/853 (0.4%) of neuropathologically non-PSP subjects were diagnosed with PSP at first assessment, while this increased to 6/853 (0.71%) at the final assessment.
The numbers and percentages of cases clinically diagnosed with graded levels of certainty for parkinsonism and PD, as well as DLB, MSA, and CBD, are given in Table 4. Among the 24 subjects who were clinically diagnosed with PSP at their last exam, 75% (18/24) had PSP at autopsy, while of the 207 cases with clinical probable PD, 9.7% (20/207) had PSP at autopsy and of the 182 subjects with clinical ParkNOS, 12.6% (23/182) had PSP at autopsy. The clinicopathologically defined PSP cases were more likely to have been diagnosed with some form of parkinsonism, as compared to the non-PSP group (∼60% vs ∼44%). Notably, however, 35/87 (∼40%) of the clinicopathologically defined PSP cases had no form of parkinsonism at first assessment while this decreased to 18/83 (21.7%) at the final assessment. At first examination, probable PD (parkinsonism with response to dopaminergic medication) was the most common clinical form of parkinsonism (21.8%) in the clinicopathological PSP group, while at last exam, parkinsonism NOS was most common (27.7%).
Calculated diagnostic performance values for identifying PSP, including sensitivity, specificity, PPV, NPV, and diagnostic accuracy, at first, last, and final clinical assessments, broken down by clinical syndrome, are given in Table 5. Specificity and NPV were extremely high, over 99% and 91%, respectively, at all assessment timepoints. In contrast, sensitivity was very low, ranging from 9.2% at first assessment to 20.7% at final assessment, while PPV was moderate, 72.7% at first assessment and 75.0% at final assessment. Notably, not a single neuropathologically defined PSP case was ever recognized during life as PSP if the clinical syndrome included dementia but lacked parkinsonism (Supplementary Data Table S3).
Table 5.
Clinical diagnostic accuracy for predicting clinicopathologically defined PSP
| PSP | Not PSP | Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|---|---|
| PSP—first movement Dx | |||||||
| Yes | 8 | 3 | 9.2% (4.1%, 17.3%) | 72.7% | 91.5% | 91.3% | |
| No | 79 | 850 | 99.6% (99.0%, 99.9%) | ||||
|
| |||||||
| Path PSP | Path non-PSP | Sensitivity | Specificity | PPV | NPV | Accuracy | |
|
| |||||||
| PSP—last movement Dx | |||||||
| Yes | 14 | 6 | 16.1% (9.1%, 25.5%) | 70.0% | 92.1% | 91.6% | |
| No | 73 | 847 | 99.3% (98.5%, 99.7%) | ||||
|
| |||||||
| Path PSP | Path non-PSP | Sensitivity | Specificity | PPV | NPV | Accuracy | |
|
| |||||||
| PSP—final movement Dx | |||||||
| Yes | 18 | 6 | 20.7% (12.7%, 30.7%) | 75.0% | 92.5% | 92.0% | |
| No | 69 | 847 | 99.3% (98.5%, 99.7%) | ||||
|
| |||||||
| Path PSP | Path non-PSP | Sensitivity | Specificity | PPV | NPV | Accuracy | |
|
| |||||||
| PSP—any movement Dx | |||||||
| Yes | 18 | 9 | 20.7% (12.7%, 30.7%) | 66.7% | 92.4% | 91.7% | |
| No | 69 | 844 | 98.9% (98.0%, 99.5%) | ||||
Analyses of the contributions of specific clinical signs and symptoms, at any exam, toward predicting neuropathologically defined, clinically affected PSP are given in Tables 6 and 7. Shown, with alternate positive cutoff scores of greater than 0 or greater than 1, are the contributions to diagnostic accuracy measures for UPDRS fall score, swallow score, speech motor score, facial expression score, and postural stability score. Shown in Table 6 are the contributions of dichotomous variables Hoehn and Yahr stage, presence or absence of downgaze palsy, square-wave jerks, and clinical diagnosis of possible REM sleep behavior disorder. The most specific clinical signs were downgaze palsy and square-wave jerks, both with ∼98% specificity; these high specificities, however, were not accompanied by similarly high sensitivities (∼15%–18%). UPDRS scores were more specific but less sensitive when using a cutoff score >1 as compared to a cutoff score >0 (Tables 6 and 7, respectively). Fall score, swallow score, and freezing of gait scores >1 were highly specific, all between 82% and 85%, but again with low sensitivities, between 26% and 37%. The most sensitive UPDRS item was a UPDRS II postural stability score >0, at ∼91% sensitivity, but with a low specificity, ∼18% (Table 6).
Table 6.
Accuracy of specific signs and symptoms for predicting clinicopathologically defined PSP
| Path PSP | Path non-PSP | Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|---|---|
| UPDRS II fall score (any exam > 0) | |||||||
| Yes | 54 | 296 | 64.3% (53.1%, 74.4%) | 15.4% | 94.7% | 64.2% | |
| No | 30 | 531 | 64.2% (60.8%, 67.5%) | ||||
| UPDRS II swallow score (any exam > 0) | |||||||
| Yes | 49 | 318 | 57.6% (46.4%, 68.3%) | 13.4% | 93.4% | 61.2% | |
| No | 36 | 510 | 61.6% (58.2%, 64.9%) | ||||
| UPDRS II freeze score (any exam > 0) | |||||||
| Yes | 24 | 171 | 28.6% (19.2%, 39.5%) | 12.3% | 91.6% | 74.7% | |
| No | 60 | 657 | 79.3% (76.4%, 82.1%) | ||||
| UPDRS III speech motor score (any exam > 0) | |||||||
| Yes | 60 | 420 | 69.8% (58.9%, 79.2%) | 12.5% | 93.9% | 50.7% | |
| No | 26 | 398 | 48.7% (45.2%, 52.1%) | ||||
| UPDRS III facial expression score (any exam > 0) | |||||||
| Yes | 63 | 443 | 73.3% (62.6%, 82.2%) | 12.5% | 94.2% | 48.5% | |
| No | 23 | 375 | 45.8% (42.4%, 49.3%) | ||||
| UPDRS III posture stability score (any exam > 0) | |||||||
| Yes | 78 | 671 | 90.7% (82.5%, 95.9%) | 10.4% | 94.7% | 24.6% | |
| No | 8 | 143 | 17.6% (15.0%, 20.4%) | ||||
| Downgaze palsy | |||||||
| Yes | 16 | 20 | 18.4% (10.9%, 28.1%) | 44.4% | 92.1% | 90.2% | |
| No | 71 | 826 | 97.6% (96.4%, 98.6%) | ||||
| Square wave jerks | |||||||
| Yes | 13 | 14 | 14.9% (8.2%, 24.2%) | 48.1% | 91.8% | 90.6% | |
| No | 74 | 833 | 98.3% (97.2%, 99.1%) | ||||
| No RBD diagnosis | |||||||
| Yes | 61 | 606 | 85.9% (75.6%, 93.0%) | 9.1% | 90.4% | 20.1% | |
| No | 10 | 94 | 13.4% (11.0%, 16.2%) | ||||
| Hoehn and Yahr stage ≥2.5* | |||||||
| Yes | 58 | 364 | 66.7% (55.7%, 76.4%) | 13.7% | 94.2% | 57.3% | |
| No | 29 | 469 | 56.3% (52.9%, 59.7%) | ||||
The cutoff was chosen based on Youden index.
Table 7.
Accuracy of specific signs and symptoms for predicting clinicopathologically defined PSP (using >1 as cutoff) (any exam)
| Path PSP | Path non-PSP | Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|---|---|
| UPDRS II fall score (any exam > 1) | |||||||
| Yes | 31 | 126 | 36.9% (26.6%, 48.1%) | 19.7% | 93.0% | 80.3% | |
| No | 53 | 701 | 84.8% (82.1%, 87.1%) | ||||
| UPDRS II swallow score (any exam > 1) | |||||||
| Yes | 29 | 147 | 34.1% (24.2%, 45.2%) | 16.5% | 92.4% | 77.7% | |
| No | 56 | 681 | 82.2% (79.4%, 84.8%) | ||||
| UPDRS II freeze score (any exam > 1) | |||||||
| Yes | 22 | 124 | 26.2% (17.2%, 36.9%) | 15.1% | 91.9% | 79.6% | |
| No | 62 | 704 | 85.0% (82.4%, 87.4%) | ||||
| UPDRS III speech motor score (any exam > 1) | |||||||
| Yes | 48 | 253 | 55.8% (44.7%, 66.5%) | 15.9% | 93.7% | 67.8% | |
| No | 38 | 565 | 69.1% (65.8%, 72.2%) | ||||
| UPDRS III facial expression score (any exam > 1) | |||||||
| Yes | 46 | 252 | 53.5% (42.4%, 64.3%) | 15.4% | 93.4% | 67.7% | |
| No | 40 | 566 | 69.2% (65.9%, 72.3%) | ||||
| UPDRS III posture stability score (any exam > 1) | |||||||
| Yes | 69 | 578 | 80.2% (70.2%, 88.0%) | 10.7% | 93.3% | 33.9% | |
| No | 17 | 236 | 29.0% (25.9%, 32.3%) | ||||
Odds ratios (ORs) for the association of a PSP clinical diagnosis, as well as for the association of specific clinical signs and symptoms, with clinicopathologically defined PSP are given in Table 8. The OR of a clinical PSP diagnosis increased from ∼28 at first exam to ∼37 at final exam. Of individual clinical signs and symptoms, those with the greatest predictive power were again downgaze palsy and square-wave jerks, with ORs of 9.3 and 10.4, respectively. The next highest OR for an individual sign was a UPDRS fall score >1, with an OR of 3.25.
Table 8.
Odds ratios for the association of diagnoses or symptoms and prediction of clinicopathologically defined PSP
| Univariate model |
Multivariable model |
|||||
|---|---|---|---|---|---|---|
| Predictor | Level | Path PSP N, total N | Odds ratio (95% CI) | p-Value | Odds ratio (95% CI) | p-Value |
| PSP diagnosis at any movement Dx | No | 69 (7.6%), 912 | 1 | |||
| Yes | 18 (66.7%), 27 | 24.43 (10.58, 56.43) | <0.001 | |||
| PSP at first movement Dx | No | 79 (8.5%), 928 | 1 | |||
| Yes | 8 (72.7%), 11 | 28.64 (7.45, 110.09) | <0.001 | |||
| PSP at Last movement Dx | No | 73 (7.9%), 919 | 1 | |||
| Yes | 14 (70.0%), 20 | 27.02 (10.08, 72.41) | <0.001 | |||
| PSP at final movement Dx | No | 69 (7.5%), 915 | 1 | |||
| Yes | 18 (75.0%), 24 | 36.78 (14.14, 95.68) | <0.001 | |||
| UPDRS II fall score (any exam > 1) | No | 53 (7.0%), 753 | 1 | 1 | ||
| Yes | 31 (19.7%), 157 | 3.25 (2.01, 5.26) | <0.001 | 2.11 (1.21, 3.68) | 0.009 | |
| UPDRS II swallow score (any exam > 1) | No | 56 (7.6%), 736 | 1 | |||
| Yes | 29 (16.5%), 176 | 2.40 (1.48, 3.88) | <0.001 | |||
| UPDRS II freeze score (any exam > 1) | No | 62 (8.1%), 765 | 1 | |||
| Yes | 22 (15.1%), 146 | 2.01 (1.19, 3.39) | 0.009 | |||
| UPDRS III speech motor score (any exam > 1) | No | 38 (6.3%), 602 | 1 | 1 | ||
| Yes | 48 (15.9%), 301 | 2.82 (1.79, 4.42) | <0.001 | 1.72 (1.02, 2.91) | 0.042 | |
| UPDRS III facial expression score (any exam > 1) | No | 40 (6.6%), 605 | 1 | |||
| Yes | 46 (15.4%), 298 | 2.58 (1.65, 4.04) | <0.001 | |||
| UPDRS III posture stability score (any exam > 1) | No | 17 (6.7%), 253 | 1 | |||
| Yes | 69 (10.7%), 646 | 1.66 (0.96, 2.88) | 0.072 | |||
| Downgaze palsy | No | 71 (7.9%), 896 | 1 | |||
| Yes | 16 (44.4%), 36 | 9.30 (4.62, 18.74) | <0.001 | |||
| Square wave jerks | No | 74 (8.2%), 906 | 1 | 1 | ||
| Yes | 13 (48.1%), 27 | 10.44 (4.73, 23.04) | <0.001 | 5.99 (2.51, 14.34) | <0.001 | |
| RBD diagnosis | Yes | 10 (9.6%), 104 | 1 | |||
| No | 61 (9.2%), 666 | 0.95 (0.47, 1.91) | 0.881 | |||
As falls occurring early in the clinical course of PSP have previously been found to be a characteristic that helps to distinguish between PSP and other causes of parkinsonism, we compared (Table 9) the elapsed time between onset of falls or frequent falls and symptom onset, in subjects clinicopathologically diagnosed with PD or clinicopathologically diagnosed with PSP. The elapsed time intervals were significantly shorter in PSP cases, for both any falls and frequent falls. For any falls, the median interval for PSP cases was 3 years while for PD cases it was 9 years. For frequent falls, the median intervals were 4 and 12 years, respectively. With an optimal interval cutoff of 6 years between fall onset and symptom onset, this gave an OR for identifying clinicopathological PSP of 6.98 (95% CI 3.81 and 12.8) and an AUC of 0.76 (p > 0.001).
Table 9.
Elapsed time between symptom onset and falls, between pathologically diagnosed PSP and clinicopathologically diagnosed PD cases
| PD (n = 188) | PSP (n = 101) | Total (n = 289) | p-Value | |
|---|---|---|---|---|
| Elapsed years between fall onset year and symptom onset year | <0.001* | |||
| Mean (SD), N | 9.9 (7.1), 155 | 3.7 (8.0), 101 | 7.6 (8.0), 244 | |
| Range | −7.0, 34.0 | −27.0, 43.0 | −27.0, 43.0 | |
| Elapsed years between frequent fall onset year and symptom onset year | <0.001* | |||
| Mean (SD), N | 11.9 (6.3), 74 | 5.9 (8.1), 48 | 9.5 (7.7), 122 | |
| Range | −7.0, 27.0 | −5.0, 43.0 | −7.0, 43.0 |
ANOVA F-test p-value.
To determine the possible contribution of comorbid pathologies to the low clinical diagnostic sensitivity for PSP, we compared several types of comorbid neuropathology in the clinicopathologically defined PSP group and the clinically affected, neuropathologically defined PSP group that had not been clinically diagnosed with PSP (Table 10). Comorbid clinicopathological diagnoses of AD, PD, DLB, vascular dementia, hippocampal sclerosis, and argyrophilic grains were all more common (except for hippocampal sclerosis) in undiagnosed PSP but the proportions were not significantly different, although it is notable that PD was almost twice as common as a comorbidity in the undiagnosed group as compared to the diagnosed group (21.5% vs 11.1%). Although the clinicopathological diagnosis of AD was not significantly more common in either group, the Braak neurofibrillary stage was significantly higher in the undiagnosed PSP group (p = 0.032), with 84% at Braak stages IV–VI as compared to 61% for the diagnosed group.
Table 10.
Comparison of comorbid neuropathology in clinically diagnosed and undiagnosed clinicopathologically defined PSP
| Not PSP (N = 69) | PSP (N = 18) | Total (N = 87) | p-Value | |
|---|---|---|---|---|
| AD, n (%) | 27 (41.5%) | 7 (38.9%) | 34 (41.0%) | 1.000* |
| Braak score, n (%) | 0.032 * | |||
| 0 | 1 (1.4%) | 0 (0.0%) | 1 (1.1%) | |
| 1 | 0 (0.0%) | 1 (5.6%) | 1 (1.1%) | |
| 2 | 3 (4.3%) | 4 (22.2%) | 7 (8.0%) | |
| 3 | 7 (10.1%) | 2 (11.1%) | 9 (10.3%) | |
| 4 | 26 (37.7%) | 2 (11.1%) | 28 (32.2%) | |
| 5 | 28 (40.6%) | 8 (44.4%) | 36 (41.4%) | |
| 6 | 4 (5.8%) | 1 (5.6%) | 5 (5.7%) | |
| PD, n (%) | 14 (21.5%) | 2 (11.1%) | 16 (19.3%) | 0.502* |
| DLB, n (%) | 4 (6.2%) | 0 (0.0%) | 4 (4.8%) | 0.572* |
| VaD, n (%) | 6 (9.2%) | 1 (5.6%) | 7 (8.4%) | 1.000* |
| HS, n (%) | 3 (4.6%) | 1 (5.6%) | 4 (4.8%) | 1.000* |
| AG, n (%) | 30 (46.2%) | 6 (33.3%) | 36 (43.4%) | 0.424* |
Fisher Exact p value.
We also considered whether cognitive impairment might contribute to the low PSP diagnostic sensitivity (Table 11). At the first clinical assessment, however, the clinically diagnosed PSP group was much more likely to be cognitively impaired (76.9% vs 45.6%), while at the last assessment the proportions were equivalent (76.9% vs 73.7%). When considering the 4 possible combinations of cognitive impairment and parkinsonism at first and last assessments, the groups with parkinsonism were much more likely to have been diagnosed with PSP (69.2% vs 52.6% at first exam and 92.3% vs 63.1% at final exam). The greatest differential was for the groups with both cognitive impairment and parkinsonism, which were much more likely to have been diagnosed than undiagnosed.
Table 11.
Initial cognitive diagnoses in clinicopathologically defined PSP and incidental PSP (iPSP)
| Clinically diagnosed PSP (n = 13) | Clinically undiagnosed PSP (n = 57) | Non-PSP (n = 686) | Total (n = 756) | p-Value | |
|---|---|---|---|---|---|
| First cognitive exam, n (%) | 0.023* | ||||
| NL | 3 (23.1%) | 31 (54.4%) | 382 (55.7%) | 416 (55.0%) | |
| MCI | 3 (23.1%) | 14 (24.6%) | 94 (13.7%) | 111 (14.7%) | |
| DEM | 7 (53.8%) | 12 (21.1%) | 210 (30.6%) | 229 (30.3%) | |
| Linked clinical and movement diagnosis group at first visit, n (%) | 0.021* | ||||
| Cog normal, no parkinsonism | 2 (15.4%) | 16 (28.1%) | 272 (39.7%) | 290 (38.4%) | |
| Cog normal, with parkinsonism | 1 (7.7%) | 15 (26.3%) | 110 (16.0%) | 126 (16.7%) | |
| Cog MCI or dementia, no parkinsonism | 2 (15.4%) | 11 (19.3%) | 138 (20.1%) | 151 (20.0%) | |
| Cog MCI or dementia, with parkinsonism | 8 (61.5%) | 15 (26.3%) | 166 (24.2%) | 189 (25.0%) | |
| Last cognitive exam, n (%) | 0.391* | ||||
| NL | 3 (23.1%) | 15 (26.3%) | 240 (35.0%) | 258 (34.1%) | |
| MCI | 1 (7.7%) | 11 (19.3%) | 119 (17.3%) | 131 (17.3%) | |
| DEM | 9 (69.2%) | 31 (54.4%) | 327 (47.7%) | 367 (48.5%) | |
| Linked clinical and movement diagnosis group at last visit, n (%) | <0.001* | ||||
| Cog normal, no parkinsonism | 0 (0.0%) | 5 (8.8%) | 183 (26.7%) | 188 (24.9%) | |
| Cog normal, with parkinsonism | 3 (23.1%) | 10 (17.5%) | 57 (8.3%) | 70 (9.3%) | |
| Cog MCI or dementia, no parkinsonism | 1 (7.7%) | 16 (28.1%) | 201 (29.3%) | 218 (28.8%) | |
| Cog MCI or dementia, with parkinsonism | 9 (69.2%) | 26 (45.6%) | 245 (35.7%) | 280 (37.0%) | |
Parkinsonism is defined as anyone who had movement diagnosis of suspected PD, possible PD, probable PD, parkinsonism NOS, PSP, DLB, MSA, and CBD.
Chi-Square p-value.
The iPSP group, by definition, had no subjects with dementia or parkinsonism, but 4 (33%) had mild cognitive impairment (MCI). Combining iPSP with clinicopathologically defined PSP, the MCI subcategorization included 3 cases with simple amnestic MCI and 12 with non-amnestic MCI. Of those clinicopathologically defined PSP cases diagnosed with dementia, 20 were classified as dementia of undetermined etiology while 13 were designated as probable AD, 10 as possible AD, 1 as DLB, 6 as vascular dementia or mixed AD with vascular dementia, 4 as frontotemporal dementia, 3 as due to medical illness, and 1 as consistent with CBD. Comparison of tufted astrocyte density estimates for prefrontal cortex found higher densities in PSP cases with dementia (median scores of 1.75 vs 1.0) but the comparison was not statistically significant.
DISCUSSION
The recent introduction of the Rainwater neuropathological criteria for the postmortem diagnosis of PSP allow an updated assessment of the neuropathologically validated prevalence and incidence of PSP, as well as a reassessment of the diagnostic accuracy of a clinical PSP diagnosis (31). We have here contributed toward both objectives, using more than 20 years of data from our longitudinal clinicopathological study, the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND).
We believe that the Rainwater histopathological criteria for PSP are an improvement over the prior Preliminary NINDS (1994) criteria in the following ways. (1) The Rainwater criteria have taken advantage of the discovery, in the years following the 1994 criteria, of the relative specificity of tufted astrocytes for PSP, while the 1994 criteria only regarded tau-positive astrocytes as “supporting” the diagnosis. The specificity of tufted astrocytes has allowed a loosening of the 1994 criteria with respect to the number of brain regions needing to be sampled. The Rainwater criteria require sampling of only 5 brain regions, substantia nigra, subthalamic nucleus, globus pallidus, peri-Rolandic cortex, and putamen, while for the 1994 criteria, a minimum of 8 brain regions were required to be sampled and stained, including globus pallidus, putamen, caudate nucleus, subthalamic nucleus, midbrain, pons, medulla, and dentate nucleus of the cerebellum, as well as an additional 5 “essential” areas to determine the presence of additional neuropathological entities, including hippocampus, parahippocampal gyrus and motor, frontal and parietal cortices. The extensive sampling of the 1994 criteria has considerable cost for neuropathology laboratories and may have restricted a full histological evaluation at some centers. (2) The Rainwater criteria are simpler in that, for all regions, only 1 or more neurofibrillary tangles or tufted astrocytes must be present, and in only 5 total regions: globus pallidus, subthalamic nucleus, and substantia nigra for neuronal tangles, and peri-Rolandic cortex and putamen for tufted astrocytes. The 1994 criteria required, for “typical PSP,” that at least 2 neurofibrillary tangles be present in at least 3 brainstem regions (globus pallidus, subthalamic nucleus and substantia nigra, or pons) as well as at least one tangle in 3 or more additional regions (striatum, oculomotor complex, medulla, and dentate nucleus). “Atypical” PSP was defined as having a lesser distribution of tangles but still these were required to be present in at least 5 brain regions. (3) The Rainwater criteria have better interobserver agreement and accuracy as compared to a “gold-standard” neuropathologist reading, with a Kappa of 0.84, sensitivity of 94%, and specificity of 96%. With the 1994 criteria, as evaluated by Litvan et al (6), “typical PSP” was correctly diagnosed by a panel of neuropathologists in only 70% of observations, and with a Kappa of only 0.69. (4) At our center, the PSP neuropathological diagnostic criteria had evolved since the 1994 criteria, requiring the presence of tufted astrocytes in at least the striatum, along with the presence of neurofibrillary tangles in at least some of the nuclei required by the 1994, without defining the minimum results required but usually at least equaling the Rainwater criteria minimum. The Rainwater criteria remove the ambiguity about how many regions must show the specific histopathology.
Upon application of the Rainwater criteria to the 103 PSP cases that were neuropathologically diagnosed in the AZSAND/BBDP database, a difficulty was encountered in that peri-Rolandic cortex had not been routinely stained for phosphorylated tau or with the Gallyas stain. The large-format sections used, however, had extensive inclusion of prefrontal cortex and this was used instead of peri-Rolandic cortex. The lack of peri-Rolandic cortex examination may have resulted in fewer cases of neuropathologically diagnosed PSP. Three cases were initially excluded, one for not having any neuronal tangles in the 3 required basal ganglia regions; the case had been diagnosed as PSP solely on the basis of having rare, tufted astrocytes in frontal and parietal cortex. Two were excluded for not having any tufted astrocytes in cortex or basal ganglia but later examination of the written reports found that this was a mistake as both cases had tufted astrocytes present in the prefrontal cortex and/or putamen. Cases were not re-examined microscopically as existing microscopic descriptions and semi-quantification were sufficiently detailed to allow application of the new criteria. Cases that were not previously diagnosed as PSP were not re-examined and therefore it is possible that some of these cases may also have met the more lenient Rainwater criteria. It is possible that the evolution of PSP neuropathological criteria toward greater leniency and a more or less “pathognomonic” status for tufted astrocytes, culminating in the Rainwater criteria, has contributed to the findings of us and other groups, that PSP is much more common than previously thought. Our usage of the Gallyas stain primarily may have decreased our sensitivity for the detection of PSP, relative to immunohistochemistry for phosphorylated tau; however, as there has never been a comprehensive comparison of these stains for the detection of PSP, this is only a conjecture.
This study found neuropathologically defined and clinically symptomatic PSP to be present in 9.1% of a large set of comprehensively assessed subjects that went on to autopsy and a clinicopathological PSP diagnosis. The incidence rate of clinicopathologically defined PSP, estimated from a set of 302 subjects who were initially without either parkinsonism or dementia, is estimated to be 780 per 100 000 per year. These prevalence and incidence figures are both orders of magnitude greater than clinically determined, non-pathologically confirmed PSP rate estimates which range, in the United States, from 5 per 100 000 up to 14.7 per 100 000, the latter rate only for those aged 80–99 (17,18,50–52). One study reported a higher incidence (34.8 per 100 000 for people aged 75–79) but relied on imprecise data from insurance claims for basal ganglia disorders (53). Our increased PSP incidence rate, compared to previous publications, is most probably due to the low clinical sensitivity for PSP, particularly when the clinical diagnosis was done by non-neurologists, as well as the reliance in previous studies on the presence of clinical parkinsonism for PSP ascertainment from clinical data. We have found that parkinsonism is not present, at first clinical presentation, in 40% of subjects later confirmed to have clinicopathological PSP (19). As there is recruitment bias in the general AZSAND population that favors enrollment of subjects likely to have MCI, AD, or PD, our incidence rate is derived from a subset of 302 subjects who were initially cognitively unimpaired and without parkinsonism and is therefore largely free of recruitment bias. Our 6% prevalence rate of clinicopathologically diagnosed PSP in these initially normal subjects closely replicates our previously published figure of 6.7% (19). Supporting our finding that PSP is much more common than previously thought from clinical studies are 2 large, unselected autopsy studies in Japan that have found PSP prevalences of 4% and 2.9% (20,21). When considering common incidental pathology types in subjects who were non-demented and without parkinsonism at death, tufted astrocytes, the pathognomonic PSP lesion, are the sixth most common finding, after neurofibrillary tangles, amyloid plaques, amyloid angiopathy, cerebral infarcts, and Lewy-type α-synucleinopathy (but not considering other miscellaneous age-related pathologies of uncertain clinical significance, such as argyrophilic grains and aging-related tau astrogliopathy).
Our study and others indicate that there is a low sensitivity for the clinical diagnosis of PSP (9,12), whether with the older NINDS criteria (4) or the newer, 2017 MDS-PSP criteria (14,15). Using the older NINDS-PSP clinical criteria, in our study, a clinical diagnosis of PSP was made, at the first movement assessment, in only 8/87 (9.2%) of the neuropathologically defined, clinically affected PSP cases, increasing to 18/87 (20.7%) at the final movement assessment. As in previous studies, specificity and negative predictive value were extremely high, over 99% and 91%; sensitivity was exceptionally low, ranging from 9.2% at first assessment to 20.7% at final assessment; and PPV was moderate, ranging from 72.7% at first assessment to 75.0% at final assessment. It is possible that if we had used the 2017 MDS clinical PSP criteria, we may have found a higher sensitivity, since the only autopsy-validated PSP diagnostic accuracy study to use the new criteria showed an improved sensitivity, at 47% (15). However, in that study, specificity dropped to 82%. We could not use the MDS 2017 criteria because the vast majority of our cases were autopsied before those criteria became available. We did not attempt, as did this previous study, to derive separate diagnostic accuracy figures for different PSP phenotypes; they had found very high accuracy for the Richardson syndrome phenotype but in our study, subjects with the full Richardson syndrome phenotype were a minority of all PSP cases defined clinicopathologically.
Analyses of the contributions of specific clinical signs and symptoms, at any exam, toward predicting neuropathologically defined, clinically affected PSP similarly indicated very high specificity for the classical PSP eye findings, as both downgaze palsy and square-wave jerks had ∼98% specificity but low sensitivities, between ∼15% and 18%. UPDRS fall score, swallow score, and freezing of gait scores >1 were also highly specific, between 82% and 85%, but with low sensitivities, between 26% and 37%. The most sensitive clinical finding other than the eye findings was a UPDRS II postural stability score >0, at ∼91% sensitivity, but again with a low specificity, ∼18%. As previously reported, falls occurring relatively early in the clinical course, especially as compared with PD, are useful in distinguishing the 2 conditions, as a cutoff interval of 6 years between symptom onset and fall onset gave an OR of 7.44 and an AUC of 0.77, when comparing to PD subjects (13).
We found that comorbid severe AD is more common in clinically undiagnosed, neuropathologically defined PSP, and may thus obscure the clinical presentation, leading to a missed PSP diagnosis. A similar situation has been hypothesized to interfere with the clinical diagnosis of DLB in the presence of severe AD (54–57).
Parkinsonism has always been considered the major clinical PSP finding but, in this study, at first examination, parkinsonism was absent in 40% of neuropathologically defined, clinically affected PSP subjects. Cognitive impairment was in fact more common than parkinsonism at first assessment but did not interfere with making a PSP diagnosis, however, not a single neuropathologically defined case of PSP with dementia lacking parkinsonism was ever clinically diagnosed with PSP. While the groups with parkinsonism were much more likely to have been diagnosed with PSP, the greatest differential was for subjects with both cognitive impairment and parkinsonism, which were much more likely to have been diagnosed than undiagnosed. The subtyping of MCI and dementia in PSP subjects illustrates the lack of specific features found, with 80% having non-amnestic MCI and more than half classified as dementia of undetermined etiology. Others have also reported dementia without parkinsonism in neuropathologically defined PSP (58).
The densities of both neuronal tangles and tufted astrocytes were lower in iPSP as compared to clinicopathological PSP, suggesting a threshold density is required for clinically evident disease expression. Additionally, cases with parkinsonism tended to have higher densities of neuronal tangles in basal ganglia areas, while cases with dementia had higher densities of both neuronal tangles and tufted astrocytes in the cortex. Cases with both parkinsonism and dementia had basal ganglia densities that were generally higher than the dementia group without parkinsonism and cortical densities that were higher than the group without dementia. Another group has previously reported greater prefrontal cortex neuronal tangle densities and immunohistochemical phosphorylated tau burden in PSP with dementia, as compared to PSP with Richardson syndrome alone, but did not find a significant difference in the numbers of tufted astrocytes (58).
Poor diagnostic accuracy for PSP has important implications for clinicians as they approach the differential diagnosis of PD, AD, and related neurodegenerative dementias in their patients. It would be especially important, given the lack of clinical accuracy for PSP and the overlap with early parkinsonism, to reassess all patients with PD, particularly within the first few years of symptom onset. Autopsy-validated studies of clinical diagnostic accuracy in early PD indicate that, as compared with patients followed for several years, misdiagnosis is common and in our center’s experience, by far the single most common cause of misdiagnosed PD is PSP (34,59). Our previous work has found that hyposmia can help distinguish PD from PSP (60). In those presenting with parkinsonism, normosmia was associated with PSP rather than PD, with a sensitivity of 93.4% and a specificity of 64.7% (60). Imaging studies, however, are not useful for differentiating PD from PSP (61) and this means that a significant fraction of those with parkinsonism and dopaminergic depletion on imaging will have PSP rather than PD. Recent work with the novel radiotracers 18F-PI-2620, 18F-THK5351, 18F-APN-1607, and others show promise but need further autopsy validation studies of their potential usefulness as biomarkers for PSP (62–65).
The most important finding of this study is that PSP is likely to be much more common than previously estimated. This study reveals a much higher incidence of PSP than found in previously published studies, and, along with other cited studies, highlights the low sensitivity of the clinical diagnosis and hence the importance of identifying specific biomarkers to distinguish PSP from PD and other neurodegenerative diseases. This will be crucial for identifying the large numbers of clinically silent PSP subjects and for the subsequent development of disease-specific therapies. We conclude that the incidence of PSP rivals that of PD and the majority of PSP cases are undetected during life.
Supplementary Material
ACKNOWLEDGMENTS
AZSAND and the Brain and Body Donation Program are supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson's Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer's Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson's Disease Consortium), the Michael J. Fox Foundation for Parkinson’s Research, and Mayo Clinic Foundation. We are extremely grateful to the efforts of the AZ SANDS staff and affiliates, and especially to the study participants, and their study partners, without whom this research would not have been possible.
Contributor Information
Erika D Driver-Dunckley, Department of Neurology, Parkinson’s Disease and Movement Disorders Center, Mayo Clinic, Scottsdale, Arizona, USA.
Nan Zhang, Department of Quantitative Health Sciences, Section of Biostatistics, Mayo Clinic, Scottsdale, Arizona, USA.
Geidy E Serrano, Banner Sun Health Research Institute, Banner Health, Sun City, Arizona, USA.
Nathaniel A Dunckley, Banner Sun Health Research Institute, Banner Health, Sun City, Arizona, USA.
Lucia I Sue, Banner Sun Health Research Institute, Banner Health, Sun City, Arizona, USA.
Holly A Shill, Department of Neurology, Barrow Neurological Institute, Phoenix, Arizona, USA.
Shyamal H Mehta, Department of Neurology, Parkinson’s Disease and Movement Disorders Center, Mayo Clinic, Scottsdale, Arizona, USA.
Christine Belden, Banner Sun Health Research Institute, Banner Health, Sun City, Arizona, USA.
Cecilia Tremblay, Banner Sun Health Research Institute, Banner Health, Sun City, Arizona, USA.
Alireza Atri, Banner Sun Health Research Institute, Banner Health, Sun City, Arizona, USA; Department of Neurology, Center for Mind/Brain Medicine, Brigham & Women’s Hospital & Harvard Medical School, Boston, Massachusetts, USA.
Charles H Adler, Department of Neurology, Parkinson’s Disease and Movement Disorders Center, Mayo Clinic, Scottsdale, Arizona, USA.
Thomas G Beach, Banner Sun Health Research Institute, Banner Health, Sun City, Arizona, USA.
FUNDING
The Arizona Study of Aging and Neurodegenerative Disorders/Brain and Body Donation Program has been supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 and P30AG072980, Arizona Alzheimer’s Disease Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium and ADHS16-162411 to SHM), and the Michael J. Fox Foundation for Parkinson’s Research.
DISCLOSURE/CONFLICT OF INTEREST
H.A.S. reports receiving research support in the past 12 months from Transposon Therapeutics, Saccadous Inc, NINDS/NIH, Jazz Pharmaceuticals, Supernus, Parkinson’s Foundation and Barrow Neurological Foundation and consulting honoraria from AbbVie and the Tremor Research Group. S.H.M. reports consulting relationships with AbbVie and Sunovion in the past 12 months. A.A. reports receiving honoraria or support for consulting; participating in independent data safety monitoring boards; providing educational lectures, programs, and materials; travel, or serving on advisory boards for Acadia, Alzheimer’s Association, Alzheimer’s Disease International, Biogen, Eisai, Lundbeck, Roche/Genentech, Novo Nordisk, and Qynapse. Book royalties from Oxford University Press for a medical book on dementia. Institutional research grant/contract funding from AZ DHS CTR040636, the Foundation for NIH, Washington University St Louis, and Gates Ventures. C.H.A. reports consulting for Avion, Cionic, CND Life Sciences, Jazz Pharmaceuticals, and XW Pharma. The other authors report no additional disclosures or conflicts of interest.
SUPPLEMENTARY DATA
Supplementary Data can be found at academic.oup.com/jnen.
REFERENCES
- 1. Steele JC, Richardson JC, Olszewski J; Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol 1964;10:333–59 [DOI] [PubMed] [Google Scholar]
- 2. Hauw JJ, Daniel SE, Dickson D, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 1994;44:2015–19 [DOI] [PubMed] [Google Scholar]
- 3. Litvan I, Hauw JJ, Bartko JJ, et al. Validity and reliability of the preliminary NINS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol 1996;55:97–105 [DOI] [PubMed] [Google Scholar]
- 4. Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): Report of the NINDS-SPSP International Workshop. Neurology 1996;47:1–9 [DOI] [PubMed] [Google Scholar]
- 5. Josephs KA, Dickson DW.. Diagnostic accuracy of progressive supranuclear palsy in the Society for Progressive Supranuclear Palsy brain bank. Mov Disord 2003;18:1018–26 [DOI] [PubMed] [Google Scholar]
- 6. Litvan I, Agid Y, Jankovic J, et al. Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steel-Richardson-Olszewski syndrome). Neurology 1996;46:922–30 [DOI] [PubMed] [Google Scholar]
- 7. Litvan I, Campbell G, Mangone CA, et al. Which clinical features differentiate progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) from related disorders? A clinicopathological study. Brain 1997;120:65–74 [DOI] [PubMed] [Google Scholar]
- 8. Osaki Y, Ben-Shlomo Y, Lees AJ, et al. Accuracy of clinical diagnosis of progressive supranuclear palsy. Mov Disord 2004;19:181–9 [DOI] [PubMed] [Google Scholar]
- 9. Birdi S, Rajput AH, Fenton M, et al. Progressive supranuclear palsy diagnosis and confounding features: Report on 16 autopsied cases. Mov Disord 2002;17:1255–64 [DOI] [PubMed] [Google Scholar]
- 10. Sakamoto R, Tsuchiya K, Mimura M.. Clinical heterogeneity in progressive supranuclear palsy: Problems of clinical diagnostic criteria of NINDS-SPSP in a retrospective study of seven Japanese autopsy cases. Neuropathology 2010;30:24–35 [DOI] [PubMed] [Google Scholar]
- 11. Respondek G, Roeber S, Kretzschmar H, et al. Accuracy of the National Institute for Neurological Disorders and Stroke/Society for Progressive Supranuclear Palsy and neuroprotection and natural history in Parkinson plus syndromes criteria for the diagnosis of progressive supranuclear palsy. Mov Disord 2013;28:504–9 [DOI] [PubMed] [Google Scholar]
- 12. Evidente VGH, Caviness JN, Sabbagh M, et al. Atypical progressive supranuclear palsy: Clinicopathological correlation in a brain bank program. Abstract. Neurology 2007;68:A48 [Google Scholar]
- 13. Respondek G, Kurz C, Arzberger T, et al. ; for the Movement Disorder Society-Endorsed PSP Study Group. Which ante mortem clinical features predict progressive supranuclear palsy pathology? Mov Disord 2017;32:995–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Höglinger GU, Respondek G, Stamelou M, et al. ; for the Movement Disorder Society-endorsed PSP Study Group. Clinical diagnosis of progressive supranuclear palsy: The Movement Disorder Society criteria. Mov Disord 2017;32:853–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ali F, Martin PR, Botha H, et al. Sensitivity and specificity of diagnostic criteria for progressive supranuclear palsy. Mov Disord 2019;34:1144–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bower JH, Maraganore DM, McDonnell SK, et al. Incidence of progressive supranuclear palsy and multiple system atrophy in Olmsted County, Minnesota, 1976 to 1990. Neurology 1997;49:1284–8 [DOI] [PubMed] [Google Scholar]
- 17. Savica R, Grossardt BR, Bower JH, et al. Incidence and pathology of synucleinopathies and tauopathies related to parkinsonism. JAMA Neurol 2013;70:859–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Swallow DMA, Zheng CS, Counsell CE.. Systematic review of prevalence studies of progressive supranuclear palsy and corticobasal syndrome. Movement Disord Clin Pract 2022;9:604–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dugger BN, Hentz JG, Adler CH, et al. Clinicopathological outcomes of prospectively followed normal elderly brain bank volunteers. J Neuropathol Exp Neurol 2014;73:244–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nogami A, Yamazaki M, Saito Y, et al. Early stage of progressive supranuclear palsy: A neuropathological study of 324 consecutive autopsy cases. J Nippon Med Sch 2015;82:266–73 [DOI] [PubMed] [Google Scholar]
- 21. Yoshida K, Hata Y, Kinoshita K, et al. Incipient progressive supranuclear palsy is more common that expected and may comprise clinicopathological subtypes: A forensic autopsy series. Acta Neuropathol 2017;133:809–23 [DOI] [PubMed] [Google Scholar]
- 22. Kovacs G, Milenkovic I, Wohrer A, et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: A community-based autopsy series. Acta Neuropathol 2013;126:365–84 [DOI] [PubMed] [Google Scholar]
- 23. Braak H, Braak E, Ohm T, et al. Silver impregnation of Alzheimer’s neurofibrillary changes counterstained for basophilic material and lipofuscin pigment. Stain Technol 1988;63:197–200 [DOI] [PubMed] [Google Scholar]
- 24. Buee L, Delacourte A.. Comparative biochemistry of tau in progressive supranuclear palsy, corticobasal degeneration, FTDP-17 and Pick’s disease. Brain Pathol 1999;9:681–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iwatsubo T, Hasegawa M, Ihara Y.. Neuronal and glial tau-positive inclusions in diverse neurologic diseases share come phosphorylation characteristics. Acta Neuropathol 1994;88:129–36 [DOI] [PubMed] [Google Scholar]
- 26. Ikeda K, Akiyama H, Arai T, et al. Glial tau pathology in neurodegenerative diseases: Their nature and comparison with neuronal tangles. Neurobiol Aging 1998;19:S85–91 [DOI] [PubMed] [Google Scholar]
- 27. Chin SS, Goldman JE.. Glial inclusions in CNS degenerative diseases. J Neuropathol Exp Neurol 1996;55:499–508 [DOI] [PubMed] [Google Scholar]
- 28. Inagaki T, Ishino H, Seno H, et al. An autopsy case of PSP with astrocytic inclusions. Jpn J Psychiatry Neurol 1994;48:85–9 [DOI] [PubMed] [Google Scholar]
- 29. Dickson DW. Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol 1999;246:II6–15 [DOI] [PubMed] [Google Scholar]
- 30. Dickson DW. Required techniques and useful molecular markers in the neuropathologic diagnosis of neurodegenerative disease. Acta Neuropathol 2005;109:14–24 [DOI] [PubMed] [Google Scholar]
- 31. Roemer SF, Grinberg LT, Crary JF, et al. Rainwater Charitable Foundation criteria for the neuropathologic diagnosis of progressive supranuclear palsy. Acta Neuropathol 2022;144:603–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beach TG, Sue LI, Walker DG, et al. The Sun Health Research Institute Brain Donation Program: Description and experience, 1987–2007. Cell Tissue Banking 2008;9:229–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beach TG, Adler CH, Sue LI, et al. Arizona study of aging and neurodegenerative disorders and brain and body donation program. Neuropathology 2015;35:354–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adler CH, Beach TG, Hentz JG, et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: Clinicopathologic study. Neurology 2014;83:406–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson's disease: Refining the diagnostic criteria. Lancet Neurol 2009;8:1150–7 [DOI] [PubMed] [Google Scholar]
- 36. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017;89:88–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thal DR, Rub U, Orantes M, et al. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002;58:1791–800 [DOI] [PubMed] [Google Scholar]
- 38. Uchihara T. Expanding morphological dimensions in neuropathology, from sequence biology to pathological sequences and clinical consequences. Neuropathology 2011;31:201–7 [DOI] [PubMed] [Google Scholar]
- 39. Feldengut S, Tredici KD, Braak H.. Paraffin sections of 70-100 micrometer: A novel technique and its benefits for studying the nervous system. J Neurosci Methods 2013;215:241–4 [DOI] [PubMed] [Google Scholar]
- 40. Braak H, Alafuzoff I, Arzberger T, et al. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006;112:389–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mirra SS, Heyman A, Mckeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–86 [DOI] [PubMed] [Google Scholar]
- 42. Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: A practical approach. Acta Neuropathol 2012;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Braak H, Braak E.. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–59 [DOI] [PubMed] [Google Scholar]
- 44. Braak H, Braak E, Bohl E, et al. Alzheimer’s disease: Amyloid plaques in cerebellum. J Neurol Sci 1989;93:277–87 [DOI] [PubMed] [Google Scholar]
- 45. Halliday G, Flowers D, Baum L.. Analysis of staining methods for different cortical plaques in Alzheimer’s disease. Acta Neuropathol 1994;87:174–86 [DOI] [PubMed] [Google Scholar]
- 46. Rowenwald A, Reusche E, Ogomori K, et al. Comparison of silver stainings and immunohistology for the detection of neurofibrillary tangles and extracellular cerebral amyloid in paraffin sections. Acta Neuropathol 1993;86:182–6 [DOI] [PubMed] [Google Scholar]
- 47. Vallet PG, Guntern R, Hof PR, et al. A comparative study of histological and immunohistochemical methods for neurofibrillary tangles and senile plaques in Alzheimer’s disease. Acta Neuropathol 1992;83:170–8 [DOI] [PubMed] [Google Scholar]
- 48. Adler CH, Beach TG, Zhang N, et al. Unified staging system for Lewy body disorders: Clinicopathologic correlations and comparison to braak staging. J Neuropathol Exp Neurol 2019;78:891–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beach TG, Adler CH, Lue L, et al. ; the Arizona Parkinson’s Disease Consortium. Unified staging system for Lewy body disorders: Correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 2009;117:613–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Street D, Whiteside D, Rittman T, et al. Prediagnostic progressive supranuclear palsy—Insights from the UK Biobank. Parkinsonism Relat Disord 2022;95:59–64 [DOI] [PubMed] [Google Scholar]
- 51. Nath U, Ben-Shlomo Y, Thomson RG, et al. The prevalence of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) in the UK. Brain 2001;124:1438–49 [DOI] [PubMed] [Google Scholar]
- 52. Stang CD, Turcano P, Mielke M, et al. Incidence and trends of progressive supranuclear palsy and corticobasal syndrome: A population-based study. J Parkinsons Dis 2020;10:179–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Viscidi E, Litvan I, Dam T, et al. Clinical features of patients with progressive supranuclear palsy in an US Insurance Claims Database. Front Neurol 2021;12:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Merdes AR, Hansen LA, Jeste DV, et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology 2003;60:1586–90 [DOI] [PubMed] [Google Scholar]
- 55. Lopez OL, Becker JT, Kaufer DI, et al. Research evaluation and prospective diagnosis of dementia with Lewy bodies. Arch Neurol 2002;59:43–6 [DOI] [PubMed] [Google Scholar]
- 56. Ballard CG, Jacoby R, Del Ser T, et al. Neuropathological substrates of psychiatric symptoms in prospectively studied patients with autopsy-confirmed dementia with Lewy bodies. Am J Psychiatry 2004;161:843–9 [DOI] [PubMed] [Google Scholar]
- 57. Del Ser T, Hachinski V, Merskey H, et al. Clinical and pathologic features of two groups of patients with dementia with Lewy bodies: Effect of coexisting Alzheimer-type lesion load. Alzheimer Dis Assoc Disord 2001;15:31–44 [DOI] [PubMed] [Google Scholar]
- 58. Sakae N, Josephs J, Litvan I, et al. Neuropathologic basis of frontotemporal dementia in progressive supranuclear palsy. Mov Disord 2019;34:1655–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Adler CH, Beach TG, Zhang N, et al. Clinical diagnostic accuracy of early/advanced Parkinson disease. Neurol Clin Pract 2021;11:e414–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shill HA, Zhang N, Driver-Dunckley E, et al. Olfaction in neuropathologically defined progressive supranuclear palsy. Mov Disord 2021;36:1700–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ba F, Martin WR.. Dopamine transport imaging as a diagnostic tool for parkinsonism and related disorders in clinical practice. Parkinsonism Relat Disord 2015;21:87–94 [DOI] [PubMed] [Google Scholar]
- 62. Brendel M, Barthel H, van Eimeren T, et al. Assessment of 18F-PI-2620 as a biomarker in progressive supranuclear palsy. JAMA Neurol 2020;77:1408–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hsu JL, Chen SH, Hsiao IT, et al. 18F-THK5351 PET imaging in patients with progressive supranuclear palsy: Associations with core domains and diagnostic certainty. Sci Rep 2020;10:19410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li L, Liu FT, Li M, et al. ; the Progressive Supranuclear Palsy Neuroimage Initiative (PSPNI). Clinical utility of 18F-APN-1607 tau PET imaging in patients with progressive supranuclear palsy. Mov Disord 2021;36:2314–23 [DOI] [PubMed] [Google Scholar]
- 65. Messerschmidt K, Barthel H, Brendel M, et al. 18F-PI-2620 tau PET improves the imaging diagnosis of progressive supranuclear palsy. J Nucl Med 2022;63:1754–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.