SUMMARY
Human pluripotent stem cells (hPSCs) are a powerful tool for disease modeling of hard-to-access tissues (such as the brain). Current protocols either direct neuronal differentiation with small molecules or use transcription-factor-mediated programming. In this study, we couple overexpression of transcription factor Neurogenin2 (Ngn2) with small molecule patterning to differentiate hPSCs into lower induced motor neurons (liMoNes/liMNs). This approach induces canonical MN markers including MN-specific Hb9/MNX1 in more than 95% of cells. liMNs resemble bona fide hPSC-derived MN, exhibit spontaneous electrical activity, express synaptic markers, and can contact muscle cells in vitro. Pooled, multiplexed single-cell RNA sequencing on 50 hPSC lines reveals reproducible populations of distinct subtypes of cervical and brachial MNs that resemble their in vivo, embryonic counterparts. Combining small molecule patterning with Ngn2 overexpression facilitates high-yield, reproducible production of disease-relevant MN subtypes, which is fundamental in propelling our knowledge of MN biology and its disruption in disease.
In brief
Limone et al. induce neuralization of hPSCs into spinal MNs by small molecule patterning and TF overexpression. Multiplexed, pooled single-cell RNA-sequencing showcases high reproducibility in dozens of cell lines. These MN villages resemble in vivo spinal MNs and produce disease-relevant MN populations.
Graphical Abstract
INTRODUCTION
Many groups have recognized the ability of stem cells to differentiate into almost any cell type of the body. This unique capability can facilitate the understanding of basic biology of tissues that are hard to access and that are specifically highly evolved in humans, such as the CNS.3,4 Most neuronal differentiation schemes mimic developmental embryonic signals by small molecule patterning. The neuralization of stem cells is achieved by manipulating bone morphogenic protein and transforming growth factor-β, commonly referred to as “dual-Smad inhibition.”5 This study further showed that different combinations of small molecules used as patterning factors could push neuronal progenitors toward distinct neuronal fates. From there, many have developed and refined differentiation protocols for specific neuronal subtypes. However, caveats remain, such as the incomplete neuralization of cultures, underlining the need for additional neuralizing factors,6 as well as the long time needed to generate mature cultures and the heterogeneity in differentiation efficiency among cell lines.7,8
To overcome these limitations, others have employed different approaches such as the overexpression of transcription factors (TFs).9 These TFs have been used to generate induced neurons (iNs) from fibroblasts,10 and the combination with subtype-specific TFs was able to generate specific types of neurons.11 These approaches have been transferred to stem cells with one of the more recent reports of Neurogenin2 (Ngn2, Neurog2, Atoh4) being able to differentiate human pluripotent stem cells (hPSCs) into glutamatergic neurons.12 These advances allowed the reproducible generation of neurons in a shorter time and fewer steps. This approach may, however, skip pivotal developmental steps part of neuronal specification, so questions have been raised regarding the identity of the generated populations and the impact of the overexpression of TFs to downstream applications.13
Previously, we have demonstrated that overexpression of Ngn2 coupled with small molecule patterning is able to enhance the regional specification of neurons to cortical-like patterned iNs (piNs).14 Additionally, small molecules have also been reported to enhance efficiency of motor neuron (MN) programming.15,16 These findings led us to hypothesize that combining Ngn2 expression with different patterning molecules could generate different neuronal cells.
We wanted to generate spinal MNs for biological modeling of degenerative motoneuron diseases, such as amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy that selectively affect these highly specialized neurons.17 MNs reside in the spinal cord and are the only neurons to exit the nervous system and contact skeletal muscles to allow us to breathe and move through a specific synaptic contact, the neuromuscular junction (NMJ). Protocols to differentiate MNs are based on decades of developmental biology studies18,19 and are extensively reviewed elsewhere.20,21 Most protocols entail the neuralization inputs described above coupled with ventralizing factors like Sonic Hedgehog and/or its agonists (Shh/SAG) and the caudalizing effects of retinoids (retinoic acid [RA])7,22–24 or, alternatively, the overexpression of a combination of TFs: Ngn2, Isl1, and Lhx3 (i.e., NILs).15,25 Both approaches have proven to be useful for investigating MN biology. However, directed differentiation produces cultures containing different cell types other than MNs with high line-to-line heterogeneity rendering disease modeling difficult. In contrast, the overexpression of three TFs produces pure cultures but very specific subtypes of MNs, limiting the scalability of these studies since several, specific combinations of TFs are needed to reproduce the diversity of MN subtypes in vitro.
Here, we report that the addition of patterning molecules during Ngn2 programming of hPSCs can lead to specification of regionally defined neuronal states. With time in culture, differentially patterned cells developed into morphologically distinct neurons that maintain regionally defined features according to developmental patterning mimicry. A reporter cell line for the MN-specific TF MNX1/Hb9 demonstrated that approximately 95% of the cells subjected to MN patterning activated this master regulator of MN development. This finding, in combination with the expression of pan-MN markers, validated the cellular identity of SAG- and RA-patterned Ngn2 cells as MN-like cells: the lower induced MNs (liMoNes/liMNs). The liMNs expressed canonical markers and resembled bona fide hiPSC-derived MNs, they were electrophysiologically active and able to form synaptic contact with muscle cells in vitro. By leveraging newly developed analysis tools for single-cell RNA sequencing (scRNA-seq) technology that enable analysis of many cell lines cultured in the same dish simultaneously. We demonstrated that our protocol produced several subtypes of disease-relevant, diaphragm- and limb-innervating MNs in a robust fashion, that is reproducible across at least 47 stem cell lines, and that these cells resemble primary MNs from the human spinal cord. This combinatorial approach addressed several shortcomings from previously published protocols and will facilitate the understanding of basic spinal MNs biology and its disruption in disease.
RESULTS
Ngn2-driven neuralization can be directed to different neuronal fates by small molecule patterning
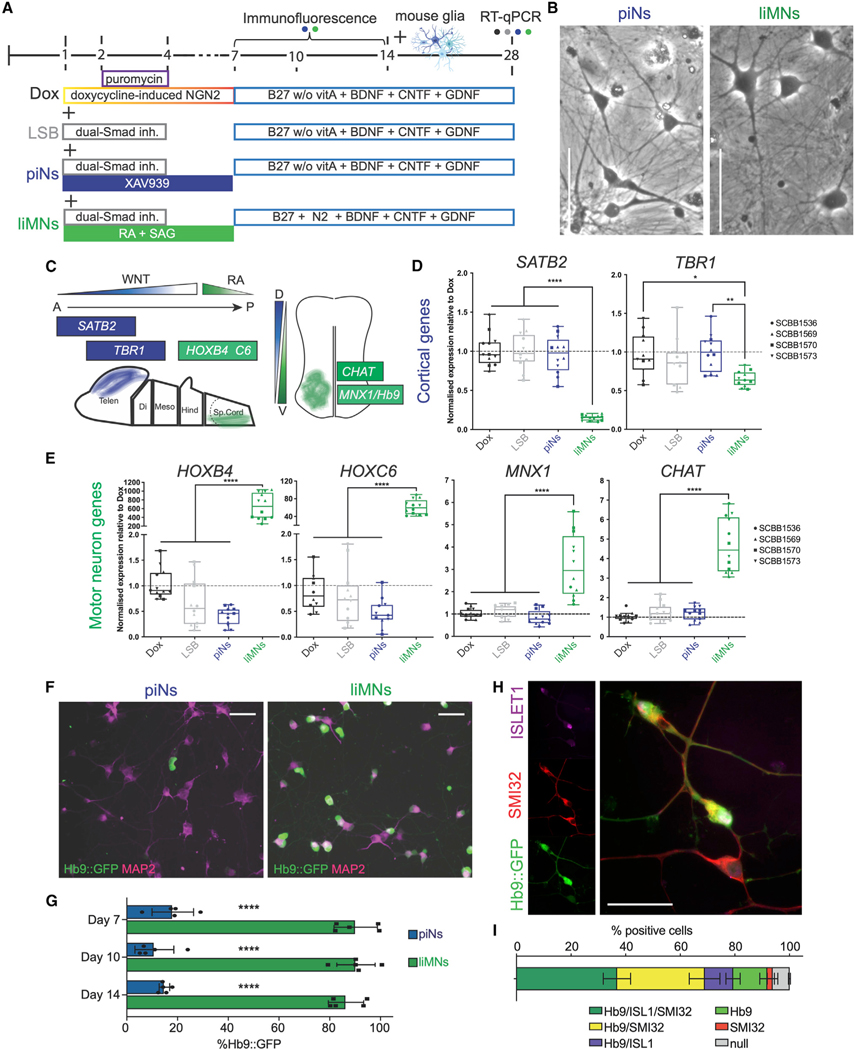
Given that the combination of patterning molecules with Ngn2 expression can generate cortical excitatory neurons,14 we wondered whether the protocol could be repurposed with alternative patterning factors to generate other types of neurons. To test this hypothesis, we used an overexpression system in which a doxycycline-inducible tetO-Ngn2-T2A-Puro/rtTA lentiviral system is used to infect hPSCs for strong overexpression of the neuralizing factor Ngn2.12 We started by substituting WNT inhibition, used to generate cortical cells (piNs),14 with ventralizing SAG and caudalizing RA to induce a ventral-posterior fate and ultimately produce liMNs (Figures 1A and 1B).
Figure 1. Ngn2-driven neuralization can be directed to different neuronal fates by small molecule patterning.
(A) Diagram of known developmental cues used to design patterning strategy. BMP, bone morphogenic protein.
(B) Differentiation schemes used for comparison of divergent Ngn2-driven trajectories: Dox, original Ngn2 overexpression from Zhang et al. 201312; LSB, Ngn2 overexpression coupled with neuralizing dual-Smad inhibition (LDN193189, SB431542); piNs, cortical-like piNs (Nehme et al. 201814); liMoNes/liMNs generated by Ngn2 overexpression and ventro-caudal patterning (RA and smoothened agonist).
(C) Genes selected as master regulators of anterior-dorsal, cortical development and ventro-caudal, spinal cord development.
(D) RT-qPCR quantification for cortical genes at day 4 (three cell lines in n = 3 technical replicates each, p values from one-way ANOVA).
(E) RT-qPCR quantification for spinal genes at day 4 (three cell lines in n = 3 technical replicates each, one-way ANOVA).
(F) Flow cytometry quantification of Hb9:GFP positive cells at day 4.
(G) Hb9:GFP intensity at day 4 of differentiation demonstrating higher total intensity of the Hb9:GFP signal in liMNs.
(H) Hb9:GFP expression day 7 after induction in piNs and liMNs, the majority of liMNs express the reporter (scale bar, 50 μm).
To test if the patterning induced regionally specified neuronal states, we selected markers pivotal for early neuronal development that are divergent between cortex and spinal cord (Figure 1C). To this end we collected RNA and performed reverse transcriptase (RT)-qPCR at day 4, a stage described as neuronal progenitor cell (NPC)-like,14 to assess the expression of these markers. While rostro-dorsalizing WNT inhibition induced the expression of master regulators of cortical development EMX1, FOXG1, OTX1 and OTX2 (Figure 1D), the caudal-ventral patterning induced the expression of posterior markers HOXB4 and HOXC6, of cholinergic master regulator ISL126 and of MNX1 (Hb9), expressed by spinal MNs in the nervous system27 (Figure 1E). Importantly, caudal-ventral patterning significantly decreased the expression of OTX1 and OTX2, TFs that regulate the schism between the cortex and posterior regions of the CNS.28 In line with previous studies, dual-Smad inhibition in combination with Ngn2 resulted in loss of pluripotency markers, OCT4 and SOX2, and acquisition of pan-neuronal markers, PAX6 and TUBB3 (Figures S1A and S1B).14
To further confirm the regional specification of NPCs, we took advantage of a reporter line that expresses green fluorescent protein (GFP) under the murine, MN-specific, Hb9 promoter29,30 inserted into human embryonic stem cell line used to validate differentiation protocols.22,31–33 Flow cytometry analysis confirmed that by day 4 after induction, more than 70% of cells treated with RA and SAG were GFP positive (Figure 1F). Strikingly, not only was the percentage of GFP+ cells higher, but the intensity of GFP signal also increased (Figures 1G and S1C), in agreement with higher levels of MNX1/Hb9 RNA. By day 7, cells subjected to RA and SAG showed strong Hb9::GFP expression whereas only a fewer, dimmer GFP-positive cells were visible in the other conditions (Figures 1H and S1D). Taken together, these data suggest that differential patterning coupled with Ngn2-overexpression leads to the specification of different neuronal fates, including MN.
Neuronal fates induced by patterned Ngn2 expression are maintained throughout differentiation
We then proceeded to confirm that regional specification was maintained long term after neurogenesis. For this purpose, we extended in vitro culturing by replating cells in neuronally supportive conditions (Figure 2A). First, we analyzed cell morphology by microscopy. Patterning produced neurons with strikingly different morphology, with piNs showing small, polarized cell bodies and MN-patterned cells showing a wider soma with a multipolar shape and one extended axon-like structure (Figures 2B, S2A, and S2B), strikingly reminiscent of the morphology of cortical pyramidal neurons and spinal, ventral horn MNs in vivo, respectively.34
Figure 2. Patterned Ngn2-induced neuronal fate is maintained throughout differentiation.
(A) Differentiation schemes for neuronal maturation after one-week of patterning: Dox, original Zhang et al. 2013; LSB, Ngn2 with dual-Smad inhibition; piNs, cortical-like piNs (Nehme et al. 2018).14.
(B) Brightfield image at day 30 of piNs and liMNs (scale bar, 100 μm).
(C) Diagram of genes specifically expressed in either anterior-dorsal cortical neurons or ventro-caudal, spinal cord MNs.
(D) RT-qPCR quantification for induction of cortical genes at day 30 (four cell lines in n = 3 technical replicates, one-way ANOVA).
(E) RT-qPCR quantification for spinal cord genes at day 28 (four cell lines in n = 3 technical replicates, one-way ANOVA).
(F) Hb9:GFP reporter expression at day 14 after induction in piNs and liMNs (scale bar, 50 μm).
(G) Quantification of Hb9:GFP reporter expression at day 7, 10 and 14 post-induction in piNs (blue) and liMNs (green) by immunofluorescence (n = 5, p values from t test at each time point).
(H) IF analysis for pan-MN SMI-32, Islet1 and Hb9:GFP reporter expression at day 7 post-induction (scale bar, 50 μm).
(I) Quantification H (n = 3 replicates).
To confirm that the regional identity specified by patterning was maintained, we collected RNA at day 30 of differentiation and investigated the expression of genes known to be specific of either glutamatergic neurons of the cortex or cholinergic MNs of the spinal cord (Figure 2C). We confirmed that caudalization repressed cortical genes SATB2 and TBR1 (Figure 2D). Expression of posterior markers HOXB4 and HOXC6 was sustained in caudalized cells and suppressed in piNs (Figure 2E). Moreover, mature ventralized cells expressed the MN-specific TF, MNX1/Hb9 and higher transcript levels of the main component of the cholinergic machinery, choline acetyltransferase (CHAT) (Figure 2E), while maintaining expression of panneuronal markers (Figure S2C). According to this polarization, expression of the Hb9::GFP reporter was also maintained throughout differentiation only in RA- and SAG-patterned cells, reaching a peak of approximately 95% at day 7 (Figures 2F, 2G, and S2E), and was then slightly downregulated as seen in early development of MNs of the spinal cord in vivo.35 To further ensure their MN identity and overcome some of the limitations of the reporter, we combined the Hb9:GFP reporter with staining for Islet 1 and SMI32, the triad recognized as the human pan-MN staining22 and confirmed that 80% of the cells co-expressed at least two of these markers (Figures 2H, 2I, and S2D). The data so far confirmed that coupling of Ngn2 overexpression with patterning factors can produce regionally specified neurons and we define the ventralized and caudalized cultures as lower induced MNs: liMoNes/liMNs.
liMNs reproducibly express canonical pan-MN markers and resemble bona fide hPSC-MN
Given that neuralization by Ngn2 overexpression can be directed to different neuronal fates and maintained during in vitro culture, we wanted to confirm the expression of key MN markers at the protein level. As early as day 14, liMNs expressed the ventral horn MN-specific marker Stathmin 2 (STMN2) (Figure 3A).36,37 By day 30, liMNs expressed cholinergic acetyltransferase (Figure 3B) and limb-innervating marker Foxp1 (Figure 3C).38 Moreover, liMNs showed reactivity for antibodies against the TF Islet1 along with SMI-32, that recognizes spinal MN-enriched neurofilament heavy chain (Figure 3D). Indeed, 60%–90% of cells expressed at least one of these markers (Figure 3E), while MN markers were robustly and reproducibly expressed by 80%–90% of cells from different cell lines (Figure 3F).
Figure 3. liMoNes reproducibly express canonical MN markers.

(A) Immunofluorescent staining for MN-specific marker Stathmin2 (STMN2) and neuronal cytoskeletal protein TUBB3 (Tuj1) at day14 (scale bar 100 μm).
(B) Immunofluorescent staining for cholinergic marker choline acetyltransferase (Chat) and neuronal cytoskeletal proteins MAP2 and TUBB3 (Tuj1) at day 30 (glial co-cultures—scale bar, 30 μm).
(C) Immunofluorescent staining for limb-innervating MN marker FOXP1 and neuronal MAP2 and TUBB3 (Tuj1) at day 30 (glial co-cultures—scale bar, 30 μm).
(D) Immunofluorescent staining for MN-enriched SMI-32, cholinergic TF islet 1 and neuronal MAP2 at day 30 (glial co-cultures—scale bar, 30 μm).
(E) Quantification for cells in B–D (n = 10).
(F) Quantification of expression of selected markers in five independently differentiated lines (five cell lines, n = 2 each).
(G) Differentiation schemes implemented to compare liMNs with bona fide hiPSC-MN derived by conventional small molecule induction (2D MN, in purple).
(H) Morphology of neuronal cells produced: piNs, liMNs and 2D-MN (scale bar, 50 μm).
(I) RT-qPCR quantification of MN markers between liMNs (green) and 2D-MN (purple) (n = 3).
We next wanted to confirm that liMNs resembled cells defined by the scientific community as bona fide hiPSC-derived MNs. We thus differentiated MNs following a conventional method using just small molecule patterning factors (2D MN).39 Briefly, stem cells were subjected to neuralizing dual-Smad inhibition followed by DAPT and SU5402 while caudalized and ventralized with RA and SAG. Differentiated neurons were separated from the mixed cultures by sorting for cell surface marker N-CAM 14 days after neuronal induction,39 and then cultured in neuronal differentiation media, under similar conditions to liMNs for 14 more days (Figures 3G, S3A, and S3B). We then compared the morphologies of the conventional 2D MNs and liMNs by imaging. We found that liMNs were morphologically similar to 2D MN, with large multipolar cell bodies, and very distinct from cortical cells (Figure 3H). Moreover, liMNs and 2D MN expressed similar patterns of pan-MN staining (Figures S3C and S3D). Remarkably, RT-qPCR analysis revealed that liMNs expressed comparable levels of other MN markers and even higher transcript levels of the limb-innervating MN marker HOXC6 (Figure 3I). These results confirmed that liMNs resemble one kind of bona fide hiPSC-derived MNs defined by the broader scientific community.
liMNs form active synaptic networks and contact muscle cells in vitro
We next set out to assess liMNs functional properties and ability to form synapses. liMNs expressed both pre- and post-synaptic molecules synaptophysin and PSD-95 (Figures 4A, S4A, and S4B) and displayed abundant staining for synapsin and axonal ankyrinG, similar to piNs (Figure S4C). Multielectrode array analyses showed that cultures have a steady increase in spiking rates over time (Figures S4D and S4E). Treating cells with the potassium-gated channel opener retigabine, a potential therapeutic agent for ALS,40,41 silenced cultures underlining the usefulness of liMNs as model for therapeutic strategies in neurodegenerative diseases (Figure 4B).
Figure 4. liMoNes can form active synaptic structures in vitro.
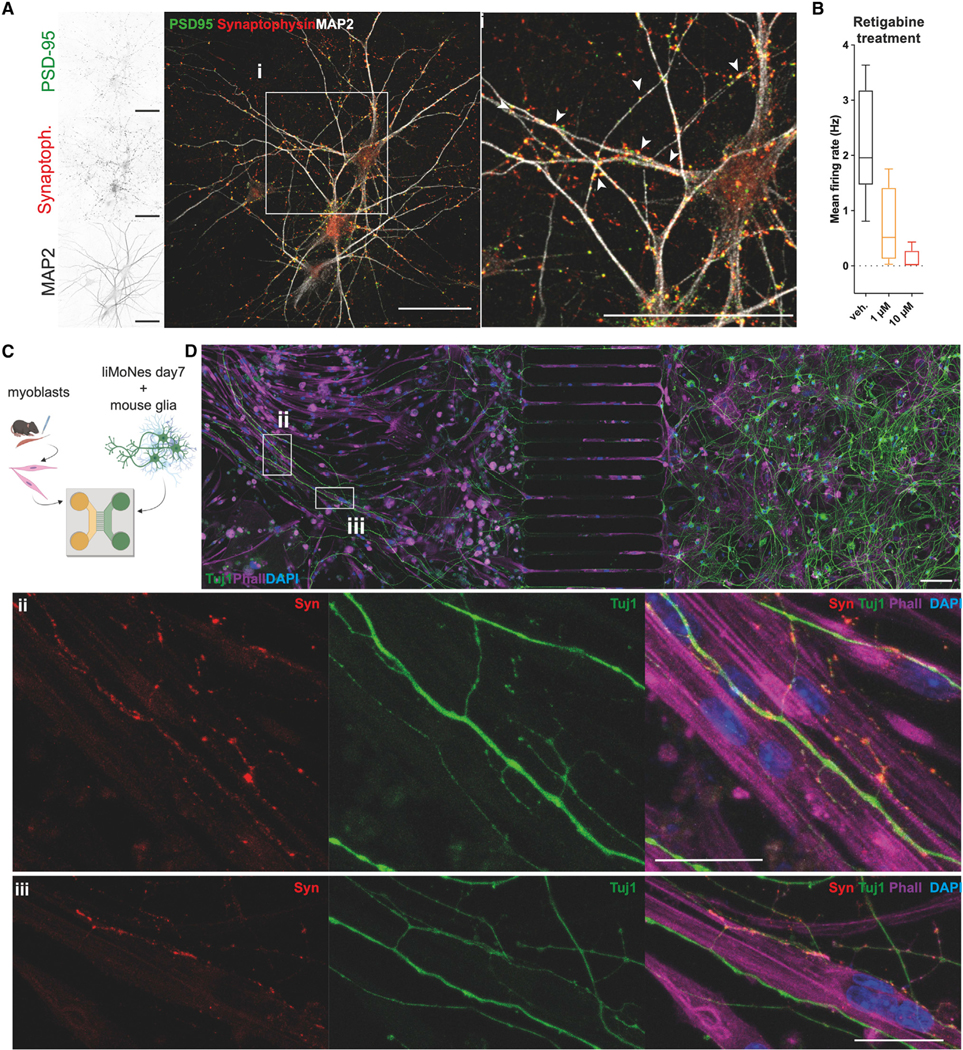
(A) Day50 liMNs express pre- and post-synaptic density proteins (scale bar, 50 μm).
(B) Mean number of spikes in day 50 cultures treated with raising concentrations of retigabine (n = 6).
(C) Diagram of co-culture experiments of liMNs and primary murine myoblasts in microfluidic devices.
(D) Immunofluorescence of co-culture of liMNs and primary murine myoblasts showing glia-liMNs co-cultures (right), where neurons extend axons through the channels (middle), contacting primary muscle cells (left). (Di–Dii) Insets of (D) showing liMNs forming synaptic-like contacts with muscles cells (scale bar, 50 μm).
MNs are the only neurons to connect with muscles through a highly specific synapse: the NMJ. To test the ability of liMNs to form NMJ-like structures we established co-cultures with murine muscle cells in compartmentalized microfluidic devices where neurons grown in one chamber can extend axons through groves that connect to muscle cells (Figure 4C). Staining showed that liMNs extended neurites to the second chamber, contact muscle cells and form structures expressing pre-synaptic protein synapsin (Figures 4Di-4Dii and S5A–S5E), a sign of an early development of contact.
scRNA-seq confirms the expression of MN-specific genes and reproducibility of the protocol
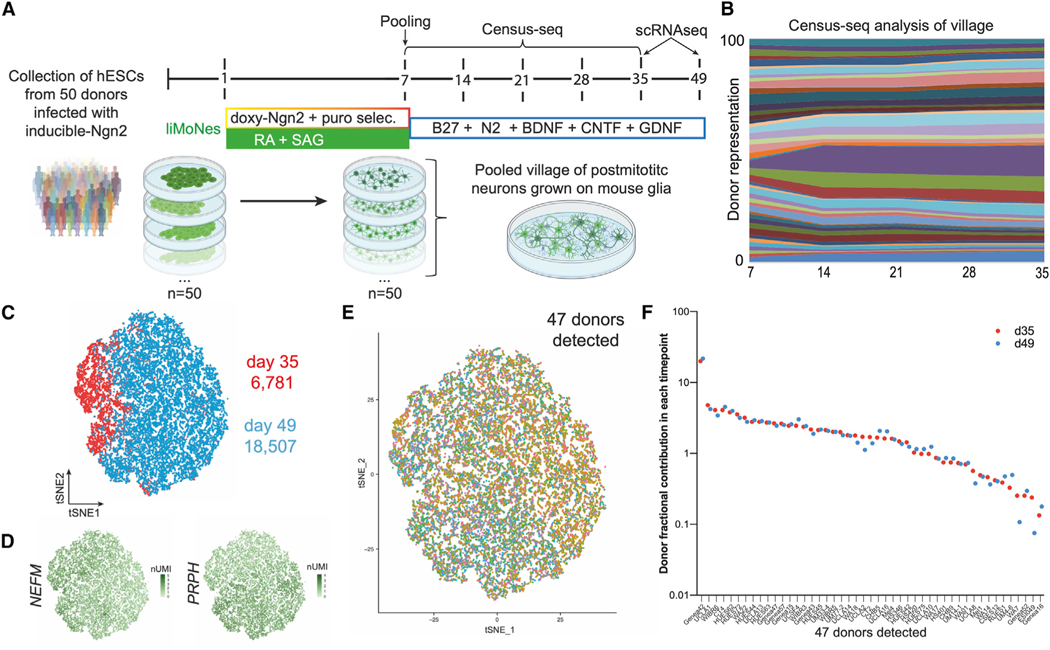
After confirming the MN-like properties of liMNs, we set out to further characterize their molecular identity and reproducibility by single cell RNA-seq. We coupled sequencing with two newly developed technologies: Census-seq and Dropulation1,2 to enable the characterization of lines from many different donors in a single experiment. These methods use the intrinsic variability of single nucleotide polymorphisms within a population as a barcode to assign identities in a mixed culture—a “village”—of multiple donors, similarly to pooled CRISPR-Cas9 barcoded screens.42–44 More precisely, Census-seq allows population-scale, quantitative identity assignment from a mixed group of donors,1 Dropulation can assign identities at a single cell level in a village for scRNA-seq studies.2 With this aim in mind, we produced liMNs villages: 50 embryonic stem cell lines, previously subjected to whole-genome sequencing, were separately differentiated into liMNs. At day 7 after induction, post-mitotic cells were pooled in equal numbers to make up villages containing all donors in one dish. Using genotypes from WGS we were able to reassign the donor identities in a mixed village (Figure 5A).
Figure 5. scRNA-seq confirms expression of MN-specific genes and reproducibility of the protocol.
(A) Pooling strategy and village construction for Census-seq and Dropulation analysis. BDNF, brain-derived neurotrophic factor; CNTF, ciliary neurotrophic factor; GDNF, glia-derived neurotrophic factor.
(B) Sandplot of Census-seq analysis showing balanced representation of 47 detected donors throughout several days after induction.
(C) t-SNE projection of scRNA-seq analysis of 25,288 cells of two timepoints of mature liMNs differentiation.
(D) t-SNE projection with expression of markers for neurons of the peripheral nervous system.
(E) t-SNE projection of 25,288 cells depicting donor’s identity of each cell from 47 donors detected by Dropulation analysis.
(F) Fraction representation of 47 donors in the two timepoints of mature liMNs differentiation.
To ensure that the donor composition remained balanced, cells were harvested once a week to collect genomic DNA for low-coverage sequencing. Census-seq analyses showed that we could detect 47 of the 50 donors originally pooled and confirmed that donor distribution remained consistent for four weeks (Figure 5B). Neurons were harvested at day 35 and day 49 for scRNA-seq and Dropulation analysis. Libraries generated from 25,288 cells demonstrated strong expression of neuronal markers, especially of the peripheral nervous system, NEFM and PRPH (Figures 5C, 5D, and S6A). liMNs did not express cycling cells markers (Figure S6B), nor markers of ventral, spinal interneuronal pools V1, V2a, V2b, V3, nor mid-dorsal spinal interneurons V0 (Figures S6C–S6E). liMNs expressed MN-enriched STMN2, NEFH, ISL1, and MNX1 (Figure S6F)22,36,37,45 and low but detectable expression of cholinergic genes ACHE, SLC5A7 (Cht1), and SLC18A3 (vAChT) (Figure S6G). Finally, we detected expression of AGRN and NRG1, expressed by MNs to form NMJs (Figure S6H).
Using the newly devised Dropulation analytical pipeline, we assigned donor identity to barcoded droplets. Initial t-distributed stochastic neighbor embedding (t-SNE) clustering showed an even distribution of each donor (Figure 5E) and we confirmed that the contribution of each donor remained constant at both timepoints (Figure 5F) underlying the robustness and reproducibility of the protocol. We, therefore, confirmed that our protocol can reproducibly generate MN-like cells from many cell lines.
Cell villages confirm polarization generated by differential patterning of Ngn2 differentiation
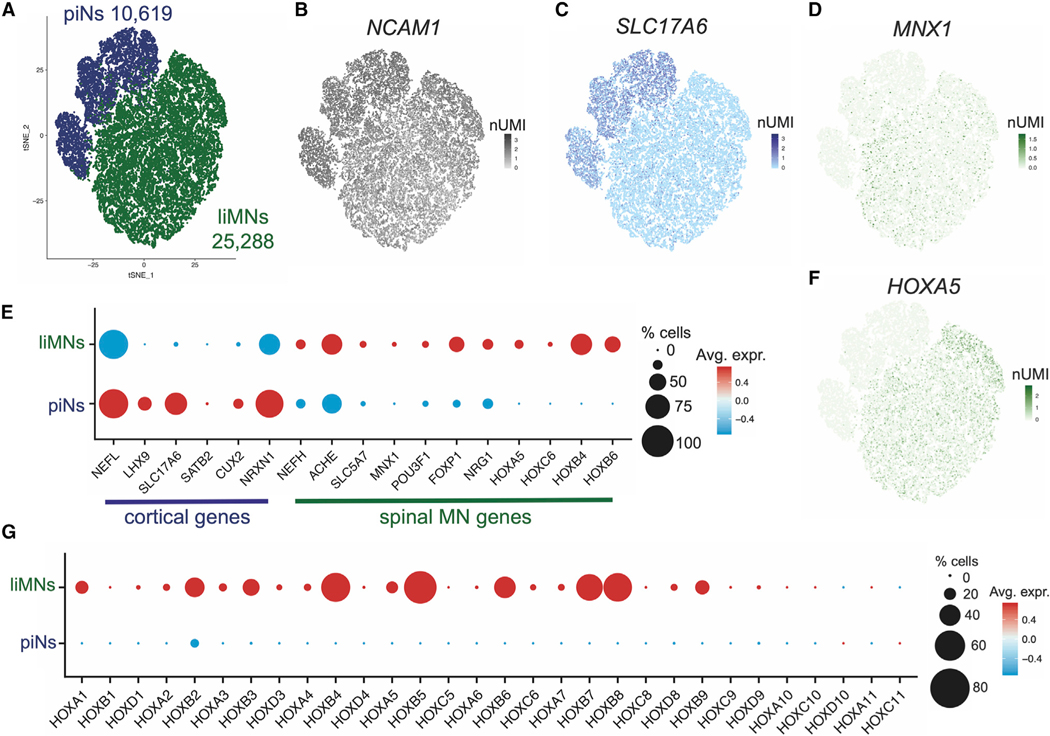
To unbiasedly confirm that differential patterning strategies could generate different neuronal fates we then compared single cell libraries from liMNs to libraries similarly generated from piNs (Figure S7A). t-SNE clustering showed a clear separation of piNs and liMNs (Figure 6A). All cells expressed neuronal markers (Figures 6B and S7B) but piNs expressed higher levels of genes of dorsal, cortical, and glutamatergic cells (Figures 6C and S7C), whereas liMNs expressed higher levels of genes of ventral, spinal, and cholinergic cells (Figures 6D and S7D), confirming that the two different patterning strategies preferentially upregulate genes connected to these distinct cellular identities in a strongly polarized manner (Figure 6E). Interestingly, HOX genes, mostly expressed in the midbrain and in the spinal cord and known markers of caudalization, were highly expressed in liMNs and barely detected in piNs (Figures 6F and 6G). We assigned donor identity to barcoded droplets with Dropulation and showed an even distribution of each donor across the different clusters (Figures S7E and S7F) underlying the robustness and reproducibility of these protocols.
Figure 6. Confirmed divergent neuronal fate of piNs and liMNs.
(A) t-SNE projection of scRNA-seq analysis of 25,288 cells of two timepoints of piNs and liMNs differentiation.
(B) t-SNE projection with expression of neuronal marker.
(C) t-SNE projection with expression of cortical-enriched marker.
(D) t-SNE projection with expression of MN-specific marker.
(E) Dotplot for differential gene expression of markers specific to either cortical excitatory neurons or spinal MNs.
(F) t-SNE projection with expression of brachial MN-specific HOX gene expression.
(G) Dotplot for gene expression of all retinoid-dependent HOX genes in piNs and liMNs.
Ventro-caudal patterning of Ngn2 can produce different MN subtypes
In vivo MNs are classified in subtypes (pools or columns) according to their position along the cord and the anatomical part of the body they innervate. Four groups lie in spinal cord areas developmentally regulated by retinoids: (1) medial motor column (MMC), along the entire spine, connects to axial musculature to maintain posture, (2) cervical spinal accessory column (SAC) innervates head and neck, (3) phrenic motor column (PMC), also cervical, innervates the diaphragm, (4) lateral motor column (LMC), at the brachial level on the cervico-thoracic boundary, connects to forelimbs and is divided into ventral-innervating, medial or dorsal-innervating, lateral LMC (Figure 7A).19 Remarkably, we were able to find markers specific to these pools in our dataset: a small group of PHOX2B-expressing SAC-like cells, wide expression of PMC-enriched ALCAM and POU3F1 (SCIP) and markers of both lateral- and medial-LMCs: FOXP1 and LHX1 (Figures S8A–S8D).
Figure 7. Ventro-caudal patterning of NGN2 can produce different MN subtypes.
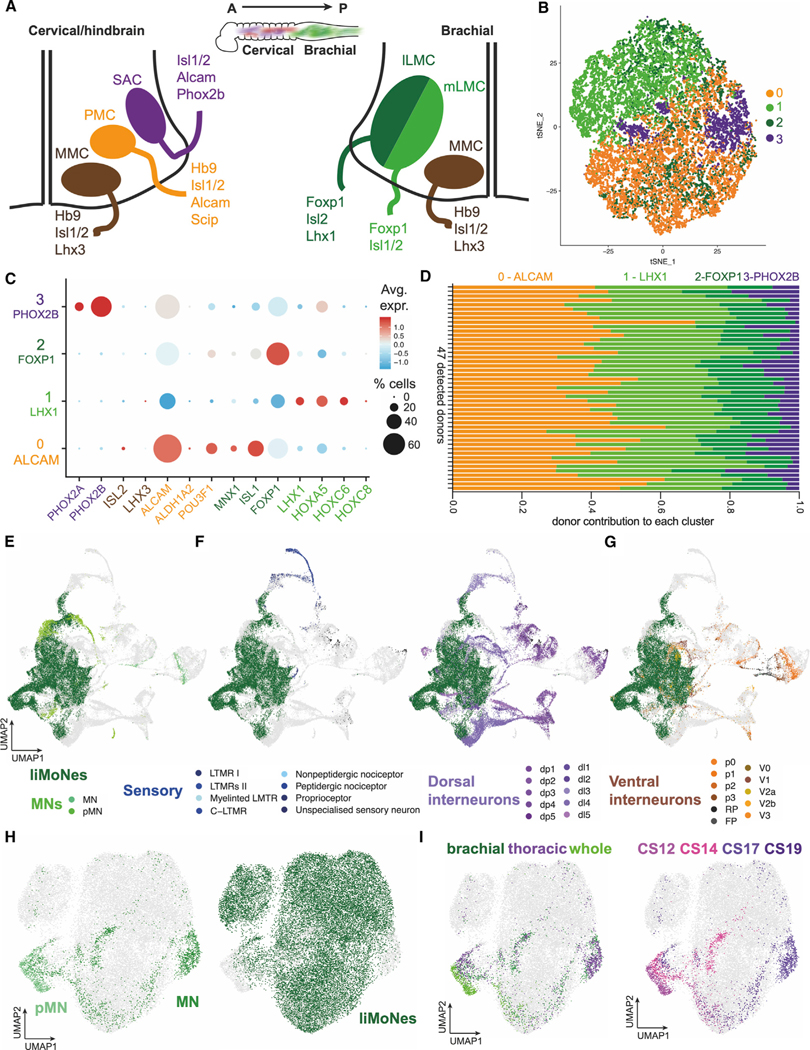
(A) Diagram of known pools of MN subtypes along mammalian spinal cord.
(B) t-SNE projection of four, unbiasedly identified subclusters in the 25,288 cells analyzed.
(C) Dotplot for differential gene expression of MN subtype-specific markers in the four cervico-brachial MN groups.
(D) Fraction of each donor’s share between the identified subclusters as calculated by Dropulation.
(E) t-SNE projection of integrated datasets: liMoNes and MNs and pMNs (progenitors) from human embryonic spinal cord Rayon et al. 2021.45
(F) t-SNE projection of integrated datasets: liMoNes, sensory neurons and dorsal interneurons from Rayon et al. 2021.45
(G) t-SNE projection of integrated datasets: liMoNes and ventral interneurons from Rayon et al. 2021.45
(H) t-SNE projection of integrated datasets with MNs only.
(I) t-SNE projection of integrated datasets with MNs only with regionality and timepoints (Carnegie stage) from Rayon et al.45 highlighted.
We wondered if the discrete expression of these markers shaped subgroups with different transcriptomic profiles. We decided to unbiasedly identify subclusters and found four groups: liMNs 0, 1, 2, and 3 (Figure 7B). Intriguingly, markers of MN pools segregated within the groups demarcating an ALCAM+ group, an LHX1+ and a FOXP1+ groups, and a small PHOX2B+ group (Figures 7C and S8E–S8G). No expression of MMC markers was found (Figure S8H), consistent with reports identifying this population as less responsive to certain patterning factors.22,46 Differential gene expression analysis for genes specific to each subgroup unbiasedly confirmed regional specification consistent with the markers described above (Figure S8I, Table S1). We observed two additional features: expression of markers of anterior digit-innervating MNs FIGN and CPNE4 in a small percentage of cells (Figure S8J)47,48; and expression of HOX genes activated in response to retinoids49 and specifically in cervical/brachial MNs50 (Figures S8K and S8L). Taking advantage of the Dropulation technology, we investigated the distribution of donors within each subcluster and surprisingly found that each of the 47 donors distributed evenly within clusters highlighting the robustness and reproducibility of the protocol (Figures 7D, S9A, and S9B).
To ensure that liMNs resembled cervico-brachial MNs, we integrated our data with a recently published scRNA-seq dataset generated from human embryonic spinal cord,45 and visualized the resulting dataset using uniform manifold approximation and projection. First, we confirmed we could identify neurons and progenitors of different spinal lineages matching the cell types identified in Rayon et al. (Figures S10A–S10D). In the integrated analysis, liMoNes clustered closely to embryonic post-mitotic MNs (Figure 7E), while they clustered separately from both sensory neurons and dorsal interneurons (Figure 7F) and partially closer to ventral interneurons (Figures 7G and S10E), further validating the MN-like fate of liMNs. We then isolated MN-like cells from the integrated dataset and analyzed them separately from the rest of the spinal cord, liMoNes and primary MN clustered separately from progenitor cells (pMNs) (Figure 7H). Consistent with HOX expression, liMoNes clustered more closely to MN of brachial origin, consistent with the more caudal position of samples in the primary human dataset and therefore low expression of more hindbrain markers (Figures S10F and S10G), and from mid-to-late stages of development (Figure 7I). Taken together, our integrative analyses with human embryonic spinal cord cells not only confirms the MN identity of liMoNes, but also demonstrates that they are composed of a plethora of MN subtypes that intrinsically recapitulates pools and columns identified in the cervical and brachial spinal cord and that these subtypes can be robustly generated in a myriad of cells lines.
DISCUSSION
In this study, we describe a rapid and efficient protocol to generate human MN-like cells from hPSCs by combining the overexpression of neuralizing factor Ngn212 and ventralizing and caudalizing small molecules patterning.31,51 We demonstrated that different patterning molecules can direct Ngn2-driven neuralization into the specification of distinct neuronal fates that are maintained during in vitro culture. In particular, we show that ventral-caudal patterning induces expression of the MN-specific TF MNX1/Hb9 in more than 90% of differentiated cells bypassing the previously used sorting methods to isolate MN from mixed cultures and in a shorter period of time. The ventro-caudalized cells expressed pan-MN markers as identified in vivo and resembled bona fide hPSC-derived MN giving them a lower MN identity—hence liMoNes/liMNs. liMNs generated electrophysiologically active cultures capable of forming early contact points with muscle cells in vitro. By leveraging newly developed single-cell RNA-seq analysis tools, we demonstrated that this protocol could successfully generate a previously reported hard-to-produce neuronal cell type by a straightforward one-step programming. Additionally, we showed that the differentiation scheme is highly scalable and reproducible across 47 cell lines, and that the generated cultures contain a diverse population of disease-relevant MN subtypes that in part resemble their human, embryonic, in vivo counterpart.
The protocol described here enabled us to overcome some of the main issues reported in previously published differentiation schemes based on small molecules patterning. Specifically, we showed how, with a single-step induction, we were able to generate in only 7 days, a pure population of post-mitotic neurons in which virtually all cells expressed the MN-specific marker MNX1/Hb9, whereas most protocols reported at least 2 weeks of differentiation to achieve partial expression of this reporter.20 Moreover, we demonstrated how the enforced expression of one TF can achieve complete neuralization of cells to avoid the heterogeneous generation of other cell types on a cell-line-to-cell-line-dependent manner7 and how this method could be replicated in dozens of pluripotent lines, allowing to increase genetic variation52. This single-step, 7-day induction protocol would allow the generation of defined MN cultures for in vitro modeling studies and avoid the time-consuming and expensive cell-sorting step to select relevant cell types from mixed ventral-caudal populations.39 Intriguingly, very few reports showed NMJ-like structures in vitro from human MNs,53–58 and so far only one has established a system that allows it in culture conditions that resemble human physiology.59 The combination of our highly pure, accelerated protocol and this report could allow further understanding of NMJs in a physiologically relevant, human context.
Our study is among the first reports to highlight the malleability of Ngn2-based reprogramming and its ability to be directed to differential states by small molecule patterning mimicking embryonic development. We have thoroughly demonstrated in a previous report that patterning can direct Ngn2 toward a cortical-like state,14 but this is the first side-by-side, systematic comparison of the ability of this programming method to diverge into different neuronal fates. Other investigators have reported that the overexpression of Ngn2 alone is able to produce an admixture of different neuronal subtypes of both the central and peripheral nervous systems,13 confirming that Ngn2-driven neuralization yields several neuronal subtypes. Here, we expand on this biology showing that small molecule patterning can direct the multipotent neuralizing ability of Ngn2 to populations of regionally specified neurons in a robust, reproducible manner.
Many molecular studies have shown how retinoids can specifically act as epigenetic modulators, open chromatin domains in neural progenitor cells consistent with spinal cord identity and aid posteriorization in MN differentiation systems.16 Moreover, transcriptomic and epigenomic studies along NIL-based MN differentiation have shown that Ngn2 acts independently of the Isl1-Lhx3 heterodimers, upregulating neuralizing factors that in turn open sites of chromatin that allow further specification into MN-fates.60 Intriguingly, others have reported that overexpression of Ngn2 in fibroblasts coupled with patterning factors could generate small populations of cholinergic neurons, hinting at the malleability of this system.61 We speculate that the addition of patterning molecules to Ngn2 programming permits the opening of chromatin at sites of MN-specific genes usually achieved by the overexpression of other TFs forming a permissive epigenetic landscape that allows specification into MN identity. Other groups have reported that, in other TF-based differentiation systems, the addition of RA can upregulate sets of genes that the TFs alone could not achieve,62 confirming that combinatorial approaches might aid specification into desired cell types.
The use of only one TF combined with small quantities of inexpensive patterning molecules renders this protocol amenable to large-scale, high-throughput studies compared with previous reports.15 The combinatorial use of multiple TFs often induces the generation of extremely specified subtypes of MNs25,33 that, even though pure and well defined, limit the ability of hPSC to differentiate into the intrinsic admixture of MNs generated by retinoids/Shh and only elicits the transcriptomic programs of restricted pools.33 Moreover, other investigators have demonstrated how combinations of multiple TFs might take longer time to develop hPSC into neurons when compared with Ngn2 alone and that the timing of overexpression could interfere with the subtypes of neurons generated.62 Here we propose that a short pulse of Ngn2 overexpression coupled with patterning molecules not only reduces the number of TF needed to direct the specification of neuralization, but also allows intrinsic developmental processes to take place and generate the myriad MN subtypes seen in spinal cord development, as shown by the similarities with our cells and primary samples. Given the differential susceptibility of subtypes of MNs to degenerate in certain diseases like ALS,63 having both resistant and susceptible populations of MNs reproducibly generated in one dish could help to further understand the dynamic process of neurodegeneration.
Limitations of the study
One of the strengths of this protocol is its high reproducibility and accelerated nature. However, the method still lacks pivotal positional, geographical, sequentially timed signals that generate the milieu of motoneurons in the spinal cord. This method thus produces MNs that do not exactly reproduce transcriptomic profiles of columns in vivo, for example: the incomplete co-expression of HB9 and Islet1 in young neurons that is only achieved later into the differentiation, the discrepancy between liMNs identities at protein and RNA levels, or the incomplete overlap of certain markers between liMoNes and their in vivo counterparts. Considering different concentrations and timing of patterning molecules and also exploring RA-independent ways of generating MNs46,64 would be an important follow up to this study. Furthermore, our microfluidic system did not show clustering of postsynaptic acetylcholine receptors (AchR) on muscle cells. Formation of mature NMJ contacts has been a primary limitation of in vitro hPSC-derived MNs and muscle co-cultures as observed in vivo.65 Toward optimization of this model, a recent study has shown that supplementation of agrin and laminin increased clustering of AchR in in vitro human co-cultures system,59 adapting this method to our protocol might provide essential steps for the further maturation of these synaptic structures for liMNs. These discrepancies are shortcomings of accelerated systems like ours; nonetheless, our approach provides a platform for the study of the biology of different MN subtypes and their functionality in health and disease in a scalable, highly reproducible manner never achieved before.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Kevin Eggan (kevin.eggan@bmrn.com).
Materials availability
This study did not generate new unique reagents.
Data and code availability
All codes and algorithms necessary for re-analysis of the single-cell RNA-sequencing data are publicly available and can be found in other publications.1,2 Raw sequencing data and count matrices have been deposited in GEO: GSE219112 and DUOS: DUOS-000121 (https://duos.broadinstitute.org/).
This paper does not report original code.
Further information requests can be directed to Kevin Eggan (kevin.eggan@bmrn.com) or Francesco Limone (francesco_limone@fas.harvard.edu).
EXPERIMENTAL MODELS AND SUBJECT DETAILS
NGN2-based differentiations
Stem cells were grown in mTeSR1 (Stem Cell Technologies, 05,850) and grown on Matrigel (Corning) coated pates at 37◦C and 5% CO2. hPSCs were infected with TetO-Ngn2-Puro, TetO-GFP and rtTA lentiviral constructs12 produced by Alstem in mTeSR medium with 1 μM RoCK inhibitor Y-27632 for 24 h hPSs were then passaged and differentiation was started when cells reached 70–80% confluency. For the first four days of differentiation cells were grown in induction medium: DMEM/F12 (Life Technologies, 11,320–033), N2 supplement (0.5%v/v, Gibco), 1X GlutaMAX (Gibco), 0.1mM Non-essential amino acid (Gibco), 0.5% glucose, doxycycline hyclate (2 μg/mL). Small molecules added: day 1 – DOX: none; LSB: 10 μM SB431543 (Custom Synthesis), 200 nM LDN193189 (Custom Synthesis); piNs: 10 μM SB431543 (Custom Synthesis), 200 nM LDN193189 (Custom Synthesis), 4 μM XAV939 (Stemgent, 04–00046); liMNs: 10 μM SB431543 (Custom Synthesis), 200 nM LDN193189 (Custom Synthesis), 2 μM retinoic acid (Sigma) and 2 μM Smoothened agonist (Custom Synthesis). Day 2–4 - DOX: puromycin (5 μg/mL); LSB: puromycin (5 μg/mL), 10 μM SB431543 (Custom Synthesis), 100 nM LDN193189 (Custom Synthesis); piNs: puromycin (5 μg/mL), 10 μM SB431543 (Custom Synthesis), 100 nM LDN193189 (Custom Synthesis), 2 μM XAV939 (Stemgent, 04–00046); liMNs: puromycin (5 μg/mL), 10 μM SB431543 (Custom Synthesis), 100 nM LDN193189 (Custom Synthesis), 1 μM retinoic acid (Sigma) and 1 μM Smoothened agonist (Custom Synthesis). On day 4 cells were dissociated using Accutase (Gibco) and replated in a 1:2 dilution to ensure puromycin selection of uninfected cells. For day 4–7, DOX, LSB and piNs cells were grown in neuronally supportive medium supplemented with small molecules as described above: Neurobasal (Life Technologies 21,103,049) supplemented with B27 supplement w/o vitA (2%v/v, Gibco), 1X GlutaMAX (Gibco), 0.1mM Non-essential amino acid (Gibco), 0.5% glucose with the addition of 10 ng/mL of BDNF, CNTF and GDNF (R&D Systems). For day 7–10, liMNs were grown with small molecules as described above in neuronally supportive medium: Neurobasal (Life Technologies 21,103,049) supplemented with B27 supplement (2%v/v, Gibco), N2 supplement (0.5%v/v, Gibco), 1X GlutaMAX (Gibco), 0.1mM Non-essential amino acid (Gibco), 0.5% glucose with the addition of 10 ng/mL of BDNF, CNTF and GDNF (R&D Systems). On day 7, cells were dissociated using accutase and replated on glial co-cultures as described previously29 in medium described above. From this time onwards, half-media change was performed every 2–3 days in neuronally supportive media described above with the only addition of 10 ng/mL of BDNF, CNTF and GDNF (R&D Systems). For most experiments, neurons were co-cultured with murine glial cells (50,000 cells/cm2) derived from postnatal brains (P0–2) as previously described,29 neurons were mixed with glia when replating day 7 cells 30,00 cells/cm2.
2D MN differentiation
Stem cells were grown in mTeSR1 (Stem Cell Technologies, 05,850) and grown on Matrigel (Corning) coated pates at 37◦C and 5% CO2. Stem cells were differentiated to bona fide 2D Motor Neurons as previously described.39,67,68 This protocol based on the principle of neuralization by dual-Smad inhibition followed by the inhibition of NOTCH/FGF pathway both under the patterning capability of retinoids and Sonic Hedgehog. Briefly, once 90–95% confluent, stem cell medium was switched to differentiation medium: 1:1 mix of Neurobasal (Life Technologies 21,103,049) and DMEM/F12 (Life Technologies, 11,320–033) supplemented with B27 supplement (2%v/v, Gibco), N2 supplement (0.5%v/v, Gibco), 1X GlutaMAX (Gibco), 0.1mM Non-essential amino acid (Gibco). For the first six days, differentiation medium was supplemented with 10 μM SB431543 (Custom Synthesis), 100nM LDN193189 (Custom Synthesis), 1 μM retinoic acid (Sigma) and 1 μM Smoothened agonist (Custom Synthesis). For the second week, differentiation medium was supplemented with: 5 μM DAPT (Custom Synthesis), 4 μM SU-5402 (Custom Synthesis), 1 μM retinoic acid (Sigma) and 1 μM Smoothened agonist (Custom Synthesis). To isolate neurons from mixed cultures we utilised an immune-panning based method previously described.39,69 At day 14, monolayers were dissociated with Accutase (Gibco) for 1 h at 37◦C. After gentle, repeated pipetting, cells were collected, spun down, and resuspended in sorting buffer and filtered. Single cell suspensions were incubated with antibody against NCAM (BD Bioscience, 557,919, 1:200) for 25 min, washed and NCAM+ cells were sorted with an BD FACS Aria II cell sorter. Sorted 2D MN were then plated on mouse glial cultures in motor neuron medium (Neurobasal (Life Technologies 21,103,049) supplemented with B27 supplement (2%v/v, Gibco), N2 supplement (0.5%v/v, Gibco), 1X GlutaMAX (Gibco), 0.1mM Non-essential amino acid (Gibco), 0.5% glucose) with the addition of 10 ng/mL of BDNF, CNTF and GDNF (R&D Systems). For most experiments, neurons were co-cultured with murine glial cells (150,000 cells/cm2) derived from postnatal brains (P0–2) as previously described.29
Co-culture of Ngn2 motor neurons and mouse myoblasts in microfluidic devices
Mouse myoblasts from hindlimb skeletal muscles of young adult mice and mouse glia form neonatal mouse brains were isolated and cultured as previously described.29,70 Microfluidic device chips (XC450, XONA Microfluidics) were designated a motor neuron compartment and a muscle compartment. The motor neuron compartment was coated with 0.1 mg/mL poly-L-ornithine (Sigma-Aldrich) in 50 mM Borate buffer, pH = 8.5 and 5 μg/mL laminin (Invitrogen), while the muscle compartment was coated with Matrigel (Corning). Day 7 Ngn2 motor neurons and mouse glia were seeded at a concentration of 100,000 neurons-200,000 glia/device. Myoblasts were seeded at a concentration of 150,000 device. Motor neurons were seeded in the motor neuron media described above with the addition of 10 ng/mL of BDNF, CNTF and GDNF (R&D Systems). For seeding and culturing the first 2 days, myoblasts were maintained in Myoblast media (DMEM/F12, 20% Fetal Bovine Serum and 10% heat-inactivated Horse Serum, and 10 ng/mL bFGF), after that, differentiation was initiated by adding myoblast differentiation media (DMEM high glucose, 5% heat-inactivated Horse Serum). Myoblast were sustained in differentiation medium for 3 days and then switched to motor neuron medium with the addition of 10 ng/mL of BDNF, CNTF and GDNF (R&D Systems) and while medium in motor neuron compared contained no neurotrophic factors to start recruitment of motor neuron axons to the muscle compartment by generation of a volumetric gradient (50 μL difference in volume between the compartments) in the device. Volumetric gradient was kept for every medium change, done every other day. Co-cultures were fixed at day 21 post-seeding for visualization of motor axon-muscle synaptic contacts.
METHOD DETAILS
FACS analyses
We used an Hb9::GFP reporter stem cell line previously described infected with the Ngn2 lentiviral constructs as described above.29 Briefly, cells were differentiated in 24 well plates and subjected to different patterning molecules. At each time point, cells were dissociated with Accutase (Gibco) as previously described, each replicate was frozen in Cryostor CS10 (STEMCELL Technologies). After all samples were collected, cells were thawed in separated tubes are resuspended in sorting buffer as described by others,39 The BD FACS Aria II cell sorted was used to quantify the percentage of Hb9:GFP+ cells in each sample after using DAPI signal to determine cell viability.
Immunofluorescence assays
Cells were washed once with PBS, fixed with 4% PFA for 20 min, washed again in PBS and blocked for 1 h in 0.1% Triton in PBS with 10% donkey serum. Fixed cells were then washed and incubated overnight with primary antibodies at 4◦C. Primary antibody solution was washed and cells were subsequently incubated with secondary antibodies (1:2000, Alexa Fluor, Life Technologies) at room temperature for 1 h, washed with PBS and stained with DAPI. Primary antibodies used: Tuj1 (R&D, MAB1195), Islet1 (Abcam, ab178400), MAP2 (Abcam, ab5392), Synapsin (Millipore, AB1543), SMI-32 (BioLegend, 801,702), Chat (Millipore, AB144P), Foxp1 (Abcam, ab16645), AnkyrinG (Millipore, MABN466), Synaptophysin (Synaptic Systems, 101,004), PSD-95 (Abcam, ab2723), STMN2 (Novus NBP49461). Images were analyzed using FIJI.
RNA extraction and RT-qPCR analyses
RNA was extracted with the miRNeasy Mini Kit (Qiagen, 217,004). cDNA was produced with iScript kit (BioRad) using 50 ng of RNA. RT-qPCR reactions were performed in triplicates using 20 ng of cDNA with SYBR Green (BioRad) and were run on a CFX96 Touch PCR Machine for 39 cycles at: 95◦C for 15s, 60◦C for 30s, 55◦C for 30s.
Western blots
For WB analyses, cells were lysed in RIPA buffer with protease inhibitors (Roche). After protein quantification by BCA assay (ThermoFisher), ten micrograms of protein were preheated in Laemmli’s buffer (BioRad), loaded in 4–20% mini-PROTEAN TGXprecast protein gels (BioRad) and gels were transferred to a PDVF membrane. Membranes were blocked in Odyssey Blocking Buffer (Li-Cor) and incubated overnight at 4◦C with primary antibodies (1:1000 dilution). After washing with TBS-T, membranes were incubated with IRDye secondary antibodies (Li-Cor) for 1 h and imaged with Odyssey CLx imaging system (Li-Cor). Primary antibodies used: Tuj1 (R&D, MAB1195), Synapsin (Millipore, AB1543), PSD-95 (Neuromab, 75–028), GAPDH (Millipore, MAB374).
Multi Electrode array analysis
Electrophysiological recordings were obtained by Axion Biosystems Multi-Electrode Array (MEA) plate system (Axion Biosystems, 12 wells or 48 wells formats) that recorded extracellular spike potential. On day 7 of differentiation, cells were detached and counted and mixed with murine glia as described above. MEA plates were previously coated with Matrigel (Corning) and cells were seeded in Neurobasal medium supplemented with ROCK inhibitor for 24 h. Recordings were performed every 2–3 days and medium was changed after recordings. Analysis was performed with AxIS (Axion Biosystems – Neuronal Metric Tool) as described by others.14,71
QUANTIFICATION AND STATISTICAL ANALYSIS
Stem cell lines, villages, single-cell RNA-sequencing, Census-seq and Dropulation
Methods for Census-seq and Dropulation are described elsewhere,1,2 we are going to provide a brief description below:
Human pluripotent cell lines and village generation1 (Mitchell et al. 2020)
Human ESC lines used in this study were part of a collection previously described.72 These lines were exome sequenced and whole genome sequenced after minimal passaging and cultured as described. Individual lines were cultured and differentiated into neurons as described. At day 6 after doxycycline induction, when cells are postmitotic, cultures were dissociated with Accutase (Gibco) and resuspended in mTeSR medium with 1 μM RoCK inhibitor Y-27632. To generate balanced “villages”, cell suspensions were counted using a Scepter 2.0 Handheld Cell Counter (Millipore Sigma) with 60 μM Scepter Cell Counting Sensor (Millipore Sigma), 0.5M viable cells from each donor cell line were mixed. At this time point 0.5M cells were harvested for Census-seq analysis and ensure balanced representation, the rest was plated for subsequent experiments.
DNA isolation and library preparation1 (Mitchell et al., 2020)
Every seven days, pellets were harvested from separate wells of the “liMNs village” after dissociation with Accutase (Gibco). Pellets were lysed and DNA precipitated and DNA was used to generate libraries using TruSeq Nano DNA Library Prep Kit (Illumina), libraries were sequenced using NextSeq 500 Sequencing System (Illumina). Generated libraries were aligned to human genome using BWA, reference genome was selected to match the genomes used to generate VCF files containing the whole-genome sequenced genotypes of each donor cell line. To exclude confounding mouse DNA from glia, a multi-organism reference was used, reads competitively aligned to both genomes and only high quality (MQ ≥ 10) were used for assignment.
Census-seq analysis1 (Mitchell et al., 2020)
The algorithms used to assign donor contribution to villages are extensively described elsewhere and their validation is outside the scope of this publication. However, briefly the aim of Census-seq algorithms is to accurately detect and precisely quantify the contribution of donors in a mixed DNA sample to monitor population dynamics over time and/or conditions. This can be achieved systematically and inexpensively by lightly sequencing genomic DNA, the algorithms attempt to determine the donors’ mixture by determining the ratio of alleles present at every SNP. The gradient-descent algorithm can then use this data to identify the donormix that maximizes the likelihood of any observed sequence data. Once the best ratio is identified, the algorithms compare the computed “most likely donor mix” to a VCF file that contains whole genome-sequencing data from all stem cell lines in the collection. These VCF files contain a filtered and refined matrix with alternate alleles at each variant for every donor’s genotype. Census-seq can use this data to find a vector of donor-specific contribution (to the mix) that can explain the allele counts detected at each site in the sequencing data provided. For each site, the allele frequency is inferred using the VCF reference files and its proportion of donor in the pool of DNA can then be calculated over the total counts for that specific site. The algorithms are then able to sum the proportion of each donor’s representation at every specific site and calculate total representation of each genotype, a.k.a. donor, in the pooled DNA, providing us an estimate of the ratio of donors in the village.
Dropulation: scRNA-sequencing and donor assignment (Wells, Nemesh, Ghosh, Mitchell et al., 2021)
For single-cell analyses, cells were harvested and prepared with 10X Chromium Single Cell 3′ Reagents V3 and sequenced on a NovaSeq 6000 (Illumina) using an S2 flow cell at 2 × 100bp. Raw sequence files were then aligned and prepared following previous Drop-seq workflow.73 Human reads were aligned to GRCh18 and filtered for high quality mapped reads (MQ ≥ 10). In order to identify donor identity of each droplet, variants were filtered through several quality controls as described previously be included in the VCF files,2,74 to summarise the goal is to only use sites that unambiguously and unequivocally can be detected as A/T or G/C. Once both the sequenced single-cell libraries and VCF reference files are filtered and QC’ed, the Dropulation algorithm is run. Dropulation analyses each droplet, hence a cell, independently and for each cell generates a number representing the likely provenance of each droplet from one donor. Each variant site is assigned a probability score for a given allele in the sequenced unique molecular identifier (UMI) calculated as the probability of the base observed compared to expected based, and 1 – probability that those reads disagree with the base sequenced. Donor identity is then assigned as the computed diploid likelihood at each UMI summed up across all sites.
This probability-based analysis allows to increase confidence in donor detection per barcode by increase the numbers of individuals in the VCF files: more individuals, more UMIs with site variants, more confident scores, higher quality donor assignments. After assigning a “likelihood score”, sites where only few donors have detected reads are ignored and scores are adjusted to allow only high confidence variant sites to be included. This second computer score is then added to the original likelihood as a weighted average score, this mixed coefficient defines the proportion of the population that presents each genotype and in adds to 1. Based on this mixed coefficient that takes into account reads mapped to each donors and the confidence to which each site can be used for this assignment, Dropulation then contains algorithms able to detect “doublets”, barcoded droplets with genetic DNAs assigned to two different donors, to avoid analysing barcodes with admixed identity but also to avoid excluding barcoded droplets with unclear donor assignment based on the coefficient previously calculated.2
Once scores are calculated, the algorithm assigns donors to single droplets. Then runs the double detection and cells that are likely doublets are filtered out. After that, donor identities are confirmed only if p value<0.05. These cells are then validated by crossing proportions of each donors as known inputs in the village and excluding any unexpected identity. Donors composing less then 0.2% of the libraries are excluded from the experiment.2
More details on the preparation of libraries and donor identification can be found in published work.2
scRNAseq analysis of villages and integrated datasets
Matrices from neuronal villages were built from 12 separate runs of 10X Chromium Single Cell 3′ Reagents V3 as described above. Any barcode with less than 400 genes and combined UMI matrices were used for downstream analysis using Seurat (v3.0.2).75 After that, barcodes were further filtered by number of genes detected 1500 < nFeature_RNA<7000 and percent of mitochondrial and ribosomal genes to reduced the number of dying cells/debris: percent.mito<20, 3 < percent.RPS<15, 5 < percent.RPS<10. The matrix was then processed via the Seurat pipeline: log-normalized by a factor of 10,000, followed by regressing out UMI counts, mitochondrial and ribosomal genes, scaled for gene expression. After quality filtering, barcodes were used to compute SNN graphs and t-SNE projections using numbers of Principal Components based on ElbowPlot analysis. SNN-graphed t-SNE projection was used to determine minimum number of clusters obtain at resolution = 0.2 (FindClusters) as described previously.69 Integration with Rayon et al. 2021 was performed using matrices and metadata available at https://github.com/briscoelab/human_single_cell. Only barcodes with available metadata concerning their cellular identity from Rayon et al. were selected to use identities assigned by peer review publication.45 The available barcodes were then loaded into Seurat v4.0.1.66 Integration with libraries previously generated from villages of liMNs was achieved using SCTransform on a merged object running the PreSCTIntegration() function according to the sctransform integration pipeline.76 Analysis of MN alone was conducted as described above by comparing liMNs generated in this study with barcodes identified as “pMN” and “MN” by Rayon et al.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
|
| ||
| Mouse monoclonal anti-Tubulin beta-III | R&D | Cat# MAB1195; RRID:AB_356859 |
| Rabbit monoclonal anti-Isletl | Abcam | Cat# ab178400 |
| Chicken polyclonal anti-MAP2 | Abcam | Cat# ab5392; RRID:AB_2138153 |
| Rabbit monoclonal anti-Synapsin | Millipore | Cat# AB1543, RRID:AB_2200400 |
| Mouse monoclonal anti-Neurofilament-H, nonphosphorylated (SMI-32) | BioLegend | Cat# 801702, RRID:AB_2715852 |
| Goat polyclonal anti-Choline Acetyltransferase | Millipore | Cat# AB144P; RRID:AB_11212924 |
| Rabbit polyclonal anti-Foxpl | Abcam | Cat# ab16645, RRID:AB_732428 |
| Mouse moloclonal anti-Ankyrin-G | Millipore | Cat# MABN466, RRID:AB_2749806 |
| Guiena pig polyclonal anti-Synaptophysin | Synaptic Systems | Cat# 101 004, RRID:AB_1210382 |
| Mouse monoclonal anti-PSD-95 | Abcam | Cat# ab2723, RRID:AB_303248 |
| Mouse monoclonal anti-PSD-95 | Neuromab | Cat# 75-028, RRID:AB_2292909 |
| Mouse monoclonal anti-GAPDH | Millipore | Cat# MAB374, RRID:AB_2107445 |
| Rabbit polyclonal anti-SCG10/STMN2 | Novus Biologicals | NBP49461 |
|
| ||
| Bacterial and virus strains | ||
|
| ||
| FUW-TetO-Ngn2-Puro | (Zhang et al.)12 | N/A |
| FUW-rtTA | (Zhang et al.)12 | N/A |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| Retigabine | Santa Cruz Biotechnology | N/A |
|
| ||
| Critical commercial assays | ||
|
| ||
| miRNeasy Mini Kit | Qiagen | 217,004 |
| iScript kit | BioRad | N/A |
| SYBR Green | BioRad | N/A |
| mini-PROTEAN® TGX™precast protein gels | BioRad | N/A |
| IRDye® secondary antibodies | Li-Cor | N/A |
| Axion Biosystems Multi-Electrode Array (MEA) plate system | Axion Biosystems | N/A |
|
| ||
| Deposited data | ||
|
| ||
| Raw and analyzed data | GEO: GSE219112DUOS: DUOS-000121 | https://duos.broadinstitute.org/ |
|
| ||
| Experimental models: Cell lines | ||
|
| ||
| Embryonic stem cell collection | Embryonic stem cell collection | Embryonic stem cell collection |
| Hues3-Hb9:GFP | HSCRB, Harvard University. (Di Giorgio, et al.)29 | N/A |
|
| ||
| Oligonucleotides | ||
|
| ||
| Pre-design qPCR Assays | IDT | https://www.idtdna.com/site/order/qpcr/predesignedassay |
|
| ||
| Software and algorithms | ||
|
| ||
| ImageJ-Fji | NIH | https://imagej-nih-gov.ezp-prod1.hul.harvard.edu/ij/, Fiji, RRID: SCR_002285 |
| GraphPad Prism | GraphPad software | https://www.graphpad.com; GraphPad Prism, RRID: SCR_002798 |
| Image Studio Lite Software | Image Studio Lite Acquisition Software LI-COR Biosciences | https://www.licor.com/bio/image-studio/; RRID: SCR_013715 |
| Seurat | (Hao and Hao et al.)66 | https://satijalab.org/seurat/index.html |
| BioRender | BioRender | http://biorender.com; RRID: SCR_018361 |
Highlights.
Overexpressing Ngn2 with RA and SAG produces liMNs/liMoNes
liMNs express MN markers, are electrophysiologically active, contact muscle in vitro
liMNs can be generated from dozens of lines and analyzed simultaneously by Dropulation
liMNs are composed of distinct MN pools that resemble primary, embryonic, human MNs
ACKNOWLEDGMENTS
The authors thank members of the Eggan, McCarroll, Nehme, and Pietiläinen groups for insights and useful discussions and Lee Rubin and members of the Rubin laboratory for support in the final steps of this project. We are grateful to the Harvard Stem Cell and Regenerative Biology Department (HSCRB) for the use of shared equipment, microscopy core, and FACS facility. We are also grateful to the Stanley Center at the Broad Institute of Harvard and MIT for support with bioinformatics and cloud space. We thank BioRender.com for useful diagrams and images used in figures throughout this publication.
We were supported by U01MH115727 to S.A.M., R.N., and K.E., as well as by the Stanley Center for Psychiatric Research at Broad Institute of MIT and Harvard, United States, and the Harvard Stem Cell and Regenerative Biology Department at Harvard University, United States.
INCLUSION AND DIVERSITY
We worked to ensure diversity in experimental samples through the selection of the cell lines. We worked to ensure diversity in experimental samples through the selection of the genomic datasets. One or more of the authors of this paper self-identifies as an under-represented ethnic minority in their field of research or within their geographical location. One or more of the authors of this paper self-identifies as a gender minority in their field of research. One or more of the authors of this paper self-identifies as a member of the LGBTQIA+ community.
Footnotes
DECLARATION OF INTERESTS
K.E. is cofounder of Q-State Biosciences, Quralis, Enclear Therapies, and is group vice president at BioMarin Pharmaceutical.
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2022.111896.
REFERENCES
- 1.Mitchell JM, Nemesh J, Ghosh S, Handsaker RE, Mello CJ, Meyer D, Raghunathan K, de Rivera H, Tegtmeyer M, Hawes D, et al. (2020). Mapping genetic effects on cellular phenotypes with “cell villages”. Preprint at bioRxiv. 10.1101/2020.06.29.174383. [DOI]
- 2.Wells MF, Nemesh J, Ghosh S, Mitchell JM, Mello CJ, Meyer D, Raghunathan K, Tegtmeyer M, Hawes D, Neumann A, et al. (2021). Natural variation in gene expression and Zika virus susceptibility revealed by villages of neural progenitor cells. Preprint at bioRxiv. 10.1101/2021.11.08.467815. [DOI] [PMC free article] [PubMed]
- 3.Penney J, Ralvenius WT, and Tsai LH (2020). Modeling Alzheimer’s disease with iPSC-derived brain cells. Mol. Psychiatry 25, 148–167. 10.1038/s41380-019-0468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Limone F, Klim JR, and Mordes DA (2022). Pluripotent stem cell strategies for rebuilding the human brain. Front Aging Neurosci 14, 1017299. 10.3389/fnagi.2022.1017299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, and Studer L. (2009). Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol 27, 275–280. 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers SM, Qi Y, Mica Y, Lee G, Zhang XJ, Niu L, Bilsland J, Cao L, Stevens E, Whiting P, et al. (2012). Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat. Biotechnol 30, 715–720. 10.1038/nbt.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulting GL, Kiskinis E, Croft GF, Amoroso MW, Oakley DH, Wainger BJ, Williams DJ, Kahler DJ, Yamaki M, Davidow L, et al. (2011). A functionally characterized test set of human induced pluripotent stem cells. Nat. Biotechnol 29, 279–286. 10.1038/nbt.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kampmann M. (2020). CRISPR-based functional genomics for neurological disease. Nat. Rev. Neurol 16, 465–480. 10.1038/s41582-020-0373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mertens J, Marchetto MC, Bardy C, and Gage FH (2016). Evaluating cell reprogramming, differentiation and conversion technologies in neuroscience. Nat. Rev. Neurosci 17, 424–437. 10.1038/nrn.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, and Wernig M. (2010). Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041. 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, and Eggan K. (2011). Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 9, 205–218. 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, Marro S, Patzke C, Acuna C, Covy J, et al. (2013). Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 78, 785–798. 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin HC, He Z, Ebert S, Schörnig M, Santel M, Nikolova MT, Weigert A, Hevers W, Kasri NN, Taverna E, et al. (2021). NGN2 induces diverse neuron types from human pluripotency. Stem Cell Rep. 16, 2118–2127. 10.1016/j.stemcr.2021.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nehme R, Zuccaro E, Ghosh SD, Li C, Sherwood JL, Pietilainen O, Barrett LE, Limone F, Worringer KA, Kommineni S, et al. (2018). Combining NGN2 programming with developmental patterning generates human excitatory neurons with NMDAR-mediated synaptic transmission. Cell Rep. 23, 2509–2523. 10.1016/j.celrep.2018.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hester ME, Murtha MJ, Song S, Rao M, Miranda CJ, Meyer K, Tian J, Boulting G, Schaffer DV, Zhu MX, et al. (2011). Rapid and efficient generation of functional motor neurons from human pluripotent stem cells using gene delivered transcription factor codes. Mol. Ther 19, 1905–1912. 10.1038/mt.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzoni EO, Mahony S, Peljto M, Patel T, Thornton SR, McCuine S, Reeder C, Boyer LA, Young RA, Gifford DK, and Wichterle H. (2013). Saltatory remodeling of Hox chromatin in response to rostrocaudal patterning signals. Nat. Neurosci 16, 1191–1198. 10.1038/nn.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han SSW, Williams LA, and Eggan KC (2011). Constructing and deconstructing stem cell models of neurological disease. Neuron 70, 626–644. 10.1016/j.neuron.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Jessell TM (2000). Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat. Rev. Genet 1, 20–29. 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 19.Stifani N. (2014). Motor neurons and the generation of spinal motor neuron diversity. Front. Cell. Neurosci 8, 293. 10.3389/fncel.2014.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sances S, Bruijn LI, Chandran S, Eggan K, Ho R, Klim JR, Livesey MR, Lowry E, Macklis JD, Rushton D, et al. (2016). Modeling ALS with motor neurons derived from human induced pluripotent stem cells. Nat. Neurosci 19, 542–553. 10.1038/nn.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis-Dusenbery BN, Williams LA, Klim JR, and Eggan K. (2014). How to make spinal motor neurons. Development 141, 491–501. 10.1242/dev.097410. [DOI] [PubMed] [Google Scholar]
- 22.Amoroso MW, Croft GF, Williams DJ, O’Keeffe S, Carrasco MA, Davis AR, Roybon L, Oakley DH, Maniatis T, Henderson CE, and Wichterle H. (2013). Accelerated high-yield generation of limb-innervating motor neurons from human stem cells. J. Neurosci 33, 574–586. 10.1523/JNEUROSCI.0906-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, et al. (2008). Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321, 1218–1221. 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 24.Kiskinis E, Sandoe J, Williams LA, Boulting GL, Moccia R, Wainger BJ, Han S, Peng T, Thams S, Mikkilineni S, et al. (2014). Pathways disrupted in human ALS motor neurons identified through genetic correction of mutant SOD1. Cell Stem Cell 14, 781–795. 10.1016/j.stem.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Santis R, Garone MG, Pagani F, de Turris V, Di Angelantonio S, and Rosa A. (2018). Direct conversion of human pluripotent stem cells into cranial motor neurons using a piggyBac vector. Stem Cell Res. 29, 189–196. 10.1016/j.scr.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, and Jessell TM (1996). Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell 84, 309–320. 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- 27.Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, and Sockanathan S. (1999). Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron 23, 659–674. 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- 28.Muhr J, Graziano E, Wilson S, Jessell TM, and Edlund T. (1999). Convergent inductive signals specify midbrain, hindbrain, and spinal cord identity in gastrula stage chick embryos. Neuron 23, 689–702. 10.1016/s0896-6273(01)80028-3. [DOI] [PubMed] [Google Scholar]
- 29.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, and Eggan K. (2007). Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat. Neurosci 10, 608–614. 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wichterle H, Lieberam I, Porter JA, and Jessell TM (2002). Directed differentiation of embryonic stem cells into motor neurons. Cell 110, 385–397. 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 31.Di Giorgio FP, Boulting GL, Bobrowicz S, and Eggan KC (2008). Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell 3, 637–648. 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Machado CB, Kanning KC, Kreis P, Stevenson D, Crossley M, Nowak M, Iacovino M, Kyba M, Chambers D, Blanc E, and Lieberam I. (2014). Reconstruction of phrenic neuron identity in embryonic stem cell-derived motor neurons. Development 141, 784–794. 10.1242/dev.097188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazzoni EO, Mahony S, Closser M, Morrison CA, Nedelec S, Williams DJ, An D, Gifford DK, and Wichterle H. (2013). Synergistic binding of transcription factors to cell-specific enhancers programs motor neuron identity. Nat. Neurosci 16, 1219–1227. 10.1038/nn.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdel-Maguid TE, and Bowsher D. (1984). Classification of neurons by dendritic branching pattern. A categorisation based on Golgi impregnation of spinal and cranial somatic and visceral afferent and efferent cells in the adult human. J. Anat 138, 689–702. [PMC free article] [PubMed] [Google Scholar]
- 35.Thaler JP, Koo SJ, Kania A, Lettieri K, Andrews S, Cox C, Jessell TM, and Pfaff SL (2004). A postmitotic role for Isl-class LIM homeodomain proteins in the assignment of visceral spinal motor neuron identity. Neuron 41, 337–350. 10.1016/s0896-6273(04)00011-x. [DOI] [PubMed] [Google Scholar]
- 36.Guerra San Juan I, Nash LA, Smith KS, Leyton-Jaimes MF, Qian M, Klim JR, Limone F, Dorr AB, Couto A, Pintacuda G, et al. (2022). Loss of mouse Stmn2 function causes motor neuropathy. Neuron 110, 1671–1688.e6. 10.1016/j.neuron.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yadav A, Matson KJE, Li L, Hua I, Gaur P, Alkaslasi M, Hasan S, Galuta A, Dedek A, Ameri S, et al. (2022). The human motoneuron expression signature is defined by ALS-related genes. Preprint at bioRxiv. 10.1101/2022.03.25.485808. [DOI]
- 38.Dasen JS, De Camilli A, Wang B, Tucker PW, and Jessell TM (2008). Hox repertoires for motor neuron diversity and connectivity gated by a single accessory factor. Cell 134, 304–316. 10.1016/j.cell.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Klim JR, Williams LA, Limone F, Guerra San Juan I, Davis-Dusenbery BN, Mordes DA, Burberry A, Steinbaugh MJ, Gamage KK, Kirchner R, et al. (2019). ALS-implicated protein TDP-43 sustains levels of STMN2, a mediator of motor neuron growth and repair. Nat. Neurosci 22, 167–179. 10.1038/s41593-018-0300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wainger BJ, Kiskinis E, Mellin C, Wiskow O, Han SSW, Sandoe J, Perez NP, Williams LA, Lee S, Boulting G, et al. (2014). Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 7, 1–11. 10.1016/j.celrep.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wainger BJ, Macklin EA, Vucic S, McIlduff CE, Paganoni S, Maragakis NJ, Bedlack R, Goyal NA, Rutkove SB, Lange DJ, et al. (2021). Effect of Ezogabine on cortical and spinal motor neuron excitability in amyotrophic lateral sclerosis: a randomized clinical trial. JAMA Neurol. 78, 186–196. 10.1001/jamaneurol.2020.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, Heckl D, Ebert BL, Root DE, Doench JG, and Zhang F. (2014). Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87. 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang T, Wei JJ, Sabatini DM, and Lander ES (2014). Genetic screens in human cells using the CRISPR-Cas9 system. Science 343, 80–84. 10.1126/science.1246981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashoti A, Limone F, van Kranenburg M, Alemany A, Baak M, Vivié J, Piccioni F, Dijkers PF, Creyghton M, Eggan K, and Geijsen N. (2022). Considerations and practical implications of performing a phenotypic CRISPR/Cas survival screen. PLoS One 17, e0263262. 10.1371/journal.pone.0263262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rayon T, Maizels RJ, Barrington C, and Briscoe J. (2021). Single-cell transcriptome profiling of the human developing spinal cord reveals a conserved genetic programme with human-specific features. Development 148, dev199711. 10.1242/dev.199711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patani R, Hollins AJ, Wishart TM, Puddifoot CA, Alvarez S, de Lera AR, Wyllie DJA, Compston DAS, Pedersen RA, Gillingwater TH, et al. (2011). Retinoid-independent motor neurogenesis from human embryonic stem cells reveals a medial columnar ground state. Nat. Commun 2, 214. 10.1038/ncomms1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mendelsohn AI, Dasen JS, and Jessell TM (2017). Divergent hox coding and evasion of retinoid signaling specifies motor neurons innervating digit muscles. Neuron 93, 792–805.e4. 10.1016/j.neuron.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blum JA, Klemm S, Shadrach JL, Guttenplan KA, Nakayama L, Kathiria A, Hoang PT, Gautier O, Kaltschmidt JA, Greenleaf WJ, and Gitler AD (2021). Single-cell transcriptomic analysis of the adult mouse spinal cord reveals molecular diversity of autonomic and skeletal motor neurons. Nat. Neurosci 24, 572–583. 10.1038/s41593-020-00795-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Philippidou P, and Dasen JS (2013). Hox genes: choreographers in neural development, architects of circuit organization. Neuron 80, 12–34. 10.1016/j.neuron.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dasen JS, Tice BC, Brenner-Morton S, and Jessell TM (2005). A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell 123, 477–491. 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 51.Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, and Zhang SC (2005). Specification of motoneurons from human embryonic stem cells. Nat. Biotechnol 23, 215–221. 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 52.Ghosh S, Nehme R, and Barrett LE (2022). Greater genetic diversity is needed in human pluripotent stem cell models. Nat Commun 13, 7301. 10.1038/s41467-022-34940-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rigamonti A, Repetti GG, Sun C, Price FD, Reny DC, Rapino F, Weisinger K, Benkler C, Peterson QP, Davidow LS, et al. (2016). Large-scale production of mature neurons from human pluripotent stem cells in a three-dimensional suspension culture system. Stem Cell Rep. 6, 993–1008. 10.1016/j.stemcr.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Demestre M, Orth M, Föhr KJ, Achberger K, Ludolph AC, Liebau S, and Boeckers TM (2015). Formation and characterisation of neuromuscular junctions between hiPSC derived motoneurons and myotubes. Stem Cell Res. 15, 328–336. 10.1016/j.scr.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Guo X, Gonzalez M, Stancescu M, Vandenburgh HH, and Hickman JJ (2011). Neuromuscular junction formation between human stem cell-derived motoneurons and human skeletal muscle in a defined system. Biomaterials 32, 9602–9611. 10.1016/j.biomaterials.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin CY, Yoshida M, Li LT, Ikenaka A, Oshima S, Nakagawa K, Sakurai H, Matsui E, Nakahata T, and Saito MK (2019). iPSC-derived functional human neuromuscular junctions model the pathophysiology of neuromuscular diseases. JCI Insight 4, e124299. 10.1172/jci.insight.124299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puttonen KA, Ruponen M, Naumenko N, Hovatta OH, Tavi P, and Koistinaho J. (2015). Generation of functional neuromuscular junctions from human pluripotent stem cell lines. Front. Cell. Neurosci 9, 473. 10.3389/fncel.2015.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Umbach JA, Adams KL, Gundersen CB, and Novitch BG (2012). Functional neuromuscular junctions formed by embryonic stem cell-derived motor neurons. PLoS One 7, e36049. 10.1371/journal.pone.0036049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stoklund Dittlau K, Krasnow EN, Fumagalli L, Vandoorne T, Baatsen P, Kerstens A, Giacomazzi G, Pavie B, Rossaert E, Beckers J, et al. (2021). Human motor units in microfluidic devices are impaired by FUS mutations and improved by HDAC6 inhibition. Stem Cell Rep. 16, 2213–2227. 10.1016/j.stemcr.2021.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Velasco S, Ibrahim MM, Kakumanu A, Garipler G, Aydin B, Al-Sayegh MA, Hirsekorn A, Abdul-Rahman F, Satija R, Ohler U, et al. (2017). A multi-step transcriptional and chromatin state cascade underlies motor neuron programming from embryonic stem cells. Cell Stem Cell 20, 205–217.e8. 10.1016/j.stem.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu ML, Zang T, Zou Y, Chang JC, Gibson JR, Huber KM, and Zhang CL (2013). Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons. Nat. Commun 4, 2183. 10.1038/ncomms3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nickolls AR, Lee MM, Espinoza DF, Szczot M, Lam RM, Wang Q, Beers J, Zou J, Nguyen MQ, Solinski HJ, et al. (2020). Transcriptional programming of human mechanosensory neuron subtypes from pluripotent stem cells. Cell Rep. 30, 932–946.e7. 10.1016/j.celrep.2019.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nijssen J, Comley LH, and Hedlund E. (2017). Motor neuron vulnerability and resistance in amyotrophic lateral sclerosis. Acta Neuropathol. 133, 863–885. 10.1007/s00401-017-1708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maury Y, Côme J, Piskorowski RA, Salah-Mohellibi N, Chevaleyre V, Peschanski M, Martinat C, and Nedelec S. (2015). Combinatorial analysis of developmental cues efficiently converts human pluripotent stem cells into multiple neuronal subtypes. Nat. Biotechnol 33, 89–96. 10.1038/nbt.3049. [DOI] [PubMed] [Google Scholar]
- 65.Jones RA, Harrison C, Eaton SL, Llavero Hurtado M, Graham LC, Alkhammash L, Oladiran OA, Gale A, Lamont DJ, Simpson H, et al. (2017). Cellular and molecular anatomy of the human neuromuscular junction. Cell Rep. 21, 2348–2356. 10.1016/j.celrep.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hao Y, Hao S, Andersen-Nissen E, Mauck WM 3rd, Zheng S, Butler A, Lee MJ, Wilk AJ, Darby C, Zager M, et al. (2021). Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29. 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fukuda A, Hazelbaker DZ, Motosugi N, Hao J, Limone F, Beccard A, Mazzucato P, Messana A, Okada C, San Juan IG, et al. (2021). De novo DNA methyltransferases DNMT3A and DNMT3B are essential for XIST silencing for erosion of dosage compensation in pluripotent stem cells. Stem Cell Rep. 16, 2138–2148. 10.1016/j.stemcr.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mordes DA, Prudencio M, Goodman LD, Klim JR, Moccia R, Limone F, Pietilainen O, Chowdhary K, Dickson DW, Rademakers R, et al. (2018). Dipeptide repeat proteins activate a heat shock response found in C9ORF72-ALS/FTLD patients. Acta Neuropathol. Commun 6, 55. 10.1186/s40478-018-0555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Limone F, Mordes DA, Couto A, Pietilainen O, Joseph BJ, Burberry A, Mitchell JM, Ghosh S, Meyer D, Therrien M, et al. (2021). Single-nucleus sequencing reveals enriched expression of genetic risk factors sensitises Motor Neurons to degeneration in ALS. Preprint at bioRxiv. 10.1101/2021.07.12.452054. [DOI] [PMC free article] [PubMed]
- 70.Shahini A, Vydiam K, Choudhury D, Rajabian N, Nguyen T, Lei P, and Andreadis ST (2018). Efficient and high yield isolation of myoblasts from skeletal muscle. Stem Cell Res. 30, 122–129. 10.1016/j.scr.2018.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hao J, Wells MF, Niu G, Guerra San Juan I, Limone F, Fukuda A, Lyeton-Jaimes MF, Joseph BJ, Qian M, Mordes DA, et al. (2021). Loss of TBK1 activity leads to TDP-43 proteinopathy through lysosomal dysfunction in human motor neurons. Preprint at bioRxiv. 10.1101/2021.10.11.464011. [DOI]
- 72.Merkle FT, Ghosh S, Kamitaki N, Mitchell J, Avior Y, Mello C, Kashin S, Mekhoubad S, Ilic D, Charlton M, et al. (2017). Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations. Nature 545, 229–233. 10.1038/nature22312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, et al. (2015). Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214. 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Limone F, Mitchell JM, Guerra San Juan I, Smith JLM, Raghunathan K, Couto A, Ghosh S, Meyer D, Mello CJ, Nemesh J, et al. (2022). Efficient generation of lower induced Motor Neurons by coupling Ngn2 expression with developmental cues. Preprint at bioRxiv. 10.1101/2022.01.12.476020. [DOI] [PMC free article] [PubMed]
- 75.Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM 3rd, Hao Y, Stoeckius M, Smibert P, and Satija R. (2019). Comprehensive integration of single-cell data. Cell 177, 1888–1902.e21. 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hafemeister C, and Satija R. (2019). Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296. 10.1186/s13059-019-1874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All codes and algorithms necessary for re-analysis of the single-cell RNA-sequencing data are publicly available and can be found in other publications.1,2 Raw sequencing data and count matrices have been deposited in GEO: GSE219112 and DUOS: DUOS-000121 (https://duos.broadinstitute.org/).
This paper does not report original code.
Further information requests can be directed to Kevin Eggan (kevin.eggan@bmrn.com) or Francesco Limone (francesco_limone@fas.harvard.edu).