Abstract
Stem cell therapy is a promising and rapidly advancing treatment strategy for a multitude of neurologic disorders. Yet, while early phase clinical trials are being pursued in many disorders, the mechanism of action often remains unclear. One important potential mechanism by which stem cells provide neuroprotection is through metabolic signaling with diseased neurons, glia, and other cell types in the nervous system microenvironment. Early studies exploring such interactions report normalization of glucose metabolism, induction of protective mitochondrial genes, and even interactions with supportive neurovasculature. Local metabolic conditions also impact stem cell biology, which can have a large impact on transplant viability and efficacy. Epigenetic changes that occur in the donor prior to collection of stem cells, and even during in vitro culture conditions, may have effects on stem cell biology that are carried into the host upon stem cell transplantation. Transplanted stem cells also face potentially toxic metabolic microenvironments at the targeted transplant site. Novel approaches for metabolically “preconditioning” stem cells prior to transplant harness metabolic machinery to optimize stem cell survival upon transplant. Ultimately, an improved understanding of the metabolic cross-talk between implanted stem cells and the local nervous system environment, in both disease and injury states, will increase the likelihood of success in translating stem cell therapy to early trials in neurological disease.
Keywords: Central nervous system, metabolism, stem cell, transplantation
Graphical Abstract
Introduction
Therapeutic options for neurologic disorders affecting the central nervous system (CNS) are at times hindered by the unknown or complicated mechanisms responsible for the underlying pathogenesis. While some CNS diseases have known genetic causes tied to a certain protein or pathway, many involve an intricate interplay between multiple cell types and metabolic processes within a complex microenvironment (Argueti-Ostrovsky et al., 2021; Guo et al., 2020; Le Gall et al., 2020; Mejzini et al., 2019). Similarly, traumatic brain and spinal cord injury, or vascular events such as stroke, induce a cascade of detrimental events that impact neurologic health (Delage et al., 2021; Mira et al., 2021; Uyeda and Muramatsu, 2020). As such, therapeutic approaches are required that offer multidimensional benefits to the diseased or injured nervous system.
Stem cell transplantation represents a promising opportunity to approach the treatment of CNS diseases and injury in a comprehensive, multifaceted manner. Many types of stem cells, including embryonic stem cells (ESCs), neural stem cells (NSCs), mesenchymal stem cells (MSCs), and induced pluripotent stem cells (iPSCs), are being evaluated in vitro, in vivo, and in translational clinical studies for their potential utility for a range of neurologic conditions (Chen and Feldman, 2017). These and other stem cell subtypes have entered the realm of early clinical trials for a variety of neurologic conditions, capitalizing on their proliferative capacity and adaptable biology. For amyotrophic lateral sclerosis (ALS), for example, intraspinal NSC transplantation has been evaluated in Phase 1 and 2 clinical trials (Feldman et al., 2014; Glass et al., 2016; Goutman et al., 2018). Additionally, several other stem cell types and delivery strategies are in various stages of development and clinical translation for ALS as well as a range of neurodegenerative conditions, including Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, and epilepsy (Bonaventura et al., 2021; De Gioia et al., 2020; Gonzalez et al., 2016; Goutman et al., 2019; Liu et al., 2021; Lunn et al., 2011; Reddy et al., 2020; Schweitzer et al., 2020; Zhao et al., 2021). Likewise, stem cell-based therapies are advancing for traumatic CNS injury (Bonilla and Zurita, 2021; Schepici et al., 2020; Silvestro et al., 2020; Younsi et al., 2021) and stroke (Azad et al., 2016; Hamblin and Lee, 2021; Kawabori et al., 2020).
For the majority of these clinical series, a precise mechanism of action for stem cells is not well established (Neal et al., 2018). Evidence is rapidly accumulating in support for metabolic drivers of pathology in nearly every neurologic disease, and thus metabolic pathways represent a promising window for stem cells to exert beneficial effects (Piers et al., 2020). Herein, we review the possible metabolic considerations associated with stem cell therapies, with particular emphasis on how stem cells impact the local environments and how metabolic implications of neurologic disease and injury states affect cell transplants.
Types of stem cells
Insight into the potential metabolic implications of the various stem cell classes used in transplantation research and translational applications first requires understanding of the origins and attributes of each stem cell type. ESCs are cells derived from the zygote or inner cell layer of the developing blastocyst, the former being totipotent (capable of differentiating to any cell type) and the latter being pluripotent (capable of differentiating to almost any cell type). In a similar vein, iPSCs are cultured cells (e.g., fibroblasts) that have been reprogrammed to express the Yamanaka factors (Oct3/4, Sox2, Klf4, c-Myc) and re-enter a state of pluripotency capable of differentiating into many cell types. NSCs, by contrast, are stem cells capable of proliferation but, upon differentiation, committed to neuroglial cell types. Similarly, MSCs are derived from a variety of tissues, such as bone marrow, and differentiate into mesodermal tissues, but have been coaxed into differentiating towards ectodermal and endodermal fates as well. Ultimately, the source and range of differentiation abilities should be considered when evaluating how stem cells may affect metabolic mechanisms in the host and vice versa.
Metabolic mechanisms by which stem cell transplants affect the host
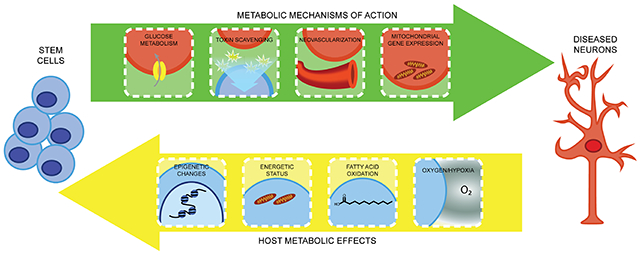
The appeal of stem cells as a therapeutic tool is tied to the many complementary opportunities for neuroprotection made possible by their inherent properties. Stem cells proliferate, providing a self-renewing resource for therapeutic application. They can also differentiate into a range of cell types, and experimental paradigms are also now available to generate many neuronal subtypes from stem cells, such as motor neurons or GABAergic neurons (Ben-Shushan et al., 2015; Gupta et al., 2018; Ren et al., 2021; Shen et al., 2021). Another clear benefit of stem cell-based strategies is the ability to affect the host by a multitude of mechanisms, simultaneously and in a sustained manner (Chen et al., 2016; Pacheco-Herrero et al., 2021; Shinozaki et al., 2021; Wei et al., 2017a). Of course, the “holy grail” of regenerative approaches is replenishing a damaged cell population using stem cells. However, particularly in the nervous system, the ability to restore and rewire native neural circuits currently faces insurmountable challenges. Alternatively, stem cell differentiation into interneurons, glia, astrocytes, and other supporting cells offers a means to harness the full biological machinery of a complete cell to attenuate the progression of neurologic diseases. In this regard, stem cells can be employed to support neuromodulation, clear toxins, alter the extracellular matrix, facilitate vascular interactions, and regulate the immune system (Figure 1). Importantly, stem cells are also capable of direct cell-cell communication (gap junctions, synapses, etc.), offer paracrine signaling and trophic support via secreted proteins and extracellular vesicles, and can be readily manipulated in vitro or in vivo to enhance expression of neuroprotective factors (Guy et al., 2019; Herman et al., 2021; McGinley et al., 2016; Willis et al., 2020).
Figure 1: Metabolic implications of stem cell therapies in the CNS.
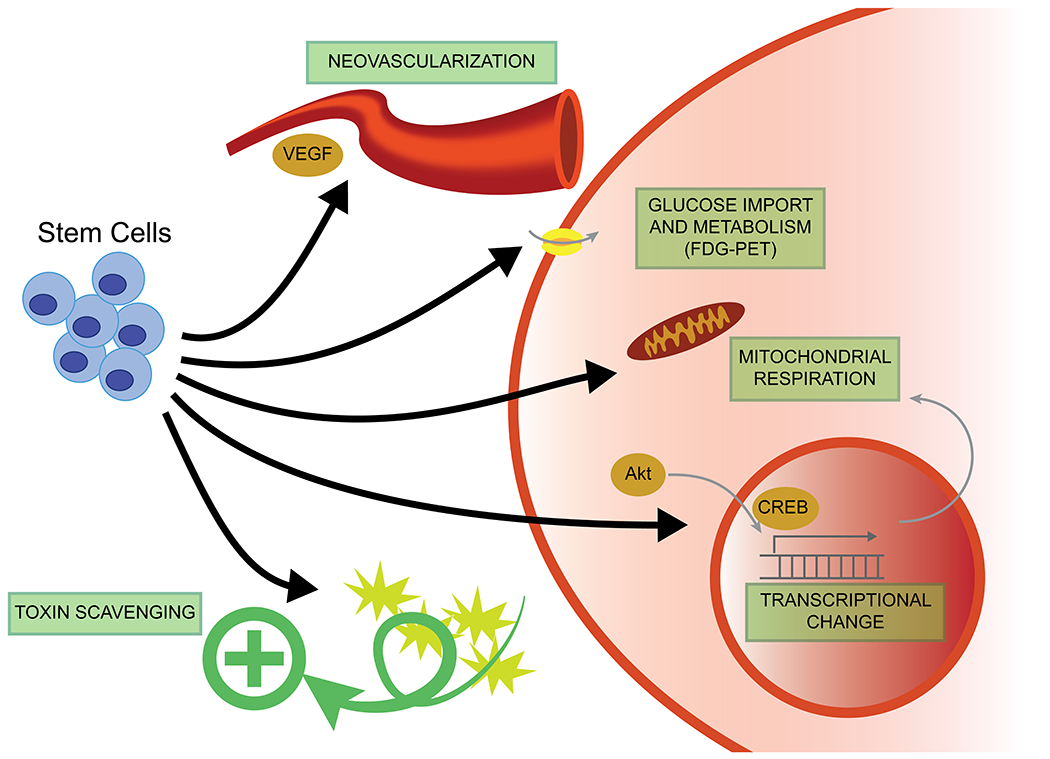
Although precise mechanisms have yet to be delineated, stem cells appear to have multimodal beneficial effects on neuronal metabolism. Stem cells may directly affect glucose metabolism and transport, which is often visualized using fluorodeoxyglucose-positron emission tomography (FDG-PET). Stem cells may also scavenge toxic metabolic byproducts. Mitochondrial proteins, which could mediate normalization of glucose metabolism and other pathways, represent a further downstream target of stem cell signaling. Secreted growth factors, such as VEGF, may also mediate metabolic repair via vascular remodeling.
One mechanism by which transplanted stem cells may benefit the host is by modulation or normalization of metabolic pathways. While the metabolic effects of stem cells have been explored in research fields such as cardiac, liver/pancreas, and hematopoietic stem cell transplants, they have not been well studied in the neurosciences. Limited reports, however, are beginning to provide insight into the implications of stem cells on glucose metabolism, mitochondrial function, and other neurovascular interactions.
Glucose metabolism
At a very basic level, the effect of stem cells on CNS glucose metabolism carries significant impact in the neurosciences. Neurons are known to rely chiefly on glycolysis and oxidative phosphorylation for their high energy demands, with minimal utilization of anaerobic forms of metabolism (Diaz-Garcia and Yellen, 2019). As a result, perturbations in glucose metabolism may have an outsized effect on neuronal function and survival. Transplanted stem cells, on the other hand, may normalize the glucose metabolism of neighboring cells and thus maximize neuronal survival in an otherwise hostile pathologic environment.
Stem cell transplantation studies in neurologic diseases have benefitted from 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) assessment of metabolic integrity. In a study of subventricular zone stem cells transplanted into Sprague-Dawley rats, striatal stem cell transplants were associated in increased FDG-PET signal, although inherent stem cell metabolism versus impact on surrounding host cells could not be parsed (Cicchetti et al., 2007). Interestingly, in a mouse model of temporal lobe epilepsy in which human ESCs were compared to GABAergic neuronal progenitors, restoration of glucose metabolism was only seen in ESC-implanted animals (Du et al., 2019). This appeared to be associated with ESC ability to differentiate down an astrocyte/glial lineage, which may represent a therapeutic strategy for epilepsy and other conditions in which normalization of the neuronal microenvironment is a central goal. Similarly, in a rat model of Huntington’s disease, transplanted mouse iPSCs resulted in improved FDG-PET signal, along with elevated expression of neuronal, astrocyte, and microglial markers (Mu et al., 2014). These studies highlight the advantages of generating diverse cell types to act on the metabolism of diseased host cells.
Evidence for metabolic benefits in the CNS are seen in a handful of studies focused on stroke. In a middle cerebral artery occlusion (MCAO) rat model of ischemic stroke, transplantation of mouse iPSC and ESCs as well as rat NSCs into the ventricular space resulted in improved glucose uptake as measured by FDG-PET within the ischemic region (Wang et al., 2013; Zhang et al., 2015a). In a similar study, human NSCs promoted a restoration of glucose metabolism as measured by FDG-PET signal, and these stem cells had better ability to reduce stroke volume when the ischemic area was more modest in size (Daadi et al., 2013). Metabolic imaging to assess stem cell transplantation in early human trials for stroke confirm transplant feasibility as well as use of FDG-PET as a promising, non-invasive method for probing transplant viability and efficacy (Kondziolka et al., 2000).
Stem cell-associated changes in glucose metabolism are also seen in traumatic conditions. In a model of traumatic brain injury, intraparenchymal injections of rat hippocampal NSCs demonstrated restoration of FDG-PET signal at the injury site (Zhang et al., 2008). By contrast, in a hemisection model of spinal cord injury, glucose content at and around the injury site more closely paralleled that of untreated controls. Instead, ATP and lactate levels appeared to diminish within the injury site in animals receiving NSC transplants (Mautes et al., 2004). These changes were hypothesized to be a result of the metabolism of the transplanted cells themselves and their adaptation to the hostile, traumatized microenvironment. It is apparent that much remains unexplored when considering the effect of stem cells on the complex metabolic cascades following traumatic injury.
In many of the above studies, it is unclear how much of the normalized PET signal is performed by the transplanted cells directly or due to effects on native tissue. Transplanted stem cells may themselves contribute to changes in metabolic readouts to some degree. Nevertheless, FDG-PET is often used as a marker for more large-scale regional brain metabolism, and restoration of this signal is suggestive of rescued neuroglial populations. This is supported by histological correlation in the above studies that demonstrate restoration of host neuron and glial counts, and that changes in FDG-PET are more globally measured when compared to cell-specific PET imaging (Daadi et al., 2013; Zhang et al., 2008).
Additionally, given that neurons are reliant on glucose and that perturbations in glucose metabolism are known to exacerbate pathology seen in most neurologic diseases, the changes in FDG-PET shown in these studies could be due to intrinsic metabolic changes induced by stem cell grafts. In other words, rather than being merely a byproduct of rescued host population cell numbers, elevated FDG-PET signal may result from stem cells altering gene expression to increase glucose uptake in a more greatly elevated, hypercompensatory manner. Further mechanistic explorations are needed to elucidate how glucose normalization is mediated (e.g., signaling that alters neuronal or glial metabolism, alteration in microvascular blood flow, induction of glucose transporters, etc.) and better understand what downstream cellular components (e.g., mitochondrion) are involved in metabolic normalization.
Mitochondrial function
Stem cells may mediate normalization of glucose metabolism by altering mitochondrially expressed proteins in resident host cells. Using the MCAO stroke model in rats, a proteomic analysis identified 39 differentially expressed proteins upon treatment with mouse iPSCs. These included many mitochondrial proteins, such as TOMM20 (translocase of outer mitochondrial membrane 20) and GALE (urine diphosphate (UDP)-galactose 4-epimerase) (Chen et al., 2021). TOMM20 is a member of the mitochondrial translocase of the outer membrane, which functions to shuttle mitochondrial-targeted proteins to the mitochondrial matrix (Omura, 1998) and has been implicated in pathophysiology of Parkinson’s disease (Franco-Iborra et al., 2018; Teixeira et al., 2016). GALE participates in the interconversion of UDP-galactose and UDP-glucose (Frey and Hegeman, 2013), important in the metabolism of galactose and generation of glucose substrates. Thus, transplanted iPSCs appear to directly affect mitochondrial physiology of host cells.
Similarly, in the APP/PS1 mouse model of Alzheimer’s disease, murine NSCs increased mitochondrial DNA and normalization of PGC-1α (peroxisome proliferator-activated receptor-gamma coactivator 1α), NRF-1 (nuclear respiratory factor 1), COXIV (cytochrome c oxidase subunit 4), and other mitochondrial proteins (Zhang et al., 2015b). PGC-1α and NRF-1 are central transcriptional regulators of mitochondrial biogenesis and cellular energy metabolism (Li et al., 2017), and this is confirmed by electron microscopy in NSC-treated animals showing normalized mitochondrial morphology and numbers. Parallel findings were likewise found using a model of Huntington’s disease treated with human adipose stem cells. Here, although mostly GABAergic neurons were formed, there appeared to be a restoration of Akt and CREB (cAMP response element-binding protein) signaling as well as PGC-1α (Lee et al., 2009). While further studies are required to identify factors mediating these stem cell-associated changes in mitochondrial function, these studies provide insight into the mechanisms by which stem cells promote neuroprotection by influencing metabolic pathways.
Neurovascular and other interactions
Stem cells may exert beneficial effects by modulating the metabolic response to pathologic injury in many other ways. For example, in a rat model of neonatal hypoxic-ischemic brain injury, transplanted MSCs appeared to attenuate the proliferation and activation of reactive astrocytes (He et al., 2019). This effect appeared to be mediated by stem cell secretion of IL-6, which suppressed 5’ adenosine monophosphate-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signaling in astrocytes. Importantly, these and the above discussed metabolic interactions could all occur simultaneously in a stem cell transplant treatment paradigm.
Transplanted stem cells may also exert beneficial effects indirectly, by first chiefly affecting neurovascular structures and blood flow, with metabolic changes a secondary benefit. In Alzheimer’s disease models, many studies have shown that stem cells are associated with increased vascular endothelial growth factor (VEGF) expression (Li et al., 2018). In turn, higher VEGF levels are associated with greater glucose metabolism and neuroprotective effects (Wang et al., 2018), which may be mediated by neovascularization (Garcia et al., 2014). Hence, secreted factors derived from transplanted cells impact the supportive structures of neuronal metabolism, and stem cells can thus provide neuroprotection by both direct and indirect mechanisms simultaneously.
While the CNS is traditionally viewed as a privileged vascular environment, the benefits of stem cell transplants may have impact systemically as well. Metabolomic analysis in the MCAO model of ischemic stroke demonstrated that improvements after rat ESC transplants were associated with increased consumption of N,N-dimethylglycine, glucose, and formate, together with reduced excretion of lactate, alanine, glutamate, 3-hydroxybutyrate, glutathione, methionine, aspartate, fatty acyl chain, choline, glycerol, myoinositol, and glycerophosphocholine, as measured in peripheral serum (Gao et al., 2020). Whether these changes result from the stem cells directly and have a downstream impact on host neurons or whether these changes simply reflect rescue of native neuronal/glial populations is unclear, but these findings represent a metabolic signature which may have value as an accessible peripheral biomarker of disease and treatment response.
Host effects on stem cell transplant metabolism
While it is often the expressed goal for stem cell transplants to impact physiology of the host, the converse is emerging as an increasingly important area of study. With this in mind, the metabolic circumstances of donor tissue from which stem cells are derived become of great importance to the transcriptional and epigenetic signatures that are carried along with cell transplants. Once stem cells reach their destination, another consideration is how stem cells are impacted by the challenging microenvironment present in neurologic injury or disease (Frederiksen et al., 2020; Nguyen et al., 2019). While they can aid in detoxifying a potentially hostile disease microenvironment and confer several additional advantages, as mentioned above, stem cells must first be amenable to surviving within the immunologic and metabolic milieu that comprises that environment. Metabolic perturbations of the host, whether due to environmental factors (diet, exposures, etc.) or intrinsic neuropathologic disease processes, likewise impact the physiology of transplanted cells. Here we will summarize early work in understanding how destination metabolic microenvironments impact transplanted cells (Figure 2), and approaches that build upon this knowledge to maximize the survival and therapeutic benefit of cell-based therapy.
Figure 2: Environmental and host metabolic influence on stem cells.
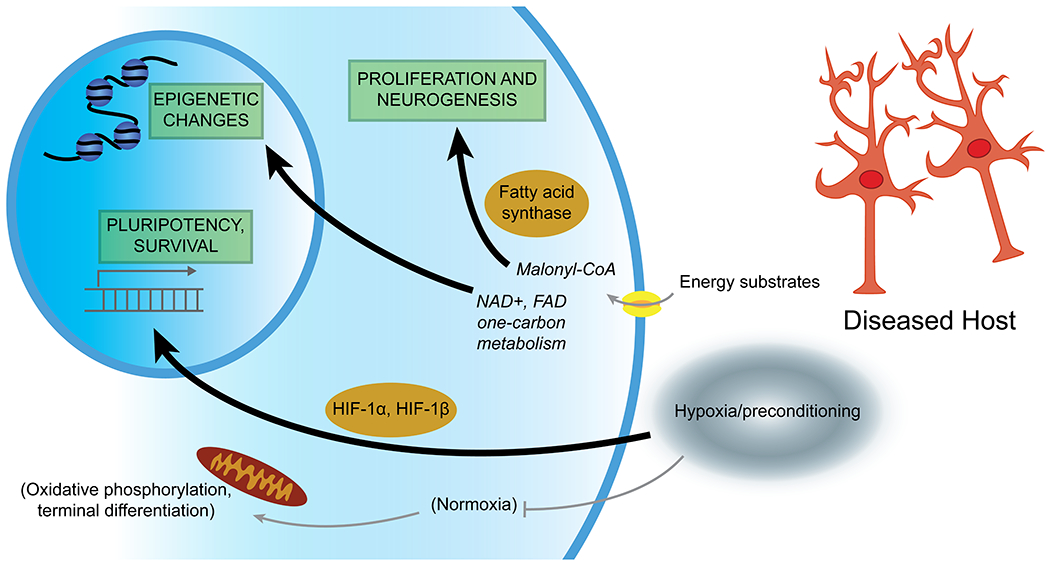
Stem cells are affected by metabolic processes both from their source (carrying resultant epigenetic changes), metabolic changes resulting from in vitro culture, and also face pathologic metabolic signaling in the target tissue. Relative levels of energy substrates appear to influence the biology of proliferation and maintenance of a pluripotency, versus commitment towards terminal differentiation. Many epigenetic alterations resulting from metabolic conditions may also be carried with transplantation and may influence downstream efficacy. “Preconditioning” of stem cells prior to transplant, for example using relative hypoxia, may be a way to improve resilience of transplanted cells and maximize therapeutic benefits.
Metabolic contributors to stem cell quiescence, pluripotency, proliferation, and differentiation
Metabolic disturbances can impact gene expression profiles of stem cells, and can thus influence the delicate balance between quiescence, self-renewal, and terminal differentiation. Interestingly, seemingly opposite dietary modifications, including both ketogenic/restricted calorie diets and high-fat diet, appear to increase stem cell self-renewal by convergent signaling onto common pathways engaged in fatty acid oxidation and peroxisome proliferator-activated receptors (Novak et al., 2021). However, high-fat diet or models of the “Western diet” appear to also impart a tendency towards inappropriate stem cell proliferation and carcinogenesis.
It is also clear that metabolic alterations have significant impact on stem cell epigenetic factors (Fawal and Davy, 2018; Ryall et al., 2015). Factors involved in oxidative phosphorylation act to modulate epigenetic changes (Tay et al., 2021). Sirtuin 1 activity, for example, is crucial for maintenance of pluripotency, participates in histone deacetylation, and is regulated by nicotinamide adenine dinucleotide (NAD+) concentrations that reflect stem cell metabolism (Correia et al., 2017; Fang et al., 2019). Histone and DNA methylation/demethylation by DNA methyltransferases and lysine-specific demethylase 1 also depends on one-carbon metabolism and the concentrations of flavin adenine dinucleotide (FAD), again impacting expression of pluripotency versus differentiation genes (Castex et al., 2017; Ryall et al., 2015). Also, embryonic exposure to hyperglycemia appears to promote chromatin reorganization, histone H3 lysine 9 trimethylation, and global DNA methylation in NSCs (Shyamasundar et al., 2013). Thus, it is important to consider this metabolic “baggage” when establishing stem cell cultures or iPSC lines. Moreover, understanding the metabolic history of cell lines may lead to optimized transplantation paradigms and downstream studies.
Metabolic pathways additionally directly contribute to stem cell survival, maintenance of pluripotency, and the switch from quiescence to proliferation (Wanet et al., 2015). Signaling that involves forkhead box class O (FOXO), mTOR, AMPK, and sirtuin signaling pathways maintain a quiescent stem cell “pool” and minimize oxidative stress; however, the signaling of these pathways may be disrupted by changes in energy availability (Rafalski et al., 2012). Interestingly, fatty acid metabolism also appears to play a central role in stem cell biology. Malonyl-CoA reduces fatty acid oxidation which then promotes exit from quiescence into proliferation (Knobloch et al., 2017), and activity of fatty acid synthase also appears to promote adult neurogenesis and proliferation (Knobloch et al., 2013). The complexities of this area of study are only just beginning to be revealed, but knowledge of metabolic contributions to proliferation and differentiation may maximize stem cell survival and could be harnessed to improve treatment outcomes.
The stem cell niche and metabolic responses to culture and transplantation
The very act of in vitro culture and manipulation can impact stem cell metabolism and subsequent performance. Endogenous stem cells appear to exist in a specific niche with defined environmental factors and metabolic pathway utilization (Ottoboni et al., 2017; Rafalski et al., 2012). For example, certain stem cell populations appear to rely on glucose and preferentially utilize glycolysis over oxidative phosphorylation (Salazar-Noratto et al., 2020). This occurs in the face of relative hypoxia, whereas the switch to oxidative phosphorylation is linked to terminal differentiation in normoxic settings (De Filippis and Delia, 2011). The ability to expand and manipulate stem cell cultures in vitro prior to implantation is often cited as an advantage for stem cell-based approaches. However, keeping in mind the metabolic switch to oxidative phosphorylation is critical when considering that most in vitro culture of stem cells occurs at atmospheric oxygen levels. This exposure to elevated oxygen levels and switch to aerobic respiration may result in fundamental changes that might prove detrimental when cells are transplanted again into damaged, hypoxic host tissues and expected to proliferate (Sandvig et al., 2017).
Furthermore, the destination for cell transplants is often hostile, with altered blood flow, impaired nutrient and toxin shuttling, and inflammatory changes. A demonstration of these interactions was demonstrated using NSC transplants performed in a compression-based model of spinal cord injury in mice (Zhang et al., 2022). At baseline, transplanted NSCs tended to differentiate towards astrocytes in the presence of an M1 proinflammatory phenotype of surrounding microglia. By contrast, spinal cord injury in aldose reductase inhibition or in aldose reductase deficient mice favored an M2 microglial phenotype, associated with differentiation of NSCs toward a neuronal phenotype and improved motor function. Aldose reductase catalyzes the conversion of excess glucose to sorbitol in the polyol pathway, and has been implicated in activation of microglia (Chang et al., 2019). Thus, metabolic factors in stem cell transplant recipients clearly influence the inflammatory milieu, which in turn impacts the differentiation and survival of transplanted stem cells. These studies underscore the need for understanding stem cell interactions with host metabolic microenvironments in order to optimize the efficacy and translation of cell-based therapies.
Stem cell preconditioning
One domain in which metabolic contributors to stem cell performance, and indeed metabolic manipulation, has had greater study is in the realm of ischemic stroke (Bernstock et al., 2017; Yu et al., 2013). It is known that stem cells enter a hostile environment of hypoxia, excitotoxicity, and inflammation when transplanted acutely after stroke. As a result, there is a high degree of cell death for both endogenous and exogenous stem cells (Othman and Tan, 2020). Efforts to combat this are described as stem cell “preconditioning” using approaches such as genetic modifications (Wei et al., 2017a; Xue et al., 2019) or engineered biomaterials (Moshayedi et al., 2016). Alternatively, simple exposure to hypoxic culture conditions appears to result in transcriptional changes that improve metabolic profiles (Wei et al., 2017b). The mechanism underlying this observation is currently under investigation and may be multifactorial. Certainly, the activation of hypoxia inducible factors HIF-1α and HIF-1β is logical, with many potential downstream metabolically active targets, including VEGF, erythropoietin (EPO), sodium-calcium exchanger-1, protein kinase D1, lactate dehydrogenase A, and uncoupling protein 2 (Dehne and Brune, 2009; Greer et al., 2012; Semenza, 2011; Zhang et al., 2019). Interestingly, given the central role of glucose in stem cell and neuronal metabolism, the glucose transporters GLUT3 and glucose-6-phosphate transporter are also induced by HIF-1α after hypoxia (Thamotharan et al., 2013). Other mediators of stem cell preconditioning include EPO (Theus et al., 2008; Wei et al., 2012) or involve an increase in the formation of connexin hemichannels and ATP release (Jaderstad et al., 2010).
Further methods to induce stem cell preconditioning include exposure to compounds such as minocycline (Sakata et al., 2012b), doxycycline (Malik et al., 2013), interleukin-6 (Sakata et al., 2012a), adjudin (Zhang et al., 2017), resveratrol (Yao et al., 2021), or sodium butyrate/nicorandil (Hosseini et al., 2018), or even direct electrical stimulation (George et al., 2017). Again, growth factor secretion and/or angiogenesis appears to be engaged in these processes and are under further study. Notably, the AMPK activator metformin also appears to impart a beneficial effect on stem cell transplants. In an endothelin-1 rat model of stroke, co-treatment with metformin and human iPSC-NSCs resulted in improved proliferation, differentiation, and reduction of human leukocyte antigen (HLA)-A expression in stem cells (Ould-Brahim et al., 2018). Reduction in HLA-A or other antigen presenting molecules may help prevent graft rejection. While detailed metabolic studies of metformin and effects on transplanted stem cells were not performed, this study underscores the complex interplay between metabolism in the periphery, the CNS, injured tissue, and stem cells.
Conclusions
Stem cell therapies for neurologic conditions impart a range of metabolic effects for the CNS as well as for the stem cells themselves (Figures 1 and 2). At baseline, the interaction between normal metabolism, impaired metabolism, and neurologic diseases is complex and poorly understood. Adding in stem cells, with their metabolic interactions with both local microenvironments as well as systemic processes, increases the combinatorial complexity of underlying metabolic and pathologic pathways. However, achieving therapeutic impact on host metabolism using the cellular capabilities of stem cells is a promising paradigm to address a wide range of neurologic conditions. Furthermore, emerging understanding regarding local environmental effects on stem cell metabolism may optimize the efficacy of stem cell treatments. It is apparent that much more detailed, high-quality research in this field is needed, and ongoing study is certain to yield great steps forward in enabling the translation of stem cell therapy for neurologic diseases and injury states. With this increased understanding of the interplay between stem cells and metabolic parameters in the brain, the success of stem cell therapy can ultimately be improved.
Acknowledgements
The authors received funding support from the National Institutes of Health (R01ES030049, R01DK129320, R01NS127188), the Andrea and Lawrence A. Wolfe Brain Health Initiative Fund, and the NeuroNetwork for Emerging Therapies at the University of Michigan. The funders had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- AMPK
AMP-activated protein kinase
- CNS
central nervous system
- COXIV
cytochrome c oxidase subunit 4
- CREB
cAMP response element-binding protein
- EPO
erythropoietin
- ESC
embryonic stem cell
- FAD
flavin adenine dinucleotide
- FDG-PET
fluorodeoxyglucose-positron emission tomography
- FOXO
forkhead box class O
- GALE
urine diphosphate-galactose 4-epimerase
- GLUT
glucose transporter
- HIF
hypoxia-inducible factor
- HLA
human leukocyte antigen
- iPSC
induced pluripotent stem cell
- PGC-1α
peroxisome proliferator-activated receptor-gamma coactivator 1α
- mTOR
mammalian target of rapamycin
- NAD+
nicotinamide adenine dinucleotide
- NRF-1
nuclear respiratory factor 1
- NSC
neural stem cell
- TOMM20
translocase of outer mitochondrial membrane 20
- UDP
urine diphosphate
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures
SAS has no relevant disclosures
KSC has no relevant disclosures
Search terms
A query in PubMed utilizing search terms “stem cell therapy” and “metabolism” and “nervous system” excluding “cancer” was performed, screening for manuscripts describing use of stem cells as a therapeutic in CNS disorders with a potential metabolic mechanism of action. In relevant articles, references were also screened for additional relevant papers.
References
- Argueti-Ostrovsky S, et al. , 2021. All Roads Lead to Rome: Different Molecular Players Converge to Common Toxic Pathways in Neurodegeneration. Cells. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad TD, et al. , 2016. Neurorestoration after stroke. Neurosurg Focus. 40, E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shushan E, et al. , 2015. Notch signaling regulates motor neuron differentiation of human embryonic stem cells. Stem Cells. 33, 403–15. [DOI] [PubMed] [Google Scholar]
- Bernstock JD, et al. , 2017. Neural stem cell transplantation in ischemic stroke: A role for preconditioning and cellular engineering. J Cereb Blood Flow Metab. 37, 2314–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventura G, et al. , 2021. Stem Cells: Innovative Therapeutic Options for Neurodegenerative Diseases? Cells. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla C, Zurita M, 2021. Cell-Based Therapies for Traumatic Brain Injury: Therapeutic Treatments and Clinical Trials. Biomedicines. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castex J, et al. , 2017. Inactivation of Lsd1 triggers senescence in trophoblast stem cells by induction of Sirt4. Cell Death Dis. 8, e2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KC, et al. , 2019. Role of aldose reductase in diabetes-induced retinal microglia activation. Chem Biol Interact. 302, 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KS, Feldman EL, Stem Cell Therapy for Amyotrophic Lateral Sclerosis. In: Boulis NM, et al. , (Eds.), Molecular and Cellular Therapies for Motor Neuron Diseases. Academic Press, Cambridge, MA, 2017, pp. 207–231. [Google Scholar]
- Chen KS, et al. , 2016. Intraspinal stem cell transplantation for amyotrophic lateral sclerosis. Ann Neurol. 79, 342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. , 2021. Quantitative proteomics revealed extensive microenvironmental changes after stem cell transplantation in ischemic stroke. Front Med. [DOI] [PubMed] [Google Scholar]
- Cicchetti F, et al. , 2007. Dual-modality in vivo monitoring of subventricular zone stem cell migration and metabolism. Contrast Media Mol Imaging. 2, 130–8. [DOI] [PubMed] [Google Scholar]
- Correia M, et al. , 2017. Sirtuins in metabolism, stemness and differentiation. Biochim Biophys Acta Gen Subj. 1861, 3444–3455. [DOI] [PubMed] [Google Scholar]
- Daadi MM, et al. , 2013. Imaging neural stem cell graft-induced structural repair in stroke. Cell Transplant. 22, 881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis L, Delia D, 2011. Hypoxia in the regulation of neural stem cells. Cell Mol Life Sci. 68, 2831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gioia R, et al. , 2020. Neural Stem Cell Transplantation for Neurodegenerative Diseases. Int J Mol Sci. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehne N, Brune B, 2009. HIF-1 in the inflammatory microenvironment. Exp Cell Res. 315, 1791–7. [DOI] [PubMed] [Google Scholar]
- Delage C, et al. , 2021. Traumatic Brain Injury: An Age-Dependent View of Post-Traumatic Neuroinflammation and Its Treatment. Pharmaceutics. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Garcia CM, Yellen G, 2019. Neurons rely on glucose rather than astrocytic lactate during stimulation. J Neurosci Res. 97, 883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R, et al. , 2019. PET imaging of metabolic changes after neural stem cells and GABA progenitor cells transplantation in a rat model of temporal lobe epilepsy. Eur J Nucl Med Mol Imaging. 46, 2392–2397. [DOI] [PubMed] [Google Scholar]
- Fang Y, et al. , 2019. Sirtuins in Metabolic and Epigenetic Regulation of Stem Cells. Trends Endocrinol Metab. 30, 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawal MA, Davy A, 2018. Impact of Metabolic Pathways and Epigenetics on Neural Stem Cells. Epigenet Insights. 11, 2516865718820946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman EL, et al. , 2014. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: phase 1 trial outcomes. Ann Neurol. 75, 363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Iborra S, et al. , 2018. Defective mitochondrial protein import contributes to complex I-induced mitochondrial dysfunction and neurodegeneration in Parkinson’s disease. Cell Death Dis. 9, 1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen HR, et al. , 2020. Non-immunogenic Induced Pluripotent Stem Cells, a Promising Way Forward for Allogenic Transplantations for Neurological Disorders. Front Genome Ed. 2, 623717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey PA, Hegeman AD, 2013. Chemical and stereochemical actions of UDP-galactose 4-epimerase. Acc Chem Res. 46, 1417–26. [DOI] [PubMed] [Google Scholar]
- Gao J, et al. , 2020. Metabolomic Profiling of the Synergistic Effects of Ginsenoside Rg1 in Combination with Neural Stem Cell Transplantation in Ischemic Stroke Rats. J Proteome Res. 19, 2676–2688. [DOI] [PubMed] [Google Scholar]
- Garcia KO, et al. , 2014. Therapeutic effects of the transplantation of VEGF overexpressing bone marrow mesenchymal stem cells in the hippocampus of murine model of Alzheimer’s disease. Front Aging Neurosci. 6, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George PM, et al. , 2017. Electrical preconditioning of stem cells with a conductive polymer scaffold enhances stroke recovery. Biomaterials. 142, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, et al. , 2016. Transplantation of spinal cord-derived neural stem cells for ALS: Analysis of phase 1 and 2 trials. Neurology. 87, 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, et al. , 2016. Neural Stem Cell Transplantation and CNS Diseases. CNS Neurol Disord Drug Targets. 15, 881–886. [DOI] [PubMed] [Google Scholar]
- Goutman SA, et al. , 2018. Long-term Phase 1/2 intraspinal stem cell transplantation outcomes in ALS. Ann Clin Transl Neurol. 5, 730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutman SA, et al. , 2019. Stem cell treatments for amyotrophic lateral sclerosis: a critical overview of early phase trials. Expert Opin Investig Drugs. 28, 525–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer SN, et al. , 2012. The updated biology of hypoxia-inducible factor. EMBO J. 31, 2448–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, et al. , 2020. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol Neurodegener. 15, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, et al. , 2018. Fibroblast growth factor 2 regulates activity and gene expression of human post-mitotic excitatory neurons. J Neurochem. 145, 188–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy R, et al. , 2019. Human Muscle Progenitor Cells Overexpressing Neurotrophic Factors Improve Neuronal Regeneration in a Sciatic Nerve Injury Mouse Model. Front Neurosci. 13, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin MH, Lee JP, 2021. Neural Stem Cells for Early Ischemic Stroke. Int J Mol Sci. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, et al. , 2019. Mesenchymal stem cells-derived IL-6 activates AMPK/mTOR signaling to inhibit the proliferation of reactive astrocytes induced by hypoxic-ischemic brain damage. Exp Neurol. 311, 15–32. [DOI] [PubMed] [Google Scholar]
- Herman S, et al. , 2021. Intranasal delivery of mesenchymal stem cells-derived extracellular vesicles for the treatment of neurological diseases. Stem Cells. 39, 1589–1600. [DOI] [PubMed] [Google Scholar]
- Hosseini SM, et al. , 2018. Preconditioned neurons with NaB and nicorandil, a favorable source for stroke cell therapy. J Cell Biochem. 119, 10301–10313. [DOI] [PubMed] [Google Scholar]
- Jaderstad J, et al. , 2010. Hypoxic preconditioning increases gap-junctional graft and host communication. Neuroreport. 21, 1126–32. [DOI] [PubMed] [Google Scholar]
- Kawabori M, et al. , 2020. Clinical Trials of Stem Cell Therapy for Cerebral Ischemic Stroke. Int J Mol Sci. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch M, et al. , 2013. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature. 493, 226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch M, et al. , 2017. A Fatty Acid Oxidation-Dependent Metabolic Shift Regulates Adult Neural Stem Cell Activity. Cell Rep. 20, 2144–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondziolka D, et al. , 2000. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 55, 565–9. [DOI] [PubMed] [Google Scholar]
- Le Gall L, et al. , 2020. Molecular and Cellular Mechanisms Affected in ALS. J Pers Med. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ST, et al. , 2009. Slowed progression in models of Huntington disease by adipose stem cell transplantation. Ann Neurol. 66, 671–81. [DOI] [PubMed] [Google Scholar]
- Li B, et al. , 2018. Regulation and effects of neurotrophic factors after neural stem cell transplantation in a transgenic mouse model of Alzheimer disease. J Neurosci Res. 96, 828–840. [DOI] [PubMed] [Google Scholar]
- Li PA, et al. , 2017. Mitochondrial biogenesis in neurodegeneration. J Neurosci Res. 95, 2025–2029. [DOI] [PubMed] [Google Scholar]
- Liu D, et al. , 2021. Cell Therapy for Neurological Disorders: The Perspective of Promising Cells. Biology (Basel). 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn JS, et al. , 2011. Stem cell technology for neurodegenerative diseases. Ann Neurol. 70, 353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik YS, et al. , 2013. Doxycycline can stimulate cytoprotection in neural stem cells with oxygen-glucose deprivation-reoxygenation injury: a potential approach to enhance effectiveness of cell transplantation therapy. Biochem Biophys Res Commun. 432, 355–8. [DOI] [PubMed] [Google Scholar]
- Mautes AE, et al. , 2004. Regional energy metabolism following short-term neural stem cell transplantation into the injured spinal cord. J Mol Neurosci. 24, 227–36. [DOI] [PubMed] [Google Scholar]
- McGinley LM, et al. , 2016. Human Cortical Neural Stem Cells Expressing Insulin-Like Growth Factor-I: A Novel Cellular Therapy for Alzheimer’s Disease. Stem Cells Transl Med. 5, 379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejzini R, et al. , 2019. ALS Genetics, Mechanisms, and Therapeutics: Where Are We Now? Front Neurosci. 13, 1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira RG, et al. , 2021. Traumatic Brain Injury: Mechanisms of Glial Response. Front Physiol. 12, 740939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshayedi P, et al. , 2016. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials. 105, 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu S, et al. , 2014. Transplantation of induced pluripotent stem cells improves functional recovery in Huntington’s disease rat model. PLoS One. 9, e101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal EG, et al. , 2018. An update on intracerebral stem cell grafts. Expert Rev Neurother. 18, 557–572. [DOI] [PubMed] [Google Scholar]
- Nguyen H, et al. , 2019. Stem cell therapy for neurological disorders: A focus on aging. Neurobiol Dis. 126, 85–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak JSS, et al. , 2021. Dietary interventions as regulators of stem cell behavior in homeostasis and disease. Genes Dev. 35, 199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura T, 1998. Mitochondria-targeting sequence, a multi-role sorting sequence recognized at all steps of protein import into mitochondria. J Biochem. 123, 1010–6. [DOI] [PubMed] [Google Scholar]
- Othman FA, Tan SC, 2020. Preconditioning Strategies to Enhance Neural Stem Cell-Based Therapy for Ischemic Stroke. Brain Sci. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottoboni L, et al. , 2017. Neural Stem Cell Plasticity: Advantages in Therapy for the Injured Central Nervous System. Front Cell Dev Biol. 5, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ould-Brahim F, et al. , 2018. Metformin Preconditioning of Human Induced Pluripotent Stem Cell-Derived Neural Stem Cells Promotes Their Engraftment and Improves Post-Stroke Regeneration and Recovery. Stem Cells Dev. 27, 1085–1096. [DOI] [PubMed] [Google Scholar]
- Pacheco-Herrero M, et al. , 2021. Current Status and Challenges of Stem Cell Treatment for Alzheimer’s Disease. J Alzheimers Dis. 84, 917–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piers TM, et al. , 2020. A locked immunometabolic switch underlies TREM2 R47H loss of function in human iPSC-derived microglia. FASEB J. 34, 2436–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafalski VA, et al. , 2012. Energy metabolism and energy-sensing pathways in mammalian embryonic and adult stem cell fate. J Cell Sci. 125, 5597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AP, et al. , 2020. Neural regeneration therapies for Alzheimer’s and Parkinson’s disease-related disorders. Biochim Biophys Acta Mol Basis Dis. 1866, 165506. [DOI] [PubMed] [Google Scholar]
- Ren J, et al. , 2021. A Step-by-Step Refined Strategy for Highly Efficient Generation of Neural Progenitors and Motor Neurons from Human Pluripotent Stem Cells. Cells. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryall JG, et al. , 2015. Metabolic Reprogramming of Stem Cell Epigenetics. Cell Stem Cell. 17, 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata H, et al. , 2012a. Interleukin 6-preconditioned neural stem cells reduce ischaemic injury in stroke mice. Brain. 135, 3298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata H, et al. , 2012b. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J Neurosci. 32, 3462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Noratto GE, et al. , 2020. Understanding and leveraging cell metabolism to enhance mesenchymal stem cell transplantation survival in tissue engineering and regenerative medicine applications. Stem Cells. 38, 22–33. [DOI] [PubMed] [Google Scholar]
- Sandvig I, et al. , 2017. Strategies to Enhance Implantation and Survival of Stem Cells After Their Injection in Ischemic Neural Tissue. Stem Cells Dev. 26, 554–565. [DOI] [PubMed] [Google Scholar]
- Schepici G, et al. , 2020. Traumatic Brain Injury and Stem Cells: An Overview of Clinical Trials, the Current Treatments and Future Therapeutic Approaches. Medicina (Kaunas). 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer JS, et al. , 2020. Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N Engl J Med. 382, 1926–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, 2011. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta. 1813, 1263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, et al. , 2021. Biomaterial Cues Regulated Differentiation of Neural Stem Cells into GABAergic Neurons through Ca(2+)/c-Jun/TLX3 Signaling Promoted by Hydroxyapatite Nanorods. Nano Lett. 21, 7371–7378. [DOI] [PubMed] [Google Scholar]
- Shinozaki M, et al. , 2021. Mechanisms of Stem Cell Therapy in Spinal Cord Injuries. Cells. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyamasundar S, et al. , 2013. Analysis of epigenetic factors in mouse embryonic neural stem cells exposed to hyperglycemia. PLoS One. 8, e65945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestro S, et al. , 2020. Stem Cells Therapy for Spinal Cord Injury: An Overview of Clinical Trials. Int J Mol Sci. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay EXY, et al. , 2021. Epigenetic plasticity and redox regulation of neural stem cell state and fate. Free Radic Biol Med. 170, 116–130. [DOI] [PubMed] [Google Scholar]
- Teixeira FR, et al. , 2016. Gsk3beta and Tomm20 are substrates of the SCFFbxo7/PARK15 ubiquitin ligase associated with Parkinson’s disease. Biochem J. 473, 3563–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamotharan S, et al. , 2013. Hypoxic adaptation engages the CBP/CREST-induced coactivator complex of Creb-HIF-1alpha in transactivating murine neuroblastic glucose transporter. Am J Physiol Endocrinol Metab. 304, E583–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theus MH, et al. , 2008. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 210, 656–70. [DOI] [PubMed] [Google Scholar]
- Uyeda A, Muramatsu R, 2020. Molecular Mechanisms of Central Nervous System Axonal Regeneration and Remyelination: A Review. Int J Mol Sci. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanet A, et al. , 2015. Connecting Mitochondria, Metabolism, and Stem Cell Fate. Stem Cells Dev. 24, 1957–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, et al. , 2013. PET demonstrates functional recovery after transplantation of induced pluripotent stem cells in a rat model of cerebral ischemic injury. J Nucl Med. 54, 785–92. [DOI] [PubMed] [Google Scholar]
- Wang Z, et al. , 2018. Association of vascular endothelial growth factor levels in CSF and cerebral glucose metabolism across the Alzheimer’s disease spectrum. Neurosci Lett. 687, 276–279. [DOI] [PubMed] [Google Scholar]
- Wei L, et al. , 2012. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 46, 635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, et al. , 2017a. Stem cell transplantation therapy for multifaceted therapeutic benefits after stroke. Prog Neurobiol. 157, 49–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei ZZ, et al. , 2017b. Priming of the Cells: Hypoxic Preconditioning for Stem Cell Therapy. Chin Med J (Engl). 130, 2361–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis CM, et al. , 2020. The neural stem cell secretome and its role in brain repair. Brain Res. 1729, 146615. [DOI] [PubMed] [Google Scholar]
- Xue WS, et al. , 2019. miR-145 protects the function of neuronal stem cells through targeting MAPK pathway in the treatment of cerebral ischemic stroke rat. Brain Res Bull. 144, 28–38. [DOI] [PubMed] [Google Scholar]
- Yao Y, et al. , 2021. Resveratrol promotes the survival and neuronal differentiation of hypoxia-conditioned neuronal progenitor cells in rats with cerebral ischemia. Front Med. 15, 472–485. [DOI] [PubMed] [Google Scholar]
- Younsi A, et al. , 2021. Long-Term Effects of Neural Precursor Cell Transplantation on Secondary Injury Processes and Functional Recovery after Severe Cervical Contusion-Compression Spinal Cord Injury. Int J Mol Sci. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SP, et al. , 2013. Preconditioning strategy in stem cell transplantation therapy. Transl Stroke Res. 4, 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, et al. , 2015a. Spatiotemporal PET Imaging of Dynamic Metabolic Changes After Therapeutic Approaches of Induced Pluripotent Stem Cells, Neuronal Stem Cells, and a Chinese Patent Medicine in Stroke. J Nucl Med. 56, 1774–9. [DOI] [PubMed] [Google Scholar]
- Zhang H, et al. , 2008. 11C-NMSP/18F-FDG microPET to monitor neural stem cell transplantation in a rat model of traumatic brain injury. Eur J Nucl Med Mol Imaging. 35, 1699–708. [DOI] [PubMed] [Google Scholar]
- Zhang K, et al. , 2022. Reducing host aldose reductase activity promotes neuronal differentiation of transplanted neural stem cells at spinal cord injury sites and facilitates locomotion recovery. Neural Regen Res. 17, 1814–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, et al. , 2017. Adjudin-preconditioned neural stem cells enhance neuroprotection after ischemia reperfusion in mice. Stem Cell Res Ther. 8, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, et al. , 2015b. Neural stem cell transplantation enhances mitochondrial biogenesis in a transgenic mouse model of Alzheimer’s disease-like pathology. Neurobiol Aging. 36, 1282–92. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. , 2019. Hypoxia conditioning enhances neuroprotective effects of aged human bone marrow mesenchymal stem cell-derived conditioned medium against cerebral ischemia in vitro. Brain Res. 1725, 146432. [DOI] [PubMed] [Google Scholar]
- Zhao L, et al. , 2021. Neural stem cell therapy for brain disease. World J Stem Cells. 13, 1278–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]


