Abstract
Backgound:
Considering the important role of the Peyer’s patches (PPs) in gut immune balance, understanding of the detailed mechanisms that control and regulate the antigens in PPs can facilitate the development of immune therapeutic strategies against the gut inflammatory diseases.
Methods:
In this review, we summarize the unique structure and function of intestinal PPs and current technologies to establish in vitro intestinal PP system focusing on M cell within the follicle-associated epithelium and IgA+ B cell models for studying mucosal immune networks. Furthermore, multidisciplinary approaches to establish more physiologically relevant PP model were proposed.
Results:
PPs are surrounded by follicle-associated epithelium containing microfold (M) cells, which serve as special gateways for luminal antigen transport across the gut epithelium. The transported antigens are processed by immune cells within PPs and then, antigen-specific mucosal immune response or mucosal tolerance is initiated, depending on the response of underlying mucosal immune cells. So far, there is no high fidelity (patho)physiological model of PPs; however, there have been several efforts to recapitulate the key steps of mucosal immunity in PPs such as antigen transport through M cells and mucosal IgA responses.
Conclusion:
Current in vitro PP models are not sufficient to recapitulate how mucosal immune system works in PPs. Advanced three-dimensional cell culture technologies would enable to recapitulate the function of PPs, and bridge the gap between animal models and human.
Keywords: Peyer’s patches, M-cell, Mucosal immunity, In vitro Peyer’s patch model
Introduction
The gastrointestinal tract is continuously exposed to food-derived antigens, pathogens, metabolites, and commensal bacteria. The lumen of gut is protected from invasion of foreign antigens by well-developed mucosal defense system comprised of a hydrated network of glycosylated mucin proteins and gut-associated lymphoid tissue (GALT) working in the immune system producing large number of secretory immunoglobulin (Ig) A (S-IgA). GALT accounts for 70% of the immune system by weight, thus, GALT is considered as one of the largest immune response-inductive sites in the body [1]. The main functions of GALTs lie in sampling and inducing adaptive immune responses against potentially harmful agents as well as non-harmful commensal microbiota [2]. GALTs are also crucial in maintaining the immune tolerance to commensal flora and dietary antigens [3]. Thus, dual function of GALTs is critical to maintain homeostasis between gut microbiota and human immune system and to prevent the development of inadvertent pathologies including auto-immune diseases. Dysregulation of GALTs drives the development of chronic diseases in gastrointestinal tracts such as inflammatory bowel disease, which damages the intestinal defense wall and disable the intestinal tissue regeneration [4, 5].
GALTs are categorized into organized GALT and diffuse GALT considering their structures [6]. The organized GALT is composed of follicles including Peyer's patches (PPs) in the small intestine, colonic patches, and isolated lymphoid follicles, which induce immune reactions. On the other hand, diffuse GALT is dominated by lymphoid cells diffusely distributed in lamina propria. It is well accepted that PPs are the major IgA inductive sites of the GALTs as shown in Fig. 1. Human PPs are composed of tens to hundreds of lymphoid follicles and concentrated in the distal part of the ileum [2]. PPs are surrounded by specialized epithelium, the follicle-associated epithelium (FAE) containing microfold (M) cells, which serve as special gateways for antigen transport from the lumen to the PPs [7]. M cells are highly specialized for phagocytosis and transcytosis to uptake large particulates and transport them into the subepithelial space. The transported antigens are processed by immune cells within PPs and then, mucosal immune responses are initiated to make antigen specific-IgA secreting B cells or tolerate, depending on the response of underlying mucosal immune cells. Accumulating studies have revealed the M cell-dependent antigen uptake serving as a central node of in the network during inflammation. These studies showed the high expression of M cell markers in inflammatory bowel disease (IBD) patients [8] and M cell-dependent antigen uptake facilitating systemic immune responses such as production of antigen-specific IgG production as well as S-IgA [9]. Considering the important role of the PPs in gut immune balance, understanding of the detailed mechanisms that control and regulate the antigens in PPs would facilitate the development of immune therapeutic strategies against the gut inflammatory diseases.
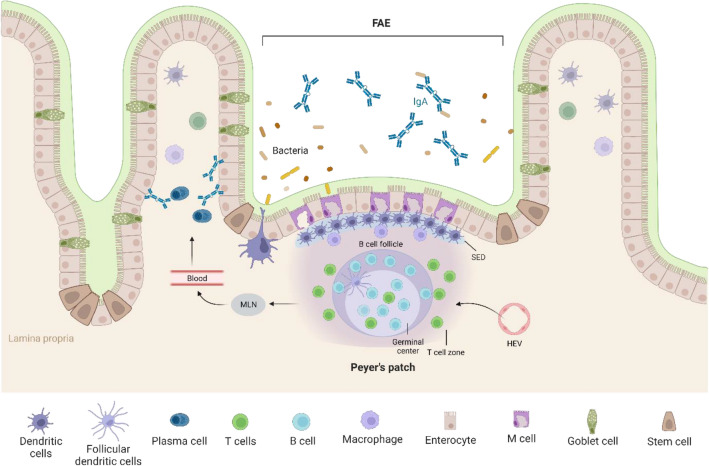
Fig. 1.
Schematic illustration of cellular composition of Peyer’s patches (PPs). PP follicles are enclosed by follicle-associated endothelium (FAE) containing M cells that shuttle luminal antigen into the PP. Subepithelial dome (SED) below the FAE contains high density of antigen-presenting cells. Lymphocytes enter PP via high endothelial venules (HEV) and form large B cell follicles and small T cell zones. The interactions between B cells and T cells at the follicle-T cell zone lead to expansion and differentiation of B cells. The activated B cells form germinal center, generating IgA-secreting plasma cells. The generated effector cells leave the PPs through efferent lymphatics and enter the circulation via mesenteric lymph nodes (MLNs) [32]. They home to the intestinal lamina propria from the blood circulation and the lamina propria plasma cells produce dimeric IgA that are transported across the epithelium. The secretory-IgA (S-IgA) can interact with bacteria in the gut lumen
However, lack of adequate human PP models challenges the deep understanding of the (patho)physiology of PPs. The anatomical and physiological features of PPs are different between animal models and human; thus, animal experiments may fail to replicate the actual behavior of human PPs. Despite a great need for in vitro human PP model, it is still challenging to recapitulating the complex structural features and cell–cell networks of in vivo PPs [2], requiring employment of advanced in vitro technologies. Organ-on-a-chips (OoCs) are biomimetic in vitro systems that emulate the functions, structure, and complex physiology of in vivo tissue in a slide-sized chip. By combining microfluidics and biomaterials technologies, OoCs enable to recapitulate the tissue-specific biological, chemical, and physical microenvironmental conditions, which was not achievable in traditional tissue culture system [10]. In this review, we will describe the current knowledges of cellular components of PPs (summarized in Table 1) and status of in vitro models recapitulating some features of in vivo PPs. Finally, we provide potential opportunities to develop novel in vitro human PP models and applications for mucosal immune modulation and intestinal tissue regeneration.
Table 1.
Components and characteristics of Peyer’s patches
| Components | Characteristics | References |
|---|---|---|
| Follicle-associated epithelium (FAE) |
- Specialized epithelial layer that overlies lymphoid follicles of PPs - Function as barrier between the intestinal lumen and PPs |
[7] |
| Microfold (M) cells |
- Specialized enterocytes, present in the FAE - Transport of luminal antigens to PPs - Low level of glycocalyx covering and flat apical surface for efficient interactions with particulate antigens - Binding the S-IgA for efficient capturing and transporting of luminal antigens - Expression of diverse glycan signatures on the apical membrane for recognition of certain bacteria or immune complexes - Presence of M cell pockets on the basolateral membrane for the migration of immune cells into the intraepithelial |
[11, 18–23] |
| Subepithelial dome (SED) |
- Areas located between the FAE and B cell follicles of PPs - Containing the high density of DCs - Direct interactions between M cells and DCs |
[29–31] |
| Large B cell follicles |
- Network of diverse populations of B cells - Antigen encounter - Complex interactions with T cells, DCs or FDCs - Initial activation of naïve B cells |
[32] |
| Germinal centers (GCs) |
- Clonal expansion and differentiation of B cells - Undergo SHM and CSR - Generation of IgA-secreting B cells |
[32–35] |
| Small T cell zone |
- Network of diverse populations of T cells - Delivering activation signals from CD4 + T cells to B cells |
[32] |
| High endothelial venules (HEVs) |
- Entry of lymphocytes to PPs - High expression of MADCAM-1, a ligand for α4β7 integrin, and CXCL13, a ligand for CXCR5 for B cell entry |
[18] |
| Efferent lymphatics | - Leave effector cells generated in the PPs to MLN | [32] |
Structure and function of Peyer’s patches
Microfold cells within the follicle-associated epithelium
The main feature of FAE is the presence of specialized enterocytes, M cells. While cells and antigens drain from organs to peripheral lymph nodes through lymphatic vessel, luminal antigens in the intestine are transported to PPs mainly by M cell-dependent transcytosis [11]. Therefore, successful mucosal immunization relies on the M-cell mediated antigen delivery to the mucosal immune induction site. M cells are derived from Lgr5-expressing stem cells residing in the FAE-associated crypts. The Lgr5+ stem cells are continuously exposed to nucleic factor-kappa B ligand (RANKL) expressed on the stromal cells in the subepithelial dome region beneath the FAE. The RANKL binding to RANK on the stem cells induces the expression of SpiB and Sox8, which are key transcription factors involved in M cell differentiation [12–14]. Another important transcription factor, Atoh8, controls the density of M cell population in FAE and is regulated by polycomb repressive complex 2 (PRC2), which is an epigenetic regulator of M cell development [15, 16]. M cells are also differentiated from enterocytes under the influence of membrane-bound lymphotoxin (LTα1β2) present on local lymphoid cells, mainly B cells. Inspired by the important role of lymphotoxin-mediated noncanonical nuclear factor (NF)-κB pathway, a recent study successfully generated mature M cells expressing glycoprotein 2 (GP2) from RANKL-treated intestinal organoid by combinational addition of retinoic acid (RA) and lymphotoxin [17].
The M cells contain low level of glycocalyx covering and have relatively flat apical surfaces with short brush borders unlike regular enterocytes. These morphological features of M cells enable to interact with particulate antigens efficiently [18]. In addition, M cells are able to bind the S-IgA, which enhances the efficiency of capturing and intracellular transporting of luminal antigens [19]. Another important feature of M cells is diverse glycan signatures on the apical membrane that are recognized by luminal microorganisms or immune complexes; for example, dectin-1 induces transcytosis of glycosylated S-IgA-antigen complexes [20], GP2 function as an uptake receptor for type-1-piliated bacteria [21], and monosialotetrahexosylganglioside supports the binding of cholera toxin [22]. The basolateral membrane of M cells has a unique intraepithelial invagination, termed M cell pockets, allowing the migration of B cells, T cells, dendritic cells (DCs), and macrophages into the intraepithelial [23]. In the absence of M cells, mucosal adaptive immunity would be impaired because efficient antigen monitoring is incapacitated. A past study confirmed the handicapped mucosal immune responses using a model of M cell deficiency [24]. M cells are a critical player in the mucosal immunosurveillance in GALT; however, active antigen transport mechanism of M cells can be exploited by pathogens. Several pathogenic bacteria including Shigella flexneri and Salmonella typhi [25] and viruses such as human influenza virus [26] use M cells as a vulnerable gateway to bypass the epithelial barrier and result in systemic infection. Furthermore, M cell population can be expanded in the intestine under inflammatory stimuli such as in Crohn’s disease [27, 28], which supports a potential role of M cells in chronic inflammatory disease in gastrointestinal tract. These studies imply that M cell-dependent antigen uptake could be a double-edged sword in the context of mucosal infection and host defense.
Subepithelial dome
The PPs contain a unique niche known as the subepithelial dome (SED) that is positioned under the FAE. DCs form a dense cell layer in the SED for antigen sampling in the lamina propria, where they perform a sentinel function for incoming pathogens. The SED is also populated by diverse populations of memory CD4+ T cells and B cells expressing IgA, IgM, and IgG isotypes [29, 30]. Some of DCs has been observed to uptake pathogens across the mucosal epithelium by opening the tight junctions between epithelial cells and sending dendrites outside the epithelium [31]. In addition, DCs are in close contact with M cells and intervene when bacteria are internalized through the M cells. The human PPs contain diverse subset of DCs, including conventional dendritic cell type 1 and type2 (cDC1 and cDC2), and plasmacytoid dendritic cell [2]. The SED DCs loaded with antigens migrate from the SED to interfollicular T-cell zones and induce conversion of naïve T cells to antigen-specific effector CD4+ T cells. The CD4+ T cells interact with B-cells in the B-cell follicles and initiate the B cell-mediated adaptive mucosal immune responses [18].
B cell follicles
The PPs are the inductive gut immune sites and contain tens to hundreds of large B cell follicles [18]. Lymphocytes enter PPs via high-endothelial venules (HEVs) located within follicles as well as T cell zones. The PPs’ HEVs have high expression of MADCAM-1, a ligand for α4β7 integrin, and display CXCL13, a ligand for CXCR5 [18]. The B cells that express the gut-homing receptors α4β7 integrin and CXCR5 migrate to PPs and differentiated into IgA-secreting plasma cells when encountering an antigen. The luminal antigens including bacteria, virus, and food-derived antigens are transported from the lumen to the SED mostly via M cells. Then, the SED DCs that internalized luminal antigens present antigen-derived peptides on the major histocompatibility complex (MHC) molecules to T and B lymphocytes, initiating antigen-specific immune responses or tolerance. The antigen-B cell receptor (BCR) ligation activates B cells to present epitopes on surface MHC-II molecules. The antigen-specific immune responses in germinal centers are induced both in T cell-dependent and T cell-independent mechanisms [32]. In T cell-dependent mechanism, antigen specific CD4+ T cells expressing CD40 interact with naïve B cells at the follicle-T cell zone. The BCR and CD40 signaling in germinal center B cells lead to extensive proliferation of antigen-specific B cells accompanied with somatic hypermutation (SHM) for affinity maturation. On the other hand, in T cell-independent mechanism, B cells are activated by multiple innate immune signaling pathways, including through triggering of Toll-like receptors. The B cells respond directly to native antigens ligated to BCRs that are present in the follicles in free form or presented by DCs. In that case, the tumor necrosis factor (TNF) family cytokines such as B cell activator factor (BAFF), a proliferation-inducing ligand (APRIL), and a peptide hormone vasoactive intestinal peptide (VIP) can trigger activation-induced cytidine deaminase (AID) expression that induces class-switch recombination (CSR).
In the presence of antigen, mature B cells diversify their antibody repertoire through SHM and CSR by expressing the AID [33, 34]. Mature B cells in PPs acquire IgA expression by undergoing CSR of germline Cμ to Cα-gene transcripts cued by chemical environment in PPs [35]. The most critical cytokine for IgA CSR is transforming growth factor-β1 (TGFβ1), which activates Cα gene promoter. DCs are induced to express αvβ8 integrin when capturing antigens, which is required for TGFβ activation [18]. In addition, DCs produce TNF cytokines and inducible nitric oxide synthase (iNOS) that enhance the T cell-dependent IgA CSR through upregulation of the expression of TGFβR in B cells. They also produce APRIL and BAFF that stimulate T cell-independent IgA CSR and induce B cell maturation and survival [36, 37]. IgA+ effector B cells generated in PPs home to the gut lamina propria, where they differentiate into IgA-secreting plasma cells. This process is enhanced by RA secreted from DCs by upregulating the expression of gut-homing receptors, including α4β7 integrin and CC-chemokine receptors (CCR9) by IgA+ effector B cells [18, 38]. While DCs play a key role in regulating gut mucosal IgA B-cell responses in PPs, follicular dendritic cells (FDCs) and T lymphocytes also contribute to IgA production of B cells. FDCs assist to initiate IgA class switching by producing BAFF, APRIL, and matrix metalloproteases that increase the level of cytokines TGF-β1 while their main role is to present antigens to B cells in the germinal center. On the other hand, when stimulated by antigen-presenting DCs, CD4+ T cells release IgA-inducing cytokines, including TGFβ1, IL-3, IL-6, and IL-10 [35]. CD40L–CD40 interactions are also necessary for T cell-dependent IgA CSR along with TGFβ1 signaling [35]. Intestinal epithelial cells also contribute to IgA CSR of B cell by producing several IgA-inducing cytokines, including IL-10, TGFβ1, BAFF and APRIL.
Modeling M cells within the follicle-associated epithelium in vitro
So far, there is no high fidelity (patho)physiological model of PPs; however, there have been several efforts to recapitulate the key steps of mucosal immunity in PPs such as antigen transport through M cells and mucosal IgA responses. In vitro M cell models enable the biochemical and molecular studies of the antigen transport in PPs in complement to immunomorphological approaches in animal models. Indeed, those models have been exploited to develop mucoadhesive polymeric carriers by M cell-targeting for discovery oral vaccines. Here, we discuss two approaches to generate FAE model containing M cells in vitro.
Induction of M-cell differentiation in vitro by cellular components
Lymphocytes located at PPs produce various signaling factors that stimulate M cell differentiation both in a secreted or membrane-bound form. Therefore, coculture system of epithelial cells and PP lymphocytes have been widely used to establish in vitro M cell model [39–44] using transwell system. First, monolayer of human intestinal epithelium is generated on the permeable membrane of transwell insert by culturing Caco-2 cells or Caco-2 sub-clone (TC-7 [41] or C2Bbe1 [42]). When intestinal monolayer is formed, M-cell differentiation is induced by addition of freshly isolated lymphoid cells from mice PPs in either the upper or lower chamber of transwell interfaced with epithelial cells [39–44]. Some studies proposed that B lymphocytes have major roles in FAE and M cell development [39, 45]; thus, human B-cell lymphoma Raji (Raji B) cells have been used as cellular components to induce M-cell differentiation instead of whole lymphoid cells from PPs [39, 45–55]. When Caco-2 cells were stimulated by Raji B cells in transwell system, epithelial cells obtained M cell-like morphology and transcytotic activity. However, transcytotic activity of Caco-2/Raji B cell model appeared to be lower than that of Caco-2/PP lymphocyte co-cultures when assessed with micro-sized particles [39]. This suggests that multiple factors and complex cellular interactions are involved in M-cell development.
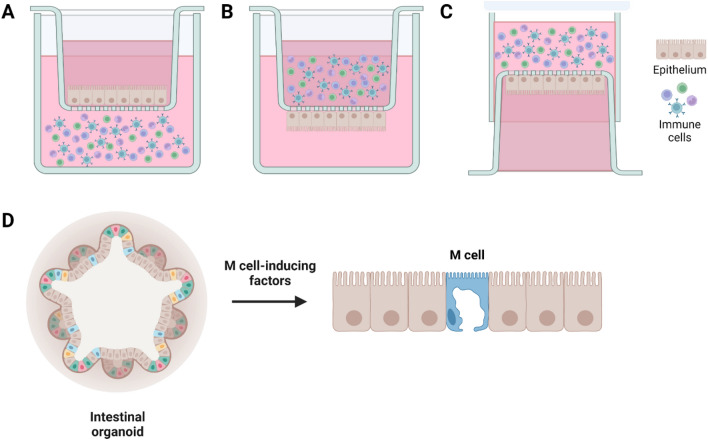
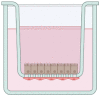
In vitro M cell co-culture models have been used as a robust cell-based platform to investigate uptake of microorganisms or particle transport. However, those models have variability in the orientation of Caco-2 and immune cells within transwell. There are three different configurations among the current M cell-like models (Fig. 2). In the normal-oriented model (Fig. 2A), Caco-2 cells are seeded on the upper face of transwell filters and the immune cells are cultured in the basal chamber, facing the basolateral side of Caco-2 cells [45]. In the inverted model (Fig. 2B), Caco-2 cells are seeded on the uppermost surface of the inverted transwell inserts and then incubated overnight until the cells were fully attached to the filters. Next day, the inserts are reoriented to the normal configurations, and then immune cells are added to the basolateral chamber of transwell [39]. The inverted model was developed to realize a more physiological condition by encouraging the direct contact between Caco-2 cells and B lymphocytes. In another inverted model (Fig. 2C), Caco-2 cells are seeded on the apical chamber in the normal orientation and the inserts are inverted and placed on a petri dish filled with medium for Caco-2 monolayers. Before seeding of immune cells, the silicon tubes are placed on the basolateral side of the filter. The immune cells were then added to the basolateral compartment [47]. Interestingly, recent study compared those three different M cell co-culture models in the aspects of particle uptake, bacterial interaction, and epithelial histology [56]. Three models all exhibited the M-cell specific truncated microvilli, demonstrating the successful M cell differentiation. The monolayer integrities of three models were determined by transepithelial electrical resistance (TEER) measurements using EVOM Epithelial Voltohmmeter with a STX2 “chopstick” electrode, and monitoring paracellular permeability of [14C]-mannitol. The inverted M cell model shown in Fig. 2C displayed the lowest transepithelial electrical resistance (TEER) and highest apparent permeability of [14C]-mannitol among three models, indicating a compromised barrier function [45]. Furthermore, it showed the highest level of particle translocation across the epithelium among three models, suggesting that the inverted model (Fig. 2C) represents the most dramatic phenotypic changes for efficient translocation of inert particles [45]. However, only M cell model shown in Fig. 2A consistently displayed the increased translocation of Salmonella typhimurium [45]. It indicates that normal-oriented model may provide more reliable result when studying pathogen transport through M cells.
Fig. 2.
Current in vitro M cell models. A–C the transwell coculture of intestinal epithelial cells with immune cells to induce the M-cell differentiation. M cells were differentiated on the epithelium through unknown chemical- or physical-interactions between the intestinal epithelial cells and immune cells. A normal-oriented model where Caco-2 cells are seeded on the upper face of transwell insert and the immune cells are cultured in the basal chamber [45]. B an inverted model where Caco-2 cells are cultured on the uppermost surface [39]. C Caco-2 cells are seeded on the apical chamber in the normal orientation and cultured inverted while immune cells are added to the basolateral compartment [47]. D Intestinal organoids were cultured under the treatment of various M cell-inducing chemical factors including RANKL [13–16, 62], TNFα [12, 61], RA or lymphotoxin [17]. Then, M cells were differentiated from the intestinal stem cells, existing in the intestinal organoids
Induction of M cell differentiation in vitro by chemical factors
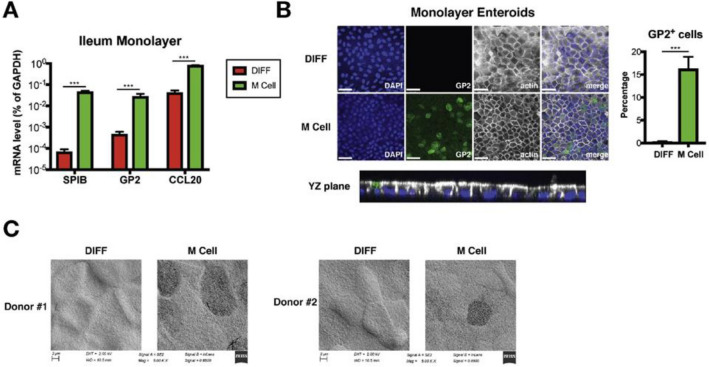
The M cell model generated by treatment of cytokines, which are involved in the induction of M cell related genes, was first developed by Wang et al. (2009) [57]. Among abundant cytokines involved in M-cell differentiation, lymphotoxin beta receptor (LTβR) and TNF receptor (TNFR) agonists were selected as candidate cytokines, considering that genetic deficiencies in these genes resulted in the reduction or loss of PPs [58, 59]. Human (Caco-2 BBe) or rat (IEC-6) intestinal epithelial cell lines were cultured on apical side of Transwell insert in the presence of lymphotoxin beta receptor (LTβR) or TNF receptor (TNFR) agonists in culture media. The treatment of LTβR and TNFR agonists induced both FAE- and M cell-specific genes in Caco-2BBe and IEC-6 monolayers as well as the reorganization of microvilli [57]. A recent study demonstrated that M cells could be generated from intestinal enteroids harboring Lgr5-expressing intestinal stem cells in the presence of RANKL [17] (Fig. 2D). The RANKL, selectively expressed by subepithelial stromal cells in PP domes, triggers the M cell differentiation from stem cells in the intestinal tissue [60]. Three-dimensional enteroids derived from murine or human small intestine were stimulated with recombinant mouse RANKL [13–16] and other signaling factors, including TNFα [12, 61], RA, and lymphotoxin [17] were additionally treated to see if M cell differentiation efficiency is enhanced. The study revealed that treatment of RA enhances the activation of RANKL-mediated M cell differentiation. Human M cells differentiated in the enteroids-derived M cell model were characterized by expression of M cell markers, including GP2, SPIB and CCL20 (Fig. 3A), GP2 staining (Fig. 3B), and M cell-like morphology such as loss of microvilli (Fig. 3C). The role of RANKL in Lgr5+ stem cell differentiation into M-cell was further demonstrated in human induced pluripotent stem cells -derived intestinal organoids (HIOs) [62]. Three-dimensional HIOs and enteroids were both dissociated into single cells and cultured on the apical compartment of transwells to from epithelial monolayers to facilitate the observation of cells. In agreement with [17], treatment of organoids with RANKL exhibited generation of cells having features of M cells [62]
Fig. 3.
Characterization of M-cell like morphology and expression of M cell markers in enteroids-derived M cell model. Human enteroids were cultured in monolayers under regular differentiation (DIFF) condition or M cell differentiation (M cell) condition. A the M cell differentiation of human ileum enteroids was examined by monitoring the change in mRNA expression level of M cell markers (SPIB, GP2, and CCL20) B GP2-positive M cells were stained (green) and the percentage of GP2-positive cells quantified, and C the loss of microvilli was observed using scanning electron microscopy analysis [17]
Modeling DC-mediated bacterial translocation in vitro
While the antigen transport from lumen to abluminal region mainly occur through M cells in PPs, DCs located at SED are responsible for M cell-independent route for bacterial uptake by protruding dendrites between FAE. To investigate the DC-mediated bacterial uptake, in vitro SED model has been developed [63]. While monolayer of Caco-2 cells was generated on the apical side of Transwell insert, mouse bone marrow–derived DCs were cultured in the basal chamber. In the SED model, it was observed that DCs directly uptake microorganisms by sending dendrites outside the epithelium between epithelial cells [63]. Intestinal epithelial model has been frequently used to explore the epithelial invasion mechanism of invasive bacteria [64], yet SED model incorporated with dendritic cells would enable a comprehensive understanding of M cell-independent host defense mechanism against bacterial invasion.
Modeling IgA production in vitro
S-IgA plays a critical role as an immunological barrier [65] acting as a first line of defense for the intestinal mucosa. S-IgA adheres to bacteria or viruses on the intestinal epithelial surface, which results in immune exclusion and neutralization of translocated bacteria, thus preserving the integrity of the intestinal barrier by preventing bacterial-induced inflammation. Given the importance of IgA in intestinal mucosal immune responses, there has been a great need of developing B cell model expressing IgA subtype. Most IgA production models in the past used peripheral blood mononuclear cells (PBMC) or B lymphocytes stimulated with cytokines, which are involved in IgA CSR [66–68]. Briere et al. [69, 70] cultured PBMC in the presence of anti-CD40 monoclonal antibody presented by a CD32-transfected fibroblastic cell line for B cell activation and IgA class switching. They found that addition of cytokines, including TGF-β, IL-2, IL-4 and/or IL-10, synergistically enhanced the production of IgA [69, 70], demonstrating that cytokines can be as therapeutic agents for IgA-deficient patients. Béniguel et al. [71] showed that PBMC and B cells can produce IgG3 or IgG1 as well as IgA in the presence of CD40L. When exogenous cytokines including IL-2 and IL-10 or IL-10 and TGF-β are additionally treated, induction of differential regulation of IgG1 and IgG3 was observed. Other studies also revealed that mitogen [72] and virulent factors including Staphylococcus aureus Cowan and lipopolysaccharide [66, 67, 73–75] can be promising stimulant factors for in vitro IgA production in human PBMC or B cell line [75]. Table 2 shows the IgA CSR factors and their working mechanism inducing IgA CSR in B cells.
Table 2.
IgA CSR factors and cell sources
| IgA CSR factor | Cell source | Characteristics | References |
|---|---|---|---|
| Transforming growth factor-β1 (TGFβ1) | FDCs, T cells, IECs |
- Activation of Cα gene promoter - Essential for IgA CSR |
[35] |
| αvβ8 integrin | DCs | - Required for TGFβ activation | [18] |
| Tumor-necrosis factor (TNF) | DCs |
- Upregulation of the expression of TGFβR by B cells - Stimulation the production of APRIL and BAFF by DCs |
[37] |
| Inducible nitric oxide synthase (iNOS) | DCs |
- Upregulation of the expression of TGFβR by B cells - Stimulation the production of APRIL and BAFF by DCs |
[37] |
| Proliferation- inducing ligand (APRIL) | DCs, FDCs, IECs |
- Induction of germline Cα gene expression and IgA CSR - Involved in B cell maturation and survival |
[35–37] |
| B cell activator factor (BAFF) | DCs, FDCs, IECs |
- Induction of germline Cα gene expression and IgA CSR - Involved in B cell maturation and survival |
[35–37] |
| Retinoic acid (RA) | DCs | - Upregulation the expression of gut-homing receptors, including α4β7 integrin and CC-chemokine receptors (CCR9) by IgA+ effector B cells | [38] |
| Matrix metalloproteases (MMP) | FDCs | - Increase the level of cytokines TGF-β1 | [35] |
| CD40L | T cells | - Essential in T cell-dependent IgA CSR | [35] |
| IL-4, IL-6, and IL-10 | T cells, IECs | - IgA-inducing cytokines | [35] |
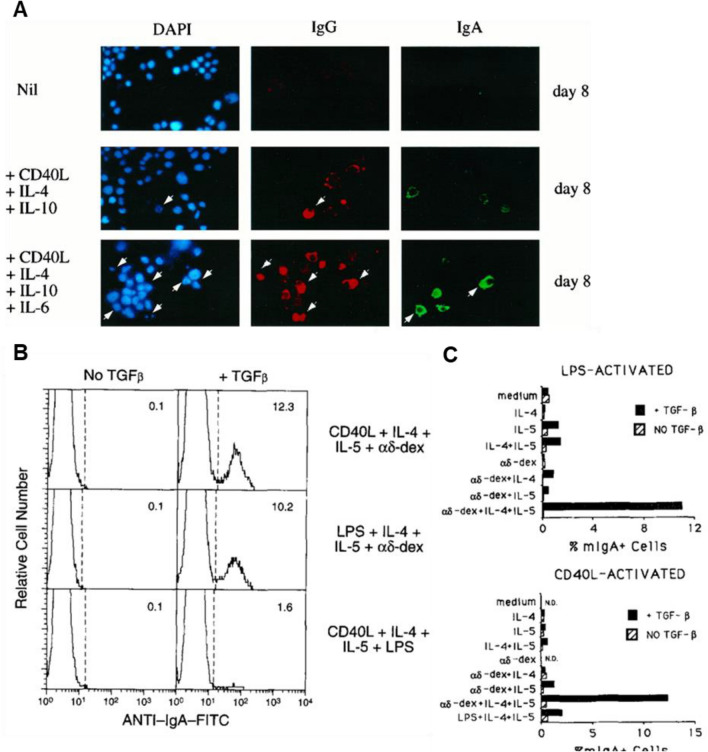
These models demonstrated the expression of IgA germline transcripts in B cells (Fig. 4A) or surface-IgA expression (Fig. 4B, C); however, there is a lack of functional assessment of the protective function of the produced IgA. For wider applications of these models, it is necessary to identify whether the S-IgA antibodies form monomer or dimer, and whether produced surface- and S-IgA can neutralize antigens. Another important feature of S-IgA is the translocation of S-IgA produced in B cell follicles to the luminal side of epithelium. To validate if IgA cross over intestinal epithelium from basal to apical side, transwell-based FAE model exhibiting epithelial polarity might offer an useful tool.
Fig. 4.
Characterization of IgA expression model. The IgA expression was monitored in coculture system of B cell in the presence of the different combinations of IgA class-switch recombination (CSR) factors. A Ig class switching of a human monoclonal B cell line, CL-01, in the presence of CD40L, IL-4, IL-10, and IL-6. Cytoplasmic IgG and IgA expressed by B cells were analyzed using fluorescence microscopic analysis [68]. B, C high-rate IgA class switching of B cells obtained from spleen induced by dual combinations of the multivalent antigen receptor crosslinker, aδ-dex, CD40L, and/or LPS, plus IL-4 + IL-5, in the presence or absence of TGF-β. The percentages of membrane IgA positive (mIgA+) cells were analyzed using flow cytometry [67]
Conclusions and perspectives
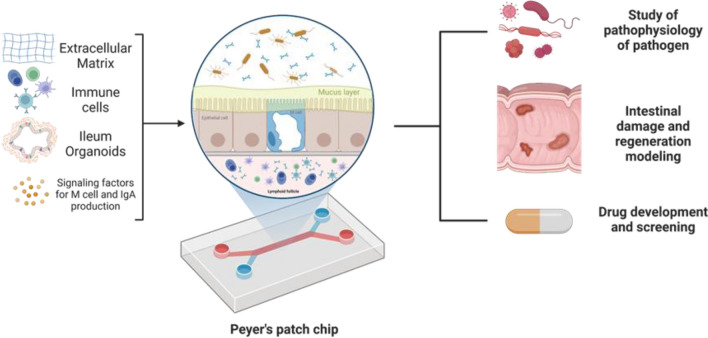
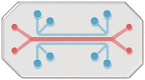
PPs transport antigen from the lumen to the underlying follicles and induce the mucosal immune responses against the antigen. Current in vitro M cell and IgA production models are not sufficient to recapitulate the how mucosal immune system works in PPs. To fully recreate the main functions of PPs, in vitro PP platform should contain FAE having functional M cells and immune cells that perform mucosal immune responses, secret cytokines and antigen-specific IgA. For this, it is a key to enable the interaction between multiple cell types including epithelial cells and immune cells in the compartmentalized. In addition, PPs contain various extracellular matrix (ECM) components and the distinctive patterns in the distribution of ECM determine the cytoarchitecture of PPs [76]. Therefore, future PP models should offer in vivo relevant ECM microenvironment to empower the functionality of cells cultured in the platform. There are various factors and cell–cell interactions involved in the differentiation of M cells and IgA+ B cells, thus the most effective combination should be further identified in the integrated PP models. The shortcomings of conventional cell culture platform might be addressed by employing advanced three-dimensional cell culture technologies including OoC and bioprinting, which enable communications between multiple types of cells controlled in separate compartment. In particular, given the environmental and cellular complexity of PPs, OoC may be a great platform to model human PPs in vitro by incorporating PPs-specific ECM, immune cells, epithelial cells, and signaling factors for the differentiation of M cells, and IgA-secreting B cells (Fig. 5). The advantages and disadvantages of current in vitro technologies that can be used to generate PP model are summarized in Table 3.
Fig. 5.
In vitro human PP model based on organ-on-a-chip technology. PPs-specific ECM, immune cells, epithelial cells, and signaling factors for the differentiation of M cells, and IgA-secreting B cells can be incorporated in the proposed PP system. The human PP model can offer a promising in vitro tool for studying infection and intestinal damage by chronic inflammation, and for discovering tissue regeneration strategy and therapeutics to control intestinal infection
Table 3.
Advantages and disadvantages of in vitro techniques for PP model
| Techniques | Advantages | Disadvantages |
|---|---|---|
|
Transwell culture |
Easy to set up | Static condition |
| Ability for co-culture | Lack of physical and biochemical cues | |
| Low cost and labor intensity | Low reproducibility | |
|
Organoid |
Patient-specific | Static condition |
| In vivo-like complexity and architecture | Low reproducibility | |
| Include cell-ECM interaction | High cost | |
| Inefficient to inoculate pathogens in luminal part | ||
|
Organ-on-a-chip |
In vivo-like complexity and architecture | Non-standard protocols |
| In vivo-like microenvironment, chemical and physical gradients | Inconsistency between chips | |
| Ability for co-culture | Low-throughput production | |
| Induce tissue-tissue interfaces | Require external pumps, tubing, connectors, and valve to operate | |
| Ability to integrate with sensors and actuators | ||
|
3D bioprinting |
Ability for co-culture | Non-standard protocols |
| High reproducibility | Challenges with cells and materials | |
| High-throughput production | ||
| Induce chemical and physical gradients |
New generation of PP models would be able to offer a great platform for discovery of drug that controls M cell development in M cell deficient or overexpressed patients, investigation of how pathogens avoid mucosal immune system in PPs, and further development of new therapeutic strategies to repair intestinal epithelium damaged in disease conditions. Many pathogens have evolved strategies to initiate infection and colonize in the mammalian mucosal tracts. Since Covid-19 pandemic caused the social, economic, and political fallouts, mucosal vaccines that trigger robust immune responses at the predominate sites of pathogen infection gained more attention than ever [77, 78]. Advanced human PP models would also serve as reliable platforms to evaluate the efficacy and safety of oral vaccine in future.
Acknowledgments
This project was supported by National Research Foundation (Grant No. 2021R1A4A3030597), Ulsan National Institute of Science and Technology, (Grant No. 1.230039.01).
Declarations
Conflict of interest
The authors have no conflicts of interests.
Ethical statement
No animal experiments were carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Murch S. Pediatric gastrointestinal and liver disease. 4. Saint Louis: WB Saunders; 2011. Gastrointestinal mucosal immunology and mechanisms of inflammation; p. 50. [Google Scholar]
- 2.Mörbe UM, Jørgensen PB, Fenton TM, von Burg N, Riis LB, Spencer J, et al. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021;14:793–802. doi: 10.1038/s41385-021-00389-4. [DOI] [PubMed] [Google Scholar]
- 3.Jiao Y, Wu L, Huntington ND, Zhang X. Crosstalk between gut microbiota and innate immunity and its implication in autoimmune diseases. Front Immunol. 2020;11:282. doi: 10.3389/fimmu.2020.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandran P, Satthaporn S, Robins A, Eremin O. Inflammatory bowel disease: dysfunction of GALT and gut bacterial flora (I) Surgeon. 2003;1:63–75. doi: 10.1016/S1479-666X(03)80118-X. [DOI] [PubMed] [Google Scholar]
- 5.Porter RJ, Arends MJ, Churchhouse AM, Din S. Inflammatory bowel disease-associated colorectal cancer: translational risks from mechanisms to medicines. J Crohns Colitis. 2021;15:2131–2141. doi: 10.1093/ecco-jcc/jjab102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knoop K, Newberry R. Isolated lymphoid follicles are dynamic reservoirs for the induction of intestinal IgA. Front Immunol. 2012;3:84. doi: 10.3389/fimmu.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owen RL, Jones AL. Epithelial cell specialization within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974;66:189–203. doi: 10.1016/S0016-5085(74)80102-2. [DOI] [PubMed] [Google Scholar]
- 8.Smillie CS, Biton M, Ordovas-Montanes J, Sullivan KM, Burgin G, Graham DB, et al. Intra-and inter-cellular rewiring of the human colon during ulcerative colitis. Cell. 2019;178:714–30.e22. doi: 10.1016/j.cell.2019.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, et al. Uptake through glycoprotein 2 of FimH+ bacteria by M cells initiates mucosal immune response. Nature. 2009;462:226–230. doi: 10.1038/nature08529. [DOI] [PubMed] [Google Scholar]
- 10.Yan J, Li Z, Guo J, Liu S, Guo J. Organ-on-a-chip: a new tool for in vitro research. Biosens Bioelectron. 2022;216:114626. doi: 10.1016/j.bios.2022.114626. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi N, Takahashi D, Takano S, Kimura S, Hase K. The Roles of Peyer's Patches and Microfold Cells in the Gut Immune System: Relevance to Autoimmune Diseases. Front Immunol. 2019;10:2345. doi: 10.3389/fimmu.2019.02345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood MB, Rios D, Williams IR. TNF-α augments RANKL-dependent intestinal M cell differentiation in enteroid cultures. Am J Physiol Cell Physiol. 2016;311:C498–C507. doi: 10.1152/ajpcell.00108.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura S, Kobayashi N, Nakamura Y, Kanaya T, Takahashi D, Fujiki R, et al. Sox8 is essential for M cell maturation to accelerate IgA response at the early stage after weaning in mice. J Exp Med. 2019;216:831–846. doi: 10.1084/jem.20181604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau Wd, Kujala P, Schneeberger K, Middendorp S, Li VSW, Barker N, et al. Peyer’s patch M cells derived from Lgr5+ stem cells require SpiB and are induced by RankL in cultured “Miniguts”. Mol Cell Biol. 2012;32:3639–47. doi: 10.1128/MCB.00434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George JJ, Oittinen M, Martin-Diaz L, Zapilko V, Iqbal S, Rintakangas T, et al. Polycomb repressive complex 2 regulates genes necessary for intestinal microfold cell (M cell) development. Cell Mol Gastroenterol Hepatol. 2021;12:873–889. doi: 10.1016/j.jcmgh.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George JJ, Martin-Diaz L, Ojanen MJT, Gasa R, Pesu M, Viiri K. PRC2 regulated Atoh8 is a regulator of intestinal microfold cell (M cell) differentiation. Int J Mol Sci. 2021;22:9355. doi: 10.3390/ijms22179355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding S, Song Y, Brulois KF, Pan J, Co JY, Ren L, et al. Retinoic acid and lymphotoxin signaling promote differentiation of human intestinal M cells. Gastroenterology. 2020;159:214–26.e1. doi: 10.1053/j.gastro.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reboldi A, Cyster JG. Peyer's patches: organizing B-cell responses at the intestinal frontier. Immunol Rev. 2016;271:230–245. doi: 10.1111/imr.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantis NJ, Cheung MC, Chintalacharuvu KR, Rey J, Corthésy B, Neutra MR. Selective adherence of IgA to Murine Peyer’s patch M cells: evidence for a novel IgA receptor. J Immunol. 2002;169:1844. doi: 10.4049/jimmunol.169.4.1844. [DOI] [PubMed] [Google Scholar]
- 20.Rochereau N, Drocourt D, Perouzel E, Pavot V, Redelinghuys P, Brown GD, et al. Dectin-1 is essential for reverse transcytosis of glycosylated SIgA-antigen complexes by intestinal M cells. PLoS Biol. 2013;11:e1001658. doi: 10.1371/journal.pbio.1001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohno H, Hase K. Glycoprotein 2 (GP2) grabbing the fimH+ bacteria into M cells for mucosal immunity. Gut Microbes. 2010;1:407–410. doi: 10.4161/gmic.1.6.14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamura YI, Kawashima R, Shirai Y, Kato R, Hamabata T, Yamamoto M, et al. Cholera toxin activates dendritic cells through dependence on GM1-ganglioside which is mediated by NF-κB translocation. Eur J Immunol. 2003;33:3205–3212. doi: 10.1002/eji.200324135. [DOI] [PubMed] [Google Scholar]
- 23.Dillon A, Lo DD. M cells: intelligent engineering of mucosal immune surveillance. Front Immunol. 2019;10:1499. doi: 10.3389/fimmu.2019.01499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rios D, Wood M, Li J, Chassaing B, Gewirtz Aa, Williams I. Antigen sampling by intestinal M cells is the principal pathway initiating mucosal IgA production to commensal enteric bacteria. Mucosal Immunol. 2016;9:907–916. doi: 10.1038/mi.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neutra MR, Frey A, Kraehenbuhl JP. Epithelial M cells: gateways for mucosal infection and immunization. Cell. 1996;86:345–348. doi: 10.1016/S0092-8674(00)80106-3. [DOI] [PubMed] [Google Scholar]
- 26.Fujimura Y, Takeda M, Ikai H, Haruma K, Akisada T, Harada T, et al. The role of M cells of human nasopharyngeal lymphoid tissue in influenza virus sampling. Virchows Arch. 2004;444:36–42. doi: 10.1007/s00428-003-0898-8. [DOI] [PubMed] [Google Scholar]
- 27.Fujimura Y, Kamoi R, Iida M. Pathogenesis of aphthoid ulcers in Crohn’s disease: correlative findings by magnifying colonoscopy, electron microscopy, and immunohistochemistry. Gut. 1996;38:724–732. doi: 10.1136/gut.38.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parnell EA, Walch EM, Lo DD. Inducible colonic M cells are dependent on TNFR2 but not Ltβr, identifying distinct signalling requirements for constitutive versus inducible M cells. J Crohns Colitis. 2017;11:751–760. doi: 10.1093/ecco-jcc/jjw212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spencer J, Finn T, Isaacson P. Human Peyer's patches: an immunohistochemical study. Gut. 1986;27:405–410. doi: 10.1136/gut.27.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjerke K, Brandtzaeg P. Immunoglobulin-and J chain-producing cells associated with lymphoid follicles in the human appendix, colon and ileum, including Peyer's patches. Clin Exp Immunol. 1986;64:432. [PMC free article] [PubMed] [Google Scholar]
- 31.Rescigno M, Rotta G, Valzasina B, Ricciardi-Castagnoli P. Dendritic cells shuttle microbes across gut epithelial monolayers. Immunobiology. 2001;204:572–581. doi: 10.1078/0171-2985-00094. [DOI] [PubMed] [Google Scholar]
- 32.Sollid LM, Iversen R. Tango of B cells with T cells in the making of secretory antibodies to gut bacteria. Nat Rev Gastroenterol Hepatol. 2023;20:120–128. doi: 10.1038/s41575-022-00674-y. [DOI] [PubMed] [Google Scholar]
- 33.Odegard VH, Schatz DG. Targeting of somatic hypermutation. Nat Rev Immunol. 2006;6:573–583. doi: 10.1038/nri1896. [DOI] [PubMed] [Google Scholar]
- 34.Stavnezer J, Guikema JEJ, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–92. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tezuka H, Abe Y, Iwata M, Takeuchi H, Ishikawa H, Matsushita M, et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- 38.Mora JR, Iwata M, Eksteen B, Song S-Y, Junt T, Senman B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 39.Kernéis S, Bogdanova A, Kraehenbuhl J-P, Pringault E. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- 40.Tyrer P, Ruth Foxwell A, Kyd J, Harvey M, Sizer P, Cripps A. Validation and quantitation of an in vitro M-cell model. Biochem Biophys Res Commun. 2002;299:377–383. doi: 10.1016/S0006-291X(02)02631-1. [DOI] [PubMed] [Google Scholar]
- 41.Hamzaoui N, Kernéis S, Caliot E, Pringault E. Expression and distribution of β1 integrins in in vitro-induced M cells: implications for Yersinia adhesion to Peyer's patch epithelium. Cell Microbiol. 2004;6:817–828. doi: 10.1111/j.1462-5822.2004.00391.x. [DOI] [PubMed] [Google Scholar]
- 42.Corr S, Hill C, Gahan CGM. An in vitro cell-culture model demonstrates internalin- and hemolysin-independent translocation of Listeria monocytogenes across M cells. Microb Pathog. 2006;41:241–250. doi: 10.1016/j.micpath.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Tyrer P, Foxwell AR, Cripps AW, Apicella MA, Kyd JM. Microbial pattern recognition receptors mediate M-cell uptake of a gram-negative bacterium. Infect Immun. 2006;74:625–631. doi: 10.1128/IAI.74.1.625-631.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadiyala I, Loo Y, Roy K, Rice J, Leong KW. Transport of chitosan–DNA nanoparticles in human intestinal M-cell model versus normal intestinal enterocytes. Eur J Pharm Sci. 2010;39:103–109. doi: 10.1016/j.ejps.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gullberg E, Leonard M, Karlsson J, Hopkins AM, Brayden D, Baird AW, et al. Expression of specific markers and particle transport in a new human intestinal M-cell model. Biochem Biophys Res Commun. 2000;279:808–813. doi: 10.1006/bbrc.2000.4038. [DOI] [PubMed] [Google Scholar]
- 46.Blanco LP, DiRita VJ. Bacterial-associated cholera toxin and GM1 binding are required for transcytosis of classical biotype Vibrio cholerae through an in vitro M cell model system. Cell Microbiol. 2006;8:982–998. doi: 10.1111/j.1462-5822.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 47.den Rieux A, Fievez V, Théate I, Mast J, Préat V, Schneider YJ. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur J Pharm Sci. 2007;30:380–91. doi: 10.1016/j.ejps.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Martinez-Argudo I, Sands C, Jepson MA. Translocation of enteropathogenic Escherichia coli across an in vitro M cell model is regulated by its type III secretion system. Cell Microbiol. 2007;9:1538–1546. doi: 10.1111/j.1462-5822.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Argudo I, Jepson MA. Salmonella translocates across an in vitro M cell model independently of SPI-1 and SPI-2. Microbiology. 2008;154:3887–3894. doi: 10.1099/mic.0.2008/021162-0. [DOI] [PubMed] [Google Scholar]
- 50.Kim SH, Seo KW, Kim J, Lee KY, Jang YS. The M cell-targeting ligand promotes antigen delivery and induces antigen-specific immune responses in mucosal vaccination. J Immunol. 2010;185:5787–5795. doi: 10.4049/jimmunol.0903184. [DOI] [PubMed] [Google Scholar]
- 51.Roberts CL, Keita ÅV, Duncan SH, O'Kennedy N, Söderholm JD, Rhodes JM, et al. Translocation of Crohn’s disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers. Gut. 2010;59:1331–1339. doi: 10.1136/gut.2009.195370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finn R, Ahmad T, Coffey ET, Brayden DJ, Baird AW, Boyd A. Translocation of Vibrio parahaemolyticus across an in vitro M cell model. FEMS Microbiol Lett. 2014;350:65–71. doi: 10.1111/1574-6968.12323. [DOI] [PubMed] [Google Scholar]
- 53.Lin S, Mukherjee S, Li J, Hou W, Pan C, Liu J. Mucosal immunity-mediated modulation of the gut microbiome by oral delivery of probiotics into Peyer’s patches. Sci Adv. 2021;7:eabf0677. doi: 10.1126/sciadv.abf0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song JG, Lee SH, Han HK. Development of an M cell targeted nanocomposite system for effective oral protein delivery: preparation, in vitro and in vivo characterization. J Nanobiotechnol. 2021;19:15. doi: 10.1186/s12951-020-00750-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoo M-K, Kang S-K, Choi J-H, Park I-K, Na H-S, Lee H-C, et al. Targeted delivery of chitosan nanoparticles to Peyer’s patch using M cell-homing peptide selected by phage display technique. Biomaterials. 2010;31:7738–7747. doi: 10.1016/j.biomaterials.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 56.Ahmad T, Gogarty M, Walsh EG, Brayden DJ. A comparison of three Peyer’s patch “M-like” cell culture models: particle uptake, bacterial interaction, and epithelial histology. Eur J Pharm Biopharm. 2017;119:426–436. doi: 10.1016/j.ejpb.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 57.Wang J, Lopez-Fraga M, Rynko A, Lo DD. TNFR and LTβR agonists induce follicle-associated epithelium and M cell specific genes in rat and human intestinal epithelial cells. Cytokine. 2009;47:69–76. doi: 10.1016/j.cyto.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tumanov AV, Kuprash DV, Lagarkova MA, Grivennikov SI, Abe K, Shakhov AN, et al. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity. 2002;17:239–250. doi: 10.1016/S1074-7613(02)00397-7. [DOI] [PubMed] [Google Scholar]
- 59.Kuprash DV, Tumanov AV, Liepinsh DJ, Koroleva EP, Drutskaya MS, Kruglov AA, et al. Novel tumor necrosis factor-knockout mice that lack Peyer's patches. Eur J Immunol. 2005;35:1592–1600. doi: 10.1002/eji.200526119. [DOI] [PubMed] [Google Scholar]
- 60.Knoop KA, Kumar N, Butler BR, Sakthivel SK, Taylor RT, Nochi T, et al. RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J Immunol. 2009;183:5738–5747. doi: 10.4049/jimmunol.0901563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ranganathan S, Doucet M, Grassel CL, Delaine-Elias B, Zachos NC, Barry EM. Evaluating Shigella flexneri pathogenesis in the human enteroid model. Infect Immun. 2019;87:e00740–e818. doi: 10.1128/IAI.00740-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Costello CM, Willsey GG, Richards AF, Kim J, Pizzuto MS, Jaconi S, et al. Transcytosis of IgA attenuates Salmonella invasion in human enteroids and intestinal organoids. Infect Immun. 2022;90:e00041–e122. doi: 10.1128/iai.00041-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 64.Gagnon M, Berner AZ, Chervet N, Chassard C, Lacroix C. Comparison of the Caco-2, HT-29 and the mucus-secreting HT29-MTX intestinal cell models to investigate Salmonella adhesion and invasion. J Microbiol Methods. 2013;94:274–279. doi: 10.1016/j.mimet.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 65.Liu D, Li J. Effect of hyperoxia on the intestinal IgA secretory component in neonatal rats and on intestinal epithelial cells in vitro. Braz J Med Biol Res. 2010;43:1034–1041. doi: 10.1590/S0100-879X2010007500106. [DOI] [PubMed] [Google Scholar]
- 66.Zan H, Cerutti A, Dramitinos P, Schaffer A, Casali P. CD40 engagement triggers switching to IgA1 and IgA2 in human B cells through induction of endogenous TGF-β: evidence for TGF-β but not IL-10-dependent direct Sμ→Sα and sequential Sμ→Sγ, Sγ→Sα DNA recombination. J Immunol. 1998;161:5217–5225. doi: 10.4049/jimmunol.161.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McIntyre TM, Kehry MR, Snapper CM. Novel in vitro model for high-rate IgA class switching. J Immunol. 1995;154:3156–3161. doi: 10.4049/jimmunol.154.7.3156. [DOI] [PubMed] [Google Scholar]
- 68.Cerutti A, Zan H, Schaffer A, Bergsagel L, Harindranath N, Max EE, et al. CD40 ligand and appropriate cytokines induce switching to IgG, IgA, and IgE and coordinated germinal center and plasmacytoid phenotypic differentiation in a human monoclonal IgM+IgD+ B cell line. J Immunol. 1998;160:2145–2157. doi: 10.4049/jimmunol.160.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brière F, Bridon JM, Chevet D, Souillet G, Bienvenu F, Guret C, et al. Interleukin 10 induces B lymphocytes from IgA-deficient patients to secrete IgA. J Clin Investig. 1994;94:97–104. doi: 10.1172/JCI117354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marconi M, Plebani A, Avanzini MA, Maccario R, Pistorio A, Duse M, et al. IL-10 and IL-4 co-operate to normalize in vitro IgA production in IgA-deficient (IgAD) patients. Clin Exp Immunol. 2001;112:528–532. doi: 10.1046/j.1365-2249.1998.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Béniguel L, Diallo TO, Remoué F, Williams DL, Cognasse F, Charrier-Mze N, et al. Differential production in vitro of antigen specific IgG1, IgG3 and IgA: a study in Schistosoma haematobium infected individuals. Parasite Immunol. 2003;25:39–44. doi: 10.1046/j.1365-3024.2003.00603.x. [DOI] [PubMed] [Google Scholar]
- 72.Kutteh WH, Koopman WJ, Conley ME, Egan ML, Mestecky J. Production of predominantly polymeric IgA by human peripheral blood lymphocytes stimulated in vitro with mitogens. J Exp Med. 1980;152:1424–1429. doi: 10.1084/jem.152.5.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Defrance T, Vanbervliet B, Brière F, Durand I, Rousset F, Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med. 1992;175:671–682. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Husain Z, Holodick N, Day C, Szymanski I, Alper CA. Increased apoptosis of CD20+ IgA+ B cells is the basis for IgA deficiency: the molecular mechanism for correction in vitro by IL-10 and CD40L. J Clin Immunol. 2006;26:113–125. doi: 10.1007/s10875-006-9001-y. [DOI] [PubMed] [Google Scholar]
- 75.Nishikawa Y, Shibata R, Ozono Y, Ichinose H, Miyazaki M, Harada T, et al. Streptococcal M protein enhances TGF-β production and increases surface IgA-positive B cells in vitro in IgA nephropathy. Nephrol Dial Transplant. 2000;15:772–777. doi: 10.1093/ndt/15.6.772. [DOI] [PubMed] [Google Scholar]
- 76.Ohtsuka A, Piazza AJ, Ermak TH, Owen RL. Correlation of extracellular matrix components with the cytoarchitecture of mouse Peyer's patches. Cell Tissue Res. 1992;269:403–410. doi: 10.1007/BF00353895. [DOI] [PubMed] [Google Scholar]
- 77.Wang S, Liu H, Zhang X, Qian F. Intranasal and oral vaccination with protein-based antigens: advantages, challenges and formulation strategies. Protein Cell. 2015;6:480–503. doi: 10.1007/s13238-015-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qi J, Zhuang J, Lv Y, Lu Y, Wu W. Exploiting or overcoming the dome trap for enhanced oral immunization and drug delivery. J Control Release. 2018;275:92–106. doi: 10.1016/j.jconrel.2018.02.021. [DOI] [PubMed] [Google Scholar]