Abstract
Objective:
This study aimed to assess Jordanians’ perception and attitudes toward COVID-19 vaccines authorized for use in Jordan. Another objective was to evaluate the population’s confidence in vaccine efficacy, their fears of the vaccines, and their perceptions and attitudes after vaccination.
Methods:
This cross-sectional study was conducted over four months (August 2021- December 2021) and included the general Jordanian population above 18 years old.
Results:
A total of 398 participants were included in the study, with the majority (around 81.0%) received at least one dose of any of COVID-19 vaccines approved for use in Jordan. Most non-vaccinated participants (67.4%) were either unwilling to receive the COVID-19 vaccine or unsure. The main reasons for receiving the vaccine were: family protection, self-protection, global efforts to fight the virus and local restrictions, with some variability between vaccinated and non-vaccinated. The major reasons for fear of COVID-19 vaccines were limited research, vaccine effectiveness, and vaccine side effects. Pfizer-BioNTech vaccine was the most trusted vaccine by vaccinated and non-vaccinated participants (47.8% and 57.9%, respectively), and Oxford-AstraZeneca was the most feared by them (42.2% and 57.9%, respectively). Internet websites (>85.0%), social media platforms (>70.0%), relatives and friends (>69.0%), and news applications (>60.0%) were the major sources of information about the COVID-19 vaccines among participants.
Conclusion:
Our results revealed that hesitation in receiving the vaccine remains a challenge in Jordan, as in other countries. The findings also show that participants, regardless of their vaccination status, had many concerns about the four types of vaccines approved for use in Jordan during the study conduction period. Moreover, the participants’ perceptions and attitudes towards the vaccines were variable between vaccinated and non-vaccinated participants and were variable for the four types of vaccines.
Keywords: COVID-19, Jordan, vaccine, attitude, perception
INTRODUCTION
The coronavirus, known as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), first appeared in December 2019 and spread globally over a few months,1 The COVID-19 caused by the virus was then declared a pandemic by the World Health Organization (WHO) on March 11th, 2020. The pandemic affected many facets of life, including healthcare systems, the economy, education, and social life. It placed billions of people in quarantine during national lockdowns.2-4
On March 2nd, 2020, the Jordanian Ministry of Health (MoH) registered the first case of confirmed COVID-19 in Jordan.5 Since then, the Jordanian government started to impose strict national regulations and announced a strict preventive lockdown on the 14th of the same month before the outbreak gained momentum.6 The very strict lockdown succeeded to reduce the spread of the outbreak in Jordan; until the 10th of June, Jordan had less than 900 confirmed cases and exactly nine deaths.6 This was the case until the second flare of the pandemic hit the country in September of the same year resulting in a dramatic increase in the numbers of new cases and deaths which nominated Jordan to have one of the highest per-capita rates of COVID-19 in the world in that period.1, 7 According to the recent WHO statistics (November 2022), there have been around 1.75 million confirmed cases in Jordan, with a death surpassing 14 thousand deaths.1
The recent advancements in rapid vaccine development have saved the situation.8-10 Several vaccines for SARS-CoV-2 have been developed and approved by the FDA’s Emergency Use Authorization, including the vaccine manufactured by Pfizer BioNTech, Moderna, and Johnson & Johnson. Later, Pfizer BioNTech received full FDA approval.11,12
Even though vaccines have proven efficacy against many transmitted diseases as one of the most effective available medical interventions that save millions of lives yearly,13 Vaccine hesitancy and refusal remain limiting factors for achieving optimal herd immunity against some diseases. Moreover, vaccine refusal has been proven to increase the risk of contracting the target disease.13 Many factors usually affect people’s general perception and attitudes toward vaccinations, including their experience with vaccines, level of education and knowledge, risk perception and trust, and perceived importance of vaccination.14 In the case of COVID-19 vaccines, additional factors have also played a role, including the speed at which the vaccines were developed in less than a year which raised some public concerns about their safety, and the uncertainty regarding the durability of the immune response gained with vaccination.15,16 Moreover, differences in efficacy and adverse effects may cause varying trust in COVID-19 vaccines. This may subsequently affect the willingness to vaccinate, particularly when people are not given a choice of vaccine they wish to receive (as has been the case in Jordan until the mid of 2021).
The Jordanian government started the vaccination campaign in January 2021. Since then and until the beginning of this study in August 2021, the percentage of Jordanians who were fully vaccinated did not exceed 25% of the total population. Hence, it was crucial to explore the population’s beliefs and perceptions toward the COVID-19 vaccines, which stand behind their attitudes and willingness to receive the vaccine. Current statistics from Jordan (October 2022) reveal an increasing percentage of the fully vaccinated population to 44.56 % of the total population (Ref: https://covidvax.live/location/jor).
This study aimed to assess the perception and attitudes of Jordanians toward COVID-19 vaccines authorised for use in Jordan. The population’s confidence in vaccines efficacy, fears of the vaccines, and the change in their perceptions and attitudes after getting vaccinated have been investigated. The level and main sources of fear related to vaccination and primary sources of information on COVID-19 vaccines have also been investigated.
METHODS
Study design and participants
This descriptive cross-sectional study was conducted in Jordan to evaluate the population’s perception and attitudes toward COVID-19 vaccines approved in Jordan and the factors standing behind them. Data collection was conducted over five months between August and December 2021. Ethical approval was taken from the Institutional Review Board at the Faculty of Pharmacy at Applied Science Private University (Approval number: 2021-PHA-26). Inclusion criteria were being older than 18 years old and residing in Jordan.
Survey development and administration
The study tool was an anonymous, self-designed, and structured survey created using Google’s online platform. The survey was developed based on an extensive literature review and with the agreement of the research team. The initial version of the survey was originally developed in English and then translated into Arabic. Before distribution, the survey was reviewed by independent experts with previous clinical research experience to assess face validity.
The survey was delivered in Arabic, and the participants were recruited through online social media platforms (including Facebook, WhatsApp, etc.). The sample size was determined via convenience sampling, and 398 participants filled the survey. The participants agreed to participate after reading a brief about the study before answering the survey. The survey was completed anonymously to minimise any potential for bias and protect participant confidentiality.
Data collection tool
The survey consisted of three main sections covering 32 questions. The first section described the participants’ sociodemographic characteristics and included gender, age, marital status, area of residence, level of education, occupation, in addition to smoking status.
The second section of the survey addressed participants’ medical history; in this section, participants were asked about a documented history of any chronic condition, their regular medications, and previous history of COVID-19 infection. Regarding the vaccines, participants were asked if they usually receive the yearly flu vaccine and if they have already received any COVID-19 vaccine approved for use in Jordan at that time including Pfizer—BioNTech, Oxford-AstraZeneca, Sinopharm-Beijing, and Gamaleya- Sputnik V.
Participants were also asked if they have any contraindication to receive the COVID-19 vaccine as per the Jordanian MoH protocol at that time. These includes having severe immune deficiency or having a history of severe allergic reactions/anaphylaxis to any medication or vaccine).
The third section of the survey intended to assess the participants’ perceptions and attitudes toward the approved COVID-19 vaccines in Jordan and their source of information about them. According to their vaccination status, participants were exposed to slightly different contexts of questions aiming at assessing the same criteria.
Non- vaccinated participants were asked if they were willing to receive the vaccine, the reasons behind their willingness to get vaccinated, and the possible fears they have from the COVID-19 vaccines. The level of trust in each of the four COVID-19 vaccines was assessed using a 4-point Likert scale, with rating ranging from 1 (never heard about the vaccine) to 4 (highly trust the vaccine). The level of fear from each of the four vaccines was also assessed using a 4-points Likert scale, ranging from 1 (does not have any fear from the vaccine) to 4 (have a high fear from the vaccine).
Vaccinated participants were asked about the type of COVID-19 vaccine they received, and the number of received doses and were requested to evaluate the severity of adverse effects they had (if any). Participants were also asked about the reasons stranded behind receiving the COVID)19 vaccine and if they have certain fears from the vaccines.
Vaccinated participants’ level of trust in each of the four vaccines was evaluated before receiving the vaccine and afterward using the same 4- points Likert scale used for non-vaccinated individuals. The level of fear from each of the vaccines was also assessed using the same 4-point Likert scale for the level of fear previously described. Participants were further asked if their level of fear from the vaccine they received had changed after being vaccinated.
All participants were last asked about the source of their information about the approved COVID-19 vaccines in Jordan.
Data analysis
Data were analysed using SPSS version 25.0 (IBM, Armonk, NY, USA). Categorical variables were reported in frequency and percentage. Continuous variables were presented as mean (standard deviation). The two-tailed P-value < 0.05 was set for the significance level.
RESULTS
Participants’ sociodemographic characteristics
The total number of participants who completed the survey was 398, with a mean age of 30.49 (10.95). More than two-thirds of the participants were females (70.6%), more than half of the participants were single (53.8%), and most of them resided in the middle of Jordan (92.7%). Around two-thirds of the participants were holders of bachelor’s degrees (66.8%). About 47.0% of the participants were employed, 20.4% were unemployed, 1.8% were retired, and 30.7% were students. Out of the employed participants (n=188), 31.9% were working in the health sector, and 20.7% in the education.
Almost three-quarters (72.6%) of the participants were non-smokers, and around a quarter (24.6%) were smoking. The detailed sociodemographic characteristics of the participants are displayed in Table 1.
Table 1.
Demographic data and participants’ characteristics (n=398)
| Parameter | n (%) |
|---|---|
| Gender | |
| Female | 281 (70.6) |
| Male | 117 (29.4) |
| Marital status | |
| Single | 214 (53.8) |
| Married | 177 (44.5) |
| Divorced | 5 (1.3) |
| Widowed | 2 (0.5) |
| Area of residence | |
| North Jordan | 21 (5.3) |
| Middle of Jordan | 369 (92.7) |
| South of Jordan | 8 (2.0) |
| Level of education | |
| Elementary | 4 (1.0) |
| Secondary | 25 (6.3) |
| College level | 55 (13.8) |
| Bachelor level | 266 (66.8) |
| Higher education | 48 (12.1) |
| Employment | |
| Employed | 188 (47.2) |
| Not employed | 81 (20.4) |
| Retired | 7 (1.8) |
| Still studying | 122 (30.7) |
| Employment sector* | |
| Health sector | 60 (31.9) |
| Education sector | 39 (20.7) |
| Financial and banking sector | 28 (14.9) |
| Agricultural sector | 0 (0.0) |
| Tourism sector | 2 (1.1) |
| Legal sector | 1 (0.5) |
| Industrial sector | 7 (3.7) |
| Craft sector | 1 (0.5) |
| Commercial sector | 11 (5.9) |
| Public services sector | 3 (1.6) |
| Press and media sector | 1 (0.5) |
| Telecommunications sector | 8 (4.3) |
| Civil society organisations sector | 2 (1.1) |
| Other | 25 (13.3) |
| Smoking status | |
| Smoker | 98 (24.6) |
| Non-smoker | 289 (72.6) |
| Ex-smoker | 11 (2.8) |
For employed participants (n=188)
Participants’ past medical history
Regarding past medical history, 16.6% of the participants suffer from one form of allergy, 7.5% have thyroid disease, 6.0% have hypertension, 6.0% have bone and joint diseases, 4.0% have diabetes, and 3.0% have asthma. Three-quarters of the participants (75.6%) denied receiving any regular medications, and less than a quarter (21.1%) reported receiving one to three medications regularly. Around Two-thirds (73.4%) of participants never got a flu vaccine. More than half of the participants (52.5%) reported not being infected with COVID-19 before, and 14.3% were ununsure whether they got infected. Less than 3% of the participants reported being infected with COVID-19 more than once. Only around 6% of the participants reported that they are not required to receive the COVID-19 vaccine due to health issues such as being immunocompromised or hypersensitive to certain products (Table 2).
Table 2.
Past medical history of the participants (n=398)
| Parameter | n (%) |
|---|---|
| Medical condition | |
| Asthma | 12 (3.0) |
| COPD | 2 (0.5) |
| Allergic diseases | 66 (16.6) |
| Thyroid diseases | 30 (7.5) |
| Cardiovascular diseases | 11 (2.8) |
| Hypertension | 24 (6.0) |
| Diabetes | 16 (4.0) |
| Kidney diseases | 4 (1.0) |
| Liver diseases | 4 (1.0) |
| Hematological diseases | 4 (1.0) |
| Bone and joint diseases | 24 (6.0) |
| Neurological diseases | 7 (1.8) |
| Gastrointestinal diseases | 10 (2.5) |
| Cancer diseases | 2 (0.5) |
| Other conditions | 14 (3.5) |
| Number of regular medications taken | |
| None | 301 (75.6) |
| 1-3 | 84 (21.1) |
| 4-6 | 9 (2.3) |
| 7-9 | 1 (0.3) |
| More than 9 | 3 (0.8) |
| Receiving the flu vaccine | |
| Yearly | 33 (8.3) |
| Most of the years | 28 (7.0) |
| Some of the years | 45 (11.3) |
| Never | 292 (73.4) |
| Been infected with COVID-19? | |
| Yes | 132 (33.2) |
| No | 209 (52.5) |
| Not sure | 57 (14.3) |
| Been re-infected with COVID-19? | |
| Yes | 10 (2.5) |
| No | 345 (86.7) |
| Not sure | 43 (10.8) |
| Being medically excluded from receiving the COVID-19 | |
| vaccine due to the following reasons? | |
| Severe immune deficiency | 10 (2.5) |
| Severe allergic reaction to medications or vaccines | 15 (3.8) |
| Receiving the COVID-19 vaccine | |
| Yes | 322 (80.9) |
| No | 76 (19.1) |
Participants’ willingness to receive the COVID-19 vaccine approved in Jordan
Among the study’s participants (n=398), the majority (n=322, 80.9%) reported receiving at least one dose of any of the COVID-19 vaccines approved for use in Jordan, while 76 participants (19.1%) falsified receiving any. Of the vaccinated participants (n=322), the majority (86.0%) reported receiving two doses of the COVID-19 vaccine, and only 14.0% received one dose (Figure 1). Of the non-vaccinated participants (n=76), an equal percentage (30.6%) were either willing to receive the COVID-19 vaccine or not sure of that, and a slightly higher percentage (36.8%) were not yet willing to receive the COVID-19 vaccine (Figure 2).
Figure 1.
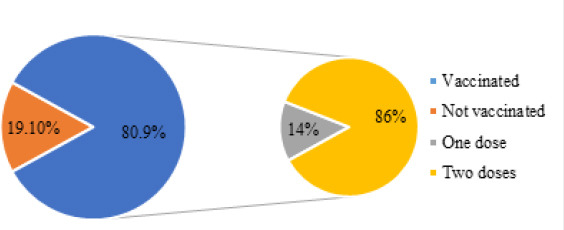
Vaccination status of the study participants (n=398) and number of doses received (n=322)
Figure 2.
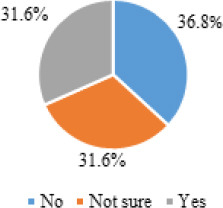
Intention to receive the COVID-19 vaccine among non-vaccinated participants (n=76)
Reasons for receiving the COVID-19 vaccines and participants’ medical history
More than three-quarters of the participants (n= 322, 80.9%) reported receiving the COVID-19 vaccine. The main reason behind receiving the COVID-19 vaccine among the vaccinated participants (n=322) was family protection (86.0%), followed by self-protection (84.5%), and global efforts to fight the virus (84.5%). On the other hand, among the non-vaccinated participants (n=76), family protection (57.9%), and local restrictions (57.9%) were the most two reported reasons (Table 3).
Table 3.
Reasons behind getting vaccinated (for vaccinated participants) or reasons that may motivate the participants to receive the COVID-19 vaccine (for non-vaccinated participants)
| Vaccinated participants (n=322) n (%) | Non-vaccinated participants (n=76) n (%) | |
|---|---|---|
| Self-protection | 272 (84.5%) | 41 (53.9%) |
| Family protection | 277 (86.0%) | 44 (57.9%) |
| Global efforts in fighting coronavirus | 272 (84.5%) | 42 (55.3%) |
| Governmental restrictions imposed on unvaccinated people | 250 (77.6%) | 38 (50.0%) |
| Working place | 169 (52.5%) | 36 (47.4%) |
| International travel restrictions | 142 (44.1%) | 34 (44.7%) |
| Local restrictions (government restrictions to enter government institutions, gyms, banks, restaurants, etc.) | 241 (74.8%) | 44 (57.9%) |
| Other reasons | 85 (26.4%) | 27 (35.5%) |
Participants’ perception and attitude toward the COVID-19 vaccines approved in Jordan
The major reasons for fear of COVID-19 vaccines among vaccinated and non-vaccinated participants were the belief that there is limited research on vaccines’ effectiveness, side effects and the possible long-term effects. Around half of the vaccinated participants and more than two-thirds of non-vaccinated participants reported their fear of the common as well as the long-term side effects of the vaccines. Around 40% of vaccinated participants (n=122) and 71% of non-vaccinated participants (n=54) reported their fear of autoimmune reactions. Around 60% of non-vaccinated participants (n=46) reported their fear of developing fertility problems due to the COVID-19 vaccine, and more than two-thirds of them (n=51) reported that their fear developed as a result of witnessing the experience of someone else who got vaccinated and suffered from adverse effects. Almost a quarter of the non-vaccinated (n=17) had the fear from vaccines due to being pregnant or lactating. The reasons for fear from the COVID-19 vaccines are reported in Table 4.
Table 4.
Major fears from COVID-19 vaccines for both vaccinated and non-vaccinated participants
| Vaccinated participants (n=322) n (%) | Non-vaccinated participants (n=76) n (%) | |
|---|---|---|
| Short-term efficacy | 192 (59.6) | 51 (67.1) |
| Insufficient studies on vaccines | 246 (76.4) | 67 (88.2) |
| Vaccines are not effective | 104 (32.3) | 50 (65.8) |
| Already have chronic conditions | 40 (12.4) | 14 (18.4) |
| Serious side effects/reactions | 80 (24.8) | 44 (57.9) |
| Common side effects | 145 (45.0) | 54 (71.1) |
| Long-term side effects | 159 (49.4) | 54 (71.1) |
| Fertility-related side effects | 102 (31.7) | 46 (60.5) |
| Autoimmune reactions | 122 (37.9) | 54 (71.1) |
| Previously had severe reaction to a medication or vaccine | 27 (8.4) | 26 (34.2) |
| Affected by other people experience | 95 (29.5) | 51 (67.1) |
| Pregnant or lactating | 16 (5.0) | 17 (22.4) |
| Other fears | 49 (15.2) | 37 (48.7) |
Vaccinated participants’ perception and attitude toward the COVID-19 vaccines approved in Jordan
Most vaccinated participants received the Pfizer-BioNTech (51.6%), followed by the Sinopharm-Beijing (38.5%) vaccine. The majority (86.0%) received two doses of the COVID-19 vaccine. The vaccine that was trusted the most among the participants was Pfizer-BioNTech (47.8% reported high trust). In comparison, the least trusted vaccine was Gamaleya-Sputnik V (9.6% reported high trust), as demonstrated in Figure 3.
Figure 3.
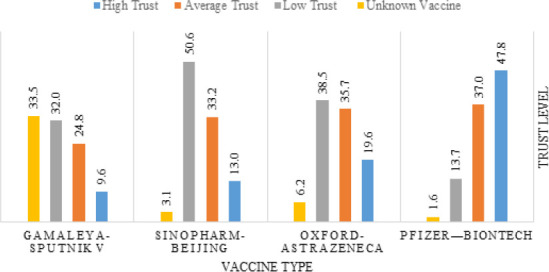
Trust level in different vaccine types among vaccinated participants (n=322)
The COVID-19 vaccine feared the most was Oxford-AstraZeneca (42.2% reported high fear), and the one that the participants feared the least was Sinopharm-Beijing (14.3% reported high fear), as demonstrated in Figure 4.
Figure 4.
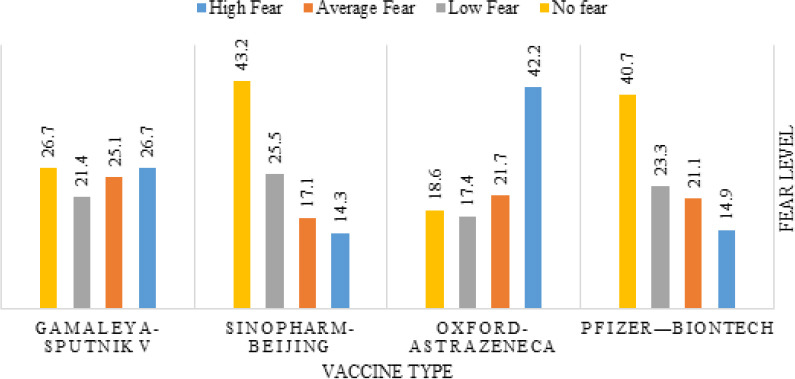
Fear level from different COVID-19 vaccine types as reported by the vaccinated participants (n=322)
Non-vaccinated participants’ perception and attitude toward the COVID-19 vaccines approved in Jordan
In general, non-vaccinated participants had lower trust levels and higher fear of all approved COVID-19 vaccines. The most trusted vaccine among them was Pfizer-BioNTech (31.6% reported high trust), while the least trusted vaccine was Gamaleya-Sputnik V (2.6% reported high trust) as demonstrated in Figure 5.
Figure 5.
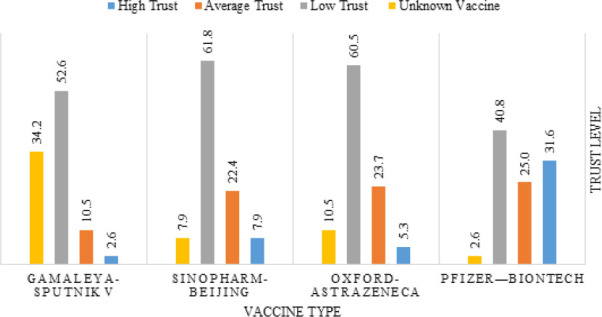
Trust level in different vaccine types- not- vaccinated participants (n=76)
The vaccines that were feared the most were Oxford-AstraZeneca (57.9% reported high fear), and Gamaleya-Sputnik V (55.3% reported high fear). In comparison, the one that the non-vaccinated participants feared the least was Sinopharm-Beijing (39.5% reported high fear), as demonstrated in Figure 6.
Figure 6.
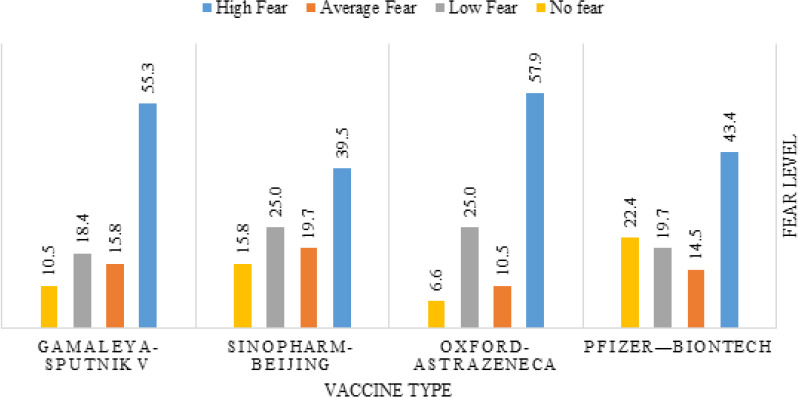
Fear level from different vaccine types among not-vaccinated participants (n= 76)
Participants’ source of information about the COVID-19 vaccines
Internet websites (>85%), social media platforms (>70%), relatives and friends (>69%), and news applications (>60%) were the major sources of information about the COVID-19 vaccines (Figure 7).
Figure 7.
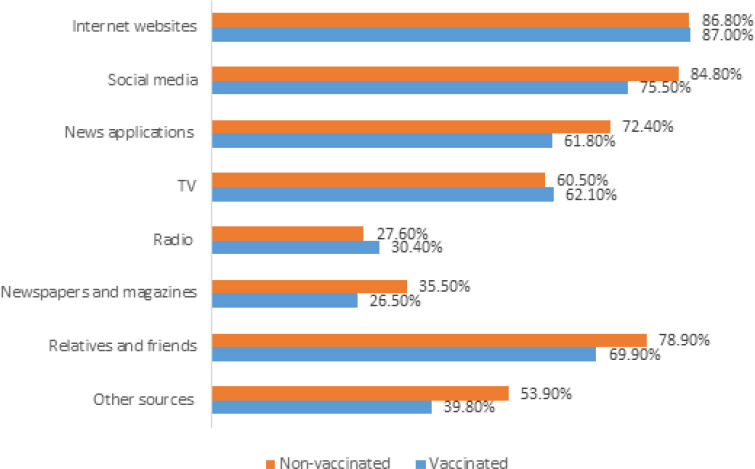
Sources of information about COVID-19 vaccines
DISCUSSION
This study aimed to assess the perception and attitudes of Jordanians toward COVID-19 vaccines authorised for use in Jordan. Vaccination is one of the most crucial strategies that the world relies on to limit the disease spread, and aid in the recovery from the devastating impacts of the pandemic. In order to achieve herd immunity, a proportion of 70% of the population need to be immuned, either naturally or by vaccination. However, many studies showed low vaccination acceptance among the population; the need for more information was one of the factors that led to vaccination hesitancy.17 As shown in the participants’ sociodemographic characteristics, age and level of education were the main factors associated with awareness of particular vaccine types in the study group. The respondents were primarily middle-aged, around 40 years old. Adding to the other factors like gender, with a high proportion of females who inhabited urban areas, predominantly the capital city of Jordan, almost half of them were employees, educated and bachelor’s degree graduates, bearing in mind that vaccine safety and efficacy might be challenging to understand by individuals with low academic background.18
These findings underscore the possible association between COVID-19 vaccine acceptability and sociodemographic characteristics, which is consistent with other studies conducted at similar times in different countries around the world and with nationwide COVID-19 vaccination campaigns.
Our results confirmed that 80.9% of participants received the COVID-19 vaccine. Fortunately, this percentage exceeds the threshold for achieving herd immunity (70%).17
The landscape of the participant’s medical history shows that a previous experience of certain types of allergies or any underlying disease like thyroid, hypertension, bone and joint, diabetes and asthma, is one of the strong correlates of vaccine acceptability to avoid the perceived likelihood of COVID-19 infection, severe symptoms, and the post COVID-19 complications.19
On the other hand, we note the controversy that COVID-19 vaccines are more often discouraged in the lowest proportion of chronically ill patients due to vaccine contraindications.20
The current study clearly shows that there may have been differences in perceptions of various authorised COVID-19 vaccines including Pfizer-BioNTech, Oxford-AstraZeneca, Sinopharm-Beijing, and Gamaleya-Sputnik V, which is shown in participants’’ trust level in each vaccine. These differences should be considered when assigning a particular vaccine to a given group of participants. Verifying that those at very high risk of COVID-19 infection will receive the vaccine(s) with the greatest public trust level, to result in increasing vaccine toleration and decreasing vaccine hesitancy in those priority groups.21
The results reveal that the majority of the vaccinated participants received the Pfizer-BioNTech vaccine (51.6%), followed by Sinopharm-Beijing (38.5%), and the rest received Oxford-AstraZeneca or Gamaleya-Sputnik V. The vaccination program’s effectiveness depends on convincing efficacy and safety data coupled with popular public acceptance and immunisation.22 However, hesitation to get vaccinated remains a notable challenge in different countries.15
Concerns about vaccine safety and side effects were on top of the reasons for hesitancy or refusal in non-vaccinated participants. These fears are sustainable, as indicated by studies conducted in the United States,18 Europe,23 and China.24 The rationale behind these fears is legitimate, as some vaccine candidate trials have been halted due to detection of undesirable side effects.25 In addition, many unvaccinated participants delayed vaccination to an indeterminate time due to a lack of trust in newly developed vaccines.
Despite the availability of services, vaccine hesitancy may result in delayed or missed vaccinations. In particular, the rapid vaccine development timeline for a COVID-19 vaccine could undermine confidence in vaccination and fuel hesitation.15 Comprehensive evidence-based efforts to address behavioral change are necessary to address the persistent problem of vaccine hesitancy.
The study results highlighted that the public knowledge and awareness of vaccines in Jordan has improved, in gratitude to the experts, health professional’s role, national authorities, and media coverage in delivering reliable information on COVID-19 vaccines, through using various channels (e.g., television, radio, newspapers, direct mail, websites, social media) for the highest possible outreach.26,27 Their activities can increase vaccination rates, and improve the sense of security as being free of any unenclosed side effects in those who have been vaccinated or who would like to be vaccinated. Subsequently, these activities may also positively impact individuals currently facing COVID-19 vaccine hesitancy.
Notwithstanding that these media channels have been a valuable tool to publicise accurate information during the COVID-19 pandemic, some platforms are also considered sources of misinformation that contribute to individuals’ hesitancy in getting vaccinated. All of these negative impacts require the government and health sectors, including pharmacists, to continuously launch a series of mass media campaigns to counter disinformation and the spread of misinformation.28
Importantly, the COVID-19 pandemic might be an ideal time to elevate the general awareness of other vaccines’ acceptance due to the public’s increased interest in vaccinations, a good opportunity that should not be missed.
Study limitations
Social media platforms were the main tool to reach a convenient sample of participants for this study, which added some selection bias; most participants were young and held a Bachelor’s degree. This may constrain the generalizability of results to the general Jordanian population. Thus, we highly urge more research targeting other age groups and reaching populations of different educational levels.
CONCLUSION
Our results revealed that hesitation in receiving the vaccine remains a challenge in Jordan, as in other countries. The findings also show that participants, regardless of their vaccination status, had many concerns about the four types of vaccines approved for use in Jordan during the study conduction period. Moreover, the participants’ perceptions and attitudes towards the vaccines were variable between vaccinated and non-vaccinated participants and were variable for the four types of vaccines.
CONFLICTS OF INTEREST
All Authors declare no conflicts of interest related to this article.
Contributor Information
Dalal Alnatour, PharmD. Faculty of Pharmacy, Applied Science Private University, Amman, Jordan. d_alnatour@asu.edu.jo.
Razan I. Nassar, MSc. Department of Clinical Pharmacy and Therapeutics, Faculty of Pharmacy, Applied Science Private University, Amman, Jordan. R_nassar@asu.edu.jo
Yara Salhi, United States of America..
Samar Thiab, PhD. Assistant Professor in Pharmaceutical Analysis, Department of Pharmaceutical Chemistry and Pharmacognosy, Faculty of Pharmacy, Applied Science Private University, Amman, Jordan. S_Thiab@asu.edu.jo.
Ahmad R. Alsayed, PhD. Department of Clinical Pharmacy and Therapeutics, Faculty of Pharmacy, Applied Science Private University, Amman 11931-166, Jordan. a_alsayed@asu.edu.jo
References
- 1.1. World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard:World Health Organization;2020.; World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard:World Health Organization. 2020 [Google Scholar]
- 2.2. Lenzen M, Li M, Malik A, et al. Global socio-economic losses and environmental gains from the Coronavirus pandemic. PLoS One. 2020;15(7):e0235654. https://doi.org/10.1371/journal.pone.0235654 [DOI] [PMC free article] [PubMed]; Lenzen M, Li M, Malik A, et al. Global socio-economic losses and environmental gains from the Coronavirus pandemic. PLoS One. 2020;15(7):e0235654. doi: 10.1371/journal.pone.0235654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.3. Betancourt JA, Rosenberg MA, Zevallos A, et al. The Impact of COVID-19 on Telemedicine Utilization Across Multiple Service Lines in the United States. Healthcare (Basel). 2020;8(4):380. https://doi.org/10.3390/healthcare8040380 [DOI] [PMC free article] [PubMed]; Betancourt JA, Rosenberg MA, Zevallos A, et al. The Impact of COVID-19 on Telemedicine Utilization Across Multiple Service Lines in the United States. Healthcare (Basel) 2020;8(4):380. doi: 10.3390/healthcare8040380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.4. Rapanta C, Botturi L, Goodyear P, et al. Online University Teaching During and After the Covid-19 Crisis:Refocusing Teacher Presence and Learning Activity. Postdigital Science and Education. 2020;2(3):923-945. https://doi.org/10.1007/s42438-020-00155-y; Rapanta C, Botturi L, Goodyear P, et al. Online University Teaching During and After the Covid-19 Crisis:Refocusing Teacher Presence and Learning Activity. Postdigital Science and Education. 2020;2(3):923–945. doi: 10.1007/s42438-020-00155-y. [DOI] [Google Scholar]
- 5.5. Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. Journal of Medical Virology. 2020;92(9):726-730. https://doi.org/10.1002/jmv.25937 [DOI] [PMC free article] [PubMed]; Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. Journal of Medical Virology. 2020;92(9):726–730. doi: 10.1002/jmv.25937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.6. Jensehaugen J. Jordan and COVID-19:Effective Response at a High Cost. PRIO Middle East Center Mideast Policy Brief No. 03-2020. 2020.; Jensehaugen J. Jordan and COVID-19:Effective Response at a High Cost. PRIO Middle East Center Mideast Policy Brief No. 03-2020. 2020 [Google Scholar]
- 7.7. Ministry of Health. COVID-19 Updates in Jordan. Ministry of Health:Ministry of Health;2020.; Ministry of Health. COVID-19 Updates in Jordan. Ministry of Health:Ministry of Health. 2020 [Google Scholar]
- 8.8. Nowakowska J, Sobocińska J, Lewicki M, et al. When science goes viral:The research response during three months of the COVID-19 outbreak. Biomed Pharmacother. 2020;129:110451. https://doi.org/10.1016/j.biopha.2020.110451 [DOI] [PMC free article] [PubMed]; Nowakowska J, Sobocińska J, Lewicki M, et al. When science goes viral:The research response during three months of the COVID-19 outbreak. Biomed Pharmacother. 2020;129:110451. doi: 10.1016/j.biopha.2020.110451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.9. Rzymski P, Nowicki M, Mullin GE, et al. Quantity does not equal quality:Scientific principles cannot be sacrificed. Int Immunopharmacol. 2020;86(1):106711. https://doi.org/10.1016/j.intimp.2020.106711 [DOI] [PMC free article] [PubMed]; Rzymski P, Nowicki M, Mullin GE, et al. Quantity does not equal quality:Scientific principles cannot be sacrificed. Int Immunopharmacol. 2020;86(1):106711. doi: 10.1016/j.intimp.2020.106711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.10. Gianola S, Jesus TS, Bargeri S, et al. Characteristics of academic publications, preprints, and registered clinical trials on the COVID-19 pandemic. PLoS One. 2020;15(10):e0240123. https://doi.org/10.1371/journal.pone.0240123 [DOI] [PMC free article] [PubMed]; Gianola S, Jesus TS, Bargeri S, et al. Characteristics of academic publications, preprints, and registered clinical trials on the COVID-19 pandemic. PLoS One. 2020;15(10):e0240123. doi: 10.1371/journal.pone.0240123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.11. Krause PA-O, Gruber MF. Emergency Use Authorization of Covid Vaccines - Safety and Efficacy Follow-up Considerations. 2020;383(19):e197. https://doi.org/10.1056/nejmp2031373 [DOI] [PubMed]; Krause PA-O, Gruber MF. Emergency Use Authorization of Covid Vaccines - Safety and Efficacy Follow-up Considerations. 2020;383(19):e197. doi: 10.1056/nejmp2031373. [DOI] [PubMed] [Google Scholar]
- 12.12. Livingston EH, Malani PN, Creech CBJJ. The Johnson &Johnson Vaccine for COVID-19. 2021;325:1575. https://doi.org/10.32388/w1nnlf [DOI] [PubMed]; Livingston EH, Malani PN, Creech CBJJ. The Johnson &Johnson Vaccine for COVID-19. 2021;325:1575. doi: 10.32388/w1nnlf. [DOI] [PubMed] [Google Scholar]
- 13.13. Phadke VK, Bednarczyk RA, Salmon DA, et al. Association Between Vaccine Refusal and Vaccine-Preventable Diseases in the United States:A Review of Measles and Pertussis. JAMA. 2016;315(11):1149. https://doi.org/10.1001/jama.2016.1353 [DOI] [PMC free article] [PubMed]; Phadke VK, Bednarczyk RA, Salmon DA, et al. Association Between Vaccine Refusal and Vaccine-Preventable Diseases in the United States:A Review of Measles and Pertussis. JAMA. 2016;315(11):1149. doi: 10.1001/jama.2016.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.14. DubéE, Laberge C, Guay M, et al. Vaccine hesitancy:an overview. Hum Vaccin Immunother. 2013;9:1763-1773. [DOI] [PMC free article] [PubMed]; Dubé E, Laberge C, Guay M, et al. Vaccine hesitancy:an overview. Hum Vaccin Immunother. 2013;9:1763–1773. doi: 10.4161/hv.24657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.15. Dror AA, Eisenbach N, Taiber S, et al. Vaccine hesitancy:the next challenge in the fight against COVID-19. Eur J Epidemiol. 2020;35:775-779. https://doi.org/10.21203/rs.3.rs-35372/v1 [DOI] [PMC free article] [PubMed]; Dror AA, Eisenbach N, Taiber S, et al. Vaccine hesitancy:the next challenge in the fight against COVID-19. Eur J Epidemiol. 2020;35:775–779. doi: 10.21203/rs.3.rs-35372/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.16. Callaghan T, Moghtaderi A, Lueck JA, et al. Correlates and disparities of COVID-19 vaccine hesitancy. 2020. https://doi.org/10.2139/ssrn.3667971; Callaghan T, Moghtaderi A, Lueck JA, et al. Correlates and disparities of COVID-19 vaccine hesitancy. 2020 doi: 10.2139/ssrn.3667971. [DOI] [Google Scholar]
- 17.17. Al-Qerem W, Jarab AS, Qarqaz R, et al. Attitudes of a sample of Jordanian young adults toward different available COVID-19 vaccines. Vacunas. 2022;23:S56-s63. https://doi.org/10.1016/j.vacun.2021.07.008 [DOI] [PMC free article] [PubMed]; Al-Qerem W, Jarab AS, Qarqaz R, et al. Attitudes of a sample of Jordanian young adults toward different available COVID-19 vaccines. Vacunas. 2022;23:S56–s63. doi: 10.1016/j.vacun.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.18. Fisher KA, Bloomstone SJ, Walder J, et al. Attitudes Toward a Potential SARS-CoV-2 Vaccine :A Survey of U.S. Adults. Ann Intern Med. 2020;173(12):964-973. https://doi.org/10.7326/m20-3569 [DOI] [PMC free article] [PubMed]; Fisher KA, Bloomstone SJ, Walder J, et al. Attitudes Toward a Potential SARS-CoV-2 Vaccine :A Survey of U. S. Adults. Ann Intern Med. 2020;173(12):964–973. doi: 10.7326/m20-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.19. Suvvari TK, Kutikuppala LVS, Tsagkaris C, et al. Post-COVID-19 complications:Multisystemic approach. J Med Virol. 2021;93(12):6451-6455. https://doi.org/10.1002/jmv.27222 [DOI] [PMC free article] [PubMed]; Suvvari TK, Kutikuppala LVS, Tsagkaris C, et al. Post-COVID-19 complications:Multisystemic approach. J Med Virol. 2021;93(12):6451–6455. doi: 10.1002/jmv.27222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.20. Štěpánek L, JanošíkováM, NakládalováM, et al. Motivation to COVID-19 Vaccination and Reasons for Hesitancy in Employees of a Czech Tertiary Care Hospital:A Cross-Sectional Survey. Vaccines (Basel). 2021;9(8):863. https://doi.org/10.3390/vaccines9080863 [DOI] [PMC free article] [PubMed]; Štěpánek L, Janošíková M, Nakládalová M, et al. Motivation to COVID-19 Vaccination and Reasons for Hesitancy in Employees of a Czech Tertiary Care Hospital:A Cross-Sectional Survey. Vaccines (Basel) 2021;9(8):863. doi: 10.3390/vaccines9080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.21. Persad G, Peek ME, Emanuel EJ. Fairly Prioritising Groups for Access to COVID-19 Vaccines. JAMA. 2020;324(16):1601-1602. https://doi.org/10.1001/jama.2020.18513 [DOI] [PubMed]; Persad G, Peek ME, Emanuel EJ. Fairly Prioritising Groups for Access to COVID-19 Vaccines. JAMA. 2020;324(16):1601–1602. doi: 10.1001/jama.2020.18513. [DOI] [PubMed] [Google Scholar]
- 22.22. Cordero DA. Rebuilding public trust:a clarified response to COVID-19 vaccine hesitancy predicament. J Public Health (Oxf). 2021;43(2):e303-e304. https://doi.org/10.1093/pubmed/fdab020 [DOI] [PMC free article] [PubMed]; Cordero DA. Rebuilding public trust:a clarified response to COVID-19 vaccine hesitancy predicament. J Public Health (Oxf) 2021;43(2):e303–e304. doi: 10.1093/pubmed/fdab020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.23. Neumann-Böhme S, Varghese NE, Sabat I, et al. Once we have it, will we use it?A European survey on willingness to be vaccinated against COVID-19. The European Journal of Health Economics. 2020;21(7):977-982. https://doi.org/10.1007/s10198-020-01208-6 [DOI] [PMC free article] [PubMed]; Neumann-Böhme S, Varghese NE, Sabat I, et al. Once we have it, will we use it?A European survey on willingness to be vaccinated against COVID-19. The European Journal of Health Economics. 2020;21(7):977–982. doi: 10.1007/s10198-020-01208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.24. Wang J, Jing R, Lai X, et al. Acceptance of COVID-19 Vaccination during the COVID-19 Pandemic in China. Vaccines (Basel). 2020;8(1):278-284. https://doi.org/10.26524/royal.37.29 [DOI] [PMC free article] [PubMed]; Wang J, Jing R, Lai X, et al. Acceptance of COVID-19 Vaccination during the COVID-19 Pandemic in China. Vaccines (Basel) 2020;8(1):278–284. doi: 10.26524/royal.37.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.25. Yorita KL, Holman RC, Sejvar JJ, et al. Infectious disease hospitalisations among infants in the United States. Pediatrics. 2008;121(2):244-252. https://doi.org/10.1542/peds.2007-1392 [DOI] [PubMed]; Yorita KL, Holman RC, Sejvar JJ, et al. Infectious disease hospitalisations among infants in the United States. Pediatrics. 2008;121(2):244–252. doi: 10.1542/peds.2007-1392. [DOI] [PubMed] [Google Scholar]
- 26.26. Brownlie J, Howson A. 'Between the demands of truth and government':health practitioners, trust and immunisation work. Soc Sci Med. 2006;62:433-443. [DOI] [PubMed]; Brownlie J, Howson A. 'Between the demands of truth and government':health practitioners, trust and immunisation work. Soc Sci Med. 2006;62:433–443. doi: 10.1016/j.socscimed.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 27.27. Larson HJ, Cooper LZ, Eskola J, et al. Addressing the vaccine confidence gap. Lancet. 2011;378(9790):526-535. https://doi.org/10.1016/s0140-6736(11)60678-8 [DOI] [PubMed]; Larson HJ, Cooper LZ, Eskola J, et al. Addressing the vaccine confidence gap. Lancet. 2011;378(9790):526–535. doi: 10.1016/s0140-6736(11)60678-8. [DOI] [PubMed] [Google Scholar]
- 28.28. Piltch-Loeb R, Savoia E, Goldberg B, et al. Examining the effect of information channel on COVID-19 vaccine acceptance. PLoS One. 2021;16(5):e0251095. https://doi.org/10.1101/2021.01.18.21250049 [DOI] [PMC free article] [PubMed]; Piltch-Loeb R, Savoia E, Goldberg B, et al. Examining the effect of information channel on COVID-19 vaccine acceptance. PLoS One. 2021;16(5):e0251095. doi: 10.1101/2021.01.18.21250049. [DOI] [PMC free article] [PubMed] [Google Scholar]


