Abstract
Fluctuations of reproductive hormones are associated with various forms of sleep disturbances and specific sleep disorders, such as insomnia or sleep-disordered breathing, across different stages of reproductive aging. During the menstrual cycle, sleep is particularly disrupted during the late luteal phase, as demonstrated by both objective and subjective measurements of sleep. Progesterone and its metabolites generally have sleep-promoting effects. A steep decline in progesterone, for example, during the late luteal phase, is associated with sleep disruption. Endogenous estrogen shows no clear correlation with sleep alterations in relation to the menstrual cycle. During pregnancy, sleep disruption is not associated with changes in estrogen or progesterone but rather with changing physiological factors, such as nocturnal micturition, gastroesophageal reflux, or musculoskeletal discomfort, all substantial factors that most likely mask any effect of hormones. Both endogenous and exogenous estrogen, as well as progesterone, are positively associated with sleep during the menopausal transition. A marked improvement of sleep disturbances is observed with perimenopausal hormone therapy. As this effect is not seen in younger women receiving contraceptive therapy, other causes of sleep disturbances, such as aging and related changes in metabolism of stress hormones, secondary effects of vasomotor symptoms, or depression, must be considered. Gonadotropins are less associated with sleep disturbances than ovarian hormones, except for during the menopausal transition where follicle-stimulating hormone is related to sleep disruption. Further, hyperandrogenism, as seen in women with polycystic ovary syndrome, is associated with sleep disturbances and specific sleep disorders, for example, obstructive sleep apnea.
Keywords: sleep, reproductive hormones, menstrual cycle, pregnancy, menopause
Sleep disturbances are reported by nearly one-third of the general population across all age groups and further increase with advancing age, affecting nearly 50% of individuals older than 65 years [1]. Important quality-of-life domains, such as physical functioning or emotional well-being, are significantly affected in patients experiencing chronic sleep disturbances [2]. While the involvement of reproductive hormones in various medical conditions, such as metabolic disorders, cardiovascular disease, and bone health is well documented, their role in sleep disturbances is less clear.
There are multiple causes of sleep disturbances in women and the pathophysiology is currently poorly understood. As women are more affected by sleep disturbances than men [3] and sleep disturbances are highly prevalent during times of hormonal changes, such as pregnancy or menopausal transition, the type, level, and changes in reproductive hormones are likely to contribute to these sleep complaints. Various clinical aspects, such as physical discomfort in pregnant women, interferences caused by the infant's sleeping rhythm in the postnatal phase, or vasomotor symptoms in perimenopausal women, are also likely to be significant contributors to sleep disturbances. A review of the relationship between female reproductive hormones and sleep disturbances is very timely since sleep disturbances constitute a growing challenge in patient care and it is critical to understand the potential contributions from the hormone environment. New wearable electronics allow for a more representative measurement of sleep in the home environment so that more and more women present with self-diagnosed sleep disorders. Also, hormone therapies, such as oral contraceptives or menopausal hormone therapies, are among the most frequently used medications worldwide. Providing background knowledge about the causal links between hormonal changes and sleep disturbances is therefore highly clinically relevant and may help in choosing appropriate treatment.
Background
Stages of Reproductive Aging
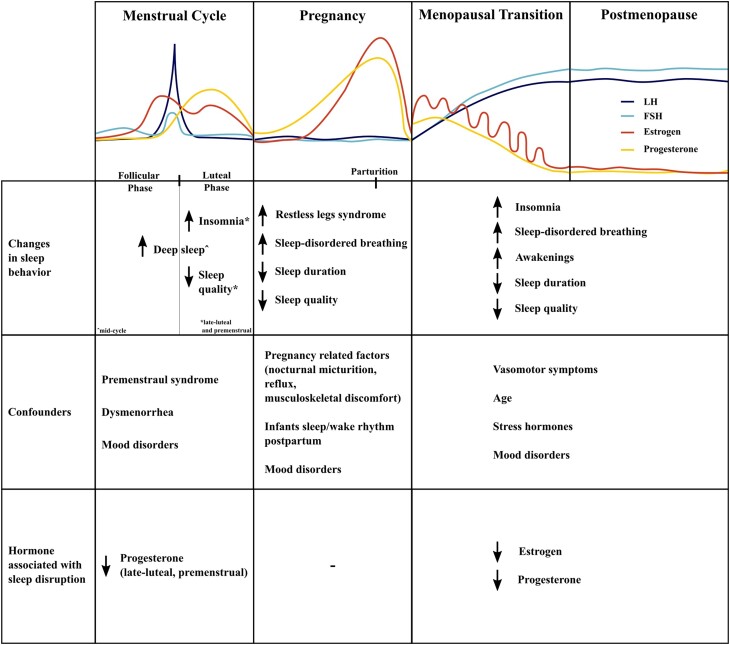
Hormone levels vary across the different stages of reproductive aging (Fig. 1), which may be divided by applying the STRAW criteria. The STRAW criteria divide the adult female life into 3 broad phases: reproductive, menopausal transition, and postmenopause [4]. These 3 phases are further subdivided into 7 stages. These life phases represent a good model to evaluate hormonal influences on sleep. The follicular phase of the menstrual cycle is predominantly controlled by follicle-stimulating hormone (FSH) and luteinizing hormone (LH) which are pulsatively released by the pituitary under regulation of the hypothalamus [5]. Both hormones control the maturation of ovarian follicles and FSH particularly induces estrogen production. With the growth of the ovarian follicle carrying the oocyte during the follicular phase, estrogen, which is generated though aromatization of androgens in the granulosa cells of these follicles, increases [6]. An estradiol level above 200 pg/mL for more than 50 hours induces the LH surge via a positive feedback mechanism, which results in ovulation [7]. Ovulation marks the end of the follicular phase and is followed by the luteal phase. The luteal phase of the menstrual cycle is characterized by rising levels of progesterone, produced in the now transformed granulosa cells of the postovulatory follicular wall forming the corpus luteum. Progesterone declines when the corpus luteum reaches the end of its natural life span in absence of a pregnancy and degenerates before the onset of menses. Additionally, part of the granulosa cells continues to aromatize androgens after ovulation which induces a second but smaller rise in estrogen during the luteal phase. Under physiologic circumstances, the menstrual cycle repeats until a woman becomes pregnant or reaches menopause.
Figure 1.
Hormone levels and sleep across stages of reproductive aging.
During pregnancy, there is a massive increase in estrogen levels until the second trimester and progesterone levels until the third trimester to about 7000 and 300 ng/mL, respectively [8], which decline abruptly after parturition [9]. In early pregnancy, up until about 8 to 10 weeks of gestation, estrogen metabolites and progesterone are produced by the corpus luteum [10]. Afterwards, the placental syncytiotrophoblast becomes a major source of progesterone und estrogen production [11]. During pregnancy, FSH and LH are at very low levels of <1 mIU/mL [12]. Prolactin levels increase throughout pregnancy until they reach about 300 ng/mL at term [8]. However, lactation only begins after delivery as the lactogenic activity of prolactin is suppressed by high levels of circulating estrogen and progesterone during pregnancy. The postpartum decline of estrogen and progesterone disinhibits the action of prolactin, thereby, resulting in lactation [13].
As women age and ovarian reserves diminish, the pattern of reproductive hormones first becomes acyclic and ultimately ovarian hormones return to very low levels as there is no longer follicular growth [14]. In the case of estrogen, this decline may be erratic and irregular, thereby causing a variety of climacteric symptoms [15]. Due to a negative feedback mechanism, FSH and LH increase as the ovarian hormones estrogen and progesterone decline [14]. There is a relative increase in androgens due to the decline of ovarian hormones and the increase of sex hormone-binding globulin (SHBG) [16]. In addition to reproductive hormones, stress hormones may play a dominant role in later reproductive stages as stress hormones increase with age [17] and also affect sleep [18].
Brief Overview of Sleep Physiology
Sleep is broadly defined as 2 states, rapid eye movement (REM) sleep and non-REM sleep, which is further divided into different stages (N1, N2, and N3 [N3 is also known as slow wave sleep]) [19, 20]. The depth of sleep progressively increases from N1 through to N3. N1 is characterized by a low-voltage, mixed-frequency electroencephalography (EEG) and is considered a transitional stage of sleep, comprising 2% to 5% of total sleep time (TST) in adults [21]. N2 sleep is characterized by the presence of sleep spindles (burst of 9-16 Hz waves, ≥ 0.5 seconds) and K-complexes (consisting of a positive-negative-positive waveform ≥0.5 seconds) on the EEG and comprises 45% to 55% of TST. N3 sleep is characterized by the presence of high amplitude, low frequency delta waves, comprising 13% to 23% of TST in adults [21]. Finally, REM sleep constitutes 20% to 25% of TST and is defined by the presence of muscle atonia in electromyography, episodic bursts of rapid eye movements, and low amplitude, mixed-frequency EEG. Non-REM and REM sleep alternate cyclically (every 90-120 minutes) across the night, with REM sleep proportions progressively increasing across the night. REM sleep facilitates learning and memory and is important for the development of the central nervous system [22, 23]. Lack of REM sleep may result in enhanced emotional reactivity [24], adverse health effects, and higher mortality [25].
Common Specific Sleep Disorders
The International Classification of Sleep disorders (ICSD-3) divides sleep disorders into 7 major categories: “Insomnia,” “Sleep-related breathing disorders,” “Sleep-related movement disorders,” “Central disorders of hypersomnolence,” “Circadian rhythm sleep-wake disorders,” “Parasomnias,” and “Other sleep disorders” [26]. Each of these are categorized into subsections with individual disorders. The following gives a brief overview of the most common relevant sleep disorders that we will investigate with regard to their potential association with female reproductive hormones. We chose the following sleep disorders due to their high prevalence.
Insomnia
Insomnia is the most common sleep disorder [27]. A meta-analysis of insomnia prevalence in the general population across different continents revealed a pooled overall prevalence of 22% and that women were 1.5 times more likely to have insomnia compared to men [28]. Besides female sex, other risk factors such as advanced age, depressive symptoms, and high stress levels have been identified [27]. According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, insomnia is classified as a dissatisfaction with sleep quantity or quality associated with one or more of the following: the difficulty of initiating sleep, the difficulty of maintaining sleep, and morning awakening with inability to return to sleep [29].
Sleep-related breathing disorders
Sleep-disordered breathing occurs in 24% of young-middle aged men and 9% of women, with a male-to-female ratio estimated between 3:1 to 5:1 in the general population [30]. It is characterized by abnormal breathing during sleep. There are different types of sleep apnea, specifically, obstructive sleep apnea (OSA) or central sleep apnea. Although men are generally more affected by sleep apnea than women [31], the prevalence in women increases as they reach menopause [32]. Sleep-disordered breathing occurs in 70% of older men and 56% of older women [30]. Additionally, pregnancy is a risk factor for sleep apnea [33]. Besides other known risk factors, such as obesity, this suggests a hormonal component in the pathophysiology of this sleep disorder.
Sleep-related movement disorders
Restless legs syndrome (RLS) is classified as a sleep-wake disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [29]. It is characterized by a continuous urge to move the legs which is most severe at night [34]. Compared with men, the prevalence in women is twice as high (9% vs 5%) [35]. Further, there is a significant increase in prevalence of RLS in pregnant women, particularly during the last trimester [36]. Periodic limb movement disorder (PLMD) is another sleep-related movement disorder where patients involuntarily move their limbs during sleep. It often occurs together with RLS [37]. Periodic limb movements are common in the elderly [38], especially females [39], but also in pregnant women [40].
Methods
The search strategy included keywords related to (i) “reproductive hormones” such as estrogen, progesterone, and their respective metabolites, FSH, LH, prolactin, and androgens (ii) any form of “sleep disturbances” both objectively and subjectively measured and specific sleep disorders such as insomnia, sleep-related breathing disorders, or sleep-related movement disorders and (iii) keywords related to “reproductive aging” such as menstrual cycle, pregnancy, postpartum, and menopause. Studies investigating the association between female reproductive hormones and general sleep disturbances or common specific sleep disorders were included. We focused on human studies. Study groups included women of reproductive age who either had a regular menstrual cycle, were taking contraceptives, or were pregnant, as well as peri- and postmenopausal women. We also considered studies which included women with known endocrine pathologies, for example polycystic ovary syndrome (PCOS) or premature ovarian insufficiency (POI). We considered studies where hormone levels were either measured directly in serum, urine, or saliva, where assumptions were made based on phase of menstrual cycle, or where hormone therapy was given. While searching for studies regarding the relationship between progesterone and sleep, we also included studies discussing the luteal phase effect on sleep. In cases where no direct hormone measurements were taken, we assumed that progesterone levels were highest during the mid-luteal phase and declined in the late luteal phase. Specific terminology regarding sleep physiology is explained in Table 1. The Study Quality Assessment Tools of the National Institutes of Health (NIH) were used to rate the quality of studies [47].
Table 1.
Specific terminology regarding sleep physiology
| Slow wave sleep | Another term for stage 3 (N3) of non-REM sleep, also referred to as deep sleep [41] |
| REM latency | Time between onset of sleep to the first REM stage, changes in REM latency are considered biological markers for a number of sleep-related disorders [42] |
| Rapid eye movement densities | Frequency of rapid eye movements, can be used as a measure of sleep need, decreases with reduction of sleep duration [43] |
| Sleep efficiency | Ratio of total sleep time to time spent in bed [44] |
| Arousals | Central nervous system activation associated with increased sympathetic tone, characterized by an abrupt EEG frequency, which may include alpha and/or theta waves and/or delta waves and/or frequencies greater than 16 Hz lasting at least 3 s [45] |
| Sleep spindles | Contribute to memory consolidation and neuronal plasticity [46] |
Results
Menstrual Cycle
In premenopausal women, sleep is influenced by the menstrual cycle [48]. The follicular phase and early luteal phase are marked by a longer duration of deep sleep compared with the time around menstruation [49]. Women reported sleeping better in their follicular and ovulatory phases compared with their premenstrual phase [50], although individual differences are reported [51].The luteal phase is characterized by less REM sleep and a higher spindle frequency [52]. Insomnia and poor sleep quality worsen in the late luteal and premenstrual phase [53]. An overview of studies examining the relationship between reproductive hormones and sleep in premenopausal women is shown in Table 2.
Table 2.
Overview of included studies examining the relationship between sleep and reproductive hormones in premenopausal women
| Author | Year | Type of study | Sample size (n) | Method of sleep disorder assessment | Method of hormone measurement | Duration of data acquisition | Multiple measurements | Overall ratinga |
|---|---|---|---|---|---|---|---|---|
| Hoffmann et al [44] | 1978 | Cross-sectional | 15 | Polysomnography, questionnaires | Blood | 1 menstrual cycle | Yes | Fair |
| Hicks and Cavanaugh [54] | 1982 | Cross-sectional | 49 | Sleep logs, questionnaires | — | 2 complete menstrual cycles | No | Poor |
| Stahl et al [55] | 1985 | Cross-sectional | 11 | Polysomnography | Blood | 1 menstrual cycle | Yes | Fair |
| Parry et al [56] | 1989 | Case-control | 16 | Sleep EEG, daily sleep logs | Blood | 1 menstrual cycle | Yes | Fair |
| Lee et al [57] | 1990 | Cross-sectional | 17 | Polysomnography, diary | Saliva | 1 menstrual cycle | Yes | Fair |
| Ishizuka et al [58] | 1994 | Cross-sectional | 5 | Polysomnography | Blood | 1 menstrual cycle | Yes | Fair |
| Armitage and Yonkers [59] | 1994 | Case study | 1 | Polysomnography, sleep diary | Blood | 1 menstrual cycle | Yes | Fair |
| Huerta et al [60] | 1995 | Cross-sectional | 151 | Questionnaires | Blood | Not stated | Yes | Fair |
| Driver et al [61] | 1996 | Cross-sectional | 9 | Polysomnography, questionnaires | Blood, urine | 32-36 days | Yes | Good |
| Lee et al [62] | 2000 | Cohort | 8 | Polysomnography | Blood | 2 consecutive nights | Yes | Good |
| Baker et al [63] | 2001 | Cross-sectional | 19 | Polysomnography, questionnaires | Blood | 6-month period | Yes | Fair |
| Baker et al [64] | 2001 | Cross-sectional | 16 | Polysomnography, questionnaires | Blood | 3-month period | Yes | Fair |
| Touzet et al [65] | 2002 | Cross-sectional | 106 | Questionnaires | Urine | 1-4 consecutive menstrual cycles | Yes | Fair |
| Baker and Driver [66] | 2004 | Cross-sectional | 26 | Questionnaires, sleep-diaries | Urine | 1 month | No | Fair |
| Kravitz et al [67] | 2005 | Cross-sectional | 630 | Questionnaire | Urine | 1 menstrual cycle | Yes | Fair |
| Hall et al [68] | 2005 | Cross-sectional | 11 | Polysomnography | Blood | 40 hours protocol | Yes | Fair |
| D'Ambrosio et al [69] | 2005 | Pre-Post | 12 | Polysomnography | Blood | 5 weeks | No | Fair |
| Hachul et al [70] | 2010 | Cross-sectional | 931 | Polysomnography, questionnaire | — | Not stated | No | Fair |
| Baker et al [71] | 2012 | Cross-sectional | 36 | Polysomnography, questionnaires | Blood, urine | 1 menstrual cycle | Yes | Fair |
| Hachul et al [72] | 2013 | Cross-sectional | 297 | Polysomnography, questionnaires | Blood | 6-month period | No | Fair |
| Sharkey et al [73] | 2014 | Cross-sectional | 27 | Polysomnography | Blood, urine | 1 menstrual cycle | Yes | Fair |
| Li et al [74] | 2015 | Cross-sectional | 19 | Actigraphy, self-report | Urine | 42 days | Yes | Fair |
| De Zambotti et al [75] | 2015 | Cross-sectional | 44 | Polysomnography, questionnaire | Blood | 1 month | No | Fair |
| Bezerra et al [76] | 2020 | Cross-sectional | 1286 | Questionnaires | — | 6-month period | No | Fair |
as rated by the NIH Quality Assessment Tools.
Estrogen
No clear association could be demonstrated between estrogen and sleep in cycling women. While studies described a positive association between estrogen and sleep efficiency in premenopausal women [74] and an inverse relationship between estradiol and number of arousals during sleep [75], other studies found higher levels of estrogen to be associated with decreased REM sleep and increased wakefulness after sleep onset [56, 71]. Further, retrieved studies were unable to detect any relation between estrogen or estrone and general sleep, wake after sleep onset and wake-index in premenopausal women [69‐73].
Progesterone
Women demonstrate more slow wave sleep during the luteal phase [63] which supports a sleep-promoting effect of progesterone. One study detected a direct correlation between progesterone and deep sleep [56] and in another study, the anovulatory group with low levels of progesterone experienced significantly more wake time during the night compared to an ovulatory group [62]. Objective measurements indicated that women experience poor sleep during the late luteal phase [44]; specifically, they reported more wakefulness after sleep onset and brief arousals in the late luteal phase [63, 71]. A sleep-promoting effect of progesterone is also supported by subjective data, as women reported decreased quality of sleep during their late luteal [44] and premenstrual phase [50, 66], when progesterone levels are declining. However, patterns of sleep disturbance may show individual differences and vary across the menstrual cycle, with some women showing no change, others showing more sleep disturbances around ovulation, and others showing sleep disturbances in the premenstrual phase [51]. REM sleep was affected by progesterone during the luteal phase. Studies discovered a shorter REM latency and an increase of the amount of REM sleep with the luteal rise of progesterone [57, 59]. Additionally, a high sleep spindle frequency during the luteal phase is described [58]. Sleep spindles are uniquely altered by menstrual phase, hypothesized to reflect the actions of progesterone metabolites on GABAA receptors in these networks [61].
Gonadotropins
Although no specific measurements related to the menstrual cycle have been published, several studies have addressed the association between gonadotropins and sleep. Polysomnographic measures indicated no association between FSH and sleep efficiency or any other sleep parameter in cycling women [56, 72]. Although FSH seemed significantly associated with early morning sleep alterations, the association no longer remained significant after statistical adjustment [60]. Few other studies discovered that objective wakefulness measured by polysomnography was associated with high FSH levels [75] and FSH levels were positively related to trouble sleeping in premenopausal women [67]. Further, FSH levels are positively correlated with partial upper airway obstruction [55]. Another study reported a significant association between FSH levels and sleep duration [65]. The authors discovered that FSH levels were 20% higher in women sleeping more than 8 hours compared with women sleeping less than 8 hours. LH pulses occurred in association with awakenings [68]. Additionally, LH levels correlated with light sleep [56]. One study found no association between LH and sleep [65] while another study found a significant correlation between sleep efficiency and LH [72].
Hormonal contraception
Objective measurements revealed that women taking hormonal contraceptives had fewer arousals [70]. Furthermore, they had a higher sleep efficiency and a lower apnea-hypopnea index [72]. In subjective reports, women taking oral contraceptives had a longer sleep duration [54]. One study discovered that women taking monophasic combined oral contraceptives had less deep sleep, but subjective sleep quality did not differ from the ratings of naturally cycling women [64]. The effect on REM latency is inconclusive, as one study found a longer REM latency in women taking hormonal contraceptives [70] and another study reported a short REM latency in these women [57]. Other studies reported more sleep disturbances or fatigue [63] and increased excessive daytime sleepiness [76] by women using hormonal contraceptives. Women with progestin-only therapies presented shorter sleep duration compared to combined therapy [76]. Another study found that women taking monophasic combined oral contraceptives had more stage 2 sleep and less deep sleep [63].
Pregnancy
Pregnancy and postpartum are both associated with more frequent sleep disruption [77]. Physiological adaptations to pregnancy cause nocturnal micturition, as early as the first trimester, and gastroesophageal reflux which may disrupt sleep [78]. Sleep quality worsens and sleep duration is shortened as pregnancy progresses [78]. Sleep disruptions during pregnancy mainly include awakenings and wakefulness during the night [36]. Musculoskeletal discomfort due to the growing uterus increases in the second and third trimester causing sleep problems [78]. Further, pregnancy is a risk factor for sleep-disordered breathing and restless legs syndrome [33]. Postpartum is a time when women experience increased difficulties with sleeping, which may on the one hand be related to the important hormonal changes following delivery and on the other hand to the specific characteristics of the neonatal period, like sleeping rhythms of newborns and the necessity to feed the child at night [36]. An overview of studies examining the relationship between reproductive hormones and sleep in pregnant and postpartum women is presented in Table 3.
Table 3.
Overview of included studies examining the relationship between sleep and reproductive hormones in pregnant and postpartum women
| Author | Year | Type of study | Sample size (n) | Method of sleep disorder assessment | Method of hormone measurement | Duration of data acquisition | Multiple measurements | Overall ratinga |
|---|---|---|---|---|---|---|---|---|
| Blyton et al [79] | 2002 | Case-control | 31 | Polysomnography, questionnaires | — | Not stated | No | Good |
| Dzaja et al [80] | 2009 | Case-control | 19 | Polysomnography, questionnaires | Blood | 36 weeks of gestation until 12 weeks postpartum | Yes | Fair |
| Wada et al [81] | 2012 | Cohort | 236 | Questionnaires | Blood | 18 months | Yes | Fair |
| Hübner et al [82] | 2013 | Case-control | 109 | Wrist actigraphy, questionnaires | Blood | Course of pregnancy and 8 weeks postpartum | Yes | Fair |
| Crowley et al [83] | 2016 | Cross-sectional | 14 | Actigraphy, questionnaire | Blood | 2 weeks | No | Fair |
| Drozdowicz-Jastrzębska et al [84] | 2017 | Cross-sectional | 84 | Questionnaire | Blood | 24-48 hours after labor | No | Fair |
| Lee et al [85] | 2017 | Case-control | 91 | Retrospective review of polysomnography for cases, questionnaires for controls | Blood | 2 years | Yes | Fair |
| Hantoushzadeh et al [86] | 2019 | Randomized parallel group | 100 | Questionnaire | — | 14 months | Yes | Good |
as rated by the NIH Quality Assessment Tools.
Estrogen
Altogether, only few studies investigated the role of estrogen in sleep disturbances during pregnancy. One cohort study discovered that longer sleep duration was inversely correlated with estradiol [81], implying a negative effect of estrogen on sleep in pregnant women. Another study demonstrated that pregnant women with restless legs syndrome (RLS) had higher levels of estrogen than pregnant women without RLS, suggesting that estrogens play a pathophysiological role in triggering RLS symptoms during pregnancy [80]. However, there are opposite results [82]. None of these studies has included physical changes related to pregnancy in their analysis.
The only available study investigating postpartum women revealed no association between estradiol levels and postpartum insomnia [84]. Unfortunately, there are no data controlling for the effect of the babies sleeping rhythm postpartum, especially when breastfeeding.
Progesterone
Pregnant women with OSA had lower levels of progesterone compared to pregnant women without OSA, suggesting that progesterone may play a protective role against OSA [85]. While progesterone itself was not associated with worse sleep quality, low serum concentrations of its metabolite allopregnanolone showed a trend to predict worse subjective sleep quality in pregnant women [83]. No association between progesterone and RLS was found during pregnancy [80]. The dramatic decrease in progesterone from the third trimester to postpartum was unrelated to changes in REM sleep parameters [62]. In a randomized clinical trial, pregnant women with previous preterm birth received either vaginal or intramuscular progesterone, but neither vaginal nor intramuscular progesterone improved sleep disturbances [86].
Gonadotropins
Data regarding FSH levels and sleep during pregnancy is limited, as studies in pregnant women focus on estrogen and progesterone. The only available study pertaining to FSH as well as LH and sleep in a group of pregnant women found that there was not an association between periodic limb movements during sleep with either FSH levels or with LH levels [80].
Prolactin
Prolactin has a positive effect on sleep, as lactation was associated with increased deep sleep [79]. Further, prolactin was positively correlated with high sleep efficiency [44]. In a group of postmenopausal women, prolactin levels were positively associated with increased sleep duration [87].
Endocrine Pathologies
Reproductive endocrine pathologies such as PCOS or POI are also associated with sleep disturbances [88, 89]. PCOS is a reproductive endocrine pathology often accompanied by excess production of androgens. These androgens can also be converted into additional estrogen via the aromatase enzyme. An excessive as well as an insufficient amount of testosterone affects sleep quality [90]. In a group of premenopausal women, a shorter sleep duration was associated with higher testosterone levels [91]. PCOS is a known risk factor for sleep apnea [92]. Even though PCOS is also associated with obesity, overweight women with PCOS have more sleep-disordered breathing than weight-matched women without PCOS [93]. As testosterone also contributes to sleep-disordered breathing [94], the excess in testosterone is one explanation for increased sleep-disordered breathing in women with PCOS. POI is the premature cessation of ovarian function in women younger than 40 years [95]. In these women, the hormone profile is analogous to postmenopausal women. Women with POI have poor sleep quality and more insomnia despite the use of hormone therapy [89].
Menopausal Transition
The prevalence of sleeping difficulties during the menopausal transition is higher compared with premenopausal women [96‐100]. Known causes are the occurrence of vasomotor symptoms and an increase in mood disorders [36]. Aging, which disrupts the normal circadian rhythm, is another cause for sleep fragmentation [78]. Additionally, the risk of sleep-disordered breathing is increased after menopause [96]. Table 4 provides an overview of studies examining the relationship between reproductive hormones and sleep in peri- and postmenopausal women.
Table 4.
Overview of included studies examining the relationship between sleep and reproductive hormones in peri- and postmenopausal women
| Author | Year | Type of study | Sample size (n) | Method of sleep disorder assessment | Method of hormone measurement | Duration of data acquisition | Multiple measurements | Overall ratinga |
|---|---|---|---|---|---|---|---|---|
| Thomson and Oswald [101] | 1977 | Controlled trial | 34 | Electrophysiological recording | — | 14 weeks | Yes | Fair |
| Furuhjelm and Carlström [102] | 1977 | Pre-Post | 209 | Self-report | Blood | 3.5 years | Yes | Poor |
| Hagen et al [103] | 1982 | Cross-sectional | 313 | Questionnaires | Blood | Not stated | No | Fair |
| Shargil [104] | 1985 | Controlled trial | 100 | Questionnaires | — | 3 years | Yes | Fair |
| Erkkola et al [105] | 1991 | Pre-Post | 249 | Interviews | — | 6 months | Yes | Fair |
| Yang et al [106] | 1995 | Pre-Post | 20 | Questionnaires | — | 3 months | No | Fair |
| Polo-Kantola et al [107] | 1999 | Cross-sectional | 63 | Polysomnography, self-report | Blood | 4 months | Yes | Good |
| Polo-Kantola et al [108] | 1999 | Randomized controlled | 66 | Polysomnography, self-report | Blood | 7 months | Yes | Fair |
| Strickler et al [109] | 2000 | Randomized controlled | 398 | Questionnaires | Blood | 12 months | Yes | Good |
| Antonijevic et al [110] | 2000 | Pre-Post | 11 | Polysomnography, questionnaire | — | Not stated | No | Fair |
| Li et al [111] | 2000 | Cross-sectional | 693 | Questionnaires, interviews | — | 3 years | No | Good |
| Hollander et al [112] | 2001 | Cohort | 436 | Questionnaire | Blood | 2 years | Yes | Good |
| Montplaisir et al [113] | 2001 | Randomized parallel group | 21 | Polysomnography, questionnaires | — | 6 months | Yes | Good |
| Barnabei et al [114] | 2002 | Randomized controlled | 2763 | Questionnaires | — | 4 years | Yes | Good |
| Netzer et al [115] | 2003 | Cross-sectional | 53 | Polysomnography | Blood | Not stated | No | Fair |
| Shahar et al [116] | 2003 | Cross-sectional | 2852 | Polysomnography, questionnaires | — | 51 months | No | Fair |
| Young et al [117] | 2003 | Cohort | 589 | Polysomnography, questionnaire | — | 8 years | Yes | Good |
| Polo-Kantola et al [118] | 2003 | Randomized controlled | 62 | Polysomnography, static-charge-sensitive bed | Blood | 7 months | Yes | Fair |
| Manber et al [119] | 2003 | Within subject | 6 | Polysomnography, sleep diary | Blood | 40 days | No | Good |
| Freeman et al [120] | 2004 | Cohort | 436 | Interviews, questionnaires | Blood | 5 years | Yes | Good |
| Ford et al [121] | 2005 | Cohort | 660 | Interviews, questionnaires | Blood | 10 years | Yes | Good |
| Gambacciani et al [122] | 2005 | Randomized controlled | 60 | Questionnaires | — | 12 weeks | Yes | Fair |
| Nielsen et al [123] | 2006 | Randomized controlled | 335 | Questionnaires | Blood | 2 years | Yes | Fair |
| Rowley et al [124] | 2006 | Pre-Post | 35 | Polysomnography | Blood | 30 days | No | Fair |
| Murphy and Campell [125] | 2007 | Cross-sectional | 10 | Polysomnography, self-report | Blood | 2 weeks and 3 nights | Yes | Fair |
| Dennerstein et al [126] | 2007 | Cohort | 204 | Interviews, questionnaires | Blood | 13 years | Yes | Good |
| Carranza-Lira et al [127] | 2007 | Pre-Post | 14 | Questionnaires | — | 3 months | No | Fair |
| Kravitz et al [128] | 2008 | Cohort | 3045 | Self-report | Blood | 7 years | Yes | Good |
| Sowers et al [129] | 2008 | Cross-sectional | 365 | Polysomnography, questionnaire | Blood | 35 days | Yes | Good |
| Pien et al [130] | 2008 | Cohort | 436 | Questionnaires | Blood | 8 years | Yes | Good |
| Schüssler et al [131] | 2008 | Randomized cross-over | 10 | Polysomnography, questionnaire | — | 2x 21 days | No | Good |
| Woods and Mitchell [132] | 2010 | Cohort | 286 | Self-report diary | Urine | 17 years | Yes | Good |
| Zheng et al [133] | 2015 | Cross-sectional | 163 | Wrist actigraphy, diaries | — | 35 days | No | Fair |
| Baker et al [134] | 2015 | Case-control | 72 | Polysomnography, diary | Blood | 2 weeks | No | Fair |
| Santoro et al [135] | 2016 | Randomized controlled | 727 | Self-report | Blood | 2 years | Yes | Fair |
| Slopien et al [136] | 2018 | Cross-sectional | 140 | Questionnaires | Blood | Not stated | No | Fair |
| Cintron et al [137] | 2018 | Randomized controlled | 727 | Questionnaire | — | 2 years | Yes | Good |
| Geiger et al [138] | 2019 | Placebo-controlled | 172 | Questionnaires, self-report | — | 12 months | Yes | Fair |
| Hatcher et al [139] | 2020 | Cohort | 762 | Questionnaire | Blood | 1 year | Yes | Good |
| Luo et al [140] | 2020 | Cohort | 458 | Questionnaires | Blood | Recruited in 2005, follow-up ongoing in 2020 | Yes | Good |
as rated by the NIH Quality Assessment Tools.
Estrogen
Polysomnographic measurements demonstrated a positive relationship between estrogen and sleep, specifically low estradiol was associated with lower sleep efficiency [125], sleep-disordered breathing [115], and a high frequency of movement arousals [107]. Subjective sleep measures from questionnaires demonstrating a positive association between estrogen and sleep found significantly lower levels of estradiol in women with insomnia [103]. In addition, decreasing estradiol levels were associated with poor sleep and trouble falling and staying asleep [112, 128]. A higher estrogen level was significantly associated with decreased awakening during the night as well as early morning awakenings [121, 132]. Two studies discovered that rather the fluctuations and degree of change in estrogen than the absolute level was associated with sleeping problems [120, 126] but in summary, the results clearly demonstrate a positive effect of estrogen on sleep in perimenopausal women.
Progesterone
During the menopausal transition, declining or low progesterone levels, resulting from the failure of a growing follicle to reach sufficient maturation for ovulation, are associated with sleep disturbances. Cycling late-reproductive-age women (aged 48-59) had lower sleep efficiency and shorter sleep time during the final 7 days of the menstrual cycle compared with the third week of the menstrual cycle [133]. In women approaching menopause, increased arousals during the luteal phase were demonstrated by more wake after sleep onset [134]. In addition, more rapid eye movement densities were displayed during the luteal phase [44]. Furthermore, low levels of progesterone were associated with sleep-disordered breathing [115]. In subjective questionnaires, lower concentrations of progesterone were associated with increased frequency of sleep disturbances and insomnia [139]. Likewise, allopregnanolone levels were negatively correlated with shallow sleep and sleep disturbances [136].
Gonadotropins
Plasma levels of FSH were significantly higher in peri- and postmenopausal women with insomnia compared to those without insomnia [103]. FSH was related to prolonged stage 2 sleep latency and prolonged slow wave sleep latency [108]. High FSH values were negatively related to sleep efficiency and associated with greater wake after sleep onset and higher apnea-hypopnea index [129]. Further, there was a negative correlation between FSH and deep sleep after menopause [44]. Increasing FSH levels were associated with more trouble falling asleep [140] as well as staying asleep, that is, awakening [132]. Likewise, subjective sleep quality was worse with high levels of FSH [129, 130]. In perimenopausal and postmenopausal women, LH was not associated with sleep disturbances [67, 112]. There was a difference in correlation between gonadotropins and poor sleep depending on reproductive age [44]. The authors discovered that in premenopause, LH and stage 3 sleep were positively correlated and after menopause, LH and number of stage shifts, number of awakenings, percentages of stages 1 and 2 and REM latency were positively correlated. Frequent stage shifts result in complaints of nonrestorative sleep [42]. This negative effect after menopause was also observed in another cross-sectional study in which higher LH levels were associated with lower sleep efficiency and higher numbers of awakenings [125].
Hormonal treatment during the menopausal transition
A recent systematic review [141] demonstrated that estrogen-only therapy, progesterone-only therapy, combined hormone therapy, selective estrogen receptor modulators, and selective tissue estrogenic activity regulators were all effective in alleviating sleep complaints. Oral estrogen-only therapy reduced insomnia and improved subjective sleep disturbances [101‐135]. Likewise, this positive effect was observed with a transdermal application of estrogen [105, 110]. In one study, the effect of a sequential transdermal application was greater than with oral administration [111]. Intranasal estradiol was also effective in reducing sleep problems [123]. The combination of estrogens and progestins was similarly successful in improving sleep disturbances [113‐137]. While most studies reported an amelioration, few studies reported a complete disappearance of sleep disturbances [102, 104]. Oral estrogen with micronized progesterone was more successful in improving sleep disturbances than oral estrogen combined with medroxyprogesterone [113, 122]. Progesterone-only treatment likewise improved sleep; both oral application [131, 142] and intranasal application [143] were successful. Hormone therapy users had less sleep-disordered breathing [116, 117] when compared to non-users. Estrogens and progestins positively influenced the apnea threshold and controlled breathing [124]. Estrogen replacement therapy decreased the occurrence and frequency of sleep apnea [118, 119] and progesterone treatment decreased breathing irregularities and arousals from sleep [72].
Discussion
Studies describing a positive relationship between endogenous estrogen and sleep are mostly limited to perimenopausal women. Likewise, the positive effect of hormone therapy on sleep during the menopausal transition is not seen in younger women taking oral contraceptive therapy. It is possible that giving hormones, that is, oral contraceptives, to young women who do not have sleep disturbances means that there is no room for improvement from the hormones. Whereas giving hormones to perimenopausal women, where the prevalence of sleep disturbances is much higher, results in an effect as there is room for improvement in this group of women. Perimenopausal estrogen therapy alleviates symptoms of insomnia by directly targeting the estrogen deficiency associated with sleep disturbances as well as other causes of sleep disturbances, such as vasomotor symptoms [141]. In younger cycling women the data were inconclusive, and in pregnant women other mechanical and physiological factors such as nocturnal micturition or gastroesophageal reflux appear to be more responsible for sleep disruption during pregnancy [78] than the absolute change in hormone level. The discrepancy in associations between reproductive hormones and sleep depending on stage of reproductive aging will on the one hand have to be attributed to different intensities and rhythms of changes in hormone levels and on the other hand to the presence of confounders such as physical changes related to pregnancy, effects of the babies’ sleeping and eating rhythms in the neonatal period, age-related changes in stress hormones as well as symptoms of the perimenopausal transition such as vasomotor symptoms. Although an effect of reproductive hormones on sleep independent of vasomotor symptoms has been established [138], the presence of these confounders could explain why the association between reproductive hormones and sleep is stronger during later stages of life.
Results concerning the associations between endogenous progesterone and sleep demonstrated that progesterone was mostly positively associated with sleep in both younger and older women, indicating a sleep-promoting effect. This was also confirmed by the fact that women slept worse during their late luteal phases when progesterone levels were declining. One explanation might be that progesterone has hypnotic anesthetic properties and induces changes in sleep comparable to those of agonistic GABAA receptor modulators [144]. Furthermore, progesterone was protective of sleep-disordered breathing in young cycling as well as pregnant and perimenopausal women. Progesterone stimulates respiration through a steroid receptor–mediated mechanism in the central nervous system [145] and may have a direct effect on upper airway dilator muscle activity [146] which could account for its positive effect on sleep-disordered breathing.
Sleeping problems were highly associated with the rate of change in estradiol [126], indicating that the dynamics of changing hormone levels seem more important than absolute levels. This is also supported by the fact that sleep is especially impaired during the late luteal phase, a phase of rapidly declining hormone levels. A more rapid rate of FSH change was associated with poor subjective sleep quality [129], and women with bilateral oophorectomy, who experience a very sudden change in hormone level, experience more severe symptoms [147], indicating that rapidly changing hormones are responsible for the occurrence of distressing sleep symptoms.
Gonadotropins have less effect on sleep disturbances than ovarian hormones. There was no correlation between FSH levels and sleep parameters in most studies. However, it should be noted that studies with large sample sizes found an association between rising FSH levels and sleep disturbances in women around the menopausal transition [128‐130]. Like the relationship between endogenous estrogen and sleep, the association between FSH and sleep disturbances seems to predominantly play a role in mid-life women around the time of menopause and not in younger cycling women. An important consideration is whether the findings concerning FSH and sleep represent an independent effect of FSH or if high FSH levels merely represent a lack of estradiol. As FSH rises when estrogen is low, one would expect opposite associations in absence of individual relationships. However, studies examining both FSH and estradiol did not simply find opposite associations between the 2 hormones and sleep, but an individual association between FSH and sleep measures was described without a co-occurring association between estrogen and sleep measures [65, 67, 75, 129], thereby indicating that FSH findings are not merely a consequence of estradiol's effect. No available literature on possible pathophysiologic mechanisms was found, but one consideration is a reflection of an overall interaction between the reproductive system and sleep system and not necessarily that FSH is the driver of sleep disturbance. The studies pertaining to LH and sleep disturbances mostly found no association between the two, neither in young cycling women nor in women past menopause.
Limitations
Available studies are currently very inhomogeneous regarding not only the timing and technique used to measure hormone levels but also with regard to the type of sleeping disorders and the definition of parameters used to evaluate each disorder. Due to different measurements of sleep, objective (eg, polysomnography) vs subjective (eg, questionnaires), it is difficult to find consistencies since the results do not necessarily align. Studies conducted with polysomnography often have low sample sizes and a low sampling frequency across the menstrual cycle, meaning that there are quite a few spurious findings in the literature that need to be replicated. Therefore, comparison of data and the development of final conclusions are hampered, and future studies should ideally base on predefined core outcome measures as well as measurement techniques.
Conclusion
Female reproductive hormones are implicated in women's sleep disturbances across different stages of reproductive aging. Most importantly, progesterone has a sleep-promoting effect. If there are no contraindications for hormone therapy and the benefits outweigh the risks, sleep disturbances during the menopausal transition can successfully be treated with estrogen and progesterone therapy. In younger women, sleep is affected by the phase of menstrual cycle. However, the associations between reproductive hormones and sleep disturbances in cycling women are less clear than in perimenopausal women; therefore, other forms of treatment are warranted.
Abbreviations
- EEG
electroencephalography
- FSH
follicle-stimulating hormone
- LH
luteinizing hormone
- PCOS
polycystic ovary syndrome
- POI
premature ovarian insufficiency
- REM
rapid eye movement
- RLS
restless legs syndrome
- TST
total sleep time
Contributor Information
Annika Haufe, Department of Reproductive Endocrinology, University Hospital Zurich, University of Zurich, CH-8091 Zürich, Switzerland.
Brigitte Leeners, Email: Brigitte.Leeners@usz.ch, Department of Reproductive Endocrinology, University Hospital Zurich, University of Zurich, CH-8091 Zürich, Switzerland.
Disclosures
The authors declare no conflict of interest related to the current work.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Ohayon MM. Epidemiological overview of sleep disorders in the general population. Sleep Med Res. 2011;2(1):1‐9. [Google Scholar]
- 2. Reimer MA, Flemons WW. Quality of life in sleep disorders. Sleep Med Rev. 2003;7(4):335‐349. [DOI] [PubMed] [Google Scholar]
- 3. Lindberg E, Janson C, Gislason T, Björnsson E, Hetta J, Boman G. Sleep disturbances in a young adult population: can gender differences be explained by differences in psychological status? Sleep. 1997;20(6):381‐387. [DOI] [PubMed] [Google Scholar]
- 4. Harlow SD, Gass M, Hall JE, et al. Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause N Y N. 2012;19(4):387‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McNeilly A, Crawford J, Taragnat C, Nicol L, McNeilly J. The differential secretion of FSH and LH: regulation through genes, feedback and packaging. Reprod Suppl. 2003;61:463‐476. [PubMed] [Google Scholar]
- 6. Gervásio CG, Bernuci MP, Silva-de-Sá MF, Rosa-e-Silva AdS. The role of androgen hormones in early follicular development. ISRN Obstet Gynecol. 2014;2014:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reed B, Carr B. The Normal Menstrual Cycle and the Control of Ovulation. August 5, 2018. In: Feingold KR, Anawalt B, Boyce Al, et al., eds. Endotext [Internet]: MDTextcom, Inc 2000. Accessed April 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK279054/
- 8. Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol. 2009;114(6):1326‐1331. [DOI] [PubMed] [Google Scholar]
- 9. Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy: I. Normal pregnancy. Am J Obstet Gynecol. 1972;112(8):1095‐1100. [DOI] [PubMed] [Google Scholar]
- 10. Pasqualini JR, Chetrite GS. The formation and transformation of hormones in maternal, placental and fetal compartments: biological implications. Horm Mol Biol Clin Investig. 2016;27(1):11‐28. [DOI] [PubMed] [Google Scholar]
- 11. Chatuphonprasert W, Jarukamjorn K, Ellinger I. Physiology and pathophysiology of steroid biosynthesis, transport and metabolism in the human placenta. Front Pharmacol. 2018;9:1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Carlo C, Bruno P, Cirillo D, Morgera R, Pellicano M, Nappi C. Increased concentrations of renin, aldosterone and Ca125 in a case of spontaneous, recurrent, familial, severe ovarian hyperstimulation syndrome. Hum Reprod Oxf Engl. 1997;12(10):2115‐2117. [DOI] [PubMed] [Google Scholar]
- 13. McNeilly AS. Lactation and the physiology of prolactin secretion. Postgrad Med J. 1975;51(594):231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hall JE. Endocrinology of the menopause. Endocrinol Metab Clin North Am. 2015;44(3):485‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Santoro N, Epperson CN, Mathews SB. Menopausal symptoms and their management. Endocrinol Metab Clin North Am. 2015;44(3):497‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brzozowska M, Lewiński A. Changes of androgens levels in menopausal women. Menopausal Rev. 2020;19(4):151‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oster H, Challet E, Ott V, et al. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr Rev. 2017;38(1):3‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumari M, Badrick E, Ferrie J, Perski A, Marmot M, Chandola T. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2009;94(12):4801‐4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specification. 1st ed.American Academy of Sleep Medicine, 2007. [Google Scholar]
- 20. Berry RB, Brooks R, Gamaldo C, et al. AASM Scoring manual updates for 2017 (version 2.4). J Clin Sleep Med. 2017;13(5):665‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirshkowitz M. Normal human sleep: an overview. Med Clin N Am. 2004;88(3):551‐565. [DOI] [PubMed] [Google Scholar]
- 22. Peever J, Fuller PM. The biology of REM sleep. Curr Biol. 2017;27(22):R1237‐R1248. [DOI] [PubMed] [Google Scholar]
- 23. Jiang F. Sleep and early brain development. Ann Nutr Metab. 2019;75(Suppl. 1):44‐54. [DOI] [PubMed] [Google Scholar]
- 24. Rosales-Lagarde A, Armony J, del Río-Portilla Y, Trejo-Martínez D, Conde R, Corsi-Cabrera M. Enhanced emotional reactivity after selective REM sleep deprivation in humans: an fMRI study. Front Behav Neurosci. 2012;6(25):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leary EB, Watson KT, Ancoli-Israel S, et al. Association of rapid eye movement sleep with mortality in middle-aged and older adults. JAMA Neurol. 2020;77(10):1241‐1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387‐1394. [DOI] [PubMed] [Google Scholar]
- 27. Mai E, Buysse DJ. Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Sleep Med Clin. 2008;3(2):167‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zeng LN, Zong QQ, Yang Y, et al. Gender difference in the prevalence of insomnia: a meta-analysis of observational studies. Front Psychiatry. 2020;11:577429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Pub; 2013. [Google Scholar]
- 30. Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12(6):481‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garvey JF, Pengo MF, Drakatos P, Kent BD. Epidemiological aspects of obstructive sleep apnea. J Thorac Dis. 2015;7(5):920‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3):608‐613. [DOI] [PubMed] [Google Scholar]
- 33. Silvestri R, Aricò I. Sleep disorders in pregnancy. Sleep Sci. 2019;12(3):232‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101‐119. [DOI] [PubMed] [Google Scholar]
- 35. Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165(11):1286‐1292. [DOI] [PubMed] [Google Scholar]
- 36. Moline ML, Broch L, Zak R, Gross V. Sleep in women across the life cycle from adulthood through menopause. Sleep Med Rev. 2003;7(2):155‐177. [DOI] [PubMed] [Google Scholar]
- 37. Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lespérance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12(1):61‐65. [DOI] [PubMed] [Google Scholar]
- 38. Hornyak M, Trenkwalder C. Restless legs syndrome and periodic limb movement disorder in the elderly. J Psychosom Res. 2004;56(5):543‐548. [DOI] [PubMed] [Google Scholar]
- 39. Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2002;53(1):547‐554. [DOI] [PubMed] [Google Scholar]
- 40. Wilson DL, Walker SP, Fung AM, O’Donoghue FJ, Barnes M, Howard ME. Periodic limb movements in sleep during pregnancy: a common but benign disorder? Sleep Biol Rhythms. 2018;16(1):11‐20. [Google Scholar]
- 41. Rechtschaffen A, Kales A, eds. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. U. S. National Institute of Neurological Diseases and Blindness, Neurological Information Network; 1968. [Google Scholar]
- 42. Shrivastava D, Jung S, Saadat M, Sirohi R, Crewson K. How to interpret the results of a sleep study. J Community Hosp Intern Med Perspect. 2014;4(5):24983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lucidi F, Devoto A, Violani C, De Gennaro L, Mastracci P, Bertini M. Rapid eye movements density as a measure of sleep need: REM density decreases linearly with the reduction of prior sleep duration. Electroencephalogr Clin Neurophysiol. 1996;99(6):556‐561. [DOI] [PubMed] [Google Scholar]
- 44. Hoffmann G, Wauters H, Pauwels E, Buytaert G, Uyttenbroeck F, Petre-Quadens O. Sleep and pituitary hormones: A comparative study between premenopause and menopause. Waking Sleeping. 1978;2(3):157‐167. [Google Scholar]
- 45. EEG Arousals: scoring rules and examples. Sleep. 1992;15(2):173‐173. [PubMed] [Google Scholar]
- 46. Lafortune M, Gagnon JF, Martin N, et al. Sleep spindles and rapid eye movement sleep as predictors of next morning cognitive performance in healthy middle-aged and older participants. J Sleep Res. 2014;23(2):159‐167. [DOI] [PubMed] [Google Scholar]
- 47. National Institutes of Health . Study Quality Assessment Tools. Published online July 2021. Accessed February 25, 2023. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 48. Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8(6):613‐622. [DOI] [PubMed] [Google Scholar]
- 49. Shilaih M, Falco L, Kuebler F, Hamvas G, Cronin M, Leeners B. Monitoring sleep patterns change across the menstrual cycle using wearable sensors. Intern J Obstet Gynecol. 2018;143(S3):348. [Google Scholar]
- 50. Brown SG, Morrison LA, Calibuso MJ, Christiansen TM. The menstrual cycle and sexual behavior: relationship to eating, exercise, sleep, and health patterns. Women Health. 2008;48(4):429‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van Reen E, Kiesner J. Individual differences in self-reported difficulty sleeping across the menstrual cycle. Arch Womens Ment Health. 2016;19(4):599‐608. [DOI] [PubMed] [Google Scholar]
- 52. Boivin DB, Shechter A. Sleep, hormones, and circadian rhythms throughout the menstrual cycle in healthy women and women with premenstrual dysphoric disorder. Int J Endocrinol. 2010;2010:259345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Baker FC, Lee KA. Menstrual cycle effects on sleep. Sleep Med Clin. 2018;13(3):283‐294. [DOI] [PubMed] [Google Scholar]
- 54. Hicks RA, Cavanaugh AM. Oral contraceptive use, the menstrual cycle, and the need for sleep. Bull Psychon Soc. 1982;19(4):215‐216. [Google Scholar]
- 55. Stahl ML, Orr WC, Males JL. Progesterone levels and sleep-related breathing during menstrual cycles of normal women. Sleep. 1985;8(3):227‐230. [DOI] [PubMed] [Google Scholar]
- 56. Parry BL, Mendelson WB, Duncan WC, Sack DA, Wehr TA. Longitudinal sleep EEG, temperature, and activity measurements across the menstrual cycle in patients with premenstrual depression and in age-matched controls. Psychiatry Res. 1989;30(3):285‐303. [DOI] [PubMed] [Google Scholar]
- 57. Lee KA, Shaver JF, Giblin EC, Woods NF. Sleep patterns related to menstrual cycle phase and premenstrual affective symptoms. Sleep. 1990;13(5):403‐409. [PubMed] [Google Scholar]
- 58. Ishizuka Y, Pollak CP, Shirakawa S, et al. Sleep spindle frequency changes during the menstrual cycle. J Sleep Res. 1994;3(1):26‐29. [DOI] [PubMed] [Google Scholar]
- 59. Armitage R, Yonkers KA. Case report: Menstrual-related very short REM latency in a healthy normal control. Sleep. 1994;17(4):345‐347. [DOI] [PubMed] [Google Scholar]
- 60. Huerta R. Symptoms at the menopausal and premenopausal years: their relationship with insulin, glucose, cortisol, FSH, prolactin, obesity and attitudes towards sexuality. Psychoneuroendocrinology. 1995;20(8):851‐864. [DOI] [PubMed] [Google Scholar]
- 61. Driver HS, Dijk DJDJ, Werth E, Biedermann K, Borbély AA. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81(2):728‐735. [DOI] [PubMed] [Google Scholar]
- 62. Lee KA, McEnany G, Zaffke ME. REM Sleep and mood state in childbearing women: sleepy or weepy? Sleep. 2000;23(7):877‐885. [PubMed] [Google Scholar]
- 63. Baker FC, Mitchell D, Driver HS. Oral contraceptives alter sleep and raise body temperature in young women. Pflugers Arch. 2001;442(5):729‐737. [DOI] [PubMed] [Google Scholar]
- 64. Baker FC, Waner JI, Vieira EF, Taylor SR, Driver HS, Mitchell D. Sleep and 24 hour body temperatures: a comparison in young men, naturally cycling women and women taking hormonal contraceptives. J Physiol. 2001;530(3):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Touzet S, Rabilloud M, Boehringer H, Barranco E, Ecochard R. Relationship between sleep and secretion of gonadotropin and ovarian hormones in women with normal cycles. Fertil Steril. 2002;77(4):738‐744. [DOI] [PubMed] [Google Scholar]
- 66. Baker FC, Driver HS. Self-reported sleep across the menstrual cycle in young, healthy women. J Psychosom Res. 2004;56(2):239‐243. [DOI] [PubMed] [Google Scholar]
- 67. Kravitz HM, Janssen I, Santoro N, et al. Relationship of day-to-day reproductive hormone levels to sleep in midlife women. Arch Intern Med. 2005;165(20):2370‐2376. [DOI] [PubMed] [Google Scholar]
- 68. Hall JE, Sullivan JP, Richardson GS. Brief wake episodes modulate sleep-inhibited luteinizing hormone secretion in the early follicular phase. J Clin Endocrinol Metab. 2005;90(4):2050‐2055. [DOI] [PubMed] [Google Scholar]
- 69. D’Ambrosio C, Stachenfeld NS, Pisani M, Mohsenin V. Sleep, breathing, and menopause: the effect of fluctuating estrogen and progesterone on sleep and breathing in women. Gend Med. 2005;2(4):238‐245. [DOI] [PubMed] [Google Scholar]
- 70. Hachul H, Andersen ML, Bittencourt LRAA, Santos-Silva R, Conway SG, Tufik S. Does the reproductive cycle influence sleep patterns in women with sleep complaints? Climacteric. 2010;13(6):594‐603. [DOI] [PubMed] [Google Scholar]
- 71. Baker FC, Sassoon SA, Kahan T, et al. Perceived poor sleep quality in the absence of polysomnographic sleep disturbance in women with severe premenstrual syndrome. J Sleep Res. 2012;21(5):535‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hachul H, Andersen ML, Bittencourt L, Santos-Silva R, Tufik S. A population-based survey on the influence of the menstrual cycle and the use of hormonal contraceptives on sleep patterns in São Paulo, Brazil. Int J Gynecol Obstet. 2013;120(2):137‐140. [DOI] [PubMed] [Google Scholar]
- 73. Sharkey KM, Crawford SL, Kim S, Joffe H. Objective sleep interruption and reproductive hormone dynamics in the menstrual cycle. Sleep Med. 2014;15(6):688‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li DX, Romans S, De Souza MJ, Murray B, Einstein G. Actigraphic and self-reported sleep quality in women: associations with ovarian hormones and mood. Sleep Med. 2015;16(10):1217‐1224. [DOI] [PubMed] [Google Scholar]
- 75. De Zambotti M, Colrain IM, Baker FC. Interaction between reproductive hormones and physiological sleep in women. J Clin Endocrinol Metab. 2015;100(4):1426‐1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bezerra AG, Andersen ML, Pires GN, et al. Hormonal contraceptive use and subjective sleep reports in women: an online survey. J Sleep Res 2020;29(6):e12983. [DOI] [PubMed] [Google Scholar]
- 77. Lord C, Sekerovic Z, Carrier J. Sleep regulation and sex hormones exposure in men and women across adulthood. Pathol Biol. 2014;62(5):302‐310. [DOI] [PubMed] [Google Scholar]
- 78. Pengo MF, Won CH, Bourjeily G, et al. Sleep in women across the life span. Chest. 2018;154(1):196‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Blyton DM, Sullivan CE, Edwards N. Lactation is associated with an increase in slow-wave sleep in women. J Sleep Res. 2002;11(4):297‐303. [DOI] [PubMed] [Google Scholar]
- 80. Dzaja A, Wehrle R, Lancel M, Pollmächer T. Elevated estradiol plasma levels in women with restless legs during pregnancy. Sleep. 2009;32(2):169‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wada K, Nagata C, Nakamura K, Iwasa S, Shiraki M, Shimizu H. Light exposure at night, sleep duration and sex hormone levels in pregnant Japanese women. Endocr J. 2012;59(5):393‐398. [DOI] [PubMed] [Google Scholar]
- 82. Hübner A, Krafft A, Gadient S, Werth E, Zimmermann R, Bassetti CL. Characteristics and determinants of restless legs syndrome in pregnancy: a prospective study. Neurology. 2013;80(8):738‐742. [DOI] [PubMed] [Google Scholar]
- 83. Crowley SK, O’Buckley TK, Schiller CE, Stuebe A, Morrow AL, Girdler SS. Blunted neuroactive steroid and HPA axis responses to stress are associated with reduced sleep quality and negative affect in pregnancy: A pilot study. Psychopharmacology (Berl). 2016;233(7):1299‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Drozdowicz-Jastrzebska E, Skalski M, Gdanska P, et al. Insomnia, postpartum depression and estradiol in women after delivery. Metab Brain Dis. 2017;32(6):1913‐1918. [DOI] [PubMed] [Google Scholar]
- 85. Lee J, Eklund EE, Lambert-Messerlian G, et al. Serum progesterone levels in pregnant women with obstructive sleep apnea: A case control study. J Womens Health. 2017;26(3):259‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hantoushzadeh S, Sheikh M, Shariat M, Mansouri R, Ghamari A, Golshahi F. The effects of progesterone therapy in pregnancy: vaginal and intramuscular administration. J Matern Fetal Neonatal Med. 2021;34(13):2033‐2040. [DOI] [PubMed] [Google Scholar]
- 87. Katz TA, Wu AH, Stanczyk FZ, et al. Determinants of prolactin in postmenopausal Chinese women in Singapore. Cancer Causes Control CCC. 2018;29(1):51‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fernandez RC, Moore VM, Ryswyk EMV, et al. Sleep disturbances in women with polycystic ovary syndrome: prevalence, pathophysiology, impact and management strategies. Nat Sci Sleep. 2018;10:45‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Benetti-Pinto CL, Menezes C, Yela DA, Cardoso TM. Sleep quality and fatigue in women with premature ovarian insufficiency receiving hormone therapy: a comparative study. Menopause. 2019;26(10):1141‐1145. [DOI] [PubMed] [Google Scholar]
- 90. Wittert G. The relationship between sleep disorders and testosterone in men. Asian J Androl. 2014;16(2):262‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nagata C, Wada K, Yamakawa M, et al. Sleep-related factors and circulating levels of sex hormones in premenopausal Japanese women. Endocr J. 2023;70(3):267‐273. [DOI] [PubMed] [Google Scholar]
- 92. Helvaci N, Karabulut E, Demir AU, Yildiz BO. Polycystic ovary syndrome and the risk of obstructive sleep apnea: a meta-analysis and review of the literature. Endocr Connect. 2017;6(7):437‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Fogel RB, Malhotra A, Pillar G, Pittman SD, Dunaif A, White DP. Increased prevalence of obstructive sleep apnea syndrome in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86(3):1175‐1180. [DOI] [PubMed] [Google Scholar]
- 94. Schneider BK, Pickett CK, Zwillich CW, et al. Influence of testosterone on breathing during sleep. J Appl Physiol. 1986;61(2):618‐623. [DOI] [PubMed] [Google Scholar]
- 95. Beck-Peccoz P, Persani L. Premature ovarian failure. Orphanet J Rare Dis. 2006;1(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Baker FC, De Zambotti M, Colrain IM, Bei B. Sleep problems during the menopausal transition: prevalence, impact, and management challenges. Nat Sci Sleep. 2018;10:73‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Takahashi K, Manabe A, Okada M, Kurioka H, Kanasaki H, Miyazaki K. Efficacy and safety of oral estriol for managing postmenopausal symptoms. Maturitas. 2000;34(2):169‐177. [DOI] [PubMed] [Google Scholar]
- 98. Terauchi M, Obayashi S, Akiyoshi M, Kato K, Matsushima E, Kubota T. Effects of oral estrogen and hypnotics on Japanese peri- and postmenopausal women with sleep disturbance. J Obstet Gynaecol Res. 2011;37(7):741‐749. [DOI] [PubMed] [Google Scholar]
- 99. Polo-Kantola P, Erkkola R, Helenius H, Irjala K, Polo O. When does estrogen replacement therapy improve sleep quality? Am J Obstet Gynecol. 1998;178(5):1002‐1009. [DOI] [PubMed] [Google Scholar]
- 100. Saletu B. Sleep, vigilance and cognition in postmenopausal women: placebo-controlled studies with 2 mg estradiol valerate, with and without 3 mg dienogest. Climacteric. 2003;6(SUPPL. 2):37‐45. [PubMed] [Google Scholar]
- 101. Thomson J, Oswald I. Effect of oestrogen on the sleep, mood, and anxiety of menopausal women. Br Med J. 1977;2(6098):1317‐1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Furuhjelm M, Carlstrom K. Treatment of climacteric and postmenopausal women with 17-β-oestradiol and norethisterone acetate. Acta Obstet Gynecol Scand. 1977;56(4):351‐361. [DOI] [PubMed] [Google Scholar]
- 103. Hagen C, Christiansen C, Christensen MS, Transbol I. Climacteric symptoms, fat mass, and plasma concentrations of LH, FSH, prl, oestradiol-17β and androstenedione in the early post-menopausal period. Acta Endocrinol (Copenh). 1982;101(1):87‐92. [DOI] [PubMed] [Google Scholar]
- 104. Shargil AA. Hormone replacement therapy in perimenopausal women with a triphasic contraceptive compound: A three-year prospective study. Int J Fertil. 1985;30(1):15‐28. [PubMed] [Google Scholar]
- 105. Erkkola R, Holma P, Jarvi T, et al. Transdermal oestrogen replacement therapy in a Finnish population. Maturitas. 1991;13(4):275‐281. [DOI] [PubMed] [Google Scholar]
- 106. Yang TS, Tsan SH, Chang SP, Ng HT. Efficacy and safety of estriol replacement therapy for climacteric women. Chin Med J Taipei. 1995;55(5):386‐391. [PubMed] [Google Scholar]
- 107. Polo-Kantola P, Erkkola R, Irjala K, Helenius H, Pullinen S, Polo O. Climacteric symptoms and sleep quality. Obstet Gynecol. 1999;94(2):219‐224. [DOI] [PubMed] [Google Scholar]
- 108. Polo-Kantola P, Erkkola R, Irjala K, Pullinen S, Virtanen I, Polo O. Effect of short-term transdermal estrogen replacement therapy on sleep: a randomized, double-blind crossover trial in postmenopausal women. Fertil Steril. 1999;71(5):873‐880. [DOI] [PubMed] [Google Scholar]
- 109. Strickler R, Stovall DW, Merritt D, Shen W, Wong M, Silfen SL. Raloxifene and estrogen effects on quality of life in healthy postmenopausal women: a placebo-controlled randomized trial. Obstet Gynecol. 2000;96(3):359‐365. [DOI] [PubMed] [Google Scholar]
- 110. Antonijevic IA, Stalla GK, Steiger A. Modulation of the sleep electroencephalogram by estrogen replacement in postmenopausal women. Am J Obstet Gynecol. 2000;182(2):277‐282. [DOI] [PubMed] [Google Scholar]
- 111. Li C, Samsioe G, Wilaman K, et al. Effects of norethisterone acetate addition to estradiol in long term HRT. Maturitas. 2000;36(2):139‐152. [DOI] [PubMed] [Google Scholar]
- 112. Hollander LE, Freeman EW, Sammela MD, Berlina JA, Grisso JA, Battistini M. Sleep quality, estradiol levels, and behavioral factors in late reproductive age women. Obstet Gynecol. 2001;98(3):391‐397. [DOI] [PubMed] [Google Scholar]
- 113. Montplaisir J, Lorrain J, Denesle R, Petit D. Sleep in menopause: differential effects of two forms of hormone replacement therapy. Menopause N Y N. 2001;8(1):10‐16. [DOI] [PubMed] [Google Scholar]
- 114. Barnabei VM, Grady D, Stovall DW, et al. Menopausal symptoms in older women and the effects of treatment with hormone therapy. Obstet Gynecol. 2002;100(6):1209‐1218. [DOI] [PubMed] [Google Scholar]
- 115. Netzer NC, Eliasson AH, Strohl KP. Women with sleep apnea have lower levels of sex hormones. Sleep Breath. 2003;7(1):25‐29. [DOI] [PubMed] [Google Scholar]
- 116. Shahar E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med. 2003;167(9):1186‐1192. [DOI] [PubMed] [Google Scholar]
- 117. Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin sleep cohort study. Am J Respir Crit Care Med. 2003;167(9):1181‐1185. [DOI] [PubMed] [Google Scholar]
- 118. Polo-Kantola P, Rauhala E, Helenius H, Erkkola R, Irjala K, Polo O. Breathing during sleep in menopause: a randomized, controlled, crossover trial with estrogen therapy. Obstet Gynecol. 2003;102(1):68‐75. [DOI] [PubMed] [Google Scholar]
- 119. Manber R, Kuo TF, Cataldo N, Colrain IM. The effects of hormone replacement therapy on sleep-disordered breathing in postmenopausal women: a pilot study. Sleep. 2003;26(2):163‐168. [PubMed] [Google Scholar]
- 120. Freeman EW, Sammel MD, Rinaudo PJ, Sheng L. Premenstrual syndrome as a predictor of menopausal symptoms. Obstet Gynecol. 2004;103(5, Part 1):960‐966. [DOI] [PubMed] [Google Scholar]
- 121. Ford K, Sowers M, Crutchfield M, Wilson A, Jannausch M. A longitudinal study of the predictors of prevalence and severity of symptoms commonly associated with menopause. Menopause N Y N. 2005;12(3):308‐317. [DOI] [PubMed] [Google Scholar]
- 122. Gambacciani M, Ciaponi M, Cappagli B, et al. Effects of low-dose, continuous combined hormone replacement therapy on sleep in symptomatic postmenopausal women. Maturitas. 2005;50(2):91‐97. [DOI] [PubMed] [Google Scholar]
- 123. Nielsen TF, Ravn P, Pitkin J, Christiansen C. Pulsed estrogen therapy improves postmenopausal quality of life: a 2-year placebo-controlled study. Maturitas. 2006;53(2):184‐190. [DOI] [PubMed] [Google Scholar]
- 124. Rowley JA, Zhou XS, Diamond MP, Badr MS. The determinants of the apnea threshold during NREM sleep in normal subjects. Sleep. 2006;29(1):95‐103. [DOI] [PubMed] [Google Scholar]
- 125. Murphy PJ, Campbell SS. Sex hormones, sleep, and core body temperature in older postmenopausal women. Sleep. 2007;30(12):1788‐1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Dennerstein L, Lehert P, Burger HG, Guthrie JR. New findings from non-linear longitudinal modelling of menopausal hormone changes. Hum Reprod Update. 2007;13(6):551‐557. [DOI] [PubMed] [Google Scholar]
- 127. Carranza-Lira S, Gooch ALMG, Saldivar N, Osterwalder MS. Climacteric symptom control after the addition of low-dose esterified conjugated estrogens to raloxifene standard doses. Int J Fertil Womens Med. 2007;52(2-3):93‐96. [PubMed] [Google Scholar]
- 128. Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31(7):979‐990. [PMC free article] [PubMed] [Google Scholar]
- 129. Sowers MF, Zheng H, Kravitz HM, et al. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep. 2008;31(10):1339‐1349. [PMC free article] [PubMed] [Google Scholar]
- 130. Pien GW, Sammel MD, Freeman EW, Lin H, DeBlasis TL. Predictors of sleep quality in women in the menopausal transition. Sleep. 2008;31(7):991‐999. [PMC free article] [PubMed] [Google Scholar]
- 131. Schüssler P, Kluge M, Yassouridis A, et al. Progesterone reduces wakefulness in sleep EEG and has no effect on cognition in healthy postmenopausal women. Psychoneuroendocrinology. 2008;33(8):1124‐1131. [DOI] [PubMed] [Google Scholar]
- 132. Woods NF, Mitchell ES. Sleep symptoms during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women's Health Study. Sleep. 2010;33(4):539‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zheng H, Harlow SD, Kravitz HM, et al. Actigraphy-defined measures of sleep and movement across the menstrual cycle in midlife menstruating women: study of Women's Health Across the Nation sleep study. Menopause. 2015;22(1):66‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Baker FC, Willoughby AR, Sassoon SA, Colrain IM, de Zambotti M. Insomnia in women approaching menopause: beyond perception. Psychoneuroendocrinology. 2015;60:96‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Santoro N, Allshouse A, Neal-Perry G, et al. Longitudinal changes in menopausal symptoms comparing women randomized to low-dose oral conjugated estrogens or transdermal estradiol plus micronized progesterone versus placebo: the Kronos Early Estrogen Prevention Study. Menopause. 2016;24(3):238‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Slopien R, Pluchino N, Warenik-Szymankiewicz A, et al. Correlation between allopregnanolone levels and depressive symptoms during late menopausal transition and early postmenopause. Gynecol Endocrinol. 2018;34(2):144‐147. [DOI] [PubMed] [Google Scholar]
- 137. Cintron D, Lahr BD, Bailey KR, et al. Effects of oral versus transdermal menopausal hormone treatments on self-reported sleep domains and their association with vasomotor symptoms in recently menopausal women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS). Menopause. 2018;25(2):145‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Geiger PJ, Eisenlohr-Moul T, Gordon JL, Rubinow DR, Girdler SS. Effects of perimenopausal transdermal estradiol on self-reported sleep, independent of its effect on vasomotor symptom bother and depressive symptoms. Menopause. 2019;26(11):1318‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Hatcher KM, Smith RL, Chiang C, Li Z, Flaws JA, Mahoney MM. Associations of phthalate exposure and endogenous hormones with self-reported sleep disruptions: results from the Midlife Women's Health Study. Menopause N Y N. 2020;27(11):1251‐1264. [DOI] [PubMed] [Google Scholar]
- 140. Luo M, Li J, Tang R, et al. Insomnia symptoms in relation to menopause among middle-aged Chinese women: findings from a longitudinal cohort study. Maturitas. 2020;141:1‐8. [DOI] [PubMed] [Google Scholar]
- 141. Haufe A, Baker FC, Leeners B. The role of ovarian hormones in the pathophysiology of perimenopausal sleep disturbances: a systematic review. Sleep Med Rev. 2022;66:101710. [DOI] [PubMed] [Google Scholar]
- 142. Caufriez A, Leproult R, L’Hermite-Baleriaux M, Kerkhofs M, Copinschi G. Progesterone prevents sleep disturbances and modulates GH, TSH, and melatonin secretion in postmenopausal women. J Clin Endocrinol Metab. 2011;96(4):E614-23. [DOI] [PubMed] [Google Scholar]
- 143. Schüssler P, Kluge M, Adamczyk M, et al. Sleep after intranasal progesterone vs. Zolpidem and placebo in postmenopausal women—a randomized, double-blind cross over study. Psychoneuroendocrinology. 2018;92:81‐86. [DOI] [PubMed] [Google Scholar]
- 144. Lancel M, Faulhaber J, Holsboer F, Rupprecht R. Progesterone induces changes in sleep comparable to those of agonistic GABA(A) receptor modulators. Am J Physiol—Endocrinol Metab. 1996;271(4):E763‐E772. [DOI] [PubMed] [Google Scholar]
- 145. Bayliss DA, Millhorn DE, Gallman EA, Cidlowski JA. Progesterone stimulates respiration through a central nervous system steroid receptor-mediated mechanism in cat. Proc Natl Acad Sci. 1987;84(21):7788‐7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Popovic RM, White DP. Upper airway muscle activity in normal women: influence of hormonal status. J Appl Physiol. 1998;84(3):1055‐1062. [DOI] [PubMed] [Google Scholar]
- 147. Hendrix SL. Bilateral oophorectomy and premature menopause. Am J Med. 2005;118(12):131‐135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.