ABSTRACT
Clostridioides difficile is the number one cause of hospital-acquired infections in the United States and one of the CDC's urgent-level pathogen threats. The inflammation caused by pathogenic C. difficile results in diarrhea and pseudomembranous colitis. Patients who undergo clinically successful treatment for this disease commonly experience recurrent infections. Current treatment options can eradicate the vegetative cell form of the bacteria but do not impact the spore form, which is impervious to antibiotics and resists conventional environmental cleaning procedures. Antibiotics used in treating C. difficile infections (CDI) often do not eradicate the pathogen and can prevent regeneration of the microbiome, leaving them vulnerable to recurrent CDI and future infections upon subsequent non-CDI-directed antibiotic therapy. Addressing the management of C. difficile spores in the gastrointestinal (GI) tract is important to make further progress in CDI treatment. Currently, no treatment options focus on reducing GI spores throughout CDI antibiotic therapy. This review focuses on colonization of the GI tract, current treatment options and potential treatment directions emphasizing germinant with antibiotic combinations to prevent recurrent disease.
Keywords: difficile, germination, recurrence, Clostridioides, microbiome, colonization resistance
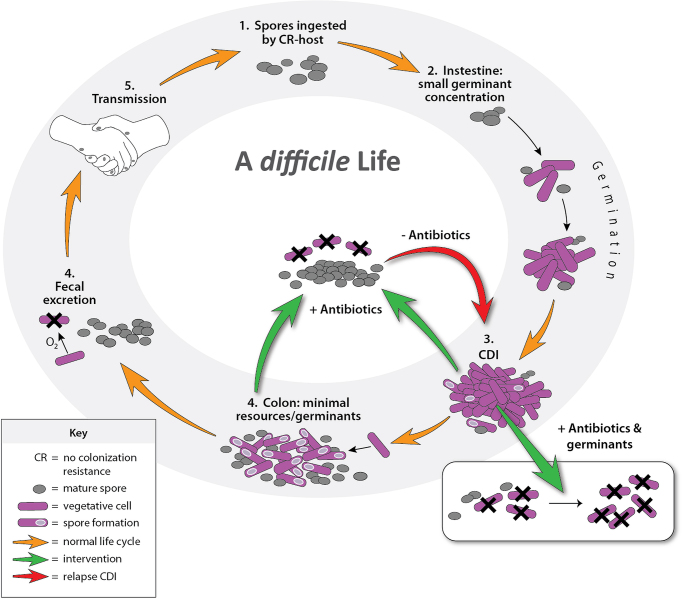
Schematic of the infectious life cycle of Clostridioidesdifficile and treatment opportunity.
INTRODUCTION
Clostridioides difficile is an anaerobic, spore and toxin forming, Gram-positive pathogen that has become the most commonly identified hospital-acquired infection (HAI) in the United States (McDonald et al. 2018). Clostridioides difficile is one of the CDC's five urgent threat level infections, requiring urgent and aggressive action. Hospital onset infections alone cost an estimated $1 billion in 2017, took 12 800 lives and caused 223 900 infections (CDC 2019). The first step in contracting a C. difficile infection (CDI) is ingesting spores through either person-to-person spread via the fecal–oral route, or direct exposure to the contaminated environment (McDonald et al. 2018). Once spores reach their ideal intestinal and colonic growth environment, they are introduced to naturally produced chemical signals, referred to as germinants, that convert them into vegetative cells. Vegetative cells then multiply and cause inflammatory disease by producing toxins. Vegetative cells revert into spores that are shed into the environment, increasing HAIs, or remain in the gastrointestinal (GI) tract to be activated later, which is a mechanism for subsequent CDI recurrence. In the treatment of CDI, antibiotics can remove vegetative cells, stopping clinical symptoms, but do not impact spores and can disrupt the normal flora that prevents C. difficile growth and CDI recurrence. The GI microbiome plays a protective role in limiting the pathogenicity of this organism, keeping clinical signs and symptoms of CDI at bay, and is often referred to as colonization resistance (CR). Disruption of CR, which results from antibiotic therapy, allows overgrowth of C. difficile leading to clinical CDI and increased spore shedding. This review will cover C. difficile acquisition and GI tract colonization, limitations and benefits of current treatment options, and potential new treatment directions emphasizing germinant with antibiotic combinations to prevent recurrent disease.
SPORE SHEDDING
The C. difficile spore is the primary vehicle used to transmit infections between patients. Mature spores are heat and ethanol resistant, not affected by antibiotics, and have an unknown lifespan in the environment, making environmental decontamination difficult. Spore shedding occurs in both clinically infected patients and asymptomatic carriers, which act as a reservoir for the pathogen. Gut carriage is estimated to be around 10% in newly admitted hospital patients (Baron et al. 2020). Asymptomatic carriage is common after completing CDI-directed antibiotic courses. Therapy results in symptom resolution and undetectable fecal spore levels, but up to 56% of patients will start asymptomatically shedding spores within 4 weeks (Sethi et al. 2010). Spores left over in the GI tract or taken up from the environment post-CDI therapy and before CR is re-established are causes of recurrence (Chilton, Pickering and Freeman 2018). High sporulation rates have been tied to recurrent CDI (rCDI), but show strain-to-strain variability and may not be related to strain type, as is typically thought for ribotype 027 epidemic strains (Burns et al. 2011; Gómez, Chaves and Orellana 2017; Tijerina et al. 2019). rCDI falls into two general categories: (i) relapse, which is a subsequent infection caused by the same strain, likely due to spores left over in the GI tract after antibiotic cessation, and (ii) re-infection, which is caused by a different strain taken up from the environment. Though relapse implies recurrence from spores left over in the GI tract, the same strain can be re-ingested from the patient's environment, making the determination between relapse and re-infection difficult. Likewise, re-infection may be caused from a colonized strain that was not originally detected but present in the patient. In rCDI patients, relapse is the most common cause and affects 52–88% (Chilton, Pickering and Freeman 2018). Differences in molecular techniques used to differentiate rCDI strains and prevalence of epidemic strains at the time of the study impact this proportion (Tang-Feldman et al. 2003; Sisto et al. 2011; Figueroa et al. 2012; Gómez, Chaves and Orellana 2017; Chilton, Pickering and Freeman 2018; Cho et al. 2020). The chance of recurrence, either relapse or re-infection, can be as high as 25% for the first, 45% for the second, and reach as high as 65% for subsequent infections (Kelly 2012). Regardless of the origin of spores in rCDI, new methods that limit spore shedding and internal spore levels need to be developed.
Spore shedding is of particular importance in the hospital environment. Hospital equipment, such as blood pressure cuffs, are not easily cleaned with strong bleach like chemicals and detergents often needed to remove spores. Contamination is ubiquitous on bed rails and floors that can be cleaned with such chemicals (Ali, Muzslay and Wilson 2015). Even residing in a room previously occupied by an individual being treated for CDI can be a risk factor for contracting the disease (hazard ratio of 2.35, P = 0.01), independent of other well-known CDI risk factors such as age, antibiotic use and proton pump inhibitors (Shaughnessy et al. 2011). New methods to decrease spore shedding and limit environmental contamination that leads to HAIs are desperately needed. One method of decontamination has been to introduce germinants into cleaning solutions (Nerandzic and Donskey 2016). Germinants cause spores to convert into vegetative cells that cannot survive in aerobic environments and are easily destroyed by cleaning solutions. One issue with this method is the type of germinants that would be needed to clean a patient's room and ensuring complete cleaning procedures. Germination is most effective when bile acids are combined with divalent cations and amino acids, which would leave behind nutrient residues on hospital surfaces. The authors also noted a delay in methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci bactericidal activity when germinants were added to quaternary ammonium, though this was short-lived and not observed after 60 min of exposure (Nerandzic and Donskey 2016). Another method being explored is germinant addition to ethanol-based hand sanitizers that resulted in 2 log10 CFU reduction in spores after 2 h (Nerandzic and Donskey 2017). This would be a convenient method of spore decontamination, though soap and warm water may be as effective, as already widely available, and it is unclear whether germinants would cause skin irritation (Oughton et al. 2009). These are promising environmental cleaning solutions that warrant further investigation. However, it may be more practical to limit the spread of spores into the environment from the source. Converting spores into vegetative cells in the presence of antibiotics through oral administration of germinants before they leave the GI tract may be a valuable approach. Taking advantage of spore biology by increasing germinant signals in the colon could transform spores into cells in vivo, which would die upon excretion into aerobic environments or be destroyed by antibiotic presence. This would interrupt the epidemiology of C. difficile by diminishing the number of spores shed into the environment that come into contact with other patients. A concern with this method is whether sudden mass germination of internal spores will cause an increase in toxin production leading to further clinical deterioration. This safety concern requires careful investigation in living systems before being used in rCDI patients.
COLONIZATION RESISTANCE
Antibiotics are a major risk factor for developing CDI as well as rCDI (Garey et al. 2008). The reason for this is thought to be from disturbances in the microbiome that remove organisms that outcompete C. difficile for resources and changes to the bile acid profile that make a more suitable environment to support vegetative cell growth. Primary bile acids, such as cholate and chenodeoxycholate, can be conjugated with amino acids such as taurine and glycine, secreted into the intestinal tract, and edited by the microbiome into secondary bile acids. Taurocholate, a conjugated primary bile acid, is the strongest known signal for germination and does not inhibit growth of C. difficile. Taurocholate can be deconjugated by bile salt hydrolases into cholate, which possesses ∼75% of taurocholate's germination power (Sorg and Sonenshein 2008; Mullish et al. 2019). Deoxycholate, the 7α-dehydroxylation product of cholate and a secondary bile acid, can induce germination but inhibits growth of C. difficile (Sorg and Sonenshein 2008). Commensal Clostridia harboring the bai operon are associated with conversion of cholate to deoxycholate and reduced risk of CDI (Solbach et al. 2018; Reed et al. 2020). Chenodeoxycholate, the other primary bile acid, and its derivatives inhibit both germination and vegetative cell growth (Sorg and Sonenshein 2008). Shifts in the bile acid pool due to antibiotic administration have been evaluated in murine models (Koenigsknecht et al. 2015; Theriot, Bowman and Young 2016). After antibiotic administration, taurocholate concentrations increase and anti-germinant and growth-inhibiting bile acids decrease. Taurocholate can reach up to 4 µg per 100 mg of cecal content in antibiotic-treated CDI susceptible mice while almost undetectable in non-antibiotic-treated mice (Koenigsknecht et al. 2015; Theriot, Bowman and Young 2016). Similar bile acid changes are seen in the colon and stool (Koenigsknecht et al. 2015). This concentration is roughly equivalent to 77.6 nM and is effective at germinating a small population of cecal spores in the absence of competitive inhibitor bile acids ex vivo (Theriot, Bowman and Young 2016). However, using Michaelis–Menten kinetics, the concentration of taurocholate to reach half maximal germination rates (Km) is reported at 2–3 mM (Sorg and Sonenshein 2010; Allen et al. 2013). The magnitude difference between the Km and taurocholate concentrations found in the cecum, colon and stool indicates a large population of spores is left dormant.
Further research is needed on whether an infection can be prevented, and possibly cured, by administering combinations of these bile acids to patients. Investigation on whether exogenous bile acids allow the natural microbiome to re-establish CR more efficiently would also be beneficial. This differs significantly from current treatments, like vancomycin, which is known to reduce CR (Lewis et al. 2015).
CURRENT CDI TREATMENTS
The Infectious Diseases Society of America and Society for Healthcare Epidemiology of America provide clinical practice guidelines and recommendations for treatment of C. difficile (Table 1). Oral vancomycin 125 mg four times daily for 10 days is a recommended treatment and most commonly used for CDI (McDonald et al. 2018). Failure to eliminate vegetative cells is unlikely because fecal vancomycin concentrations reach several magnitudes higher than the minimum inhibitory concentration (MIC) for C. difficile, similar to the other recommended therapy fidaxomicin (Gonzales et al. 2010; Louie et al. 2011). In addition, vancomycin fecal concentrations above the MIC can remain elevated for 4–5 days after treatment compared with metronidazole that is only detectable during therapy (Abujamel et al. 2013). The main concern with vancomycin is the drastic change to the microbiome that impacts CR and collateral vancomycin resistance to other pathologic organisms that can fill the void (Edlund et al. 1997; Louie et al. 2009; Lewis et al. 2015; Isaac et al. 2017).
Table 1.
Recommendations for the treatment of C. difficile infection in adults.*
| Clinical definition | Supportive clinical data | Recommended treatmenta | Strength of recommendation/quality of evidence | Costb |
|---|---|---|---|---|
| Initial episode, non-severe | Leukocytosis with a white blood cell count of ≤15 000 cells/mL and a serum creatinine level <1.5 mg/dL | VAN 125 mg given four times daily for 10 days, OR | Strong/high | $ |
| FDX 200 mg given twice daily for 10 days | Strong/high | $$$ | ||
| Alternate if above agents are unavailable: metronidazole, 500 mg three times per day by mouth for 10 days | Weak/high | $ | ||
| Initial episode, severec | Leukocytosis with a white blood cell count of ≥15 000 cells/mL or a serum creatinine level >1.5 mg/dL | VAN, 125 mg four times per day by mouth for 10 days, OR | Strong/high | $ |
| FDX 200 mg given twice daily for 10 days | Strong/high | $$$ | ||
| Initial episode, fulminant | Hypotension or shock, ileus, megacolon | VAN, 500 mg four times per day by mouth or by nasogastric tube. If ileus, consider adding rectal instillation of VAN. Intravenously administered metronidazole (500 mg every 8 h) should be administered together with oral or rectal VAN, particularly if ileus is present. | Strong/moderate (oral VAN); weak/low (rectal VAN); strong/moderate (intravenous metronidazole) | $$ |
| First recurrence | VAN 125 mg given four times daily for 10 days if metronidazole was used for the initial episode, OR | Weak/low | $ | |
| Use a prolonged tapered and pulsed VAN regimen if a standard regimen was used for the initial episode (e.g. 125 mg four times per day for 10–14 days, two times per day for a week, once per day for a week and then every 2 or 3 days for 2–8 weeks), OR | Weak/low | $ | ||
| FDX 200 mg given twice daily for 10 days if VAN was used for the initial episode | Weak/moderate | $$$ | ||
| Second or subsequent recurrence | VAN in a tapered and pulsed regimen, OR | Weak/low | $ | |
| VAN, 125 mg four times per day by mouth for 10 days followed by rifaximin 400 mg three times daily for 20 days, OR | Weak/low | $$$ | ||
| FDX 200 mg given twice daily for 10 days, OR | Weak/low | $$$ | ||
| Fecal microbiota transplantationd | Strong/moderate | $$ |
Abbreviations: FDX, fidaxomicin; VAN, vancomycin.
This table was adapted with permission from the IDSA guidelines.
All randomized trials have compared 10-day treatment courses, but some patients (particularly those treated with metronidazole) may have delayed response to treatment and clinicians should consider extending treatment duration to 14 days in those circumstances.
Costs are based off of AWP for the recommended treatment duration: $ = 0–1000, $$ = 1000–2500, $$$ >2500.
The criteria proposed for defining severe or fulminant Clostridioides difficile infection (CDI) are based on expert opinion. These may need to be reviewed in the future upon publication of prospectively validated severity scores for patients with CDI.
The opinion of the panel is that appropriate antibiotic treatments for at least two recurrences (i.e. three CDI episodes) should be tried prior to offering fecal microbiota transplantation.
Reducing the residual spore burden is the rationale for tapering and pulsed vancomycin dosing in rCDI. Extending vancomycin exposure time allows for residual spores to germinate and lower concentrations of antibiotic may allow partial return of CR, though results have been mixed and the treatment approach is labeled as a weak recommendation with low-quality evidence in the IDSA guideline (Gentry et al. 2017; Sirbu et al. 2017; McDonald et al. 2018). Additionally, vancomycin has not been shown to reliably reduce spore formation or toxin production in cultures compared with tetracyclines and fidaxomicin and has difficulty eliminating cells in stationary phases of growth, which is when spores and toxins are made (Babakhani et al. 2012; Bouillaut et al. 2015; Aldape et al. 2017). Vancomycin also takes longer to inhibit the outgrowth of newly germinated spores compared with fidaxomicin, which may allow time for toxin production during treatment (Allen et al. 2013). The ability of tetracyclines and fidaxomicin to reliably inhibit spore and toxin production and their limited impact on CR are likely why tetracyclines have low association, and can be protective, in developing CDI and why fidaxomicin has better rCDI treatment outcomes (Cornely et al. 2012; Tariq et al. 2018). However, these drugs, as with all antibiotics, do not impact spores that are already present in the GI tract. Lastly, from a patient's perspective, taking a medication four times a day can be very cumbersome and is inversely associated with medication adherence (Claxton, Cramer and Pierce 2001).
In two phase 3 randomized, double blind trials, oral fidaxomicin 200 mg twice daily for 10 days showed superiority over vancomycin in preventing rCDI as a secondary outcome within 28 days of treatment discontinuation (Cornely et al. 2012). The per protocol recurrence rate for fidaxomicin compared with vancomycin in patients without a prior episode was 11.7% vs 22.6% (P < 0.001). In patients with a prior episode, fidaxomicin saw 19.7% of patients experience recurrence compared with 35.5% for vancomycin (P = 0.045). Though relapse rates differed, the primary outcome of clinical cure as assessed by resolution of diarrhea at the end of 10 days of treatment was similar between the two groups (Crook et al. 2012). Interestingly, this reduction in rCDI may be strain specific, as rCDI rates do not differ when treating the epidemic NAP1/BI/027 strain (Louie et al. 2011). Fidaxomicin's lower recurrence rate is likely attributable to its narrower spectrum of activity compared with vancomycin, preserving aspects of CR (Louie et al. 2012). Fidaxomicin also has the ability to associate with spores, persisting on the surface through hydrophobic interactions that may last until the spore germinates, keeping the antibiotic in close proximity (Chilton et al. 2016). Vancomycin does not adhere to spores in the same manner as fidaxomicin (Chilton et al. 2016). In addition, decreases in spore and toxin production associated with fidaxomicin, an inhibitor of bacterial RNA polymerase, may contribute to its increased efficacy.
As C. difficile toxins A and B are a significant contributor to disease severity, anti-toxin therapeutics have been developed. A single dose of bezlotoxumab 10 mg/kg IV, a monoclonal antibody directed against toxin B, after a CDI-directed antibiotic course has shown similar efficacy in reducing rCDI to fidaxomicin. In two phase 3 randomized trials, the combined recurrence rate for bezlotoxumab was 17% compared with 27% for standard of care (Wilcox et al. 2017). Unfortunately, this study was not designed to determine whether the combination of fidaxomicin with bezlotoxumab could further decrease rCDI, and this approach would be cost prohibitive in its broad application to patients. The development of an antibody toward toxin A, actoxumab, was halted due to its lack of effect against rCDI (Wilcox et al. 2017). It is unknown why using an antibody directed toward toxin B reduces recurrence, while toxin A is ineffective. It could be related to the potency of the toxins, with toxin B possessing 100–1000× the toxicity of A in vitro, and inhibition of this toxin alone may be enough to diminish clinical symptoms (Chaves-Olarte et al. 1997). Further information on how toxin B sequestration effects the life cycle of C. difficile is needed. Information on spore shedding in this population would also be beneficial pertaining to contact precautions and prevention of HAIs.
Fecal microbiota transplantation (FMT), the instillation of healthy donor feces into a patient's GI tract after CDI directed antibiotic therapy, is recommended for patients with multiple rCDI episodes who have failed appropriate antibiotic treatment. Restoring CR with FMT helps reverse the damage caused by multiple rounds of antibiotic treatment, removing the niche that C. difficile needs to grow. Efficacy for FMT varies, but is close to 90% in patients who have had multiple rCDI episodes, the highest risk population for rCDI (Mattila et al. 2012). The high success rate of this procedure likely involves changing the bile acid profile, in conjunction with resource competition, as bacteria in FMT possessing bile salt hydrolases or the bai operon have been tied to efficacy (Solbach et al. 2018; Mullish et al. 2019). Changing the bile acid profile from a taurocholate rich environment to one of anti-germinating and growth inhibiting bile acids likely prevents the level of growth needed to cause clinical disease. Information on spore shedding post-FMT is limited, but likely to occur as asymptomatic carriage post-antibiotic treatment for CDI is common (Sethi et al. 2010). Antibiotic administration after FMT has resulted in CDI, suggesting patients were not decolonized or that spores persisted in their environment until ingested during subsequent dysbiosis (Mattila et al. 2012; Allegretti et al. 2019). A major concern with FMT is transmitting infectious agents between patients. One example includes extended spectrum beta-lactamase E. coli bacteremia that resulted in the death of a patient (DeFilipp et al. 2019). Developing the framework within a hospital system to screen donor stool for transplantation can be another barrier. However, this has been partially alleviated through commercially available pre-screened and ready-to-use preparations.
Another promising non-antibiotic treatment being explored, similar to FMT restoration of CR, is oral administration of non-toxigenic C. difficile (NTCD) spores post-CDI-directed antibiotic therapy. In a phase 2, randomized, double blind, placebo-controlled trial, patients were given differing doses of NTCD strain M3 spores for 7 or 14 days (Gerding et al. 2015). Patients were first treated with courses of vancomycin or metronidazole, two of the CDI-directed antibiotics with the highest recurrence rates, and given NTCD spores or placebo starting 2 days after completion of antibiotics. Patients given placebo experienced higher rates of rCDI at 30% compared with 11% of patients in the NTCD group (OR 0.28 95%, CI 0.11–0.68, P = 0.006). The adverse effect profile of spore administration was minimal, and often less than that of placebo. It should be noted that the majority of patients treated were not rCDI patients and multiple rCDI patients were excluded from participation. Lastly, this form of CR, which is likely due to resource competition, may be particularly cost effective to produce through culture. The same durability that allows spores to survive in the environment should afford a long shelf life for hospital pharmacies. The results of this trial are encouraging though more information is needed, particularly in multiple rCDI patients.
OPPORTUNITIES FOR NEW TREATMENT APPROACHES
Spore shedding and persistence in the GI tract are the greatest barriers in preventing rCDI. This was partially elucidated in an murine model with genetically modified strains of C. difficile that are unable to produce spores (Deakin et al. 2012). Spore negative strains do not cause recurrence, yet colonizing other mice with wild-type, spore-producing strains did. Hypotheses that increased germination rates may be responsible for more severe disease have been refuted (Carlson et al. 2015). More stringent control over germination likely allows spores to accumulate during both asymptomatic carriage and active infections. These germination resistant spores may outlast CDI antibiotic therapy and re-establish infection before CR returns. Spores that readily germinate would turn into viable cells during the treatment process and be eliminated by antibiotics. Likewise, if spores are too resistant to germination, they may not be able to outpace regeneration of CR post-CDI antibiotic therapy. This creates an optimal window for germination and outgrowth, after antibiotics wash out from the GI tract following treatment and before CR is replenished. In the case of vancomycin treatment, this optimal window would be between day 5 and 21 after discontinuing therapy in many patients, though the 21-day window may be extended in select patients (Abujamel et al. 2013). Replenishing CR with FMT removes this window, but may put patients at risk for CDI during future antibiotic treatment, as spores are likely still present. A solution to these problems might be found in using supraphysiologic germinant concentrations to decolonize patients. Figure 1 is a conceptual illustration of the germinant-antibiotic approach to disrupt the C. difficile spore-cell life cycle.
Figure 1.
Schematic of the infectious life cycle of C. difficile and treatment opportunities.
Administering germinants to patients during CDI antibiotic therapy may sufficiently diminish the spore burden to allow CR to regenerate. This approach would leave behind only hyperdormant spores, decrease total antibiotic administration time and may provide a decolonization method for rCDI patients. Additionally, decreased spore shedding in the hospital environment would likely lead to reductions in HAIs. The primary concern with this method is a sudden increase in toxin production and subsequent decline in clinical status. However, understanding that certain antibiotics, such as fidaxomicin and tetracyclines, decrease toxin production, and bezlotoxumab binds toxin B to neutralize its toxigenic effect should mitigate these concerns. In addition to inducing germination, taurocholate, at concentrations as low as 5 mM, has been found to mitigate cytotoxic effects of toxins A and B ex vivo (Darkoh et al. 2013). Decreased activity occurred without changing toxin concentration, although the mechanism remains unclear (Darkoh et al. 2013). Administering germinants could also be timed effectively, allowing for vegetative cells to be removed with antibiotics before germinating spores, limiting the active bacterial load. Toxin production occurs during stationary phases of growth, when vegetative cell burden is high, and may be related to quorum sensing (Darkoh et al. 2015). Careful investigation is needed to determine whether newly germinated spores at levels found within the host are able to produce toxins in antibiotic presence.
Additional considerations are the types of germinants that would be needed for transition from spore to vegetative cell. Taurocholate is taken up in the small intestine and recycled in the bile acid pool. Creation of a non-absorbable version of taurocholate would be beneficial to assure that the dose is reaching the colon in significant amounts. Alternatively, inhibitors of the apical sodium-dependent bile acid transporter, responsible for bile acid uptake, have recently been developed to aid insulin responses in type 2 diabetes and prevent itching in primary biliary cholangitis (Hegade et al. 2017; Al-Dury and Marschall 2018). Oral administration of taurocholate has been tested for other indications and was well tolerated when given at doses as high as 1 g every 8 h over 2 days, though the number of patients tested was small (Plusa and Clark 1991). Calcium, a strong co-germinant, is only 25% bioavailable when given as calcium carbonate, and should not have issue reaching significant concentrations to induce germination (Hunt and Johnson 2007). The specific co-germinants required vary between strains, but usually include a divalent cation and amino acids, both of which should be safe to administer to patients (Shrestha and Sorg 2018).
The final consideration in using germination to eradicate spores is hyperdormancy, or spores that do not readily vegetate in response to germinants. This may be due to the spore's outermost layer, the exosporium. The bile acid receptor CspC implicated in initiating germination is hypothesized to be in the spore coat or outer membrane, both located under the exosporium (Kochan et al. 2018). In addition, exosporium removal increases the spore's ability to form colonies (Calderón et al. 2018). The exosporium aids in chemical and heat resistance and increases hydrophobicity, which may allow it to adhere to environmental surfaces and colonic mucosa (Paredes-Sabja and Sarker 2012; Calderón et al. 2018). This layer also degrades over time, which may aid in preventing germination until antibiotics are washed out of the GI tract (Pizarro-Guajardo, Calderón-Romero and Paredes-Sabja 2016). This raises concerns, as previous experiments looking at germination efficiency often include multiple washing steps before the introduction of germinants. Unfortunately, it would not be possible to remove this layer in vivo with conventional methods such as sonication, centrifugation or enzyme digestion. However, hyperdormant spores may not germinate in sufficient numbers or in a timely manner to outpace the regeneration of CR.
CONCLUSION
Spore shedding and colonization of the GI tract are significant barriers for preventing HAIs and treating rCDI patients. Options to remove toxigenic spores from a patient's GI tract are severely limited and progressive CDI antibiotic usage may prevent re-establishment of CR. FMT has been very successful at preventing rCDI, but its efficacy is removed through subsequent antibiotic treatment. Currently, no therapy exists to address this issue. Turning spores into vegetative cells with germinants for antibiotic targeting is an alternative approach with potential to decrease the prevalence of CDI. Future studies should investigate the effect and safety of germinants with antibiotics in animal models of CDI to determine whether combination therapy has potential benefit.
Supplementary Material
Contributor Information
Noah Budi, School of Pharmacy, University of Wisconsin-Madison, Madison, WI, USA, 53705.
Nasia Safdar, Division of Infectious Diseases, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA, 53726.
Warren E Rose, School of Pharmacy, University of Wisconsin-Madison, Madison, WI, USA, 53705.
Conflicts of interests
None declared.
REFERENCES
- Abujamel T, Cadnum JL, Jury LAet al. . Defining the vulnerable period for re-establishment of Clostridium difficilecolonization after treatment of C. difficile infection with oral vancomycin or metronidazole. PLoS One. 2013;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Dury S, Marschall HU. Ileal bile acid transporter inhibition for the treatment of chronic constipation, cholestatic pruritus, and NASH. Front Pharmacol. 2018;9:931. Published 2018 Aug 21, DOI: 10.3389/fphar.2018.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldape MJ, Packham AE, Heeney DDet al. . Fidaxomicin reduces early toxin A and B production and sporulation in Clostridium difficilein vitro. J Med Microbiol. 2017;66:1393–9. [DOI] [PubMed] [Google Scholar]
- Ali S, Muzslay M, Wilson P. A novel quantitative sampling technique for detection and monitoring of Clostridium difficile contamination in the clinical environment. J Clin Microbiol. 2015;53:2570–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegretti JR, Kao D, Phelps Eet al. . Risk of Clostridium difficile infection with systemic antimicrobial therapy following successful fecal microbiota transplant: should we recommend anti-Clostridium difficile antibiotic prophylaxis?. Dig Dis Sci. 2019;64:1668–71. [DOI] [PubMed] [Google Scholar]
- Allen CA, Babakhani F, Sears Pet al. . Both fidaxomicin and vancomycin inhibit outgrowth of Clostridium difficile spores. Antimicrob Agents Chemother. 2013;57:664–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babakhani F, Bouillaut L, Gomez Aet al. . Fidaxomicin inhibits spore production in Clostridium difficile. Clin Infect Dis. 2012;55:S162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron SW, Ostrowsky BE, Nori Pet al. . Screening of Clostridioides difficile carriers in an urban academic medical center: understanding implications of disease. Infect Control Hosp Epidemiol. 2020;41:149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillaut L, McBride S, Sorg JAet al. . Effects of surotomycin on Clostridium difficile viability and toxin production in vitro. Antimicrob Agents Chemother. 2015;59:4199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns DA, Heeg D, Cartman STet al. . Reconsidering the sporulation characteristics of hypervirulent Clostridium difficile BI/NAP1/027. PLoS One. 2011;6:e24894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón P, Castro P, Reyes Ret al. . Clostridium difficile exosporium cysteine-rich proteins are essential for the morphogenesis of the exosporium layer, spore resistance, and affect C. difficile pathogenesis. PLoS Pathog. 2018;14:e1007199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson PE Jr, Kaiser AM, McColm SAet al. . Variation in germination of Clostridium difficile clinical isolates correlates to disease severity. Anaerobe. 2015;33:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Services, CDC. 2019. [Google Scholar]
- Chaves-Olarte E, Weidmann M, Von Eichel-Streiber Cet al. . Toxins A and B from Clostridium difficile differ with respect to enzymatic potencies, cellular substrate specificities, and surface binding to cultured cells. J Clin Invest. 1997;100:1734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton CH, Crowther GS, Ashwin Het al. . Association of fidaxomicin with C. difficile spores: effects of persistence on subsequent spore recovery, outgrowth and toxin production. PLoS One. 2016;11:e0161200, DOI: 10.1371/journal.pone.0161200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton CH, Pickering DS, Freeman J. Microbiologic factors affecting Clostridium difficile recurrence. Clin Microbiol Infect. 2018;24:476–82. [DOI] [PubMed] [Google Scholar]
- Cho J, Cunningham S, Pu Met al. . Clostridioides difficile whole genome sequencing differentiates relapse with the same strain from reinfection with a new strain[published online ahead of print, 2020 Feb 17]. Clin Infect Dis. 2020;ciaa159, DOI: 10.1093/cid/ciaa159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–310. [DOI] [PubMed] [Google Scholar]
- Cornely OA, Miller MA, Louie TJet al. . Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55:154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook DW, Walker AS, Kean Yet al. . Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55:S93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darkoh C, Brown EL, Kaplan HBet al. . Bile salt inhibition of host cell damage by Clostridium difficile toxins. PLoS One. 2013;8:e79631. Published 2013 Nov 11, DOI: 10.1371/journal.pone.0079631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darkoh C, Dupont HL, Norris SJet al. . Toxin synthesis by Clostridium difficile is regulated through quorum signaling. mBio. 2015;6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin LJ, Clare S, Fagan RPet al. . The Clostridium difficile spo0A gene is a persistence and transmission factor. Infect Immun. 2012;80:2704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFilipp Z, Bloom PP, Torres Soto Met al. . Drug-resistantE. colibacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381:2043–50. [DOI] [PubMed] [Google Scholar]
- Edlund C, Barkholt L, Olsson-Liljequist Bet al. . Effect of vancomycin on intestinal flora of patients who previously received antimicrobial therapy. Clin Infect Dis. 1997;25:729–32. [DOI] [PubMed] [Google Scholar]
- Figueroa I, Johnson S, Sambol SPet al. . Relapse versus reinfection: recurrent Clostridium difficile infection following treatment with fidaxomicin or vancomycin. Clin Infect Dis. 2012;55:104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey KW, Sethi S, Yadav Yet al. . Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. 2008;70:298–304. [DOI] [PubMed] [Google Scholar]
- Gentry CA, Giancola SE, Thind Set al. . A propensity-matched analysis between standard versus tapered oral vancomycin courses for the management of recurrent Clostridium difficile infection. Open Forum Infect Dis. 2017;4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerding DN, Meyer T, Lee Cet al. . Administration of spores of nontoxigenic Clostridium difficile strain M3 for prevention of recurrent C difficile infection: a Randomized clinical trial. JAMA. 2015;313:1719–27. [DOI] [PubMed] [Google Scholar]
- Gonzales M, Pepin J, Frost EHet al. . Faecal pharmacokinetics of orally administered vancomycin in patients with suspected Clostridium difficile infection. BMC Infect Dis. 2010;10:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez S, Chaves F, Orellana MA. Clinical, epidemiological and microbiological characteristics of relapse and re-infection in Clostridium difficile infection. Anaerobe. 2017;48:147–51. [DOI] [PubMed] [Google Scholar]
- Hegade VS, Kendrick SF, Dobbins RLet al. . Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114–23. [DOI] [PubMed] [Google Scholar]
- Hunt CD, Johnson LAK. Calcium requirements: new estimations for men and women by cross-sectional statistical analyses of calcium balance data from metabolic studies. Am J Clin Nutr. 2007;86:1054–63. [DOI] [PubMed] [Google Scholar]
- Isaac S, Scher JU, Djukovic Aet al. . Short- and long-term effects of oral vancomycin on the human intestinal microbiota. J Antimicrob Chemother. 2017;72:128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection?. Clin Microbiol Infect. 2012;18:21–7. [DOI] [PubMed] [Google Scholar]
- Kochan TJ, Foley MH, Shoshiev MSet al. . Updates to Clostridium difficile spore germination. J Bacteriol. 2018;200:e00218–18.. Published 2018 Jul 25, DOI: 10.1128/JB.00218-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsknecht MJ, Theriot CM, Bergin ILet al. . Dynamics and establishment of Clostridium difficile infection in the murine gastrointestinal tract. Infect Immun. 2015;83:934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BB, Buffie CG, Carter RAet al. . Loss of Microbiota-Mediated colonization resistance to Clostridium difficile infection with oral vancomycin compared with metronidazole. J Infect Dis. 2015;212:1656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie TJ, Cannon K, Byrne Bet al. . Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Infect Dis. 2012;55:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie TJ, Emery J, Krulicki Wet al. . OPT-80 eliminates Clostridium difficile and is sparing of bacteroides species during treatment of C. difficile infection. Antimicrob Agents Chemother. 2009;53:261–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie TJ, Miller MA, Mullane KMet al. . Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–31. [DOI] [PubMed] [Google Scholar]
- Mattila E, Uusitalo-Seppälä R, Wuorela Met al. . Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology. 2012;142:490–6. [DOI] [PubMed] [Google Scholar]
- McDonald LC, Gerding DN, Johnson Set al. . Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:e1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullish BH, Mcdonald JAK, Pechlivanis Aet al. . Microbial bile salt hydrolases mediate the efficacy of faecal microbiota transplant in the treatment of recurrent Clostridioides difficile infection. Gut. 2019;68:1791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerandzic MM, Donskey CJ. A quaternary ammonium disinfectant containing germinants reduces clostridium difficile spores on surfaces by inducing susceptibility to environmental stressors. Open Forum Infect Dis. 2016;3:ofw196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerandzic MM, Donskey CJ. Sensitizing Clostridium difficile spores with germinants on skin and environmental surfaces represents a new strategy for reducing spores via ambient mechanisms. Pathog Immun. 2017;2:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oughton MT, Loo VG, Dendukuri Net al. . Hand hygiene with soap and water is superior to alcohol rub and antiseptic wipes for removal of Clostridium difficile. Infect Control Hosp Epidemiol. 2009;30:939–44. [DOI] [PubMed] [Google Scholar]
- Paredes-Sabja D, Sarker MR. Adherence of Clostridium difficile spores to Caco-2 cells in culture. J Med Microbiol. 2012;61:1208–18. [DOI] [PubMed] [Google Scholar]
- Pizarro-Guajardo M, Calderón-Romero P, Paredes-Sabja D. Ultrastructure variability of the exosporium layer of Clostridium difficile spores from sporulating cultures and biofilms. Appl Environ Microbiol. 2016;82:5892–8.. Published 2016 Sep 16, DOI: 10.1128/AEM.01463-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plusa SM, Clark NW. Prevention of postoperative renal dysfunction in patients with obstructive jaundice: a comparison of mannitol-induced diuresis and oral sodium taurocholate. J R Coll Surg Edinb. 1991;36:303–5. [PubMed] [Google Scholar]
- Reed AD, Nethery MA, Stewart Aet al. . Strain-dependent inhibition of Clostridioides difficile by commensal clostridia carrying the bile acid-inducible (bai) operon. J Bacteriol. 2020;202:e00039–20.. Published 2020 May 11, DOI: 10.1128/JB.00039-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi AK, Al-Nassir WN, Nerandzic MMet al. . Persistence of skin contamination and environmental shedding of Clostridium difficile during and after treatment of C. difficile infection. Infect Control Hosp Epidemiol. 2010;31:21–7. [DOI] [PubMed] [Google Scholar]
- Shaughnessy MK, Micielli RL, DePestel DDet al. . Evaluation of hospital room assignment and acquisition of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2011;32:201–6. [DOI] [PubMed] [Google Scholar]
- Shrestha R, Sorg JA. Hierarchical recognition of amino acid co-germinants during Clostridioides difficile spore germination. Anaerobe. 2018;49:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu BD, Soriano MM, Manzo Cet al. . Vancomycin taper and pulse regimen with careful follow-up for patients with recurrent Clostridium difficile infection. Clin Infect Dis. 2017;65:1396–9. [DOI] [PubMed] [Google Scholar]
- Sisto F, Scaltrito MM, Zago Met al. . Molecular analysis of relapses or reinfections of Clostridium difficile-associated diarrhea. New Microbiol. 2011;34:399–402. [PubMed] [Google Scholar]
- Solbach P, Chhatwal P, Woltemate Set al. . BaiCD gene cluster abundance is negatively correlated with Clostridium difficile infection. PLoS One. 2018;13:e0196977. Published 2018 May 8, DOI: 10.1371/journal.pone.0196977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg JA, Sonenshein AL. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol. 2008;190:2505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg JA, Sonenshein AL. Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. J Bacteriol. 2010;192:4983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Feldman Y, Mayo S, Silva Jet al. . Molecular analysis of Clostridium difficile strains isolated from 18 cases of recurrent Clostridium difficile-associated diarrhea. J Clin Microbiol. 2003;41:3413–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariq R, Cho J, Kapoor Set al. . Low risk of primary Clostridium difficile infection with tetracyclines: a systematic review and metaanalysis. Clin Infect Dis. 2018;66:514–22. [DOI] [PubMed] [Google Scholar]
- Theriot CM, Bowman AA, Young VB. Antibiotic-induced alterations of the gut microbiota alter secondary bile acid production and allow for Clostridium difficile spore germination and outgrowth in the large intestine. mSphere. 2016;1:e00045–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijerina L, Villarreal L, Baines SDet al. . High sporulation and overexpression of virulence factors in biofilms and reduced susceptibility to vancomycin and linezolid in recurrent Clostridioides difficile infection isolates. PLoS One. 2019;14:e0220671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox MH, Gerding DN, Poxton IRet al. . Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.