Abstract
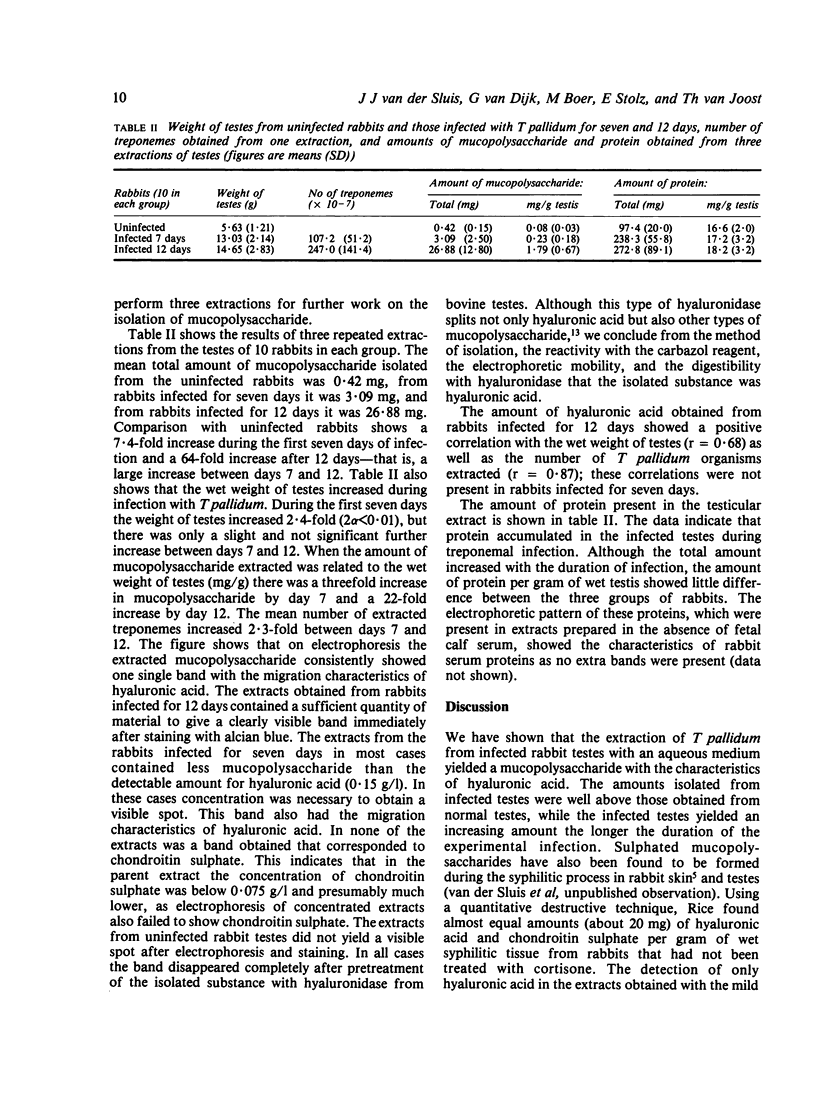
The amount and nature of mucopolysaccharides present in extraction fluids routinely obtained in the isolation procedure of Treponema pallidum from infected rabbit testes was investigated. The mean quantity of mucopolysaccharides extracted from both testes of groups of 10 rabbits was 3.09 mg after infection for seven days and 26.88 mg after infection for 12 days, while from the testes of uninfected rabbits a mean of 0.42 mg was obtained. On electrophoresis the isolated mucopolysaccharides showed only one single band with the migration characteristics of hyaluronic acid. This band disappeared completely after pretreatment with hyaluronidase from bovine testes, which showed that during infection with T pallidum increasing amounts of hyaluronic acid accumulate. They can, at least in part, be extracted by a gentle extraction procedure, suggesting that this material binds loosely. The amount of hyaluronic acid isolated 12 days after infection showed positive correlations with the wet weight of testes as well as the number of treponemes isolated; seven days after infection such correlations were not present.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Baker-Zander S., Sell S. A histopathologic and immunologic study of the course of syphilis in the experimentally infected rabbit. Demonstration of long-lasting cellular immunity. Am J Pathol. 1980 Nov;101(2):387–414. [PMC free article] [PubMed] [Google Scholar]
- Breen M., Weinstein H. G., Andersen M., Veis A. Microanalysis and characterization of acidic glycosaminoglycans in human tissues. Anal Biochem. 1970 May;35(1):146–159. doi: 10.1016/0003-2697(70)90020-5. [DOI] [PubMed] [Google Scholar]
- CHRISTIANSEN S. Protective layer covering pathogenic treponemata. Lancet. 1963 Feb 23;1(7278):423–425. doi: 10.1016/s0140-6736(63)92309-2. [DOI] [PubMed] [Google Scholar]
- CONN H. J. R.S. Breed, bacterial taxonomist. Science. 1956 Aug 10;124(3215):256–256. doi: 10.1126/science.124.3215.256. [DOI] [PubMed] [Google Scholar]
- Fieldsteel A. H., Cox D. L., Moeckli R. A. Cultivation of virulent Treponema pallidum in tissue culture. Infect Immun. 1981 May;32(2):908–915. doi: 10.1128/iai.32.2.908-915.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J. Pathogenesis and immunology of Treponema pallidum. Annu Rev Microbiol. 1981;35:29–54. doi: 10.1146/annurev.mi.35.100181.000333. [DOI] [PubMed] [Google Scholar]
- Pennock C. A. A review and selection of simple laboratory methods used for the study of glycosaminoglycan excretion and the diagnosis of the mucopolysaccharidoses. J Clin Pathol. 1976 Feb;29(2):111–123. doi: 10.1136/jcp.29.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT V., DAMMIN G. J. Morphologic and histochemical sequences in syphilitic and in tuberculous orchitis in the rabbit. Am J Syph Gonorrhea Vener Dis. 1954 May;38(3):189–202. [PubMed] [Google Scholar]
- TURNER T. B., HOLLANDER D. H. Studies on the mechanism of action of cortisone in experimental syphilis. Am J Syph Gonorrhea Vener Dis. 1954 Sep;38(5):371–387. [PubMed] [Google Scholar]
- Zeigler J. A., Jones A. M., Jones R. H., Kubica K. M. Demonstration of extracellular material at the surface of pathogenic T. pallidum cells. Br J Vener Dis. 1976 Feb;52(1):1–8. doi: 10.1136/sti.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]



