Abstract
Objective:
While outcome from mild traumatic brain injury (mTBI) is generally favorable, concern remains over potential negative long-term effects, including impaired cognition. This study examined the link between cognitive performance and remote mTBIs within the Long-term Impact of Military-relevant Brain Injury Consortium-Chronic Effects of Neurotrauma Consortium (LIMBIC-CENC) multicenter, observational study of Veterans and Service Members (SMs) with combat exposure.
Method:
Baseline data of the participants passing all cognitive performance validity tests (n=1,310) were used to conduct cross-sectional analysis. Using multivariable regression models that adjusted for covariates, including age and estimated pre-exposure intellectual function, positive mTBI history groups, 1–2 lifetime mTBIs (non-repetitive, n=614) and 3+ lifetime mTBIs (repetitive; n=440) were compared to TBI negative controls (n=256) on each of seven cognitive domains computed by averaging Z-scores of prespecified component tests. Significance levels were adjusted for multiple comparisons.
Results:
Neither of the mTBI positive groups differed from the mTBI negative control group on any of the cognitive domains in multivariable analyses. Findings were also consistently negative across sensitivity analyses (e.g., mTBIs as a continuous variable, number of blast-related mTBIs, or years since first and last mTBI).
Conclusions:
Our findings demonstrate that the average Veteran or SM who experienced one or more mTBIs does not have post-acute objective cognitive deficits due to mTBIs alone. A holistic healthcare approach including comorbidity assessment is indicated for patients reporting chronic cognitive difficulties after mTBI(s), and strategies for addressing misattribution may be beneficial. Future study is recommended with longitudinal designs to assess within-subjects decline from potential neurodegeneration.
Keywords: traumatic brain injury, concussion, cognition, neuropsychological testing, military
BACKGROUND
Traumatic brain injury (TBI) is defined as a traumatically induced structural injury and/or physiological disruption of brain function as a result of an external force that is indicated by new onset or worsening of at least one of the following clinical signs immediately following the event: loss of consciousness (LOC), memory gap consistent with post-traumatic amnesia (PTA), alteration of mental state (e.g., dazed, confused), or neurological deficits (American Congress of Rehabilitation Medicine, 1993; Work Group, 2021). Expert consensus definitions by the American Congress of Rehabilitation Medicine (ACRM) and the Departments of Veterans Affairs and Defense working group on a common definition further classify TBI severity as mild, moderate, or severe, with classification as mild if LOC does not exceed 30 minutes, initial Glasgow Coma Score is not below 13, PTA does not exceed 24 hours, and there is no evidence of traumatic intracranial hemorrhage on clinical imaging (American Congress of Rehabilitation Medicine, 1993; Work Group, 2021). Mild TBI (mTBI), often termed concussion, is by far the most common severity category in both civilian and military populations. Since 2000, more than 430,000 United States (U.S.) Service Members (SMs) have sustained a documented TBI, with the vast majority (>80%) of cases categorized as mTBI (TBI Center of Excellence, 2021). The Veterans Health Administration conducts mTBI screening on all returning Post-9/11 Veterans, but only those reporting active symptoms receive a comprehensive TBI evaluation. Therefore, actual case prevalence is likely higher due to commonly occurring, undocumented mTBI history (Donnelly et al., 2011)
Cognitive dysfunction is a clinical hallmark of TBI, manifesting behaviorally, symptomatically, and/or as reduced performance on objective cognitive tests. After severe TBI, acute neurocognitive dysfunction is usually pronounced and obvious with a period of frank disorientation and confusion or worse. Objective evidence of reduced cognition is also commonly measurable within the first several weeks after mTBI (Karr et al., 2014). Over time, cognitive performance normally improves to varying degrees. In severe TBI, full recovery is atypical and chronically-reduced cognition is common (Dikmen et al., 2009; J. Ponsford et al., 2008; Ruttan et al., 2008); whereas with mTBI, most patients have excellent, if not full, recoveries within several days to months. In general, objective, late cognitive deficits after mTBI have been elusive to document at the group level, with multiple, meta-analytic reviews concluding insufficient evidence for deleterious effects of mTBI on objective cognitive assessment beyond the first few months post-injury (Belanger et al., 2005; Binder et al., 1997; Frencham et al., 2005; Karr et al., 2014; O’Neil et al., 2014; Rohling et al., 2011; Schretlen & Shapiro, 2003).
Nevertheless, 20% or more of patients with mTBI experience persistent post-concussion symptoms (PPCS) (Lagacé-Legendre et al., 2021), previously referred to as ‘post-concussive syndrome’ (Brenner et al., 2009). PPCS typically include cognitive symptoms, such as decreased concentration, memory, and processing speed, in addition to somatic and emotional symptoms, such as headache, dizziness, disordered sleep, fatigue, irritability, increased arousal, and sensitivity to light and sound (Brenner et al., 2009; Stein & McAllister, 2009). PPCS are especially common among U.S. Veterans and SMs who sustained mTBI in the post-911 wars, including Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) (Cifu et al., 2013; Hendricks et al., 2013; Taylor et al., 2012). Despite the common occurrence of chronic cognitive subjective symptomology after mTBI, extant research shows a lack of a consistent relationship with cognitive performance on formal neuropsychological examination, including samples of military personnel (Mac Donald et al., 2017). The Veterans Affairs (VA) /Department of Defense (DoD) clinical practice guideline (Work Group, 2021), the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), and other standardized guides, make a distinction between mTBI as an injurious historical event and PPCS with or without mild cognitive disorder (Cnossen et al., 2017; Iverson, 2019).
Despite the lack of convincing objective evidence that mTBI chronically alters cognitive performance, there remains justifiable concern that mTBI may lead to not only PPCS, but also chronic neurocognitive impairment. Many argue that TBI should be considered an evolving, chronic condition, even if initial severity was mild (Wilson et al., 2017). One explanation for negative findings in most studies may be the subtle nature of cognitive performance effects that may be present in only a small subset of patients. As studies examining control group comparators do not consider premorbid cognitive performance, they may fail to detect mild within-subject reductions. Another confounder in detecting cognitive impairment in the chronic stage of mTBI may be that other common comorbidities adversely impact performance on tests of cognition. These include psychiatric disorders (Vasterling et al. 2012), sleep abnormalities (Anderson & Jordan, 2021), and pain (Khalid & Tubbs, 2017). Sleep is an especially important variable to consider, given its known impact on cognitive performance (Fortier-Brochu et al., 2012), and the prevalence of insomnia, sleep apnea, and other sleep disorders among those with a history of mTBI (J. L. Ponsford et al., 2013; Schreiber et al., 2008). Comorbid posttraumatic stress disorder (PTSD) and chronic pain are also important considerations as many symptoms and deficits that accompany PTSD and chronic pain are non-specific and overlap with mTBI (Iverson, 2006; Otis et al., 2011; Porter et al., 2018). Additionally suboptimal effort and secondary gain may accompany self-reports of cognitive difficulty (Armistead-Jehle et al., 2016).
There is emerging evidence that repeated mTBI may confer additional risk for both persisting symptoms and cognitive compromise. In civilians, repetitive mTBIs often encountered in sports, including football, boxing, and soccer (Guskiewicz et al., 2003; Safinia et al., 2016), may be related to impairment over longer time periods, (Guskiewicz et al., 2003; Miller et al., 2013), or serve as risk factors for developing other conditions, including neurodegenerative disorders (Vincent et al., 2014). A recent animal study demonstrated that repetitive TBIs can result in worse and more persistent neurological deficits compared to a single TBI (Bachstetter et al., 2020). A meta-analysis concluded that multiple concussions appear to be a risk factor for cognitive impairment in some individuals (Manley et al., 2017). However, the literature remains inconclusive, as many other studies have failed to demonstrate an effect of repeated mTBI on objective cognitive performance (Belanger et al., 2010) (Cooper et al., 2018; Macciocchi et al., 2001). Another factor specific to military populations with mixed research evidence is the concern that blast-related mTBI has more enduring long-term effects on cognition compared to blunt mTBI (Belanger et al., 2009; Martindale et al., 2020).
Taken together, there is inconclusive research regarding the risk of chronic, neurocognitive decrements after a single mTBI or even repeated mTBIs. The Long-term Impact of Military-relevant Brain Injury Consortium (LIMBIC)-Chronic Effects of Neurotrauma Consortium’s (LIMBIC-CENC) multicenter, prospective longitudinal study (PLS) was designed to address this critical research gap and to improve understanding of the long-term effects of mTBI within the military and veteran populations (Walker et al., 2016; Walker, Hirsch, et al., 2018). The current study utilized the baseline (at enrollment) evaluations from the large LIMBIC-CENC PLS dataset. Our specific aim was to determine the amount of variance explained by mTBI history alone on current, objective, cognitive performance in domains that have been demonstrated to be impacted in both civilian and military-related mild TBI including episodic and working memory, attention, processing speed, executive functioning and fine motor dexterity (Barker-Collo et al., 2015; Storzbach et al., 2015; Wilde et al., 2010). The main hypothesis was, compared to those reporting totally negative mTBI histories, those with three or more lifetime mTBIs (repetitive mTBI) have lower performance on these cognitive domains when adjusting for comorbidities and other potential contributing factors. Other aspects of mTBI history that our analysis considered were the total number of mTBIs measured continuously, the mTBI(s) mechanism (blast versus blunt), and elapsed time between mTBI event(s) and time of testing.
METHODS
Design:
This cross-sectional study analyzed data collected at the enrollment visit for the ongoing LIMBIC-CENC multicenter PLS. Details of the LIMBIC-CENC PLS objectives, recruitment processes, eligibility criteria, and overall methods have been previously described (Sickinger et al., 2018; Walker et al., 2016; Walker, Hirsch, et al., 2018). Briefly, the PLS is a longitudinal, observational study that has established a large multicenter cohort of cohort of current and former U.S. SMs with combat exposure and continues to enroll new participants at eleven geographically dispersed sites. While most participants experienced one or more lifetime mTBIs, slightly less than 20% report completely negative lifetime TBI histories. The overall study objective is to answer questions about the long-term effects of mTBI including the relationship to other factors and sequelae of combat exposure. At the baseline visit after enrollment, all participants underwent comprehensive assessment that included an extensive neuropsychological test battery, symptom inventories, and biometrics. Participants were primarily recruited through mass mailings and non-paid advertisements. All individuals 18 years of age or older with a history of combat exposure are eligible to participate in this study. The only exclusions were a history of moderate to severe TBI or major neurologic or psychiatric disorder, such as stroke or schizophrenia, with significant decrease in functional status. Common mental health comorbidities, such as PTSD and depression, were permitted. The parent study, including the database registry and all secondary analyses, was approved by the local Institutional Review Boards at each of the PLS enrollment sites. All participants provide written consent prior to any study procedures.
Sample selection
At the time of this data extraction, 1,551 participants had enrolled in the PLS and completed baseline assessments. For the current analyses, only data collected at baseline assessment were utilized, and participants who failed any of the three tests of performance validity described further below were excluded.
Lifetime Mild TBI history:
Clinical diagnosis of all lifetime mTBI(s) was obtained through a rigorous, standardized process. Each participant’s potential concussive events (PCEs) were cataloged using a modified version of the Ohio State University TBI Identification (OSU TBI-ID) (Corrigan & Bogner, 2007). Each PCE was then assessed via a validated retrospective Concussion Diagnostic Interview, yielding a preliminary algorithm-generated TBI diagnosis (No mTBI, mTBI with PTA, or mTBI without PTA) (Walker et al., 2015). Every algorithm rating was then reviewed, checked against available medical records, and vetted with a centralized, expert committee to yield a final determination that adhered to the VA/DoD common definition of mTBI (Management of Concussion/mTBI Working Group, 2009), which is also consistent with the American Congress of Rehabilitation Medicine definition of mTBI (American Congress of Rehabilitation Medicine, 1993). From this TBI categorization data, positive lifetime mTBI histories were subclassified for these analyses as either 1) non-TBI control, 2) 1–2 lifetime mTBIs (non-repetitive mTBI), or 3) 3+ lifetime mTBIs (repetitive mTBI). The classification for non-repetitive versus repetitive mTBI was based on evidence from prior LIMBIC-CENC PLS studies showing differences between these groups in postural stability (Walker, Nowak, et al., 2018) and brain-based biomarkers (Kenney et al., 2018), and to achieve approximately equal group sizes for better statistical power. For planned sensitivity analyses, we alternatively replaced the mTBI frequency categories by its continuous version, the total number of lifetime mTBIs, and we separately analyzed the total number of lifetime mTBIs that were of blast-related mechanism. We also considered years since first and last mTBI as covariates.
Years since index event:
For mTBI positive participants, the self-described worst mTBI served as the index event unless there were zero lifetime mTBIs (or all mTBIs were before any military combat) in which case the self-identified worst PCE during a combat deployment was used for a control comparison.
Cognitive Performance Testing:
The extensive LIMBIC-CENC cognitive test battery includes a spectrum of traditional neuropsychological tests and the recently developed computerized NIH Toolbox Cognition Battery (NIHTB-CB). All instruments used are recommended as part of the NIH Common Data Elements for mTBI (Hicks et al., 2013). All neuropsychology test administrators were trained to conduct each assessment following the standardized procedures established by the test developer prior to study enrollment. Administrators were required to submit a video recording of themselves conducting the neuropsychology battery on a test subject to assess fidelity of assessment implementation. Regular auditing was performed on all neuropsychology data to ensure there were no transcription errors from case report form to database entry and to detect and remedy if needed any study wide or site-specific scoring drift.
To minimize spurious findings, we utilized a hypothesis-driven approach to preselect a comprehensive and multidimensional set of primary neurocognitive outcomes. Through expert consensus among the study group, we identified seven domains of neurocognition that would be most representative of TBI-related impairment consisting of: Episodic Memory, Attention, Processing Speed, Working Memory, Executive Functioning, Verbal Fluency, and Fine Motor and Dexterity. From the battery of traditional and NIHTB-CB tests, we parsimoniously selected specific subtests or indices scores for each domain. The components of each domain are listed in Table 1 and further detail on the test instruments and measures is provided below.
Table 1.
Pre-specified Neurocognitive Outcomes
| Domains | Component Test Scores |
|---|---|
| Episodic memory | CVLT-II: Trials 1–5 total; Long Delay Free Recall BVMT-R: Total Recall NIH-TB-CB Picture Sequence |
| Attention | TMT Part A |
| Processing speed | WAIS-IV Processing Speed Index NIH-TB-CB Pattern Comparison |
| Working Memory | WAIS-IV Working Memory Index NIH-TB-CB List Sorting |
| Executive functioning | TMT Part B WAIS-IV Visual Puzzles NIH-TB-CB Dimensional change card sort test NIH-TB-CB Flanker Inhibitory control |
| Verbal Fluency | D-KEFS VFT: Letter fluency; Category fluency |
| Fine Motor & Dexterity | Grooved Pegboard: Dominant; Non-dominant |
Abbreviations: CVLT-II = California Verbal Learning Test-II; BVMT-R = Brief Visuospatial Memory Test-Revised; NIH TB-CB = NIH Toolbox Cognition Battery; TMT = Trail Making Test; WAIS-IV = Wechsler Adult Intelligence Scale 4th Edition; DKEFS = the Delis–Kaplan Executive Function System
California Verbal Learning Test-II (CVLT-II):
Verbal episodic memory was measured using the Trials 1–5 Total Recall score and Long Delay Free Recall score (Delis et al., 2000). For each of the five trials, participants hear a list of 16 words, in the same order, and then are asked to recall the words in any order after each trial (Total Recall), and then again after 30 minutes (Long Delay Free Recall). Test-retest reliability for the standard forms, which are the ones used in the current study, ranged from 0.80 (Total Trials 1–5 Recall) to 0.83 (Long-delay Free Recall), and there is also evidence of discriminability (Woods et al., 2006).
Brief Visuospatial Memory Test-Revised (BVMT-R):
Visual episodic memory was assessed using the Trials 1–3 Total score and Delayed Recall score (R. Benedict, 1997). During each of the three 10-second learning trials, participants study a 2 X 3 array of six figures and are then asked to reproduce each figure in its correct location. After a 25-minute delay, participants are asked again to draw each figure in its correct location from memory. There is evidence of construct and criterion-related validity (R. H. B. Benedict et al., 1996). Reliability coefficients are high (0.96 to 0.97) for the three learning trials and for Delayed Recall (0.97). The test-retest reliability coefficients range from 0.60 (Trial 1) to 0.84 (Trial 3) (R. Benedict, 1997).
NIHTB-CB:
The NIHTB-CB consists of multiple subtests that span most of the same neurocognitive domains as a traditional neuropsychological test battery (Weintraub et al., 2013). We prespecified the Picture Sequence test for episodic memory, Pattern Comparison for processing speed, List Sorting for working memory, and both the Dimensional Change Card Sort and Flanker Inhibitory Control for executive functioning.
NIH-TB-CB Picture Sequence test ‒
Each picture in a thematic series is presented individually, centered on the screen, as an audio file describes its content. The picture is then moved to a fixed place on the screen. After all pictures are presented, the participant moves them into the originally presented sequence. Excellent test-retest reliability (0.77–0.84) has been reported, as well as evidence of convergent and discriminant validity (Dikmen et al., 2014).
NIH-TB-CB Pattern Comparison Processing Speed –
Participants view two visual patterns and press a button to indicate whether they are the same or not the same. Good test-retest reliability (0.73–0.74) has been demonstrated, as well as evidence of convergent and discriminant validity (Carlozzi et al., 2014).
NIH-TB-CB List Sorting –
\This test was designed to assess working memory. It entails sorting and sequencing visually and auditorily presented stimuli. There is evidence of convergent and discriminant validity, as well as excellent test-retest reliability with an overall value of 0.77 (Tulsky et al., 2014).
NIH-TB-CB Dimensional change card sort test –
This test is a measure of cognitive flexibility. Two target pictures are presented that vary along two dimensions (e.g., shape and color) and participants must match a series of pictures to the target pictures, first according to one dimension (e.g., color) and then according to the other dimension (e.g., shape). “Switch” trials are also employed, in which the participant must switch the dimension being matched. Excellent test-retest reliability (.81-.85) has been reported (Zelazo et al., 2014), as well as convergent and discriminant validity.
NIH-TB-CB Flanker Inhibitory control –
In this task, participants are required to indicate the left–right orientation of a centrally presented stimulus while inhibiting attention to the potentially incongruent stimuli that surround it. On some trials, the orientation of the flanking stimuli is congruent with the orientation of the central stimulus, and on others it is incongruent. Performance on the incongruent trials provides a measure of inhibitory control. This task has excellent test-retest reliability (.83-.85) as well as the expected convergent and discriminant validity (Zelazo et al., 2014).
Trail Making Test (TMT):
The TMT Part A was used to measure simple attention, and Part B was used to measure executive function (Reitan & Wolfson, 1994). Both parts of the TMT consist of 25 circles distributed across a sheet of paper. In Part A, the circles are numbered 1 – 25, and the participant is asked to draw lines to connect the numbers in ascending order. In Part B, the circles include both numbers and letters and the participant is asked to alternate between numbers and letters.
TMT Part B is known to be sensitive to a wide range of cognitive difficulties (Lamberty et al., 1994). Test-retest reliability is high for Trails A (0.83) and B (0.90) (DesRosiers & Kavanagh, 1987) in head injured patients. For intervals of 3 weeks to 1 year, test-retest reliability is moderate to high for Part A (r=0.36 to 0.79) and Part B (r=0.44 to 0.89) (Bornstein et al., 1987; Dikmen et al., 1999; Matarazzo et al., 1974). In terms of executive functioning, Part B is more associated with cognitive flexibility than set-shifting (Kortte et al., 2002).
Wechsler Adult Intelligence Scale 4th Edition (WAIS-IV):
All subtests of the widely used WAIS-IV have demonstrated good psychometric properties (Wechsler, D. et al., 2008).
Processing speed was measured by the WAIS-IV Processing Speed Index, a composite of the timed tasks of Symbol Search and Coding (Wechsler, D. et al., 2008). During the Symbol Search task, the participant decides whether either of the two target symbols match any of the symbols in a search group. For the Coding task, the participant is presented with a series of boxes and must draw the symbol that corresponds to the number in each box based on a coding legend presented at the top of the page.
Working memory was measured with the WAIS-IV Working Memory Index, a composite of the Digit Span and Letter-Number Sequencing tasks (Wechsler, D. et al., 2008). The Digit Span test consists of the examiner reading lists of numbers, and, based on instruction, the participant repeats the number sequence in the same order, backwards, or in ascending order. For Letter-Number Sequencing, the examiner reads a list of numbers and letters, and the participant recalls the list of numbers in ascending order and the letters in alphabetical order.
The WAIS-IV Visual Puzzles task was used to measure executive functioning / nonverbal reasoning. The participant views a puzzle and selects three options that, when combined, reconstruct the puzzle (Wechsler, D. et al., 2008).
Delis–Kaplan Executive Function System Verbal Fluency Test:
Verbal fluency was evaluated using the Letter Fluency and Category Fluency tasks (Delis et al., 2001), which assess the ability to generate words quickly according to specified rules. Per the manual, this subtest has adequate psychometric properties including internal consistency and stability. Both types of fluency have been found to be sensitive to mild TBI (Raskin & Rearick, 1996). A meta-analysis found that category fluency was more sensitive to lesions of the temporal lobes, while both letter and category fluency was associated with frontal lesions (Henry & Crawford, 2004).
Grooved Pegboard:
This widely used timed task was used to measure fine motor and dexterity skills linked to neurocognition (Klove, 1963). It has been found as a reliable source for testing manual dexterity. Retest reliability of this assessment is adequate (.72-.74) (Yancosek & Howell, 2009). Moderate/high associations have been reported with measures of attention (Schear & Sato, 1989; Strenge et al., 2002) and perceptual speed (Schear & Sato, 1989). Weak/modest associations have been noted between pegboard scores and daily functioning after head injury (Farmer & Eakman, 1995).
Performance Validity:
A review of performance validity testing and expert consensus panel in neuropsychology both recommend stand-alone and embedded performance validity indicators for cognitive testing (Lippa, 2018; Sweet et al., 2021). In the current study, performance validity was assessed by one stand-alone instrument, the Medical Symptom Validity Test (MSVT), applying the developer recommended cut-off scores (Green et al., 2011) and two embedded measures, the Reliable Digit Span from the WAIS-IV (Schroeder et al., 2012) and the Forced Choice Recognition trial from the CVLT-II (Woods et al., 2006). These measures of performance validity have been tested previously among an OEF/OIF/OND Veteran population with mild TBI (Clark et al., 2014). Participants were excluded from analyses if they scored below recommended cut-offs on any one of these three performance validity tests (PVTs).
Medications:
Lists of current medications were collected at the time of cognitive performance testing and categorized into pharmacologic classes. For the purposes of the current analyses, we grouped relevant medication classes into one of the following three major categories: antidepressant, cognitive-enhancing (Stimulant, Cholinesterase Inhibitor, NMDA Receptor Antagonist), and or potential cognitive impairing (Antipsychotic, Alpha Adrenergic Agonist, Anti-Epileptic Agent, Anticholinergic, Antihistamine, Barbiturate, Benzodiazepine, Beta-Adrenergic Blocker, Cannabinoid, Central Alpha 2 Adrenergic Agonist, Nonbenzodiazepine Anxiolytic, Nonbenzodiazepine Hypnotic, Opioid Agonist, and central acting Muscle Relaxant).
Candidate covariates
Based on our literature review and expert consensus among the author group, selected demographics and measures were included as candidate covariates that we hypothesized could potentially contribute to cognitive performance. These covariates also included population-specific variables of interest such as military status and branch.
Demographics:
Standardized demographic data was collected at baseline as queried in the Centers for Disease Control and Prevention-developed Behavioral Risk Factor Surveillance System (BRFSS) (Rolle-Lake & Robbins, 2020) and supplemented with military-specific information. We selected age, gender, race, ethnicity, education level, and marital status into the candidate covariates.
General intellectual functioning:
The NIHTB-CB Picture Vocabulary task score was used to estimate pre-exposure intellectual function. This task assesses auditory comprehension of single words with different levels of difficulty and measured with an auditory word-picture matching paradigm (Gershon et al., 2014). Excellent test-retest reliability (0.80) has been demonstrated, as well as evidence of convergent and discriminant validity.
Military Occupational:
Military status (current versus former), military service branch, total number of months on combat deployments, and combat intensity via section D of the Deployment Risk and Resiliency Inventory, Version 2 (DRRI-2) (Vogt et al., 2013). Additionally, during the lifetime TBI interview process, we queried the total number of controlled military-related blast exposures and for analyses classified as none, low (1–9), medium (10–89), or heavy (>89) because of heavily bimodal distribution at the floor and ceiling (99 or more).
Psychosocial:
The following were evaluated as covariates: PTSD status via the PTSD Checklist for the Diagnostic and Statistical Manual, 5th edition (PCL-5) with total score > 33 indicating clinically significant PTSD symptoms (Blevins et al., 2015); depression via the Patient Health Questionnaire-9, a widely used 9-item rating of current (prior two-week) depression symptoms with total scores ranging from 0 (None) to 27 (Severe) (Kroenke et al., 2001); illicit drug use via the Drug Abuse Screening Test 10 questionnaire (Yudko et al., 2007); alcohol consumption within last three months via the Alcohol Use Disorders Test Consumption questionnaire categorized as none, non-hazardous use, or hazardous use (Kuitunen-Paul & Roerecke, 2018); social support via section O of the DRRI-2.
Other Comorbidities:
Subjective sleep quality via the Pittsburgh Sleep Quality Index (Buysse et al., 1989); sleep apnea risk via the STOP-BANG questionnaire with high risk classified as STOP-BANG ≥ 3 (Chung et al., 2016); pain-related dysfunction via the TBI Quality of Life Pain Inhibition short form (Lange et al., 2016); neurobehavioral somatic symptoms using the Somatosensory (range: 0 to 28) and Vestibular (range: 0–12) sub-scales of the Neurobehavioral Symptom Inventory, where higher scores indicate higher severity (Meterko et al., 2012); self-reported history of Attention Deficit (Hyperactivity) Disorder (AD[H]D); and measured body mass index (BMI) were entered as relevant covariates.
Statistical methods:
To derive the final Z-scores for the seven cognitive domains of interest, Z-scores within the study sample were first calculated for all component neurocognitive tests using raw component test scores, their sample mean, and sample standard deviation. Subjects were included if at least one of the seven scores was non-missing. These component Z-scores were then averaged for each subject (i.e., sum of non-missing component Z-scores divided by number of non-missing component scores), following the definition of the domain, to generate the domain score of the subject. Pearson’s correlation coefficients were calculated pairwise between the seven domains to preliminarily assess their interrelationship.
Univariable linear regression models assessed differences in each of the cognitive domain outcomes across the three main mTBI groups of interest (non-mTBI control as the reference versus 1–2 and 3+ lifetime mTBIs). Similarly, we also ran seven sets of univariable regression models for each of the candidate covariates, which confirmed significant relationships for every selected covariate on at least one cognitive domain (results not shown). Given this, and rather than selecting a separate set of covariates for each cognitive domain outcome, we used the entire set of candidate covariates described earlier as a uniform adjustment in the multivariable models.
Multivariable linear regression was the primary approach for examining the conditional relationship between mTBI groups and each of the seven neurocognitive domain outcomes separately while adjusting for the covariates. Regression coefficients and 95% confidence intervals (CIs) were reported. The coefficient with leading “10×” means the interpretation of the coefficient is based on an increment of 10 times the original unit of the variable. In addition to the primary analysis focusing on the three mTBI groups, we also performed a sensitivity analysis replacing the three mTBI groups with the number of mTBIs as a continuous variable, and also with total number of blast-related mTBIs. A subset analysis was conducted among participants with mTBI history to study time since first and last mTBI events. We also compared medication use across TBI groups using chi-squared or Kruskal-Wallis test for categorical or skew continuous variable, respectively.
As we analyzed seven cognitive outcomes, we used Holm’s adjustment for multiple comparisons to adjust the p-values (Holm, 1979). The Holm’s method compares each p-value to a threshold of 0.05/(7-k), where k is the rank of the raw p-value among the 7 raw p-values. Since it is difficult to implement different significance thresholds across multiple predictors and outcomes, we instead reported “adjusted p-values” by multiplying each raw p-value by (7-k) to enable uniform comparisons with the single conventional 0.05 significance threshold. We reported both sets of p-values, raw (“p-values”) and after Holm’s adjustment (“adjusted p-values”). We focused on comparing adjusted p-values with 0.05 to determine statistical significance. Note that the 95% CIs reported with each coefficient were not adjusted for multiple comparisons.
We used the generalized variance inflation factor (GVIF) to assess multicollinearity among covariates in our multivariable model settings. Multicollinearity was considered tolerable if the GVIF was <2.4, which is equivalent to a conventional VIF<5 (Gareth James, Daniela Witten, Trevor Hastie, and Robert Tibshirani, 2014). All statistical analyses were implemented using R v. 4.0.3.(R: A Language and Environment for Statistical Computing., n.d.)
Transparency and Openness (TOP)
The study and its investigators adhered to the highest level of TOP guidelines in conducting and reporting this study.
Availability of data and material:
Data used in this study are available to the public through the Federal Interagency Brain Injury Research (FITBIR) Informatics System.
RESULTS
Participants:
The available sample included 1,551particiapants and of those, 241 failed at least one of the three PVTs and were excluded, resulting in a final analysis sample size of 1,310. Further exclusions based on missing data varied by outcome domain and are depicted in the consort diagram (Figure 1). Table 2 shows the demographic and clinical characteristics of the entire sample and each of the main mTBI groups of interest: TBI negative controls (no TBIs, n=256), non-repetitive mTBI (1–2 lifetime mTBIs; n=614), and repetitive mTBI (3+ mTBIs; n=440). Overall, the sample’s mean age at enrollment was 39.7 years and their mean time since index event was 9.7 years. Across TBI groups, time from index event ranged from 8.9 years for repetitive mTBI to 10.2 for controls. Time since index event for each TBI group is also presented graphically as box plots in Figure 2. Overall, the sample was diverse racially (27% non-White) and ethnically (17% Hispanic), was predominantly male (87%), educated with at least some college or technical school (86%), had an enlisted rank (84%), and served in the Army (65%). Regarding medications, there were no differences across TBI groups for being on an antidepressant (p=0.24) or a cognitive-enhancing medication (p=0.28). The repetitive mTBI group was receiving more medication classes that might compromise cognition (p=0.003), but the mean difference (0.2) and effect size (Cohen’s d=0.18) versus the TBI negative controls were very small.
Figure 1.
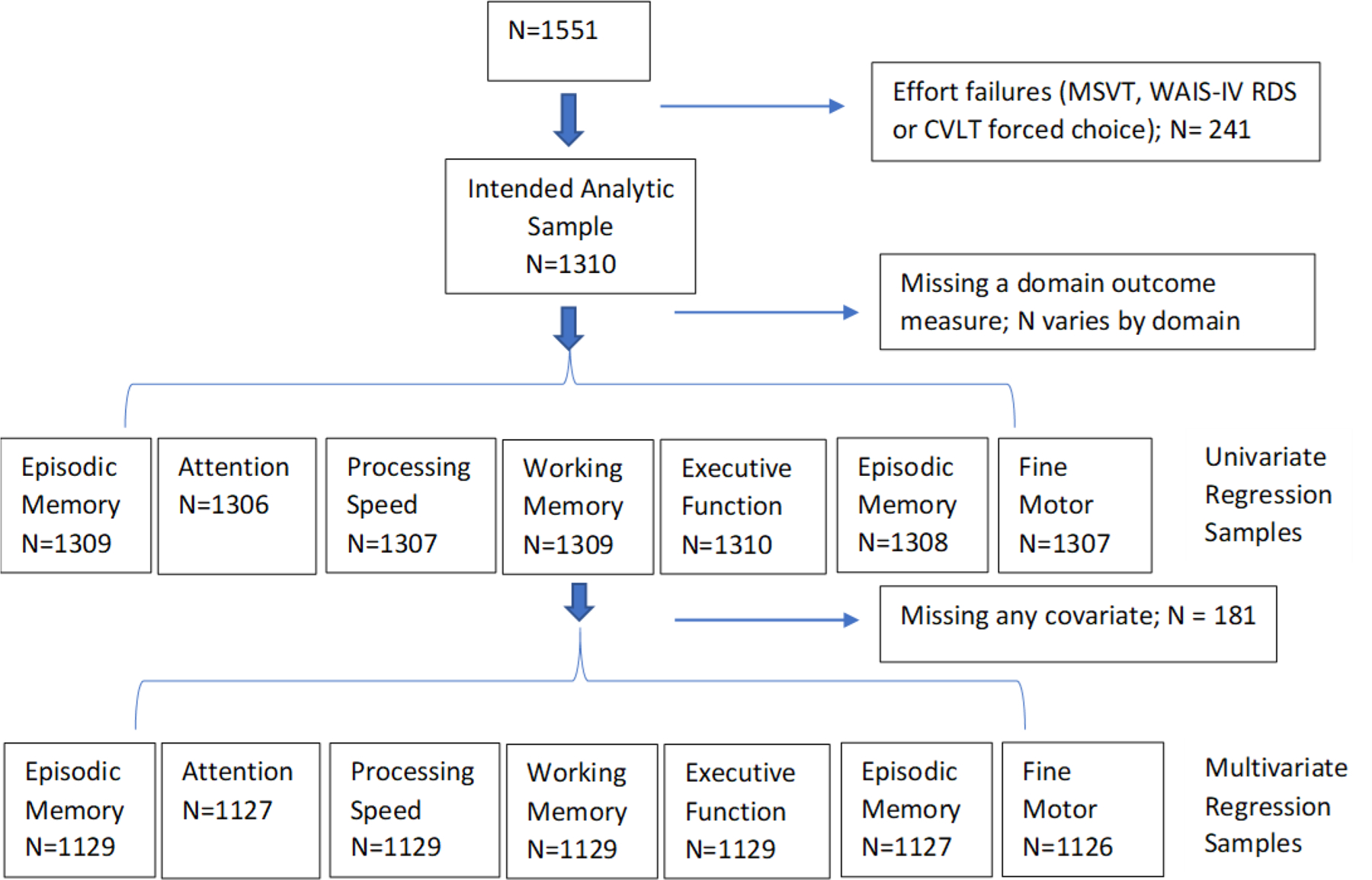
Consort Diagram
Abbreviations: MSVT= Medical Symptom Validity Test; WAIS-IV= Wechsler Adult Intelligence Scale 4th Edition; RDS= Reliable Digit Span CVLT-II = California Verbal Learning Test-II
Figure 2.
Years Since Index Event Abbreviation: mTBI = mild traumatic brain injury.
Cognitive performance across mTBI groups and their unadjusted comparisons
The mean Z-scores for the seven cognitive performance measures are shown in the top portion of Table 3 stratified by mTBI group (0, 1–2, 3+ lifetime mTBIs). The strength of correlation between the seven cognitive domains within our sample was generally in the ‘moderate’ range (Cohen, 1988), with values ranging from r=0.19 to r=0.49 (full results are available online in Supplemental Table S1). The bottom portion of Table 3 displays the results of the univariate regression models that compare the mTBI positive groups to the TBI negative controls. Neither mTBI positive group (repetitive, non-repetitive) was significantly different from the TBI negative control group for any of the respective cognitive domain Z-scores after applying the Holm’s correction for the seven comparisons (all adjusted p-values > 0.05). Coefficients for the comparison of 1–2 mTBIs to no TBIs ranged from −0.09 (Episodic Memory and Processing Speed) to 0.02 (Verbal Fluency). Coefficients for the comparison of 3+ mTBIs to no TBIs ranged from −0.17 (Fine Motor and Dexterity) to 0.18 (Verbal Fluency). For the non-repetitive TBI group (1–2 mTBIs) versus controls, adjusted p-values ranged from p=0.09 (Executive Function) to 1 (two domains). For repetitive mTBI (3+ mTBIs) versus controls, p-values ranged from p=0.06 (Verbal Fluency) to 1 (three domains).
Table 3.
Unadjusted Mean (SD) Z-scores for cognitive performance (top) with univariate linear regression models (bottom)
| No mTBI (N=256) |
1–2 mTBIs (N=614) |
3+ mTBIs (N=440) |
Missing | |||||
|---|---|---|---|---|---|---|---|---|
| Episodic Memory | 0.06 (0.77) | -0.03 (0.78) | 0.01 (0.76) | 1 | ||||
| Attention | 0.03 (0.99) | -0.05 (1.08) | 0.05 (0.88) | 4 | ||||
| Processing Speed | 0.09 (0.83) | 0.00 (0.81) | -0.06 (0.72) | 3 | ||||
| Working Memory | -0.03 (0.74) | -0.02 (0.78) | 0.04 (0.76) | 1 | ||||
| Executive Functioning | 0.06 (0.52) | -0.05 (0.63) | 0.04 (0.64) | 0 | ||||
| Verbal Fluency | -0.07 (0.86) | -0.05 (0.87) | 0.11 (0.88) | 2 | ||||
| Fine Motor & Dexterity | 0.13 (0.86) | -0.02 (0.96) | -0.04 (0.93) | 3 | ||||
|
Coefficient
(95% CI) |
p-value |
Adjusted
p-value |
Coefficient
(95% CI) |
p-value |
Adjusted
p-value |
N | ||
| Episodic Memory | - | -0.09 (-0.20,0.02) | 0.12 | 0.60 | -0.05 (-0.17,0.07) | 0.44 | 1 | 1309 |
| Attention | - | -0.08 (-0.22,0.07) | 0.31 | 0.93 | 0.02 (-0.14,0.17) | 0.83 | 1 | 1306 |
| Processing Speed | - | -0.09 (-0.20, 0.03) | 0.13 | 0.60 | -0.15 (-0.27,-0.03) | 0.015* | 0.09 | 1307 |
| Working Memory | - | 0.01 (-0.10,0.12) | 0.87 | 1 | 0.08 (-0.04,0.19) | 0.20 | 0.80 | 1309 |
| Executive Functioning | - | -0.11 (-0.20,-0.02) | 0.013* | 0.09 | -0.02 (-0.12, 0.07) | 0.65 | 1 | 1310 |
| Verbal Fluency | - | 0.02 (-0.11,0.15) | 0.74 | 1 | 0.18 ( 0.05,0.32) | 0.008* | 0.06 | 1308 |
| Fine Motor & Dexterity | - | -0.15 (-0.29,-0.02) | 0.028* | 0.17 | -0.17 (-0.32,-0.03) | 0.020* | 0.10 | 1307 |
* p-value < 0.05
Note: Higher positive values indicate better performance
Interpretation examples:
For the Executive Functioning outcome, those with 1–2 mTBIs have lower scores compared to those who have none, although this difference was not statistically significant after adjusting for multiple comparisons (coefficient = −0.11, 95% CI: −0.20,−0.02, p-value=0.013).
The “10 ×” is interpreted as “10 units increase” in DRRI-2, instead of the conventional “every 1 unit increase”. For example, when outcome is Fine Motor Dexterity, for every 10 units increase in DRRI-2, the domain scores decreased 0.04 unit (coefficient = −0.04, 95% CI: −0.07,−0.01, p-value=0.015).
Multivariable regression analyses for groups with 1–2 and 3+ lifetime mTBIs versus TBI negative
Table 4 displays the results of the multivariable regression analyses that compare the cognitive performance between the two TBI positive groups and the TBI negative controls while adjusting for covariates. Because of the use of seven cognitive domains for the primary outcome, Table 4 is divided into 4A and 4B. The top rows in both 4A and 4B show the findings for the regression coefficients and significance testing comparing mTBI groups, which is the independent variable of interest. Below that, the remainder of the rows in the table report coefficients and significant testing findings for the covariates.
Table 4A.
Multivariable linear regression models for Veterans and Service Members with repetitive and non-repetitive mTBI versus those with no TBI history
| Episodic Memory | Attention | Processing Speed | Working Memory | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Coefficient (95% CI) |
p-value | Coefficient (95% CI) |
p-value | Coefficient (95% CI) |
p-value | Coefficient (95% CI) |
p-value | ||||
| Raw | Adjust | Raw | Adjust | Raw | Adjust | Raw | Adjust | |||||
| mTBI Positive Groups versus TBI Negative Controls | ||||||||||||
| 1–2 mTBIs | −0.06 (−0.17, 0.05) | 0.26 | 1 | −0.09 (−0.24, 0.07) | 0.26 | 1 | −0.01 (−0.12, 0.10) | 0.90 | 1 | 0.02 (−0.08, 0.13) | 0.70 | 1 |
| 3+ mTBIs | −0.03 (−0.16, 0.09) | 0.60 | 1 | 0.04 (−0.14, 0.22) | 0.65 | 1 | −0.04 (−0.17, 0.09) | 0.54 | 1 | 0.02 (−0.10, 0.14) | 0.76 | 1 |
| Covariates | ||||||||||||
| Age | −0.03 (−0.03,−0.02) | <0.001 | 0.01 | −0.03 (−0.04, −0.02) | <0.001 | 0.01 | −0.03 (−0.04,−0.03) | <0.001 | 0.01 | −0.02 (−0.03,−0.02) | <0.001 | 0.01 |
| Gender: Male | −0.29 (−0.42,−0.15) | <0.001 | 0.01 | −0.11 (−0.30, 0.09) | 0.29 | 0.75 | −0.36 (−0.50,−0.22) | <0.001 | 0.01 | 0.10 (−0.03, 0.23) | 0.14 | 0.56 |
| Race/Ethnicity: Non-Hispanic White/Asian | 0.07 (−0.02, 0.16) | 0.15 | 0.52 | 0.17 ( 0.04, 0.30) | 0.013 | 0.06 | 0.07 (−0.02, 0.17) | 0.13 | 0.52 | 0.13 ( 0.04, 0.21) | 0.006 | 0.04 |
| Married:Married/couple | 0.07 (−0.03, 0.17) | 0.14 | 0.84 | 0.01 (−0.13, 0.15) | 0.91 | 1 | 0.09 (−0.02, 0.19) | 0.10 | 0.7 | 0.05 (−0.05, 0.15) | 0.31 | 1 |
| Never married | 0.04 (−0.09, 0.17) | 0.60 | 1 | −0.04 (−0.22, 0.14) | 0.65 | 1 | −0.04 (−0.17, 0.09) | 0.55 | 1 | 0.03 (−0.10, 0.15) | 0.68 | 1 |
|
Education: Some College |
−0.04 (−0.17, 0.08) | 0.52 | 0.69 | 0.11 (−0.07, 0.29) | 0.23 | 0.69 | 0.10 (−0.02, 0.23) | 0.11 | 0.55 | 0.24 ( 0.12, 0.36) | <0.001 | 0.01 |
| College Graduate | −0.02 (−0.15, 0.11) | 0.73 | 0.73 | 0.10 (−0.09, 0.28) | 0.29 | 0.58 | 0.21 ( 0.08, 0.34) | 0.002 | 0.01 | 0.21 ( 0.09, 0.34) | <0.001 | 0.01 |
| BMI | 0.00 (−0.01, 0.01) | 0.46 | 1 | 0.00 (−0.01, 0.01) | 0.76 | 1 | 0.00 (−0.01, 0.01) | 0.44 | 1 | 0.00 (−0.01, 0.01) | 0.98 | 1 |
| AD(H)D: Yes | −0.16 (−0.32,−0.01) | 0.038 | 0.27 | 0.05 (−0.16, 0.27) | 0.63 | 1 | −0.05 (−0.20, 0.10) | 0.52 | 1 | −0.05 (−0.19, 0.10) | 0.53 | 1 |
| Service Branch:Army | −0.02 (−0.13, 0.09) | 0.73 | 1 | −0.10 (−0.25, 0.06) | 0.22 | 1 | −0.03 (−0.14, 0.08) | 0.62 | 1 | −0.06 (−0.17, 0.04) | 0.22 | 1 |
| Marine Corps | −0.01 (−0.16, 0.13) | 0.85 | 1 | 0.11 (−0.09, 0.32) | 0.29 | 1 | 0.02 (−0.13, 0.16) | 0.81 | 1 | 0.00 (−0.14, 0.14) | 0.98 | 1 |
| Current Military:Yes | 0.10 ( 0.00, 0.21) | 0.06 | 0.36 | 0.09 (−0.06, 0.25) | 0.23 | 1 | 0.03 (−0.08, 0.13) | 0.65 | 1 | 0.13 ( 0.03, 0.23) | 0.015 | 0.1 |
| Deployed Time (yr) | −0.01 (−0.05, 0.04) | 0.76 | 1 | −0.01 (−0.07, 0.04) | 0.63 | 1 | 0.04 (−0.01, 0.08) | 0.09 | 0.63 | 0.02 (−0.02, 0.06) | 0.42 | 1 |
| Combat Intensity (10 × DRRI-2-C) | 0.00 (−0.03, 0.03) | 0.99 | 1 | 0.04 (−0.01, 0.09) | 0.10 | 0.7 | 0.01 (−0.02, 0.05) | 0.50 | 1 | −0.02 (−0.05, 0.01) | 0.27 | 1 |
| Depression (PHQ-9) | 0.00 (−0.01, 0.01) | 0.94 | 1 | −0.01 (−0.02, 0.01) | 0.42 | 1 | 0.00 (−0.01, 0.01) | 0.94 | 1 | 0.00 (−0.01, 0.01) | 0.86 | 1 |
| Pain (TBI-QOL) | 0.00 ( 0.00, 0.01) | 0.26 | 1 | 0.00 (−0.01, 0.01) | 0.51 | 1 | 0.00 ( 0.00, 0.01) | 0.27 | 1 | 0.00 (−0.01, 0.00) | 0.37 | 1 |
| Estimate Intellect (NIHTB-CB pic vocab) | 0.02 ( 0.02, 0.03) | <0.001 | 0.01 | 0.01 ( 0.00, 0.02) | 0.007 | 0.01 | 0.01 ( 0.01, 0.02) | <0.001 | 0.01 | 0.03 ( 0.02, 0.03) | <0.001 | 0.01 |
| PTSD (PCL-5) | 0.00 (−0.01, 0.00) | 0.033 | 0.2 | 0.00 (−0.01, 0.00) | 0.11 | 0.44 | 0.00 (−0.01, 0.00) | 0.22 | 0.63 | 0.00 (−0.01, 0.00) | 0.041 | 0.21 |
| Hazardous Alcohol Use (AUDIT-C): Yes, non-hazardous | −0.03 (−0.12, 0.06) | 0.54 | 1 | −0.01 (−0.14, 0.11) | 0.83 | 1 | −0.03 (−0.12, 0.06) | 0.51 | 1 | 0.03 (−0.06, 0.12) | 0.48 | 1 |
| Not user | −0.10 (−0.22, 0.02) | 0.09 | 0.54 | 0.00 (−0.17, 0.17) | 0.97 | 1 | −0.11 (−0.23, 0.01) | 0.07 | 0.49 | 0.03 (−0.09, 0.14) | 0.62 | 1 |
| Sleep Problems (PSQI) | 0.01 (−0.01, 0.02) | 0.41 | 1 | 0.00 (−0.01, 0.02) | 0.60 | 1 | −0.01 (−0.02, 0.01) | 0.39 | 1 | 0.01 ( 0.00, 0.02) | 0.023 | 0.16 |
| Sleep Apnea High Risk (STOP-BANG) | −0.08 (−0.19, 0.02) | 0.12 | 0.84 | 0.06 (−0.09, 0.21) | 0.43 | 1 | −0.06 (−0.17, 0.04) | 0.24 | 1 | −0.04 (−0.14, 0.06) | 0.43 | 1 |
| Controlled Blast Exposure: Low (1–9) | −0.01 (−0.15, 0.14) | 0.93 | 1 | 0.08 (−0.12, 0.28) | 0.44 | 1 | 0.00 (−0.14, 0.15) | 0.95 | 1 | −0.04 (−0.18, 0.10) | 0.61 | 1 |
| Medium (10–89) | −0.03 (−0.17, 0.11) | 0.67 | 1 | −0.04 (−0.24, 0.16) | 0.72 | 1 | −0.03 (−0.17, 0.11) | 0.68 | 1 | −0.02 (−0.16, 0.11) | 0.76 | 1 |
| Heavy (>89) | −0.04 (−0.19, 0.10) | 0.56 | 1 | −0.03 (−0.23, 0.17) | 0.76 | 1 | 0.03 (−0.12, 0.17) | 0.73 | 1 | −0.09 (−0.22, 0.05) | 0.23 | 1 |
|
Somatosensory Symptoms (NSI) |
0.00 (−0.01, 0.02) | 0.92 | 1 | 0.00 (−0.02, 0.02) | 0.88 | 1 | 0.00 (−0.02, 0.01) | 0.54 | 1 | −0.01 (−0.02, 0.01) | 0.36 | 1 |
| Vestibular Symptoms (NSI) | −0.01 (−0.04, 0.02) | 0.36 | 1 | −0.01 (−0.05, 0.03) | 0.54 | 1 | −0.02 (−0.05, 0.01) | 0.11 | 0.66 | −0.01 (−0.04, 0.02) | 0.55 | 1 |
Notes: Bolded covariates are significant after P < 0.05 corrected for multiple comparisons. Number of observations used to fit model, in order, are: 1129,1127,1129,1129
Abbreviations: ADHD = Attention Deficit Hyperactivity Disorder; AUDIT-C= Alcohol Use Disorders Test Consumption; DAST-10= Drug Abuse Screening Test- 10; DRRI-2= Deployment Risk and Resiliency Inventory, Version 2; NIH TB-CB = NIH Toolbox Cognition battery; NSI= Neurobehavioral Symptom Inventory PCL-5= PTSD Checklist for the Diagnostic and Statistical Manual, 5th edition; PSQI= Pittsburgh Sleep Quality Index; PHQ-9=Patient Health Questionnaire-9; TBI QoL= Traumatic Brain Injury Quality of Life.
Table 4B.
Multivariable linear regression models for repetitive and non-repetitive mTBI versus TBI negative (continued)
| Executive Function | Verbal Fluency | Fine Motor & Dexterity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| variable | Coefficient (95% CI) |
p-value | Coefficient (95% CI) |
p-value | Coefficient (95% CI) |
p-value | |||
| Raw | Adjust | Raw | Adjust | Raw | Adjust | ||||
| mTBI Positive Groups versus TBI Negative Controls | |||||||||
| 1–2 mTBIs | −0.08 (−0.16, 0.00) | 0.06 | 0.42 | 0.05 (−0.08, 0.18) | 0.42 | 1 | −0.02 (−0.16, 0.12) | 0.77 | 1 |
| 3+ mTBIs | −0.04 (−0.14, 0.05) | 0.39 | 1 | 0.17 ( 0.03, 0.32) | 0.022 | 0.15 | 0.01 (−0.15, 0.16) | 0.95 | 1 |
| Covariates | |||||||||
| Age | −0.02 (−0.02,−0.01) | <0.001 | 0.01 | −0.01 (−0.02,−0.01) | <0.001 | 0.01 | −0.03 (−0.04,−0.02) | <0.001 | 0.01 |
| Gender: Male | −0.06 (−0.17, 0.04) | 0.25 | 0.75 | 0.04 (−0.13, 0.20) | 0.67 | 0.75 | −0.27 (−0.44,−0.10) | 0.002 | 0.01 |
| Race/Ethnicity: Non−Hispanic White/Asian | 0.15 ( 0.08, 0.22) | <0.001 | 0.01 | 0.00 (−0.11, 0.11) | 0.94 | 0.94 | 0.05 (−0.07, 0.16) | 0.41 | 0.82 |
| Married: Married/couple | 0.01 (−0.07, 0.09) | 0.82 | 1 | −0.05 (−0.17, 0.07) | 0.38 | 1 | −0.05 (−0.17, 0.08) | 0.47 | 1 |
| Never married | −0.05 (−0.15, 0.05) | 0.34 | 1 | 0.01 (−0.14, 0.16) | 0.90 | 1 | −0.19 (−0.35,−0.03) | 0.022 | 0.15 |
| Education: Some College | 0.07 (−0.03, 0.17) | 0.16 | 0.64 | 0.07 (−0.08, 0.22) | 0.34 | 0.69 | 0.21 ( 0.06, 0.37) | 0.007 | 0.04 |
| College Graduate | 0.10 ( 0.00, 0.20) | 0.06 | 0.18 | 0.22 ( 0.06, 0.37) | 0.007 | 0.03 | 0.37 ( 0.21, 0.54) | <0.001 | 0.01 |
| BMI | 0.00 (−0.01, 0.00) | 0.24 | 1 | 0.01 ( 0.00, 0.02) | 0.06 | 0.36 | −0.01 (−0.03, 0.00) | 0.007 | 0.05 |
| ADD: Yes | −0.10 (−0.22, 0.01) | 0.09 | 0.54 | −0.04 (−0.22, 0.14) | 0.66 | 1 | 0.15 (−0.04, 0.34) | 0.12 | 0.6 |
| Service Branch:Army | −0.04 (−0.12, 0.04) | 0.34 | 1 | −0.08 (−0.21, 0.04) | 0.20 | 1 | −0.02 (−0.15, 0.11) | 0.76 | 1 |
| Marine Corps | −0.05 (−0.16, 0.06) | 0.38 | 1 | −0.09 (−0.27, 0.08) | 0.28 | 1 | −0.02 (−0.20, 0.16) | 0.79 | 1 |
| Current Military:Yes | 0.01 (−0.07, 0.09) | 0.82 | 1 | 0.06 (−0.07, 0.19) | 0.37 | 1 | 0.00 (−0.14, 0.13) | 0.97 | 1 |
| Deployed Time (yr) | 0.00 (−0.03, 0.03) | 0.88 | 1 | 0.01 (−0.04, 0.06) | 0.60 | 1 | −0.01 (−0.06, 0.04) | 0.74 | 1 |
| Combat Intensity (10 × DRRI−2-C) | 0.02 (−0.01, 0.04) | 0.21 | 1 | 0.01 (−0.03, 0.05) | 0.50 | 1 | −0.02 (−0.06, 0.02) | 0.31 | 1 |
| Depression (PHQ9) | −0.01 (−0.02, 0.00) | 0.16 | 1 | 0.00 (−0.02, 0.01) | 0.95 | 1 | 0.00 (−0.02, 0.01) | 0.72 | 1 |
| Pain (TBIQOL) | 0.00 ( 0.00, 0.01) | 0.71 | 1 | 0.00 (−0.01, 0.01) | 0.74 | 1 | 0.00 ( 0.00, 0.01) | 0.22 | 1 |
| Estimate Intellect (NIHTB-CB pic vocab) | 0.02 ( 0.01, 0.02) | <0.001 | 0.01 | 0.03 ( 0.03, 0.04) | <0.001 | 0.01 | 0.01 ( 0.01, 0.02) | <0.001 | 0.01 |
| PTSD (PCL5) | 0.00 (−0.01, 0.00) | 0.27 | 0.63 | 0.00 (−0.01, 0.00) | 0.21 | 0.63 | −0.01 (−0.01, 0.00) | 0.026 | 0.18 |
| ETOH: Yes, non-hazardous | −0.05 (−0.12, 0.02) | 0.18 | 1 | 0.00 (−0.11, 0.10) | 0.95 | 1 | 0.01 (−0.10, 0.12) | 0.85 | 1 |
| Not user | −0.07 (−0.16, 0.02) | 0.13 | 0.65 | −0.03 (−0.17, 0.12) | 0.73 | 1 | −0.04 (−0.19, 0.11) | 0.61 | 1 |
| Sleep Problems (PSQI) | 0.00 (−0.01, 0.01) | 0.73 | 1 | 0.00 (−0.02, 0.01) | 0.74 | 1 | 0.00 (−0.02, 0.01) | 0.96 | 1 |
| Sleep Apnea High Risk (STOP−BANG) | 0.04 (−0.04, 0.12) | 0.30 | 1 | −0.04 (−0.16, 0.09) | 0.57 | 1 | −0.03 (−0.16, 0.10) | 0.62 | 1 |
| Controlled Blast Exposure: Low (1−9) | 0.01 (−0.10, 0.12) | 0.91 | 1 | −0.12 (−0.29, 0.05) | 0.17 | 1 | −0.07 (−0.25, 0.11) | 0.44 | 1 |
| Medium (10–89) | 0.00 (−0.11, 0.11) | 0.96 | 1 | −0.29 (−0.46,−0.12) | <0.001 | 0.01 | 0.03 (−0.14, 0.21) | 0.70 | 1 |
| Heavy (>89) | −0.02 (−0.12, 0.09) | 0.79 | 1 | −0.18 (−0.35,−0.01) | 0.038 | 0.27 | −0.12 (−0.29, 0.06) | 0.20 | 1 |
|
Somatosensory Symptoms (NSI) |
−0.01 (−0.02, 0.00) | 0.07 | 0.49 | 0.00 (−0.02, 0.02) | 0.96 | 1 | 0.00 (−0.02, 0.01) | 0.65 | 1 |
| Vestibular Symptoms (NSI) | 0.00 (−0.02, 0.02) | 0.88 | 1 | −0.03 (−0.06, 0.01) | 0.14 | 0.7 | −0.05 (−0.09,−0.02) | 0.003 | 0.02 |
Notes: Bolded covariates are significant after P < 0.05 corrected for multiple comparisons.
Number of observations used to fit model, in order, are: 1129,1127,1129,1129
Abbreviations: ADHD = Attention Deficit Hyperactivity Disorder; AUDIT-C= Alcohol Use Disorders Test Consumption; DAST-10= Drug Abuse Screening Test- 10; DRRI-2= Deployment Risk and Resiliency Inventory, Version 2; NIH TB-CB = NIH Toolbox Cognition battery; NSI= Neurobehavioral Symptom Inventory; PCL-5= PTSD Checklist for the Diagnostic and Statistical Manual, 5th edition; PSQI= Pittsburgh Sleep Quality Index; PHQ-9=Patient Health Questionnaire-9; TBI QoL= Traumatic Brain Injury Quality of Life;
Neither of the mTBI positive groups, 1–2 lifetime mTBIs (non-repetitive) or 3+ lifetime mTBIs (repetitive), were significantly different from the mTBI negative control group on any cognitive outcome after adjusting for covariates and multiple comparisons. Coefficients for the comparison of 1–2 mTBIs to no TBIs ranged from −0.09 (Attention) to 0.05 (Verbal Fluency) and all adjusted p-values were 1 except for Executive Function (p=0.42). Coefficients for the comparison of 3+ mTBIs to no TBIs ranged from −0.04 (Processing Speed and Executive Function) to 0.17 (Verbal Fluency) and the only adjusted p-value under 1 was Verbal Fluency (adjusted p=0.15). There was no presence of multicollinearity in any model (all GVIF < 2.4, full results available on-line in supplementary Table S2). Covariates with significant findings after adjusting for multiple comparisons (adjusted p<0.05) were age, sex, race, education, estimated pre-exposure intellectual function, controlled blast, and vestibular symptoms. Age and estimated intellect were consistently related across all outcomes (adjusted p=0.01). Females performed better than males on three domains: episodic memory, processing speed, and fine motor and dexterity.
Sensitivity analyses for continuous number of mTBIs, number of blast-related mTBIs, and time since first and last mTBI.
The multivariable models described above were repeated in sensitivity analyses replacing the 3 group mTBI categorical variable with the number of mTBIs as a continuous variable (Table S3) and separately by total number of blast-related mTBIs (Table S4). We also conducted a sensitivity analysis repeating the above multivariable regression models within the mTBI subgroup, comparing 1–2 mTBIs versus 3+ mTBIs and also controlling for time since first and time since last mTBI as additional covariates (Table S6A and S6B). Similar to the primary analysis, none of these sensitivity analyses showed significant relationships of mTBIs with any of the seven cognitive domain outcomes after adjustment for multiple comparisons, with all of the adjusted p-value for mTBIs above 0.05 even before adjustment except for total number of blast-related mTBIs on Verbal Fluency (raw p=0.028; adjusted p=0.2) and repetitive versus non-repetitive mTBI on Verbal Fluency (raw p=0.015; adjusted p=0.1). For all of these additional models, all GVIF values were below 2.4, indicating no evidence of multicollinearity (Table S5).
DISCUSSION
This study examined whether remote mTBI(s) were associated with lower cognitive functioning relative to a negative mTBI history. Using cross-sectional analyses of a large, well-characterized sample of combat-exposed Veterans and SMs and objective neuropsychological measures, we found no differences in group cognitive performance for either those with non-repetitive (1–2 lifetime) or repetitive (3+ lifetime) mTBIs versus TBI negative controls. Additional sensitivity analyses also showed non-significance for all other TBI explanators examined, namely number of mTBIs as a continuous variable, number of blast-related mTBIs, and years since first and last mTBI. These findings align with prior studies and provide some of the strongest evidence to date that in unselected mTBI cases, lower cognitive performance does not chronically persist relative to controls (Belanger et al., 2005; Binder et al., 1997; Frencham et al., 2005; Karr et al., 2014; Rohling et al., 2011; Schretlen & Shapiro, 2003). Although not part of our primary analyses, our negative findings for blast-related mTBI also provide strong evidence against blast-related mTBI having a unique deleterious chronic effect on cognition. Clinically, our findings demonstrate that the average Veteran or SM who experiences one or more mTBIs should not expect to have chronically lingering challenges with cognitive ability due to mTBI alone.
Of all the covariates examined in our models, only age and estimated pre-exposure intellectual function were consistently associated with statistically significant differences across all seven cognitive domains (all adjusted p=0.01). The strong association with lower scores as age increased was expected given our use of raw scores rather than population-normed scores to calculate the sample-based Z-scores. As also expected, higher estimated pre-exposure intellectual function (NIHTB-CB Picture Vocabulary test) was related to higher scores on other cognitive measures, and education level had a significant effect on four domains. Additionally, females scored better than males on measures of episodic memory, processing speed, and fine motor skills. These sex-related findings are consistent with the literature in the general population, especially for episodic memory (Asperholm et al., 2019) and processing speed (Camarata & Woodcock, 2006).
While providing an encouraging message to Veterans, SMs, and providers, these negative findings do not rule out the potential for a vulnerable subset of individuals who do experience chronic objective cognitive changes after mTBI. Future study is recommended to assess rates of cognitive impairment using thresholds of cognitive performance as a function of TBI group membership while adjusting for other factors. If present, that might be better elucidated with a within-subjects, before-after, longitudinal study design. Additionally, our cross-sectional design did not directly address the question of greater cognitive decline over time from neurodegeneration or other delayed mTBI manifestation. We plan to test both of these hypotheses in future LIMBIC-CENC PLS analyses as we continue to reassess the cohort longitudinally and examine any within-subjects changes over time, including those who may sustain a new mTBI.
The physical and mental health symptom measure covariates showed less significance in the models than anticipated, but results were consistent with smaller studies that also measured performance validity (Wisdom et al., 2014). A separate, concurrent LIMBIC-CENC analysis is focusing more specifically on the interaction between PTSD and cognitive performance and may yield discrete findings. Vestibular symptoms on the NSI had a significant effect on fine motor scores, with higher self-reported symptom levels associated with poorer performance. This finding raises the question of how much this task (i.e., completion times on the Grooved Pegboard test) relates to neurocognition versus vestibular or motor control problems of any etiology. The lack of stronger evidence for significance of other symptom measures such as pain, sleep, and depression may in part be explained by our exclusion of PVT failures. Model overfitting may be another explanation (see further below).
The current study’s findings should be considered together with the literature at large to best judge clinical implications. In comparison to TBI negative controls, individuals with remote mTBI history, especially those with repetitive mTBI, are well-documented to have greater self-reported cognitive difficulties (Walker, Hirsch, et al., 2018). Our new findings add to mounting evidence that most individuals with remote mTBI(s) do not have objective cognitive deficits that might explain their self-reported cognitive difficulties (Soble et al., 2013). This highlights the importance of a holistic treatment approach to include stress management techniques and treatment of other commonly comorbid conditions associated with cognitive symptoms such as PTSD, pain, insomnia, and depression. Providers should acknowledge, address and show empathy for patients’ postconcussive experiences. However, it is important that providers do not suggest to their patients that mTBI results in long term, severe cognitive deficits; in some cases, it is possible that a focus on the TBI diagnosis may be unhelpful or even counter-productive (e.g., if fear of mTBI-related cognitive deficits prevents engagement in safe and healthy activities, or discourages other evidence-based treatment for comorbidities such as PTSD). For patients endorsing persisting problems with cognitive functioning, cognitive rehabilitation is still considered the standard of care, but it is crucial to focus on compensatory strategies and other behavioral training rather than on improving objective cognitive functioning deficits which are not as likely as self-reported cognitive symptoms (i.e., perceived cognitive deficits) (Cooper et al., 2017). Psychotherapy also seems well-suited to address stress-related symptoms and provide reinforcement for use of cognitive compensatory strategies (Cooper et al., 2015). Notwithstanding the lower than expected association of symptom measures to objective cognitive performance in the current study, addressing modifiable conditions such as depression/anxiety, PTSD, insomnia/sleep disorders, and pain would likely have indirect benefit, if not direct, on subjective cognitive symptoms and possibly objective cognitive performance as well.
The clinical implications of this study’s findings have heightened importance because of the ubiquity of stories on concussion in the news and popular press indicating concussions likely will lead to a deteriorating course of cognitive and behavioral difficulties, which has created unique challenges for healthcare providers, particularly neuropsychologists who perform cognitive evaluations, in their efforts to treat those with a history of concussion(s) (Ahmed & Hall, 2017; Baugh et al., 2017, 2021). As has been empirically demonstrated, beliefs, attributions and expectations may adversely impact recovery from concussion (Ozen & Fernandes, 2011). As a provider, it is extremely difficult to counter the effects of the media with empirically-balanced information, such as that found in this study and many others. This is thought to occur because people associate concussion with negative outcomes and therefore experience a type of “diagnosis threat” (Suhr & Gunstad, 2002). From a clinical perspective, it is important to note that attributional styles are mutable and can be successfully addressed (Peters et al., 2011) . However, the traditional model of neuropsychological assessment and subsequent feedback, which may provide reassurance in other clinical populations, often is ineffective in individuals with tightly held beliefs/attributions about concussion. Cross-disciplinary, behavioral approaches that draw upon elements of treatment for other chronic conditions might best serve any patients who may have negative beliefs and sequalae from concussion(s). For example, Belanger et al. (Belanger et al., 2020) advocate for a three-phased approach including: assessment and education, targeted interventions directed at specific symptoms and comorbidities, and psychotherapy to address any mental health issues. Future research is needed to test such approaches in longitudinal studies.
Future research should also consider repetitive low-level exposures as it relates to cognition. If there is cumulative brain damage from repetitive, sub-concussive, low-level blast exposures as evidence from other evaluation techniques suggests (Belding et al., 2021; Goldstein et al., 2012; Stone et al., 2020; Tate et al., 2013), it was not apparent on our cognitive testing. Using our original one-item measure, the only significant reduction was for verbal fluency in those with a medium level (10–89) of controlled blast exposures compared to none, but it was not significant for the high level (90+) group. The LIMBIC-CENC PLS has added a questionnaire on athletic head impact exposures and a consensus-developed questionnaire for cumulative military blast exposures for which information is still being collected on enrolled participants. Future research incorporating these instruments may provide additional insights.
Key strengths of this study include the very large and ethnically and racially diverse sample, the inclusion of mTBI-negative but deployed controls, and the exclusion of cases with profiles of invalid testing. Our study has the largest sample size to date with comprehensive neurocognitive testing in Veterans and SMs with blast-related mTBI. This is important because extant literature has demonstrated inconsistent findings on the enduring deleterious effects of blast-related mTBI versus blunt mTBI including on cognition (Belanger et al., 2009; Martindale et al., 2020). The use of dissimilar controls and/or inclusion of cases with diminished or feigned effort are both important sources of potential bias toward positive associations between mTBI and cognitive decline. Notably, our mTBI negative controls were recruited through the same processes as the mTBI positive participants which provided a relevant control group with similar background characteristics and experiences. For assuring valid performance effort, we utilized an aggressive approach by excluding any failures of either a gold-standard stand-alone test or either of two additional well-accepted embedded measures. Another strength was the a-priori designation of seven cognitive domain composite scores for the dependent variable and appropriately adjusting for Type 1 error. Due to the contrasting constructs of various neurocognitive processes, single composite scores have pitfalls for both validity and sensitivity. On the other hand, single cognitive test scores are prone to reliability concerns and comparing an excessive number of cognitive test scores increases risk of Type 1 error. The large sample size also permitted us to analyze and statistically adjust for the many covariates that might influence the relationship between remote mTBI history and cognitive performance. Finally, the use of measures recommended as NIH NINDS TBI common data elements allows other studies to compare their results to ours (Hicks et al., 2013; Report Viewer | NINDS Common Data Elements, n.d.)
There are some limitations to this study. Foremost is the cross-sectional design, which precludes understanding of later cognitive changes which may be due to TBI or other comorbidities as well as subtle changes that may be related to other aspects of deployment. The lack of difference in cognitive performance may be, in part, related to benefit gained from treatment since our participants had access to specialized brain injury rehabilitation services in the Military and/or Veterans healthcare system. Additionally, although we used a validated structured interview method with layers of quality assurance, the retrospective identification of historical mTBI is prone to recall bias. Although our use of pre-selected cognitive domain composite scores sharpened our scientific rigor, it is possible we may have not selected the most sensitive measures to capture cognitive effects. The relationships between the seven domains in our sample showed ‘moderate’ correlations, suggesting that further collapsing of domains may be possible. While our consideration of multiple potential confounders is a strength, regression models are increasingly prone to overfitting as the number of covariates increases (Babyak, 2004). It seems unlikely that overfitting contributed to our null findings given that there were also no significant differences in preliminary univariable analyses comparing mTBI groups on the cognitive outcomes. We also did not implement any automated covariate selection methods in constructing our multivariable models.
Constraints on Generalizability:
Given the LIMBIC-CENC research mission, our study sample consists entirely of current and former military SMs who often sustained mTBI during combat deployment and/or blast-related mTBI, so the findings may not generalize well to civilians. Because most of our recruitment/enrollment sites are based at VA medical facilities, these findings may not generalize to sizeable population of former SMs who receive medical care entirely at non-VA medical centers. Our study sample, which mirrored the broader U.S. military population in gender make up, was predominantly male, so our findings may also not generalize well to women.
Conclusion:
This study found no evidence that remote mTBI(s) influence objective cognitive performance among SMs and Veterans with combat exposure history who passed performance validity measures. This suggests that self-reported concerns about cognitive difficulties in persons in the chronic phase following mTBI are likely best treated with a holistic approach that considers common comorbid conditions and situational factors that could be associated with longstanding cognitive complaints. Strategies for addressing misattribution may also be beneficial and further research on behavioral interventions for patients with tightly held negative beliefs on the impact of their remote concussions is also recommended. Given the limitations of our cross-sectional design and between-subjects comparison, future study with the LIMBIC-CENC PLS cohort will be performed using longitudinal design to assess within-subjects changes and before-after data for participants with incipient mTBI as well as assessing rates of cognitive impairment.
Supplementary Material
Table 2.
Characteristics of Sample and mTBI groups.
| Variable | Type/Level | Total N=1310 | No mTBI: N=256 | 1–2 mTBIs: N=614 | 3+ mTBIs: N=440 | Missing |
|---|---|---|---|---|---|---|
| Age | [mean (SD)] Years | 39.7 (9.7) | 39.6 (10.2) | 39.3 (10.0) | 40.3 (8.9) | 0 |
| Index Event Date | [mean (SD)] Years since | 9.9 (4.9) | 10.1 (5.4) | 9.7 (5.0) | 10.3 (4.4) | 0 |
| Gender | Female | 169 (13%) | 58 (23%) | 78 (13%) | 33 (8%) | 0 |
| Male | 1141 (87%) | 198 (77%) | 536 (87%) | 407 (92%) | ||
| Race | American Indian or Alaska Native | 8 (1%) | 2 (1%) | 2 (0%) | 4 (1%) | 0 |
| Asian | 22 (2%) | 9 (4%) | 6 (1%) | 7 (2%) | ||
| Black or African American | 242 (18%) | 61 (24%) | 127 (21%) | 54 (12%) | ||
| Pacific Islander | 10 (1%) | 2 (1%) | 3 (0%) | 5 (1%) | ||
| White | 953 (73%) | 175 (68%) | 437 (71%) | 341 (78%) | ||
| Other | 59 (5%) | 4 (2%) | 31 (5%) | 24 (5%) | ||
| Don’t know/Not sure/Refused | 16 (1%) | 3 (1%) | 8 (1%) | 5 (1%) | ||
| Ethnicity | Hispanic or Latino | 229 (17%) | 49 (19%) | 115 (19%) | 65 (15%) | 0 |
| Not Hispanic or Latino | 1066 (81%) | 205 (80%) | 495 (81%) | 366 (83%) | ||
| Don’t know/Not sure/Refused | 15 (1%) | 2 (1%) | 4 (1%) | 9 (2%) | ||
| Race/Ethnicity Dichotomized | White or Asian Non-Hispanic | 801 (62%) | 146 (57%) | 359 (59%) | 296 (69%) | 21 |
| All others (e.g. Black, Hispanic) | 488 (38%) | 108 (43%) | 247 (41%) | 133 (31%) | ||
| Married | Divorced/Separated/Widowed | 315 (24%) | 64 (25%) | 160 (26%) | 91 (21%) | 1 |
| Married/Couple | 768 (59%) | 143 (56%) | 344 (56%) | 281 (64%) | ||
| Never married | 226 (17%) | 49 (19%) | 110 (18%) | 67 (15%) | ||
| Education | Any High school, no college | 184 (14%) | 37 (14%) | 102 (17%) | 45 (10%) | 0 |
| Some college or technical school | 533 (41%) | 98 (38%) | 246 (40%) | 189 (43%) | ||
| College (4+ years) graduate | 593 (45%) | 121 (47%) | 266 (43%) | 206 (47%) | ||
| BMI | [mean (SD)] | 29.9 (5.1) | 29.7 (5.5) | 30.0 (5.1) | 29.9 (5.0) | 11 |
| AD(H)D history | No | 1200 (92%) | 240 (94%) | 565 (92%) | 395 (90%) | 6 |
| Yes | 104 (8%) | 14 (6%) | 47 (8%) | 43 (10%) | ||
| Military Rank | Enlisted | 1099 (84%) | 213 (84%) | 529 (86%) | 357 (81%) | 4 |
| Officer | 207 (16%) | 42 (16%) | 83 (14%) | 82 (19%) | ||
| Service Branch | Army | 856 (65%) | 162 (63%) | 402 (65%) | 292 (66%) | 0 |
| Marine Corps | 192 (15%) | 27 (11%) | 97 (16%) | 68 (15%) | ||
| all others | 262 (20%) | 67 (26%) | 115 (19%) | 80 (18%) | ||
| Military Status | Veteran | 1068 (82%) | 220 (86%) | 520 (85%) | 328 (75%) | 3 |
| Active/Reserve | 239 (18%) | 35 (14%) | 93 (15%) | 111 (25%) | ||
| Deployed Time | [mean (SD)] Months | 19.7 (13.2) | 17.4 (11.2) | 18.8 (12.6) | 22.2 (14.7) | 27 |
| Combat Intensity | [mean (SD)] DRRI-2-D | 37.7 (15.3) | 30.2 (12.3) | 37.9 (15.3) | 41.9 (15.4) | 4 |
| Social Support | [mean (SD)] DRRI-2-S | 38.6 (8.6) | 39.6 (8.5) | 38.5 (8.6) | 38.1 (8.6) | 0 |
| Depression | [mean (SD)] PHQ-9 | 7.7 (5.9) | 5.8 (5.5) | 7.9 (6.1) | 8.7 (5.8) | 15 |
| Pain | [mean (SD)] TBI-QoL Pain Intensity | 21.7 (10.4) | 17.5 (9.1) | 21.9 (10.5) | 24.0 (10.4) | 69 |
| Pre-exposure Intellectual Function Estimate | [mean (SD)] NIH-TB-CB Picture Vocabulary | 108.5 (8.2) | 107.4 (8.7) | 107.7 (8.2) | 110.3 (7.6) | 54 |
| PTSD symptoms | [mean (SD)] PCL-5 | 25.5 (18.9) | 18.6 (17.0) | 26.1 (19.4) | 28.7 (18.3) | 14 |
| Alcohol use (Audit-C) (past three months) | User at Hazardous level | 474 (36%) | 79 (31%) | 221 (36%) | 174 (40%) | 7 |
| User Non-hazardous level | 603 (46%) | 136 (53%) | 269 (44%) | 198 (45%) | ||
| Abstinent | 226 (17%) | 40 (16%) | 120 (20%) | 66 (15%) | ||
| Drug Use (DAST-10) (past year) | None | 1093 (84%) | 217 (85%) | 508 (83%) | 368 (84%) | 7 |
| Yes | 210 (16%) | 37 (15%) | 103 (17%) | 70 (16%) | ||
| Sleep Quality | [mean (SD)] PSQI | 9.8 (4.6) | 8.2 (4.6) | 9.7 (4.5) | 10.7 (4.3) | 32 |
| Sleep Apnea (High Risk on STOP-BANG) | No | 498 (39%) | 130 (52%) | 232 (39%) | 136 (32%) | 34 |
| Yes | 778 (61%) | 122 (48%) | 366 (61%) | 290 (68%) | ||
| Controlled Blast Exposures | None (0) | 497 (38%) | 136 (53%) | 231 (38%) | 130 (30%) | 0 |
| Low (1–9) | 338 (26%) | 52 (20%) | 171 (28%) | 115 (26%) | ||
| Medium (10–89) | 320 (24%) | 46 (18%) | 144 (23%) | 130 (30%) | ||
| Heavy (>89) | 497 (38%) | 136 (53%) | 231 (38%) | 130 (30%) | ||
| Somatosensory Symptoms | [mean (SD)] NSI SS Domain | 6.1 (4.7) | 3.9 (4.0) | 6.0 (4.6) | 7.5 (4.8) | 9 |
| Vestibular Symptoms | [mean (SD)] NSI V Domain | 2.0 (2.1) | 0.9 (1.4) | 1.9 (2.1) | 2.6 (2.3) | 7 |
Abbreviations: ADHD = Attention Deficit Hyperactivity Disorder; AUDIT-C= Alcohol Use Disorders Test Consumption; DAST-10= Drug Abuse Screening Test- 10;
DRRI-2= Deployment Risk and Resiliency Inventory, Version 2; NIH TB-CB = NIH Toolbox Cognition battery; NSI= Neurobehavioral Symptom Inventory;
PCL-5= PTSD Checklist for the Diagnostic and Statistical Manual, 5th edition; PSQI= Pittsburgh Sleep Quality Index; PHQ-9=Patient Health Questionnaire-9;
TBI QoL= Traumatic Brain Injury Quality of Life.
Key Points:
Question:
Is cognitive performance altered long-term after mild traumatic brain injury (mTBI) relative to non-TBI controls?
Findings:
Among combat-exposed Veterans and Service Members (SMs), neither of the mTBI positive groups, non-repetitive (1–2) or repetitive (>3), differed from the TBI negative controls on any cognitive testing domain when adjusting for other factors.
Importance:
Remote mTBI alone, even if repetitive, does not cause objective cognitive problems in the average Veteran or SM. A holistic healthcare approach including comorbidity assessment is indicated for patients reporting chronic cognitive difficulties after mTBI(s), and strategies for addressing misattribution may be beneficial.
Next steps:
Future study is recommended with longitudinal designs to assess any potential within-subjects decline from possible neurodegeneration.
ACKNOWLEDGEMENTS:
Funding:
This work was supported by the Assistant Secretary of Defense for Health Affairs endorsed by the Department of Defense, through the Psychological Health/Traumatic Brain Injury Research Program Long-Term Impact of Military-Relevant Brain Injury Consortium (LIMBIC) Award/W81XWH-18-PH/TBIRP-LIMBIC under Awards No. W81XWH1920067 and W81XWH-13-2-0095, and by the U.S. Department of Veterans Affairs Awards No. I01 CX002097, I01 CX002096, I01 HX003155, I01 RX003444, I01 RX003443, I01 RX003442, I01 CX001135, I01 CX001246, I01 RX001774, I01 RX 001135, I01 RX 002076, I01 RX 001880, I01 RX 002172, I01 RX 002173, I01 RX 002171, I01 RX 002174, and I01 RX 002170, I01 CX001820. The U.S. Army Medical Research Acquisition Activity, 839 Chandler Street, Fort Detrick MD 21702–5014 is the awarding and administering acquisition office. Additional funding was provided by VA Health Services Research and Development (IK6HX002608). This investigation was also supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR002538 (formerly 5UL1TR001067-05, 8UL1TR000105 and UL1RR025764).
Footnotes
Conflicts of interest/Competing interests: The authors have no conflicts of interest to disclose.
Disclaimer: The views, opinions, interpretations, conclusions and recommendations expressed in this manuscript are those of the authors and do not reflect the official policy of the Department of the Navy, Department of the Army, Department of Defense, Department of Veterans Affairs or the U.S. Government.
Ethics approval: This study was approved by the local Institutional Review Boards at all eleven prospective longitudinal study enrollment sites.
Consent to participate: All study participants signed informed consent document prior to undergoing study procedures.
Consent for publication: Consent form signed by all participants included consent for publication of their deidentified data.
Availability of data and material: Available to public in the Federal Interagency Brain Injury Research (FITBIR) Informatics System.
Code availability: not applicable
Contributor Information
William C. Walker, Dept. of Physical Medicine and Rehabilitation (PM&R), School of Medicine, Virginia Commonwealth University, Richmond, VA. PM&R Service, Richmond Veterans Affairs Medical Center (VAMC)
Maya E O’Neil, VA Portland Health Care System; Dept. of Psychiatry and Dept. of Medical Informatics & Clinical Epidemiology, Oregon Health and Science University, Portland, OR..
Zhining Ou, Division of Epidemiology, Department of Internal Medicine, School of Medicine, University of Utah. Salt Lake City, UT
Terri K. Pogoda, Center for Healthcare Organization and Implementation Research, VA Boston Healthcare System, Boston, MA; Department of Health Law, Policy & Management, Boston University School of Public Health, Boston, MA.
Heather G. Belanger, Special Operations Command (SOCOM), Surgeon General’s Office, St Michael’s Inc, Departments of Psychology, and Psychiatry and Behavioral Neurosciences, University of South Florida, James A Haley Veterans Hospital Research Service, Tampa, FL
Randall S. Scheibel, Michael E. DeBakey Veterans Affairs Medical Center, Houston, TX; H. Ben Taub Department of Physical Medicine and Rehabilitation, Baylor College of Medicine, Houston, TX
Angela P. Presson, Division of Epidemiology, Department of Internal Medicine, School of Medicine, University of Utah. Salt Lake City, UT
Shannon R. Miles, Mental Health and Behavioral Sciences Service, James A Haley Veterans’ Hospital, Tampa, FL; Psychiatry and Behavioral Neurosciences, Morsani College of Medicine, University of South Florida, Tampa, FL
Elisabeth A. Wilde, George E. Wahlen Veterans Affairs Salt Lake City Healthcare System; Department of Neurology, University of Utah, Salt Lake City, UT; H. Ben Taub Department of Physical Medicine and Rehabilitation, Baylor College of Medicine, Houston, TX.
David F. Tate, George E. Wahlen Veterans Affairs Salt Lake City Healthcare System; Department of Neurology, University of Utah, Salt Lake City, UT;.
Maya Troyanskaya, H. Ben Taub Department of Physical Medicine and Rehabilitation, Baylor College of Medicine. Houston, TX.; Michael E. DeBakey Veterans Affairs Medical Center Houston, TX..
Mary Jo Pugh, George E. Wahlen Veterans Affairs Salt Lake City Healthcare System; Department of Medicine, University of Utah, Salt Lake City, UT.
Amy Jak, VA San Diego Healthcare System; University of California, San Diego, Department of Psychiatry, San Diego, CA
David X. Cifu, Department of Physical Medicine and Rehabilitation (PM&R), School of Medicine, Virginia Commonwealth University, Richmond, VA; PM&R Service, Richmond Veterans Affairs Medical Center (VAMC), Richmond, VA.
REFERENCES
- Ahmed OH, & Hall EE (2017). “It was only a mild concussion”: Exploring the description of sports concussion in online news articles. Physical Therapy in Sport: Official Journal of the Association of Chartered Physiotherapists in Sports Medicine, 23, 7–13. 10.1016/j.ptsp.2016.07.003 [DOI] [PubMed] [Google Scholar]
- American Congress of Rehabilitation Medicine, M. T. B. I. C. (1993). Definition of mild traumatic brain injury. J Head Trauma Rehabil, 8, 86–87. [Google Scholar]
- American Psychiatric Association, A. P., & Association, A. P. (2013). Diagnostic and statistical manual of mental disorders: DSM-5 Washington, DC: American Psychiatric Association. [Google Scholar]
- Anderson JFI, & Jordan AS (2021). An observational study of the association between sleep disturbance, fatigue and cognition in the post-acute period after mild traumatic brain injury in prospectively studied premorbidly healthy adults. Neuropsychological Rehabilitation, 31(9), 1444–1465. 10.1080/09602011.2020.1781665 [DOI] [PubMed] [Google Scholar]
- Armistead-Jehle P, Cooper DB, & Vanderploeg RD (2016). The role of performance validity tests in the assessment of cognitive functioning after military concussion: A replication and extension. Applied Neuropsychology. Adult, 23(4), 264–273. 10.1080/23279095.2015.1055564 [DOI] [PubMed] [Google Scholar]
- Asperholm M, Högman N, Rafi J, & Herlitz A (2019). What did you do yesterday? A meta-analysis of sex differences in episodic memory. Psychological Bulletin, 145(8), 785–821. 10.1037/bul0000197 [DOI] [PubMed] [Google Scholar]
- Babyak MA (2004). What you see may not be what you get: A brief, nontechnical introduction to overfitting in regression-type models. Psychosomatic Medicine, 66(3), 411–421. 10.1097/01.psy.0000127692.23278.a9 [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Morganti JM, Bodnar CN, Webster SJ, Higgins EK, Roberts KN, Snider H, Meier SE, Nation GK, Goulding DS, Hamm M, Powell DK, Vandsburger M, Van Eldik LJ, & Abisambra JF (2020). The effects of mild closed head injuries on tauopathy and cognitive deficits in rodents: Primary results in wild type and rTg4510 mice, and a systematic review. Experimental Neurology, 326, 113180. 10.1016/j.expneurol.2020.113180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker-Collo S, Jones K, Theadom A, Starkey N, Dowell A, McPherson K, Ameratunga S, Dudley M, Te Ao B, Feigin V, & BIONIC Research Group. (2015). Neuropsychological outcome and its correlates in the first year after adult mild traumatic brain injury: A population-based New Zealand study. Brain Injury, 29(13–14), 1604–1616. 10.3109/02699052.2015.1075143 [DOI] [PubMed] [Google Scholar]
- Baugh CM, Gedlaman MA, Daneshvar DH, & Kroshus E (2021). Factors Influencing College Football Players’ Beliefs About Incurring Football-Related Dementia. Orthopaedic Journal of Sports Medicine, 9(4), 23259671211001130. 10.1177/23259671211001129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh CM, Kroshus E, Kiernan PT, Mendel D, & Meehan WP (2017). Football Players’ Perceptions of Future Risk of Concussion and Concussion-Related Health Outcomes. Journal of Neurotrauma, 34(4), 790–797. 10.1089/neu.2016.4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger HG, Curtiss G, Demery JA, Lebowitz BK, & Vanderploeg RD (2005). Factors moderating neuropsychological outcomes following mild traumatic brain injury: A meta-analysis. Journal of the International Neuropsychological Society: JINS, 11(3), 215–227. 10.1017/S1355617705050277 [DOI] [PubMed] [Google Scholar]
- Belanger HG, Kretzmer T, Yoash-Gantz R, Pickett T, & Tupler LA (2009). Cognitive sequelae of blast-related versus other mechanisms of brain trauma. Journal of the International Neuropsychological Society: JINS, 15(1), 1–8. 10.1017/S1355617708090036 [DOI] [PubMed] [Google Scholar]
- Belanger HG, Spiegel E, & Vanderploeg RD (2010). Neuropsychological performance following a history of multiple self-reported concussions: A meta-analysis. Journal of the International Neuropsychological Society: JINS, 16(2), 262–267. 10.1017/S1355617709991287 [DOI] [PubMed] [Google Scholar]
- Belanger HG, Wortzel HS, Vanderploeg RD, & Cooper DB (2020). A model for intervening with veterans and service members who are concerned about developing Chronic Traumatic Encephalopathy (CTE). The Clinical Neuropsychologist, 34(6), 1105–1123. 10.1080/13854046.2019.1699166 [DOI] [PubMed] [Google Scholar]
- Belding JN, Englert RM, Fitzmaurice S, Jackson JR, Koenig HG, Hunter MA, Thomsen CJ, & da Silva UO (2021). Potential Health and Performance Effects of High-Level and Low-Level Blast: A Scoping Review of Two Decades of Research. Frontiers in Neurology, 12, 628782. 10.3389/fneur.2021.628782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict R (1997). Brief Visuospatial Memory Test-Revised. In Psychological Assessment Resources 10.1037/1040-3590.8.2.145 [DOI]
- Benedict RHB, Schretlen D, Groninger L, Dobraski M, & Shpritz B (1996). Revision of the Brief Visuospatial Memory Test: Studies of normal performance, reliability, and validity. Psychological Assessment, 8(2), 145–153. 10.1037/1040-3590.8.2.145 [DOI] [Google Scholar]
- Binder LM, Rohling ML, & Larrabee GJ (1997). A review of mild head trauma. Part I: Meta-analytic review of neuropsychological studies. Journal of Clinical and Experimental Neuropsychology, 19(3), 421–431. 10.1080/01688639708403870 [DOI] [PubMed] [Google Scholar]
- Blevins CA, Weathers FW, Davis MT, Witte TK, & Domino JL (2015). The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation. Journal of Traumatic Stress, 28(6), 489–498. 10.1002/jts.22059 [DOI] [PubMed] [Google Scholar]
- Bornstein RA, Baker GB, & Douglass AB (1987). Short-term retest reliability of the Halstead-Reitan Battery in a normal sample. The Journal of Nervous and Mental Disease, 175(4), 229–232. 10.1097/00005053-198704000-00007 [DOI] [PubMed] [Google Scholar]
- Brenner LA, Vanderploeg RD, & Terrio H (2009). Assessment and diagnosis of mild traumatic brain injury, posttraumatic stress disorder, and other polytrauma conditions: Burden of adversity hypothesis. Rehabilitation Psychology, 54(3), 239–246. 10.1037/a0016908 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Camarata S, & Woodcock R (2006). Sex differences in processing speed: Developmental effects in males and females. Intelligence, 34(3), 231–252. 10.1016/j.intell.2005.12.001 [DOI] [Google Scholar]
- Carlozzi NE, Tulsky DS, Chiaravalloti ND, Beaumont JL, Weintraub S, Conway K, & Gershon RC (2014). NIH Toolbox Cognitive Battery (NIHTB-CB): The NIHTB Pattern Comparison Processing Speed Test. Journal of the International Neuropsychological Society: JINS, 20(6), 630–641. 10.1017/S1355617714000319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung F, Abdullah HR, & Liao P (2016). STOP-Bang Questionnaire: A Practical Approach to Screen for Obstructive Sleep Apnea. Chest, 149(3), 631–638. 10.1378/chest.15-0903 [DOI] [PubMed] [Google Scholar]
- Cifu DX, Taylor BC, Carne WF, Bidelspach D, Sayer NA, Scholten J, & Campbell EH (2013). Traumatic brain injury, posttraumatic stress disorder, and pain diagnoses in OIF/OEF/OND Veterans. Journal of Rehabilitation Research and Development, 50(9), 1169–1176. 10.1682/JRRD.2013.01.0006 [DOI] [PubMed] [Google Scholar]
- Clark AL, Amick MM, Fortier C, Milberg WP, & McGlinchey RE (2014). Poor performance validity predicts clinical characteristics and cognitive test performance of OEF/OIF/OND Veterans in a research setting. The Clinical Neuropsychologist, 28(5), 802–825. 10.1080/13854046.2014.904928 [DOI] [PubMed] [Google Scholar]
- Cnossen MC, Winkler EA, Yue JK, Okonkwo DO, Valadka AB, Steyerberg EW, Lingsma HF, Manley GT, & TRACK-TBI Investigators. (2017). Development of a Prediction Model for Post-Concussive Symptoms following Mild Traumatic Brain Injury: A TRACK-TBI Pilot Study. Journal of Neurotrauma, 34(16), 2396–2409. 10.1089/neu.2016.4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Set Correlation and Contingency Tables. Applied Psychological Measurement, 12(4), 425–434. 10.1177/014662168801200410 [DOI] [Google Scholar]
- Cooper DB, Bowles AO, Kennedy JE, Curtiss G, French LM, Tate DF, & Vanderploeg RD (2017). Cognitive Rehabilitation for Military Service Members With Mild Traumatic Brain Injury: A Randomized Clinical Trial. The Journal of Head Trauma Rehabilitation, 32(3), E1–E15. 10.1097/HTR.0000000000000254 [DOI] [PubMed] [Google Scholar]
- Cooper DB, Bunner AE, Kennedy JE, Balldin V, Tate DF, Eapen BC, & Jaramillo CA (2015). Treatment of persistent post-concussive symptoms after mild traumatic brain injury: A systematic review of cognitive rehabilitation and behavioral health interventions in military service members and veterans. Brain Imaging and Behavior, 9(3), 403–420. 10.1007/s11682-015-9440-2 [DOI] [PubMed] [Google Scholar]
- Cooper DB, Curtiss G, Armistead-Jehle P, Belanger HG, Tate DF, Reid M, Bowles AO, Velez CS, Kennedy JE, & Vanderploeg RD (2018). Neuropsychological Performance and Subjective Symptom Reporting in Military Service Members With a History of Multiple Concussions: Comparison With a Single Concussion, Posttraumatic Stress Disorder, and Orthopedic Trauma. The Journal of Head Trauma Rehabilitation, 33(2), 81–90. 10.1097/HTR.0000000000000375 [DOI] [PubMed] [Google Scholar]
- Corrigan JD, & Bogner J (2007). Initial reliability and validity of the Ohio State University TBI Identification Method. The Journal of Head Trauma Rehabilitation, 22(6), 318–329. 10.1097/01.HTR.0000300227.67748.77 [DOI] [PubMed] [Google Scholar]
- Defense and Veterans Brain Injury Center. (2019). DoD Worldwide Numbers for TBI http://dvbic.dcoe.mil/dod-worldwide-numbers-tbi
- Delis DC, Kaplan E, & Kramer JH (2001). Delis-Kaplan executive function system. Pearson. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, & Ober BA (2000). California Verbal Learning Test (2nd ed.). Psychological Corporation. [Google Scholar]
- DesRosiers G, & Kavanagh D (1987). Cognitive assessment in closed head injury: Stability, validity and parallel forms for two neuropsychological measures of recovery. International Journal of Clinical Neuropsychology, 9(4), 162–173. [Google Scholar]
- Dikmen SS, Bauer PJ, Weintraub S, Mungas D, Slotkin J, Beaumont JL, Gershon R, Temkin NR, & Heaton RK (2014). Measuring episodic memory across the lifespan: NIH Toolbox Picture Sequence Memory Test. Journal of the International Neuropsychological Society: JINS, 20(6), 611–619. 10.1017/S1355617714000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikmen SS, Corrigan JD, Levin HS, Machamer J, Stiers W, & Weisskopf MG (2009). Cognitive outcome following traumatic brain injury. The Journal of Head Trauma Rehabilitation, 24(6), 430–438. 10.1097/HTR.0b013e3181c133e9 [DOI] [PubMed] [Google Scholar]
- Dikmen SS, Heaton RK, Grant I, & Temkin NR (1999). Test-retest reliability and practice effects of expanded Halstead-Reitan Neuropsychological Test Battery. Journal of the International Neuropsychological Society: JINS, 5(4), 346–356. [PubMed] [Google Scholar]
- Donnelly KT, Donnelly JP, Dunnam M, Warner GC, Kittleson CJ, Constance JE, Bradshaw CB, & Alt M (2011). Reliability, sensitivity, and specificity of the VA traumatic brain injury screening tool. The Journal of Head Trauma Rehabilitation, 26(6), 439–453. 10.1097/HTR.0b013e3182005de3 [DOI] [PubMed] [Google Scholar]
- Farmer JE, & Eakman AM (1995). The relationship between neuropsychological functioning and instrumental activities of daily living following acquired brain injury. Applied Neuropsychology, 2(3–4), 107–115. 10.1080/09084282.1995.9645347 [DOI] [PubMed] [Google Scholar]
- Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, & Morin CM (2012). Insomnia and daytime cognitive performance: A meta-analysis. Sleep Medicine Reviews, 16(1), 83–94. 10.1016/j.smrv.2011.03.008 [DOI] [PubMed] [Google Scholar]
- Frencham KAR, Fox AM, & Maybery MT (2005). Neuropsychological studies of mild traumatic brain injury: A meta-analytic review of research since 1995. Journal of Clinical and Experimental Neuropsychology, 27(3), 334–351. 10.1080/13803390490520328 [DOI] [PubMed] [Google Scholar]
- James Gareth, Witten Daniela, Hastie Trevor, and Tibshirani Robert. (2014). An Introduction to Statistical Learning: With Applications in R Springer Publishing Company, Incorporated. [Google Scholar]
- Gershon RC, Cook KF, Mungas D, Manly JJ, Slotkin J, Beaumont JL, & Weintraub S (2014). Language measures of the NIH Toolbox Cognition Battery. Journal of the International Neuropsychological Society: JINS, 20(6), 642–651. 10.1017/S1355617714000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LE, Fisher AM, Tagge CA, Zhang X-L, Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, Goletiani CJ, Maglakelidze GM, Casey N, Moncaster JA, Minaeva O, Moir RD, Nowinski CJ, Stern RA, Cantu RC, … McKee AC (2012). Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Science Translational Medicine, 4(134), 134ra60. 10.1126/scitranslmed.3003716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P, Montijo J, & Brockhaus R (2011). High specificity of the Word Memory Test and Medical Symptom Validity Test in groups with severe verbal memory impairment. Applied Neuropsychology, 18(2), 86–94. 10.1080/09084282.2010.523389 [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, McCrea M, Marshall SW, Cantu RC, Randolph C, Barr W, Onate JA, & Kelly JP (2003). Cumulative effects associated with recurrent concussion in collegiate football players: The NCAA Concussion Study. JAMA, 290(19), 2549–2555. 10.1001/jama.290.19.2549 [DOI] [PubMed] [Google Scholar]
- Hendricks AM, Amara J, Baker E, Charns MP, Gardner JA, Iverson KM, Kimerling R, Krengel M, Meterko M, Pogoda TK, Stolzmann KL, & Lew HL (2013). Screening for mild traumatic brain injury in OEF-OIF deployed US military: An empirical assessment of VHA’s experience. Brain Injury, 27(2), 125–134. 10.3109/02699052.2012.729284 [DOI] [PubMed] [Google Scholar]
- Henry JD, & Crawford JR (2004). A meta-analytic review of verbal fluency performance in patients with traumatic brain injury. Neuropsychology, 18(4), 621–628. 10.1037/0894-4105.18.4.621 [DOI] [PubMed] [Google Scholar]
- Hicks R, Giacino J, Harrison-Felix C, Manley G, Valadka A, & Wilde EA (2013). Progress in developing common data elements for traumatic brain injury research: Version two--the end of the beginning. Journal of Neurotrauma, 30(22), 1852–1861. 10.1089/neu.2013.2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S (1979). A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics, 6(2), 65–70. [Google Scholar]
- Iverson GL (2006). Misdiagnosis of the persistent postconcussion syndrome in patients with depression. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 21(4), 303–310. 10.1016/j.acn.2005.12.008 [DOI] [PubMed] [Google Scholar]
- Iverson GL (2019). Network Analysis and Precision Rehabilitation for the Post-concussion Syndrome. Frontiers in Neurology, 10, 489. 10.3389/fneur.2019.00489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr JE, Areshenkoff CN, & Garcia-Barrera MA (2014). The neuropsychological outcomes of concussion: A systematic review of meta-analyses on the cognitive sequelae of mild traumatic brain injury. Neuropsychology, 28(3), 321–336. 10.1037/neu0000037 [DOI] [PubMed] [Google Scholar]
- Kenney K, Qu B-X, Lai C, Devoto C, Motamedi V, Walker WC, Levin HS, Nolen T, Wilde EA, Diaz-Arrastia R, Gill J, & Investigators, the C. M. O. S. (2018). Higher exosomal phosphorylated tau and total tau among veterans with combat-related repetitive chronic mild traumatic brain injury. Brain Injury, 32(10), 1276–1284. 10.1080/02699052.2018.1483530 [DOI] [PubMed] [Google Scholar]
- Khalid S, & Tubbs RS (2017). Neuroanatomy and Neuropsychology of Pain. Cureus, 9(10), e1754. 10.7759/cureus.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klove H (1963). Grooved Pegboard Test Lafayette, IN: Lafayette Instrument. [Google Scholar]
- Kortte KB, Horner MD, & Windham WK (2002). The trail making test, part B: Cognitive flexibility or ability to maintain set? Applied Neuropsychology, 9(2), 106–109. 10.1207/S15324826AN0902_5 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuitunen-Paul S, & Roerecke M (2018). Alcohol Use Disorders Identification Test (AUDIT) and mortality risk: A systematic review and meta-analysis. Journal of Epidemiology and Community Health, 72(9), 856–863. 10.1136/jech-2017-210078 [DOI] [PubMed] [Google Scholar]
- Lagacé-Legendre C, Boucher V, Robert S, Tardif P-A, Ouellet M-C, de Guise E, Boulard G, Frémont P, Émond M, Moore L, & Le Sage N (2021). Persistent Postconcussion Symptoms: An Expert Consensus-Based Definition Using the Delphi Method. The Journal of Head Trauma Rehabilitation, 36(2), 96–102. 10.1097/HTR.0000000000000613 [DOI] [PubMed] [Google Scholar]
- Lamberty GJ, Putnam SH, Chatel DM, Bieliauskas LA, & et al. (1994). Derived Trail Making Test indices: A preliminary report. Neuropsychiatry, Neuropsychology, & Behavioral Neurology, 7(3), 230–234. [Google Scholar]
- Lange RT, Brickell TA, Bailie JM, Tulsky DS, & French LM (2016). Clinical Utility and Psychometric Properties of the Traumatic Brain Injury Quality of Life Scale (TBI-QOL) in US Military Service Members. The Journal of Head Trauma Rehabilitation, 31(1), 62–78. 10.1097/HTR.0000000000000149 [DOI] [PubMed] [Google Scholar]
- Lippa SM (2018). Performance validity testing in neuropsychology: A clinical guide, critical review, and update on a rapidly evolving literature. The Clinical Neuropsychologist, 32(3), 391–421. 10.1080/13854046.2017.1406146 [DOI] [PubMed] [Google Scholar]
- Mac Donald CL, Barber J, Jordan M, Johnson AM, Dikmen S, Fann JR, & Temkin N (2017). Early Clinical Predictors of 5-Year Outcome After Concussive Blast Traumatic Brain Injury. JAMA Neurology, 74(7), 821–829. 10.1001/jamaneurol.2017.0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macciocchi SN, Barth JT, Littlefield L, & Cantu RC (2001). Multiple Concussions and Neuropsychological Functioning in Collegiate Football Players. Journal of Athletic Training, 36(3), 303–306. [PMC free article] [PubMed] [Google Scholar]
- Management of Concussion/mTBI Working Group. (2009). VA/DoD Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. Journal of Rehabilitation Research and Development, 46(6), CP1–68. [PubMed] [Google Scholar]
- Manley G, Gardner AJ, Schneider KJ, Guskiewicz KM, Bailes J, Cantu RC, Castellani RJ, Turner M, Jordan BD, Randolph C, Dvořák J, Hayden KA, Tator CH, McCrory P, & Iverson GL (2017). A systematic review of potential long-term effects of sport-related concussion. British Journal of Sports Medicine, 51(12), 969–977. 10.1136/bjsports-2017-097791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale SL, Ord AS, & Rowland JA (2020). Influence of blast exposure on cognitive functioning in combat veterans. Neuropsychology 10.1037/neu0000672 [DOI] [PMC free article] [PubMed]
- Matarazzo JD, Wiens AN, Matarazzo RG, & Goldstein SG (1974). Psychometric and clinical test-retest reliability of the Halstead impairment index in a sample of healthy, young, normal men. The Journal of Nervous and Mental Disease, 158(1), 37–49. 10.1097/00005053-197401000-00006 [DOI] [PubMed] [Google Scholar]
- Meterko M, Baker E, Stolzmann KL, Hendricks AM, Cicerone KD, & Lew HL (2012). Psychometric assessment of the Neurobehavioral Symptom Inventory-22: The structure of persistent postconcussive symptoms following deployment-related mild traumatic brain injury among veterans. The Journal of Head Trauma Rehabilitation, 27(1), 55–62. 10.1097/HTR.0b013e318230fb17 [DOI] [PubMed] [Google Scholar]
- Miller KJ, Ivins BJ, & Schwab KA (2013). Self-reported mild TBI and postconcussive symptoms in a peacetime active duty military population: Effect of multiple TBI history versus single mild TBI. The Journal of Head Trauma Rehabilitation, 28(1), 31–38. 10.1097/HTR.0b013e318255ceae [DOI] [PubMed] [Google Scholar]
- O’Neil ME, Carlson KF, Storzbach D, Brenner LA, Freeman M, Quiñones AR, Motu’apuaka M, & Kansagara D (2014). Factors associated with mild traumatic brain injury in veterans and military personnel: A systematic review. Journal of the International Neuropsychological Society: JINS, 20(3), 249–261. 10.1017/S1355617714000204 [DOI] [PubMed] [Google Scholar]
- Otis JD, McGlinchey R, Vasterling JJ, & Kerns RD (2011). Complicating factors associated with mild traumatic brain injury: Impact on pain and posttraumatic stress disorder treatment. Journal of Clinical Psychology in Medical Settings, 18(2), 145–154. 10.1007/s10880-011-9239-2 [DOI] [PubMed] [Google Scholar]
- Ozen LJ, & Fernandes MA (2011). Effects of “diagnosis threat” on cognitive and affective functioning long after mild head injury. Journal of the International Neuropsychological Society: JINS, 17(2), 219–229. 10.1017/S135561771000144X [DOI] [PubMed] [Google Scholar]
- Peters KD, Constans JI, & Mathews A (2011). Experimental modification of attribution processes. Journal of Abnormal Psychology, 120(1), 168–173. 10.1037/a0021899 [DOI] [PubMed] [Google Scholar]
- Ponsford J, Draper K, & Schönberger M (2008). Functional outcome 10 years after traumatic brain injury: Its relationship with demographic, injury severity, and cognitive and emotional status. Journal of the International Neuropsychological Society: JINS, 14(2), 233–242. 10.1017/S1355617708080272 [DOI] [PubMed] [Google Scholar]
- Ponsford JL, Parcell DL, Sinclair KL, Roper M, & Rajaratnam SMW (2013). Changes in sleep patterns following traumatic brain injury: A controlled study. Neurorehabilitation and Neural Repair, 27(7), 613–621. 10.1177/1545968313481283 [DOI] [PubMed] [Google Scholar]
- Porter KE, Stein MB, Martis B, Avallone KM, McSweeney LB, Smith ER, Simon NM, Gargan S, Liberzon I, Hoge CW, & Rauch SAM (2018). Postconcussive symptoms (PCS) following combat-related traumatic brain injury (TBI) in Veterans with posttraumatic stress disorder (PTSD): Influence of TBI, PTSD, and depression on symptoms measured by the Neurobehavioral Symptom Inventory (NSI). Journal of Psychiatric Research, 102, 8–13. 10.1016/j.jpsychires.2018.03.004 [DOI] [PubMed] [Google Scholar]
- R: A language and environment for statistical computing. (n.d.). Foundation for Statistical Computing. https://www.R-project.org/
- Raskin SA, & Rearick E (1996). Verbal fluency in individuals with mild traumatic brain injury. Neuropsychology, 10(3), 416–422. 10.1037/0894-4105.10.3.416 [DOI] [Google Scholar]
- Reitan RM, & Wolfson D (1994). A selective and critical review of neuropsychological deficits and the frontal lobes. Neuropsychology Review, 4(3), 161–198. 10.1007/BF01874891 [DOI] [PubMed] [Google Scholar]
- Report Viewer | NINDS Common Data Elements. (n.d.) Retrieved November 25, 2019, from https://www.commondataelements.ninds.nih.gov/report-viewer/24612/Pittsburgh%20Sleep%20Quality%20Index%20(PSQI)
- Rohling ML, Binder LM, Demakis GJ, Larrabee GJ, Ploetz DM, & Langhinrichsen-Rohling J (2011). A meta-analysis of neuropsychological outcome after mild traumatic brain injury: Re-analyses and reconsiderations of Binder et al. (1997), Frencham et al. (2005), and Pertab et al. (2009). The Clinical Neuropsychologist, 25(4), 608–623. 10.1080/13854046.2011.565076 [DOI] [PubMed] [Google Scholar]
- Rolle-Lake L, & Robbins E (2020). Behavioral Risk Factor Surveillance System. In StatPearls StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK553031/ [PubMed] [Google Scholar]
- Ruttan L, Martin K, Liu A, Colella B, & Green RE (2008). Long-term cognitive outcome in moderate to severe traumatic brain injury: A meta-analysis examining timed and untimed tests at 1 and 4.5 or more years after injury. Archives of Physical Medicine and Rehabilitation, 89(12 Suppl), S69–76. 10.1016/j.apmr.2008.07.007 [DOI] [PubMed] [Google Scholar]
- Safinia C, Bershad EM, Clark HB, SantaCruz K, Alakbarova N, Suarez JI, & Divani AA (2016). Chronic Traumatic Encephalopathy in Athletes Involved with High-impact Sports. Journal of Vascular and Interventional Neurology, 9(2), 34–48. [PMC free article] [PubMed] [Google Scholar]
- Schear JM, & Sato SD (1989). Effects of visual acuity and visual motor speed and dexterity on cognitive test performance. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 4(1), 25–32. [PubMed] [Google Scholar]
- Schreiber S, Barkai G, Gur-Hartman T, Peles E, Tov N, Dolberg OT, & Pick CG (2008). Long-lasting sleep patterns of adult patients with minor traumatic brain injury (mTBI) and non-mTBI subjects. Sleep Medicine, 9(5), 481–487. 10.1016/j.sleep.2007.04.014 [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, & Shapiro AM (2003). A quantitative review of the effects of traumatic brain injury on cognitive functioning. International Review of Psychiatry (Abingdon, England), 15(4), 341–349. 10.1080/09540260310001606728 [DOI] [PubMed] [Google Scholar]
- Schroeder RW, Twumasi-Ankrah P, Baade LE, & Marshall PS (2012). Reliable Digit Span: A systematic review and cross-validation study. Assessment, 19(1), 21–30. 10.1177/1073191111428764 [DOI] [PubMed] [Google Scholar]
- Sickinger K, Walker WC, Agyemang AA, Cifu DX, Lewis TL, & Carne W (2018). Recruiting for a multicentre DoD and VA longitudinal study: Lessons learned. Brain Injury, 32(10), 1218–1225. 10.1080/02699052.2018.1492740 [DOI] [PubMed] [Google Scholar]
- Soble JR, Spanierman LB, & Fitzgerald Smith J (2013). Neuropsychological functioning of combat veterans with posttraumatic stress disorder and mild traumatic brain injury. Journal of Clinical and Experimental Neuropsychology, 35(5), 551–561. 10.1080/13803395.2013.798398 [DOI] [PubMed] [Google Scholar]
- Stein MB, & McAllister TW (2009). Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. The American Journal of Psychiatry, 166(7), 768–776. 10.1176/appi.ajp.2009.08101604 [DOI] [PubMed] [Google Scholar]
- Stone JR, Avants BB, Tustison NJ, Wassermann EM, Gill J, Polejaeva E, Dell KC, Carr W, Yarnell AM, LoPresti ML, Walker P, O’Brien M, Domeisen N, Quick A, Modica CM, Hughes JD, Haran FJ, Goforth C, & Ahlers ST (2020). Functional and Structural Neuroimaging Correlates of Repetitive Low-Level Blast Exposure in Career Breachers. Journal of Neurotrauma, 37(23), 2468–2481. 10.1089/neu.2020.7141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storzbach D, O’Neil ME, Roost S-M, Kowalski H, Iverson GL, Binder LM, Fann JR, & Huckans M (2015). Comparing the Neuropsychological Test Performance of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) Veterans with and without Blast Exposure, Mild Traumatic Brain Injury, and Posttraumatic Stress Symptoms. Journal of the International Neuropsychological Society: JINS, 21(5), 353–363. 10.1017/S1355617715000326 [DOI] [PubMed] [Google Scholar]
- Strenge H, Niederberger U, & Seelhorst U (2002). Correlation between tests of attention and performance on grooved and Purdue pegboards in normal subjects. Perceptual and Motor Skills, 95(2), 507–514. 10.2466/pms.2002.95.2.507 [DOI] [PubMed] [Google Scholar]
- Suhr JA, & Gunstad J (2002). “Diagnosis Threat”: The effect of negative expectations on cognitive performance in head injury. Journal of Clinical and Experimental Neuropsychology, 24(4), 448–457. 10.1076/jcen.24.4.448.1039 [DOI] [PubMed] [Google Scholar]
- Sweet JJ, Heilbronner RL, Morgan JE, Larrabee GJ, Rohling ML, Boone KB, Kirkwood MW, Schroeder RW, Suhr JA, & Conference Participants. (2021). American Academy of Clinical Neuropsychology (AACN) 2021 consensus statement on validity assessment: Update of the 2009 AACN consensus conference statement on neuropsychological assessment of effort, response bias, and malingering. The Clinical Neuropsychologist, 35(6), 1053–1106. 10.1080/13854046.2021.1896036 [DOI] [PubMed] [Google Scholar]
- Tate CM, Wang KKW, Eonta S, Zhang Y, Carr W, Tortella FC, Hayes RL, & Kamimori GH (2013). Serum brain biomarker level, neurocognitive performance, and self-reported symptom changes in soldiers repeatedly exposed to low-level blast: A breacher pilot study. Journal of Neurotrauma, 30(19), 1620–1630. 10.1089/neu.2012.2683 [DOI] [PubMed] [Google Scholar]
- Taylor BC, Hagel EM, Carlson KF, Cifu DX, Cutting A, Bidelspach DE, & Sayer NA (2012). Prevalence and costs of co-occurring traumatic brain injury with and without psychiatric disturbance and pain among Afghanistan and Iraq War Veteran V.A. users. Medical Care, 50(4), 342–346. 10.1097/MLR.0b013e318245a558 [DOI] [PubMed] [Google Scholar]
- TBI Center of Excellence. (2021). DOD TBI Worldwide Numbers. Defense Center of Excellence for Traumatic Brain Injuryry Center http://dvbic.dcoe.mil/dod-worldwide-numbers-tbi
- Tulsky DS, Carlozzi N, Chiaravalloti ND, Beaumont JL, Kisala PA, Mungas D, Conway K, & Gershon R (2014). NIH Toolbox Cognition Battery (NIHTB-CB): List sorting test to measure working memory. Journal of the International Neuropsychological Society: JINS, 20(6), 599–610. 10.1017/S135561771400040X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AS, Roebuck-Spencer TM, & Cernich A (2014). Cognitive changes and dementia risk after traumatic brain injury: Implications for aging military personnel. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 10(3 Suppl), S174–187. 10.1016/j.jalz.2014.04.006 [DOI] [PubMed] [Google Scholar]
- Vogt D, Smith BN, King LA, King DW, Knight J, & Vasterling JJ (2013). Deployment risk and resilience inventory-2 (DRRI-2): An updated tool for assessing psychosocial risk and resilience factors among service members and veterans. Journal of Traumatic Stress, 26(6), 710–717. 10.1002/jts.21868 [DOI] [PubMed] [Google Scholar]
- Walker WC, Carne W, Franke LM, Nolen T, Dikmen SD, Cifu DX, Wilson K, Belanger HG, & Williams R (2016). The Chronic Effects of Neurotrauma Consortium (CENC) multi-centre observational study: Description of study and characteristics of early participants. Brain Injury, 30(12), 1469–1480. 10.1080/02699052.2016.1219061 [DOI] [PubMed] [Google Scholar]
- Walker WC, Cifu DX, Hudak AM, Goldberg G, Kunz RD, & Sima AP (2015). Structured interview for mild traumatic brain injury after military blast: Inter-rater agreement and development of diagnostic algorithm. Journal of Neurotrauma, 32(7), 464–473. 10.1089/neu.2014.3433 [DOI] [PubMed] [Google Scholar]
- Walker WC, Hirsch S, Carne W, Nolen T, Cifu DX, Wilde EA, Levin HS, Brearly TW, Eapen BC, & Williams R (2018). Chronic Effects of Neurotrauma Consortium (CENC) multicentre study interim analysis: Differences between participants with positive versus negative mild TBI histories. Brain Injury, 32(9), 1079–1089. 10.1080/02699052.2018.1479041 [DOI] [PubMed] [Google Scholar]
- Walker WC, Nowak KJ, Kenney K, Franke LM, Eapen BC, Skop K, Levin H, Agyemang AA, Tate DF, Wilde EA, Hinds S, & Nolen TL (2018). Is balance performance reduced after mild traumatic brain injury?: Interim analysis from chronic effects of neurotrauma consortium (CENC) multi-centre study. Brain Injury, 32(10), 1156–1168. 10.1080/02699052.2018.1483529 [DOI] [PubMed] [Google Scholar]
- Wechsler D, Coalson DL, & Raiford SE (2008). WAIS-IV technical and interpretive manual Pearson. [Google Scholar]
- Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, Carlozzi NE, Slotkin J, Blitz D, Wallner-Allen K, Fox NA, Beaumont JL, Mungas D, Nowinski CJ, Richler J, Deocampo JA, Anderson JE, Manly JJ, Borosh B, … Gershon RC (2013). Cognition assessment using the NIH Toolbox. Neurology, 80(11 Suppl 3), S54–64. 10.1212/WNL.0b013e3182872ded [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, Whiteneck GG, Bogner J, Bushnik T, Cifu DX, Dikmen S, French L, Giacino JT, Hart T, Malec JF, Millis SR, Novack TA, Sherer M, Tulsky DS, Vanderploeg RD, & von Steinbuechel N (2010). Recommendations for the use of common outcome measures in traumatic brain injury research. Archives of Physical Medicine and Rehabilitation, 91(11), 1650–1660.e17. 10.1016/j.apmr.2010.06.033 [DOI] [PubMed] [Google Scholar]
- Wilson L, Stewart W, Dams-O’Connor K, Diaz-Arrastia R, Horton L, Menon DK, & Polinder S (2017). The chronic and evolving neurological consequences of traumatic brain injury. The Lancet. Neurology, 16(10), 813–825. 10.1016/S1474-4422(17)30279-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom NM, Pastorek NJ, Miller BI, Booth JE, Romesser JM, Linck JF, & Sim AH (2014). PTSD and cognitive functioning: Importance of including performance validity testing. The Clinical Neuropsychologist, 28(1), 128–145. 10.1080/13854046.2013.863977 [DOI] [PubMed] [Google Scholar]
- Woods SP, Delis DC, Scott JC, Kramer JH, & Holdnack JA (2006). The California Verbal Learning Test--second edition: Test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 21(5), 413–420. 10.1016/j.acn.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Work Group. (2021). VA/DoD Clinical Practice Guidelines. Management and Rehabilitation of Post-Acute Mild Traumatic Brain Injury (mTBI). Version 3.0. Department of Veterans Affairs (US) and Department of Defense https://www.healthquality.va.gov/guidelines/Rehab/mtbi/VADoDmTBICPGFinal508.pdf
- Yancosek KE, & Howell D (2009). A narrative review of dexterity assessments. Journal of Hand Therapy: Official Journal of the American Society of Hand Therapists, 22(3), 258–269; quiz 270. 10.1016/j.jht.2008.11.004 [DOI] [PubMed] [Google Scholar]
- Yudko E, Lozhkina O, & Fouts A (2007). A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. Journal of Substance Abuse Treatment, 32(2), 189–198. 10.1016/j.jsat.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Anderson JE, Richler J, Wallner-Allen K, Beaumont JL, Conway KP, Gershon R, & Weintraub S (2014). NIH Toolbox Cognition Battery (CB): Validation of Executive Function Measures in Adults. Journal of the International Neuropsychological Society : JINS, 20(6), 620–629. 10.1017/S1355617714000472 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


