In Heitmeier et al., 1 Figure 2 was published incorrectly. In Figure 2H, the x axis currently reads “Factor Xia activity (%)” but should read: “Inhibition of FXIa activity (%)”. Also, the figure legend is missing the word “activity”, when describing panel 2H.
FIGURE 2.
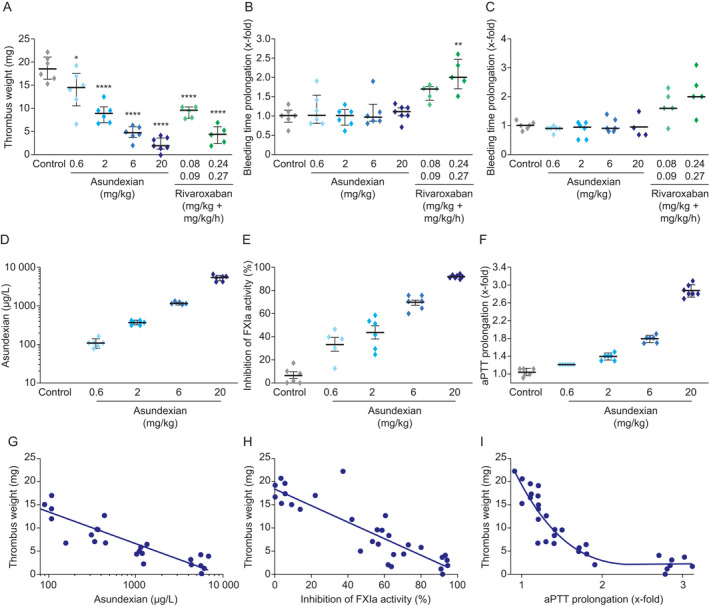
Effects of asundexian versus rivaroxaban on thrombus weight following FeCl2 ‐induced damage to the carotid artery (A), ear bleeding time (B) and gum bleeding time (C) in rabbits. Plasma concentrations of asundexian (D), inhibition of FXIa activity (E) and aPTT prolongation (F) according to asundexian dose, and correlation of plasma concentrations (G), Inhibition of FXIa activity (%) (H), and aPTT prolongation (I) with thrombus weight. Asundexian was administered as an intravenous bolus, whereas rivaroxaban was administered as an intravenous bolus followed by continuous infusion. Results are presented as individual values and median with 25th and 75th percentiles (Parts A–F). *p < .05, ***p < .001. aPTT, activated partial thromboplastin time; FXIa, activated coagulation factor XI.
The correct figure and caption text is given below.
The author regrets the error.
REFERENCE
- 1. Heitmeier S, Visser M, Tersteegen A, et al. Pharmacological profile of asundexian, a novel, orally bioavailable inhibitor of factor Xia. J Thromb Haemost. 2022;20(6):1400‐1411. doi: 10.1111/jth.15700 [DOI] [PMC free article] [PubMed] [Google Scholar]


