CONSPECTUS:
Since its discovery in 1965, the inorganic drug cisplatin has become a mainstay of cancer therapies and has inspired many platinum (Pt)-based compounds to solve various issues of toxicity and limitations associated with the original cisplatin. However, many of these drugs/prodrugs continue to be plagued by an array of side effects, limited circulation, and half-life and off-target effects. To solve this issue, we have constructed an array of platinum-based prodrugs on a Pt(IV) skeleton, which provides more favorable geometry and hydrophobicity, easier functionalization, and ultimately better targeting abilities. Each of these Pt(IV) prodrugs aims to either combine cisplatin with other agents for a combination therapeutic effect or improve the targeting of cisplatin itself, all for the more effective treatment of specific cancers. Our developed prodrugs include Platin-A, which combines cisplatin with the anti-inflammatory agent aspirin, Platin-M, which is functionalized with a mitochondria-targeting moiety, and Platin-B and Platin-Cbl, which combine cisplatin with components to combat cellular resistance to chemotherapy. At the same time, however, we recognize the crucial role of nanotechnology in improving the efficacy of cisplatin prodrugs and other inorganic compounds for the treatment of cancers. We describe several key benefits provided by nanomedicine that vastly improve the reach and utility of cisplatin prodrugs, including the ability of biodegradable polymeric nanoparticles (NPs) to deliver these agents with precision to the mitochondria, transport drugs across the blood–brain barrier, and target cisplatin prodrugs to specific cancers using various ligands. In addition, we highlight our progress in the engineering of innovative new polymers to improve the release patterns, pharmacokinetics, and dosages of cancer therapies. In this Account, we aim to describe the growing need for collaboration between the fields of inorganic chemistry and nanotechnology and how new advancements can not only improve on traditional chemotherapeutic agents but also expand their reach to entirely new subsets of cancers. In addition to detailing the design and principles behind our modifications of cisplatin and the efficacy of these new prodrugs against aggressive, cisplatin-resistant, or metastatic cancers, we also shed light on nanotechnology’s essential role in protecting inorganic drugs and the human body from one another for more effective disease treatment without the off-target effects with which it is normally associated. We hope that this perspective into the important intersection between inorganic medicinal chemistry and nanotechnology will inspire future research on cisplatin prodrugs and other inorganic agents, innovative polymer and NP design, and the ways in which these two fields can greatly advance cancer treatment.
Graphical Abstract
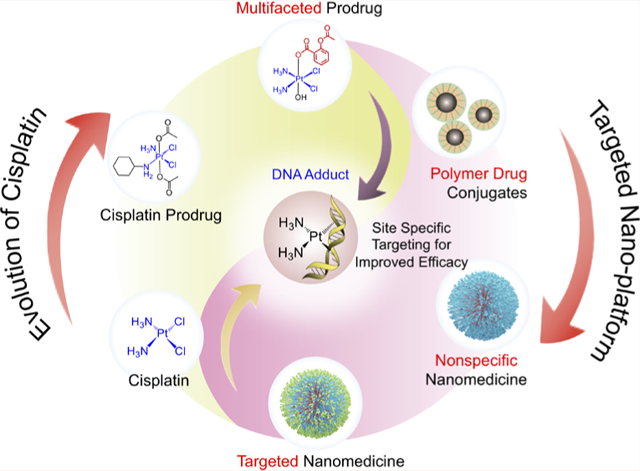
1. INTRODUCTION
Inorganic materials have been proven to be revolutionary discoveries for the advancement of medicine. Many inorganic compounds are crafted by and used in nature, yet they are able to be utilized by scientists and chemically modified and functionalized to give an even more diverse set of applications through the field of inorganic medicinal chemistry.1 By incorporating these drugs into our medicinal approach to disease treatment, they have shown great efficacy for a variety of purposes, whether it be for reaching specific intracellular targets or treating various cancers and other diseases.2 Through the process of functionalization, drugs that were normally labile, highly reactive, and either too hydrophobic or hydrophilic were able to be tamed and improved further.3 However, these drugs often continue to exhibit side effects and unspecific targeting and reactivity, all causing undue harm to patients and preventing medicinal chemistry from taking full advantage of their immense potential.
Perhaps some of the most impactful inorganic materials have originated from metals and their role in medicinal chemistry. Metal ions are naturally involved in biological processes because of their ability to form coordination complexes, and metal-lodrugs can show activity against various diseases as well as aid in diagnostic methods through imaging (Figure 1A).2,4,5
Figure 1.
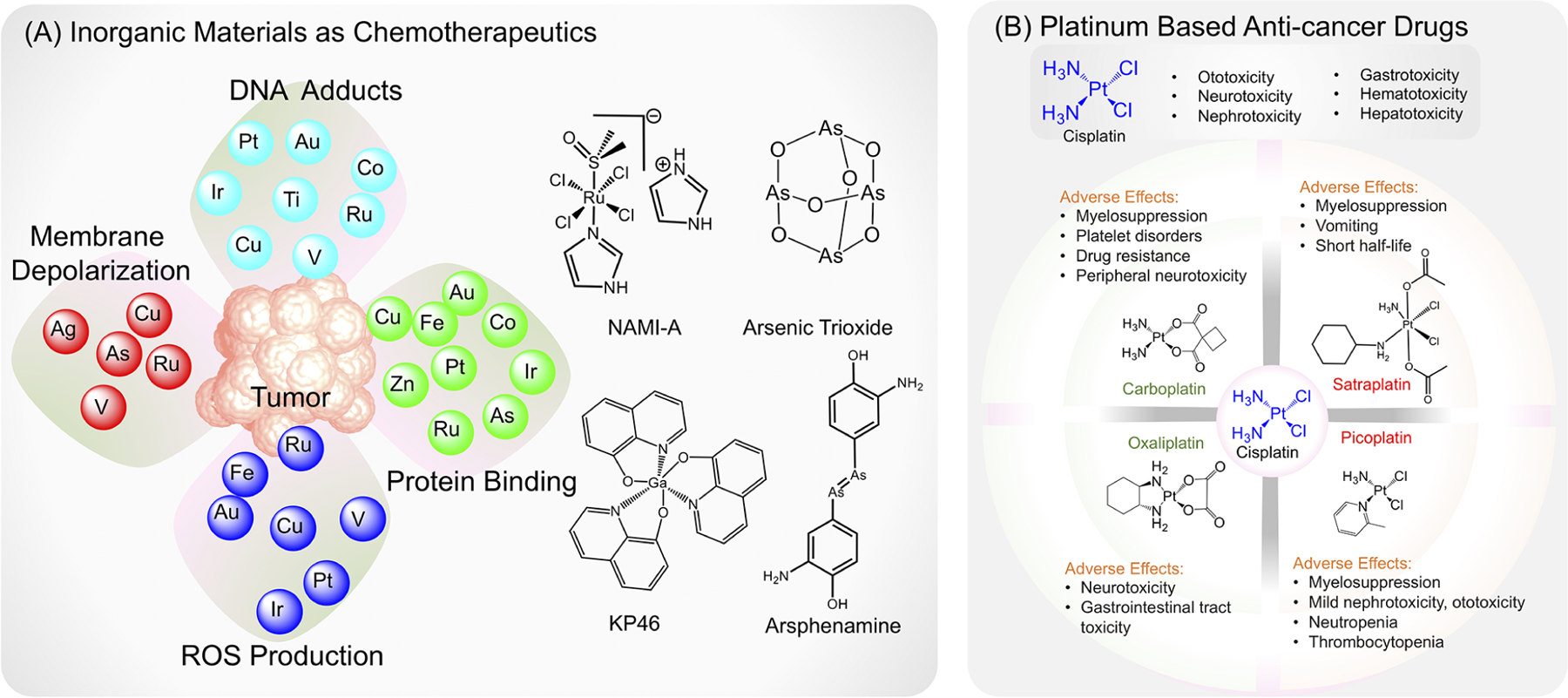
(A) Anticancer properties of various metals and inorganic agents. (B) Structures of different platinum-based compounds and associated side effects.
Metals and metal-based compounds have pervaded cancer therapies for several decades. Noble metals such as rhodium and ruthenium have been shown to be effective in the treatment of specific cancers. Ruthenium compounds such as NAMI-A, a RuCl4-based compound (Figure 1A), have been proven to be effective against lung cancer metastases,6 and rhodium(III) complexes containing bidentate aromatic ligands and other functionalization have shown cytotoxicity against breast cancers and leukemias.7 These rhodium(III) complexes have been able to be further functionalized for increased specificity toward malignant cells using phenanthroline ligands. Further yet, gold and silver complexes and nanoparticles (NPs) have shown great efficacy against various tumors, including ovarian cancers, prostate cancers, breast cancers, and leukemias.8,9
Perhaps the most well-known anticancer application of metals is that of platinum, which lies in the center of the widely used cisplatin (Figure 1B). Unlike most organic drugs, cisplatin is simple in structure and ubiquitous throughout many cancer treatment regimens but is often reactive and labile and continues to possess side effects and systemic issues, many of which make chemotherapy just as painful and difficult for cancer patients as the disease itself (Figure 1B).10
The discovery of cisplatin by Dr. Barnett Rosenberg in 1965 initially began as an experiment to determine if an electrical field could affect cell division before it was found that the platinum used in the electrodes was the actual component impacting cell division.11 Almost as soon as Rosenberg and colleagues found that cisplatin was effective against a sarcoma mouse model, they also discovered that the compound was highly toxic.12 Though researchers were nervous about the prospect of treating cancer or other diseases with heavy metals, they soon began to consider the idea of cisplatin cancer therapy after witnessing its impressive efficacy against several types of tumors, and the drug was approved for use in cancer patients by the U.S. Food and Drug Administration in 1978. Researchers also began discovering that, chemically, much of cisplatin’s lability and reactivity was due to the two chlorines in the compound’s structure.13 They began replacing or substituting these groups to create compounds such as oxaliplatin and carboplatin, which were soon incorporated into treatments as well (Figure 1B).14,15 However, as with the original cisplatin, these modified agents continued to cause significant neurotoxicity and peripheral neuropathy, leading to numbness and sensory loss in patients.16 In addition, many of these compounds have issues with distribution and the half-life within patients. For example, satraplatin, an orally administrable Pt(IV) agent containing acetate groups, initially showed anticancer effects against cisplatin-resistant hormone-refractory prostate cancer but was found to have an extremely short half-life of only 6.3 min in human whole blood (Figure 1B).17
Though many traditional Pt compounds including cisplatin and early functionalized versions were based on platinum with a +2 oxidation state [Pt(II)], there are several benefits that arise when creating cisplatin prodrugs with a Pt(IV) state because they are able to carry additional ligands that can alter the compounds’ hydrophobicity and targeting abilities, and they have a favorable electronic configuration that renders them more inert (Figure 2).18 Furthermore, the shift from square-planar geometry to octahedral geometry associated with Pt(IV) compounds is also favorable for further functionalization and use as prodrugs.19,20
Figure 2.
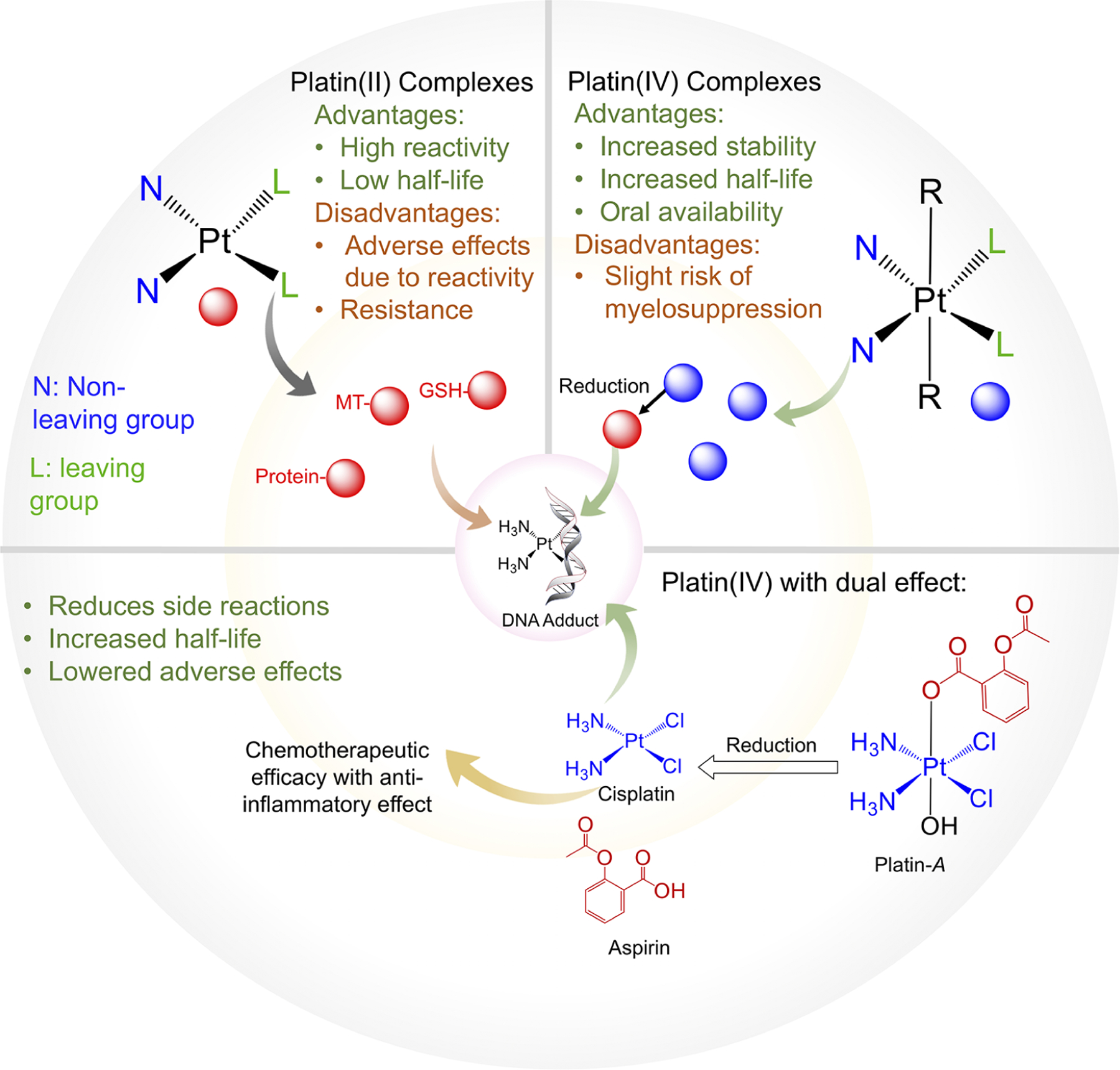
Comparison between Pt(II) and Pt(IV) complexes, including the benefits of Pt(IV) compounds that allow for an improved half-life and circulation, fewer off-target effects, and improved stability, as demonstrated by the developed prodrug Platin-A.
As an early example from our group, through the design and synthesis of Platin-A, a simple Pt(IV) prodrug, we were able to address many of the aforementioned issues with cisplatin and previously developed prodrugs (Figure 2).21–23 Platin-A was designed to release both cisplatin and aspirin concurrently for cancer treatment.22,23 The key priority in this new prodrug was to lessen the toxicity of cisplatin through the delivery of the anti-inflammatory agent aspirin. Cisplatin itself was found to cause significant nephrotoxicity and ototoxicity, and the addition of aspirin to the compound not only reduced these off-target effects but provided improved and synergistic anticancer properties to the compound.23 In this Account, we will also document such a possibility by combining four aspirin and three cisplatin moieties in a dendron conjugated polymer, allowing for synergistic and spatiotemporal delivery of cisplatin and aspirin.24,25 Furthermore, through animal testing, the single prodrug containing aspirin was found to significantly reduce the toxicity normally associated with cisplatin, including ototoxicity, nephrotoxicity, neurotoxicity, and associated nausea.23 Though current inorganic materials for cancer therapy still come with several drawbacks, including limited efficacy and side effects, our creation of Platin-A has laid the groundwork for future cisplatin modifications and the development of other modified inorganic agents for use with NP delivery systems.
2. DEVELOPMENT OF CONTROLLED-RELEASE POLYMER-BASED DELIVERY SYSTEMS
Although the advancements in cancer treatment made by inorganic chemistry are undeniable, it is through a second crucial field—nanotechnology—that we are able to solve the persisting slate of issues with traditional inorganic drugs and truly unlock the full potential of these compounds for medicinal treatment and the improvement of human health.26,27 As new viral diseases, chemotherapy-resistant cancers, and other medical challenges continue to come to our attention, the need has never been stronger to intersect the fields of inorganic chemistry and nanomedicine for the advancement of translational sciences and medicine. The COVID-19 vaccines from Moderna and Pfizer are a prime example of this intersection because they utilize lipid nanoparticles to stabilize, protect, and transport the mRNA contents of the vaccine. The unprecedented and incredibly quick development of these vaccines would largely not have been possible without the joining of traditional fields with nanotechnology.
While inorganic drugs are nonspecific and often too reactive, they also suffer from a brief half-life, rapid metabolism and excretion, and recognition and opsonization by components of the immune system, all pitfalls that can be alleviated through nanomedicine approaches such as the creation of nanoparticle-based delivery systems. Through organic polymer synthesis for the creation of new drug-loaded nanoformulations, researchers have been able to vastly improve the applications and reach of therapies. In essence, nanotechnology can function to protect both the drug and the human body from one another through encapsulating a drug, protecting it from harsh bodily conditions, and delivering it to a specific target, all while protecting the human from off-target effects and drug toxicity in the process.
Some of the earliest polymer-based carriers of anticancer drugs were N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers. These polymers influenced other early drug-carrier systems and greatly improved the efficacy of chemotherapy as compared to standard low-molecular-weight drugs by improving the circulation time and allowing therapeutics to preferentially accumulate in tumors.28 When conjugated to HPMA copolymers, traditional anticancer compounds such as doxorubicin and meso-chlorin e6 mono(N-2-aminoethylamide) were found to be effective against cancer cells resistant to the free version of the drugs and offered synergistic effects when combined with traditional photodynamic therapy.29 In addition, these polymers offered improved active targeting mechanisms for better drug delivery through the conjugation of antibodies that targeted antigens present on cancers such as ovarian carcinoma. These HPMA-based constructs also carry the potential for clinical translation. One such drug, ProLindac, a diaminocyclohexane platinum polymer prodrug that utilizes an HPMA-based polymer, demonstrated improved efficacy and tolerability over the free drug on which it is based, oxaliplatin, and makes use of a pH-sensitive linker to release oxaliplatin in low-pH environments such as those found in tumors (Figure 3A).30,31 The advent of HPMA-based polymers demonstrates the ability of polymer engineering to solve issues related to drug distribution, controlled release, and tumor targeting.
Figure 3.
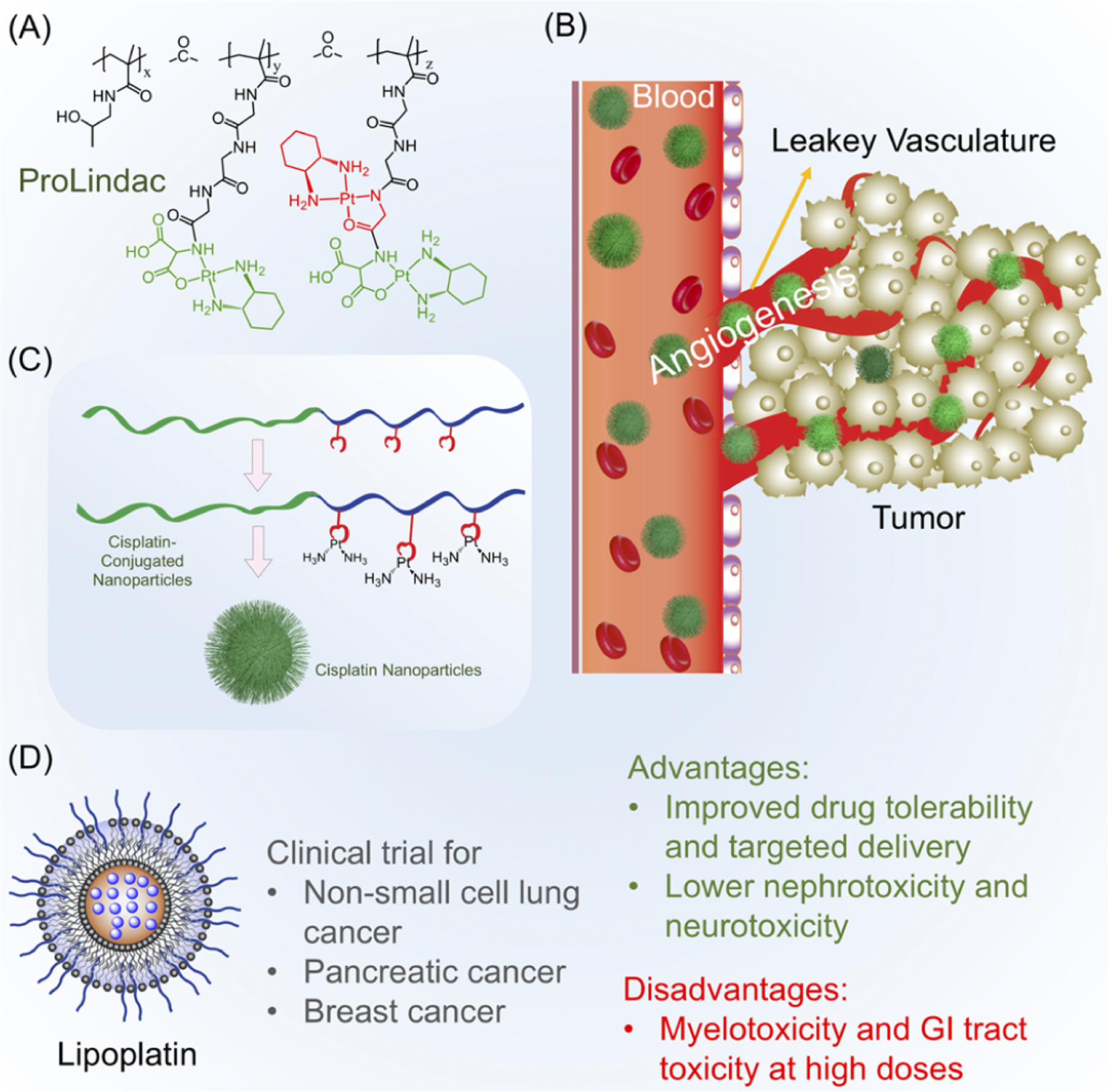
(A) Example of controlled-release polymer-based-drug ProLindac, which allows for the delivery and release of oxaliplatin. (B) Illustration of the enhanced permeability and retention (EPR) effect, which allows for the preferential accumulation of size-optimized NPs at the tumor site. (C) Representation of cisplatin-conjugated polymers and the formation of polymeric NPs for the gradual release of cisplatin. (D) Example of FDA-approved nanomedicine Lipoplatin, which demonstrates improved safety and delivery over those of traditional cisplatin.
As researchers began looking toward nanotechnology as a potential treatment modality for various diseases such as cancers, the concept of the enhanced permeability and retention (EPR) effect came into play, as Maeda and other researchers presented the idea that macromolecular entities such as protein-conjugated drugs, NPs, or liposomes of particular sizes would be able to preferentially accumulate at the sites of tumors or tissue injury and retain at an appreciable amount (Figure 3B).32 It was hypothesized that the accumulation of these nanomaterials at tumor sites was due to factors such as increased blood flow, angiogenesis, and more permeable vasculature (Figure 3B).33 In addition to HPMA-based polymers, several other polymer- and macromolecule-based delivery systems were developed to improve the efficacy of cisplatin and other inorganic anticancer drugs while relying on principles such as the EPR effect. For example, a diblock copolymer containing poly(ethylene glycol) (PEG) and poly(caprolactone) (PCL) was conjugated to cisplatin and used to form nanoparticles that allowed for the controlled release of cisplatin from the polymer scaffold over the course of 6 to 7 days, greatly improving the drug’s availability (Figure 3C).34 These polymers were also designed to be glutathione-resistant, preventing the detoxification of cisplatin via cytoplasmic thiols.35 These principles were further extended to allow the polymers and NPs to carry multiple drugs for combination therapy. A similar triple-block polymer incorporating an outer layer of PEG, a middle layer of PCL for the inclusion of doxorubicin, and an inner layer of carboxylic-functionalized PCL for conjugation to cisplatin was able to self-assemble into stable nanoparticles and accomplished several goals, including full growth inhibition of GSH-overexpressing breast cancer cells, lower effective doses of the drugs, and prevention of the normally antagonistic relationship between doxorubicin and cisplatin.36
Several nanomedicine applications are already being used in clinical practice following FDA approval, demonstrating the potential of these nanoparticles to translate into clinical trials and medical practice. Doxil and lipoplatin, for example, both improve upon the original drugs on which they are based, doxorubicin and cisplatin, by reducing their off-target effects and toxicity (Figure 3D).37,38 While Doxil consists of doxorubicin encapsulation in the core of a liposome coated with methoxypoly(ethylene glycol), lipoplatin similarly consists of cisplatin loaded into a lipid bilayer functionalized with poly(ethylene glycol). Both formulations show marked improvement over their original counterparts in terms of lower reactivity and improved systemic circulation time, a trend common with nanoformulations of other inorganic drugs.37,38 Although some engineered polymer prodrugs such as ProLindac are unable to progress in clinical trials, new systems and methods of platinum drug delivery are continuing to be developed, including backbone-shattering polymeric nanoparticles and near-infrared light-response nanocarriers.39,40 However, many of these continue to utilize the original form of cisplatin, and relatively few of these newly developed nanotechnology-based treatments make use of platin prodrugs,41 meaning that similar toxicities and side effects associated with cisplatin may arise.
Much of our recent work has focused on the dual development of both cisplatin prodrugs and poly(lactic-co-glycolic acid) (PLGA)- and PEG-based polymeric, biodegradable NPs for the efficient and targeted drug delivery for a host of cancers. Through innovative polymer engineering and synthesis of a suite of cisplatin prodrugs, we have been able to overcome several issues that persist with traditional chemotherapy.
3. INTERSECTION BETWEEN INORGANIC CHEMISTRY AND NANOMEDICINE FOR CANCER TREATMENT
Over the past 10 years, our laboratory has improved existing inorganic compounds and drugs using both functionalization and incorporation into nanoparticle-based drug-delivery systems.22–25,42–48 Much of this work involves the inorganic drug cisplatin, which for many years has served as a staple of chemotherapy regimens and cancer treatment as a whole. However, its ceiling remains much higher because it exhibits high reactivity, off-target effects, and a low half-life, all issues that we have been able to improve through chemical modifications and the incorporation of newly created cisplatin prodrugs into targeted nanoparticles. The creation of these cisplatin prodrugs has also been focused on achieving cisplatin’s chemotherapeutic effect while overcoming tumor site inflammation, targeting cisplatin to the mitochondria, and overcoming cisplatin resistance in cancer cells. A majority of the new properties and effects that arise with these cisplatin prodrugs are simply unattainable with the original cisplatin itself because of its nature as a systemic treatment, clear toxicity, and numerous side effects.
As we have worked to create new Pt(IV) compounds, we have also made use of the principles of nanotechnology and nanomedicine to develop polymeric delivery vehicles to further improve the utility of our prodrugs. The origins of nanotechnology can be traced back to a 1959 talk by physicist Richard Feynman titled “There’s Plenty of Room at the Bottom: An Invitation to Enter a New Field of Physics”, in which he outlined a few key tenets of the field that are still important today when discussing nanomedicine: the direct manipulation and arrangement of atoms, the unique properties that arise from materials on a small scale, and the construction of materials from the bottom up. In the decades following Feynman’s talk, the principles of polymer synthesis and self-assembly were to focus of nanomedicine, and they continue to inform our approach today. Along with proposed mechanisms of passive targeting such as the EPR effect came ideas of active targeting through ligand attachment to the polymers used to construct NPs. As we have worked to develop nanomedicine-based solutions to numerous diseases and conditions, we have kept in mind these basic principles when designing our NPs so that they can both advance the utility of the inorganic drugs we develop and possess their own targeting and therapeutic properties.
Using NPs, we have taken our developed cisplatin prodrugs and a wide range of commercially available inorganic and organic drugs and helped to deliver them with precision across biological barriers and to a host of cellular and molecular targets. These include targeting mitochondria and receptors expressed on specific tumors as well as crossing biological barriers such as the gut epithelium barrier and the blood–brain barrier (BBB) to access the central nervous system (CNS), all to treat a much broader array of conditions, from cancers and heart diseases to viral infections and neurological conditions. Furthermore, we have expanded the utility of both the inorganic agents and NPs to be used as diagnostic and imaging tools and have developed innovative new nanoparticles that possess therapeutic abilities themselves.49,50
Our synthesis of cisplatin prodrugs was specifically tailored for the future use of these prodrugs with polymeric nanoparticle designs. To accomplish this, we outlined and considered three key principles for improving the interactions between cisplatin and the delivery vehicles (Figure 4A). First, we created cisplatin prodrugs such that NPs would be able to engage in the physical entrapment of these compounds. By utilizing amphiphilic diblock polymers that created NPs with a hydrophilic exterior and hydrophobic core and concurrently synthesizing Pt(IV) molecules functionalized with hydrophobic moieties, our prodrugs showed increased loading into the core of biodegradable nanoparticles for future delivery and controlled release. Second, we reviewed the idea of host–guest chemistry and the importance of maintaining the structure and integrity of drug-loaded nanoparticles through noncovalent bonding. However, we recognized that these interactions are limited to specific types of molecules and are generally less stable, necessitating other methods of developing stable nanoparticles. Finally, we identified the potential of chemical conjugation for opening new therapeutic and targeting possibilities. For example, azide drugs could quickly and conveniently be conjugated to alkyne-containing polymers through click chemistry methods, allowing for more stable controlled-release polymers. In addition, various antibody fragments and peptides could be conjugated to the nanoparticle surface for the targeting of specific antigens and receptors on biological barriers or target tissues, such as our use of maleimide–thiol chemistry to link immunoglobulin fragments to the nanoparticle surface.51
Figure 4.
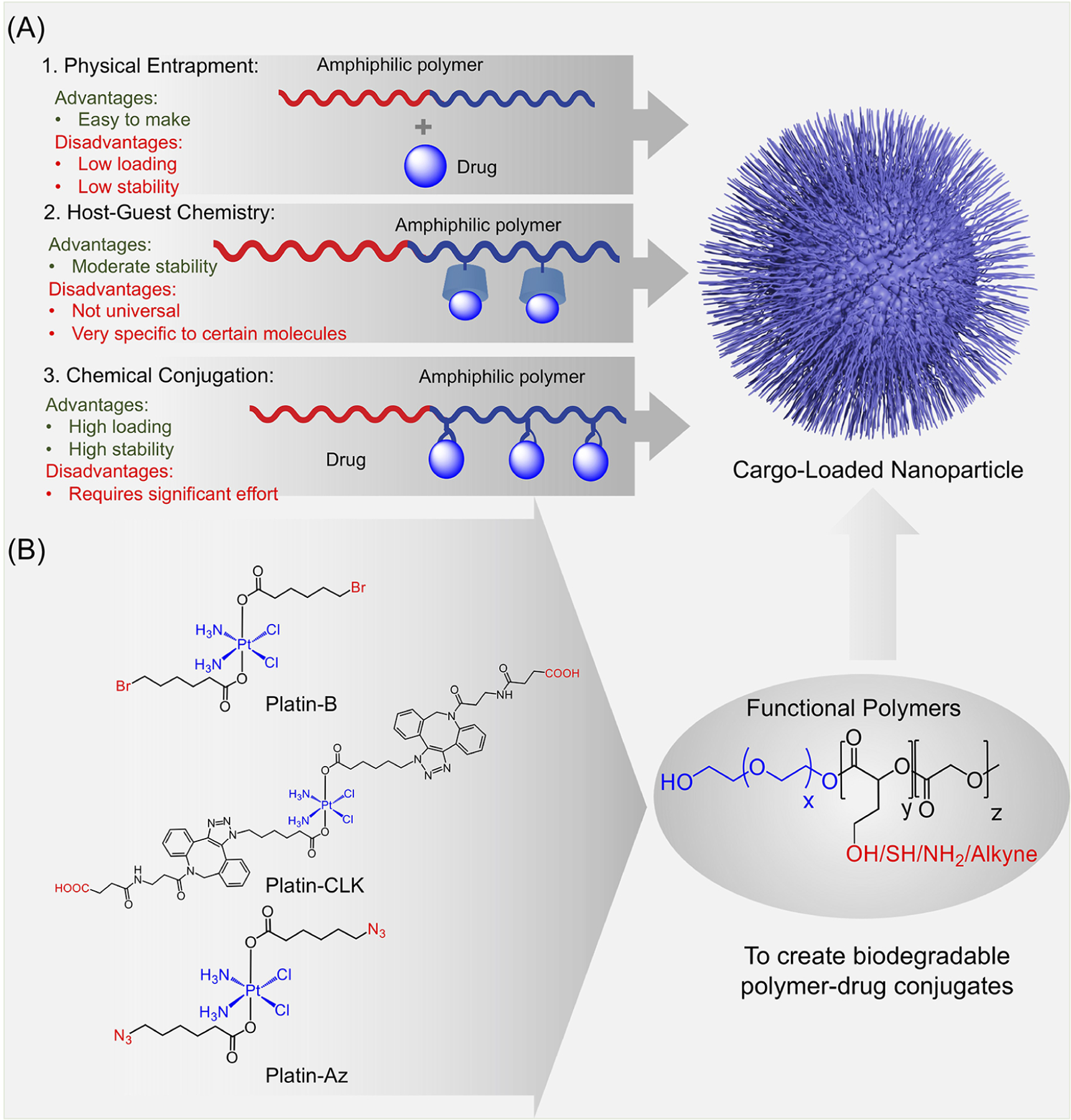
(A) Principles influencing the construction of functional polymer–prodrug combinations, including physical entrapment, host–guest chemistry, and chemical conjugation. (B) Cisplatin prodrugs Platin-Az, Platin-CLK, and Platin-B produced from novel synthetic strategies for the quick conjugation of additional moieties which can promote the formation of cargo-loaded NPs.
In combining inorganic cancer drugs and nanoparticle systems, we have learned four main lessons that can greatly benefit future work in the field.
3.1. New Synthetic Strategies
The creation of functionalized Pt(IV) compounds has allowed us to take advantage of new synthetic strategies. To explore new methods of circumventing cancer resistance to cisplatin, our laboratory constructed Platin-B, a cisplatin prodrug containing a pipobroman-mimicking alkylating agent (Figure 4B).45 Pipobroman itself is able to use its terminal bromides to alkylate DNA bases and proteins, so adding 6-bromohexanoic acid, a portion resembling pipobroman’s active structural component, to the Pt(IV) skeleton was theorized to help form DNA adducts within cancer cells. In addition, the moiety was expected to prevent the cisplatin prodrug from causing the quick sequestration and breakdown that cisplatin itself suffers and was able to do so by interacting with glutathione using the 6-bromohexanoic acid component. Platin-B was able to show increased toxicity to cisplatin-resistant cancer cells by forming adducts with glutathione and interacting with both nuclear and mitochondrial DNA. In comparison to controlling compounds such as cisplatin and butyroplatin, Platin-B possessed a significantly lower IC50 toward the cisplatin-resistant ovarian cancer cell line A2780/CP70. The enhanced efficacy of Platin-B can be attributed to several factors, including its increased uptake into cells due to its lipophilicity, its ability to preferentially form adducts with mitochondrial DNA over nuclear DNA, and its increased tendency to bind to hexokinase 2, which normally plays a role in preventing cancer cell apoptosis.
Two new Pt(IV) compounds, Platin-Az and Platin-CLK, were developed to demonstrate the importance of click-chemistry-based reactions with minimal steps (Figure 4B).21 As previously discussed, Pt(IV) complexes offer two axial sites for various ligands and are conveniently kinetically inert. However, to avoid the reduction of the Pt(IV) complex, instead of utilizing a copper(I)-based click chemistry approach, a new strain-promoted alkyne–azide cycloaddition (SPAAC) approach was used to install azide groups, thus creating the Platin-Az complex with terminal azides to serve as a precursor for a range of different reactions and prodrugs. This allowed for a one-step addition of an azadibenzocyclooctyne carboxylic acid functionality and the production of Platin-CLK, a model for many other possible Pt(IV) prodrugs with low toxicity.
Pt(II) and Pt(IV) compounds demonstrate the convenience of developing new prodrugs by utilizing halide (bromide) or azide groups on the original complexes to add new functionalities.
3.2. Mitochondrial Targeting
Around the time of the development of Platin-A, our group also developed a mitochondria-targeted cisplatin prodrug, Platin-M.43,52 The creation of this new prodrug was aimed at combating the issue of tumor chemoresistance to cisplatin treatment, which largely arises from the repair of platin-DNA adducts in the nucleus by nucleotide excision repair (NER) and other mechanisms. Therefore, the targeting of cisplatin to the mitochondria and thus the mitochondrial DNA presented a promising opportunity to bypass chemoresistance mechanisms and avoid off-target effects while still targeting cancer cells because the mitochondrial base excision repair system cannot repair platin-DNA adducts. The targeting of the mitochondria was done through the chemical addition of a triphenyl-phosphonium (TPP) cation moiety whose positive charge takes advantage of the hyperpolarized mitochondrial membrane (Figure 5A).
Figure 5.
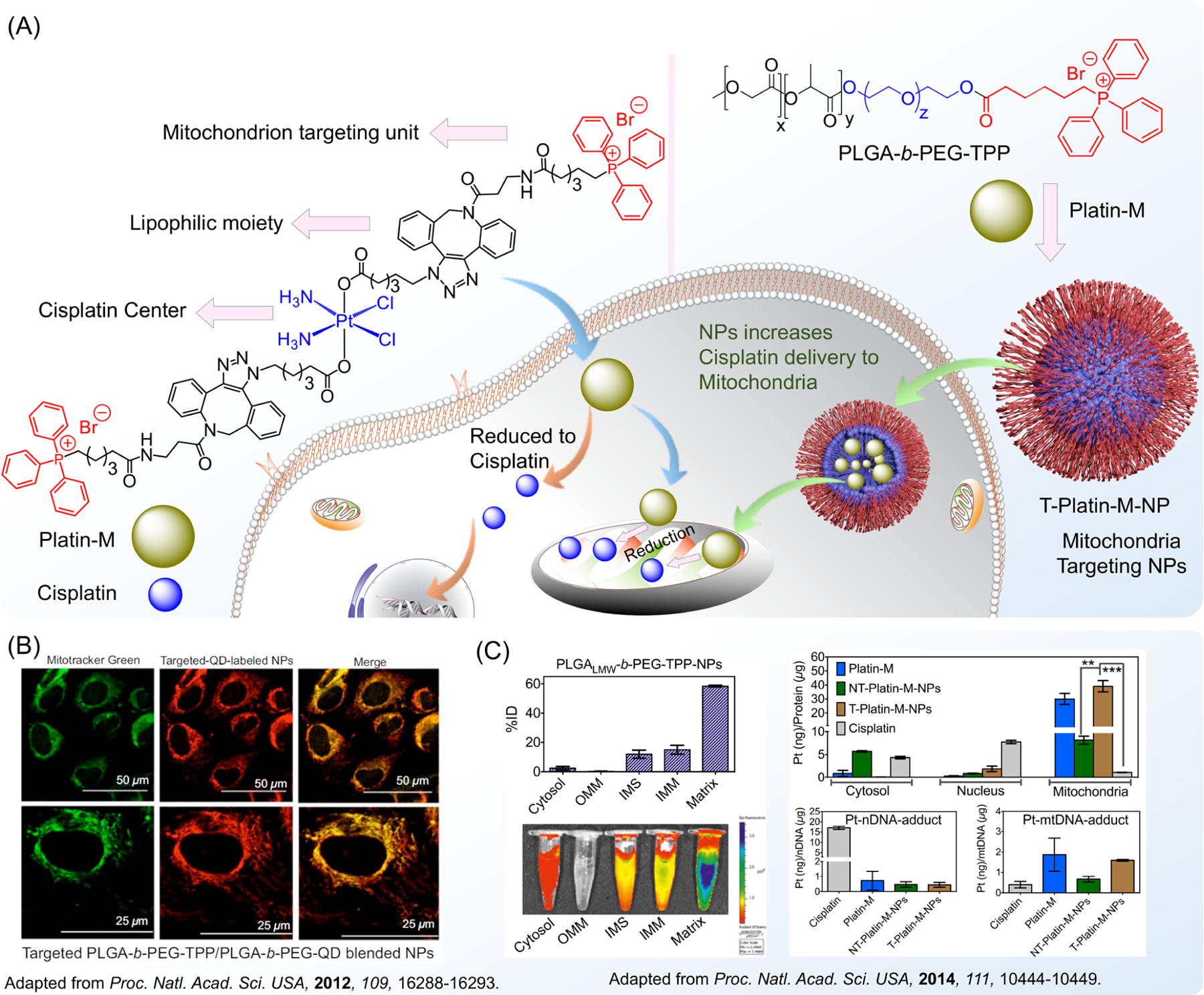
(A) Structural basis of the mitochondrion-targeting properties of cisplatin prodrugs and polymeric nanoparticles, allowing for improved delivery of drugs to mitochondria and the targeting of mitochondrial DNA. (B) Ability of mitochondrion-targeted nanoparticles to accumulate in the mitochondrial matrix. (C) Demonstration of cisplatin delivery to the mitochondria in the form of a prodrug and a nanoparticle and the formation of mitochondrial DNA adducts. Adapted with permission from refs 42 and 43. Copyright 2012 and 2014 National Academy of Sciences.
In addition to testing in mice, the distribution and therapeutic abilities of Platin-M-loaded nanoparticles were investigated in a canine brain cancer model, further substantiating the improved targeting, safety, and efficacy of this new cisplatin prodrug.52 While the new Platin-M-loaded nanoparticle was found to be more effective in the large animal model than carboplatin, a platin-based compound commonly used to treat aggressive glioblastoma, it was also found to pose no observable toxicity in the blood, brain, and other organs. The synthetic modification conducted to create Platin-M from cisplatin was in itself a step forward in functionalization processes in the scientific community. The synthesis took place through the previously described SPAAC approach, and this copper-free click-chemistry-based synthesis presented a convenient method through which countless other Pt(IV) prodrugs could be created.46
To further improve the mitochondrial targeting of cancer therapies, it was also vital to develop these mitochondria-targeting abilities in nanoparticle delivery vehicles for widespread use with a variety of anticancer agents such as the cisplatin prodrugs. This targeting of mitochondria through nanomaterials is vital to the better delivery of drugs, particularly for conditions related to metabolic alterations in cancers and mitochondrial dysfunctions that may cause oxidative stress and inflammation, and to target agents to hard-to-repair mitochondrial DNA rather than nuclear DNA. Although mitochondrial targeting has been a part of our initial modification and improvement of inorganic drugs, such as through the aforementioned creation of Platin-M and Mito-magneto, our development of mitochondria-targeted nanoparticles as drug-delivery vehicles has opened up countless new doors for drug delivery and disease treatment. To start, nanoparticles targeted to the hyperpolarized mitochondrial membrane through the attachment of TPP cations to polymers that comprise the NP have allowed for the delivery of many of our cisplatin prodrugs, including Platin-M and Platin-Cbl, to the mitochondria for better targeting of mitochondrial DNA (Figure 5B).42,43,47 This nanoparticle, based on the PLGA-b-PEG-TPP polymer, possesses a delocalized positive surface charge and a hydrophobic core conducive to drug loading and transport. Polymers constructed from high-molecular-weight and low-molecular-weight PLGA-COOH, along with 10% of the PLGA-b-PEG-quantum dot (QD), produced nanoparticles with hydrodynamic diameters of 51.3 and 143.2 nm, respectively.43 These size measurements indicate the mitochondria-targeted nanoparticles’ suitability for a wide variety of purposes throughout the body, including effective drug loading and cellular uptake as well as prolonged circulation. This same nanoparticle has also been able to encapsulate a host of other hydrophobic inorganic drugs and other agents, such as Mito-DCA,53,54 antioxidant coenzyme Q10 (CoQ10),55,56 and a prodrug of the anti-inflammatory agent aspirin.55,57
Another utilization of the engineered mitochondria-targeted polymeric nanoparticles for cancer treatment is cancer immunotherapy that relies on boosting or activating certain subsets of a patient’s immune system, an area in which the nanoparticle delivery of inorganic agents can prove useful. While old methods of dendritic cell-based immunotherapy struggled in generating tumor antigen-specific T-cell responses, we were able to utilize a nanoparticle-delivered inorganic photosensitizer to allow breast cancer cells to activate dendritic cells and lead to a more robust immune response and T-cell stimulation (Figure 6A).44,58,59 This photosensitizer, zinc phthalocyanine (ZnPc), was able to be packaged into a mitochondria-targeted nanoparticle and was found to cause treated breast cancer cells to stimulate the ex vivo dendritic cell production of interferon gamma, a cytokine with an important role in differentiating T cells into cytotoxic CD8 T cells. Therefore, these dendritic cells have the potential to be activated and administered to patients because they were shown to produce interferon gamma as helper T cells, as natural killer cells do during an immune response toward a tumor (Figure 6B). The effective mitochondrial delivery of ZnPc was enabled by the targeted nanoparticle and lays the groundwork for future vaccines based on cancer cell supernatants and activated dendritic cells.
Figure 6.
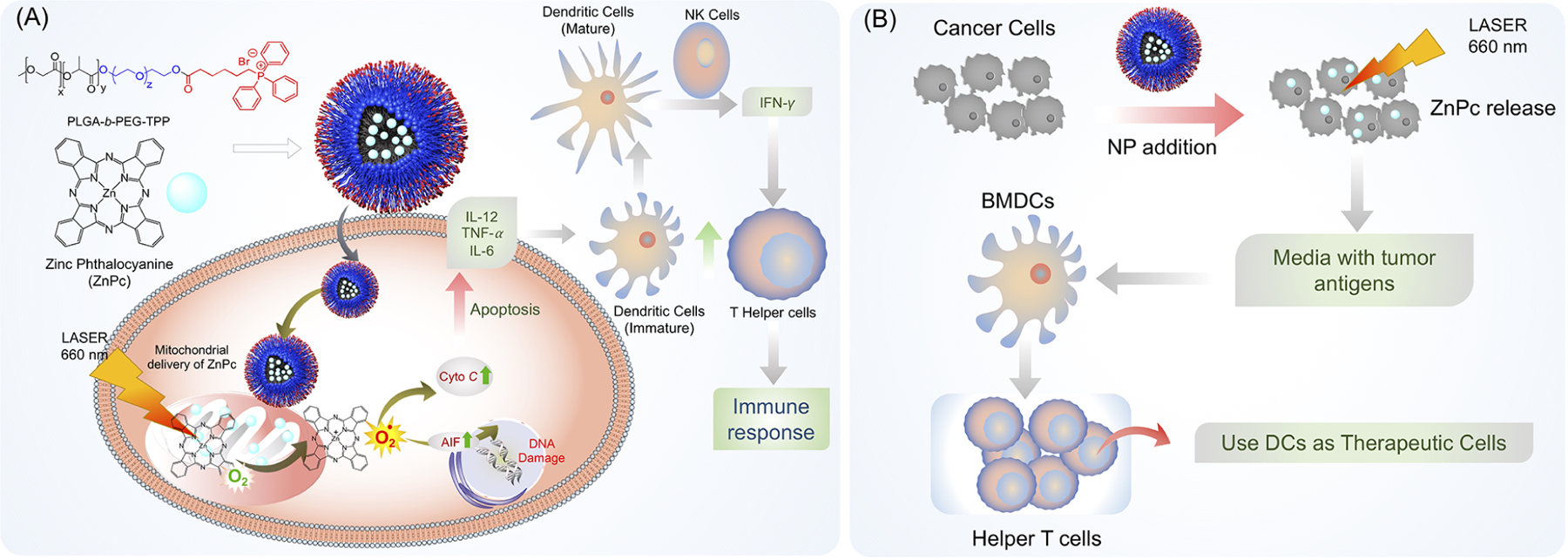
(A) Schematic of zinc phthalocyanine photosensitizer delivery to the mitochondria via a mitochondrion-targeted nanoparticle, followed by the local generation of singlet oxygen, leading to the production of tumor antigens and inflammatory cytokines and the eventual activation of dendritic cells, NK cells, and T helper cells. (B) Proposed scheme for the potential utilization of ex-vivo-stimulated DCs for cancer immunotherapy, in which ZnPc is used to stimulate tumor antigen production, which is then used to activate a patient’s dendritic cells outside of the body before they are reintroduced into the body as a cancer therapeutic.
3.3. Nanoparticle-Based Cisplatin Prodrug Combination Therapies
Another of our developed cisplatin prodrugs, Platin-Cbl, combined cisplatin with chlorambucil (Cbl) to further target glutathione, a key cellular defense molecule that prevents polarization of the mitochondrial membrane and apoptosis (Figure 7A).47 This new prodrug was found to more effectively treat cisplatin-resistant cancers by taking advantage of synergistic effects between cisplatin and Cbl, and this dual-drug therapy was conveniently offered through a single modified inorganic molecule. This new prodrug added to cisplatin’s DNA cross-linking abilities by adding the capacity of Cbl to alkylate DNA to the molecule, allowing for a greater ability to induce apoptosis in cancer cells.
Figure 7.
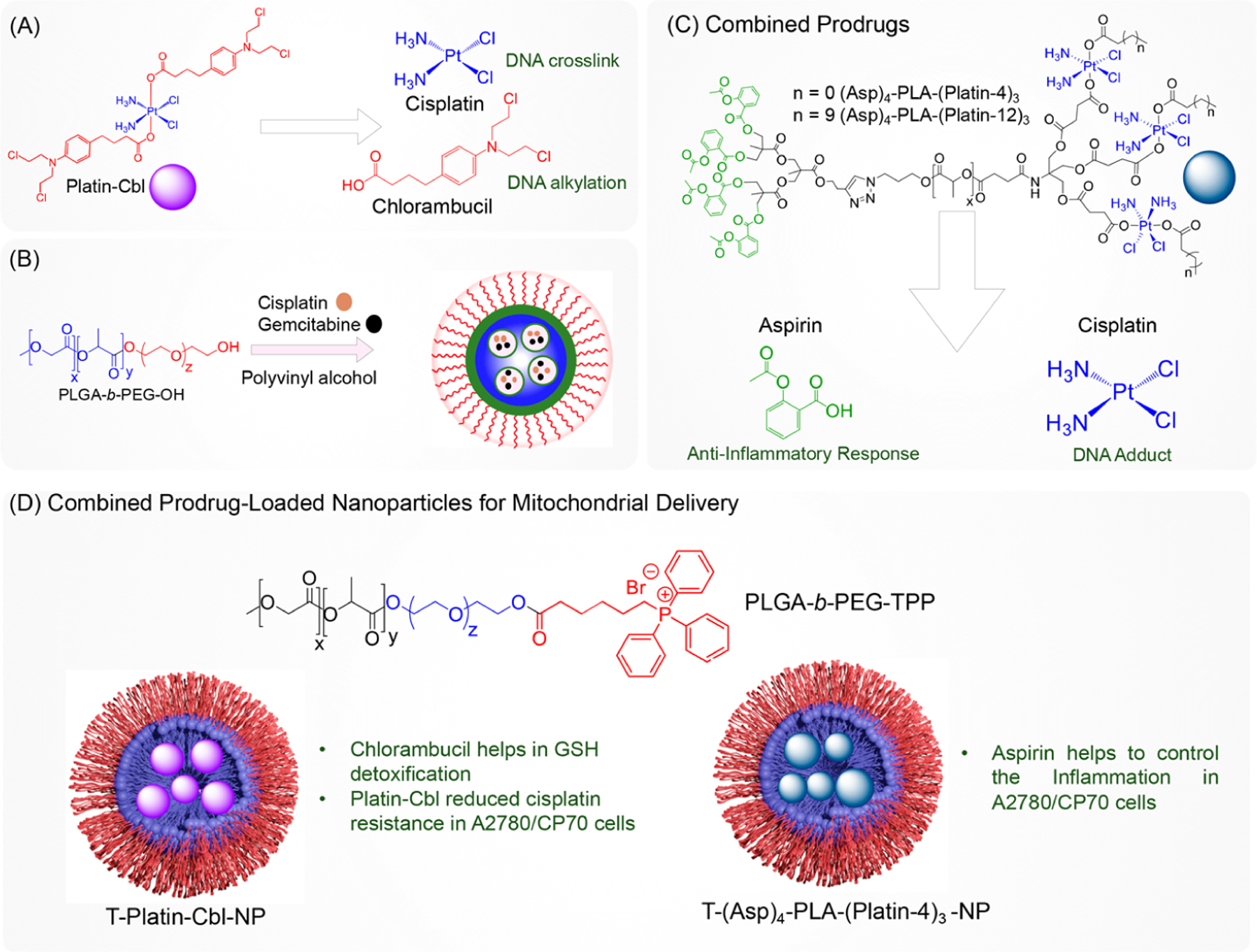
(A) Synthesis of the cisplatin prodrug containing an additional drug moiety, (B) physical entrapment of two drugs in a nanodelivery vehicle, (C) incorporation of a fixed ratio of the cisplatin prodrug and other agents into single polymer for spatiotemporal controlled release, and (D) polymeric nanoparticles with ability to deliver drugs such as cisplatin, chlorambucil, or aspirin to the mitochondria of cisplatin-resistant cancer cells.
However, the model presented by Platin-Cbl of a single platinum compound containing multiple drug moieties is not always applicable to other drug combinations. The need for polymers, rather than prodrugs, that combine multiple therapeutics comes from the issue of differential drug ratios that may arise with traditional combination treatments. For example, we previously loaded both cisplatin and gemcitabine into PLGA-b-PEG-OH-based nanoparticles for the improved treatment of chemo-resistant ovarian cancer cells (Figure 7B), but a convenient alternative to this would be to engineer polymers that contain multiple drugs conjugated to the backbone.48 One of our earliest cisplatin prodrugs, Platin-A, offered the combination of cisplatin and aspirin on a single platinum-based compound but was unable to be loaded into polymeric nanoparticles because of issues with its ability to load inside the hydrophobic core of the nanoparticle. However, if cisplatin and aspirin were independently loaded into a single nanoparticle formulation, then several issues would likely arise, including greatly contrasting release kinetics and a failure to offer the necessary drug ratio needed for effective disease treatment, poor drug loading and targeting, differential drug leaching from the nanoparticle, and issues with a poor half-life and circulation time.
Innovative engineering of polymers can help to fix these key issues that arise when attempting to use a combination of inorganic agents and other drugs to treat metastatic and chemotherapy-resistant cancers. This in turn would allow for control over the effective doses and ratios of drugs used in combination, the challenge of delivering them to target tissues, and the varying distribution and pharmacokinetics of drugs within the body.
To solve the issues mentioned above with Platin-A and the prospect of loading unassociated cisplatin and aspirin into nanoparticles, we designed a biodegradable polymer containing predetermined ratios and combinations of therapeutic drugs that can be used to form a nanoparticle for the gradual release of these drugs at specific target sites (Figure 7C).25 Although traditional polymers such as the poly(lactide)-b-poly(ethylene glycol) (PLA-b-PEG) block copolymer are effective at forming drug-loaded nanoparticles for stable drug delivery and release for cancers and other diseases, they are limited in their ability to attach to multiple surface ligands or drug moieties, particularly when these conjugated components have varying lipophilicities. To address this, we synthesized a form of PLA with an increased number of terminal functionalities to create a dendritic polymer with branching at the terminal ends of PLA. The dendrons at each end of PLA contained branches with a specific amount of a drug. In this case, one end of the polymer was functionalized with a prodrug of the anti-inflammatory agent aspirin and the other end was functionalized with a cisplatin prodrug. The two drugs were thus able to be combined in a specific stoichiometric ratio in the synthesis process of the polymer, and this dendritic polymer was blended with the poly(d,l-lactic-co-glycolic acid)-block-(PLGA-b)-poly(ethylene glycol) (PEG)-OH polymer (PLGA-b-PEG-OH) to create a nanoparticle containing both anti-inflammatory and chemotherapeutic properties (Figure 7D). The controlled, gradual release kinetics of the aspirin and cisplatin prodrugs was observed, with the cisplatin prodrugs being released faster because of their aliphatic ester linkages to the polymer and the possibility of release via reduction, and the slow release of the aspirin prodrug was due to its aromatic ester linkages to the polymer. To test the ability of this dendritic polymer-based nanoparticle to treat castration-resistant prostate cancer (CRPC), which is a particularly aggressive and resistant form of the already common cancer, we utilized a blended nanoparticle constructed from a modified PLA polymer containing aspirin and the cisplatin prodrug Platin-12 in a 4:3 ratio combined with the PLGA-b-PEG-GLU (Figure 8B) polymer containing the prostate-specific membrane antigen (PSMA)-targeting peptide GLU.24 This nanoparticle was able to demonstrate increased accumulation in and toxicity toward PSMA-expressing LNCaP cells as well as PC3 cells and anti-inflammatory properties in vivo. In mice with LNCaP xenografts, animals treated with the therapeutic nanoparticle showed significant tumor reduction and no recurrence, which was not present with nontargeted nanoparticles or traditional therapies.
Figure 8.
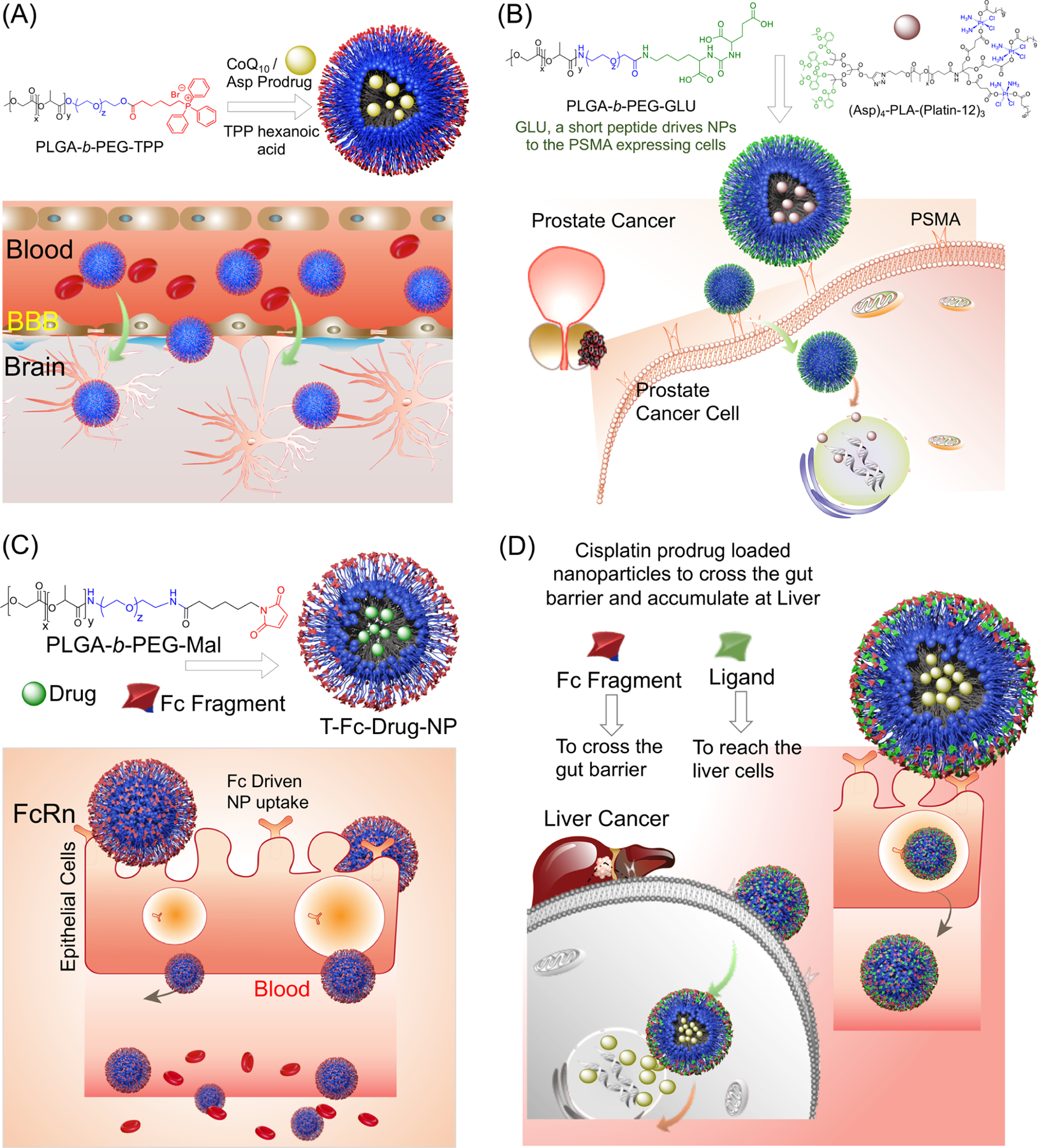
Nanoparticle-based delivery to various biological barriers and specific cancers, including (A) blood–brain barrier-penetrating nanoparticles utilizing the PLGA-b-PEG-TPP polymer and the incorporated CoQ10 and aspirin prodrug for the treatment of brain cancers and associated conditions; (B) prostate-cancer-targeting nanoparticle delivery of the cisplatin prodrug using the interaction between the GLU peptide incorporated into the polymer and PSMA expressed on prostate cancer cells; (C) gut-epithelium-targeting nanoplatform for the oral delivery of various drugs into the bloodstream via FcRn-mediated uptake; and (D) proposed liver-cancer-targeting nanoformulation with blended ligand-containing polymers.
3.4. Targeting of Specific Tumors and Biological Barriers
Among the most crucial developments of our polymeric biodegradable nanoparticle drug-delivery systems is their ability to target specific tumors and cross biological barriers through the conjugation of various ligands to the nanoparticle surface or through properties intrinsic to the nanoparticle itself. These nanoparticles thus open up new ways to treat cancers and other diseases that were previously unattainable by the free drugs themselves.
One of the most crucial and selective barriers is the blood–brain barrier, and one of the most important creations of our laboratory is the development of a blood–brain barrier (BBB)-crossing nanoparticle (Figure 8A). The blood–brain barrier plays a role in the failure of many traditional inorganic drugs to pass into the brain to treat various cancers, neurodegenerative diseases, and viruses, making the creation of BBB-penetrating nanomedicines an urgent goal. Nanoparticles synthesized from the PLGA-b-PEG-TPP polymer with small amounts of TPP-(CH2)5-COOH were found to effectively penetrate and deliver payloads across the BBB without damaging the crucial integrity of the barrier itself.42,55 Their polymer composition also allowed them to target mitochondria once inside cells because of the positive surface charge of the nanoparticle provided by the TPP cations, in addition to their BBB penetration. This opened the door to delivering agents such as aspirin and CoQ10 to mitigate inflammation, oxidative stress, and general mitochondrial dysfunction caused by various diseases and conditions in the CNS, such as brain tumors, Alzheimer’s disease, and Parkinson’s disease.55 To study the efficacy of these BBB-penetrating nanoparticles in delivering neuroprotectants to brain cells, SOD1G93A rats overexpressing mutations in superoxide dismutase (SOD1) were used as a model in which the organisms possessed higher-than-normal levels of reactive oxygen species (ROS) and decreased ATP production in the brain. Within rats treated with CoQ10- and (Asp)4-loaded brain-distributing nanoparticles, isolated neurons and astrocytes were found to have significantly increased ATP production and decreased ROS production without causing any undue inflammation or barrier damage.55 Furthermore, these principles of nanoparticle-mediated drug delivery across the BBB can be extended to other diseases, including viral diseases such as HIV. In treating HIV viral reservoirs in the CNS to prevent HIV-associated neurocognitive disorders, our BBB-penetrating nanoparticle has also shown the ability to encapsulate a select antiretroviral agents (efavirenz, darunavir, and elvitegravir) and deliver them into the brain for the treatment of microglia and astrocytes impacted by HIV. When paired with the previously devised CoQ10- and (Asp)4-loaded BBB-penetrating nanoparticles, the combination therapy of antiretroviral-loaded nanoparticles was able to effectively treat HIV and the oxidative stress and inflammation resulting from intravenous drug use in mice.
Through the conjugation of ligands to the surface of nanoparticles, we have demonstrated the ability of drug-loaded nanoparticles to target specific tumor types, such as prostate cancer. Chemotherapy is known to wreak havoc on the body and cause countless off-target side effects, making more targeted drug delivery to specific tumors a top priority in cancer research. On their own, common inorganic drugs such as cisplatin led to systemic consequences that can be reduced through more targeted delivery to specific tissues or cell types. One benefit offered by nanoparticle-based treatments is the opportunity to attach various ligands or antigens for the direct targeting of specific tumor types. Our development of a nanoparticle functionalized with an aptamer-targeting PSMA has allowed for the targeting of PSMA-expressing prostate cancer cells, and this targeting effect was also repeated with a nanoparticle surface-conjugated PSMA-targeting peptide as well (Figure 8B).24,60 These active targeting mechanisms allowed for the nanoparticles to deliver cisplatin prodrugs more effectively.
The creation of orally deliverable nanoparticle-based therapeutics is another area of interest because oral treatments often boost the treatment feasibility and patient compliance and can ease the burden of chemotherapy. To demonstrate the ability of an orally administered nanoparticle to deliver a hydrophobic agent into the bloodstream, we created a proof-of-concept nanoparticle with surface-conjugated IgG Fc fragments.51 This drug-loaded nanoparticle was able to effectively bind to FcRn receptors on epithelial cells in the gut lumen and transport the loaded hydrophobic drug into the bloodstream for controlled release over the course of several days (Figure 8C). The simple conjugation of this antibody fragment was made possible through the use of PLGA-b-PEG-Mal polymers containing a terminal maleimide portion, which allowed for sulfhydryl immobilization and the attachment of the Fc fragment to the nanoparticle surface through thiol–ene chemistry. The ability of these nanoparticles to transport hydrophobic agents into the bloodstream opens the door for use with a wide range of chemotherapeutics, along with the possibility of creating blended nanoparticles with additional polymers targeted to specific tumors. The ability of polymeric nanoparticles to target these specific tissues and tumors can be extended to combat diseases, such as liver cancer (Figure 8D).
Ultimately, one of the most fundamental reasons that nanomedicine has been able to take inorganic drugs to new heights is the tunability of these nanoparticle platforms, a principle shown throughout our work in the field. Our studies delivering inorganic agents via biodegradable, polymeric nanoparticles exemplify several key benefits of nanomedicine that arise from fine-tuning the properties of NPs: controlled and prolonged drug release, improved half-life, optimal biodistribution, appropriate size and surface charge, unique composition and blending of polymers, and control of active and passive targeting.
4. CONCLUSIONS AND PERSPECTIVE
Through the work presented in this Account, we believe we have made significant progress in fostering the intersection between inorganic materials and polymer-based nanoparticles to advance cancer treatment. Moving forward, we believe that the knowledge we have gained through these efforts to combine inorganic medicinal chemistry and nanomedicine can have a major impact on translational and medicinal sciences in the future. Through our work by developing cisplatin prodrugs and other inorganic materials for therapeutic purposes, we have found that the functionalization of these drugs has helped them achieve properties that were impossible with the original form of cisplatin or the other agents. Likewise, the delivery of these new drugs in fine-tuned nanoparticle platforms has allowed for the selection of particular tissues and intracellular targets and drug delivery across barriers previously deemed impenetrable. As our group continues to work on the development and analysis of various cisplatin prodrugs and other inorganic materials, we are also simultaneously improving the nanomedicine-based approaches for better drug delivery and the treatment of a wide range of diseases.
Though both fields have progressed considerably in recent years, we still recognize the following challenges and goals that exist and must be addressed through future work:
Solving issues related to the nanotechnology-mediated selective delivery of drugs and prodrugs to tumors. Although the targeting of NPs and prodrugs to tumors has greatly improved through the aforementioned developments, certain challenges remain with these forms of drug delivery. These include the need to ensure that as high a proportion of NPs as possible reach the tumor site rather than other distal sites and organs. In addition, there is a need to ensure the cellular interaction of delivered prodrugs and uptake into tumor cells, particularly with more aggressive metastatic tumors. Finally, as nanotechnology-based drug-delivery systems increase in complexity, it may be difficult to appropriately translate pharmacokinetic and biodistribution properties from animal models to humans.
The expansion of existing platinum prodrugs to a wider range of applications. Though our current slate of Pt(IV) prodrugs has shown great efficacy against a number of cancers, their reach can be expanded by examining the specific activity of each prodrug and comparing it to the key characteristics of a specific tumor type. For example, many cases of pancreatic cancer demonstrate cisplatin resistance due to high NER activity, indicating that combination therapies involving our mitochondria-targeted Platin-M molecule are worth exploring because of its ability to disrupt mitochondrial DNA. Conversely, cases of glioblastoma may potentially respond to platin-based therapy, but high doses of cisplatin are needed because of the presence of the blood–brain barrier. Therefore, the use of lower doses of Platin-M could be utilized inside our BBB-penetrating nanoparticle for more effective and less toxic treatments for cases of glioblastoma.
The synthesis of cisplatin prodrugs that provide new abilities or metabolic targets. As we continue to study the impact of our current cisplatin prodrugs, we believe that new platinum-based compounds can be developed for more specific targeting of cellular processes that may make it easier to treat certain cancers. By combining cisplatin with various metabolic inhibitors of processes such as glycolysis, fatty acid oxidation, and oxidative phosphorylation, we can design a series of platinum prodrugs that combat various types of tumors based on their metabolic preferences and flexibility. These prodrugs can be used in combination and delivered via our nanoparticles to treat particularly aggressive tumors or cancers that alter their metabolism based on current treatments.
Understanding the connection between the structure and behavior of polymeric nanoparticles. A central question that remains is the mechanism by which our brain-accumulating nanoparticles are able to cross the blood–brain barrier and deliver drugs to the CNS. Elucidating the reasons behind this property and other characteristics of the nanoparticles will be crucial to designing new nanoformulations that more effectively deliver inorganic drugs and expand their utility. In addition, although much of our testing has relied on the intravenous administration of nanoparticles, we have investigated the impact of differing treatment modalities on the distribution and efficacy of the nanoparticles and will continue doing so to discover why certain methods are more effective for particular diseases.
Altogether, we believe that these goals will provide even stronger evidence that the modification of inorganic drugs and organic polymer- and nanoparticle-based delivery can greatly improve the utility of these agents in combating cancers.
ACKNOWLEDGMENTS
S.D. acknowledges funding from the Sylvester Comprehensive Cancer Center, Department of Defense Prostate Cancer Idea Award (W81XWH-12-1-0406), the Florida Department of Health Bankhead-Coley Cancer Research Award (8BC10), the Florida Department of Health Zika Research Grant Initiative (award number 7ZK28), the Barth Syndrome Foundation, the American Heart Association National Scientist Development Award (14SDG18690009), the Ralph E. Powe Junior Faculty Enhancement Award from ORAU, the NHLBI High Priority Bridge Award (R56HL121392), the Georgia Research Alliance, and the NCI-funded Sylvester Comprehensive Cancer Center (support grant 1P30CA240139) for supporting our laboratory projects on nanomedicine and anticancer therapeutics. We extend our acknowledgement to our laboratory previous trainees and collaborators who participated in our projects. S.D. extends her gratitude to her academic mentors who helped her to realize the potential of metals in medicine.
Biographies
Anuj S. Shah is currently an M.D. candidate at the University of Miami Miller School of Medicine. He received his B.S. degree from the University of Miami in 2021, majoring in microbiology and immunology. He has been working under Prof. Shanta Dhar since 2018, investigating the development of nanotherapeutics for cancers and viral diseases.
Bapurao Surnar is currently working as an assistant scientist under Dr. Shanta Dhar in the Department of Biochemistry and Molecular Biology, University of Miami. He worked as a postdoctoral fellow in the same laboratory from 2017 to 2020. He received his Ph.D. in polymer chemistry in 2017 from the Indian Institute of Science Education and Research (IISER), Pune, India. He is currently working on the development of new nanomedicine platforms for the treatment of prostate, breast, and brain cancer and emerging viral diseases.
Nagesh Kolishetti is currently an assistant professor at Herbert Wertheim College of Medicine, Florida International University (FIU). He received his Ph.D. from the Indian Institute of Science under the supervision of Prof. S. Ramakrishnan. He worked as a postdoctoral fellow at the University of Massachusetts with Prof. Rudolf Faust from 2006 to 2008, Brigham and Women’s Hospital, Harvard Medical School with Prof. Omid Farokhzad in close collaboration with Prof. Robert Langer of the Massachusetts Institute of Technology from 2008 to 2010, and the University of Georgia with Prof. Geert-Jan Boons from 2010 to 2014. He then moved to industry and worked as a research director of chemistry at Partikula LLC. In 2017, he joined FIU, and his current scientific interests are focused on polymers, drug delivery, formulations, and nanomedicine.
Shanta Dhar is an associate professor of biochemistry and molecular biology in the University of Miami Miller School of Medicine. She also holds the position of Assistant Director of Technology and Innovation at Sylvester Comprehensive Cancer Center. Before moving to Miami, she was an assistant professor of Chemistry at the University of Georgia. She received her Ph.D. from the Indian Institute of Science studying ternary copper(II) complexes for photocleaving DNA under visible light under the supervision of Prof. Akhil R. Chakravarty. She conducted her first postdoctoral research training at Johns Hopkins University under the supervision of Prof. Marc M. Greenberg. During her second postdoctoral training, she worked as an Anna Fuller Fellow of Molecular Oncology at the Koch Institute, Massachusetts Institute of Technology under the supervision of Prof. Stephen J. Lippard. Her research program uses sophisticated design approaches at the interface of chemistry, biology, and nanotechnology and the synthesis and engineering of biocompatible nanomaterials for various diseases including but not limited to cancer, cardiovascular diseases, viral diseases, and diseases of the central nervous system.
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/accountsmr.1c00178
The authors declare no competing financial interest.
Contributor Information
Anuj S. Shah, Department of Biochemistry and Molecular Biology, University of Miami Miller School of Medicine, Miami, Florida 33136, United States
Bapurao Surnar, Department of Biochemistry and Molecular Biology and Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, Miami, Florida 33136, United States;.
Nagesh Kolishetti, Department of Immunology & Nano-Medicine, Herbert Wertheim College of Medicine, Florida International University, Miami, Florida 33199, United States;.
Shanta Dhar, Department of Biochemistry and Molecular Biology and Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, Miami, Florida 33136, United States;.
REFERENCES
- (1).Farrell N Biomedical uses and applications of inorganic chemistry. An overview. Coord. Chem. Rev 2002, 232 (1), 1–4. [Google Scholar]
- (2).Sadler PJ; Guo Z Inorganic chemistry and medicine. Chem. Aust 1999, 66 (10), 6–11. [Google Scholar]
- (3).Pooja D; Panyaram S; Kulhari H; Reddy B; Rachamalla SS; Sistla R Natural polysaccharide functionalized gold nanoparticles as biocompatible drug delivery carrier. Int. J. Biol. Macromol 2015, 80, 48–56. [DOI] [PubMed] [Google Scholar]
- (4).Ibers JA; Holm RH Modeling Coordination Sites in Metallobiomolecules. Science 1980, 209 (4453), 223–235. [DOI] [PubMed] [Google Scholar]
- (5).Rosenberg B Platinum coordination complexes in cancer chemotherapy. Naturwissenschaften 1973, 60 (9), 399–406. [DOI] [PubMed] [Google Scholar]
- (6).Bergamo A; Gaiddon C; Schellens JH; Beijnen JH; Sava G Approaching tumour therapy beyond platinum drugs: status of the art and perspectives of ruthenium drug candidates. J. Inorg. Biochem 2012, 106 (1), 90–9. [DOI] [PubMed] [Google Scholar]
- (7).Geldmacher Y; Oleszak M; Sheldrick WS Rhodium(III) and iridium(III) complexes as anticancer agents. Inorg. Chim. Acta 2012, 393, 84–102. [Google Scholar]
- (8).Jain S; Hirst DG; O’Sullivan JM Gold nanoparticles as novel agents for cancer therapy. Br J. Radiol 2012, 85 (1010), 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Boisselier E; Astruc D Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies, and toxicity. Chem. Soc. Rev 2009, 38, 1759–1782. [DOI] [PubMed] [Google Scholar]
- (10).Florea A-M; Büsselberg D Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011, 3 (1), 1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Rosenberg B; Van Camp L; Krigas T Inhibition of cell division in escherichia coli by electrolysis products from a platinum electrode. Nature 1965, 205 (4972), 698–699. [DOI] [PubMed] [Google Scholar]
- (12).Rosenberg B; VanCamp L The successful regression of large solid sarcoma 180 tumors by platinum compounds. Cancer Res. 1970, 30 (6), 1799. [PubMed] [Google Scholar]
- (13).Jordan P; Carmo-Fonseca M Molecular mechanisms involved in cisplatin cytotoxicity. Cell. Mol. Life Sci 2000, 57 (8), 1229–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Raymond E; Chaney SG; Taamma A; Cvitkovic E Oxaliplatin: A review of preclinical and clinical studies. Ann. Oncol 1998, 9 (10), 1053–1071. [DOI] [PubMed] [Google Scholar]
- (15).Wagstaff AJ; Ward A; Benfield P; Heel RC Carboplatin. Drugs 1989, 37 (2), 162–190. [DOI] [PubMed] [Google Scholar]
- (16).Oun R; Moussa YE; Wheate NJ The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47 (19), 6645–6653. [DOI] [PubMed] [Google Scholar]
- (17).Carr JL; Tingle MD; McKeage MJ Rapid biotransformation of satraplatin by human red blood cells in vitro. Cancer Chemother. Pharmacol 2002, 50 (1), 9–15. [DOI] [PubMed] [Google Scholar]
- (18).Huttunen KM; Raunio H; Rautio J Prodrugs–from serendipity to rational design. Pharmacol Rev. 2011, 63 (3), 750–71. [DOI] [PubMed] [Google Scholar]
- (19).Dhar S; Gu FX; Langer R; Farokhzad OC; Lippard SJ Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA–PEG nanoparticles. Proc. Natl. Acad. Sci. U. S. A 2008, 105 (45), 17356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Dhar S; Liu Z; Thomale J; Dai H; Lippard SJ Targeted single-wall carbon nanotube-mediated Pt(IV) prodrug delivery using folate as a homing device. J. Am. Chem. Soc 2008, 130 (34), 11467–11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Basu U; Banik B; Wen R; Pathak RK; Dhar S The Platin-X series: Activation, targeting, and delivery. Dalton Trans. 2016, 45 (33), 12992–13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Pathak RK; Marrache S; Choi JH; Berding TB; Dhar S The prodrug platin-A: simultaneous release of cisplatin and aspirin. Angew. Chem., Int. Ed. Engl 2014, 53 (7), 1963–7. [DOI] [PubMed] [Google Scholar]
- (23).Surnar B; Kolishetti N; Basu U; Ahmad A; Goka E; Marples B; Kolb D; Lippman ME; Dhar S Reduction of cisplatin-induced ototoxicity without compromising its antitumor activity. Biochemistry 2018, 57 (46), 6500–6513. [DOI] [PubMed] [Google Scholar]
- (24).Pathak RK; Basu U; Ahmad A; Sarkar S; Kumar A; Surnar B; Ansari S; Wilczek K; Ivan ME; Marples B; Kolishetti N; Dhar S A designer bow-tie combination therapeutic platform: An approach to resistant cancer treatment by simultaneous delivery of cytotoxic and anti-inflammatory agents and radiation. Biomaterials 2018, 187, 117–129. [DOI] [PubMed] [Google Scholar]
- (25).Pathak RK; Dhar S A nanoparticle cocktail: Temporal release of predefined drug combinations. J. Am. Chem. Soc 2015, 137 (26), 8324–8327. [DOI] [PubMed] [Google Scholar]
- (26).Barry NPE; Sadler PJ Challenges for metals in medicine: How nanotechnology may help to shape the future. ACS Nano 2013, 7 (7), 5654–5659. [DOI] [PubMed] [Google Scholar]
- (27).Farokhzad OC; Langer R Impact of nanotechnology on drug delivery. ACS Nano 2009, 3 (1), 16–20. [DOI] [PubMed] [Google Scholar]
- (28).Kopecek J; Kopecková P; Minko T; Lu Z HPMA copolymer-anticancer drug conjugates: Design, activity, and mechanism of action. Eur. J. Pharm. Biopharm 2000, 50 (1), 61–81. [DOI] [PubMed] [Google Scholar]
- (29).Kopecek J; Kopecková P HPMA copolymers: origins, early developments, present, and future. Adv. Drug Deliv. Rev 2010, 62 (2), 122–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Nowotnik DP; Cvitkovic E ProLindac (AP5346): A review of the development of an HPMA DACH platinum polymer therapeutic. Adv. Drug Deliv. Rev 2009, 61 (13), 1214–9. [DOI] [PubMed] [Google Scholar]
- (31).Nowotnik DP AP5346 (ProLindac™), A DACH platinum polymer conjugate in phase II trials against ovarian cancer. Curr. Bioact. Compd 2011, 7 (1), 21–26. [Google Scholar]
- (32).Maeda H; Wu J; Sawa T; Matsumura Y; Hori K Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65 (1), 271–284. [DOI] [PubMed] [Google Scholar]
- (33).Fang J; Nakamura H; Maeda H The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv Rev 2011, 63 (3), 136–151. [DOI] [PubMed] [Google Scholar]
- (34).Surnar B; Subash PP; Jayakannan M Biodegradable block copolymer scaffolds for loading and delivering cisplatin anticancer drug. Z. Anog. Allg. Chem 2014, 640 (6), 1119–1126. [Google Scholar]
- (35).Surnar B; Sharma K; Jayakannan M Core–shell polymer nanoparticles for prevention of GSH drug detoxification and cisplatin delivery to breast cancer cells. Nanoscale 2015, 7 (42), 17964–17979. [DOI] [PubMed] [Google Scholar]
- (36).Surnar B; Jayakannan M Triple block nanocarrier platform for synergistic cancer therapy of antagonistic drugs. Biomacromolecules 2016, 17 (12), 4075–4085. [DOI] [PubMed] [Google Scholar]
- (37).Safra T; Muggia F; Jeffers S; Tsao-Wei DD; Groshen S; Lyass O; Henderson R; Berry G; Gabizon A Pegylated liposomal doxorubicin (doxil): Reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann. Oncol 2000, 11 (8), 1029–1034. [DOI] [PubMed] [Google Scholar]
- (38).Boulikas T Clinical overview on Lipoplatin: a successful liposomal formulation of cisplatin. Expert Opin. Investig. Drugs 2009, 18 (8), 1197–1218. [DOI] [PubMed] [Google Scholar]
- (39).Cong Y; Xiao H; Xiong H; Wang Z; Ding J; Li C; Chen X; Liang XJ; Zhou D; Huang Y Dual drug backboned shattering polymeric theranostic nanomedicine for synergistic eradication of patient-derived lung cancer. Adv. Mater 2018, 30 (11), 1706220. [DOI] [PubMed] [Google Scholar]
- (40).Li F; Li T; Cao W; Wang L; Xu H Near-infrared light stimuli-responsive synergistic therapy nanoplatforms based on the coordination of tellurium-containing block polymer and cisplatin for cancer treatment. Biomaterials 2017, 133, 208–218. [DOI] [PubMed] [Google Scholar]
- (41).Zhang Z; Wu Y; Kuang G; Liu S; Zhou D; Chen X; Jing X; Huang Y Pt(iv) prodrug-backboned micelle and DCA loaded nanofibers for enhanced local cancer treatment. J. Mater. Chem. B 2017, 5 (11), 2115–2125. [DOI] [PubMed] [Google Scholar]
- (42).Marrache S; Dhar S Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. Proc. Natl. Acad. Sci. U. S. A 2012, 109 (40), 16288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Marrache S; Pathak RK; Dhar S Detouring of cisplatin to access mitochondrial genome for overcoming resistance. Proc. Natl. Acad. Sci. U. S. A 2014, 111 (29), 10444–10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Marrache S; Tundup S; Harn DA; Dhar S Ex vivo programming of dendritic cells by mitochondria-targeted nanoparticles to produce interferon-gamma for cancer immunotherapy. ACS Nano 2013, 7 (8), 7392–402. [DOI] [PubMed] [Google Scholar]
- (45).Pathak RK; Dhar S Unique use of alkylation for chemo-redox activity by a Pt(IV) prodrug. Che. Eur. J 2016, 22 (9), 3029–3036. [DOI] [PubMed] [Google Scholar]
- (46).Pathak RK; McNitt CD; Popik VV; Dhar S Copper-free click-chemistry platform to functionalize cisplatin prodrugs. Chem. Eur. J 2014, 20 (23), 6861–6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Pathak RK; Wen R; Kolishetti N; Dhar S A Prodrug of two approved drugs, cisplatin and chlorambucil, for chemo war against cancer. Mol. Cancer Ther 2017, 16 (4), 625–636. [DOI] [PubMed] [Google Scholar]
- (48).Hung SW; Marrache S; Cummins S; Bhutia YD; Mody H; Hooks SB; Dhar S; Govindarajan R Defective hCNT1 transport contributes to gemcitabine chemoresistance in ovarian cancer subtypes: Overcoming transport defects using a nanoparticle approach. Cancer Lett. 2015, 359 (2), 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Banik B; Askins BW; Dhar S Mito-magneto: A tool for nanoparticle mediated mitochondria isolation. Nanoscale 2016, 8 (47), 19581–19591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Marrache S; Dhar S Biodegradable synthetic high-density lipoprotein nanoparticles for atherosclerosis. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (23), 9445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Surnar B; Kamran MZ; Shah AS; Basu U; Kolishetti N; Deo S; Jayaweera DT; Daunert S; Dhar S Orally administrable therapeutic synthetic nanoparticle for zika virus. ACS Nano 2019, 13 (10), 11034–11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Feldhaeusser B; Platt SR; Marrache S; Kolishetti N; Pathak RK; Montgomery DJ; Reno LR; Howerth E; Dhar S Evaluation of nanoparticle delivered cisplatin in beagles. Nanoscale 2015, 7 (33), 13822–30. [DOI] [PubMed] [Google Scholar]
- (53).Pathak RK; Marrache S; Harn DA; Dhar S Mito-DCA: a mitochondria targeted molecular scaffold for efficacious delivery of metabolic modulator dichloroacetate. ACS Chem. Biol 2014, 9 (5), 1178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Kolb D; Kolishetti N; Surnar B; Sarkar S; Guin S; Shah AS; Dhar S Metabolic modulation of the tumor microenvironment leads to multiple checkpoint inhibition and immune cell infiltration. ACS Nano 2020, 14 (9), 11055–11066. [DOI] [PubMed] [Google Scholar]
- (55).Surnar B; Basu U; Banik B; Ahmad A; Marples B; Kolishetti N; Dhar S Nanotechnology-mediated crossing of two impermeable membranes to modulate the stars of the neurovascular unit for neuroprotection. Proc. Natl. Acad. Sci. U. S. A 2018, 115 (52), E12333–E12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Velichkovska M; Surnar B; Nair M; Dhar S; Toborek M Targeted mitochondrial CoQ10 delivery attenuates antiretroviral-drug-induced senescence of neural progenitor cells. Mol. Pharmaceut 2019, 16 (2), 724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Kalathil AA; Kumar A; Banik B; Ruiter TA; Pathak RK; Dhar S New formulation of old aspirin for better delivery. Chem. Commun 2016, 52 (1), 140–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Marrache S; Tundup S; Harn DA; Dhar S Ex vivo generation of functional immune cells by mitochondria-targeted photosensitization of cancer cells. Methods Mol. Biol 2015, 1265, 113–22. [DOI] [PubMed] [Google Scholar]
- (59).Marrache S; Choi JH; Tundup S; Zaver D; Harn DA; Dhar S Immune stimulating photoactive hybrid nanoparticles for metastatic breast cancer. Integr. Biol 2013, 5 (1), 215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Dhar S; Kolishetti N; Lippard SJ; Farokhzad OC Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc. Natl. Acad. Sci. U.S.A 2011, 108 (5), 1850–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]


