Abstract
Immunization strategies against tuberculosis (TB) that confer better protection than neonatal vaccination with the 101-year-old Bacille Calmette-Guerin (BCG) are urgently needed to control the epidemic, but clinical development is hampered by a lack of established immune correlates of protection (CoPs). Two phase 2b clinical trials offer the first opportunity to discover human CoPs against TB. Adolescent BCG re-vaccination showed partial protection against Mycobacterium tuberculosis (Mtb) infection, as measured by sustained IFNγ release assay (IGRA) conversion. Adult M72/AS01E vaccination showed partial protection against pulmonary TB. We describe two collaborative research programs to discover CoPs against TB and ensure rigorous, streamlined use of available samples, involving international immunology experts in TB and state-of-the-art technologies, sponsors and funders. Hypotheses covering immune responses thought to be important in protection against TB have been defined and prioritized. A statistical framework to integrate the data analysis strategy was developed. Exploratory analyses will be performed to generate novel hypotheses.
Keywords: tuberculosis, BCG, M72/AS01E, correlates of protection, systems vaccinology
TB epidemiology
Tuberculosis disease (TB) is caused by Mycobacterium tuberculosis (Mtb), a mycobacterium transmitted by aerosol, which predominantly affects the lungs but can spread to any other organ [1]. Phylogenetic studies suggest that Mtb and humans co-evolved, possibly for the last 70,000 years [2]. For instance, TB may have killed up to 20% of the European and North American populations between the 17th and 19th century and was still responsible for the most annual deaths from a single pathogen globally until 2019 [3]. In 2020, global TB notification rates dropped to 5.8 million cases due to massive disruptions in testing and reporting caused by the COVID-19 pandemic, while the number of deaths increased, compared to 2019, to a total of 1.5 million [4].
Exposure to Mtb does not necessarily result in an established infection, but it is estimated that one-in-four humans are, or have been, infected with Mtb [5]. The population with viable Mtb infection is difficult to estimate, since no diagnostic test can directly detect Mtb in healthy individuals. Mtb infection status is inferred from the measurement of immune sensitization to Mtb antigens by tuberculin skin tests (TST) or, more recently, IFNγ release assays (IGRA) [6]. Recent epidemiological models suggest that once Mtb infection is established, a large proportion of individuals may clear or effectively control infection, possibly associated with TST and/or IGRA reversion to negative, while a smaller proportion may progress to TB disease over their lifetime [7]. Progression to disease is thought to typically occur relatively rapidly, within two years of primary infection; late progression may be associated with re-infection or weakening of the immune response several years after primary infection.
Novel and improved diagnostics, treatments and vaccines are urgently needed to address and ultimately end the TB epidemic [4]. Advocacy, political and economic commitments are also essential to address the common distorted perception that TB is an issue of the past, or that a deadly disease affecting mostly the poor is not a global emergency.
TB vaccines
Bacille Calmette-Guerin (BCG) is the only available vaccine against TB [3]. This 101-year-old live, attenuated vaccine is routinely administered at birth in most countries [8], because it affords >80% protection against severe and disseminated TB disease, which has high mortality rates in children below 2 years of age [9]. Newborn BCG vaccination also confers partial protection against Mtb infection and pulmonary TB disease, but efficacy estimates vary greatly depending on age, geographical location, and previous sensitization to mycobacteria [9, 10]. BCG saves lives, especially when given in early life, via both pathogen-specific and pathogen-agnostic immunity that is under intense mechanistic investigation [11, 12], but has not stopped the TB epidemic; therefore, novel vaccines or immunization strategies are urgently needed.
While more efficacious and safer newborn BCG replacement vaccines are being tested to protect this vulnerable population, epidemiological modelling suggests that prevention of TB disease (POD) vaccines in adolescents and adults would have greater impact on TB transmission, and TB control in the general population [13]. Although the predicted impact of prevention of Mtb infection (POI) vaccines is lower compared to POD [14], measuring POI (or prevention of sustained infection, POSI) is also considered an innovative clinical trial end-point to efficiently provide proof-of-concept, since Mtb infection occurs much more often than TB disease [15]. However, PO(S)I vaccine efficacy may not necessarily predict POD, since immune responses required to prevent establishment of Mtb infection might be different from those required to prevent TB disease. Furthermore, partial prevention of infection may not translate to prevention of disease, since only a small minority of those who become infected typically progress to disease, and disease may preferentially occur in the subpopulation in whom infection occurred despite vaccination [1].
Rational design and clinical development of new TB vaccines have been hampered by a lack of immune correlates of protection (CoPs) [16]. In 2018, two phase 2 randomized placebo-controlled clinical trials reported partial efficacy of novel TB vaccination strategies, which, for the first time, provide the opportunity to discover CoPs against established Mtb infection and TB disease.
In the first trial, which aimed to assess POI, BCG re-vaccination of IGRA-negative adolescents provided 45.4% (95% CI, 6.4 to 68.1) protection against sustained IGRA conversion, defined as conversion to a positive test without reversion to negative status 3- and 6-months post-conversion [17], which is suggestive of established Mtb infection. Immunogenicity analyses showed significant boosting of BCG-specific Th1 and Th22 cells, as well as modest induction of NK responses after re-vaccination [18]. Antibody responses to BCG were not measured in this trial.
In the second, a POD trial, vaccination of IGRA positive adults with the investigational M72/AS01E vaccine provided 49.7% (95% CI, 2.1 to 74.2) protection against microbiologically-confirmed pulmonary TB disease [19, 20]. Vaccination with M72/AS01E induced robust M72-specific IgG and Th1 cellular responses [20], as well as NK cell responses [21].
Key features of these trials that affect the design and potential outcomes of the CoPs analysis are summarized in Table 1.
Table 1:
Summary of BCG revaccination and M72/AS01E vaccine trials
| BCG revaccination POSI trial | M72/AS01E POD trial | |
|---|---|---|
| Intervention | BCG vaccine, 1 intradermal injection of 5×105 CFU at Day 0 | M72/AS01E, 2 intra-muscular injections of 10μg M72 |
| Formulation | Live attenuated M. bovis (Danish strain 1331), reconstituted in Sauton diluent without adjuvant, ~4000 antigens | Subunit vaccine (M72: recombinant fusion protein of Mtb32A and Mtb39A) with adjuvant (Adjuvant System containing MPL, QS-21 and liposome (25 μg MPL and 25 μg QS-21) |
| Population | 659* HIV negative, IGRA negative adolescents, randomized 1:1 to BCG re-vaccination or placebo | 3575 HIV negative, IGRA positive adults, randomized 1:1 to M72/AS01E vaccination or placebo |
| Efficacy | 45.4 % (95% CI 6.4 to 68.1) | 49.7 (95% CI 2.1 to 74.2) |
| Case Definition | Sustained IGRA conversion (secondary endpoint) | Culture or PCR-confirmed pulmonary TB without HIV (primary endpoint) |
| Endpoints | 57 total 21/312 in BCG arm 36/310 in placebo arm |
39 total 13/1626 in M72/AS01E arm 26/1663 in placebo arm |
| Vaccine-reactive immune responses in placebo arm | detectable, high variability |
virtually undetectable, low variability |
| Samples for primary CoP analysis: PBMC Plasma Fixed cells from whole blood RNA from whole blood |
2 vials at Day 0, 70, Month 6 2 vials at Day 0, 70, Month 6 2 vials at Day 0, 3 (placebo) or 7 (BCG), Month 6 1 vial at Day 0, 3 (placebo) or 7 (BCG), Month 6 |
6 vials at Day 0, 37, Month 6 2 vials at Day 0, 37, Month 6 2 vials at Day 0, 37, Month 6 1 vial at Day 0, 37, Month 6 |
Abbreviations: CFU = colony forming unit; MPL = 3-O-desacyl-4-monophosphoryl lipid A; QS-21 = Quillaja saponaria Molina, fraction 21
H4:IC31 recipients not included in CoP analyses
TB immune correlates program
The TB Immune Correlates Program was initiated in 2018 for BCG-induced CoPs and in 2020 for M72/AS01E-induced CoPs. The Program provides the strategy and governance structure to enable discovery of CoPs from established Mtb infection (inferred from sustained IGRA conversion in the BCG re-vaccination trial) and TB disease (microbiologically confirmed in the M72/AS01E trial) through a highly collaborative approach. The Program aims to: 1) Define hypotheses, biomarkers, and assays to be employed for the discovery of CoPs; 2) Develop the statistical framework and integrated data analysis strategy to evaluate the pre-specified hypotheses; 3) Pursue additional hypothesis-generating exploratory efforts; and 4) Ensure rigorous, efficient and streamlined use of the precious samples stored from these trials.
We hypothesize that vaccination induced a variety of immune responses comprising multiple immune cell subsets and, effector mechanisms, which synergistically contributed to the control of Mtb growth following infection, resulting in reduced rates of sustained IGRA conversion (or increased rates of IGRA reversion) in the BCG trial, or reduced rates of TB disease (M72/AS01E trial) in vaccine compared to placebo recipients. Within this expectation, we aim to test a parsimonious set of pre-defined immunological hypotheses that are informed by the published literature, while allowing generation of additional hypotheses across a broad set of immunological compartments and mechanisms in a manner that rigorously controls the chance of false discovery. While the differences in trial designs and outcomes (Table 1) justify distinct hypotheses and experimental approaches, the overall alignment between the programs may enable identification of commonalities between the CoPs for POD and POSI.
Common between the two trials is the severe limitation of available samples (Table 1), particularly for cellular assays [peripheral blood mononuclear cells (PBMC)]. To ensure feasibility and robustness, and to reduce the number of outcomes tested, it was deemed necessary to apply a staged approach, whereby pilot studies are conducted on a limited set of samples [excluding samples from participants who met the respective clinical endpoint (i.e., cases)] to down-select hypotheses and assays for the primary analysis comparing endpoint cases and non-cases. To preserve statistical power in the primary analyses, results from the pilot study will be used to select assays that exhibit the characteristics required of a CoPs biomarker (see below). Hypotheses will be pre-specified and prioritized in the primary analysis. Exploratory analyses will follow to generate novel hypotheses for validation in ongoing or planned larger trials of BCG re-vaccination and M72/AS01E.
There are several key stakeholders of the TB Immune Correlates Program. Open calls-for-ideas were issued by the Leadership Team, separately for the BCG Program and the M72 Program. For both Programs, proposals from a large number of international investigators were evaluated by the Scientific Advisory Committee, and recommendations were made to the Funders, who further prioritized the most promising approaches to manage available resources. Established Biospecimen Governance Committees (including clinical trial sponsors and clinical site representatives) reviewed and approved sample access for the selected assays to the Principal Investigators (PIs) included in the BCG and M72 Correlates Study Groups. Results generated from the pilot studies will be evaluated and prioritized with a harmonized statistical approach, with analysis conducted by an independent statistical team [the Vaccines and Immunology Statistical Center (VISC) at Fred Hutchinson Cancer Center; Fiore-Gartland & Gilbert].
Hypotheses and experimental approach
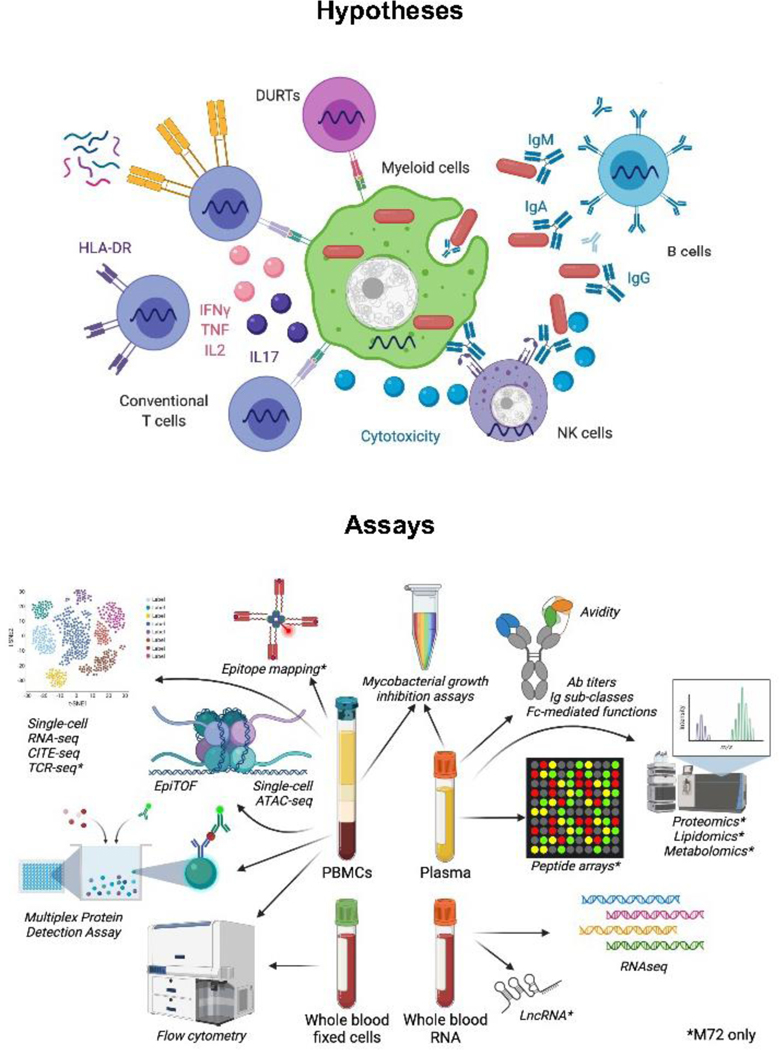
Immune responses to Mtb are complex, include many immune cell subsets and effector mechanisms of the immune system and are affected by the extra-cellular milieu and pre-existing immunity [22, 23]. Available evidence from pre-clinical models, particularly non-human primates (NHP), as well as cohort studies investigating immune correlates of risk for TB were considered to delineate the “immunological space” to be covered by the pilot studies and to outline the primary hypotheses to be tested using several state-of-the-art technological approaches (Figure 1 and Table 2). Primary hypotheses and outcomes will be further refined based on results from the pilot studies.
Figure 1: Hypotheses and experimental approach.
Immune responses thought to be important for protection against TB have been identified and prioritized based on current knowledge. A systems immunology approach including several state-of-the-art assays will be employed to measure pre-selected outcomes as well as to generate new hypotheses (see text and Table 2 for more details). Most assays will be performed for both the BCG and M72/AS01E programs, those performed only for the M72/AS01E programs are denoted by *.
Table 2:
Experimental approaches used to measure outcomes within each immune compartment
| Immune compartment | Outcome measure | Assay |
|---|---|---|
| Antigen-specific T-cell responses | Functional, activation and memory profiles | - PBMC-ICS and flow cytometry - CITE-seq and Seq-Well S3 or 10x Genomics on sorted T cells* |
| Recognized epitopes | - Tetramer staining and flow cytometry* - IFNγ ELISpot* |
|
| TCR repertoire and gene expression | - Single-cell TCR sequencing on sorted cells (SMART-Seq2 or Seq-Well S3 or 10x Genomics)* - Immunoseq* |
|
| Secretion of immunomodulatory factors | - Multiplex Protein Detection Assay* | |
| Humoral responses | Ab titers | - Binding Ab multiplex assay (BAMA) |
| Ab sub-classes | ||
| Ab avidity | - Biolayer Interferometry (BLI) | |
| Fc-mediated functions | - Ab-dependent NK cell activation - Ab-dependent cellular phagocytosis - Ab-dependent complement deposition - Ab-dependent neutrophil phagocytosis - Neutrophil extracellular traps - Ab-depended dendritic cell phagocytosis - Fc receptor binding array |
|
| Ab specificity | - Linear peptide array* - Mtb proteome microarrays* - Phage immunoprecipitation* |
|
| Donor-unrestricted T cells | Functional, activation and memory profiles | - PBMC-ICS and flow cytometry |
| TCR repertoire and gene expression | - Single-cell TCR sequencing on sorted cells (SMART-Seq2 or Seq-Well S3 or 10x Genomics)* - Immunoseq* - CITE-seq and Seq-Well S3 or 10xGenomics on sorted T cells* |
|
| Phenotype and absolute counts | - Flow cytometry on fixed whole blood cells | |
| Trained innate immunity | Epigenetic profiles | - EpiTOF (mass cytometry) - Single-cell ATAC-seq - Long non-coding RNA qPCR* |
| Functional responses | - PBMC-ICS and flow cytometry - CITE-seq and Seq-Well S3 or 10x Genomics on bulk stimulated PBMC - Secreted immunomodulatory factors in response to heterologous stimuli (O-Link) |
|
| Cooperation between immune compartments | Mycobacterial growth inhibition | - Heterologous macrophage MGIA with autologous plasma - Heterologous whole blood MGIA with autologous plasma - Autologous PBMC and plasma MGIA* |
| Innate immunity / Milieu | Bulk PBMC functional, activation and memory profiles | - CITE-seq and Seq-Well S3 or 10xGenomics* on bulk stimulated PBMC |
| Immunophenotype and absolute counts | - Flow cytometry on fixed whole blood cells | |
| Gene expression profiles and transcriptomic TB signatures | - RNA sequencing on whole blood | |
| Apolipoproteins and complement | - Targeted LC/MS* | |
| Lipidomics | - LC-MS/MS* | |
| Proteomics | - LC-MS/MS* | |
| Metabolomics | - GC-MS* |
Abbreviations: PBMC = peripheral blood mononuclear cells; ICS = intra-cellular cytokine staining; TCR = T cell receptor; Ab = antibody; MGIA = mycobacterial growth inhibition assay; LC/MS = liquid chromatography and mass spectrometry; LC-MS/MS = liquid chromatography-tandem mass spectrometry; GC-MS = gas chromatography and mass spectrometry.
M72 only
CD4 T cells are considered the cornerstone of immunity against Mtb. Their antigen-specificity, functional and phenotypic profiles, differentiation and activation status, as well the capacity to home to the lung parenchyma are all considered key features of successful anti-Mtb immune responses. Two independent NHP studies using alternative BCG vaccination routes, mucosal or intra-venous, recently showed protection against Mtb infection and TB disease [24, 25]. In both studies, increased abundance of mycobacteria-specific Th1/Th17 cells in the lung was associated with protection. No CoPs were identified in peripheral blood in the mucosal BCG study [24], while further experiments and analyses are ongoing in the intra-venous NHP model [26]. Even more recently, another NHP study showed that frequencies of Th1/Th17 and T1/T17 and cytotoxic T cells measured within individual granulomas are associated with differential control of Mtb infection [27].
These data support the primary hypothesis that mycobacteria-specific CD4 T cells displaying a hybrid Th1/Th17 phenotype are the main mediators of a protective immune response to Mtb.
Antigen-specific T cell responses will be measured primarily by intra-cellular cytokine staining and flow cytometry after antigen stimulation of PBMC from both trials [28] (McElrath & Andersen-Nissen).
For the M72 Program, additional approaches include sorting of M72-specific T cells and single-cell analyses including DNA-tagged antibodies, TCR sequencing and RNA sequencing using different platforms (Musvosvi, Scriba & Shalek, McElrath & Andersen-Nissen). Since M72/AS01E only contains two Mtb antigens, further epitope mapping will be performed (Ernst & Altin), as well as broader measurements of immunomodulatory factors secreted upon PBMC stimulation with M72 by multiplex protein detection assay (McElrath & Andersen-Nissen).
Pathogen-specific antibodies are the primary CoPs for many effective vaccines, and multiple antibody functions beyond neutralization have been implicated in protection [29]. Studies conducted over a century ago showed some benefit of transferring serum from immunized horses to patients with TB, but antibodies to Mtb have not been consistently associated with protection (reviewed in [30, 31]). Recent studies using modern techniques have reinvigorated the hypothesis that antibodies may play a role in mycobacterial control, predominantly through Fc-receptor (FcR)-mediated functionality that can lead to killing of Mtb in infected cells [32]. The postulated beneficial role of NK cells reviewed in [33]) may well be linked to FcR-mediated effector functions.
These data support the co-primary hypothesis that antibody-dependent NK-cell activation contributes to control of Mtb.
Antibody sub-classes may also be important for protection, presumably via a different mechanism to antibody-dependent NK-cell activation. An NHP study that assessed protection against Mtb following mucosal BCG vaccination suggested that mycobacteria-specific IgA responses in bronchoalveolar lavage may correlate with protection [24]. In a different study, robust IgM responses induced in the blood and lungs by intravenous BCG vaccination of NHP were associated with protection and reduced mycobacterial burden [26].
Antibodies will be profiled for both Programs using several approaches, which include measurement of titers, sub-classes and avidity (Tomaras), as well as identification of Fc-mediated functions (Tomaras, Alter) and antibody-mediated mycobacterial growth inhibition (Alter). Peptides recognized by antibodies will be identified by phage immunoprecipitation (BCG, Rajan & Javid), peptide array (M72, Tomaras) and Mtb proteome microarrays (M72, Yee).
“Training” of monocytes/macrophages, and possibly NK cells, following innate immune activation by BCG or other pathogenic products has been well characterized [34] and has been associated with broad pathogen-agnostic protection against unrelated infections in humans [35]. AS01, the adjuvant used in the M72 vaccine, is known to potently activate the immune system [36]. It must, however, be acknowledged that trained immunity, as currently understood, may not be sufficiently long-lived to explain the durability of protection, which was observed up to 2 years after BCG re-vaccination and up to 3 years after M72/AS01E vaccination.
Nevertheless, investigation of the role of innate – including trained – immunity is warranted considering its possible role in inducing and supporting the development of long-lasting adaptive immune responses.
Since epigenetic re-programming is a key feature underlying trained immunity, it will be measured by EpiTOF [37] (Utz & Khatri) and single-cell ATAC-seq (BCG: Barreiro; M72: Pulendran). Immune-gene priming long non-coding RNAs regulate the deposition of H3K4me3 at the promoters of immune genes [38] and will be measured by qPCR (M72, Mhlanga & Netea). Functional responses to heterologous stimuli will be measured by O-Link in the supernatant of stimulated PBMC in both trials (Utz & Khatri).
Technological advances have also contributed to a much more refined understanding of non-classical, so-called donor-unrestricted T (DURT) cells, which recognize non-protein-based antigens, and their role in mycobacterial control [39]. It was recently shown that the frequencies of DURT cells were not modulated by primary vaccination or re-vaccination with BCG [40]; however, the effects of BCG re-vaccination on their functional or phenotypic attributes have not yet been fully explored. Presentation not only of peptides but also non-protein-based antigens by the non-polymorphic antigen-presenting molecules CD1, MR1 and HLA-E may contribute to the pool of protective mycobacterial T-cell responses induced by BCG re-vaccination. While clear correlations with Mtb infection have not been described for these T cell subsets, enrichment of mucosa-associated invariant T (MAIT) cells has been observed in exposed individuals who remain uninfected [41], supporting the hypothesis that BCG-induced DURTs contribute to the early control of Mtb infection.
DURT cell frequencies and absolute counts will be measured in fixed whole blood by flow cytometry (Nemes & Scriba) and their function by intra-cellular cytokine staining after PBMC stimulation (McElrath & Andersen-Nissen).
In addition to generating outcome measures related to our primary biological hypotheses, several assays that provide an unbiased view of vaccine-induced changes and are thus likely to generate new hypotheses, or define the systemic milieu in which immune responses are induced by vaccination, will be employed.
A mycobacteria growth inhibition assay [42, 43] will be used (Joosten & Ottenhoff) as a functional readout to determine whether M72/AS01E vaccination enhances overall mycobacterial growth control or even killing in vitro, as well as the relative contribution of cell-mediated and antibody-mediated immunity on this outcome.
Another hypothesis is that RNA sequencing (RNA-seq) analysis of whole blood, as well as single cells, will identify novel gene expression profiles, cellular subsets or pathways associated with protection against infection and/or disease (BCG: Shalek, M72: Musvosvi, Scriba & Shalek, McElrath & Andersen-Nissen). The whole blood RNA-seq dataset from the M72/AS01E trial will also be mined to test the hypotheses that blood transcriptomic signatures of risk of TB, which allow identification of individuals in early stages of disease progression, or with subclinical disease [44–46], are elevated in M72 trial participants who developed TB within the first year [46] compared to non-cases (Scriba & Fiore-Gartland). Further, blood transcriptomic signatures associated with protection identified in animal models [47, 48] will be assessed for increased expression in non-cases compared to cases (Duffy & Nemeth).
To test the hypothesis that vaccination with M72/AS01E elicits a multimolecular biosignature in participant plasma correlating with protection from active TB [49–51], lipidomics, metabolomics and proteomics approaches (Tafesse & Lewinsohn) as well as targeted measurements of apolipoproteins and complement proteins (Steen & Levy) will be undertaken.
Finally, phenotyping of whole blood leucocyte populations provides a snapshot of immune status in the absence of stimulation. Baseline expression of the activation marker HLA-DR on T cells [52], increased myeloid to lymphoid cell ratio [53] and reduced abundance of NK cells [54] have all been associated with increased risk of TB and will be assessed as biomarkers of risk of TB, or as CoPs. Whole blood immunophenotyping and absolute counts will be performed by flow cytometry [55] (Nemes and Scriba).
Statistical considerations
The primary objective of the statistical analyses for the Pilot Study is to describe and rank assay readouts based on their potential to predict sustained infection or TB disease in future case-cohort analyses. Participants were selected for the Pilot Study at random from among the vaccine and placebo recipients that did not meet any of the trial endpoint criteria. Each lab will analyze a baseline and post-immunization (BCG: Day 70 post-BCG; M72/AS01E: Day 37, one week post-2nd injection) sample from a subset of vaccine (n=64) and placebo (n=22) recipients.
A head-to-head analysis will be conducted by the statistical team to quantify vaccine-induced immune responses, characterize performances of the assays, and identify a set of low-dimensional biomarkers. These analyses will inform decisions about which assays to prioritize for the Primary analysis of cases and non-cases.
Assay and readout performance will be evaluated using the following criteria:
Broad, biologically-relevant dynamic range among vaccine recipients after vaccination
Broad, biologically-relevant dynamic range among all participants at baseline
Low intra-individual temporal variability among placebo recipients
Large shift in the distribution among vaccine vs. placebo recipients (i.e., evidence of a vaccine-induced response)
Low technical measurement error
Low covariation among readouts within each assay, with a low-dimensional representation of the measured response
Low covariation of readouts across assays, reducing overlap and redundancy in immunological space
Broad coverage of relevant immune functions
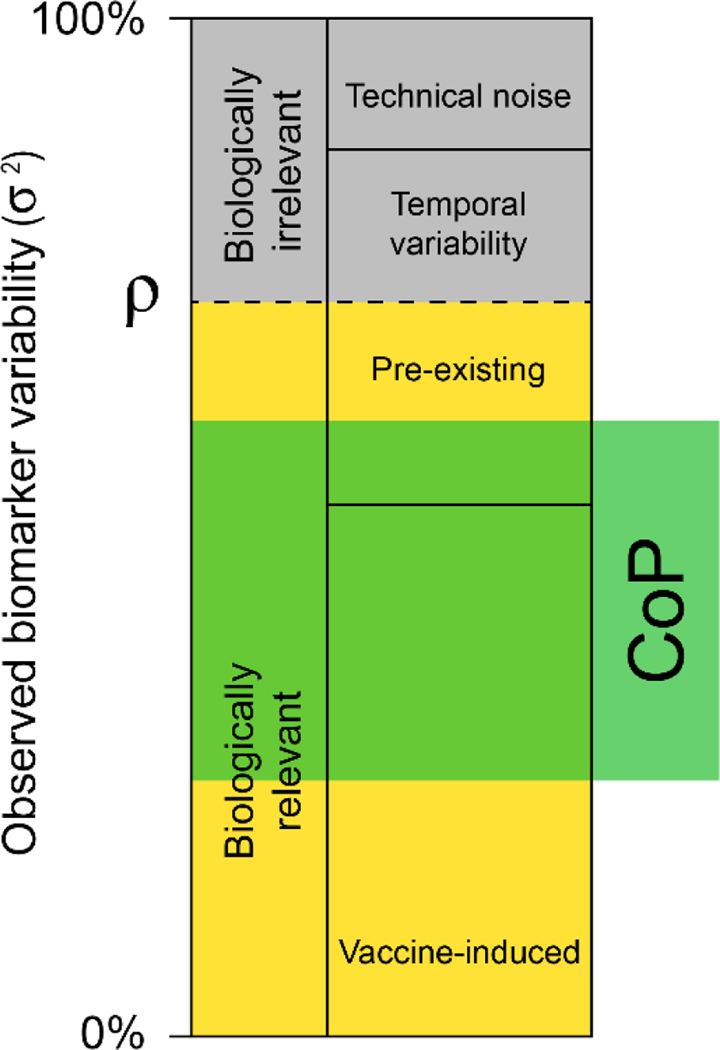
With these criteria we will attempt to deconstruct the variance of each readout into the components of vaccine-induced and non-vaccine-induced variation. We will also seek to select assays and readouts that maximize the proportion of variability that could possibly correlate with TB risk or vaccine protection (Figure 2).
Figure 2: Partitioning of biomarker variability.
The inter-vaccinee variance of each biomarker is made up of biologically relevant and irrelevant components. Irrelevant components contain measurement error and types of temporal variability that cannot be correlated with protection; measurement error can be estimated from technical replicates while temporal variability can be estimated from longitudinal sampling of placebos. Pre-existing and vaccine-induced variability in the marker can both be correlated with protection. The biologically relevant proportion of variation (ρ) can be affected by pre-existing factors like host-genetics (e.g., HLA, TLR SNPs), microbiome, or pre-existing immunity to Mtb.
The sample size required to evaluate these outcomes in the Pilot studies, considering the expected variability of baseline BCG- and M72-specific responses as well as the number of cases available for the final analysis of each trial (Table 1), was determined to be 24 BCG and 12 placebo recipients for the BCG Program, and 40 M72/AS01E and 10 placebo recipients for the M72 Program [56].
The goal of the Primary statistical analysis will be to evaluate each biomarker and combinations of biomarkers as correlates of risk (CoRs) and CoPs using a “case-cohort” design, including cases and non-cases from the randomized vaccine and placebo treatment groups.
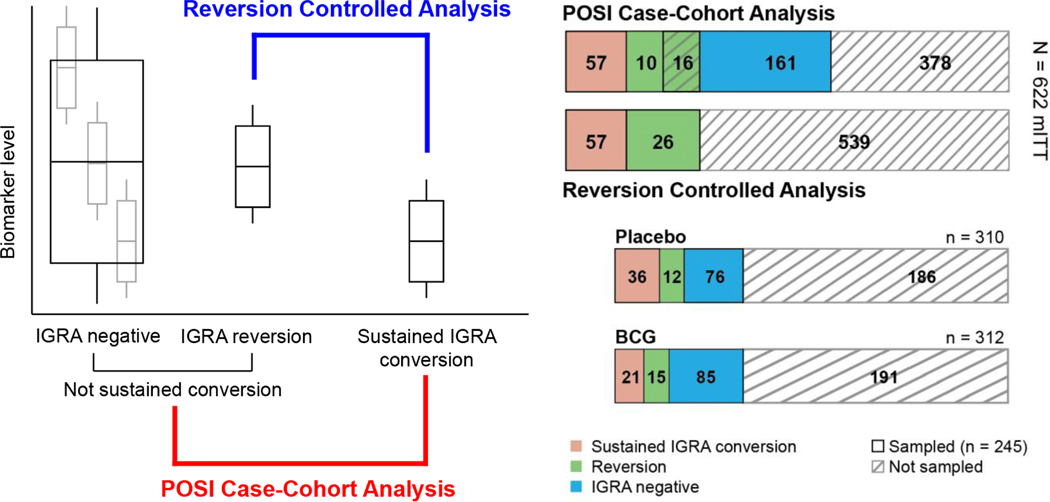
For the BCG Program, two different case-cohort analyses will be performed (Figure 3). In the “modified intent-to-treat (mITT)-controlled analysis” sustained IGRA converters will be compared to participants who did not display sustained IGRA conversion, i.e., a combination of participants who remained IGRA-negative throughout the study and those who showed initial IGRA conversion, but subsequently reverted to IGRA-negative (“IGRA reverters”). In the “reversion-controlled analysis” sustained IGRA converters will be compared to IGRA reverters only. The all mITT-controlled analysis includes participants who remained IGRA negative throughout the study, who likely include a combination of non-exposed individuals as well as exposed individuals in whom vaccine-induced responses or natural immunity were able to prevent initial infection. The latter group is impossible to unequivocally identify in this study since exposure (e.g., to household members with active disease) was not measured for participants. The reversion-controlled analysis on the other hand includes subjects who were clearly exposed since they showed initial IGRA conversion, but then presumably were able to control infection, allowing them to revert to IGRA negative. This analysis therefore controls for exposure, although it does exclude exposed participants who do not convert. In both types of analysis, immune markers that are enriched in vaccine recipients who do not become sustained converters may be identified as putative CoPs. The total number of participants in each of the groups is shown in Figure 3 (n=57 cases and n=187 non-cases).
Figure 3:
Sampling strategy for the BCG re-vaccination prevention of sustained infection (POSI) case-cohort immune correlates study. The correlates analysis will include two distinct comparisons: (1) Sustained IGRA conversion vs. no sustained conversion (POSI Case-Cohort Analysis), and (2) Sustained IGRA conversion vs. IGRA reversion (Reversion Controlled Analysis). The POSI Case-Cohort Analysis samples controls from participants without a sustained IGRA conversion, including a mixture of potentially exposed, unexposed and IGRA converted-reverted. In contrast, the Reversion Controlled Analysis conditions on initial conversion, excluding unexposed individuals and focusing on the phenotypic differences that distinguish reversion from sustained infection.
For the M72/AS01E Program, all cases (n=39) meeting the primary TB disease endpoint definition will be included [20]. A higher non-case to case ratio of samples has been proposed for the M72 study (5:1, n=195 non-cases) compared to the BCG study (3:1) to increase statistical power [57] and adapt for the fewer number of cases in the M72 study (Table 1). Since the BCG study was conducted at two clinical sites in the same province in South Africa [58], no site-specific considerations were required for the selection of non-cases. However, the M72/AS01E trial was conducted at 11 clinical sites across three African countries and significant differences in immunogenicity were noted when comparing participants recruited in South Africa and Kenya [20]. Recruitment site will therefore be an important variable to consider in the selection of non-cases.
CoPs will be evaluated using a range of statistical methods including covariate-adjusted regression to evaluate correlates of risk within each treatment group, a vaccine efficacy modification or “principal stratification” framework to estimate vaccine efficacy as a function of the vaccine-induced biomarker [56, 59], a causal mediation framework to estimate the proportion of the efficacy that can be explained by the biomarker [60] and a machine learning framework, which frames the analysis as a multivariate classification of cases vs. non-cases [61]. Analyses will leverage existing code that was developed and implemented for studying CoRs and CoPs in the US government COVID-19 vaccine trials, including an open-source statistical analysis plan and open-source codebase [62]. Similarly, all Primary analyses will be pre-specified in a statistical analysis plan [63].
Exploratory analyses will also be conducted to evaluate a broader set of potential biomarkers and to infer mechanistic insights from their attributions to protective immunity. With multi-scale cross-cell type “omics” data generated in the case-cohort studies, an integrative multivariate framework that directly models data from several platforms will provide an insightful view of the cross-talk between immune cell types while simultaneously identifying new candidate biomarkers. Specifically, supervised integration models will identify a set of biomarkers that maximize the covariance with phenotypic outcomes while considering interactions among multiple data modalities. In addition, a module-based method transforming genes/proteins/metabolites into pathways/cellular responses based on prior biological knowledge will be integrated into the multivariate modeling to improve data interpretation. As there are many new emerging multi-omic predictive algorithms [64, 65], we are benchmarking existing algorithms holding robust performance and applicable to multi-scale cross-cell type data modalities. Moreover, network analyses through partial correlation networks or Gaussian/mixed graphical models from data integration will also be performed to infer potential functional linkages between immune responses and vaccine efficacy. These descriptive analyses will generate novel hypotheses that may be evaluated in future studies.
Progress and hurdles
The BCG Program was launched in late 2018, pilot studies have been completed, data review is expected in October 2022, followed by a swift selection of the assays that will be included for the Primary analysis, which should be completed in 2023. The M72/AS01E CoPs Program was launched in early 2020 and Pilot studies are expected to start in late 2022. Both TB Immune Correlates Programs have been heavily affected by disruptions caused by the COVID-19 pandemic, including closure of laboratories in 2020 and general institutional de-prioritization of non-COVID-related research. Additionally, recent changes in South African data privacy legislation (the Protection of Personal Information Act [POPI Act], https://popia.co.za/) resulted in significant delays in the fulfilment of regulatory and contractual requirements due to the unfamiliarity of participating research institutions with the requirements set forth in the act.
The decision to issue open calls for proposals and to establish two large consortia of international investigators was made to ensure that the best possible scientific expertise and state-of-the-art technologies were deployed in the fight against TB, with a spirit of collaboration and data sharing. The hurdles in fulfilling regulatory and contractual requirements involving multiple partners in a timely fashion were initially under-estimated and resulted in significant delays.
Concluding remarks
The year 2018 was dubbed “the year of TB vaccines” reflecting the publication of vaccine efficacy results for BCG revaccination (POSI) and M72/AS01E (POD). The scientific community has been waiting in hopeful anticipation for the identification of immune correlates of protection against TB, which can be discovered now that randomized placebo-controlled clinical trials of partially efficacious vaccines have been completed. The COVID-19 pandemic has greatly affected TB patient management and research programs around the world, and caused much frustration within the TB research community, which has been chronically under-funded and de-prioritized, despite focusing on the infectious disease that has killed most humans (and continues to do so) in history. The speed and success of COVID-19 research is inspiring, and provides new hope that when vaccine developers, funders, and scientists establish collaborative partnerships with strong political and public support the unimaginable (several COVID-19 vaccines developed at warp speed and identification of the first correlates of protection months thereafter) can happen [62, 66]. This sense of urgency and scale now needs to be applied to TB vaccines.
Translation Insight
A CoPs for one or both of these TB vaccines has enormous potential to accelerate vaccine development and the clinical development pathway. A “mechanistic CoPs” [67], established by these studies and validated in follow-up experiments, can inform the field about the roles of host immune responses in Mtb infection and disease progression, thereby facilitating iterative vaccine design and prioritization of vaccines in the clinical pipeline. However, a CoPs does not need to be mechanistic to be clinically valuable; a validated statistical CoPs - with known or unknown protective mechanisms - can be used to de-risk clinical development and accelerate vaccine licensure through “immuno-bridging”. With a validated CoPs a vaccine could even be licensed on the basis of meeting specific immunogenicity criteria developed from CoPs studies of a previously licensed vaccine [68]. Therefore, establishing a CoPs for PO(S)I or POD based on the BCG and M72 studies could have implications for TB vaccine development well beyond these two vaccines. Candidate CoPs identified by the studies described here will require independent validation, which is possible by leveraging a larger BCG re-vaccination POSI trial ongoing in South Africa (NCT04152161) and a phase 3 trial with M72/AS01E, which is currently being planned.
Financial disclosure/acknowledgments
MC is an employee of the GSK group of companies and holds shares in the GSK group of companies. OL is a named inventor on several patents related to human in vitro systems and vaccine adjuvants. All other authors declare no financial conflicts of interest.
We would like to thank our Scientific Advisory Committee (Alvaro Borges, Rhea Coler, Mark Davis, Tom Evans, Helen Fletcher, Sarah Fortune, Willem Hanekom, Anne Kasmar, Shabaana Khader, James Kublin, Sarah Mudrak, Mario Roederer, Lew Schrager, Robert Seder, Divya Shah, Kevin Urdahl and Robert Wilkinson) for their advice and support in identifying the laboratory partners that make up the BCG and M72 Correlates Study Groups.
We thank the members of the Biospecimen Access Oversight Committees for the Aeras C-040–404 and C-041–972 studies (Alexander Schmidt, Ann Ginsberg, Dereck Tait, Willem Hanekom, Jim Tartaglia, Morten Ruhwald, Mark Hatherill, Heather Siefers, Dominick Laddy and Lewis Schrager) for approving the use of specimens from these studies for the Immune Correlates Program.
We would like to thank NIAID and the Bill & Melinda Gates Foundation for their funding of the TB Immune Correlates Program.
We are grateful to the Clinical Trial teams for these studies, all study site personnel, and especially the trial participants for providing this unique opportunity to identify correlates of protection for TB vaccines.
Footnotes
BCG Correlates PIs Study Team:
Galit Alter
Erica Andersen-Nissen
Luis Barreiro
One Dintwe
Andrew Fiore-Gartland
Ollivier Hyrien
Claire Imbratta
Babak Javid
Purvesh Khatri
Hadar Malca
Elisa Nemes
Gerlinde Obermoser
Jayant Rajan
Thomas Scriba
S. Moses Dennison
Alex K. Shalek
Georgia Tomaras
Nancy Tran
PJ Utz
M72 Correlates PIs Study Team:
John Aitchison
Galit Alter
Erica Andersen-Nissen
One Dintwe
Fergal Duffy
Joel Ernst
Andrew Fiore-Gartland
Ollivier Hyrien
Claire Imbratta
Simone A. Joosten
Purvesh Khatri
Ofer Levy
David Lewinsohn
Holden Maecker
Hadar Malca
Musa Mhlanga
Munyaradzi Musvosvi
Elisa Nemes
Mihai Netea
Tom H.M. Ottenhoff
Bali Pulendran
Thomas Scriba
S. Moses Dennison
Alex K. Shalek
Hanno Steen
Fikadu G. Tafesse
Georgia Tomaras
Nancy Tran
PJ Utz
Angela Yee
References
- 1.Pai M, et al. , Tuberculosis. Nat Rev Dis Primers, 2016. 2: p. 16076. [DOI] [PubMed] [Google Scholar]
- 2.Comas I, et al. , Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet, 2013. 45(10): p. 1176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufmann SHE, Vaccine Development Against Tuberculosis Over the Last 140 Years: Failure as Part of Success. Front Microbiol, 2021. 12: p. 750124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO, Global tuberculosis report. 2021. [Google Scholar]
- 5.Houben RM and Dodd PJ, The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med, 2016. 13(10): p. e1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pai M, et al. , Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev, 2014. 27(1): p. 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emery JC, et al. , Self-clearance of Mycobacterium tuberculosis infection: implications for lifetime risk and population at-risk of tuberculosis disease. Proc Biol Sci, 2021. 288(1943): p. 20201635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lancione S, et al. , Tracking changes in national BCG vaccination policies and practices using the BCG World Atlas. BMJ Glob Health, 2022. 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangtani P, et al. , Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis, 2014. 58(4): p. 470–80. [DOI] [PubMed] [Google Scholar]
- 10.Roy A, et al. , Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ, 2014. 349: p. g4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angelidou A, et al. , BCG as a Case Study for Precision Vaccine Development: Lessons From Vaccine Heterogeneity, Trained Immunity, and Immune Ontogeny. Front Microbiol, 2020. 11: p. 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brook B, et al. , A place for neutrophils in the beneficial pathogen-agnostic effects of the BCG vaccine. Vaccine, 2022. 40(11): p. 1534–1539. [DOI] [PubMed] [Google Scholar]
- 13.Knight GM, et al. , Impact and cost-effectiveness of new tuberculosis vaccines in low- and middle-income countries. Proc Natl Acad Sci U S A, 2014. 111(43): p. 15520–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris RC, et al. , Potential impact of tuberculosis vaccines in China, South Africa, and India. Sci Transl Med, 2020. 12(564). [DOI] [PubMed] [Google Scholar]
- 15.Ellis RD, et al. , Innovative clinical trial designs to rationalize TB vaccine development. Tuberculosis (Edinb), 2015. 95(3): p. 352–7. [DOI] [PubMed] [Google Scholar]
- 16.Andersen P.and Scriba TJ, Moving tuberculosis vaccines from theory to practice. Nat Rev Immunol, 2019. [DOI] [PubMed] [Google Scholar]
- 17.Nemes E, et al. , Prevention of M. tuberculosis Infection with H4:IC31 Vaccine or BCG Revaccination. N Engl J Med, 2018. 379(2): p. 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rozot V, et al. , Multidimensional analyses reveal modulation of adaptive and innate immune subsets by tuberculosis vaccines. Commun Biol, 2020. 3(1): p. 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Der Meeren O, et al. , Phase 2b Controlled Trial of M72/AS01E Vaccine to Prevent Tuberculosis. New England Journal of Medicine, 2018. 379(17): p. 1621–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tait DR, et al. , Final Analysis of a Trial of M72/AS01E Vaccine to Prevent Tuberculosis. N Engl J Med, 2019. [DOI] [PubMed] [Google Scholar]
- 21.Penn-Nicholson A, et al. , Safety and immunogenicity of candidate vaccine M72/AS01E in adolescents in a TB endemic setting. Vaccine, 2015. 33(32): p. 4025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scriba TJ, Netea MG, and Ginsberg AM, Key recent advances in TB vaccine development and understanding of protective immune responses against Mycobacterium tuberculosis. Semin Immunol, 2020. 50: p. 101431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen SE, et al. , It Takes a Village: The Multifaceted Immune Response to Mycobacterium tuberculosis Infection and Vaccine-Induced Immunity. Front Immunol, 2022. 13: p. 840225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dijkman K, et al. , Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat Med, 2019. 25(2): p. 255–262. [DOI] [PubMed] [Google Scholar]
- 25.Darrah PA, et al. , Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature, 2020. 577(7788): p. 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irvine EB, et al. , Robust IgM responses following intravenous vaccination with Bacille Calmette-Guerin associate with prevention of Mycobacterium tuberculosis infection in macaques. Nat Immunol, 2021. 22(12): p. 1515–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gideon HP, et al. , Multimodal profiling of lung granulomas in macaques reveals cellular correlates of tuberculosis control. Immunity, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dintwe O, et al. , OMIP-056: Evaluation of Human Conventional T Cells, Donor-Unrestricted T Cells, and NK Cells Including Memory Phenotype by Intracellular Cytokine Staining. Cytometry A, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plotkin SA, Correlates of protection induced by vaccination. Clin Vaccine Immunol, 2010. 17(7): p. 1055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glatman-Freedman A.and Casadevall A, Serum therapy for tuberculosis revisited: reappraisal of the role of antibody-mediated immunity against Mycobacterium tuberculosis. Clin Microbiol Rev, 1998. 11(3): p. 514–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rijnink WF, Ottenhoff THM, and Joosten SA, B-Cells and Antibodies as Contributors to Effector Immune Responses in Tuberculosis. Front Immunol, 2021. 12: p. 640168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu LL, et al. , A Functional Role for Antibodies in Tuberculosis. Cell, 2016. 167(2): p. 433–443 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemes E, et al. , Targeting Unconventional Host Components for Vaccination-Induced Protection Against TB. Front Immunol, 2020. 11: p. 1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khader SA, et al. , Targeting innate immunity for tuberculosis vaccination. J Clin Invest, 2019. 129(9): p. 3482–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prentice S, et al. , BCG-induced non-specific effects on heterologous infectious disease in Ugandan neonates: an investigator-blind randomised controlled trial. Lancet Infect Dis, 2021. 21(7): p. 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Didierlaurent AM, et al. , Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J Immunol, 2014. 193(4): p. 1920–30. [DOI] [PubMed] [Google Scholar]
- 37.Cheung P, et al. , Single-cell epigenetics - Chromatin modification atlas unveiled by mass cytometry. Clin Immunol, 2018. 196: p. 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fanucchi S, et al. , Immune genes are primed for robust transcription by proximal long noncoding RNAs located in nuclear compartments. Nat Genet, 2019. 51(1): p. 138–150. [DOI] [PubMed] [Google Scholar]
- 39.Joosten SA, et al. , Harnessing donor unrestricted T-cells for new vaccines against tuberculosis. Vaccine, 2019. 37(23): p. 3022–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gela A, et al. , Effects of BCG vaccination on donor unrestricted T cells in two prospective cohort studies. EBioMedicine, 2022. 76: p. 103839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vorkas CK, et al. , Mucosal-associated invariant and gammadelta T cell subsets respond to initial Mycobacterium tuberculosis infection. JCI Insight, 2018. 3(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brennan MJ, et al. , The Cross-Species Mycobacterial Growth Inhibition Assay (MGIA) Project, 2010–2014. Clin Vaccine Immunol, 2017. 24(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joosten SA, et al. , Mycobacterial growth inhibition is associated with trained innate immunity. J Clin Invest, 2018. 128(5): p. 1837–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zak DE, et al. , A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet, 2016. 387(10035): p. 2312–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Penn-Nicholson A, et al. , RISK6, a 6-gene transcriptomic signature of TB disease risk, diagnosis and treatment response. Sci Rep, 2020. 10(1): p. 8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scriba TJ, et al. , Biomarker-guided tuberculosis preventive therapy (CORTIS): a randomised controlled trial. Lancet Infect Dis, 2021. 21(3): p. 354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duffy FJ, et al. , A contained Mycobacterium tuberculosis mouse infection model predicts active disease and containment in humans. J Infect Dis, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansen SG, et al. , Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat Med, 2018. 24(2): p. 130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiner J 3rd, et al. , Metabolite changes in blood predict the onset of tuberculosis. Nat Commun, 2018. 9(1): p. 5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duffy FJ, et al. , Immunometabolic Signatures Predict Risk of Progression to Active Tuberculosis and Disease Outcome. Front Immunol, 2019. 10: p. 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Penn-Nicholson A, et al. , Discovery and validation of a prognostic proteomic signature for tuberculosis progression: A prospective cohort study. PLoS Med, 2019. 16(4): p. e1002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fletcher HA, et al. , T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat Commun, 2016. 7: p. 11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fletcher HA, et al. , Human newborn bacille Calmette-Guerin vaccination and risk of tuberculosis disease: a case-control study. BMC Med, 2016. 14: p. 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roy Chowdhury R, et al. , A multi-cohort study of the immune factors associated with M. tuberculosis infection outcomes. Nature, 2018. 560(7720): p. 644–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nemes E, et al. , Differential leukocyte counting and immunophenotyping in cryopreserved ex vivo whole blood. Cytometry A, 2015. 87(2): p. 157–65. [DOI] [PubMed] [Google Scholar]
- 56.Gilbert PB and Huang Y, Predicting Overall Vaccine Efficacy in a New Setting by Re-Calibrating Baseline Covariate and Intermediate Response Endpoint Effect Modifiers of Type-Specific Vaccine Efficacy. Epidemiol Methods, 2016. 5(1): p. 93–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gilbert PB, Janes HE, and Huang Y, Power/sample size calculations for assessing correlates of risk in clinical efficacy trials. Stat Med, 2016. 35(21): p. 3745–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nemes E, et al. , Optimization and interpretation of serial QuantiFERON testing to measure acquisition of Mycobacterium tuberculosis Infection. Am J Respir Crit Care Med, 2017. 196(5): p. 638–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhuang Y, Huang Y, and Gilbert PB, Evaluation of treatment effect modification by biomarkers measured pre- and post-randomization in the presence of non-monotone missingness. Biostatistics, 2022. 23(2): p. 541–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cowling BJ, et al. , Influenza Hemagglutination-inhibition Antibody Titer as a Mediator of Vaccine-induced Protection for Influenza B. Clin Infect Dis, 2019. 68(10): p. 1713–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Price BL, Gilbert PB, and van der Laan MJ, Estimation of the optimal surrogate based on a randomized trial. Biometrics, 2018. 74(4): p. 1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gilbert PB, et al. , Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science, 2022. 375(6576): p. 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.USG COVID-19 Response Team / Coronavirus Prevention Network (CoVPN) Biostatistics Team, P.B.G., Fong Youyi, Benkeser David, Andriesen Jessica, Bhavesh BorateBhavesh Borate, Marco Carone, Lindsay N Carpp CarppLindsay N., Diaz Ivan, Fay Michael P., Fiore-GartlandAndrew Fiore-Gartland Andrew, Hejazi Nima S., Huang Ying, Huang Yunda, Hyrien Ollivier, Janes Holly E., Juraska Michal, Li Kendrick, Luedtke Alex, Nason Martha, Randhawa April K., van der Laan Lars, Williamson Brian, Zhang Wenbo, Follmann Dean, USG COVID-19 Response Team / CoVPN Vaccine Efficacy Trial Immune Correlates Statistical Analysis Plan, in Online resource, Figshare. 2022. [Google Scholar]
- 64.Rappoport N.and Shamir R, Multi-omic and multi-view clustering algorithms: review and cancer benchmark. Nucleic Acids Res, 2018. 46(20): p. 10546–10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duan R, et al. , Evaluation and comparison of multi-omics data integration methods for cancer subtyping. PLoS Comput Biol, 2021. 17(8): p. e1009224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bok K, et al. , Accelerated COVID-19 vaccine development: milestones, lessons, and prospects. Immunity, 2021. 54(8): p. 1636–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Plotkin SA and Gilbert PB, Nomenclature for immune correlates of protection after vaccination. Clin Infect Dis, 2012. 54(11): p. 1615–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gilbert PB, et al. , Bridging Efficacy of a Tetravalent Dengue Vaccine from Children/Adolescents to Adults in Highly Endemic Countries Based on Neutralizing Antibody Response. Am J Trop Med Hyg, 2019. 101(1): p. 164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]