Abstract
This communication discloses the first examples of aza-Wacker cyclizations of sulfamate esters. Within the realm of related cyclization reactions, this protocol is differential in that it forms 6-membered rings in good yield and uses catalytic amounts of palladium (0) rather than palladium (II) salts. These reactions scale well, and their products are demonstrated to be valuable synthetic intermediates.
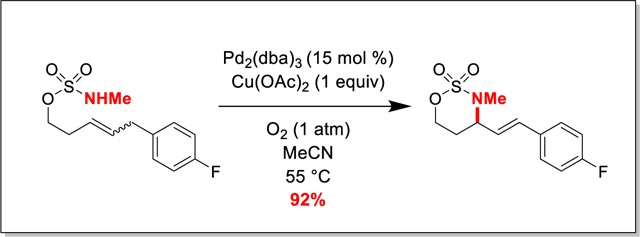
Graphical Abstract
The oxidative functionalization of unsaturated moieties remains an area of vigorous research activity.1–5 Within the realm of alkene functionalization, Wacker-type cyclizations of alcohols have been extensively investigated.6–15 In sharp contrast, the analogous cyclization of nitrogen moieties onto alkenes, the aza-Wacker reaction, remains relatively under-explored.16–18 Inspired by the pioneering studies of Åkermark, Bäckvall, Zetterberg19–22 and Hegedus23–25 as well as important recent contributions from Stoltz,26 Zhang,27–29 and Stahl,30–34 among others,35–41 we chose the aza-Wacker cyclization as a point of focus. Elegant aza-Wacker cyclization reactions have been disclosed with protected amines,36, 37, 42 amides,26, 28, 43 aminals,35 and hemiaminals.33 With few exceptions,33, 44 the majority of these protocols have been developed with alkenyl amines and require the nitrogen functionality to be native to the molecule. Furthermore, examples of aza-Wacker protocols that form heterocyclic rings containing more than 5 atoms remain extremely limited.31, 37, 45–49 As the alcohol functional group is ubiquitous in organic molecules, a general protocol where a nitrogen containing auxiliary could be affixed to an alkenyl alcohol and then oxidatively cyclized would be highly desirable. We envisioned a reaction where diverse sulfamate auxiliaries would be easily appended to alcohols and then cyclized to form six-membered and larger rings in an aza-Wacker type process.50–53
In this communication, we disclose the first examples of aza-Wacker cyclizations of sulfamate esters. These reactions reliably yield valuable 6-membered oxathiazinane heterocycles with pendant unsaturation. The oxathiazinane moiety is a masked 1,3-amino alcohol, a motif found in myriad biologically active compounds (Figure 1), and can be used as a synthetic intermediate for a variety of transformations.54–58 Our protocol, catalytic in palladium and reliant on Cu(OAc)2/O2 as stoichiometric oxidants, offers convenient access to these important synthetic intermediates (Scheme 1).
Figure 1.
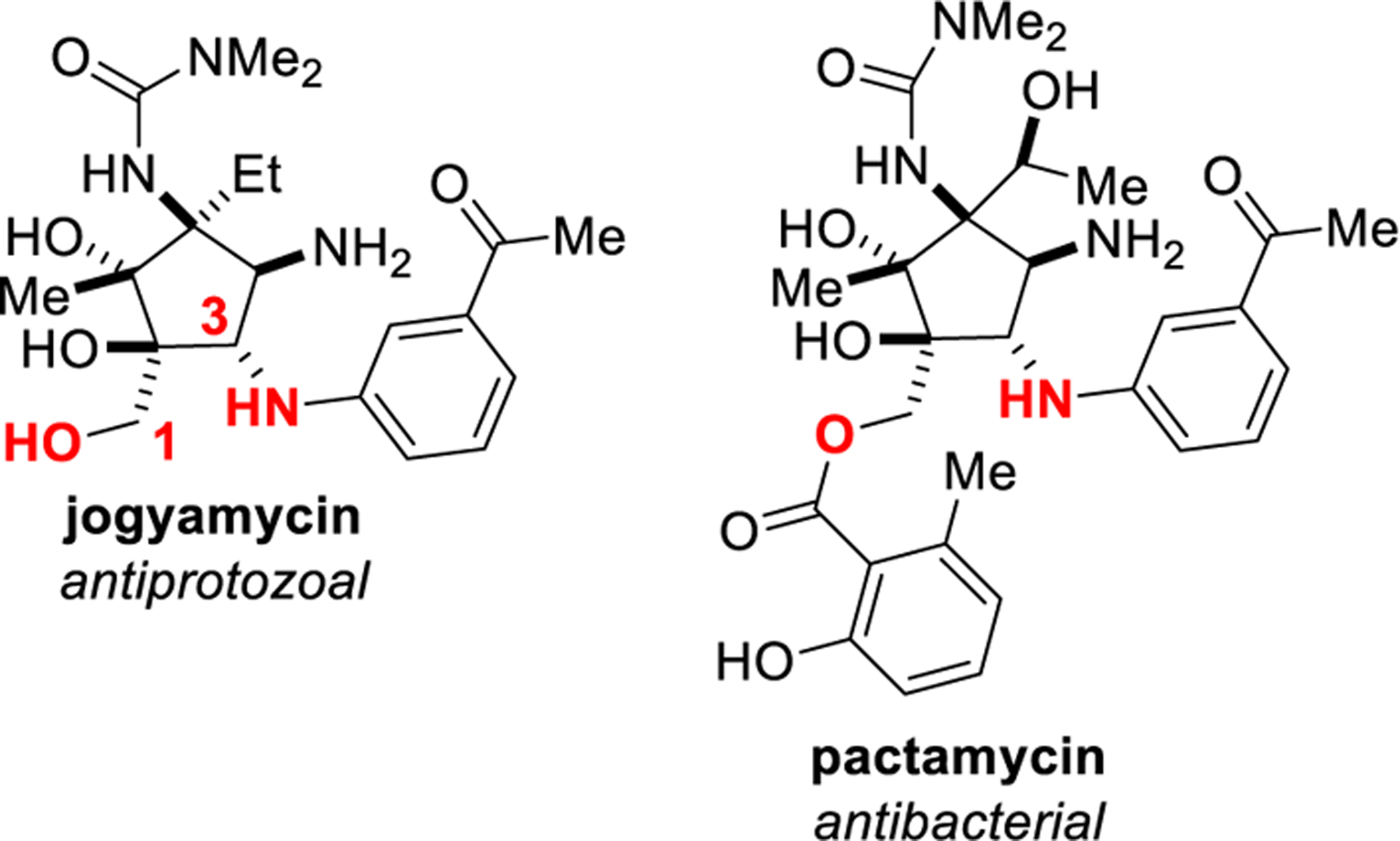
1,3-amino alcohols are vital structural elements in biologically active molecules.
Scheme 1.
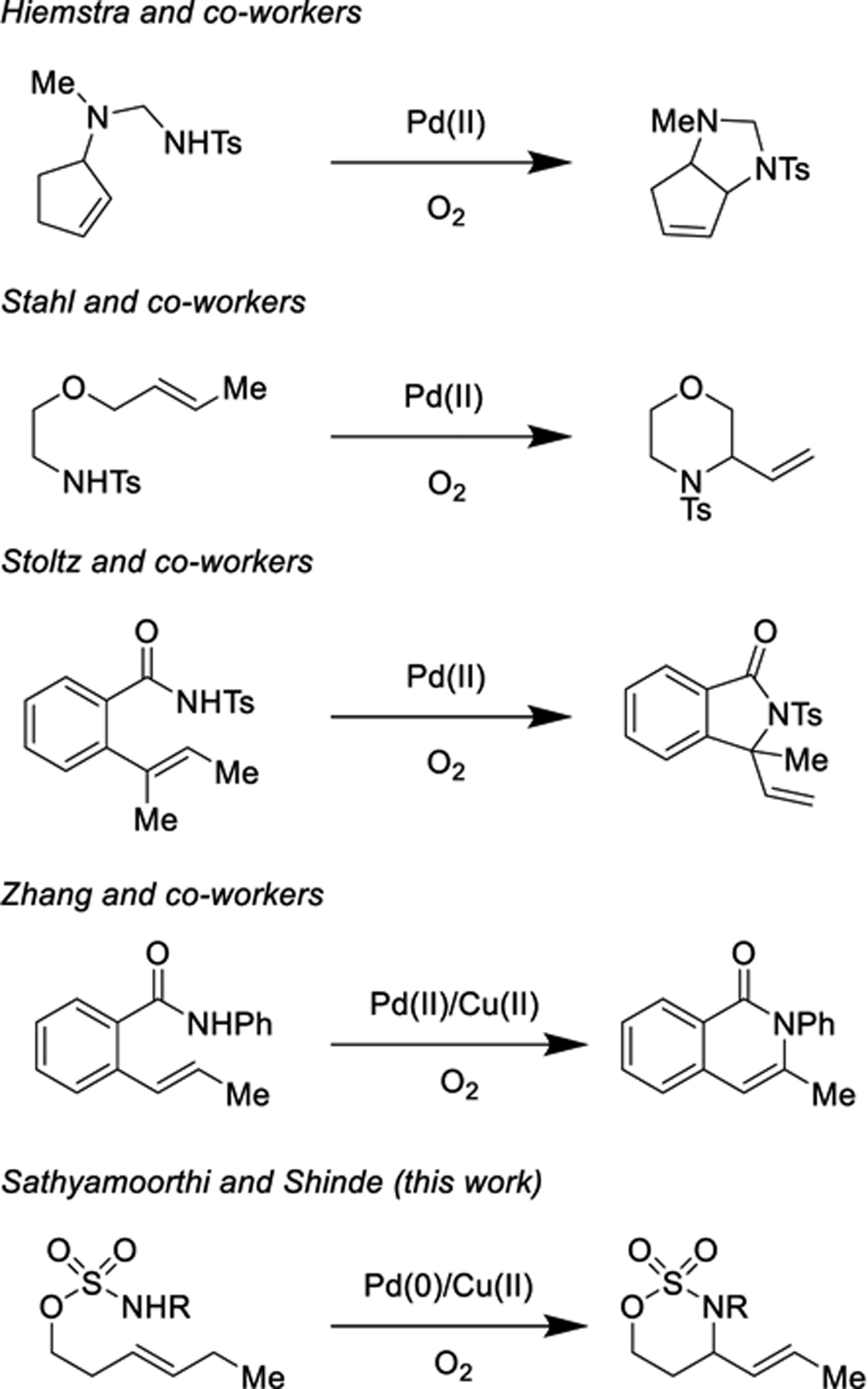
Literature precedent inspires an aza-Wacker cyclinzation of sulfamate esters.
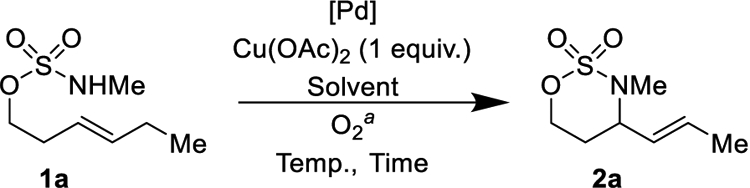
Optimization of an oxidative cyclization of alkenyl sulfamate esters was performed with (E)-hex-3-en-1-yl methylsulfamate 1a, prepared in a single step from commercially available trans-3-hexen-1-ol (Table 1). Using Andersson’s protocol for oxidative tosylamine cyclization Pd(OAc)2/O2 in DMSO, (See Supporting Information for reaction conditions),36 the conversion of 1a into 2a was approximately 30%. While modest, this important result gave us hope that this reaction could be further developed. Augmenting these conditions with Cu(OAc)259 boosted the yield of 2a to 46% (Table 1, Entry 1). Switching solvents from DMSO to THF (Table 1, Entry 2), toluene (Table 1, Entry 3), or methanol (Table 1, Entry 4) was deleterious. In CH3CN, the yield of 2a was similar to that in DMSO (Table 1, Entry 5). Increasing the pressure of O2 from 1 atm to 4 atm31 did little (Table 1, Entry 6) to increase yield. Switching to palladium chloride salts was markedly deleterious (Table 1, Entries 7–8). In contrast to other aza-Wacker reactions, we saw no advantage with Pd(TFA)2 relative to Pd(OAc)2 (Table 1, Entry 9). Increasing the reaction temperature from 55 °C to 80 °C (Table 1, Entry 10) conferred a modest 5% boost in yield. At 55 °C, increasing the reaction time from 17 h to 26 h was similarly beneficial (Table 1, Entry 11). In conjunction with Pd(OAc)2, bidentate ligands [(PhSO)2, DPPE, bipyridine (Table 1, Entry 12 and Supplementary Table 1)] and monodentate ligands [PPh3, P(OiPr)3, IMes, pyridine (Supplementary Table 1)] were invariably deleterious. To our great surprise, switching to catalytic Pd2(dba)3 (Table 1, Entries 13–14) improved the reaction yields dramatically. To our knowledge, this is the first disclosure of an aza-Wacker cyclization that employs a Pd(0) pre-catalyst.
Table 1.
Optimization of reaction conditions.
| ||||
|---|---|---|---|---|
| Entry | [Pd] | Solvent | Temp., Time | 2/1b |
| 1c | Pd(OAc)2 (10%) | DMSO | 55° C, 17 h | 46:54 |
| 2 | Pd(OAc)2 (10%) | THF | 55° C, 17 h | NR |
| 3 | Pd(OAc)2 (10%) | Toluene | 55° C, 17 h | NR |
| 4d | Pd(OAc)2 (10%) | MeOH | 55° C, 17 h | 40:25 |
| 5 | Pd(OAc)2 (10%) | CH3CN | 55° C, 17 h | 50:50 |
| 6 | Pd(OAc)2 (10%) | CH3CN | 55° C, 17 h | 53:39 |
| 7 | Pd(CH3CN)2(Cl)2 (10%) | CH3CN | 55° C, 17 h | 30:30 |
| 8 | Pd(DPPF)Cl2 (10%) | CH3CN | 55° C, 17 h | NR |
| 9 | Pd(TFA)2 (10%) | CH3CN | 55° C, 17 h | 50:30 |
| 10 | Pd(OAc)2 (10%) | CH3CN | 80° C, 17 h | 56:28 |
| 11 | Pd(OAc)2 (10%) | CH3CN | 55° C, 26 h | 56:30 |
| 12 | White Catalyste | CH3CN | 55° C, 17 h | 10:90 |
| 13 | Pd2(dba)3 (10%) | CH3CN | 55° C, 17 h | 62:07 |
| 14 | Pd2(dba)3 (15%) | CH3CN | 55° C, 17 h | 70:00f |
1 atm, unless mentioned otherwise
yields estimated by 1H NMR integration against an internal standard (1,3,5-trimethoxybenzene)
Without copper, conversion is ~30%; without O2, a similar drop in yield is observed
O2 pressure = 4 atm
1,2-Bis(phenylsulfinyl)ethane palladium (II) acetate (10 mol%)
At present, we are unable to account for the decreased mass balance
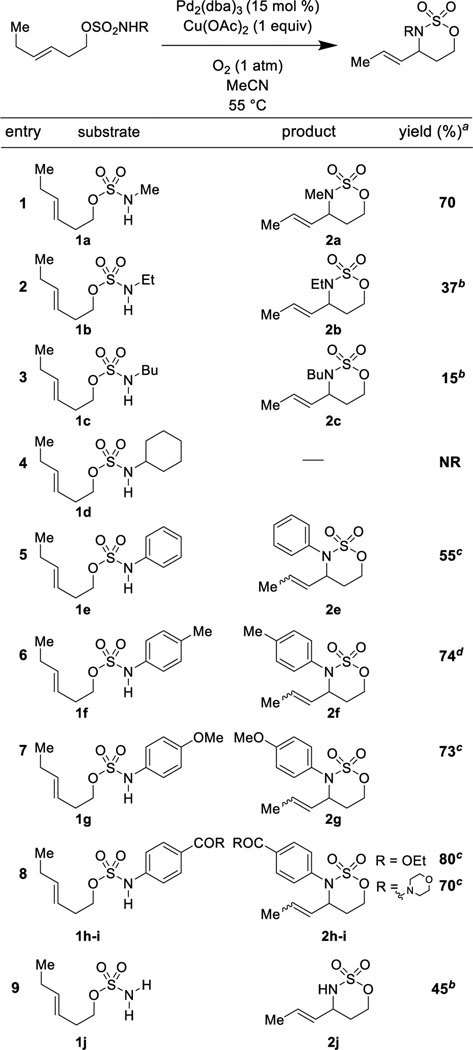
Systematically varying the substituent attached to the sulfamate nitrogen (Table 2) revealed that the reaction is exquisitely sensitive to the steric bulk of alkyl substituents (Table 2, Entries 1–4). In contrast, diverse electron rich and electron deficient aryl substituents on the nitrogen are well tolerated (Table 2, Entries 5–8). We hypothesize that cis/trans isomerism in some of the products arises from reversible formation of a palladium π-allyl complex from the reaction of Pd2(dba)3 with the product oxathiazinanes.60, 61 In the absence of any substituent on the nitrogen, the oxidative cyclization proceeded, albeit in lower yield than with alkyl or aryl substitution (Table 2, Entry 9). Such products are alternatively accessible via C-H amination processes51, 62 and are not the focus of this study. Our protocol is complementary to C–H amination methods in that it allows convenient, one-step access to N-alkylated and N-arylated oxathiazinane heterocycles without the formation of competing aziridination side products. It should also be noted that while unsubstituted oxathiazinane heterocycles can be easily alkylated, we know of only example of oxathiazinane N-arylation.63
Table 2.
Structure-Reactivity relationship of sulfamate esters.
|
isolated yield, unless otherwise mentioned
yield estimated by 1H NMR integration against an internal standard (1,3,5-trimethoxybenzene)
isolated as a 10:1 mixture of trans/cis isomers
isolated as a 5:1 mixture of trans/cis isomers
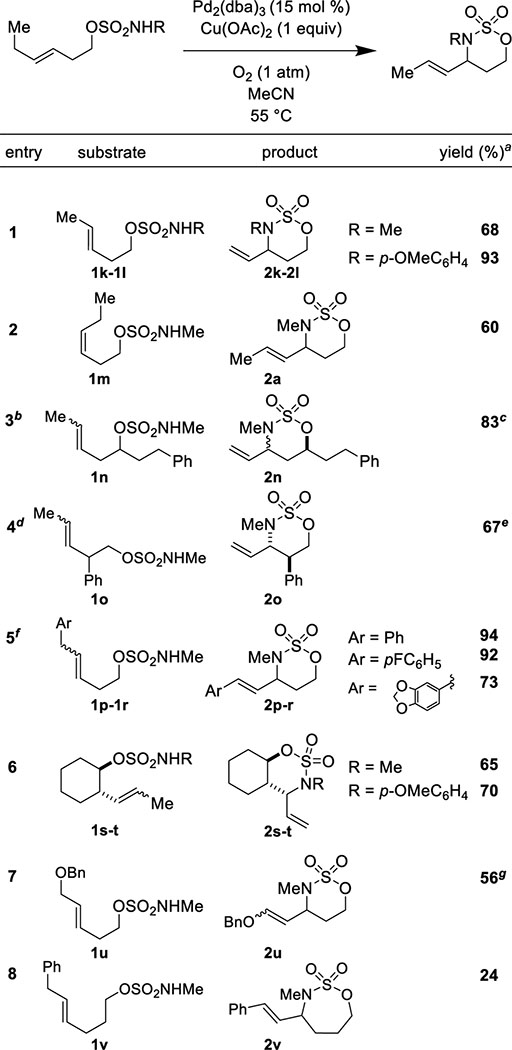
A variety of alkenyl sulfamates were prepared and tested with our optimized reaction protocol of catalytic Pd2(dba)3 and stoichiometric Cu(OAc)2 heated in CH3CN under 1 atm of O2 (Table 3). 6-membered rings reliably formed with synthetically useful yields (56% to >90%). The diastereoselectivity of the reaction was found to be highly substrate dependent and ranged from reasonable (4:1, Table 3, Entry 3) to excellent (>20:1, Table 3, Entry 4). Seven membered ring formation was also possible, albeit in significantly lower yield (Table 3, Entry 8). Both cis and trans disubstituted alkenes engaged effectively. Overall, our reaction protocol was found to be compatible with a range of functional groups, including ethyl esters, morpholine amides, benzyl ethers, fluorinated arenes, and 1,3-benzodioxoles.
TABLE 3.
Diverse alkenyl sulfamates engage productively.
|
isolated yield, unless otherwise mentioned
1:1 mixture of cis/trans isomers
isolated as a 4:1 mixture of syn/anti diastereomers
1:1 mixture of cis/trans isomers
isolated as a single diastereomer
Ar = Ph, 4.6:1 mixture of trans/cis isomers; Ar = pFC6H5, 5.9:1 mixture of trans/cis isomers; Ar = 1,3-benzodioxole, 5:1 mixture of trans/cis isomers
isolated as a 1.6:1 mixture of trans/cis isomers
Under our optimized protocol, even when the scale was increased 20–45 times, the reactions continued to proceed with synthetically useful efficiency (Scheme 2). At a scale of 1.30 g (4.58 mmol, ~20-fold increase), (E)-hex-3-en-1-yl (4-methoxyphenyl)sulfamate cyclized with comparable yield to the reaction at 0.2 mmol (Scheme 2a). On smaller scale, a 10:1 mixture of trans/cis products formed; on larger scale, a 6:1 mixture of trans and cis products was isolated (Scheme 2a). At a scale of 1.74 g (9.09 mmol, ~45-fold increase), (E)-hex-3-en-1-yl methylsulfamate cyclized with a yield of 56% (Scheme 2b).
Scheme 2.
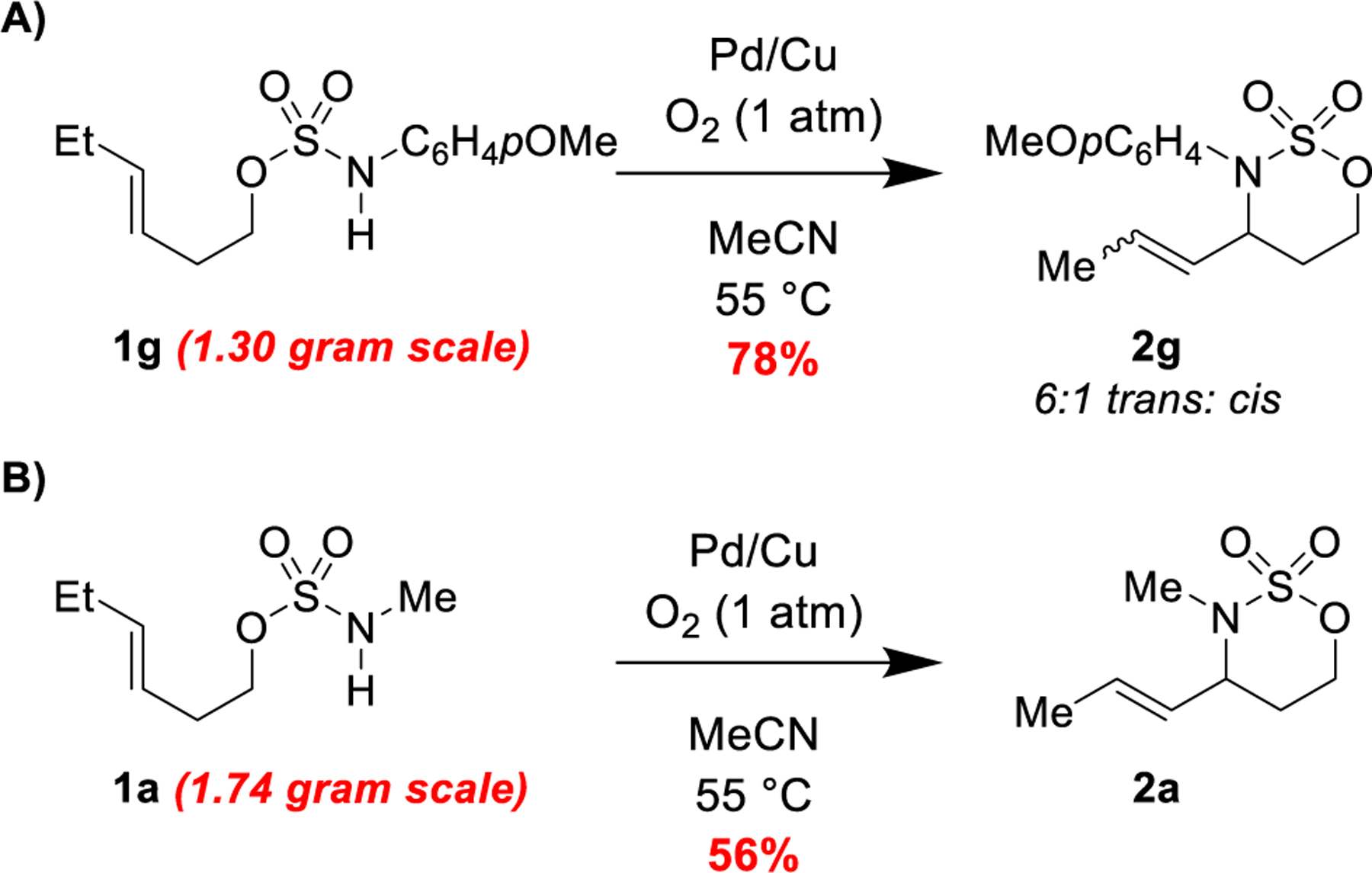
Oxdative cyclization scales successfully under standard conditions.
The resulting unsaturated [1,2,3]-oxathiazinane-2,2-dioxide heterocycles are versatile synthetic intermediates for a variety of transformations (Scheme 3). Hydrogenation to form a fully saturated [1,2,3]-oxathiazinane-2,2-dioxide with 10% Pd/C under 1 atm of H2 proceeded in an excellent yield of 95% (Scheme 3a). Oxidation of the alkene with mCPBA to form the epoxide was also viable (Scheme 3b). The alkoxy-sulfonyl auxiliary was liberated via a smooth reaction with 2-naphthol (Scheme 3c).
Scheme 3.
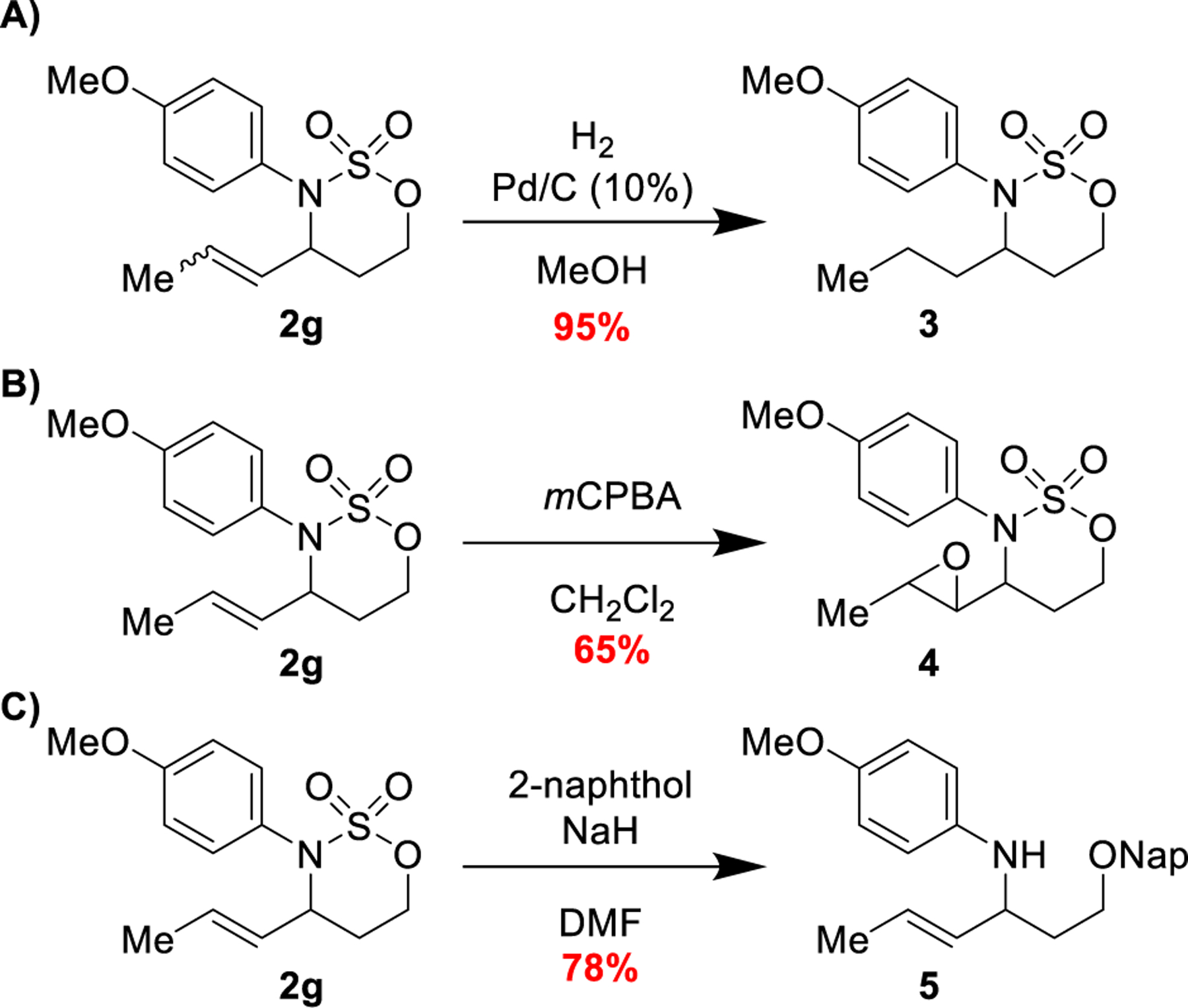
Alkenyl oxathiazinane heterocycles are versatile synthons.
In summary, we report the first examples of aza-Wacker cyclizations of sulfamate esters. The reactions are catalytic in Pd2(dba)3 and utilize Cu(OAc)2 and O2 as terminal oxidants. Our protocol is compatible with both N-alkyl and N-aryl sulfamates and tolerates a range of important functional groups. These reactions proceed in good yields on scales both large and small, and the resulting alkenyl oxathiazinane heterocycles are shown to be valuable synthetic intermediates. In time, we expect that this reaction will find use in both academic and industrial organic chemistry.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by start-up funding provided jointly by the University of Kansas Office of the Provost and the Department of Medicinal Chemistry as well as a grant from the COBRE Protein Structure and Function Small Grants Program. Support for the NMR instrumentation was provided by the NIH Shared Instrumentation Grant #S10RR014767. We deeply thank Professors Robert Hanzlik, Barbara Timmermann, Apurba Dutta, Mark Farrell, and Ryan Altman (University of Kansas) for generous donations of equipment and reagents. We are indebted to Dr. Justin Douglas, Sarah Neunswander (University of Kansas) and Dr. Stephen Lynch (Stanford University) for providing advice regarding NMR assignments as well as Lawrence Seib (University of Kansas) for high resolution mass spectrometry data. Professor Robert A. Pascal, Jr. (Tulane University) is also gratefully acknowledged for providing much advice and encouragement.
Dedicated to Professor Richard N. Zare
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental Procedures, Characterization data, and NMR spectra (PDF)
Contributor Information
Anand H. Shinde, Department of Medicinal Chemistry, University of Kansas, Lawrence, KS 66047
Shyam Sathyamoorthi, Department of Medicinal Chemistry, University of Kansas, Lawrence, KS 66047.
REFERENCES
- 1.Zeni G; Larock RC, Synthesis of Heterocycles via Palladium-Catalyzed Oxidative Addition. Chem. Rev 2006, 106, 4644–4680. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y; Cornwall RG; Du H; Zhao B; Shi Y, Catalytic Diamination of Olefins via N–N Bond Activation. Acc. Chem. Res 2014, 47, 3665–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Duill ML; Engle KM, Protodepalladation as a Strategic Elementary Step in Catalysis. Synthesis 2018, 50, 4699–4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa D, Additions to non-activated alkenes: Recent advances. Arab. J. Chem 2020, 13, 799–834. [Google Scholar]
- 5.Ma K; Martin BS; Yin X; Dai M, Natural product syntheses via carbonylative cyclizations. Nat. Prod. Rep 2019, 36, 174–219. [DOI] [PubMed] [Google Scholar]
- 6.Hosokawa T; Hirata M; Murahashi S-I; Sonoda A, Palladium(II)-catalyzed cyclization of γ,δ-unsaturated alcohols synthesis of 2-vinyltetrahydrofurans. Tetrahedron Lett 1976, 17, 1821–1824. [Google Scholar]
- 7.Hosokawa T; Miyagi S; Murahashi S; Sonoda A, Oxidative cyclization of 2-allylphenols by palladium(II) acetate. Changes in product distribution. J. Org. Chem, 1978, 43, 2752–2757. [Google Scholar]
- 8.Semmelhack MF; Kim CR; Dobler W; Meier M, Controlled beta-hydride elimination during tetrahydropyran formation with Pd(II); diastereoselective formation of the tetrahydropyran ring of tetronomycin. Tetrahedron Lett 1989, 30, 4925–4928. [Google Scholar]
- 9.Trend RM; Ramtohul YK; Ferreira EM; Stoltz BM, Palladium-Catalyzed Oxidative Wacker Cyclizations in Nonpolar Organic Solvents with Molecular Oxygen: A Stepping Stone to Asymmetric Aerobic Cyclizations. Angew. Chem. Int. Ed 2003, 42, 2892–2895. [DOI] [PubMed] [Google Scholar]
- 10.Muñiz K, Palladium-Carbene Catalysts for Aerobic, Intramolecular Wacker-Type Cyclisation Reactions. Adv. Synth. Catal 2004, 346, 1425–1428. [Google Scholar]
- 11.Hayashi T; Yamasaki K; Mimura M; Uozumi Y, Deuterium-Labeling Studies Establishing Stereochemistry at the Oxypalladation Step in Wacker-Type Oxidative Cyclization of an o-Allylphenol. J. Am. Chem. Soc 2004, 126, 3036–3037. [DOI] [PubMed] [Google Scholar]
- 12.Zeni G; Larock RC, Synthesis of Heterocycles via Palladium π-Olefin and π-Alkyne Chemistry. Chem. Rev 2004, 104, 2285–2310. [DOI] [PubMed] [Google Scholar]
- 13.Beccalli EM; Broggini G; Martinelli M; Sottocornola S, C−C, C−O, C−N Bond Formation on sp2 Carbon by Pd(II)-Catalyzed Reactions Involving Oxidant Agents. Chem. Rev 2007, 107, 5318–5365. [DOI] [PubMed] [Google Scholar]
- 14.Doháňošová J; Gracza T, Asymmetric Palladium-Catalysed Intramolecular Wacker-Type Cyclisations of Unsaturated Alcohols and Amino Alcohols. Molecules 2013, 18, 6173–6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michel Brian W., L. D. S., Sigman Matthew W., Wacker Oxidation, The. In Organic Reactions,2014; pp 75–414. [Google Scholar]
- 16.McDonald RI; Liu G; Stahl SS, Palladium(II)-Catalyzed Alkene Functionalization via Nucleopalladation: Stereochemical Pathways and Enantioselective Catalytic Applications. Chem. Rev 2011, 111, 2981–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D; Weinstein AB; White PB; Stahl SS, Ligand-Promoted Palladium-Catalyzed Aerobic Oxidation Reactions. Chem. Rev 2018, 118, 2636–2679. [DOI] [PubMed] [Google Scholar]
- 18.Kotov V; Scarborough CC; Stahl SS, Palladium-Catalyzed Aerobic Oxidative Amination of Alkenes: Development of Intra- and Intermolecular Aza-Wacker Reactions. Inorg. Chem 2007, 46, 1910–1923. [DOI] [PubMed] [Google Scholar]
- 19.Åkermark B; Bäckvall E, J.; Siirala-Hanseń, K.; Sjöberg, K.; Zetterberg, K., The steric course of the palladium promoted amination of simple olefins. Tetrahedron Lett 1974, 15, 1363–1366. [Google Scholar]
- 20.Åkermark B; Bäckvall JE; Hegedus LS; Zetterberg K; Siirala-Hansén K; Sjöberg K, Palladium-promoted addition of amines to isolated double bonds. J. Organomet. Chem 1974, 72, 127–138. [Google Scholar]
- 21.Åkermark B; Bäckvall J-E, Competitive palladium-promoted amination of butenes. Tetrahedron Lett 1975, 16, 819–822. [Google Scholar]
- 22.Aekermark B; Zetterberg K, Palladium-promoted amination of olefins. Direct proof for the trans stereochemistry. J. Am. Chem. Soc 1984, 106, 5560–5561. [Google Scholar]
- 23.Hegedus LS; Allen GF; Bozell JJ; Waterman EL, Palladium-assisted intramolecular amination of olefins. Synthesis of nitrogen heterocycles. J. Am. Chem. Soc 1978, 100, 5800–5807. [Google Scholar]
- 24.Hegedus LS; McKearin JM, Palladium-catalyzed cyclization of .omega.-olefinic tosamides. Synthesis of nonaromatic nitrogen heterocycles. J. Am. Chem. Soc 1982, 104, 2444–2451. [Google Scholar]
- 25.Hegedus LS; Akermark B; Zetterberg K; Olsson LF, Palladium-assisted amination of olefins. A mechanistic study. J. Am. Chem. Soc 1984, 106, 7122–7126. [Google Scholar]
- 26.Trend RM; Ramtohul YK; Stoltz BM, Oxidative Cyclizations in a Nonpolar Solvent Using Molecular Oxygen and Studies on the Stereochemistry of Oxypalladation. J. Am. Chem. Soc 2005, 127, 17778–17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang F; Wu Z; Zhang W, Pd-catalyzed asymmetric aza-Wacker-type cyclization reaction of olefinic tosylamides. Tetrahedron Lett 2010, 51, 5124–5126. [Google Scholar]
- 28.Yang G; Zhang W, Regioselective Pd-Catalyzed Aerobic Aza-Wacker Cyclization for Preparation of Isoindolinones and Isoquinolin-1(2H)-ones. Org. Lett 2012, 14, 268–271. [DOI] [PubMed] [Google Scholar]
- 29.Yang G; Shen C; Zhang W, An Asymmetric Aerobic Aza-Wacker-Type Cyclization: Synthesis of Isoindolinones Bearing Tetrasubstituted Carbon Stereocenters. Angew. Chem. Int. Ed 2012, 51, 9141–9145. [DOI] [PubMed] [Google Scholar]
- 30.Redford JE; McDonald RI; Rigsby ML; Wiensch JD; Stahl SS, Stereoselective Synthesis of cis-2,5-Disubstituted Pyrrolidines via Wacker-Type Aerobic Oxidative Cyclization of Alkenes with tert-Butanesulfinamide Nucleophiles. Org. Lett 2012, 14, 1242–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Z; Stahl SS, Intramolecular Pd(II)-Catalyzed Aerobic Oxidative Amination of Alkenes: Synthesis of Six-Membered N-Heterocycles. Org. Lett 2012, 14, 1234–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye X; White PB; Stahl SS, Mechanistic Studies of Wacker-Type Amidocyclization of Alkenes Catalyzed by (IMes)Pd(TFA)2(H2O): Kinetic and Stereochemical Implications of Proton Transfer. J. Org. Chem, 2013, 78, 2083–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinstein AB; Schuman DP; Tan ZX; Stahl SS, Synthesis of Vicinal Aminoalcohols by Stereoselective Aza-Wacker Cyclizations: Access to (−)-Acosamine by Redox Relay. Angew. Chem. Int. Ed 2013, 52, 11867–11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White PB; Jaworski JN; Zhu GH; Stahl SS, Diazafluorenone-Promoted Oxidation Catalysis: Insights into the Role of Bidentate Ligands in Pd-Catalyzed Aerobic Aza-Wacker Reactions. ACS Catalysis 2016, 6, 3340–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Benthem RATM; Hiemstra H; Longarela GR; Speckamp WN, Formamide as a superior nitrogen nucleophile in palladium(II) mediated synthesis of imidazolidines. Tetrahedron Lett 1994, 35, 9281–9284. [Google Scholar]
- 36.Rönn M; Bäckvall J-E; Andersson PG, Palladium(II)-Catalyzed Cyclization Using Molecular Oxygen as Reoxidant. Tetrahedron Lett 1995, 36, 7749–7752. [Google Scholar]
- 37.Larock RC; Hightower TR; Hasvold LA; Peterson KP, Palladium(II)-Catalyzed Cyclization of Olefinic Tosylamides. J. Org. Chem, 1996, 61, 3584–3585. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z; Zhang J; Tan J; Wang Z, A Facile Access to Pyrroles from Amino Acids via an Aza-Wacker Cyclization. J. Org. Chem, 2008, 73, 5180–5182. [DOI] [PubMed] [Google Scholar]
- 39.Youn SW; Lee SR, Unusual 1,2-aryl migration in Pd(ii)-catalyzed aza-Wacker-type cyclization of 2-alkenylanilines. Org. Biomol. Chem 2015, 13, 4652–4656. [DOI] [PubMed] [Google Scholar]
- 40.Xiong P; Xu F; Qian X-Y; Yohannes Y; Song J; Lu X; Xu H-C, Copper-Catalyzed Intramolecular Oxidative Amination of Unactivated Internal Alkenes. Chem. Eur. J 2016, 22, 4379–4383. [DOI] [PubMed] [Google Scholar]
- 41.Yi X; Hu X, Formal Aza-Wacker Cyclization by Tandem Electrochemical Oxidation and Copper Catalysis. Angew. Chem. Int. Ed 2019, 58, 4700–4704. [DOI] [PubMed] [Google Scholar]
- 42.McDonald RI; Stahl SS, Modular Synthesis of 1,2-Diamine Derivatives by Palladium-Catalyzed Aerobic Oxidative Cyclization of Allylic Sulfamides. Angew. Chem. Int. Ed 2010, 49, 5529–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andersson PG; Baeckvall JE, Palladium-catalyzed tandem cyclization of 4,6- and 5,7-diene amides. A new route toward the pyrrolizidine and indolizidine alkaloids. J. Am. Chem. Soc 1992, 114, 8696–8698. [Google Scholar]
- 44.Malkov AV; Lee DS; Barłóg M; Elsegood MRJ; Kočovský P, Palladium-Catalyzed Stereoselective Intramolecular Oxidative Amidation of Alkenes in the Synthesis of 1,3- and 1,4-Amino Alcohols and 1,3-Diamines. Chem. Eur. J 2014, 20, 4901–4905. [DOI] [PubMed] [Google Scholar]
- 45.Tamaru Y; Hojo M; Higashimura H; Yoshida Z, Urea as the most reactive and versatile nitrogen nucleophile for the palladium(2+)-catalyzed cyclization of unsaturated amines. J. Am. Chem. Soc 1988, 110, 3994–4002. [Google Scholar]
- 46.Fraunhoffer KJ; White MC, syn-1,2-Amino Alcohols via Diastereoselective Allylic C−H Amination. J. Am. Chem. Soc 2007, 129, 7274–7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atkinson D; Kabeshov MA; Edgar M; Malkov AV, Intramolecular Carbonyl Nitroso Ene Reaction Catalyzed by Iron(III) Chloride/Hydrogen Peroxide as an Efficient Tool for Direct Allylic Amination. Adv. Synth. Catal 2011, 353, 3347–3351. [Google Scholar]
- 48.Frazier CP; Engelking JR; Read de Alaniz J, Copper-Catalyzed Aerobic Oxidation of Hydroxamic Acids Leads to a Mild and Versatile Acylnitroso Ene Reaction. J. Am. Chem. Soc 2011, 133, 10430–10433. [DOI] [PubMed] [Google Scholar]
- 49.Derrien N; Sharley JS; Rubtsov AE; Malkov AV, Oxidative Azo–Ene Cyclization. Org. Lett 2017, 19, 234–237. [DOI] [PubMed] [Google Scholar]
- 50.Espino CG; Wehn PM; Chow J; Du Bois J, Synthesis of 1,3-Difunctionalized Amine Derivatives through Selective C−H Bond Oxidation. J. Am. Chem. Soc 2001, 123, 6935–6936. [Google Scholar]
- 51.Paradine SM; Griffin JR; Zhao J; Petronico AL; Miller SM; Christina White M, A manganese catalyst for highly reactive yet chemoselective intramolecular C(sp3)–H amination. Nat. Chem 2015, 7, 987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sathyamoorthi S; Banerjee S; Du Bois J; Burns NZ; Zare RN, Site-selective bromination of sp3 C–H bonds. Chemical Science 2018, 9, 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Short MA; Blackburn JM; Roizen JL, Sulfamate Esters Guide Selective Radical-Mediated Chlorination of Aliphatic C−H Bonds. Angew. Chem. Int. Ed 2018, 57, 296–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wehn PM; Du Bois J, Exploring New Uses for C−H Amination: Ni-Catalyzed Cross-Coupling of Cyclic Sulfamates. Org. Lett 2005, 7, 4685–4688. [DOI] [PubMed] [Google Scholar]
- 55.Fleming JJ; McReynolds MD; Du Bois J, (+)-Saxitoxin: A First and Second Generation Stereoselective Synthesis. J. Am. Chem. Soc 2007, 129, 9964–9975. [DOI] [PubMed] [Google Scholar]
- 56.Burke EG; Schomaker JM, Oxidative Allene Amination for the Synthesis of Azetidin-3-ones. Angew. Chem. Int. Ed 2015, 54, 12097–12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu L; Gerstner NC; Oxtoby LJ; Guzei IA; Schomaker JM, Fluorinated Amine Stereotriads via Allene Amination. Org. Lett 2017, 19, 3239–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Su JY; Olson DE; Ting SI; Du Bois J, Synthetic Studies Toward Pactamycin Highlighting Oxidative C–H and Alkene Amination Technologies. J. Org. Chem, 2018, 83, 7121–7134. [DOI] [PubMed] [Google Scholar]
- 59.Hosokawa T; Uno T; Murahashi S-I, Palladium(II)-catalysed oxidative cyclisation of 2-allylphenols in the presence of copper(II) acetate and molecular oxygen. Oxidation state of palladium in the Wacker-type reaction. J. Chem. Soc., Chem. Commun, 1979, 475–476. [Google Scholar]
- 60.Tsuji J; Minami I, New synthetic reactions of allyl alkyl carbonates, allyl .beta.-keto carboxylates, and allyl vinylic carbonates catalyzed by palladium complexes. Acc. Chem. Res 1987, 20, 140–145. [Google Scholar]
- 61.Trost BM, Metal catalyzed allylic alkylation: its development in the Trost laboratories. Tetrahedron 2015, 71, 5708–5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harvey ME; Musaev DG; Du Bois J, A Diruthenium Catalyst for Selective, Intramolecular Allylic C–H Amination: Reaction Development and Mechanistic Insight Gained through Experiment and Theory. J. Am. Chem. Soc 2011, 133, 17207–17216. [DOI] [PubMed] [Google Scholar]
- 63.Su JY Synthetic studies toward pactamycin using oxidative alkene and C-H amination technologies. Doctoral Dissertation, Stanford University, Stanford, CA, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


