Abstract
Mycosis fungoides, an uncommon form of cutaneous T-cell lymphoma, arises in the skin and frequently progresses to generalized lymphadenopathy. Although the cause of cutaneous T-cell lymphoma is unknown, chronic immunosuppression may play a role. A few cases have been reported in renal transplant recipients; however, ours appears to be the 1st report of cutaneous T-cell lymphoma in a cardiac transplant recipient. In our patient, cutaneous manifestations of the disease were noted less than 1 year after transplantation. Seven years after transplantation, Sézary syndrome, a variant form of mycosis fungoides, was diagnosed by tissue biopsy and flow cytometry analysis. Photopheresis improved symptoms but was not well tolerated because of hemodynamic sequelae. Psoralen and ultraviolet A therapy also improved the patient's skin condition, but a generalized lymphadenopathy developed. The maintenance immunosuppressive regimen was changed from cyclosporine (3 mg/kg/day) and azathioprine to cyclosporine (1.5 mg/kg/day) and cyclophosphamide. Although effective in the short-term, the results of this therapeutic strategy could not be fully evaluated because the patient died of acute myocardial infarction.
Key words: Cyclosporins/adverse effects; heart transplantation; herpes-viridae infections/complications; immunosuppression/adverse effects; lymphoma, T-cell, cutaneous; mycosis fungoides; photopheresis; PUVA therapy; Sezary syndrome; skin neoplasms
Mycosis fungoides (MF) is a form of cutaneous T-cell lymphoma (CTCL) that originates in the skin-homing helper memory T-cells and can progress to involve the lymph nodes and internal organs. The disease is characterized by pleomorphic skin lesions that can involve any body surface. Hematogenous spread of the malignant cells may lead to Sézary syndrome, the leukemic variant of mycosis fungoides that is characterized by a diffuse exfoliative erythroderma and abnormal circulating cells.
The incidence of lymphoproliferative disorders in solid-organ transplant recipients is 25 to 50 times greater than in the general population; however, T-cell lymphomas are rare in transplant recipients. 1 Here, we report what is, to the best of our knowledge, the 1st case in the English literature of CTCL in a cardiac transplant recipient; and we discuss the management of this unique presentation.
Case Report
In September 1988, a 65-year-old man with a 6-year history of progressive dilated cardiomyopathy and atrial fibrillation underwent orthotopic cardiac transplantation. Standard triple-agent immunosuppressive therapy (cyclosporine, azathioprine, and prednisone) was begun after surgery. During the early postoperative period, the patient experienced a single rejection episode that was successfully treated with steroid pulse therapy. The immunosuppressive regimen was adjusted, and the dose of cyclosporine was tapered to maintain serum levels at 150 to 250 mg/dL during the 1st year.
At a routine examination 4 months after transplantation, scattered red lesions were noted on the skin of the patient's arms and back. These lesions were attributed to a hypersensitivity to methocarbamol, which was discontinued. Two years after transplantation, the patient developed axillary lymphadenopathy and a progressive salmon-colored, pruritic, macular rash on his hands, arms, and back. Initially, the rash was attributed to a drug hypersensitivity; however, changing medications did not affect the rash. At the same time, a staphylococcal infection developed at a catheterization site in the patient's left groin.
In January 1993 (5 years after transplantation), raised lesions were detected on the skin of the left forearm and forehead. Results from biopsy specimens showed squamous cell carcinoma of the forearm and solar keratosis of the forehead. The patient had a generalized violaceous macular rash of the upper torso and extremities, along with inguinal and axillary lympha-denopathy. Serologic studies indicated a monoclonal gammopathy of the IgM kappa chain (IgM, 1160 mg/dL).
In October 1995, routine laboratory studies showed a high leukocyte count (12,000 cells/mL; 64% lymphocytes; 95% CD4+, 2% CD8+). Physical examination revealed erythroderma and scaling on the trunk (Fig. 1A), tinea pedis of the lower extremities, and occasional purpuric papules on the extremities. Although inguinal and axillary adenopathies were noted (Fig. 1B), no biopsy specimens were obtained at those sites because of the patient's immunocompromised status. A skin biopsy specimen from the thigh showed a superficial perivascular infiltration of small- to medium-sized lymphoid vessels with moderate to marked atypical nuclear changes (Fig. 2A). A focal proliferation of markedly atypical small lymphoid cells was seen in the dermis along with overlying Pautrier's microabscesses (Fig. 2B). Minimal epidermotropism was seen. These findings were consistent with a diagnosis of mycosis fungoides. In immunophenotypic studies of frozen sections, the neoplastic cells reacted strongly with the T-cell markers CD2, CD3, and CD5. In addition, approximately 10% of the cells reacted with CD7, whereas only a few cells reacted with CD30.
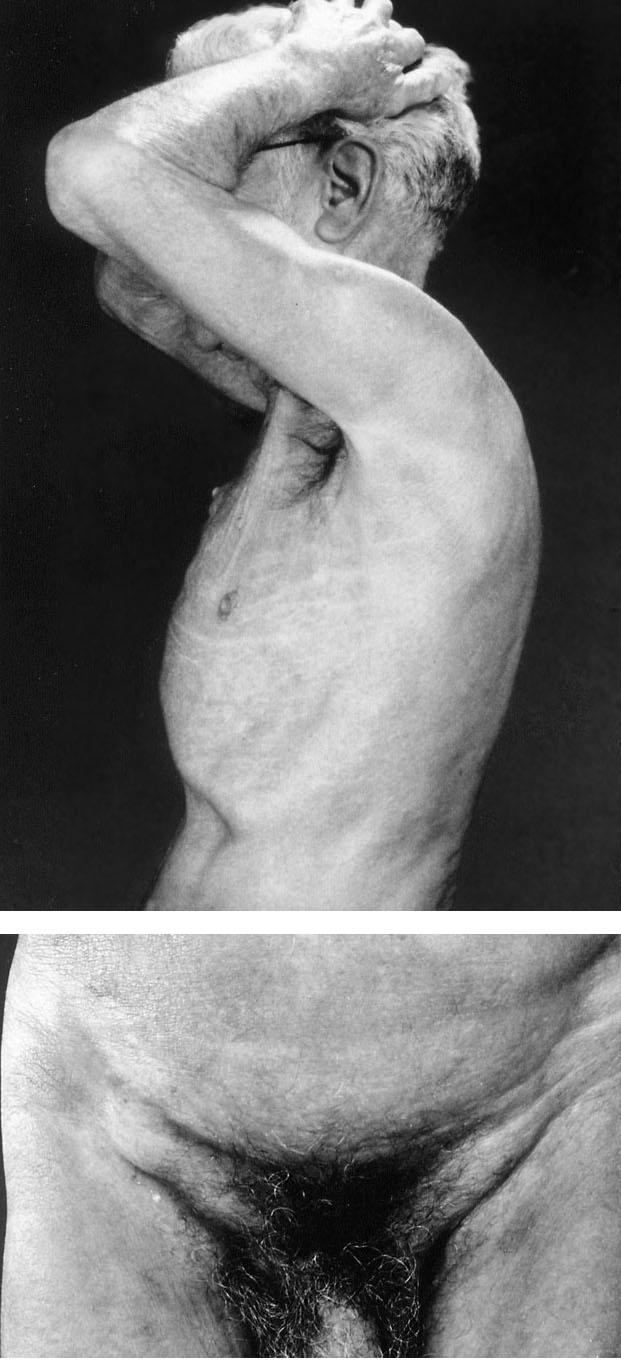
Fig. 1 Generalized erythroderma (A) and inguinal adenopathy (B) present 7 years after cardiac transplantation.
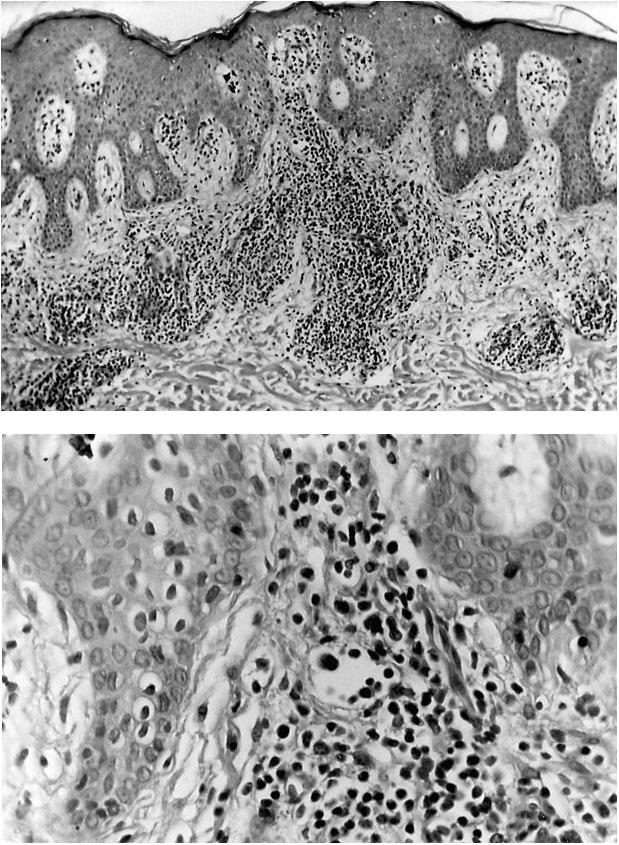
Fig. 2 A) Photomicrograph (H+E, orig. ×100) of a lesion from a skin biopsy of the thigh showing a superficial perivascular infiltrate of small- to medium-sized lymphocytes and epider-motropism. B) Magnification (orig. ×200) of the photomicro-graph in (A) showing moderate to marked atypia of the lymph-ocytes in the papillary dermis.
Examination of a peripheral blood smear revealed multiple atypical lymphocytes that exhibited convoluted and cerebriform nuclei. The nuclei had condensed chromatin, and the cytoplasm was slightly basophilic. No blasts were seen. Flow cytometry studies showed an abnormal lymphocyte population: 92% CD4+ cells and 5% CD8+ cells (ratio 17:1). Reverse transcriptase-polymerase chain reaction indicated that the Vβ gene of the T-cell receptor was overexpressed in T lymphocytes from the blood and skin (Figs. 3 and 4). Genetic rearrangement of the T-cell β chain was confirmed by Southern blot hybridization to the constant V gene probe.
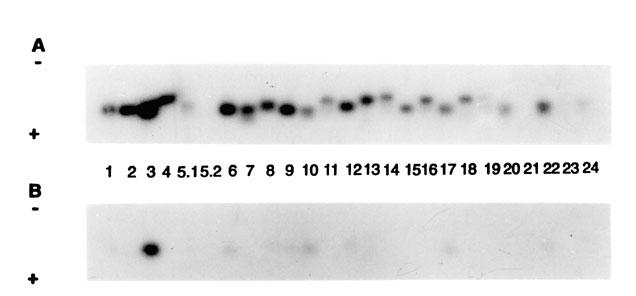
Fig. 3 Autoradiograph of T-cell Vβ gene expression in blood (A) and skin (B), as obtained by Southern blot analysis.
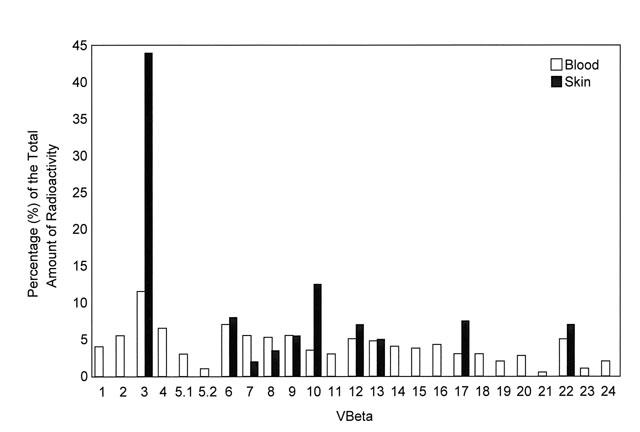
Fig. 4 Histogram of the Vβ repertoire of the T-cell receptor in blood and skin. Each relative signal is expressed as a percentage of the total amount of radioactivity.
Viral studies revealed no evidence of infection with human T-cell lymphotropic virus-type 1 (HTLV-1) and no antibodies to hepatitis virus. The ratio of IgG to Epstein-Barr virus (EBV) was 1:640; that of IgM to EBV, 1:10.
Lymphocytosis was noted on bone marrow aspirates and biopsy specimens. Most lymphocytes were similar in morphology to those seen on the peripheral blood smear. The differential cell count from bone marrow included: 25% abnormal lymphocytes, 7.5% normal lymphocytes, 1% plasma cells, 1% promyelocytes, 35.5% segmented neutrophils and bands, and 22.5% normoblasts (myeloid/erythroid ratio, 2:1). Examination of a decalcified bone marrow biopsy core showed a small amount of marrow containing a 25% cellular infiltrate, which in turn contained trilineage marrow elements including megakaryocytes and a few small lymphoid-appearing cells with irregular nuclei scattered throughout. The presence of T cells in the infiltrate was confirmed immunohistochemically: cells stained positively for the markers CD3, PPD4, and UCHL1. This interstitial pattern of involvement by leukemic cells was consistent with Sézary syndrome. The patient's disease was graded as stage IVB (T3LN4M1). His skin score progressed from 167 to 200.
In January 1996, the patient began monthly extracorporeal photopheresis therapy. Each treatment session was preceded by treatment with 50 mg of 8-methoxypsoralen ultra. The patient's pruritus improved, but the treatments were not well tolerated because of fluid overload. Photopheresis was discontinued after 4 months; the patient's skin score at that time was 370. Psoralen and ultraviolet A (PUVA) therapy (0.5 joules, 3 times/week) was then initiated. After 14 weeks, the patient's skin score decreased to 200 and then, 2 months later, to 90. During this time, the patient received antibiotic therapy for a staphylococcal infection of the skin and topical triamcinolone cream for relief of symptoms. While undergoing PUVA therapy, the patient developed a prominent adenopathy of the bilateral cervical lymphatic chains and of the supraclavicular, axillary, inguinal, and femoral regions. Computed tomographic studies indicated a nonspecific infiltrate in the left lung base and adenopathy of the axillary, inguinal, and porta hepatis regions.
In November 1996, the patient's immunosuppressive regimen was changed: the cyclosporine dose was reduced from 3 mg/kg/day to 1.5 mg/kg/day and azathioprine was replaced with cyclophosphamide (50 mg/day). The patient then refused chemotherapy and was prescribed a regimen of total skin radiotherapy. However, before starting the radiotherapy regimen, the patient developed an intractable ventricular fibrillation that progressed to cardiac arrest and led to his death. Findings at autopsy indicated an acute thrombosis of the right coronary artery, an infarct of the left ventricular anteroseptal wall, and cardiac allograft rejection (Texas Heart Institute grade 5; International Society for Heart and Lung Transplantation grade 3A).
Discussion
The incidence of lymphoproliferative disorders in solid-organ transplant recipients is 1% to 6%, significantly higher than in the general population. 1–3 Non-Hodgkin's lymphomas are rare in transplant patients and are usually of B-cell origin. Although a few cases of T-cell lymphomas have been reported in renal transplant recipients, 4,5 the case we have described here is, to our knowledge, the 1st reported case of CTCL in a cardiac transplant patient.
Lymphomas usually develop about 1 year after transplantation in cyclosporine-treated patients and 4 years after transplantation in non-cyclosporine-treated patients. 6,7 In the general population, the time from onset of symptoms to a pathologist's diagnosis of MF is about 6 years. 8 In the case presented here, cutaneous changes were noted by 4 months after transplantation, but lymphoma was not diagnosed until 7 years later. We are not able to determine retro-spectively whether the initial cutaneous lesions were early manifestations of CTCL or a hypersensitivity reaction to a drug. The rarity of CTCL in both the transplant and general populations probably contributed to the delay in diagnosis in this case.
The cause of CTCL is unknown. Several groups have reported that HTLV contributes to the development of CTCL. 9,10 Epstein-Barr virus has also been reported as a causative agent. 11 Viral studies in our patient did not indicate exposure to HTLV but did indicate exposure to EBV. We have previously reported on 2 patients in whom CTCL developed within months after cardiac bypass surgery; these patients were also negative for exposure to HTLV-1. 12 In a previous study, 13 we found no evidence of infection with HTLV-1 in 19 patients with mycosis fungoides, as indicated by the absence of the tax gene on polymerase chain reaction. Others 14 have confirmed this finding. We have also reported that CTCL may be accompanied or exacerbated by a coexisting Staphylococcus aureus infection. 15
Some studies suggest that patients treated with cyclosporine may be predisposed to lymphoproliferative diseases. 6,7,16,17 Indeed, cyclosporine is associated with worsening or onset of CTCL, 18 and we believe that immunosuppressive therapy probably contributed to the development of CTCL in our patient. Starzl and colleagues 19 1st reported that cyclosporine-associated lymphomas could be reversed in patients undergoing cyclosporine-steroid therapy by reducing or discontinuing cyclosporine. Chen and coworkers 20 successfully treated 10 of 18 transplant patients after reducing cyclosporine and eliminating azathioprine therapy. In contrast, Cooper's group 21 reported partial regression of CTCL in 2 of 11 nontransplant patients after administration of high-dose cyclosporine (7.5 mg/kg, twice daily). When cyclosporine therapy was discontinued in these patients, the disease progressed; a 2nd regression was noted when cyclosporine therapy was reinitiated. Lymphoproliferative disorders in cardiac transplant recipients have been successfully treated with cyclophosphamide-doxorubicin-vincristine-prednisone chemotherapy. 22 We have successfully treated 2 cardiac transplant patients with lymphoproliferative disorders by substituting cyclo-phosphamide for azathioprine (unpublished data). We changed the immunosuppressive regimen in the patient presented here by discontinuing azathioprine, adding cyclophosphamide, and lowering the dose of cyclosporine. The success of this regimen could not be assessed, however, because the patient died of other causes.
The need for immunosuppression in our patient limited our options for treating his CTCL/Sézary syndrome. Although very effective, alpha interferon was not a therapeutic option because it may predispose patients to heart failure or graft rejection. In addition, alpha interferon is often poorly tolerated in older patients. We chose photopheresis because it has been used successfully to treat rejection in cardiac transplant patients. Furthermore, photopheresis has led to complete remission in 15% of patients with Sézary syndrome 23 and has improved symptoms in up to 80% of patients with erythroderma. 24,25 However, photopheresis caused fluid shifts and pulmonary edema in our patient. Subsequent PUVA therapy, in which ultraviolet A rays were directed to the skin instead of the blood, improved symptoms in our patient. The use of antibiotic agents to treat the staphylococcal infection and of topical steroids also improved the skin condition. This improvement, however, was accompanied by progressive adenopathy, which suggested a change in the homing of lymphoma cells from the skin to the lymph nodes.
Transplantation physicians should be aware of the possibility of CTCL in cardiac transplant patients. If CTCL had been detected earlier in our patient, modulation of immunosuppressive therapy might have led to a more rapid and complete remission. We were unable to evaluate fully the efficacy of our treatment regimen in this patient, but it is clear that CTCL is a challenging disease to treat, especially in the unique patient population of cardiac transplant recipients. Future studies of transplantation immunology should address themselves to the presentation of lymphoid cancers and the role of viruses and multimodality immunosuppressive therapy.
Acknowledgment
T-cell studies were funded in part by a grant to one of the authors (M.D.) from the Ladies' Leukemia League.
Footnotes
Address for reprints: O.H. Frazier, MD, Texas Heart Institute, P.O. Box 20345, Houston, TX 77225-0345
References
- 1.Penn I. Cancer in immunosuppressed patients. Transplant Proc 1984;16:492–4. [PubMed]
- 2.Penn I. Why do immunosuppressed patients develop cancer? Crit Rev Oncog 1989;1:27–52. [PubMed]
- 3.Nalesnik MA, Makowka L, Starzl TE. The diagnosis and treatment of posttransplant lymphoproliferative disorders. Curr Prob Surg 1988;25:367–472. [DOI] [PubMed]
- 4.Euvrard S, Noble CP, Kanitakis J, Ffrench M, Berger F, Delecluse HJ, et al. Brief report: successive occurrence of T-cell and B-cell lymphomas after renal transplantation in a patient with multiple cutaneous squamous-cell carcinomas. N Engl J Med 1992;327:1924–6. [DOI] [PubMed]
- 5.Raferty MJ, Tidman MJ, Koffman G, Ogg CS, Macdonald DM, Cameron JS. Posttransplantation T cell lymphoma of the skin. Transplantation 1988;46:475–7. [DOI] [PubMed]
- 6.Penn I. Cancers following cyclosporine therapy. Transplantation 1987;43:32–5. [DOI] [PubMed]
- 7.Penn I. The changing pattern of posttransplant malignancies. Transplant Proc 1991;23(1 Pt 2):1101–3. [PubMed]
- 8.Hoppe RT, Wood GS, Abel EA. Mycosis fungoides and Sezary syndrome: pathology, staging, and treatment. Curr Probl Cancer 1990;14:293–371. [DOI] [PubMed]
- 9.Pancake BA, Zucker-Franklin D, Coutavas EE. The cutaneous T cell lymphoma, mycosis fungoides, is a human T cell lymphotropic virus-associated disease. A study of 50 patients. J Clin Invest 1995;95:547–54. [DOI] [PMC free article] [PubMed]
- 10.D'Incan M, Bignon YJ, Souteyrand P, Roger H, Rance N, Dastugue B. Polymerase chain reaction (PCR) detection of an HTLV-1 DNA sequence in the genome of cutaneous T-cell lymphoma (CTCL) patients [abstract]. J Invest Dermatol 1990;94:393.
- 11.McGregor JM, Yu CC, Lu QL, Cotter FE, Levison DA, MacDonald DM. Posttransplant cutaneous lymphoma. J Am Acad Dermatol 1993;29:549–54. [DOI] [PubMed]
- 12.Grossman D, Duvic M. Cutaneous T-cell lymphoma after blood transfusion [letter]. Lancet 1993;342:1483. [DOI] [PubMed]
- 13.Sarris AH, Daliani D, Ulmer R, Crow M, Broxmeyer HE, Pugh W, et al. Interferon-inducible protein-10 and the pathogenesis of cutaneous T-cell lymphomas. Leuk Lymphoma 1996;24:103–10. [DOI] [PubMed]
- 14.Li G, Vowels BR, Benoit BM, Rook AH, Lessin SR. Failure to detect human T-lymphotropic virus type-I proviral DNA in cell lines and tissues from patients with cutaneous T-cell lymphoma. J Invest Dermatol 1996;107:308–13. [DOI] [PubMed]
- 15.Jackow CM, Cather JC, Hearne V, Asano AT, Musser JM, Duvic M. Association of erythrodermic cutaneous T-cell lymphoma, superantigen-positive Staphylococcus aureus, and oligoclonal T-cell receptor V beta gene expansion [published erratum appears in Blood 1997;89:3496]. Blood 1997;89:32–40. [PubMed]
- 16.Demetris AJ, Nalesnik MA, Kunz HW, Gill TJ 3rd, Shino-zuka H. Sequential analyses of the development of lymphoproliferative disorders in rats receiving cyclosporine. Transplantation 1984;38:239–46. [DOI] [PubMed]
- 17.Reitz BA, Bieber CP. Cancer after the use of cyclosporin-A in animals. Cancer Survey 1982;1:613–19.
- 18.Pielop JA, Jones D, Duvic M. Transient CD30-positive nodal transformation of cutaneous T-cell lymphoma associated with cyclosporine treatment. Int J Dermatol. In press. [DOI] [PubMed]
- 19.Starzl TE, Nalesnik MA, Porter KA, Ho M, Iwatsuki S, Griffith BP, et al. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporine-steroid therapy. Lancet 1984;1:583–7. [DOI] [PMC free article] [PubMed]
- 20.Chen JM, Barr ML, Chadburn A, Frizzera G, Schenkel FA, Sciacca RR, et al. Management of lymphoproliferative disorders after cardiac transplantation. Ann Thorac Surg 1993; 56:527–38. [DOI] [PubMed]
- 21.Cooper DL, Braverman IM, Sarris AH, Durivage HJ, Saidman BH, Davis CA, Hait WN. Cyclosporine treatment of refractory T-cell lymphomas. Cancer 1993;71:2335–41. [DOI] [PubMed]
- 22.Garrett TJ, Chadburn A, Barr ML, Drusin RE, Chen JM, Schulman LL, et al. Posttransplantation lymphoproliferative disorders treated with cyclophosphamide-doxorubicin-vincristine-prednisone chemotherapy. Cancer 1993;72: 2782–5. [DOI] [PubMed]
- 23.Duvic M, Hester JP, Lemak NA. Photopheresis therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol 1996;35: 573–9. [DOI] [PubMed]
- 24.Edelson R, Berger C, Gasparro FP, Jegasothy B, Heald P, Wintroub B, et al. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy. Preliminary results. N Engl J Med 1987;316:297–303. [DOI] [PubMed]
- 25.Heald P, Rook A, Perez M, Wintroub B, Knobler R, Jegasothy B, et al. Treatment of erythrodermic cutaneous T-cell lymphoma with extracorporeal photochemotherapy. J Am Acad Dermatol 1992;27:427–33. [DOI] [PubMed]


