Abstract
Inflammatory bowel disease has an enormous impact on public health, medical systems, economies, and social conditions. Biologic therapy has ameliorated the treatment and clinical course of patients with inflammatory bowel disease. The efficacy and safety profiles of currently available therapies are still less that optimal in numerous ways, highlighting the requirement for new therapeutic targets. A bunch of new drug studies are underway in inflammatory bowel disease with promising results. This is an outlined guideline of clinical diagnosis and pharmaceutical therapy of inflammatory bowel disease. Outline delineates the overall recommendations on the modern principles of desirable practice to bolster the adoption of best implementations and exploration as well as inflammatory bowel disease patient, gastroenterologist, and other healthcare provider education. Inflammatory bowel disease encompasses Crohn’s disease and ulcerative colitis, the two unsolved medical inflammatory bowel disease-subtypes condition with no drug for cure. The signs and symptoms on first presentation relate to the anatomical localization and severity of the disease and less with the resulting diagnosis that can clinically and histologically be non-definitive to interpret and establish criteria, specifically in colonic inflammatory bowel disease when the establishment is inconclusive is classified as indeterminate colitis. Conservative pharmaceuticals and accessible avenues do not depend on the disease phenotype. The first line management is to manage symptoms and stabilize active disease; at the same time maintenance therapy is indicated. Nutrition and diet do not play a primary therapeutic role but is warranted as supportive care. There is need of special guideline that explore solution of groundwork gap in terms of access limitations to inflammatory bowel disease care, particularly in developing countries and the irregular representation of socioeconomic stratification with a strategic plan, for the unanswered questions and perspective for the future, especially during the surfaced global COVID-19 pandemic caused by coronavirus SARS-CoV2 impacting on both the patient’s psychological functioning and endoscopy services. Establishment of a global registry system and accumulated experiences have led to consensus for inflammatory bowel disease management under the COVID-19 pandemic. Painstakingly, the pandemic has influenced medical care systems for these patients. I briefly herein viewpoint summarize among other updates the telemedicine roles during the pandemic and how operationally inflammatory bowel disease centers managed patients and ensured quality of care. In conclusion: inflammatory bowel disease has become a global emergent disease. Serious medical errors are public health problem observed in developing nations i.e., to distinguish inflammatory bowel disease and infectious and parasitic diseases. Refractory inflammatory bowel disease is a still significant challenge in the management of patients with Crohn’s disease and ulcerative colitis. There are gaps in knowledge and future research directions on the recent newly registered pharmaceuticals. The main clinical outcomes for inflammatory bowel disease were maintained during the COVID-19 pandemic period.
Keywords: Inflammatory bowel disease, Ulcerative colitis, Crohn’s disease, Crohn’s colitis, Indeterminate colitis, Clinical diagnosis guideline, Molecular diagnostics, Medical Treatment guidelines, uneven representation of socioeconomic strata, inflammatory bowel disease care during COVID-19
1. Background
Inflammatory bowel disease (IBD) includes Crohn’s disease (CD) and ulcerative colitis (UC) are global emergent disease that are highly heterogeneous, debilitating, incurable, persistent, relapsing/ worsening, immune-arbitrated inflammatory pathologies of the digestive system canal [1]. UC causes inflammation and ulceration confined to the mucosal layer and the submucosae compartment of the colon and rectum [2]. CC compared to UC is segmental causing inflammation impacting the whole digestive system tract from the mouth to the anus. Further, CC cause inflammation deeper and can involve all the four colon layers that may engage other organs through fistulation [3,4].
Even though IBD that encompasses CD and UC, the two known digestive system pathologies have been considered to affect discrete of Western and Northern European ancestry, the epidemiology is changing dramatically with the steadily escalating incidence in hitherto low-incidence regions including resource-limited countries. The population of demographics of IBD in the United Kingdom, United States of America, Canada, and France are also changing, with increases in none-White born races and ethnicities [5–7]. It is consequently crucial to entirely apprehend the epidemiology and progression of IBD in discrete racial and ethnic groups, and the effects of race, culture, and ethnicity of access to care, use of resources, and disease related outcomes.
There are established guidelines for the diagnosis and pharmaceutical management of IBD [8,9] which includes international clinical practice tool recommendations that incorporates various best practices, and other evidences has widely been issued [10,11]. In the past two decades there have vastly been advances in research, i.e., molecular diagnostics and pharmacological evolution for IBD management [12,13]. The aim of this overview is to provide disease guidance consensus for healthcare professionals managing IBD, to ensure that investigation, diagnosis, treatment, and monitoring decisions are based on the best available common consent evidence, and to promote and ameliorate best accepted practice.
Etiobiopathogeneses of IBD are not yet fully elucidated of the opinion to be multifactorial [1,14,15] involving complex interaction linking the genetic, environmental and/or microbial factors and the immune responses [16]. The signaling pathway processes are arbitrated via intrinsic of the autoignition counter response to self-antigens [17–20].
Countries with strong economy i.e., US, Canada and Europe have continued to promote ambulatory regimens healthcare conveyance [21–24], third world countries have inadequate healthcare system services rendering limitations to getting the required quality of care [25] because of restricted capital and doctors and nurses luck knowledge because they are not trained about IBD [26]. In addition, for cost-effectiveness considerations and recommendations by the World Health Organization (WHO) and the World Gastroenterology Organization (WGO) [27,28], third world nations will wrestle in the inflexible economy. There is need of special guideline, currently not available that explore solution of infrastructure limitation in terms of access to IBD care in resource-limited countries and the inconsistency representation of socioeconomic stratification with a unique plan regarding how to surge this evolving pandemic [29]. The level of primary care and referral hospitals in third world nations face significant infrastructural insufficiencies and lack of regular clinical supervision and laboratory assessments and monitoring patients [30,29]. Patients face difficulty affording pharmaceuticals per the recommended and approval guidelines in the favor of wealthy countries.
2. Method
Information exploration guidelines regarding recommendation for the diagnosis and treatment of IBD was executed utilizing preestablished protocols in accordance with the quality of reporting meta-analyses of observational studies (MOOSE) [31, 32], MEDLINE and EMBASE were searched between 1980–2021. Further, Cumulative Index of Nursing and Allied Health Literature (CINAHL), PubMed, Google search engine, Cochrane Database and IBD-associated society organizations i.e. American Society for Gastrointestinal Endoscopy (ASGE), American College of Gastroenterology (ACG), British Society of Gastroenterology (BSG), International Foundation for Gastrointestinal Disorders (IFGD), American Gastroenterological Association (AGA), American Society of Colon and Rectal Surgeons (ASCRS), Society of American Gastrointestinal and Endoscopic Surgeons (SAGE), World Health Organization (WHO), United States Food and Drug Administration (USFDA), International Organization for the Study of Inflammatory Bowel Disease (IOIBD),European Medicines Agency (EMA), European Crohn’s and Colitis (ECC), American Crohn’s and Colitis (CCFA), CAG=Canadian Association of Gastroenterology (CAG) were also used.
3. Clinical Diagnosis
Standardized diagnostic test for IBD do not exist to date [33,34]. The common state-of-art-diagnosis of IBD relies on accumulation of clinical, radiologic, endoscopic, and histopathologic interpretation/ classification [35, 36]. This imprecise anthology is ambiguous in up to 15% of colonic IBD patients and are labeled as ‘‘indeterminate colitis’’ (IC) because the explication benchmark for CC and UC are unspecified [37,38]. There is another 15% of the colitides cases that are offered pouch surgery i.e., restorative proctocolectomy with ileal pouch-anal anastomosis (RPC-IPAA) for their ultimate UC diagnosis will have their initial UC diagnosis changed to ileal CD based on the subsequent clinical and histopathology changes indicate development of CD in the ileal pouch an indication that authentic CC was not diagnosed prior to colectomy [39,40]. Fifty percent of these patients with subsequent pouch CD will have their pouch extirpated or diverted [41,42].
4. Crohn’s disease
Crohn’s disease is diagnosed in at least four patients per 100000 live births in the United States and Canada. The incidence and prevalence is evolving internationally [29,43,44] specifically in the third world nations [30,29].. Clinically, CD differs from UC in that it may result in inflammation involving all the four intestinal walls i.e., mucosa, submucosa, muscularis and serosa and can also transpire in any parts of the digestive system tract, from the mouth, esophagus, stomach, duodenum, small intestine, colon, and rectum [36]. Further, CD may also penetrate to other systemic organs outside the GI tract through fistulation [3,4,45]. The clinical features for diagnosing CD includes an imprecise mixture of categorization system mentioned in section three above of IBD clinical diagnosis, and histopathological interpretation showing important, transmural, or granulomatous, asymmetric features [46,47]. Computed tomography (CT) enterography of the abdomen is the leading recommended and preferred first-line radiologic test used in the evaluation and/or assessment of CD. The diagnostic accuracy of magnetic resonance enterography / enteroclysis is equivalent to that of CT scans and prevents liability exposure to ionizing radiation. Endoscopic score metrics are the benchmark tool used to estimate the CD activity and often are used in the clinical setting trials to compute proof of the efficacy and safety of various drugs on causing and continuing remission and epithelial convalescence. There are many recommended scoring systems in the guideline but the most commonly used to measure clinical disease severity (CDS) include short inflammatory bowel disease questionnaire (SIBDQ, HBI- Harvey-Bradshaw index (HBI), and Lehmann score and Crohn’s Disease Activity Index (CDAI) [46,47].
5. Ulcerative Colitis
Most UC onset is in early adolescence [48]. Unmanaged UC results in chronic inflammation and ulcerations in the epithelial and submucosal layers restricted to the colon and rectum [36,48]. Approximately 15% of the patients may encounter toxic fulminant UC that may be admitted to hospital as emergence [48,49]. Establishing the UC diagnosis and disease state of a patient sample gastrointestinal pathologists depend on microscopic visual inspection and interpretation of distinct and/or stained tissue sections [50,51]. These performances provide with a compelling degree of discussion [52] and are abundance with exceptions [52,53]. Tutoring in pathology subspecialties is needed to improve and achieve the standard of care to avoid diagnostic ambiguity and delay [54,55]. Regardless of these illustrious benchmarks, ineradicable positions appear in which dispassionately cannot be conventionally dependable and where remarkable discrepancy of opinion occurs not to mention expert specialists [56]. Fundamental guideline overview and the consortium specialized review, disease activities of UC are summarized based on the Witts criteria and Truelove and Mayo Clinic score [57,58]. Mayo Clinic scores of 6–12 with an endoscopic subscore of 2 or 3 are aforethought moderate to severe disease. This guideline is explicated as hospital admitted patients with the following Truelove and Witts criteria: ≥6 hematochezia (bloody diarrhea) movements/day with at least 1 marker of inseparable toxicity, including heart beat/rate >90 beats/min, body temperature >37.8°C, blood hemoglobin <10.5 g/dL, and/or ESR (erythrocyte sedimentation rate) −30 mm/h [58].
6. Indeterminate Colitis
An estimated 15% of patients with colonic IBD cannot be delineated, especially during early stage of the disease and are termed ‘‘indeterminate colitis’’ (IC) due to non-definitive foundation of benchmark for UC and CC confounding effective treatment regimens and appropriate surgeries [37,59]. Elucidation of molecular biomolecules and different cellular signaling pathway mechanisms driving IBD heterogeneity is target to the future pharmaceutical inhibitor development to advancing patient care and quality of life [60–63]. Here, the central medical challenge is the discrimination of colonic IBD into the specific subtypes with accuracy. There are reports showing that human alpha defensin 5 (DEFA5, alias HD5) in the colon crypt mucosa with aberrant expression of Paneth cell-like cells (PCLCs) in areas identified with an ectopic colonic ileal metaplasia that is diagnostic of CC with a positive predictive value of 96% [64,65,103].
7. Clinical Presentation
The clinical presentation of IBD depends on the site and extent of mucosal inflammation, Quiscent, mild, moderate, and severe. The frequency of presenting symptoms is presented in Table 1 and in Table 2 are manifestations of IBD [164–172].
Table 1.
| Symptom | CD (%) | UC (%) |
|---|---|---|
|
| ||
| Abdominal pain | 62–95 | 33–76 |
| Diarrhea | 52–78 | 67–93 |
| Weight loss | 43–92 | 22–55 |
| Hematochezia | 14–60 | 52–97 |
| Delayed growth | 30–33 | 6 |
| Fever | 11–48 | 4–34 |
| Perianal disease | 25 | 0 |
| Extraintestinal manifestation | 15–25 | 2–16 |
Table 2.
| System | Manifestation |
|---|---|
|
| |
| Generalized | Fever |
| Weight loss | |
| Malaise | |
| Anorexia | |
| Fatigue | |
| Nausea/ vomiting | |
| Ocular | Uveitis |
| Episcleritis | |
| Iritis | |
| Conjunctivitis | |
| Oral | Cheilitis |
| Stomatitis | |
| Aphthae | |
| Pulmonary | Pulmonary vasculitis |
| Fibrosing alveolitis | |
| Vascular | Vasculitis |
| Thrombosis | |
| Hepatobiliary | Primary sclerosing Cholangitis |
| Hepatitis | |
| Cholangitis | |
| Jaundice | |
| Pancreatic | Pancreatitis |
| Gastrointestinal | Abdominal Pain |
| Nausea/ vomiting | |
| Diarrhea | |
| Hematochezia | |
| Renal/ Urinary | Nephrolithiasis |
| Obstructive hydronephrosis | |
| Enterovesical fistula | |
| UTI | |
| Amyloidosis | |
| Hematologic | Iron deficiency anemia Anemia of chronic disease Thrombocytosis Vitamin B12 deficiency Autoimmune hemolytic anemia |
| Endocrine | Decreased growth velocity Delayed sexual maturation |
| Integumentary | Erythema nodosum Pyoderma gangrenosum Perianal disease |
| Musculoskeletal | Osteopenia and osteoporosis Arthritis / Arthralgias Ankylosing spondylitis |
8. Molecular Diagnostic Advances in IBD
Molecular diagnostics research focuses on improving the diagnosis for patients with the predominantly colonic IBD subtypes of UC and CC with accuracy [36,66,67]. Current reported data indicate possible proteomic signatures that discriminate between UC and CC patients and predict the outcome of IC patients for their eventual progress to either authentic UC or CC [64,65]. Analytical evidence demonstrate that colonic apparent crypt cell-like cells (CCLCs) secreted human alpha defensin 5 (DEFA5, alias HD5) is aberrantly expressed in CC as compared to UC [66, 68]. Both the CCLCs and DEFA5 are not analogous of colon. Therefore presence of DEFA5 in areas of the colonic mucosa with aberrant expansion of CCLCs identifies an area of ectopic colonic ileal metaplasia, positive for Paneth cell markers, that is consistent with the diagnosis of CC [68]. Detection of DEFA5 in the biopsy tissues from the otherwise inexact diagnosed IC patients; and that UC patients who underwent RPC-IPAA surgery and later diagnosed with CD were more accurately differentiated from those which retained their original UC diagnosis by detection of DEFA5 [39]. Together, this multipronged evidenced approach suggests that a DEFA5 bioassay, can be adopted in the clinical practice to facilitate translational accessory for accurate IBD diagnosis, initial assessment prior to prescription of disease subtype appropriate pharmaceutical management and/or more importantly surgical options in case of conventional drug refractory.
9. Core Tip
To date there is no drug for cure for IBD. Optimized studies support the concept of the presence of multiple antibodies against enteric bacterial antigens in IBD [103]. Whether the production and releasing of antibodies is a result of barrier dysfunction induced by inflammation or a serologic finding secondary to IBD is discussible. Histopathology and clinical evaluation show that CD and UC, the two major classifications of IBD subtypes, are indeed discrete entities and have disparate causes and distinguishable mechanisms of tissue destruction/ damage and management [69,70]. The foundational inquiry is why the systemic innate immune process responds aggressively to indigenous innocuous, inextinguishable bacteria (the commensals), deliverance complex mixes of tissue by-product signatures (cytokines, chemokine, growth factors) and other substances that cause inflammation (antibody–antigen reaction against mucosal resistance). There has been a speculation that the trigger(s) of IBD may be identical, and the phenotype i.e., UC and CC are determined by the patient’s own immunity (level of strength) [16].
10. Treatment
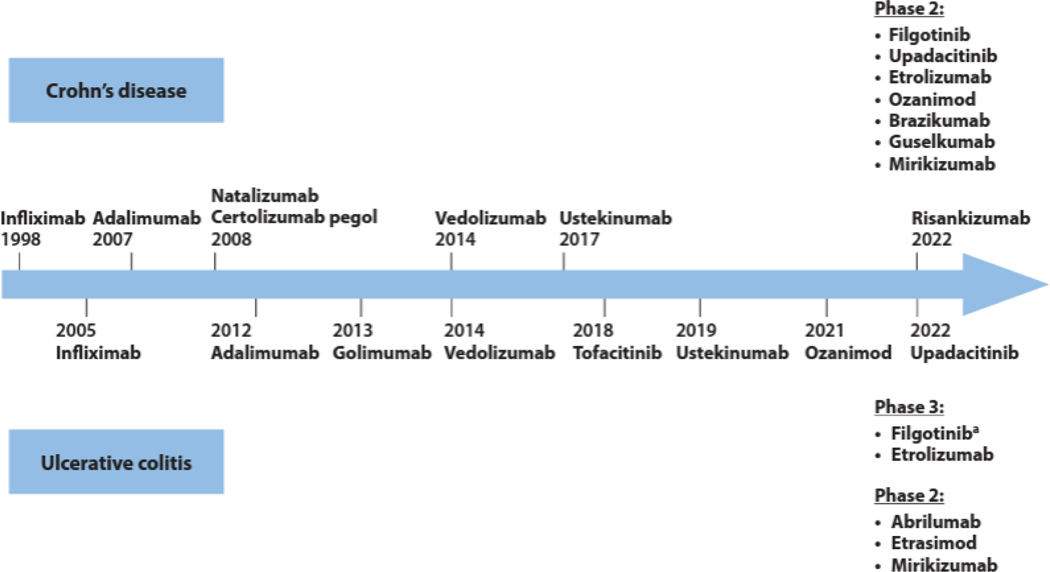
There are several international and individual national recommendation options in the treatment of IBD, of which some are specific for CD [8,71–73], UC specific [33,60,74–77] and/or both UC and CD [13, 78–80]. There are different recommended pharmaceutical classes for long-term treatment of moderate to severe IBD prescribed for promotion of remission and for prolonged maintenance remedy if they are effectual efficacious [77]. This specialized clinical practice is regarded professional and/or accepted by medical experts in this guideline and it is assumed that if a drug (excluding corticosteroids and cyclosporine) is initiated for and is efficacious for resulting of remission or response, it will be continued for maintenance of mucosal healing and remission [77]. There has been encouraging timeline of drugs approved by the United States Food and Drug Administration for the management of IBD, and published abstracts and articles for positive phase 2 and 3 clinical trials, Figure 1.
Figure 1.
Timeline of drugs approved by the United States Food and Drug Administration for the management of IBD encompassing ulcerative colitis and Crohn’s disease, and published abstracts and articles for positive phase 2 and 3 clinical trials [163].
11. Pharmacological Treatment
The guideline by the American Gastroenterological Association (AGA) Institute’s Clinical Guidelines Committee approved by the AGA Governing Board is supported by a practical review that advises extended synthesis of the evidence-based from which these propositions were worked out [81]. Pharmacologically, IBD has no known cure to date [1]. The purpose of conservative clinical management of IBD is to induce and maintain remission in patients with dynamic/active disease [82,83]. Treatment strategies consist of symptomatic conservative pharmacological therapy and inevitably surgery depending on disease location, severity, and patients’ treatment history [84]. The conventional step-up approach consists of first-line therapy with classical treatment approaches such as aminosalicylates, corticosteroids, and immunomodulators (e.g., azathiopurine, 6-mercaptopurine) [85]. Due to variability in cellular processes that underlie the natural history of CC making diagnosis difficult, delayed, and appropriate treatment is often confounded [1,86]. In evaluating the best evidenced practice for optimizing treatment of IBD, the convergence is on anti-inflammatory drugs and therapies that restrain the immune system. The intestinal luminal innate immune response continues to be the important key focus of drug development and therapies [87,88]. The concept of dysbiosis of the anti-inflammatory, immune-, and microbiome-modulating has surfaced as a potential pathogenetic focus in IBD with a burgeoning interest in influencing the microbiome as a means of managing the disease in the therapeutic armamentarium [89,90]. Synchronous evolution of our understanding of the basic biology of IBD and triggers, there is an increasing appreciation for disconnect between patients’ symptomatology and IBD. As clinical trials have concurrently addressed both symptom scores and mucosal healing, physician scientists have gained a wide range appreciation for the fact that several symptoms may not be caused by active inflammation, and therefore focusing only on immunomodulatory therapies would not serve patients’ needs adequately. Furthermore, there is an emerging understanding of the significance of stress and psychological health in symptom experience and required therapy. The available conservative pharmacological treatment greatly reduces signs and symptoms, of which otherwise can be life threatening and fatal, bring about long-term remission [91,92]. With the correct diagnosis and treatment, people with the IBD can manage symptoms and improve their health quality of life (HQoL) [93,94].
Cytokines, chemokines, and growth factors are engaged in luminal intestinal homeostasis and pathological processes related triggers with IBD. The biological effects of secretagogues including several involved in the pathology of CD and UC, occur because of receptor-mediated signaling via the Janus kinase (JAK) and signal transducer and activator of transcription (STAT) DNA-binding families of proteins [95, 96].
More recently, newer biologics, cell-based therapies have emerged and been introduced, raining new aspirations that outcomes can be ameliorated. Despite this, in refractory cases, many patients are recommended a proctocolectomy requiring a stoma [12,41,97,98]. Since there is no drug for sure for IBD, the goal of IBD treatment is to reduce the inflammation that causes patient symptoms. In the best cases, this may lead not only to symptom relief but also to long-term remission, lower risks of adverse complexities and improved patient quality of life. Medical therapy has advanced dramatically in the last ten years with the establishment of targeted biologic therapies, the upsurge of older treatments, including rugs such as immunomodulators and 5-aminosalicylic acid (5-ASA), and a better understanding of the mucosal immune system and the genetics involved in the etiopathogenesis triggers of IBD. The therapeutic paradigm involves a step-up approach, moving to contentious powerful therapies only when milder therapies with fewer potential adverse side effects fail or when patients declare themselves to have an intrusive disease.
Anti-inflammatory pharmaceuticals:
Anti-inflammatory pharmaceuticals are often the first step in the line of treatment of IBD. Anti-inflammatories include corticosteroids and aminosalicylates, such as mesalamine (Asacol HD, Delzicol, others), balsalazide (Colazal) and olsalazine (Dipentum). Which medication indicated depends on the anatomical area of gastrointestinal tract (GI) that is impacted.
Immune system suppressors:
These medicines work in diverse ways to minimize the aggressive immune response that releases inflammation-inducing chemicals into the system. When released, these chemicals create an anti-body-antigen reaction against the mucosal resistance sequence of the patient that can damage the luminal inner lining of the digestive tract. Some examples of immunosuppressant drugs commonly used include azathioprine (Azasan, Imuran), mercaptopurine (Purinethol, Purixan) and methotrexate (Trexall).
Biologics:
In the past twenty years, biological care with monoclonal antibodies targeting TNFα has become a groundwork of managing patients with IBD. Biologics are used routinely and are meant to overcome the limitations of therapies and ensure that individual patients can be treated with optimal drugs that are safe and precisely target IBD [99,100]. Pharmaceutic drug monitoring to guide the use of biologic therapy has widely been reported in AGA guidelines [101,102]. The guideline is predetermined for the multipurpose use of gastroenterology healthcare providers, primary care providers, surgeons, patients, and policymakers. The regulatory pathway of a biologic drug needs flourishing clinical trials that substantiate clinical efficacy as well as approval from regulatory agencies such as the United States Food and Drug Administration (FDA) and European Medicines Agency (EMA) [103,104]. Biotherapeutics, or biologics, are drug products fabricated using living instrumentation design systems. Their production typically involves genetically modified/ engineered animal, plant, or bacterial cells. Since their introduction, biologics have had a substantial impact on the clinical management of systemic inflammatory conditions such as IBD [105,106]. Biologics are a newer category of therapy in which treatment is directed toward mitigation or neutralizing chemicals in the body that are inflammation triggers. Examples include infliximab (Remicade), adalimumab (Humira), golimumab (Simponi), certolizumab (Cimzia), vedolizumab (Entyvio) and ustekinumab (Stelara).
Biosimilars:
These are biological drugs like but different to the original biological agent called “originator biologic.” The advent of monoclonal antibodies, anti-TNF-α agents, has dramatically improved the therapeutic approach to patients with immune-mediated IBD [107]. These includes infliximab, adalimumab, and certolizumab in CD and infliximab, adalimumab, and golimumab which are effective and safe therapies to induce disease remission, achieve mucosal healing and improve UC patients’ HQoL [108,109]. Biosimilars represent a great opportunity to decrease health care costs and increase the access to effective medications to a larger number of patients particularly to those who are struggling economically is great advantage [3]. The bioequivalence of biosimilars is based on robust preclinical study evidence, but the clinical data supporting their efficaciousness and safeness in IBD mostly come from single-arm, noncontrolled cohort studies [110,111]. However, these molecules result are costly leading in high direct costs for the health care system, particularly in the long term [29]. A biosimilar is a biological brand that is highly like risk management plan (RMP), with indistinguishable clinically revealing disparities in terms of efficacy, safety, and purity [112,113]. Biosimilars and generic drugs both represent pursuit of a prize towards brand-name/trademark drugs. Even though a developed generic is an exact copy similar of the original small-molecule medicine, it is not feasible to manufacture identical copies of a biologic [114,115]. Since biologics are difficult to replicate, biosimilars are developed using alternate approaches such that the final product is almost identical to the RMP with respect to the initial amino acid sequence [116,117]. Due to the inherent variability of the living bacteria-based systems used in the development of biosimilar drugs, there is microheterogeneity between biosimilar and RMP [118].
In the mise-en-scène of biosimilars, regulatory agencies only need to make sure that high similarity and/or comparability is substantiated between the biosimilar and its RMP before a biosimilar candidate can officially be sanctioned and marketed, resulting in a simpler approval pathway [119]. The anticipation with biosimilars is that their entry into the market drives competition between drug developing companies, to compete and reduce prices comparably to how the generic market has, and to increase overall patient access to appropriate biologic treatments in the long-term. Currently, only two biosimilars have been approved for use in IBD in the United States: infliximab-dyyb and adalimumab-atto [120,121], Table 3. However, several anti-TNF biosimilars development have either been posed, are being evaluated in final-stage clinical trials, or are awaiting official approval from federal regulatory agencies i.e., The U.S. Food and Drug Administration (FDA).
Table 3.
Clinical studies on infliximab-dyyb induction in inflammatory bowel disease.
| Study | Population | Results | Definition of Outcome Response (Remission | Reference |
|---|---|---|---|---|
| Comparison Randomized 8 vs. 12 wks IFX maintenance | CD = 103 | 15 Point decrease in PCDAI Remission: PCDAI ≤10 | 8wks group: 56% 12 wks group: 24% (p = 0.001) | Hyams et al., [122] |
|
| ||||
| Comparison Randomized 10 vs. 60 wks IFX maintenance | CD = 40 | Remission: PCDAI <5 | 10 wks group: 83% 60 wks group: 61% | Ruemmele et al., [123] |
|
| ||||
| Comparison Randomized 8 vs. 12 wks IFX maintenance | UC = 60 |
Response: Decreased in Mayo score By ≥ 30% and ≥3 points Clinical remission: Mayo score ≤2 with no individual subscore >1 and PUCAI <10 |
8wks group: 38% 12 wks group: 18% (p = 0.146) 54 wks group: 28.6% | Hyams et al., [124] |
|
| ||||
| Comparison randomized high dose ADA (40 mg or 20 mg/body weight ≥40kg or 40 kg; n = 95 or Low dose (20 mg or 10 mg/body weight ≥40 kg or 40 kg; n = 95) | Moderate to severe CD = 188 | Response: Decreased in PCDAI ≥15 | High dose: 59% Low dose: 48% (p = 0.073) |
Hyams et al., [125] |
| High dose: 39% Low dose: 28% (p = 0.075) | ||||
| Remission: PCDAI ≤10 | High dose: 42% Low dose: 28% (p = 0.075) | |||
|
| ||||
| Prospective observational | CD = 46 UC = 32 |
Clinical remission rates at week 14 79% (CD), 59% (UC) Significant decrease in CRP, calprotectin | No adverse events reported | Jahnsen et al., [126] |
|
| ||||
| Prospective multicenter | CD = 32 UC = 42 |
Clinical response at week 54 87.5% (CD), 100% (UC) Clinical remission rates at week 54 75% (CD), 50% (UC) | Adverse events in 11% of UC pts | Jung et al., [127] |
|
| ||||
| Prospective multicenter, national cohort | CD = 126 UC = 84 | Clinical response at week 14 81.4% (CD), 77% (UC) Clinical remission rates at week 14 53.6% (CD), 58.6% (UC) | Adverse events in 17.1% in all pts | Gecse et al., [128] |
|
| ||||
| Switch from RPM to Infleximab-dyyb | Pediatric CD = 32 UC = 7 |
Clinical remission rates 88% (CD), 57% (UC) Decreased in PCDAI, CRP, ESR | No adverse events reported | Sieczkowska et al., [129] |
|
| ||||
| Prospective observational, Cohort switch | CD = 57 UC = 26 |
Clinical response at week 16 Calprotectin | No adverse events reported | Smits et al., [130] |
Abbreviations: IBD: Inflammatory bowel disease. CD: Crohn’s disease. UC: Ulcerative colitis. CRP: C-reactive protein. IFX: Infliximab. ADA: Adalimumab. PCDAI: Pediatric Crohn’s Disease Activity. PUCAI: Pediatric Ulcerative colitis Activity Index. WKS: Weeks. DAI: Disease Activity Index. ESR: Erythrocyte sedimentation rate. HBI: Harvey Bradshaw Index. OCDAL: Pediatric Crohn’s Disease Activity Index.
Antibiotics:
All kinds of antibiotics may be used in addition to other medications or when infection is a concern, especially in cases of perianal CD. Commonly prescribed antibiotics include ciprofloxacin (Cipro xr, Cetraxal, Otiprio) and metronidazole (Flagyl).
Other medications and supplements:
In addition to controlling inflammation, some medications may be indicated to alleviate symptoms depending on the disease activity and severity of IBD. In medical practice, one or more of the following are in addition commonly prescribed:
Anti-diarrheal medications:
A fiber supplement such as psyllium powder (Metamucil) or methylcellulose (Citrucel) to assuage mild to moderate diarrhea. For more-severe diarrhea, loperamide (Imodium A-D) may be efficacious alternative.
Analgesics:
There are several pain reliever meds recommended for mild pain, i.e., acetaminophen (Tylenol, others). However, ibuprofen (Advil, Motrin IB, & others), naproxen sodium (Aleve) and diclofenac sodium likely will provoke the disease and worsen making symptoms become severe.
Vitamins and supplements:
Vitamin deficiencies are common in patients suffering from IBD and is considered a potential pathogenic factor in their development [131,132]. Patients with IBD should be monitored and compensated for nutritional deficiencies [131–135].
Dietary Therapy in IBD:
Based on science, both epidemiological studies and experimental studies there is no doubt that there are numerous associations that link IBD and diet [136–138]. Diet has partly been realized to contribute and play a role in the pathogenesis in inflammation, with research advances showing the effect of dietary exposures on the intestinal microbiome as well as mucosal integrity [139,140]. Specifically, the culture in industrialization of food and westernization of dietary practices such as fast food is suspected to play a key role in increasing incidence of IBD [141,142]. The use and ability of dietary intervention has shown to be beneficial to reduce inflammation as is illustrated in the efficacy of exclusive enteral nutrition (EEN) to induce remission in IBD, specifically in CD [143,144]. EEN, which is a balanced nutritionally complete liquid formula with the exclusion of all solid contents. Outlining areas of health coaching and dietary treatment options, the current knowledge about patient inspirations for pursuing dietary therapy and improvement for the decision-making process are emerging [145, 146]. When weight loss is severe enteral or parenteral nutrition is indicated to treat IBD. This will ameliorate generic nutrition and allow the bowel to rest. Bowel rest can slow inflammation in the short term. About 60% of patients with CC develop strictures, containing various degree of inflammation, fibrosis, and hyperplasia formation bowel obstruction [147]. Inflammatory strictures may benefit from the complementary use of pharmacologic anti-inflammatory and nutritional treatment to attain histologic and clinical remission. In a stenosis or stricture in the bowel, low-residue diet. This will help to minimize the chance that undigested food will get stuck in the luminal stricture part of the bowel continuity and lead to obstruction.
Costs in IBD Management:
The economic implications of IBD are enormous [84]. Hospital admission rates and costs for IBD show an increasing trend assessed by specific pharmaceutical and disease features [148, 149]. In the US alone, the estimated annual direct treatment costs are greater than $6.8 billion, and indirect costs amount to an additional $5.5 billion [150,151]. Infliximab (Remicade) and adalimumab (Humira) introduction and maintenance therapies were cost-effective in comparison to standard care in patients suffering from moderate or severe CD; however, in patients with conventional-drug refractory CD, fistulizing CD and for maintenance of surgically induced remission incremental cost-effectiveness ratio (ICERs) were above acceptable cost-effectiveness thresholds [29,152]. In mild UC, induction of remission using high dose mesalazine (5-aminosalicylic acid (5-ASA)) is reported dominant in comparison to standard dose. In UC refractory to conventional evidence-based practice treatments, prescribing infliximab and adalimumab induction and maintenance treatment were not economically sound as compared to recommended standard care; however, ICERs for treatment with vedolizumab (Entyvio) and pouch surgery, RPC-IPAA are favorable to date. While biologic agents significantly ameliorate outcomes, they sustained significant inflated costs and hence are not economical, particularly for use as maintenance therapy [29,152]. The worthwhileness of biologic drugs may refine as market prices fall and with the introduction of biosimilars. Future research endeavors should focus on identifying optimal therapeutic strategies reflecting practical routine clinical practice, integrate indirect costs and evaluate estimates of lifespan costs and benefits.
Management of Patients with IBD during the COVID-19 Pandemic
Coronavirus disease 2019 (COVID-19) pandemic has been a global tragedy that changed the traditional management plan of all diseases, with no exception of patients with IBD [11,153–155]. Bravely, the main clinical outcomes were maintained during the COVID-19 pandemic period largely because scheduled visits were replaced by phone calls and virtual consultations [156]. Virtual clinic follow-up using the contact center service (CCS) on the reorganization of a high-volume IBD centers and on the continuity of care during the COVID-19 outbreak. This approach could be implemented after the pandemic to optimize the resources of the IBD centers [156,157]. This has led to substantial changes causing interruption of non-essential endoscopic procedures and outpatient visits challenged, particularly on the assessment of disease activity by increasing the risk of relapse, disease complications, delays of new IBD diagnosis and detection of early post-operative recurrence of de novo CD [158]. The interventional performance of routine endoscopy was largely suspended in many IBD Clinic and Centers worldwide where undesirable acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread [153]. Experts highlight different scenarios in which endoscopy should still be performed imperatively in unique circumstances in patients with IBD, as well as suggestion instructions regarding the use of personal protective equipment [153,159] for carry out safe procedures and the possible risks of postponing endoscopy in IBD and a post-pandemic plan for access to endoscopy as summarized by Iacucci et al. [153]. Clinical Practice Update (CPU) from expert emerge evidence that provide timely council about the treatment of patients with IBD during the CONVID-19. Admittedly, comments herewith provide perspective on a topic of high clinical importance that underwent internal peer review by the Clinical Practice Updates Committees and external peer review through standard procedures of Gastroenterology [11,153]. We are reminded however that as the understanding of the novel coronavirus progresses, IBD-specific issues and guidance may change beyond reasonable doubt [11,160]. Management of patient attending outpatient clinic with IBD in remission in the setting of asymptomatic SARS-CoV-2 infection or confirmed or suspected COVID-19 without systemic hyperinflammation syndrome are summarized in Tables 4, 5 and 6 [11,154,161,162].
Table 4.
Management of patients attending outpatient clinic with quiescent inflammatory bowel disease in the scenario of asymptomatic severe acute respiratory syndrome coronavirus 2 infection or confirmed or suspected coronavirus disease 2019 [11, 154,161,162].
| Management | |
|---|---|
| Asymptomatic infection with Taper or SARS-CoV-2 | (1) Budesonide, aminosalycilates, antibiotics, and topical therapy may be maintained; (2) Hold immunomodulators tofacitinib, and biologics for 2 wk; (3) withdraw systemic corticosteroids (prednisone); and (4) Monitoring for 2 wk for COVID-19 symptoms. |
| Mild COVID-19 | (1) Budesonide, aminosalycilates, antibiotics, and topical therapy may be maintained; (2) Hold immunomodulators, tofacitinib, and biologics for 2 wk; and (3) Taper or withdraw systemic corticosteroids (prednisone) |
| COVID-19 with pulmonary immune-involvement without SHS | (1) Budesonide, aminosalycilates, antibiotics, and topical therapy may be maintained; (2) Hold immunomodulators, tofacitinib, and biologics for 2 wk; and (3) Taper or discontinue systemic corticosteroids |
Immunomodulators refer to thiopurines and methotrexate. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; COVID-19: Coronavirus disease 2019; SHS: Systemic hyperinflammation syndrome.
Table 5.
Management of patients attending outpatient clinic with mildly active inflammatory bowel disease in the scenario of the asymptomatic severe acute respiratory syndrome coronavirus 2 infection or confirmed or suspected coronavirus disease 2019 [11,15,4,161,162].
| Management | |
|---|---|
| Asymptomatic infection or with SARS-CoV-2 | (1) Budesonide, aminosalycilates, antibiotics, and topical therapy may be used if needed; (2) Holds immunomodulators, tofacitinib, and biologics for 2 wk; (3) Taper withdraw corticosteroids (prednisone < 20 mg/d); and (4) Monitoring for 2 wk for COVID-19 to present. |
| Mild COVID-19 | (1) Budesonide, aminosalycilates, antibiotics, and topical therapy may be used if needed; (2) Hold immunomodulators, tofacitinib, and biologics for 2 wk; (3) Taper or withdraw systemic corticosteroids); and (4) Monitoring for 2 wk for COVID-19 symptoms to disappear. |
| COVID-19 with pulmonary unit involvement without SHS | (1) Budesonide, aminosalycilates, antibiotics, and topical therapy may be used if necessary; (2) Hold immunomodulators, tofacitinib, and biologics for at least 2 wk or COVID-19 resolves; and (3) Taper or withdraw systemic corticosteroids. |
Table 6.
Management of patients attending outpatient clinic with moderately to severely active inflammatory bowel disease in the scenario of asymptomatic severe acute respiratory syndrome coronavirus 2 infection or confirmed or suspected coronavirus disease 2019 [11, 154,161,162].
| Management | |
|---|---|
| Asymptomatic infection necessary with SARS-CoV-2 | 1) Restrict the use of prednisone ≤ 40 mg/d if necessary; (2) Avoid immunomodulators and to facitinib; with SARS-CoV-2 (3) Escalate to biologics as (preferably in monotherapy); and (4) Thromboprophylaxis |
| Mild COVID-19 | 1) Restrict the use of prednisone ≤ 40 mg/d if necessary; (2) Avoid starting or stopping, if in use, immunomodulators, and tofacitinib; (3) Escalate to biologics and dose optimization as necessary (preferably in monotherapy); and (4) Thromboprophylaxis |
| COVID-19 with pulmonary Escalate involvement without SHS with infectious | (1) Restrict the use of prednisone ≤ 40 mg/d if necessary; (2) Avoid starting or stopping pulmonary involvement immunomodulators, and tofacitinib; (3) to biologics and dose optimization as without SHS necessary (preferably) in monotherapy based on balance of benefits and risks; consultation diseases expert for possible COVID-19 treatment with antiviral or experimental anticitokine therapy; and (4) Thromboprophylaxis |
Immunomodulators refer to thiopurines and methotrexate. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; COVID-19: Coronavirus disease 2019; SHS: Systemic hyperinflammation syndrome
Conclusion:
IBD has become a global emergent disease. Refractory IBD is still a significant challenge in the management of patients with Crohn’s disease and ulcerative colitis. There are gaps in knowledge and future research directions on the recent newly registered pharmaceuticals. Serious medical errors are public health problem observed in developing nations around the world i.e., to distinguish IBD from bacterial or infectious parasitic diseases e.g., amoebiasis and shigellosis inadvertently contributes to severe delay in diagnosis and treatment. The main clinical outcomes for IBD were maintained during the COVID-19 pandemic period.
Funding Statement:
Amosy M’Koma received the Vanderbilt-Ingram Cancer Center Gastrointestinal ((VICC-GI) SPORE Career Enhancement Award - P50CA236733.
NIH/NIDDK-R21DK095186; Research Foundation, The American Society of Colon and Rectal Surgeons - LPG086,
Footnotes
Conflicts of Interest:
Amosy M’Koma received Honoraria fees for Educational Presentation from The International Colorectal Research summit (Korean society of coloproctology) 2021, Seoul, Korea, and Lipscomb University Health Sciences, Nashville, Tennessee, United States 2019. In addition, he is an inventor of two Patents: (i) Assay methods for diagnosing and treating inflammatory bowel disease. US Patent US11427852B2, 2022 and (ii) Targeted DEFA5 antibody and assay methods for diagnosing and treating inflammatory bowel disease. US Patent 16/622,259, 2021). https://patents.google.com/?inventor=Amosy+M%27KOMA This does not alter our adherence to the Medical Research Archives, European Society of Medicine journal’s policies on sharing educational data and materials in science.
References
- 1.M’Koma A E, The Multifactorial Etiopathogeneses Interplay of Inflammatory Bowel Disease: An Overview. Gastrointest Disord 2018, 1 (1), 75–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conrad K; Roggenbuck D; Laass MW, Diagnosis and classification of ulcerative colitis. Autoimmun Rev 2014, 13 (4–5), 463–6. [DOI] [PubMed] [Google Scholar]
- 3.Nosti PA; Stahl TJ; Sokol AI, Surgical repair of rectovaginal fistulas in patients with Crohn’s disease. European journal of obstetrics, gynecology, and reproductive biology 2013. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen OH; Rogler G; Hahnloser D; Thomsen OO, Diagnosis and management of fistulizing Crohn’s disease. Nat Clin Pract Gastroenterol Hepatol 2009, 6 (2), 92–106. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan GG, The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015, 12 (12), 720–7. [DOI] [PubMed] [Google Scholar]
- 6.Windsor JW; Kaplan GG, Evolving Epidemiology of IBD. Curr Gastroenterol Rep 2019, 21 (8), 40. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan GG; Windsor JW, The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2021, 18 (1), 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuoka K; Kobayashi T; Ueno F; Matsui T; Hirai F; Inoue N; Kato J; Kobayashi K; Kobayashi K; Koganei K; Kunisaki R; Motoya S; Nagahori M; Nakase H; Omata F; Saruta M; Watanabe T; Tanaka T; Kanai T; Noguchi Y; Takahashi KI; Watanabe K; Hibi T; Suzuki Y; Watanabe M; Sugano K; Shimosegawa T, Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol 2018, 53 (3), 305–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapasi R; Glatter J; Lamb CA; Acheson AG; Andrews C; Arnott ID; Barrett KJ; Bell G; Bhatnagar G; Bloom S; Brookes MJ; Brown SR; Burch N; Burman A; Crook K; Cummings JF; Davies J; Demick A; Epstein J; Faiz O; Feakins R; Fletcher M; Garrick V; Jaffray B; Johnson M; Keetarut K; Limdi J; Meade U; Muhammed R; Murdock A; Posford N; Rowse G; Shaw I; St Clair Jones A; Taylor S; Weaver S; Younge L; Hawthorne AB, Consensus standards of healthcare for adults and children with inflammatory bowel disease in the UK. Frontline Gastroenterol 2020, 11 (3), 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amiot A; Bouguen G; Bonnaud G; Bouhnik Y; Hagege H; Peyrin-Biroulet L; French National Consensus Clinical guidelines for the management of, I. B. D. s. g., Clinical guidelines for the management of inflammatory bowel disease: Update of a French national consensus. Dig Liver Dis 2021, 53 (1), 35–43. [DOI] [PubMed] [Google Scholar]
- 11.Rubin DT; Feuerstein JD; Wang AY; Cohen RD, AGA Clinical Practice Update on Management of Inflammatory Bowel Disease During the COVID-19 Pandemic: Expert Commentary. Gastroenterology 2020, 159 (1), 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.M’Koma AE; Wise PE; Muldoon RL; Schwartz DA; Washington MK; Herline AJ, Evolution of the restorative proctocolectomy and its effects on gastrointestinal hormones. Int J Colorectal Dis 2007, 22 (10), 1143–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen B; Kochhar G; Navaneethan U; Farraye FA; Schwartz DA; Iacucci M; Bernstein CN; Dryden G; Cross R; Bruining DH; Kobayashi T; Lukas M; Shergill A; Bortlik M; Lan N; Lukas M; Tang SJ; Kotze PG; Kiran RP; Dulai PS; El-Hachem S; Coelho-Prabhu N; Thakkar S; Mao R; Chen G; Zhang S; Suarez BG; Lama YG; Silverberg MS; Sandborn WJ, Practical guidelines on endoscopic treatment for Crohn’s disease strictures: a consensus statement from the Global Interventional Inflammatory Bowel Disease Group. Lancet Gastroenterol Hepatol 2020, 5 (4), 393–405. [DOI] [PubMed] [Google Scholar]
- 14.Rahimi R; Nikfar S; Rezaie A; Abdollahi M, A meta-analysis of antibiotic therapy for active ulcerative colitis. Dig Dis Sci 2007, 52 (11), 2920–5. [DOI] [PubMed] [Google Scholar]
- 15.Heller F; Fuss IJ; Nieuwenhuis EE; Blumberg RS; Strober W, Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity 2002, 17 (5), 629–38. [DOI] [PubMed] [Google Scholar]
- 16.Esmaily H; Sanei Y; Abdollahi M, Autoantibodies and an immune-based rat model of inflammatory bowel disease. World J Gastroenterol 2013, 19 (43), 7569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strober W; Fuss IJ; Blumberg RS, The immunology of mucosal models of inflammation. Annu Rev Immunol 2002, 20, 495–549. [DOI] [PubMed] [Google Scholar]
- 18.Weber MS; Steinman L; Zamvil SS, Statins--treatment option for central nervous system autoimmune disease? Neurotherapeutics 2007, 4 (4), 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steward-Tharp SM; Song YJ; Siegel RM; O’Shea JJ, New insights into T cell biology and T cell-directed therapy for autoimmunity, inflammation, and immunosuppression. Ann N Y Acad Sci 2010, 1183, 123–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludwig RJ; Vanhoorelbeke K; Leypoldt F; Kaya Z; Bieber K; McLachlan SM; Komorowski L; Luo J; Cabral-Marques O; Hammers CM; Lindstrom JM; Lamprecht P; Fischer A; Riemekasten G; Tersteeg C; Sondermann P; Rapoport B; Wandinger KP; Probst C; El Beidaq A; Schmidt E; Verkman A; Manz RA; Nimmerjahn F, Mechanisms of Autoantibody-Induced Pathology. Front Immunol 2017, 8, 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longobardi T; Jacobs P; Bernstein CN, Utilization of health care resources by individuals with inflammatory bowel disease in the United States: a profile of time since diagnosis. Am J Gastroenterol 2004, 99 (4), 650–5. [DOI] [PubMed] [Google Scholar]
- 22.McGlynn EA; Asch SM; Adams J; Keesey J; Hicks J; DeCristofaro A; Kerr EA, The quality of health care delivered to adults in the United States. N Engl J Med 2003, 348 (26), 2635–45. [DOI] [PubMed] [Google Scholar]
- 23.Kappelman MD; Dorn SD; Peterson E; Runge T; Allen JI, Quality of care for gastrointestinal conditions: a primer for gastroenterologists. Am J Gastroenterol 2011, 106 (7), 1182–7. [DOI] [PubMed] [Google Scholar]
- 24.Crandall WV; Margolis PA; Kappelman MD; King EC; Pratt JM; Boyle BM; Duffy LF; Grunow JE; Kim SC; Leibowitz I; Schoen BT; Colletti RB; ImproveCareNow C, Improved outcomes in a quality improvement collaborative for pediatric inflammatory bowel disease. Pediatrics 2012, 129 (4), e1030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogler G; Bernstein CN; Sood A; Goh KL; Yamamoto-Furusho JK; Abbas Z; Fried M, Role of biological therapy for inflammatory bowel disease in developing countries. Gut 2012, 61 (5), 706–12. [DOI] [PubMed] [Google Scholar]
- 26.Herman AM HA, James SD, Ballard BR, M’Koma AE, Inflammatory Bowel Disease OnLine Web-Based Guide to Health Professionals and Patients in Developing and African Nations. Japanise Journal of Gastroenterology and Hepatology 2020, 3 (2), 1–11. [PMC free article] [PubMed] [Google Scholar]
- 27.Organization WH, Threshold values for intervention cost-effectiveness by Region. 2008. [Google Scholar]
- 28.Organization WH, Cost effectiveness and strategic planning (WHO-CHOICE) 2021. [Google Scholar]
- 29.M’Koma A E, Inflammatory Bowel Disease: An Expanding Global Health Problem. Clinical Medicine Insights Gastroenterology 2013, (6), 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herman AM HA, James SD, Ballard BR, M’Koma AE, Inflammatory Bowel Disease OnLine Web-Based Guide to Health Professionals and Patients in Developing and African Nations. Japanise Journal of Gastroenterology and Hepatology 2020. 3(2): p. 1–11. [PMC free article] [PubMed] [Google Scholar]
- 31.Stroup DF; Berlin JA; Morton SC; Olkin I; Williamson GD; Rennie D; Moher D; Becker BJ; Sipe TA; Thacker SB, Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama 2000, 283 (15), 2008–12. [DOI] [PubMed] [Google Scholar]
- 32.Greenland S, Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987, 9, 1–30. [DOI] [PubMed] [Google Scholar]
- 33.Loginov AS; Parfenov AI; Sivash ES; Tsvetkov VF; Zinov’ev OI, [Crohn’s disease. The problem of early diagnosis]. Ter Arkh 1992, 64 (4), 82–5. [PubMed] [Google Scholar]
- 34.Griffiths AM, Challenging question: can we diagnose Crohn’s disease without histology? Dig Dis 2013, 31 (2), 202–6. [DOI] [PubMed] [Google Scholar]
- 35.Van Assche G; Dignass A; Bokemeyer B; Danese S; Gionchetti P; Moser G; Beaugerie L; Gomollon F; Hauser W; Herrlinger K; Oldenburg B; Panes J; Portela F; Rogler G; Stein J; Tilg H; Travis S; Lindsay JO; European C. s.; Colitis O, Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3: special situations. J Crohns Colitis 2013, 7 (1), 1–33. [DOI] [PubMed] [Google Scholar]
- 36.M’Koma AE, Diagnosis of inflammatory bowel disease: Potential role of molecular biometrics. World journal of gastrointestinal surgery 2014, 6 (11), 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burakoff R, Indeterminate colitis: clinical spectrum of disease. J Clin Gastroenterol 2004, 38 (5 Suppl 1), S41–3. [DOI] [PubMed] [Google Scholar]
- 38.Tremaine WJ, Is indeterminate colitis determinable? Curr Gastroenterol Rep 2012, 14 (2), 162–5. [DOI] [PubMed] [Google Scholar]
- 39.James SD HA, Um JW, Ballard BR, Smoot DT, M’Koma AE, The MYTHS of de novo Crohn’s Disease After Restorative Proctocolectomy with Ileal Pouch-anal Anastomosis for Ulcerative Colitis. Japenese Journal of Gastroenterology and Hepatology 2020, 3 (2), 1–10. [PMC free article] [PubMed] [Google Scholar]
- 40.Jarchin L; Spencer EA; Khaitov S; Greenstein A; Jossen J; Lai J; Dunkin D; Pittman N; Benkov K; Dubinsky MC, De Novo Crohn’s Disease of the Pouch in Children Undergoing Ileal Pouch-Anal Anastomosis for Ulcerative Colitis. J Pediatr Gastroenterol Nutr 2019, 69 (4), 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.James SD; Hawkins AT; M’Koma AE, Adenocarcinoma at the Ileostomy Site After a Proctocolectomy for Ulcerative Colitis and/or Familial Adenomatous Polyposis: An Overview. Ostomy/wound management 2018, 64 (6), 30–40. [PMC free article] [PubMed] [Google Scholar]
- 42.Brown CJ; Maclean AR; Cohen Z; Macrae HM; O’Connor BI; McLeod RS, Crohn’s disease and indeterminate colitis and the ileal pouch-anal anastomosis: outcomes and patterns of failure. Dis Colon Rectum 2005, 48 (8), 1542–9. [DOI] [PubMed] [Google Scholar]
- 43.Burisch J; Munkholm P, Inflammatory bowel disease epidemiology. Curr Opin Gastroenterol 2013, 29 (4), 357–62. [DOI] [PubMed] [Google Scholar]
- 44.Molodecky NA; Soon IS; Rabi DM; Ghali WA; Ferris M; Chernoff G; Benchimol EI; Panaccione R; Ghosh S; Barkema HW; Kaplan GG, Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142 (1), 46–54 e42. [DOI] [PubMed] [Google Scholar]
- 45.Lopez N; Ramamoorthy S; Sandborn WJ, Recent advances in the management of perianal fistulizing Crohn’s disease: lessons for the clinic. Expert Rev Gastroenterol Hepatol 2019, 13 (6), 563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gajendran M; Loganathan P; Catinella AP; Hashash JG, A comprehensive review and update on Crohn’s disease. Dis Mon 2018, 64 (2), 20–57. [DOI] [PubMed] [Google Scholar]
- 47.Gajendran M; Bauer AJ; Buchholz BM; Watson AR; Koutroubakis IE; Hashash JG; Ramos-Rivers C; Shah N; Lee KK; Cruz RJ; Regueiro M; Zuckerbraun B; Schwartz M; Swoger J; Barrie A; Harrison J; Hartman DJ; Salgado J; Rivers WM; Click B; Anderson AM; Umapathy C; Babichenko D; Dunn MA; Binion DG, Ileocecal Anastomosis Type Significantly Influences Long-Term Functional Status, Quality of Life, and Healthcare Utilization in Postoperative Crohn’s Disease Patients Independent of Inflammation Recurrence. Am J Gastroenterol 2018, 113 (4), 576–583. [DOI] [PubMed] [Google Scholar]
- 48.Fumery M; Singh S; Dulai PS; Gower-Rousseau C; Peyrin-Biroulet L; Sandborn WJ, Natural History of Adult Ulcerative Colitis in Population-based Cohorts: A Systematic Review. Clin Gastroenterol Hepatol 2018, 16 (3), 343–356 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Narula N; Kim BJ; Davis CH; Dewhurst WL; Samp LA; Aloia TA, A proactive outreach intervention that decreases readmission after hepatectomy. Surgery 2018, 163 (4), 703–708. [DOI] [PubMed] [Google Scholar]
- 50.Theodossi A; Spiegelhalter DJ; Jass J; Firth J; Dixon M; Leader M; Levison DA; Lindley R; Filipe I; Price A; et al. , Observer variation and discriminatory value of biopsy features in inflammatory bowel disease. Gut 1994, 35 (7), 961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seldenrijk CA; Morson BC; Meuwissen SG; Schipper NW; Lindeman J; Meijer CJ, Histopathological evaluation of colonic mucosal biopsy specimens in chronic inflammatory bowel disease: diagnostic implications. Gut 1991, 32 (12), 1514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rizzardi AE; Johnson AT; Vogel RI; Pambuccian SE; Henriksen J; Skubitz AP; Metzger GJ; Schmechel SC, Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagnostic pathology 2012, 7, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gavrielides MA; Gallas BD; Lenz P; Badano A; Hewitt SM, Observer variability in the interpretation of HER2/neu immunohistochemical expression with unaided and computer-aided digital microscopy. Arch Pathol Lab Med 2011, 135 (2), 233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sayed IM; Suarez K; Lim E; Singh S; Pereira M; Ibeawuchi SR; Katkar G; Dunkel Y; Mittal Y; Chattopadhyay R; Guma M; Boland BS; Dulai PS; Sandborn WJ; Ghosh P; Das S, Host engulfment pathway controls inflammation in inflammatory bowel disease. FEBS J 2020, 287 (18), 3967–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mosli M; Sabbahi H; Alyousef H; Abdulhaq M; Hadadi A; Aljahdali E; Jawa H; Bazarah S; Qari Y, Risk Stratification of Patients with Crohn’s Disease: A Retrospective Analysis of Clinical Decision Making and Its Impact on Long-Term Outcome. Dig Dis 2018, 36 (1), 49–55. [DOI] [PubMed] [Google Scholar]
- 56.Staradub VL; Messenger KA; Hao N; Wiley EL; Morrow M, Changes in breast cancer therapy because of pathology second opinions. Ann Surg Oncol 2002, 9 (10), 982–7. [DOI] [PubMed] [Google Scholar]
- 57.Dassopoulos T; Cohen RD; Scherl EJ; Schwartz RM; Kosinski L; Regueiro MD, Ulcerative Colitis Care Pathway. Gastroenterology 2015, 149 (1), 238–45. [DOI] [PubMed] [Google Scholar]
- 58.Truelove SC; Horler AR; Richards WC, Serial biopsy in ulcerative colitis. Br Med J 1955, 2 (4956), 1590–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitchell PJ; Rabau MY; Haboubi NY, Indeterminate colitis. Tech Coloproctol 2007, 11 (2), 91–6. [DOI] [PubMed] [Google Scholar]
- 60.Adamina M; Bonovas S; Raine T; Spinelli A; Warusavitarne J; Armuzzi A; Bachmann O; Bager P; Biancone L; Bokemeyer B; Bossuyt P; Burisch J; Collins P; Doherty G; El-Hussuna A; Ellul P; Fiorino G; Frei-Lanter C; Furfaro F; Gingert C; Gionchetti P; Gisbert JP; Gomollon F; Gonzalez Lorenzo M; Gordon H; Hlavaty T; Juillerat P; Katsanos K; Kopylov U; Krustins E; Kucharzik T; Lytras T; Maaser C; Magro F; Marshall JK; Myrelid P; Pellino G; Rosa I; Sabino J; Savarino E; Stassen L; Torres J; Uzzan M; Vavricka S; Verstockt B; Zmora O, ECCO Guidelines on Therapeutics in Crohn’s Disease: Surgical Treatment. J Crohns Colitis 2020, 14 (2), 155–168. [DOI] [PubMed] [Google Scholar]
- 61.Chang S; Hudesman D, First-Line Biologics or Small Molecules in Inflammatory Bowel Disease: a Practical Guide for the Clinician. Curr Gastroenterol Rep 2020, 22 (2), 7. [DOI] [PubMed] [Google Scholar]
- 62.Bischoff SC; Escher J; Hebuterne X; Klek S; Krznaric Z; Schneider S; Shamir R; Stardelova K; Wierdsma N; Wiskin AE; Forbes A, ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin Nutr 2020, 39 (3), 632–653. [DOI] [PubMed] [Google Scholar]
- 63.Lega S; Pin A; Arrigo S; Cifaldi C; Girardelli M; Bianco AM; Malamisura M; Angelino G; Faraci S; Rea F; Romeo EF; Aloi M; Romano C; Barabino A; Martelossi S; Tommasini A; Di Matteo G; Cancrini C; De Angelis P; Finocchi A; Bramuzzo M, Diagnostic Approach to Monogenic Inflammatory Bowel Disease in Clinical Practice: A Ten-Year Multicentric Experience. Inflammatory bowel diseases 2020, 26 (5), 720–727. [DOI] [PubMed] [Google Scholar]
- 64.M’Koma A, Wise PE, Seeley EH, Washington MK, Schwartz DA, Muldoon RL, Herline AJ, Caprioli RM, Human Alpha Defensins are Differentially Expressed Between the Inflammatory Colitides. Gastroenterology 2010, 138 (5, Suppl 1), S–525. [Google Scholar]
- 65.Seeley EH; Washington MK; Caprioli RM; M’Koma AE, Proteomic patterns of colonic mucosal tissues delineate Crohn’s colitis and ulcerative colitis. Proteomics Clin Appl 2013, 7 (7–8), 541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams AD; Korolkova OY; Sakwe AM; Geiger TM; James SD; Muldoon RL; Herline AJ; Goodwin JS; Izban MG; Washington MK; Smoot DT; Ballard BR; Gazouli M; M’Koma AE, Human alpha defensin 5 is a candidate biomarker to delineate inflammatory bowel disease. PloS one 2017, 12 (8), e0179710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams AD SA, Smoot DT, Washington MK, Ballard BR, Geiger TM, M’Koma AE, Indeterminate Colitis Precision into Crohn’s Colitis and Ulcerative Colitis Using Molecular Biometrics. In Annual Scientific Meeting, The American Society of Colon and Rectal Surgeons, www.fascrs.org, Ed. The American Society of Colon and Rectal Surgeons: Los Angeles Convention Center, 2016; p 30. [Google Scholar]
- 68.Rana T KO, Rachakonda G, Williams AD, Hawkins AT, James SD, Sakwe AM, Hui N, Wang L, Yu C, Goodwin JS, Izban MG, Offodile RS, Washington MK, Ballard BR, Smoot DT, Shi XZ, Forbes, Shanker A, M’Koma AE, Linking bacterial enterotoxins and alpha Defensin 5 expansion in the Crohn’s colitis: A new insight into the etiopathogenetic and differentiation triggers driving colonic inflammatory bowel disease. PloS one 2021, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Podolsky DK, Inflammatory bowel disease. N Engl J Med 2002, 347 (6), 417–29. [DOI] [PubMed] [Google Scholar]
- 70.Hyams JS; Davis P; Grancher K; Lerer T; Justinich CJ; Markowitz J, Clinical outcome of ulcerative colitis in children. J Pediatr 1996, 129 (1), 81–8. [DOI] [PubMed] [Google Scholar]
- 71.Lamb CA; Kennedy NA; Raine T; Hendy PA; Smith PJ; Limdi JK; Hayee B; Lomer MCE; Parkes GC; Selinger C; Barrett KJ; Davies RJ; Bennett C; Gittens S; Dunlop MG; Faiz O; Fraser A; Garrick V; Johnston PD; Parkes M; Sanderson J; Terry H; group I. B. D. g. e. c.; Gaya DR; Iqbal TH; Taylor SA; Smith M; Brookes M; Hansen R; Hawthorne AB, British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl 3), s1–s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panaccione R; Steinhart AH; Bressler B; Khanna R; Marshall JK; Targownik L; Afif W; Bitton A; Borgaonkar M; Chauhan U; Halloran B; Jones J; Kennedy E; Leontiadis GI; Loftus EV Jr.; Meddings J; Moayyedi P; Murthy S; Plamondon S; Rosenfeld G; Schwartz D; Seow CH; Williams C; Bernstein CN, Canadian Association of Gastroenterology Clinical Practice Guideline for the Management of Luminal Crohn’s Disease. J Can Assoc Gastroenterol 2019, 2 (3), e1–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mack DR; Benchimol EI; Critch J; deBruyn J; Tse F; Moayyedi P; Church P; Deslandres C; El-Matary W; Huynh H; Jantchou P; Lawrence S; Otley A; Sherlock M; Walters T; Kappelman MD; Sadowski D; Marshall JK; Griffiths A, Canadian Association of Gastroenterology Clinical Practice Guideline for the Medical Management of Pediatric Luminal Crohn’s Disease. J Can Assoc Gastroenterol 2019, 2 (3), e35–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Turner D; Ruemmele FM; Orlanski-Meyer E; Griffiths AM; de Carpi JM; Bronsky J; Veres G; Aloi M; Strisciuglio C; Braegger CP; Assa A; Romano C; Hussey S; Stanton M; Pakarinen M; de Ridder L; Katsanos K; Croft N; Navas-Lopez V; Wilson DC; Lawrence S; Russell RK, Management of Paediatric Ulcerative Colitis, Part 1: Ambulatory Care-An Evidence-based Guideline From European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2018, 67 (2), 257–291. [DOI] [PubMed] [Google Scholar]
- 75.Rubin DT; Ananthakrishnan AN; Siegel CA; Sauer BG; Long MD, ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol 2019, 114 (3), 384–413. [DOI] [PubMed] [Google Scholar]
- 76.Turner D; Ruemmele FM; Orlanski-Meyer E; Griffiths AM; de Carpi JM; Bronsky J; Veres G; Aloi M; Strisciuglio C; Braegger CP; Assa A; Romano C; Hussey S; Stanton M; Pakarinen M; de Ridder L; Katsanos KH; Croft N; Navas-Lopez VM; Wilson DC; Lawrence S; Russell RK, Management of Paediatric Ulcerative Colitis, Part 2: Acute Severe Colitis-An Evidence-based Consensus Guideline From the European Crohn’s and Colitis Organization and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2018, 67 (2), 292–310. [DOI] [PubMed] [Google Scholar]
- 77.Feuerstein JD; Isaacs KL; Schneider Y; Siddique SM; Falck-Ytter Y; Singh S; Committee AGAICG, AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020, 158 (5), 1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pellino G; Keller DS; Sampietro GM; Annese V; Carvello M; Celentano V; Coco C; Colombo F; Cracco N; Di Candido F; Franceschi M; Laureti S; Mattioli G; Pio L; Sciaudone G; Sica G; Villanacci V; Zinicola R; Leone S; Danese S; Spinelli A; Delaini G; Selvaggi F; the Italian Society of Colorectal, S., Inflammatory bowel disease (IBD) position statement of the Italian Society of Colorectal Surgery (SICCR): general principles of IBD management. Tech Coloproctol 2020, 24 (2), 105–126. [DOI] [PubMed] [Google Scholar]
- 79.Kaur M; Dalal RL; Shaffer S; Schwartz DA; Rubin DT, Inpatient Management of Inflammatory Bowel Disease-Related Complications. Clin Gastroenterol Hepatol 2020, 18 (6), 1346–1355. [DOI] [PubMed] [Google Scholar]
- 80.Amiot A; Bouguen G; Bonnaud G; Bouhnik Y; Hagege H; Peyrin-Biroulet L; French National Consensus Clinical guidelines for the management of, I. B. D. s. g., Clinical guidelines for the management of inflammatory bowel disease: Update of a French national consensus. Dig Liver Dis 2020. [Google Scholar]
- 81.Singh S; Allegretti JR; Siddique SM; Terdiman JP, AGA Technical Review on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020, 158 (5), 1465–1496 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dignass A; Van Assche G; Lindsay JO; Lemann M; Soderholm J; Colombel JF; Danese S; D’Hoore A; Gassull M; Gomollon F; Hommes DW; Michetti P; O’Morain C; Oresland T; Windsor A; Stange EF; Travis SP; European C. s.; Colitis, O., The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis 2010, 4 (1), 28–62. [DOI] [PubMed] [Google Scholar]
- 83.Dignass A; Lindsay JO; Sturm A; Windsor A; Colombel JF; Allez M; D’Haens G; D’Hoore A; Mantzaris G; Novacek G; Oresland T; Reinisch W; Sans M; Stange E; Vermeire S; Travis S; Van Assche G, Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis 2012, 6 (10), 991–1030. [DOI] [PubMed] [Google Scholar]
- 84.Bodger K, Cost effectiveness of treatments for inflammatory bowel disease. Pharmacoeconomics 2011, 29 (5), 387–401. [DOI] [PubMed] [Google Scholar]
- 85.Hanauer SB, Top-down versus step-up approaches to chronic inflammatory bowel disease: presumed innocent or presumed guilty. Nat Clin Pract Gastroenterol Hepatol 2005, 2 (11), 493. [DOI] [PubMed] [Google Scholar]
- 86.Spekhorst LM; Visschedijk MC; Alberts R; Festen EA; van der Wouden EJ; Dijkstra G; Weersma RK; Dutch Initiative on C; Colitis, Performance of the Montreal classification for inflammatory bowel diseases. World J Gastroenterol 2014, 20 (41), 15374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salaritabar A; Darvishi B; Hadjiakhoondi F; Manayi A; Sureda A; Nabavi SF; Fitzpatrick LR; Nabavi SM; Bishayee A, Therapeutic potential of flavonoids in inflammatory bowel disease: A comprehensive review. World J Gastroenterol 2017, 23 (28), 5097–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Castellanos JG; Longman RS, Innate lymphoid cells link gut microbes with mucosal T cell immunity. Gut Microbes 2020, 11 (2), 231236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ni J; Wu GD; Albenberg L; Tomov VT, Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol 2017, 14 (10), 573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stange EF; Schroeder BO, Microbiota and mucosal defense in IBD: an update. Expert Rev Gastroenterol Hepatol 2019, 13 (10), 963–976. [DOI] [PubMed] [Google Scholar]
- 91.Uhlig HH; Powrie F, Translating Immunology into Therapeutic Concepts for Inflammatory Bowel Disease. Annu Rev Immunol 2018, 36, 755–781. [DOI] [PubMed] [Google Scholar]
- 92.Jeong DY; Kim S; Son MJ; Son CY; Kim JY; Kronbichler A; Lee KH; Shin JI, Induction and maintenance treatment of inflammatory bowel disease: A comprehensive review. Autoimmun Rev 2019, 18 (5), 439–454. [DOI] [PubMed] [Google Scholar]
- 93.Ghosh S; Mitchell R, Impact of inflammatory bowel disease on quality of life: Results of the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) patient survey. J Crohns Colitis 2007, 1 (1), 10–20. [DOI] [PubMed] [Google Scholar]
- 94.Fukuda T; Naganuma M; Sugimoto S; Nanki K; Mizuno S; Mutaguchi M; Nakazato Y; Inoue N; Ogata H; Iwao Y; Kanai T, The risk factor of clinical relapse in ulcerative colitis patients with low dose 5-aminosalicylic acid as maintenance therapy: A report from the IBD registry. PloS one 2017, 12 (11), e0187737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Salas A; Hernandez-Rocha C; Duijvestein M; Faubion W; McGovern D; Vermeire S; Vetrano S; Vande Casteele N, JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2020, 17 (6), 323–337. [DOI] [PubMed] [Google Scholar]
- 96.Coskun M; Salem M; Pedersen J; Nielsen OH, Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol Res 2013, 76, 1–8. [DOI] [PubMed] [Google Scholar]
- 97.M’Koma AE MH, Adunyah SE, Inflammatory bowel disease-associated colorectal cancer: proctocolectomy andmucosectomy does not necessarily eliminate pouch related cancer incidences. Int J colorect Dis 2011, 26, 533–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Orlanski-Meyer E; Topf-Olivestone C; Ledder O; Dotan I; Folmer-Hansen L; Kindermann A; Assa A; Kolho KL; Kolacek S; Carroll MW; Strisciuglio C; Aloi M; Hansen R; Navon D; Winter HS; Navas-Lopez VM; de Ridder L; Smets F; Weiss B; Turner D, Outcomes Following Pouch Formation in Paediatric Ulcerative Colitis: A Study From the Porto Group of ESPGHAN. J Pediatr Gastroenterol Nutr 2020, 71 (3), 346–353. [DOI] [PubMed] [Google Scholar]
- 99.Bosani M; Ardizzone S; Porro GB, Biologic targeting in the treatment of inflammatory bowel diseases. Biologics : targets & therapy 2009, 3, 77–97. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.Florholmen JR; Johnsen KM; Meyer R; Olsen T; Moe OK; Tandberg P; Gundersen MD; Kvamme JM; Johnsen K; Loitegard T; Raschpichler G; Vold C; Sorbye SW; Goll R, Discovery and validation of mucosal TNF expression combined with histological score - a biomarker for personalized treatment in ulcerative colitis. BMC Gastroenterol 2020, 20 (1), 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ko CW; Singh S; Feuerstein JD; Falck-Ytter C; Falck-Ytter Y; Cross RK; American Gastroenterological Association Institute Clinical Guidelines, C., AGA Clinical Practice Guidelines on the Management of Mild-to-Moderate Ulcerative Colitis. Gastroenterology 2019, 156 (3), 748–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Feuerstein JD; Nguyen GC; Kupfer SS; Falck-Ytter Y; Singh S; American Gastroenterological Association Institute Clinical Guidelines, C., American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology 2017, 153 (3), 827–834. [DOI] [PubMed] [Google Scholar]
- 103.Danese S; Vuitton L; Peyrin-Biroulet L, Biologic agents for IBD: practical insights. Nat Rev Gastroenterol Hepatol 2015, 12 (9), 537–45. [DOI] [PubMed] [Google Scholar]
- 104.Morrow T; Felcone LH, Defining the difference: What Makes Biologics Unique. Biotechnol Healthc 2004, 1 (4), 24–9. [PMC free article] [PubMed] [Google Scholar]
- 105.Kuek A; Hazleman BL; Ostor AJ, Immunemediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgrad Med J 2007, 83 (978), 251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rossen NG; Fuentes S; van der Spek MJ; Tijssen JG; Hartman JH; Duflou A; Lowenberg M; van den Brink GR; Mathus-Vliegen EM; de Vos WM; Zoetendal EG; D’Haens GR; Ponsioen CY, Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology 2015, 149 (1), 110–118 e4. [DOI] [PubMed] [Google Scholar]
- 107.Meyer A; Rudant J; Drouin J; Weill A; Carbonnel F; Coste J, Effectiveness and Safety of Reference Infliximab and Biosimilar in Crohn Disease: A French Equivalence Study. Ann Intern Med 2019, 170 (2), 99–107. [DOI] [PubMed] [Google Scholar]
- 108.Cholapranee A; Hazlewood GS; Kaplan GG; Peyrin-Biroulet L; Ananthakrishnan AN, Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther 2017, 45 (10), 1291–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Krogsgaard LR; Lyngesen M; Bytzer P, Systematic review: quality of trials on the symptomatic effects of the low FODMAP diet for irritable bowel syndrome. Aliment Pharmacol Ther 2017, 45 (12), 1506–1513. [DOI] [PubMed] [Google Scholar]
- 110.Torres J; Bonovas S; Doherty G; Kucharzik T; Gisbert JP; Raine T; Adamina M; Armuzzi A; Bachmann O; Bager P; Biancone L; Bokemeyer B; Bossuyt P; Burisch J; Collins P; El-Hussuna A; Ellul P; Frei-Lanter C; Furfaro F; Gingert C; Gionchetti P; Gomollon F; Gonzalez-Lorenzo M; Gordon H; Hlavaty T; Juillerat P; Katsanos K; Kopylov U; Krustins E; Lytras T; Maaser C; Magro F; Marshall JK; Myrelid P; Pellino G; Rosa I; Sabino J; Savarino E; Spinelli A; Stassen L; Uzzan M; Vavricka S; Verstockt B; Warusavitarne J; Zmora O; Fiorino G, ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J Crohns Colitis 2020, 14 (1), 4–22. [DOI] [PubMed] [Google Scholar]
- 111.Lukas M; Malickova K; Kolar M; Bortlik M; Vasatko M; Machkova N; Hruba V; Duricova D; Lukas M, Switching From Originator Adalimumab to the Biosimilar SB5 in Patients With Inflammatory Bowel Disease: Short-term Experience From a Single Tertiary Clinical Centre. J Crohns Colitis 2020, 14 (7), 915–919. [DOI] [PubMed] [Google Scholar]
- 112.Rubinstein E, Letter--A Challenge to the Pharmacy Profession to Raise Its Voice on Health Care Reform. J Manag Care Spec Pharm 2017, 23 (12), 1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rubinstein E, Letter--Draft FDA Guidance “Considerations in Demonstrating Interchangeability with a Reference Product”: Overview and Presentation-Related Concerns. J Manag Care Spec Pharm 2017, 23 (3), 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alten R, [Biosimilars in rheumatology. Development and results of clinical trials]. Z Rheumatol 2015, 74 (8), 682–8. [DOI] [PubMed] [Google Scholar]
- 115.Alten R; Cronstein BN, Clinical trial development for biosimilars. Semin Arthritis Rheum 2015, 44 (6 Suppl), S2–8. [DOI] [PubMed] [Google Scholar]
- 116.Avila-Ribeiro P; Fiorino G; Danese S, The Experience with Biosimilars of Infliximab in Inflammatory Bowel Disease. Curr Pharm Des 2017, 23 (44), 6759–6769. [DOI] [PubMed] [Google Scholar]
- 117.Jacobs I; Singh E; Sewell KL; Al-Sabbagh A; Shane LG, Patient attitudes and understanding about biosimilars: an international cross-sectional survey. Patient Prefer Adherence 2016, 10, 937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Al-Sabbagh A; Olech E; McClellan JE; Kirchhoff CF, Development of biosimilars. Semin Arthritis Rheum 2016, 45 (5 Suppl), S11–8. [DOI] [PubMed] [Google Scholar]
- 119.Lagasse HA; Alexaki A; Simhadri VL; Katagiri NH; Jankowski W; Sauna ZE; Kimchi-Sarfaty C, Recent advances in (therapeutic protein) drug development. F1000Res 2017, 6, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McCamish M; Woollett G, The rise of the biosimilar. Expert Rev Clin Pharmacol 2012, 5 (6), 597–9. [DOI] [PubMed] [Google Scholar]
- 121.Zheng MK; Shih DQ; Chen GC, Insights on the use of biosimilars in the treatment of inflammatory bowel disease. World J Gastroenterol 2017, 23 (11), 1932–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hyams JS; Griffiths A; Markowitz J; Baldassano RN; Faubion WA Jr.; Colletti RB; Dubinsky M; Kierkus J; Rosh J; Wang Y; Huang B; Bittle B; Marshall M; Lazar A, Safety and efficacy of adalimumab for moderate to severe Crohn’s disease in children. Gastroenterology 2012, 143 (2), 365–74 e2. [DOI] [PubMed] [Google Scholar]
- 123.Ruemmele FM; Lachaux A; Cezard JP; Morali A; Maurage C; Ginies JL; Viola S; Goulet O; Lamireau T; Scaillon M; Breton A; Sarles J; Groupe Francophone d’Hepatologie G. e. N. P., Efficacy of infliximab in pediatric Crohn’s disease: a randomized multicenter open-label trial comparing scheduled to on demand maintenance therapy. Inflammatory bowel diseases 2009, 15 (3), 388–94. [DOI] [PubMed] [Google Scholar]
- 124.Hyams J; Damaraju L; Blank M; Johanns J; Guzzo C; Winter HS; Kugathasan S; Cohen S; Markowitz J; Escher JC; Veereman-Wauters G; Crandall W; Baldassano R; Griffiths A; Group TS, Induction and maintenance therapy with infliximab for children with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol 2012, 10 (4), 391–9 e1. [DOI] [PubMed] [Google Scholar]
- 125.Hyams J; Crandall W; Kugathasan S; Griffiths A; Olson A; Johanns J; Liu G; Travers S; Heuschkel R; Markowitz J; Cohen S; Winter H; Veereman-Wauters G; Ferry G; Baldassano R; Group RS, Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology 2007, 132 (3), 863–73; quiz 1165–6. [DOI] [PubMed] [Google Scholar]
- 126.Jahnsen J; Detlie TE; Vatn S; Ricanek P, Biosimilar infliximab (CT-P13) in the treatment of inflammatory bowel disease: A Norwegian observational study. Expert Rev Gastroenterol Hepatol 2015, 9 Suppl 1, 45–52. [DOI] [PubMed] [Google Scholar]
- 127.Bohm M; Kario K; Kandzari DE; Mahfoud F; Weber MA; Schmieder RE; Tsioufis K; Pocock S; Konstantinidis D; Choi JW; East C; Lee DP; Ma A; Ewen S; Cohen DL; Wilensky R; Devireddy CM; Lea J; Schmid A; Weil J; Agdirlioglu T; Reedus D; Jefferson BK; Reyes D; D’Souza R; Sharp ASP; Sharif F; Fahy M; DeBruin V; Cohen SA; Brar S; Townsend RR; Investigators SH-OMP, Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet 2020, 395 (10234), 1444–1451. [DOI] [PubMed] [Google Scholar]
- 128.Gecse KB; Lovasz BD; Farkas K; Banai J; Bene L; Gasztonyi B; Golovics PA; Kristof T; Lakatos L; Csontos AA; Juhasz M; Nagy F; Palatka K; Papp M; Patai A; Lakner L; Salamon A; Szamosi T; Szepes Z; Toth GT; Vincze A; Szalay B; Molnar T; Lakatos PL, Efficacy and Safety of the Biosimilar Infliximab CT-P13 Treatment in Inflammatory Bowel Diseases: A Prospective, Multicentre, Nationwide Cohort. J Crohns Colitis 2016, 10 (2), 133–40. [DOI] [PubMed] [Google Scholar]
- 129.Sieczkowska J; Jarzebicka D; Banaszkiewicz A; Plocek A; Gawronska A; Toporowska-Kowalska E; Oracz G; Meglicka M; Kierkus J, Switching Between Infliximab Originator and Biosimilar in Paediatric Patients with Inflammatory Bowel Disease. Preliminary Observations. J Crohns Colitis 2016, 10 (2), 127–32. [DOI] [PubMed] [Google Scholar]
- 130.Smits LJ; Derikx LA; de Jong DJ; Boshuizen RS; van Esch AA; Drenth JP; Hoentjen F, Clinical Outcomes Following a Switch from Remicade(R) to the Biosimilar CT-P13 in Inflammatory Bowel Disease Patients: A Prospective Observational Cohort Study. J Crohns Colitis 2016, 10 (11), 1287–1293. [DOI] [PubMed] [Google Scholar]
- 131.M’Koma AE; Lindquist K; Liljeqvist L, Biochemical laboratory data in patients before and after restorative proctocolectomy. A study on 83 patients with a follow-up of 36 months. Ann Chir 1994, 48 (6), 525–34. [PubMed] [Google Scholar]
- 132.Kaitha S; Bashir M; Ali T, Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol 2015, 6 (3), 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.M’Koma AE; Wise PE; Schwartz DA; Muldoon RL; Herline AJ, Prevalence and outcome of anemia after restorative proctocolectomy: a clinical literature review. Dis Colon Rectum 2009, 52 (4), 726–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.M’Koma AE, Follow-up results of hematology data before and after restorative proctocolectomy. Clinical outcome. Dis Colon Rectum 1994, 37 (9), 932–7. [DOI] [PubMed] [Google Scholar]
- 135.M’Koma AE, Observation on essential biochemical data profile in connection with restorative proctocolectomy in humans. Vitamin B12 and fat absorption cited. Dissertation Thesis 2001, 1–126. [Google Scholar]
- 136.Marion-Letellier R; Savoye G; Ghosh S, IBD: In Food We Trust. J Crohns Colitis 2016, 10 (11), 1351–1361. [DOI] [PubMed] [Google Scholar]
- 137.Cabre E; Domenech E, Impact of environmental and dietary factors on the course of inflammatory bowel disease. World J Gastroenterol 2012, 18 (29), 3814–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cabre E; Manosa M; Gassull MA, Omega-3 fatty acids and inflammatory bowel diseases - a systematic review. Br J Nutr 2012, 107 Suppl 2, S240–52. [DOI] [PubMed] [Google Scholar]
- 139.Rizzello F; Spisni E; Giovanardi E; Imbesi V; Salice M; Alvisi P; Valerii MC; Gionchetti P, Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients 2019, 11 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reddavide R; Rotolo O; Caruso MG; Stasi E; Notarnicola M; Miraglia C; Nouvenne A; Meschi T; De’ Angelis GL; Di Mario F; Leandro G, The role of diet in the prevention and treatment of Inflammatory Bowel Diseases. Acta Biomed 2018, 89 (9-S), 60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Solomon SM, Genome editing in animals: why FDA regulation matters. Nat Biotechnol 2020, 38 (2), 142–143. [DOI] [PubMed] [Google Scholar]
- 142.Hartman C; Eliakim R; Shamir R, Nutritional status and nutritional therapy in inflammatory bowel diseases. World J Gastroenterol 2009, 15 (21), 2570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yu Y; Chen KC; Chen J, Exclusive enteral nutrition versus corticosteroids for treatment of pediatric Crohn’s disease: a meta-analysis. World J Pediatr 2019, 15 (1), 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Herrador-Lopez M; Martin-Masot R; Navas-Lopez VM, EEN Yesterday and Today … CDED Today and Tomorrow. Nutrients 2020, 12 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Owczarek D; Rodacki T; Domagala-Rodacka R; Cibor D; Mach T, Diet and nutritional factors in inflammatory bowel diseases. World J Gastroenterol 2016, 22 (3), 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aleksandrova K; Romero-Mosquera B; Hernandez V, Diet, Gut Microbiome and Epigenetics: Emerging Links with Inflammatory Bowel Diseases and Prospects for Management and Prevention. Nutrients 2017, 9 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lewis RT; Maron DJ, Anorectal Crohn’s disease. Surg Clin North Am 2010, 90 (1), 83–97, Table of Contents. [DOI] [PubMed] [Google Scholar]
- 148.Petryszyn PW; Witczak I, Costs in inflammatory bowel diseases. Prz Gastroenterol 2016, 11 (1), 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bernstein CN; Papineau N; Zajaczkowski J; Rawsthorne P; Okrusko G; Blanchard JF, Direct hospital costs for patients with inflammatory bowel disease in a Canadian tertiary care university hospital. Am J Gastroenterol 2000, 95 (3), 677–83. [DOI] [PubMed] [Google Scholar]
- 150.Kuenzig ME; Benchimol EI; Lee L; Targownik LE; Singh H; Kaplan GG; Bernstein CN; Bitton A; Nguyen GC; Lee K; Cooke-Lauder J; Murthy SK, The Impact of Inflammatory Bowel Disease in Canada 2018: Direct Costs and Health Services Utilization. J Can Assoc Gastroenterol 2019, 2 (Suppl 1), S17–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vadstrup K; Alulis S; Borsi A; Elkjaer Stallknecht S; Nielsen A; Rikke Jorgensen T; Wennerstrom C; Qvist N; Munkholm P, Societal costs attributable to Crohn’s disease and ulcerative colitis within the first 5 years after diagnosis: a Danish nationwide cost-of-illness study 2002–2016. Scand J Gastroenterol 2020, 55 (1), 41–46. [DOI] [PubMed] [Google Scholar]
- 152.Pillai N; Dusheiko M; Burnand B; Pittet V, A systematic review of cost-effectiveness studies comparing conventional, biological and surgical interventions for inflammatory bowel disease. PloS one 2017, 12 (10), e0185500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Iacucci M; Cannatelli R; Labarile N; Mao R; Panaccione R; Danese S; Kochhar GS; Ghosh S; Shen B, Endoscopy in inflammatory bowel diseases during the COVID-19 pandemic and post-pandemic period. Lancet Gastroenterol Hepatol 2020, 5 (6), 598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lichtenstein GR; Rubin DT, Coronavirus and Patients With Inflammatory Bowel Disease: Management Strategies for the Practicing Clinician. Am J Gastroenterol 2020, 115 (10), 1566–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gajendran M; Perisetti A; Aziz M; Raghavapuram S; Bansal P; Tharian B; Goyal H, Inflammatory bowel disease amid the COVID-19 pandemic: impact, management strategies, and lessons learned. Ann Gastroenterol 2020, 33 (6), 591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nardone OM; Rispo A; Testa A; Imperatore N; Pellegrini L; Guarino AD; Ricciolino S; Patturelli M; De Palma G; Castiglione F, The impact of a dedicated contact centre on the clinical outcome of patients with inflammatory bowel disease during the COVID-19 outbreak. Therap Adv Gastroenterol 2020, 13, 1756284820959586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Taxonera C; Alba C; Olivares D; Martin M; Ventero A; Canas M, Innovation in IBD Care During the COVID-19 Pandemic: Results of a Cross-Sectional Survey on Patient-Reported Experience Measures. Inflammatory bowel diseases 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nardone OM; Rispo A; Castiglione F, Noninvasive monitoring of inflammatory bowel disease in the post COVID-19 era. Dig Liver Dis 2020, 52 (11), 1236–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Aysha AA; Rentsch C; Prentice R; Johnson D; Bryant RV; Ward MG; Costello SP; Lewindon P; Ghaly S; Connor SJ; Begun J; Christensen B, Practical management of inflammatory bowel disease patients during the COVID-19 pandemic: expert commentary from the Gastroenterological Society of Australia Inflammatory Bowel Disease faculty. Intern Med J 2020, 50 (7), 798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sultan S; Lim JK; Altayar O; Davitkov P; Feuerstein JD; Siddique SM; Falck-Ytter Y; El-Serag HB; ewilson@gastro.org, A. G. A. I. E. a., AGA Rapid Recommendations for Gastrointestinal Procedures During the COVID-19 Pandemic. Gastroenterology 2020, 159 (2), 739–758 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rubin DT; Abreu MT; Rai V; Siegel CA; International Organization for the Study of Inflammatory Bowel, D., Management of Patients With Crohn’s Disease and Ulcerative Colitis During the Coronavirus Disease-2019 Pandemic: Results of an International Meeting. Gastroenterology 2020, 159 (1), 6–13 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Magro F; Rahier JF; Abreu C; MacMahon E; Hart A; van der Woude CJ; Gordon H; Adamina M; Viget N; Vavricka S; Kucharzik T; Leone S; Siegmund B; Danese S; Peyrin-Biroulet L, Inflammatory Bowel Disease Management During the COVID-19 Outbreak: The Ten Do’s and Don’ts from the ECCO-COVID Taskforce. J Crohns Colitis 2020, 14 (14 Suppl 3), S798–S806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Santiago P; Braga-Neto MB; Edward V. Loftus EV, Novel Therapies for Patients With Inflammatory Bowel Disease. Gastroenterology & Hepatology Volume 18, Issue 8 August 2022. [PMC free article] [PubMed] [Google Scholar]
- 164.Mamula P, Markowitz JE, Baldassano RN. Inflammatory bowel disease in early childhood and adolescence: special considerations. Gastroenterol Clin North Am 2003. Sep;32(3):967–95. [DOI] [PubMed] [Google Scholar]
- 165.Fish D, Kugathasan S. Inflammatory bowel disease. Adolesc Med Clin. 2004. Feb; 15(1):67–90 [DOI] [PubMed] [Google Scholar]
- 166.Baldassano RN, Piccoli DA. Inflammatory bowel disease in pediatric and adolescent patients. Gastroenterol Clin North Am 1999. Jun;28(2):445–58. [DOI] [PubMed] [Google Scholar]
- 167.Andres PG, Friedman LS. Epidemiology and the natural course of inflammatory bowel disease. Gastroenterol Clin North Am 1999. Jun;28(2):255–81. [DOI] [PubMed] [Google Scholar]
- 168.Raj V, Lichtenstein DR. Hepatobiliary manifestations of inflammatory bowel disease. Gastroenterol Clin North Am 1999. Jun;28(2):491–513. [DOI] [PubMed] [Google Scholar]
- 169.M’Koma AE. Serum biochemical evaluation of patients with functional pouches ten to 20 years after restorative proctocolectomy. Int J Colorectal Dis 2006. Oct;21(7):711–20. [DOI] [PubMed] [Google Scholar]
- 170.M’Koma AE, Lindquist K, Liljeqvist L. Observations in the blood lipid profile in patients undergoing restorative proctocolectomy. Int J Surg Investig 2000;2(3):227–35 [PubMed] [Google Scholar]
- 171.M’Koma AE, Lindquist K, Liljeqvist L. A study of plasma immunoglobulins profile in connection with restorative proctocolectomy. Int J Surg Investig 2000;2(2):125–31. [PubMed] [Google Scholar]
- 172.Hawkins AT, Um JW, M’Koma AE.Adaptive Returns of Deficient Systemic Plasma Immunoglobulin G Levels as Rehabilitation Biomarker After Emergency Colectomy for Fulminant Ulcerative Colitis. Clin Med Insights Gastroenterol 2017 Dec 13;10:1179552217746692. [DOI] [PMC free article] [PubMed] [Google Scholar]