Abstract
Background
In low- and middle-income countries (L&MICs), the biggest contributing factors to the global burden of disease in childhood are deaths due to respiratory illness and diarrhoea, both of which are closely related to use of water, sanitation, and hygiene (WASH) services by households. However, current estimates of the health impacts of WASH interventions use self-reported morbidity, which may fail to capture longer-term or more severe impacts. Reported mortality is thought to be less prone to bias than other reported measures. This study aimed to answer the question: What are the impacts of WASH interventions on reported childhood mortality in L&MICs?
Methods and findings
We conducted a systematic review and meta-analysis, using a published protocol. Systematic searches of 11 academic databases and trial registries, plus organisational repositories, were undertaken to locate studies of WASH interventions, which were published in peer review journals or other sources (e.g., organisational reports and working papers). Intervention studies of WASH improvements implemented under endemic disease circumstances in L&MICs were eligible, which reported findings at any time until March 2020. We used the participant flow data supplied in response to journal editors’ calls for greater transparency. Data were collected by two authors working independently.
We included evidence from 24 randomized and 11 nonrandomized studies of WASH interventions from all global regions, incorporating 2,600 deaths. Effects of 48 WASH treatment arms were included in analysis. We critically appraised and synthesised evidence using meta-analysis to improve statistical power. We found WASH interventions are associated with a significant reduction of 17% in the odds of all-cause mortality in childhood (OR = 0.83, 95% CI = 0.74, 0.92, evidence from 38 interventions), and a significant reduction in diarrhoea mortality of 45% (OR = 0.55, 95% CI = 0.35, 0.84; 10 interventions).
Further analysis by WASH technology indicated interventions providing improved water in quantity to households were most consistently associated with reductions in all-cause mortality. Community-wide sanitation was most consistently associated with reductions in diarrhoea mortality. Around one-half of the included studies were assessed as being at “moderate risk of bias” in attributing mortality in childhood to the WASH intervention, and no studies were found to be at “low risk of bias.” The review should be updated to incorporate additional published and unpublished participant flow data.
Conclusions
The findings are congruent with theories of infectious disease transmission. Washing with water presents a barrier to respiratory illness and diarrhoea, which are the two biggest contributors to all-cause mortality in childhood in L&MICs. Community-wide sanitation halts the spread of diarrhoea. We observed that evidence synthesis can provide new findings, going beyond the underlying data from trials to generate crucial insights for policy. Transparent reporting in trials creates opportunities for research synthesis to answer questions about mortality, which individual studies of interventions cannot be reliably designed to address.
Hugh Sharma Waddington and colleagues investigate the impacts of water, sanitation and hygiene interventions on all-cause and diarrhoea-related mortality in low- and middle-income countries.
Author summary
Why was this study done?
The biggest contributor to the global burden of infectious disease in childhood in developing countries is mortality due to respiratory and diarrhoeal infections, both of which are closely linked to deficient water, sanitation, and hygiene (WASH) availability and use by households.
Multiple systematic reviews and meta-analyses of WASH-related morbidity have been conducted, but there is a shortage of rigorous, systematic evidence on the effectiveness of WASH interventions in reducing mortality.
What did the researchers do and find?
We conducted a systematic review and meta-analysis of the impacts of WASH interventions on all-cause and diarrhoea-related mortality in L&MICs, incorporating evidence from 35 studies comprising 48 distinct WASH intervention arms.
We found significant effects on all-cause mortality among children aged under 5 of interventions to improve the quantity of water available (34% reduction), hygiene promotion when water supplies were accessible to households (29% reduction), and community-wide sanitation (21% reduction).
We also found significant effects of WASH interventions on diarrhoea mortality among under 5s (45% reduction), which were significantly larger when provided to communities that were at the lowest rungs of the sanitation ladder, compared to those that already had improved WASH.
What do these findings mean?
Interventions to prevent water-related mortality in childhood in endemic disease circumstances provide adequate water supplies to households, enabling domestic hygiene and safe excreta disposal in the household and community.
Systematic reviews can provide new evidence for decision making, but the approach we present is reliant on trial authors and journals adhering to agreed standards of reporting.
Introduction
Diarrhoeal diseases and respiratory infections are thought to kill 4.1 million people each year [1,2]. Half of these deaths are of infants and young children aged less than 5 years old [3], around 1.2 million of whom live in circumstances without adequate drinking water, sanitation, and hygiene (WASH) in low- and middle-income countries (L&MICs) [4]. The global burden of disease (GBD) for communicable causes is weighted heavily by mortality in childhood, the two biggest single causes of which are diarrhoea and respiratory infection. Approximately 90% of the total diarrhoea GBD and 99% of the total respiratory infection GBD are due to years of life lost (YLL) (Table A in S1 Annex).
Unfortunately, studies of the effects of WASH interventions on diarrhoea and other causes of mortality are beset by such ethical and logistical difficulties that, with few exceptions (e.g., [5]), practically none were carried out until recently (e.g., [6–8]). For example, it could not be ethical to design a prospective study to measure mortality as a primary outcome when lifesaving oral rehydration solution (ORS) is widely available and affordable. As a result, and in accordance with the recommendation of the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) procedure [9], the focus shifted from mortality to morbidity—mainly from diarrhoea—as a more accessible outcome.
GBD estimates of WASH-related mortality are presently calculated using estimated coefficients on diarrhoea morbidity impacts from systematic reviews and meta-analyses. Estimates vary widely (Table B in S1 Annex), suggesting great imprecision affecting our measurement of the gravity of the diarrhoea problem, globally or in any specific context. Of the 44 systematic reviews included in a recent WASH sector-wide interventions evidence map [10], half of which concerned effects of WASH provision on diarrhoea, none had synthesised the evidence on mortality in childhood. The most recent systematic evidence on WASH interventions and diarrhoeal illness was reported in The Lancet in July 2022 [11].
A common finding in existing reviews is that bundling WASH together does not produce additive effects in comparison with single water, sanitation, or hygiene technologies [12]. One possible reason for this finding is bias in reporting. For example, the most common method of collecting health outcomes data in impact evaluations of WASH interventions is through participant report [10]. However, data on reported illness have been shown to be biased in open (also called “unblinded”) trials [13–16]. Perhaps carers might misrepresent illness to minimise the time spent with enumerators when data are collected repeatedly over time [17,18]. Social desirability bias may also arise where participants are inadvertently induced to report favourably. Briscoe and colleagues [19] highlighted how diarrhoeal illness becomes normalised among highly exposed groups over time, which leads to underreporting, a problem we might expect to become worse when reporting is done by someone other than the patient, in this case the child’s carer. Or illness may be acknowledged differently by sex [20], if girls who complain about pain are less likely than boys to be pacified by their carers and, therefore, report less illness. In other words, we may not see additive effects of multiple WASH technologies provided together if bias in the reporting of disease outcomes, rather than diarrhoea epidemiology, is driving the findings.
The key advantage of randomised controlled trials (RCTs) over other methods is the clarity with which randomisation balances unobservable differences across groups in expectation, not in any single trial, but over multiple draws from the population [21]. Thus, the “gold standard” for evidence on health impacts from these studies uses meta-analysis of findings from multiple studies [22]. However, meta-analysis can also magnify biases, because it is harder to identify errors where they pervade the whole data set. Some approach is clearly needed to address reporting bias. Of great potential concern is publication bias, the phenomenon whereby studies are more likely to be published if they find significant effects, a factor that is made more likely when they are funded by private manufacturers, as has been common in trials of water treatment (chlorine, water filters) and hygiene (soap) [23].
In this paper, we present a different approach to estimate the health effects of WASH interventions. There is a large number of trials of WASH interventions, sufficient numbers on which to estimate global effects on mortality, even when the individual studies themselves did not aim to do so. We conducted a systematic review of the effects of WASH interventions on child mortality in L&MIC contexts, drawing on a number of sources including losses to follow-up due to mortality as reported in participant flows. It is an established finding that study participants do not misreport death, even in open studies [15,16]. This might be because death of a child is a rare and salient event. The crucial advantage of this approach, therefore, is that reported mortality is less prone to bias.
We sought to answer four review questions:
What are the effects of interventions promoting improved water supply, water treatment and storage, sanitation, and hygiene in L&MICs on all-cause mortality, and to what extent do these effects vary by contextual factors?
What are the effects of WASH interventions in L&MICs on diarrhoea mortality, and to what extent do they vary by contextual factors?
What are the predicted effects of WASH interventions at different baseline mortality levels?
To what extent are the findings robust to potential biases at the individual study and review levels?
Methods
Search and selection of studies
This review was registered with Prospero under registration number CRD42020210694 and is reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline (S1 PRISMA Checklist). A full description of the procedures followed for searches, study inclusion, outcomes data collection, analysis, and reporting is presented in the published protocol [24]. Searches for literature were done as part of an evidence and gap map [10]. Studies selected were published at any time until March 2020. Eleven academic databases and trial registries (e.g., Cochrane, Econlit, Medline, OpenTrials, Scholar, Web of Science) and sources of nonpeer-reviewed literature including databases and organisational repositories were searched (e.g., 3ie Repositories, J-PAL, IRC International Water and Sanitation Center, UNICEF, the World Bank, and the regional development banks). We used reference snowballing, including bibliographic backreferencing and forward citation tracking of studies and existing reviews. As a measure to reduce publication bias, studies published in any format were eligible, and searches done of repositories of this information. As a measure to avoid language bias, studies published in English, French, Spanish, and Portuguese were included, and searches done of repositories of this information. A priority search algorithm based on machine learning was used in filtering studies at title and abstract stage using EPPI-reviewer software [25]. Selection of studies was done by two authors working independently.
Eligible studies were RCTs and nonrandomised studies of interventions (NRSI) promoting access to or use of WASH technologies to households in L&MICs in endemic disease circumstances. We included new or improved water supplies, drinking water treatment and storage, sanitation, and hygiene technologies, including those enabling or promoting handwashing at key times and other beneficial household practices (e.g., the washing of food, clothing, and fomites). We excluded trial arms with a major non-WASH component (e.g., nutrition interventions). We classified WASH interventions according to the “main WASH” technology provided, which was either water supply, water treatment and storage, sanitation, or hygiene technologies provided or promoted alone, or multiple combinations of WASH technologies. It was also possible to characterise interventions by whether they provided any improvements in water supply, water treatment, sanitation, and/or hygiene alone or in combination with others, which we refer to as “any WASH.” This was due to problems in clearly identifying all the components of an intervention. For example, a debate among practitioners suggested that hand hygiene messaging is usually incorporated in community-led total sanitation (CLTS) [26].
Counterfactual conditions were categorised as “improved” or “unimproved” according to the WHO/UNICEF Joint Monitoring Programme (JMP) classification. Improved water supplies were defined where the majority of households in the control sample used drinking water from an improved source (e.g., piped water to the household, a community standpipe, or protected spring) within a 30-minute round trip including waiting time. For sanitation, the counterfactual scenario was defined as “improved” if the majority of controls had a sewer connection to the home or an improved pit latrine was used by a single household. Where insufficient information was reported about the counterfactual scenario to categorise baseline water supply or sanitation use, the figures were imputed from online data provided by the JMP for the relevant country, year, and location.
A risk-of-bias tool was developed for WASH impact evaluations that drew on Cochrane’s tools for RCTs [27], cluster RCTs [28], and nonrandomised studies of interventions [29], and a tool for appraising quasi-experiments [30]. Six bias domains were assessed: confounding, selection bias, departures from intended interventions, missing data, outcome measurement bias, and reporting bias. The studies were assessed on the likelihood of bias in estimating effects of WASH access on mortality in children aged 5 years or under. This may or may not have been a primary research question in the papers themselves; hence, our ratings do not provide risk-of-bias assessments for the study overall. The risk-of-bias assessments were done by two researchers working independently, at the outcome level for each included study arm, as recommended by Cochrane [22] and the Campbell Collaboration [31]. Template data collection forms are available in the study protocol [24]. Data extracted from included studies are provided in Table C in S1 Annex. The dataset used in analysis is provided in S1 Dataset.
Measuring mortality outcomes
The primary outcomes for the review were all-cause mortality and mortality due to diarrhoeal illness. Outcomes data were collected independently by two researchers from two sources. The first source was the few studies that reported mortality alongside statistical information [6–8,32,33]. Mortality data were also recoverable from studies that reported losses to follow-up (attrition) in sample populations. Participant flow diagrams were reviewed in all studies of WASH technologies in L&MICs to obtain crude mortality rates for field trials by intervention group. These studies, therefore, formed the major source of evidence on all-cause mortality. Some studies also reported cause-specific mortality rates, including diarrhoea and other infections, defined by carers in verbal autopsy and/or clinicians, or collected from vital registries.
Mortality rates were computed over a standard period, as mortality measurements increase over longer exposure periods. Age-specific (e.g., under 2) mortality rates were defined where these data were available [6–8,34], or, if they were not, crude mortality rates were taken over the data collection period. Intervention effects were measured as the odds ratio (OR) of the mortality rates, and their 95% confidence intervals. Where studies reported multiple intervention arms against a single control arm, we split the control sample assuming an equal mortality rate for each comparison. We applied a continuity correction in study arms where there were no deaths, by adding 0.5 to all frequencies, which can cause bias in meta-analysis of rare events [35]. These studies were assessed as being at “high risk of bias” in the outcome measurement domain [36–40].
Evidence synthesis approach
Overall pooled effects were estimated for all-cause mortality (review question 1) and diarrhoea mortality (review question 2) using Stata. We assessed the consistency of the pooled effects using I-squared and tau-squared statistics to measure the relative and absolute heterogeneity between studies. We tested for effect moderators in meta-analysis and meta-regression analysis, including the WASH intervention technology provided to study participants, water supply and sanitation conditions in the counterfactual group, participant characteristics (age and if from immunocompromised group), and study characteristics (season of data collection and length of follow-up). We report forest plots showing country and WASH technologies for each analysis (we also report the same forest plots by study author in Figs A-G in S1 Annex). To aid interpretation of the meta-regression coefficients, we calculated OR prediction values at the means, minima and maxima of the dichotomous variables, and the mean and interquartile range of the continuous variable. Moderator variables were prespecified based on theory and previous reviews, with the exceptions of the moderator analysis by baseline mortality rate and the negative control. We used meta-regression plots to assess the predicted effects of the interventions by baseline mortality rate (review question 3).
We evaluated the likelihood that potential biases could cast doubt on the results (review question 4). The effects of WASH improvements on mortality are largely expected to occur by blocking transmission of infectious diseases, primarily faeco-oral and respiratory infections, in childhood. People who survive beyond the age of 5 are thought to have developed sufficiently robust immunity to these diseases; hence, the effects of WASH improvements on mortality among older groups is expected to be far weaker. Therefore, as a negative control [41,42], meta-analysis was estimated for those studies that reported all-cause mortality among a “placebo population” of participants aged over 5 years. We also assessed the sensitivity of the pooled effects to exclusion of each single effect, examined whether there was a correlation between risk-of-bias rating and the estimated effect, and tested for small-study effects (publication bias) at the review level using graphical inspection of funnel plots and regression tests.
Results
Description of searches and included studies
From 13,500 deduplicated records, 684 full-text reports of WASH intervention studies were screened, of which 35 were identified that reported mortality outcomes, 30 of which were measured among children aged 5 or under (Fig 1). We were not able to incorporate trials that met the review inclusion criteria but did not report participant flows (e.g., [43]). We found 24 RCTs that measured mortality, all of which were published in peer review journals. RCTs were of water treatment and storage, sanitation, and/or hygiene interventions, which mainly used cluster design, with clustering at the community level. We found no RCTs of water supply provision or promotion that reported mortality estimates. Several studies used prospective nonrandomised trial designs [33,36,44], five analysed cohort data [38,45–48], one used a matched pipeline approach [49], and two used repeated cross-section data with double-differences [50,51]. Six of the studies were designed retrospectively after the WASH intervention had been conducted [47–51]. The effect of the WASH intervention was not calculable for one nonrandomised study [51]. All RCTs were reported in English. Of the nine included nonrandomised studies of interventions, which were published in peer review journals and reports, three were in French [33], Spanish [49], or Portuguese [44]. The studies were published from 1985 onwards, the majority in the 2010s. The evidence is representative of all lower-income global regions and many relevant contexts, including rural, urban, and peri-urban informal settlements.
Fig 1. PRISMA study search flow.
We included in meta-analysis 38 WASH study arms examining all-cause mortality in childhood, of which 26 were from RCTs, and 10 examining diarrhoea mortality, of which 6 were from RCTs. For six studies, we could also extract seven estimates of effects on mortality among adults and/or children aged over 5 [32,39,47,52–54]. In five studies comprising seven study arms, mortality was only reported for all age groups combined [39,40,55–57]. There was a total of 168,500 participants in the included studies of all-cause mortality and 2,600 deaths. When including the natural experiment by Galiani and colleagues [50], we estimated there were 165,000 more child deaths.
We grouped the interventions by WASH technology provided. Many concerned direct hardware provision—water supplies, filters, handwashing stations, and/or latrines—and health messaging. Thus, the WASH technology provided in six studies was household water treatment by chlorine alone [6,8,37,40,54,57] or alongside safe storage devices [56,58]. Three studies evaluated filter provision with safe storage [59–61], and two evaluated UV irradiation (solar disinfection (SODIS)) [62,63]. A further 11 studies incorporated arms evaluating hygiene promotion alone [6,8,34,36,39,40,45,58,64–66]. Others included arms combining household water treatment with handwashing promotion [38,40,65] or alongside handwashing and sanitation [6–8]. A water supply improvement was provided alone in three nonrandomised studies [46,48,67], another concerned improved water supply and sewage connections [50], three were of water supplies and latrines [44,47,49], and one other was of water supply, latrines, and handwashing promotion [33]. Three study arms were evaluated of latrine provision or promotion alone [8,8,52], but ten studies evaluated sanitation alongside other WASH technology improvements [6–8,32,33,44,47,49,50,53]. For example, the Total Sanitation Campaign in India provided hygiene education alongside CLTS, subsidies, and sanitation marketing [53]. There were also concerns about reliability of or distance to the water supply in a few studies [8,65], which may have affected ability of study participants to practice improved hygiene.
Counterfactual groups often received standard WASH access although, occasionally, they received another intervention; for example, all participants received hygiene education in one study [56]. Most counterfactual samples were assessed as using improved water supplies [7,34,36–38,40,44,45,48–50,53,57,58,63,64,66]. In a few instances, counterfactuals received piped water inside the compound [36,50], otherwise it was sourced by household members from outside. In one study of continuous water supply provision (“safely managed drinking water”), counterfactuals received water for only a few hours a week on average [48]. In under half of cases, sanitation was classified as being improved [6,36,38,40,44,48–50,56,63–65]. In all others, the majority of households openly defaecated, or used shared facilities or unimproved facilities like pits without concrete slabs. Imputations were made where it was not clear exactly what types of water and sanitation services were used by households in the counterfactual scenario [33,34,37,38,44–46,49,50,52,55,58,61].
Assessment of biases at the study level
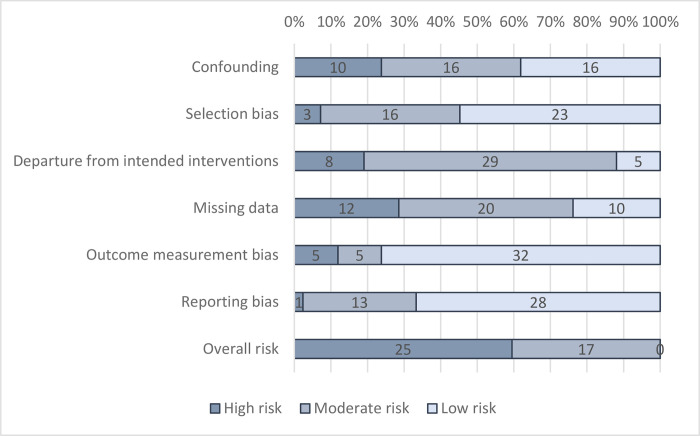
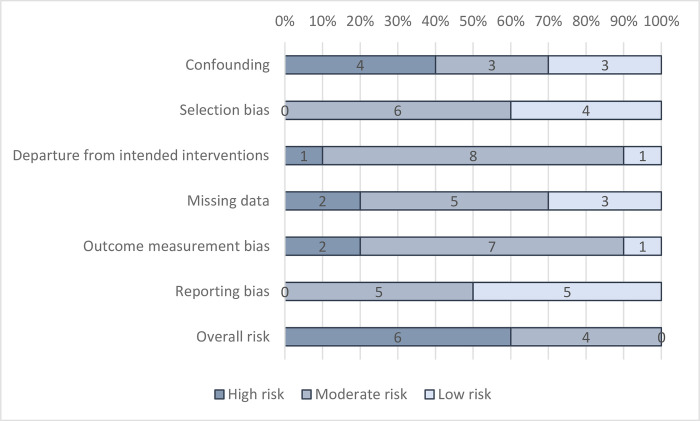
In general, just under half of studies (40%) were found to be at “moderate risk of bias” overall in attributing changes to the intervention, for all-cause mortality (Fig 2) and mortality due to diarrhoea (Fig 3). No studies were at “low risk of bias.”
Fig 2. Risk-of-bias assessment: All-cause mortality.
Fig 3. Risk-of-bias assessment: Diarrhoea mortality.
One-third of RCTs reported using adequate allocation sequence and concealment and demonstrated baseline covariate balance, to satisfy a “low risk” rating on confounding. In some cases, data were collected on WASH at pretest, but balance was not presented for all relevant variables, such as sanitation and hygiene access. Three NRSI were assessed as being at “moderate risk of bias” in confounding. These were all studies of water supply improvements including privatised water provision in Argentinean municipalities [50], improved water supply reliability in India [48], and piped water supply and latrines in India [47]. In all cases, participation was largely determined by programme placement, which is thought less problematic to address than self-selection into programmes by participants. In Argentina, it was the local government’s decision to implement a central government policy allowing for privatisation of the water supply [50]. For piped water in India, all households in a community were simultaneously connected to the water supply by the NGO Gram Vikas [47]. For the study examining the reliability of water supplies in India, all households were connected to the municipal supply [58]. Participation was then carefully modelled using a rich set of covariates measured at baseline and based on factors thought to influence programme targeting. Each study also presented null findings for a negative control (placebo outcome): mortality due to noninfectious causes [50] and the incidence of bruising and scrapes [47,48].
Where participants were recruited before allocation in cluster-RCTs, or where recruiters were blinded to allocation, the studies were judged to be at “low risk” of selection bias. Where recruitment was done afterwards by those potentially with knowledge of allocation or where individuals needed to be recruited later due to attrition (losses to follow-up during the trial), the study was judged to be at risk of bias. Studies were also assessed as being at “high risk of bias” when overall attrition rates were greater than 20%, or differential attrition greater than 10 percentage points, or where no information was provided about reasons for dropouts by intervention group, tests for covariate balance, or robustness of findings. Selection bias and attrition bias were deemed less problematic where studies used census data [50] or vital registration [44].
In general, departures from intended interventions due to contamination (controls receive the treatment) or spillover effects (control outcomes are caused by treatment outcomes) were judged unlikely to be problematic in many studies, which used cluster randomisation and reported geographical separation of groups. Of specific relevance to mortality estimates, studies providing ORS to severely ill children and/or encouraging mothers to attend health clinic were judged to have high risk of bias in the outcome measure.
Regarding outcome measurement, all-cause mortality was usually categorised as being a reliable measure even when self-reported with long recall, owing to the salience and rarity of the event; the longest recall was 6 years [65], the shortest 2 days [38], and usually, it was 12 months or less. However, there is greater suspicion about cause-specific mortality where reporting is through verbal autopsy by the child’s carer. If cause-specific mortality was measured, assessment was therefore made as to whether it was verified by a clinician or taken from vital registration, in which case it was assessed as being at “low risk of bias.” While observational studies of WASH provision have verified cause of death through consultation with a clinician [5], no RCTs and only two NRSI used vital registration data [44,50]. One study [44] was assessed as at “low risk” of outcome reporting bias for diarrhoea mortality, while another was assessed as at “high risk of bias” because the study did not attribute cause-specific mortality to diarrhoea, using infectious and parasitic disease mortality instead [50]. In all other studies, the cause of death was given by verbal autopsy.
Nearly all trials were preregistered, four reported publishing a protocol with preanalysis plan [6–8,60], and three blinded data analysts [6–8]. In addition, one NRSI was deemed to have “low risk of bias” on reporting, because it published a baseline report with preanalysis plan [68].
Impacts of WASH on all-cause mortality (review question 1)
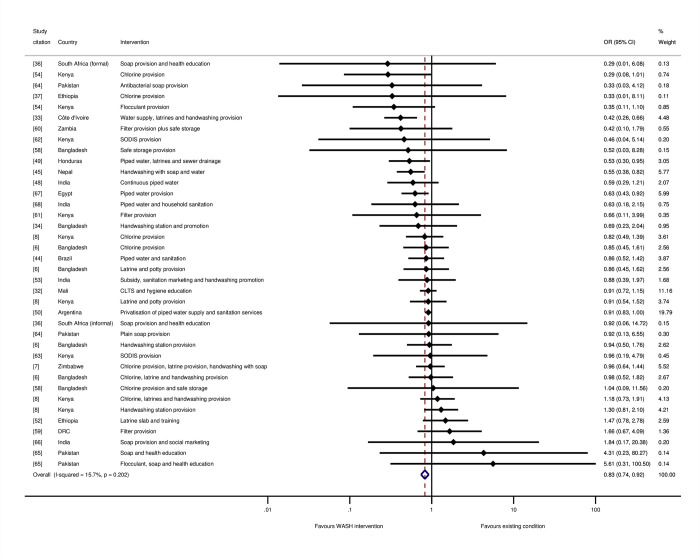
We conducted meta-analysis across intervention arms reporting all-cause mortality in children aged under 5 years (Fig 4). WASH interventions typically reduced the odds of all-cause mortality in childhood by 17% overall (OR = 0.83, 95% CI = 0.74, 0.92, 38 estimates). There was a small degree of estimated relative heterogeneity (I-squared = 16%) and absolute heterogeneity (tau-squared = 0.01).
Fig 4. Effects on all-cause mortality in childhood of WASH interventions.
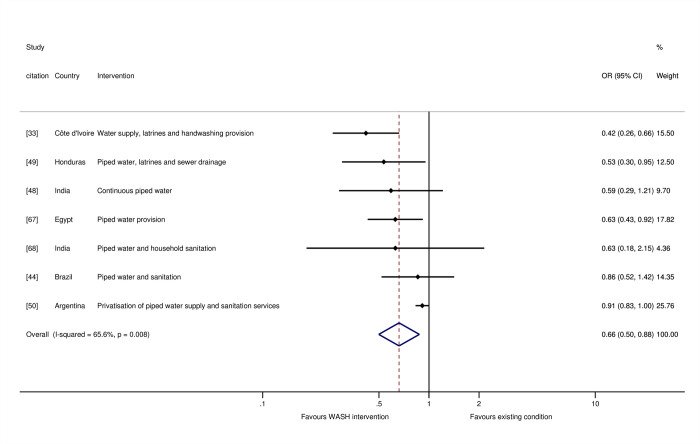
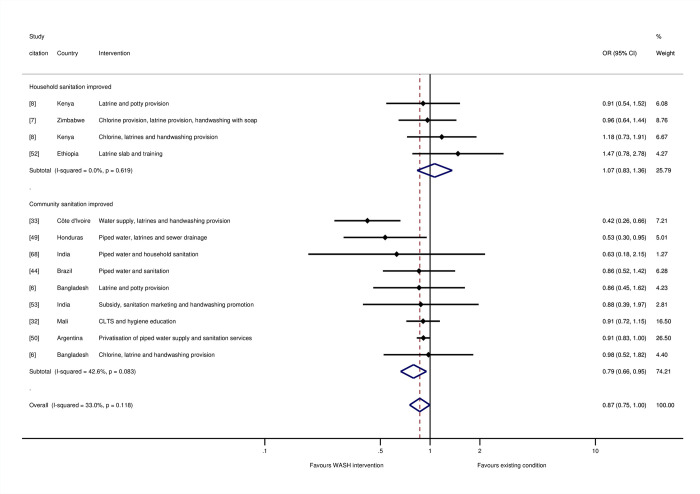
For the stratified meta-analyses by WASH technology, trial arms incorporating “any WASH”—that is, any single water supply, water treatment, sanitation, or hygiene technology, whether provided alone or alongside any other WASH technology—were meta-analysed. We found a 34% reduction in the odds of mortality for water supply interventions (OR = 0.66, 95% CI = 0.50, 0.88; I-squared = 66%; 7 estimates) (Fig 5). Four of these were studies where the risk of bias was high [33,44,49,67], while three were at “moderate risk of bias” [47,48,50]. For sanitation, we estimated 13% reduction in mortality overall (OR = 0.87, 95% CI = 0.75, 1.00; I-squared = 33%; 13 estimates). Four of the studies were assessed as being at “high risk of bias” [33,44,49,52], and seven were at “moderate risk of bias.” We tested for a threshold effect of sanitation improvement—that is, whether there needed to be a certain share of households in a community covered before the benefits of sanitation were realised [69]. When sanitation interventions targeted the whole community rather than individual households, or if households were targeted for sanitation improvements in circumstances when most of the community already used improved sanitation facilities, there were greater effects on mortality among children participating in the study (Fig 6). There was an estimated 21% reduction in the odds of mortality when sanitation was being improved community-wide (OR = 0.79, 95% CI = 0.66, 0.95; I-squared = 43%; 8 estimates), but no effect of sanitation where it was provided to specific households, where the majority of community members did not already use improved sanitation (OR = 1.07, 95% CI = 0.83, 1.36; I-squared = 0%; 4 estimates).
Fig 5. Effects on all-cause mortality in childhood of water supply interventions.
Fig 6. Effects on all-cause mortality in childhood of sanitation interventions.
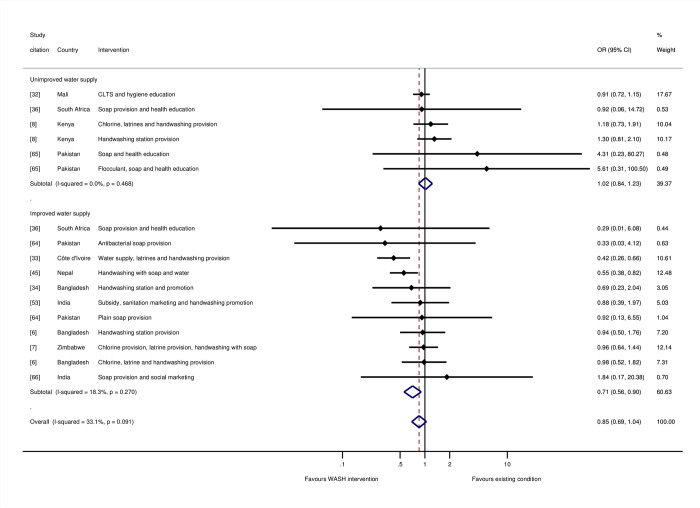
The overall effect of hygiene promotion was not statistically significant (OR = 0.85, 95% CI = 0.69, 1.04; I-squared = 33%; 17 estimates). Five of the studies were assessed as being at “high risk of bias” [33,36,45,65,66], and seven were at “moderate risk of bias.” Further analysis was done to test the hypothesis that hygiene promotion would be more effective when done under conditions of improved water supply, or, if not, when water supply was an intervention component alongside hygiene, and there were no concerns about the reliability of or distance to the water supply. The results suggested that this was indeed the case: There was no estimated effect of hygiene in circumstances where water supplies were not already improved (OR = 1.02, 95% CI = 0.84, 1.23; I-squared = 0%; 6 estimates). In contrast, there was a 29% reduction in the odds of mortality, when hygiene was provided in circumstances where the water supply was also being improved or had been improved previously (OR = 0.71, 95% CI = 0.56, 0.90; I-squared = 18%; 11 estimates) (Fig 7).
Fig 7. Effects on all-cause mortality in childhood of hygiene interventions.
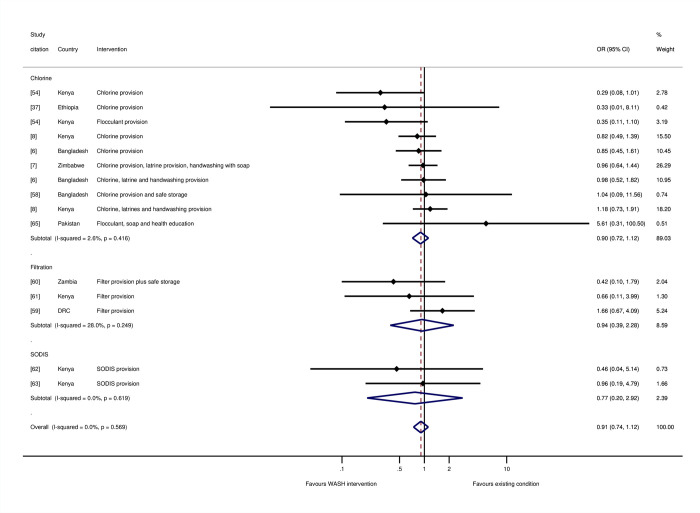
There were no significant effects on mortality of household water treatment and storage overall (OR = 0.93, 95% CI = 0.75, 1.14; I-squared = 0%; 15 estimates), or for individual water treatment technologies including chlorination (OR = 0.90, 95% CI = 0.72, 1.12; I-squared = 2%; 10 estimates), filtration (OR = 0.94, 95% CI = 0.39, 2.28; I-squared = 28%; 3 estimates), or SODIS (OR = 0.77, 95% CI = 0.20, 2.92; I-squared = 0%; 2 estimates) (Fig 8). Five of the studies were assessed as being at “high risk of bias” [59,61–63,65], and seven were at “moderate risk of bias.”
Fig 8. Effects on all-cause mortality in childhood of drinking water treatment and storage.
Meta-analysis by the “main WASH” technology that was provided suggested reductions in odds of death in childhood that were of the same magnitude, but not statistically significant for any single technology provided alone. But there was a significant reduction in mortality where multiple WASH technologies were promoted or provided of 16% (OR = 0.84, 95% CI = 0.71, 0.99, I-squared = 41%, 11 estimates). Five of the seven studies with the largest effects of multiple WASH technologies incorporated a water supply improvement [33,44,47,49,50], usually piped water to the household or yard.
We estimated meta-regressions to explore further whether the variation in effects by WASH technology intervention, and the other contextual factors we had identified from theory might explain differences across studies (Table 1). The regression pooled data from study participants of any age, incorporating the 14 additional estimates measured among all population groups or adults and children aged over 5. The reductions in mortality were significantly larger when interventions were conducted in circumstances where participants were children aged under 5 years, or data collection was limited to the summer rainy season. Where the study collected data over a shorter follow-up period, the effect on mortality was also significantly larger. Impacts on mortality were significantly greater when water supply improvements were made. The explanatory power of the regression was high (R-squared = 76%), and there was very little residual heterogeneity (I-squared = 0%; tau-squared < 0.01). The findings suggested a predicted value of 12% reduction in odds of mortality at the data means, which include study participants of any age (OR = 0.88) (Table 1, Panel 3). The maximum value of 74% reduction in odds of mortality (OR = 0.26) is for children aged 5 or under from immunocompromised groups who would receive all WASH interventions, with measurement made against comparators living in very poor communities with unimproved sanitation services, during the summer rainy season at 6-month intervention follow-up.
Table 1. Meta-regression analysis of all-cause mortality with prediction values.
| OR | 95% Conf. Interval | p-value | ||
|---|---|---|---|---|
| Panel 1: Intervention technology | ||||
| 1 = Water supply improvement | 0.60 | 0.41 | 0.89 | 0.01 |
| 1 = Water treatment and/or storage | 1.13 | 0.85 | 1.51 | 0.40 |
| 1 = Hygiene improvement | 1.14 | 0.80 | 1.61 | 0.46 |
| 1 = Hygiene and improved water supply | 0.82 | 0.63 | 1.07 | 0.14 |
| 1 = Household sanitation | 1.06 | 0.82 | 1.38 | 0.64 |
| 1 = Community-wide sanitation | 0.87 | 0.63 | 1.22 | 0.41 |
| Panel 2: Contextual factors | ||||
| 1 = Sanitation unimproved at baseline | 0.80 | 0.55 | 1.16 | 0.23 |
| 1 = Children aged under 5 | 0.72 | 0.56 | 0.93 | 0.01 |
| 1 = Immunocompromised group | 0.72 | 0.33 | 1.55 | 0.39 |
| 1 = Summer/rainy season | 0.58 | 0.31 | 1.08 | 0.09 |
| Follow-up period (years) | 1.06 | 1.00 | 1.12 | 0.06 |
| Constant | 0.96 | 0.63 | 1.43 | 0.81 |
| Panel 3: Prediction values of OR | ||||
| Data means | 0.88 | |||
| Minimum values of variables | 1.07 | |||
| Maximum values of variables | 0.26 | |||
| Panel 4: Test information | ||||
| Number of observations | 52 | |||
| Tau-squared | 0.01 | |||
| I-squared | 0% | |||
| Adjusted R-squared | 76% | |||
Impacts of WASH on diarrhoea mortality (review question 2)
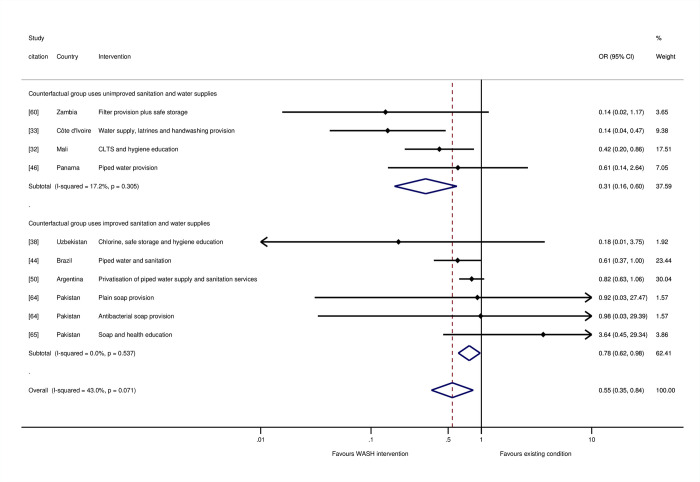
The meta-analysis of diarrhoea mortality in childhood indicated WASH provision and promotion lead to a significant reduction in the odds of death due to diarrhoea by 45% (OR = 0.55, 95% CI = 0.35, 0.84; 10 estimates) (Fig 9). Six of the studies were assessed as being at “high risk of bias” [33,38,44,46,50,65], and three were at “moderate risk” [32,60,64]. The relatively high degree of absolute and relative heterogeneity in findings (I-squared = 43%, tau-squared = 0.15) suggested additional analysis was needed of factors that could explain the variation across study contexts.
Fig 9. Effects on diarrhoea mortality in childhood of WASH interventions.
One of those factors is the degree of movement up the WASH ladders. We tested this hypothesis in moderator analysis according to the type of water supply and sanitation facilities used in the counterfactual group. When the WASH interventions were provided when counterfactuals were using no or unimproved sanitation and water supplies and, therefore, exposed to very high risk of environmental contamination by pathogens, there was an estimated 69% reduction in diarrhoea mortality in childhood (OR = 0.31, 95% CI = 0.16, 0.60, I-squared = 17%, 4 estimates). But for interventions provided in circumstances when most people already had access to improved sanitation, there was only a 22% reduction in odds of mortality (OR = 0.78, 95% CI = 0.62, 0.98, I-squared = 0%, 6 estimates) (Fig 9). The impacts of WASH interventions on childhood diarrhoea mortality were significantly greater (p < 0.01) when counterfactual groups lacked access to improved water supply and sanitation—and most people were therefore using unimproved facilities, or none at all and openly defaecating—than when most people in counterfactual groups were using improved facilities.
The largest effects on diarrhoea mortality were from studies of multiple WASH technologies: two contained a component that aimed to provide latrines to all households in intervention communities [32,33], and two involved water supply improvements [33] or hygiene promotion when water supplies were already improved [38]. With regard to the two studies of latrine provision or promotion to whole communities, both were provided alongside hygiene promotion, but only in Côte d’Ivoire was the water supply also improved [33]. In the case of Mali [32], hygiene promotion was given as part of CLTS when water supplies were limited. Another longitudinal follow-up study of an RCT of hygiene improvement, which was rated at “high risk of bias,” was conducted among communities where some households had access to running water for only 2 hours each week [65], suggesting these households had limited opportunities for adherence to improved hygiene practices.
Few studies of household water treatment in endemic circumstances have reported diarrhoea mortality outcomes. Among the studies examining household water treatment, only one was of an approach that has been found to reduce diarrhoea morbidity; the study was of filtration [60], and it found large but statistically insignificant impacts in children from immunocompromised populations (HIV–positive mothers). The other was a study of chlorine provision alongside safe storage and hygiene education [38]. Meta-regression analysis suggested interventions providing community-wide sanitation, and hygiene promotion in circumstances when water supplies were improved were associated with significantly larger impacts on diarrhoea mortality (Table D in S1 Annex).
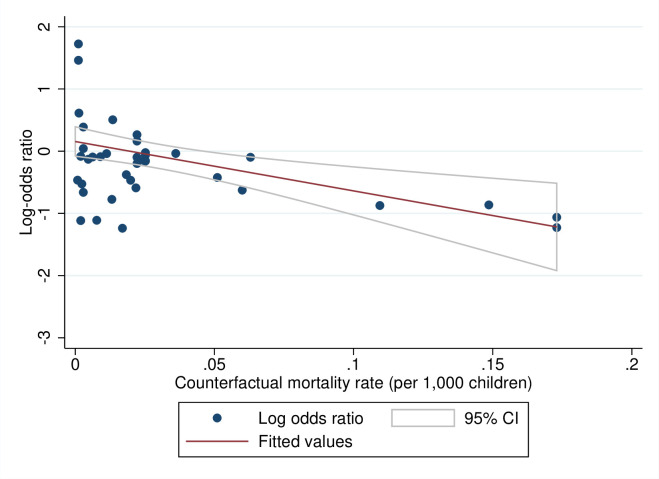
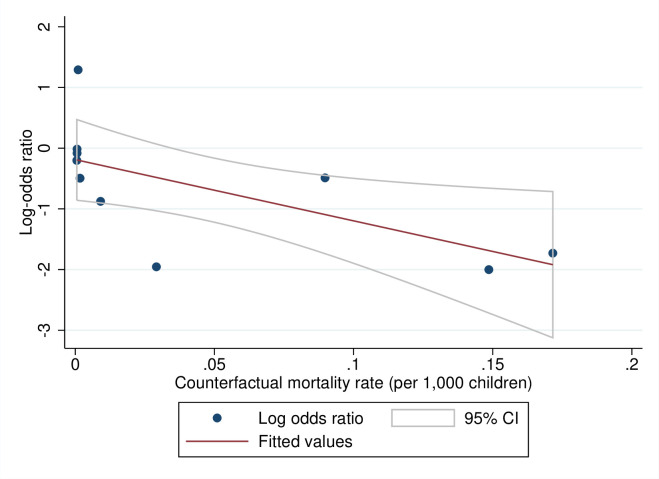
Predicted effects of WASH interventions by baseline mortality rates (review question 3)
We tested for a theoretical relationship between the contextual starting values and programme effectiveness—that is, one might expect higher returns from a lower base—by plotting the relationships between the baseline mortality rate measured in the counterfactual group and the log-odds ratios for all-cause (Fig 10) and diarrhoea mortality (Fig 11). The results suggested that, at higher baseline mortality rates, WASH interventions tended to have larger effects on mortality. For example, where the crude mortality rate was 75 per 1,000 live children, as it is in many African countries and communities in South Asia, the estimated reduction in odds of all-cause mortality in childhood was 33% (OR = 0.67, 95% CI = 0.47, 0.86). At the same baseline mortality rate, there was a reduction of 61% in the odds of diarrhoea mortality (OR = 0.39; 95% CI = 0.20, 0.67).
Fig 10. Meta-regression plot of all-cause mortality in childhood against prevalence.
Fig 11. Meta-regression plot of diarrhoea mortality in childhood against prevalence.
Note: Fitted values are from inverse-variance weighted regression.
Evaluation of biases at the review level (review question 4)
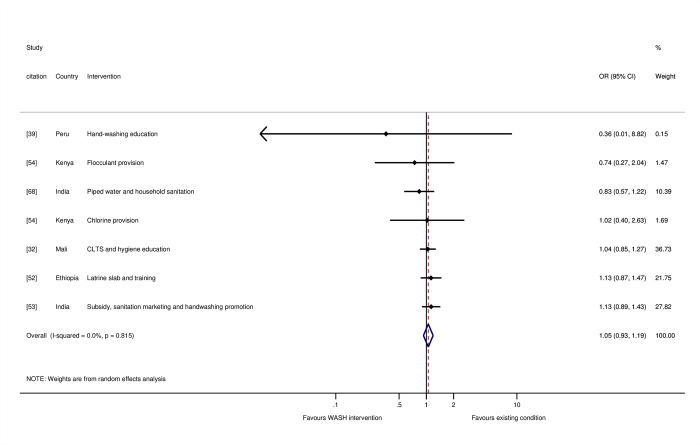
In this section, we present findings from a negative control (placebo population), analysis of small study effects, and the results of sensitivity analyses. Using meta-analysis to power studies adequately with small effect sizes does not necessarily generate effects that are statistically significant if there is no underlying causal relationship [70]. The meta-analysis of studies reporting all-cause mortality did not suggest WASH interventions affected mortality when participants were restricted to adults and children aged over 5 (OR = 1.05, 95% CI = 0.93, 1.19, I-squared = 0%, 7 estimates) (Fig 12). The study with the largest effect on mortality was of health messaging among 10-year-old school children [39]. Several of the studies were of chlorination [54,56,57]. We might expect to see effects on maternal mortality due to sepsis, which improved WASH—particularly in places of birth like health facilities—is thought to alleviate [71]. None of the interventions provided a WASH improvement in a health facility.
Fig 12. Effects on all-cause mortality for study participants aged over 5 years.
Since the mortality data were largely collected from participant flow diagrams, the fact that mortality estimates are available at all is indicative of the good standards of reporting in the studies included in this review. This suggested publication bias was likely to be limited, most clearly for prospective trials of WASH interventions, as found in the analysis of small study effects (Figs H and I in S1 Annex). We tested the sensitivity of the findings to exclusion of particular studies. For example, the pooled effect estimate might be influenced by studies with large samples [50] or those conducted among extremely poor or vulnerable groups [32,60]. The overall findings, and the findings for particular WASH technologies or circumstances, were not significantly affected by exclusion of these, or any other individual studies. We also examined whether there was a correlation between risk-of-bias rating and the estimated effect on mortality. The effects assessed at “high risk of bias” incorporated studies that did not distinguish under 5s from other population groups [40,57]. The meta-analysis of NRSI at “high risk of bias” found a greater reduction in odds of all-cause mortality than other studies (OR = 0.58, 95% CI = 0.48, 0.70; I-squared = 0%; 8 estimates) (Fig J in S1 Annex). In contrast, we found no significant change in the odds of death for RCTs that had “high risk of bias” in measuring the effect on mortality in children aged under 5 years (OR = 1.41; 95% CI = 0.99, 2.01; I-squared = 0%; 15 estimates) (Fig K in S1 Annex).
Discussion
Summary of main findings
This systematic review and meta-analysis estimated the impacts of WASH interventions on children’s mortality by pooling data, collected mainly from reported participant flows in multiple studies, for all-cause and diarrhoea mortality. The approach helped overcome two critical issues in primary study research. Firstly, it is difficult to design prospective impact evaluations like RCTs to estimate precise effects on mortality. And, secondly, mortality is thought to be reported with less bias than other, more accessible outcome measures like morbidity. The findings suggested WASH interventions cause large and statistically significant reductions in the odds of mortality in childhood in endemic circumstances. For mortality due to any cause, we estimated around one-in-five deaths are averted by WASH interventions. For severe diarrhoea disease, we estimated a reduction in odds of mortality by nearly half. However, these averages concealed important heterogeneity in effects. Further analysis suggested that the reduction in all-cause mortality was most consistently established where the interventions provided an improved water supply.
Since many of the studies examining water improvements were of piped water to the household or yard, the analysis therefore suggested a mechanism through which water affects mortality: by enabling domestic hygienic practices around handwashing, food preparation, and cleanliness. Indeed, where hygiene was promoted, the analysis suggested it was only effective in circumstances where there was likely to be sufficient water available. In other words, when people have more water to wash in, they are able to wash properly, which significantly improves the survival chances of their children. Effects in individual studies of hygiene also appeared related to water supply access. For example, in Côte d’Ivoire [33], hygiene education was provided alongside village water pumps that gave 76 cubic metres per day for a community of 400 people, equivalent to 190 litres per capita per day. The study with the smallest effect on diarrhoea mortality was conducted among communities where some households had access to running water for only 2 hours each week [65].
Latrine promotion to whole communities was most consistently associated with the reductions in diarrhoea mortality in childhood, although we note the small number of intervention effects available (n = 2). Thus, when sanitation is available and used by the majority of people in a community, it lessens children’s interactions with faeces in the public realm, reducing infection transmission and mortality. In contrast, the effect on all-cause or diarrhoea mortality of household water treatment was not significant. Few studies have estimated the effects of water treatment and storage on diarrhoea mortality, and only one used a method (filtration) thought to be efficacious in removing common causes of enteric infection in low-income settings [60]. Most of the studies of household water treatment evaluated chlorination, which is not thought efficacious in removing common diarrhoea pathogens in low-income settings like cryptosporidium [72].
The analysis suggested WASH interventions were most effective when they were given in circumstances of high environmental risk, where most households openly defaecated or used unimproved water supply and sanitation amenities, and the baseline mortality rate was consequently higher. WASH interventions were also more effective in the summer rainy season. Diarrhoea mortality in South Asia and sub-Saharan Africa has been shown as largely associated with E. coli infection in infants and cryptosporidium in children [73], both of which are expected to be more prevalent in warmer conditions. Shorter trials, which are usually conducted in the peak diarrhoea season when the intervention is most efficacious, also tended to have significantly larger effects on all-cause mortality. Because the season of data collection was accounted for in meta-regression analysis, this suggested there may be other reasons for studies with longer follow-ups to have smaller effects, such as problems in maintaining the WASH technology and/or reduced adherence over time.
Meta-regression analysis suggested approximately three-quarters of deaths in childhood can be averted when WASH interventions are provided to immunocompromised groups, living in very poor communities who otherwise have unimproved sanitation services, during the peak diarrhoea season. We found no evidence of publication bias due to small-study effects in trials of WASH interventions, presumably because mortality was not defined as an outcome in these studies.
What the study adds to existing research
These results support predictions from theory. One would expect a stronger relationship between improved WASH access and diarrhoea mortality than all-cause mortality, as we have found. Inadequate WASH may cause death in young children through other routes such as respiratory infection and undernutrition, but diarrhoea is thought most closely related to WASH amenities; in contrast, respiratory illness and nutrition are also affected by the quality of indoor air and nutrient intake [4]. The findings are therefore consistent with the principal causes of mortality in childhood: Domestic hygiene is the common factor that can block transmission of faeco-oral and respiratory infections [74]; community-wide sanitation breaks transmission of diarrhoea from open defaecation in the public and domestic domains [75]. These effects would tend to be greater over a counterfactual where existing water supply and sanitation services are not available or unimproved, so that community members are not able to practice handwashing and are openly defaecating or using facilities that are either shared between two or more households or ones that do not adequately separate excreta from the environment.
Therefore, the significantly greater impacts of WASH interventions in contexts where environmental contamination and the baseline mortality are high are consistent with the WASH ladders concept: Where the WASH improvement is from a lower base or enhances access to WASH together, one would expect bigger effects on health. The review’s findings of null effects on all-cause mortality for study participants aged over 5 years is also consistent with the maturation of immunity systems with age, causing older children and adults to be less susceptible to infectious disease mortality than under 5s [76]. This is in contrast to reviews that have found significant effects on diarrhoea morbidity for those aged over 5 years too [77]. The F-diagram includes six intermediate transmission vectors (fluids, fields, flies, fingers, food, and fomites), of which only the fluids route is addressed through water quality [78]. While we did not find significant effects, on all-cause or diarrhoea mortality, of water treatment interventions, which act on water quality, where drinking water of quality is used to prepare food, it may help reduce food-borne disease transmission, thought particularly important for weaning children [79].
Nonrandomised studies at “high risk of bias” can produce inflated effects, as we found here, because p-hacking would tend to increase effect size magnitudes. However, we estimated the opposite effect for RCTs—that “high risk of bias” is associated with smaller effects on mortality—a finding that is consistent with site selection bias [80,81]. In other words, trials that are more carefully conducted and reported are of interventions that also tend to be designed and implemented appropriately to the local context, and therefore adhered to, hence being more effective. An example is when interventions promote handwashing (e.g., education, social marketing, and soap provision) in contexts where the quantity of water available to households is sufficient to practice domestic cleanliness; or, if it is thought not to be, improvements in water supply access or reliability are made too.
Findings in relation to other systematic reviews
The evidence presented here, that water supply and community-wide sanitation save children’s lives in L&MICs, is consistent with findings from an early review [82], but in several respects is quite different from later ones. These have not tended to find significant effects on diarrhoea morbidity of interventions that aim to improve access to water in quantity for household use. The most recent review by WHO suggests that clean drinking water provided at the point-of-use, most consistently through filtration, reduces reported diarrhoeal illness by around one-half [11]. Reviews of morbidity have found that household water treatment appears to be more effective when a protective container is also provided [83], as it may be, for example, in household filtration devices when drinking water is accessed through a straw or tap. Reviews have also found smaller or null effects for household water treatment technologies like chlorination, when studies were double-blinded [13,23,83], or when methods were used to correct for lack of blinding [84,85]. Hand hygiene interventions have been found to have varying effects on diarrhoeal illness [74,86,87], and a review is being published to update the evidence on respiratory infection [88]. The difference between our findings for mortality and the reviews of morbidity might arise because of the contexts in which the studies have been conducted and, specifically, the availability of treatment. However, many of the papers and contexts included in this review are also represented in the reviews of morbidity.
A few other published reviews provide estimates of mortality reduction due to factors associated with WASH provision. Morris and colleagues [89] reviewed evidence on cause-specific mortality among under 5s, estimating 22% of deaths were due to diarrhoea and 20% to pneumonia. Benova and colleagues [90] estimated significant reductions in maternal mortality that appeared most closely related to water supply access (OR = 0.42, 95% CI = 0.29, 0.83, I-squared = 0%, 2 estimates).
Limitations of the study
The reporting of children’s deaths through interviews with mothers is susceptible to some biases and omissions, which have been investigated and documented in the literature [91,92]. Omissions are relatively common in the reporting of deaths occurring 10 to 15 years before a survey takes place, but there is no evidence of underreporting of deaths for more recent time periods. As for biases, there is no evidence that mothers from a variety of countries tend to underreport deaths occurring soon after birth or deaths of girls. Given the relatively shorter recall period employed in the studies considered in our review, we believe underreporting of deaths is unlikely. It is also not obvious why underreporting of deaths should differ between treated and untreated groups.
Hence, regarding the quality of the evidence collected here, reported mortality is not thought to be a biased measure per se. All-cause mortality data can also be triangulated with corresponding data from other sources, such as vital registration, and even the possible effect of other diseases, such as respiratory infections [93]. Cause-specific death rates are thought less reliable [16], dependent as they are on a verbal autopsy interview with the bereaved family of the patient, who may be too distraught to give an unbiased, let alone a coherent account of the patient’s last days. But, like all-cause mortality, verbal autopsy can be triangulated with, or done by, a physician, which we incorporated in the risk-of-bias assessment. Vital registration and verbal autopsy estimates are also used in GBD calculations.
A potentially more serious source of bias is differential attrition. During survey interviews, deaths will not be reported for mothers who migrated or died. To the extent that WASH interventions affect migration and adult mortality rates, child mortality rates might be downwards biased in intervention areas. In other words, a potential source of bias affecting the crude death rate calculations used in this study is that they are right-censored: that is, where data are collected contemporaneously among participants regardless of age, children born into the study or who migrate out and younger children will have completed shorter durations than older children; the data on pre- and neonatal mortality may also be censored by maternal deaths in pregnancy or labour. This causes downwards bias in the estimate of mortality in any single trial arm, although the bias may be less problematic in randomised trials with contemporaneous data collection across arms. A final source of bias in mortality estimates is where severely ill children were given ORS or encouraged to attend health clinic [37,40,56–58,60,64]. Hence, for all of these reasons, the results should be interpreted as providing lower-bound estimates of the impacts of WASH on mortality in childhood.
The evidence synthesis combined a variety of WASH technologies, promotional interventions, and counterfactuals. The stratified meta-analysis and meta-regressions incorporated information about the WASH technologies and counterfactual scenarios. However, inconsistency across interventions is an important potential limitation of meta-analyses of general WASH improvements. For example, in addition to direct provision, we included many promotional approaches, including hygiene social marketing [34], CLTS [32] and latrine promotion with subsidies [53], the decentralisation of water services to local government [67], and the privatisation of local water supply and sanitation provision [50]. This may be addressed through systematic analysis of adherence to measure likely exposures to improved WASH technologies [94], and as more studies and participant flows become available for stratified analysis of particular interventions.
What the findings imply for policy and research
In 2016, the United Nations proclaimed 2018 to 2028 the International Decade for Action on Water for Sustainable Development (https://www.unwater.org/new-decade-water/). Our results provide evidentiary support for greater attention to ensuring populations can access and use improved water supplies for domestic hygiene and sanitation. We present evidence that suggests these interventions may significantly improve survival in early childhood from infection. Even though the review was restricted to endemic disease circumstances, the findings may also be relevant for epidemic disease control including Coronavirus 2019 [95]. It is well known that water supplies and sanitation are pro-poor and gender-inclusive interventions due to the time-savings and safety they may enable [96–98]. Our results suggest significant contributions could be made to reducing the global disease burden in childhood from improvements in water supplies and community-wide sanitation where access is particularly inadequate, especially in sub-Saharan Africa and parts of South Asia. They also suggest that hygiene interventions are effective where children’s carers have sufficient water to wash in.
Transparent study reporting is crucial for accountability and learning by enabling effects for all relevant outcomes to be measured. A common source of bias in WASH trials is caused by differential losses to follow-up out of the study (attrition). How much attrition there is, and the reasons for it—for example, participant deaths—should be known. Reporting standards are well known in health research due to the work of the Consolidated Standards of Reporting Trials (CONSORT) Group [99,100], and standards have been published in development economics too [101]. Many authors and journals do now report this information, but there are lags in practices across the research communities producing WASH trials. According to a recent survey, participant flows have been reported in around half of studies in environmental health, especially in the last decade when this information is a condition of publication in reputable journals and features on related checklists, but they are rarely provided in studies in development economics [10].
Water is an important enabling factor for practising hand and food hygiene and some forms of sanitation (e.g., flush toilets), but articles do not typically report data on distance to the water source, or water consumption (litres per capita per day) and how it is used (e.g., whether consumed or used in bathing). This information is crucial for understanding mechanisms and, therefore, the generalisability of the findings. Three studies provided information on distance to the water supply [8,32,55], one of which also reported water consumption [32]. In addition, it was not always clear exactly which interventions were provided to participants, not just the nature of the water supply improvement but whether hand or food hygiene were promoted. Therefore, a final recommendation is for more transparent reporting about the conditions being compared, including clearer information about the WASH technology itself that is being promoted and the counterfactual scenario. For example, if hygiene messaging is part of the intervention, it should be clearly indicated in the article title or abstract.
Conclusions and suggested research directions
We found large and consistent effects of water supply interventions on all-cause mortality in childhood and of community-wide sanitation interventions on diarrhoea mortality. The contribution of this synthesis—to use participant flow data to provide estimates of changes in child mortality associated with WASH interventions—has been enabled by studies that use agreed standards of reporting such as CONSORT. There is potentially a large number of estimates of mortality in childhood from studies that do not use these methods of reporting, as a recent meta-analysis of household water treatment has indicated [102]. Going forward, the challenge will be for an author collaborative to provide sufficient incentives to obtain unpublished participant flow data, to ensure that future systematic reviews and meta-analyses are representative of the complete data available on mortality in WASH intervention studies. There is also a need for more rigorous studies of water supply improvements. Although prospective evaluations of water supply interventions are being done (e.g., [103]), we are only aware of one published randomised field trial of a water supply improvement in Ghana [104] and one study that randomised encouragement of subsidies for household connections in Morocco [105]. If services are allocated by administrative area or according to a threshold rule (e.g., the share of community members currently covered by a service), it may be possible to use a discontinuity design, an approach that has been shown to generate the same effect estimates as RCTs, when applied prospectively or retrospectively [106]. We are hopeful that the evidence presented in this review, and the evidence we are calling for, will prove useful for those taking decisions about what WASH improvements are needed in the second half of the International Decade for Action on Water.
Supporting information
(DOCX)
Table A. The global burden of infectious disease is mainly due to mortality (per 100,000). Table B. Global Burden of Disease: A moving target? Table C. Description of studies included in this systematic review by WASH technology. Table D. Meta-regression analysis of diarrhoea mortality in childhood by WASH intervention technology. Fig A. All-cause mortality. Fig B. All-cause mortality: Water supply interventions. Fig C. All-cause mortality: Sanitation interventions. Fig D. All-cause mortality: Hygiene interventions. Fig E. All-cause mortality: Drinking water treatment and storage interventions. Fig F. Diarrhoea mortality. Fig G. Effects on all-cause mortality for participants aged over 5 years. Fig H. Funnel graphs with regression lines: All-cause mortality. Fig I. Funnel graphs with regression lines: Diarrhoea mortality. Fig J. Effects on all-cause mortality for nonrandomised studies of interventions at “high risk of bias.” Fig K. Effects on all-cause mortality for randomised controlled trials at “high risk of bias.”
(DOCX)
(DTA)
Acknowledgments
Helpful comments were given by participants at UNC Water and Health 2021, the What Works Global Summit 2021, the LSHTM-IFS WASH Economics Conference 2022, the University of Salford, Paul Hunter, Britta Augsburg, Oliver Cumming and colleagues in the Environmental Health Group at LSHTM.
Abbreviations
- CLTS
community-led total sanitation
- GBD
global burden of disease
- GRADE
Grading of Recommendations, Assessment, Development, and Evaluations
- JMP
Joint Monitoring Programme
- L&MICs
low- and middle-income countries
- NRSI
nonrandomised studies of interventions
- OR
odds ratio
- ORS
oral rehydration solution
- RCT
randomised controlled trial
- SODIS
solar disinfection
- WASH
water, sanitation, and hygiene
- YLL
years of life lost
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–1210. doi: 10.1016/S0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troeger C, Blacker BF, Khalil IA, Rao PC, Cao S, Zimsen SR, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1211–1228. doi: 10.1016/S1473-3099(18)30362-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, et al. Global burden of childhood pneumonia and diarrhoea. Lancet Lond Engl. 2013. Apr 20;381(9875):1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prüss-Ustün A, Wolf J, Bartram J, Clasen T, Cumming O, Freeman MC, et al. Burden of disease from inadequate water, sanitation and hygiene for selected adverse health outcomes: An updated analysis with a focus on low- and middle-income countries. Int J Hyg Environ Health. 2019. Jun;222(5):765–777. doi: 10.1016/j.ijheh.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Victora CG, Smith PG, Vaughan JP, Nobre LC, Lombard C, Teixeira AMB, et al. Water supply, sanitation and housing in relation to the risk of infant mortality from diarrhoea. Int J Epidemiol. 1988;17(3):651–654. doi: 10.1093/ije/17.3.651 [DOI] [PubMed] [Google Scholar]
- 6.Luby SP, Rahman M, Arnold BF, Unicomb L, Ashraf S, Winch PJ, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. Lancet Glob Health. 2018;6(3):e302–e315. doi: 10.1016/S2214-109X(17)30490-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humphrey JH, Mbuya MN, Ntozini R, Moulton LH, Stoltzfus RJ, Tavengwa NV, et al. Independent and combined effects of improved water, sanitation, and hygiene, and improved complementary feeding, on child stunting and anaemia in rural Zimbabwe: a cluster-randomised trial. Lancet Glob Health. 2019;7(1):e132–e147. doi: 10.1016/S2214-109X(18)30374-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Null C, Stewart CP, Pickering AJ, Dentz HN, Arnold BF, Arnold CD, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster-randomised controlled trial. Lancet Glob Health. 2018. Mar 1;6(3):e316–e329. doi: 10.1016/S2214-109X(18)30005-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 10.Chirgwin H, Cairncross S, Zehra D, Sharma WH. Interventions promoting uptake of water, sanitation and hygiene (WASH) technologies in low-and middle-income countries: An evidence and gap map of effectiveness studies. Campbell Syst Rev. 2021;17(4):e1194. doi: 10.1002/cl2.1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf J, Hubbard S, Brauer M, Ambelu A, Arnold BF, Bain R, et al. Effectiveness of interventions to improve drinking water, sanitation, and handwashing with soap on risk of diarrhoeal disease in children in low-income and middle-income settings: a systematic review and meta-analysis. Lancet. 2022;400(10345):48–59. doi: 10.1016/S0140-6736(22)00937-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fewtrell L, Colford JMJ. Water, Sanitation and Hygiene: Interventions and Diarrhoea [Internet]. Washington, DC: World Bank; 2004. Jul [cited 2022 Sep 15]. Available from: https://openknowledge.worldbank.org/handle/10986/13742 [Google Scholar]
- 13.Schmidt WP, Cairncross S. Household water treatment in poor populations: is there enough evidence for scaling up now? Environ Sci Technol. 2009;43(4):986–992. doi: 10.1021/es802232w [DOI] [PubMed] [Google Scholar]
- 14.Zwane AP, Zinman J, Van Dusen E, Pariente W, Null C, Miguel E, et al. Being surveyed can change later behavior and related parameter estimates. Proc Natl Acad Sci. 2011;108(5):1821–1826. doi: 10.1073/pnas.1000776108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336(7644):601–605. doi: 10.1136/bmj.39465.451748.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta-epidemiological studies. Health Technol Assess. 2012;16(35):1–82. doi: 10.3310/hta16350 [DOI] [PubMed] [Google Scholar]
- 17.Clasen T. Comments from Dr. Thomas Clasen on a draft of the GiveWell Water Quality Report, November 15, 2013. [Internet]. GiveWell; 2013 [cited 2019 Jan 1]. Available from: https://files.givewell.org/files/DWDA%202009/Interventions/Water/Water%20Purification%20Assessment/eBeer-clasen-comments.pdf [Google Scholar]
- 18.Schmidt WP, Arnold BF, Boisson S, Genser B, Luby SP, Barreto ML, et al. Epidemiological methods in diarrhoea studies—an update. Int J Epidemiol. 2011. Dec;40(6):1678–1692. doi: 10.1093/ije/dyr152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briscoe J, Feachem RG, Rahaman MM. Evaluating health impact: water supply, sanitation, and hygiene education. Ottawa, ON, CA: IDRC; 1986. [Google Scholar]
- 20.Imo State Evaluation Team. Evaluating water and sanitation projects: lessons from Imo State, Nigeria. Health Policy Plan. 1989:40–49. [Google Scholar]
- 21.Deaton A, Cartwright N. Understanding and misunderstanding randomized controlled trials. Soc Sci Med. 2018;210:2–21. doi: 10.1016/j.socscimed.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021) [Internet]. Cochrane. 2021. [cited 2021 May 31]. Available from: https://training.cochrane.org/handbook/current [Google Scholar]
- 23.Waddington H, Snilstveit B, White H, Fewtrell L. Water, sanitation and hygiene interventions to combat childhood diarrhoea in developing countries. New Delhi Int Initiat Impact Eval. 2009. [Google Scholar]
- 24.Sharma Waddington H, Cairncross S. PROTOCOL: Water, sanitation and hygiene for reducing childhood mortality in low-and middle-income countries. Campbell Syst Rev. 2021;17(1):e1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas J, Brunton J, Graziosi S. EPPI-Reviewer 4.0: software for research synthesis. EPPI-Centre Software. London: Social Science Research Unit. Inst Educ Univ Lond. 2010;20(18):8–11. [Google Scholar]
- 26.Bongartz P. CLTS Update February 2012 [Internet]. Institute of Development Studies. 2012. [cited 2021 Nov 1]. Available from: https://archive.ids.ac.uk/clts/sites/communityledtotalsanitation.org/files/CLTS_Update_Feb_2012.pdf [Google Scholar]
- 27.Higgins JP, Sterne JA, Savovic J, Page MJ, Hróbjartsson A, Boutron I, et al. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev. 2016;10(Suppl 1):29–31. [Google Scholar]
- 28.Eldridge S, Campbell M, Campbell M, Drahota-Towns A, Giraudeau B, Higgins J, et al. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0): additional considerations for cluster-randomized trials. 2016. [cited 2021 Jan 1]. Available from: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials [Google Scholar]
- 29.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016:355. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waddington H, White H, Snilstveit B, Hombrados JG, Vojtkova M, Davies P, et al. How to do a good systematic review of effects in international development: a tool kit. J Dev Eff. 2012;4(3):359–387. [Google Scholar]
- 31.Collaboration Campbell. Campbell Collaboration Systematic Reviews: Policies and Guidelines [Internet]. The Campbell Collaboration. 2021. [cited 2022 Sep 15]. Available from: https://campbellcollaboration.org/library/campbell-collaboration-systematic-reviews-policies-and-guidelines.html [Google Scholar]
- 32.Pickering AJ, Djebbari H, Lopez C, Coulibaly M, Alzua ML. Effect of a community-led sanitation intervention on child diarrhoea and child growth in rural Mali: a cluster-randomised controlled trial. Lancet Glob Health. 2015. Nov 1;3(11):e701–e711. doi: 10.1016/S2214-109X(15)00144-8 [DOI] [PubMed] [Google Scholar]
- 33.Messou E, Sangaré SV, Josseran R, Le Corre C, Guélain J. Effect of hygiene measures, water sanitation and oral rehydration therapy on diarrhea in children less than five years old in the south of Ivory Coast. Bull Soc Pathol Exot. 1997;90(1):44–47. [PubMed] [Google Scholar]
- 34.Ram PK, Nasreen S, Kamm K, Allen J, Kumar S, Rahman MA, et al. Impact of an Intensive Perinatal Handwashing Promotion Intervention on Maternal Handwashing Behavior in the Neonatal Period: Findings from a Randomized Controlled Trial in Rural Bangladesh. BioMed Res Int [Internet]. 2017. [cited 2022 Sep 15]. Available from: https://www.hindawi.com/journals/bmri/2017/6081470/ doi: 10.1155/2017/6081470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Efthimiou O. Practical guide to the meta-analysis of rare events. Evid Based Ment Health. 2018. May;21(2):72–76. doi: 10.1136/eb-2018-102911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cole EC, Hawkley M, Rubino JR, Crookston BT, McCue K, Dixon J, et al. Comprehensive family hygiene promotion in peri-urban Cape Town: Gastrointestinal and respiratory illness and skin infection reduction in children aged under 5. South Afr J Child Health. 2012;6(4):109–117. [Google Scholar]
- 37.Mengistie B, Berhane Y, Worku A. Household Water Chlorination Reduces Incidence of Diarrhea among Under-Five Children in Rural Ethiopia: A Cluster Randomized Controlled Trial. PLoS ONE. 2013. Oct 23;8(10):e77887. doi: 10.1371/journal.pone.0077887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semenza JC, Roberts L, Henderson A, Bogan J, Rubin CH. Water distribution system and diarrheal disease transmission: a case study in Uzbekistan. Am J Trop Med Hyg. 1998;59(6):941–946. doi: 10.4269/ajtmh.1998.59.941 [DOI] [PubMed] [Google Scholar]
- 39.Gyorkos TW, Maheu-Giroux M, Blouin B, Casapia M. Impact of health education on soil-transmitted helminth infections in schoolchildren of the Peruvian Amazon: a cluster-randomized controlled trial. PLoS Negl Trop Dis. 2013;7(9):e2397. doi: 10.1371/journal.pntd.0002397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luby SP, Agboatwalla M, Painter J, Altaf A, Billhimer W, Keswick B, et al. Combining drinking water treatment and hand washing for diarrhoea prevention, a cluster randomised controlled trial. Trop Med Int Health. 2006;11(4):479–489. doi: 10.1111/j.1365-3156.2006.01592.x [DOI] [PubMed] [Google Scholar]
- 41.Lipsitch M, Tchetgen ET, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21(3):383. doi: 10.1097/EDE.0b013e3181d61eeb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnold BF, Ercumen A. Negative Control Outcomes: A Tool to Detect Bias in Randomized Trials. JAMA. 2016. Dec 27;316(24):2597–2598. doi: 10.1001/jama.2016.17700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kremer M, Leino J, Miguel E, Zwane AP. Spring Cleaning: Rural Water Impacts, Valuation, and Property Rights Institutions*. Q J Econ. 2011. Feb 1;126(1):145–205. doi: 10.1093/qje/qjq010 [DOI] [PubMed] [Google Scholar]
- 44.Rasella D. Impacto do Programa Água para Todos (PAT) sobre a morbi-mortalidade por diarreia em crianças do Estado da Bahia, Brasil. Cad Saúde Pública. 2003;29:40–50. [DOI] [PubMed] [Google Scholar]
- 45.Rhee V, Mullany LC, Khatry SK, Katz J, LeClerq SC, Darmstadt GL, et al. Maternal and Birth Attendant Hand Washing and Neonatal Mortality in Southern Nepal. Arch Pediatr Adolesc Med. 2008. Jul 7;162(7):603–608. doi: 10.1001/archpedi.162.7.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryder R, Reeves W, Singh N, Hall C, Kapikian A, Gomez B, et al. The childhood health effects of an improved water supply system on a remote Panamanian island. Am J Trop Med Hyg. 1985;34(5):021–924. doi: 10.4269/ajtmh.1985.34.921 [DOI] [PubMed] [Google Scholar]
- 47.Reese H, Routray P, Torondel B, Sinharoy SS, Mishra S, Freeman MC, et al. Assessing longer-term effectiveness of a combined household-level piped water and sanitation intervention on child diarrhoea, acute respiratory infection, soil-transmitted helminth infection and nutritional status: a matched cohort study in rural Odisha, India. Int J Epidemiol. 2019. Dec 1;48(6):1757–1767. doi: 10.1093/ije/dyz157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ercumen A, Arnold BF, Kumpel E, Burt Z, Ray I, Nelson K, et al. Upgrading a piped water supply from intermittent to continuous delivery and association with waterborne illness: a matched cohort study in urban India. PLoS Med. 2015;12(10):e1001892. doi: 10.1371/journal.pmed.1001892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Instituto Apoyo. Evaluacion de impacto y sostenibilidad de los proyectos de foncodes. Instituto Apoyo, Tegucigalpa. [Internet]. Instituto Apoyo, Tegucigalpa. 2000. [cited 2021 Feb 8]. Available from: https://fdocuments.ec/document/sexta-evaluacin-expost-del-foncodes-world-rubio-en-la-definicin-del-diseo.html?page=7 [Google Scholar]
- 50.Galiani S, Gertler P, Schargrodsky E. Water for life: The impact of the privatization of water services on child mortality. J Polit Econ. 2005;113(1):83–120. [Google Scholar]
- 51.Granados C, Sánchez F. Water reforms, decentralization and child mortality in Colombia, 1990–2005. World Dev. 2014;53:68–79. [Google Scholar]
- 52.Gebre T, Ayele B, Zerihun M, House JI, Stoller NE, Zhou Z, et al. Latrine Promotion for Trachoma: Assessment of Mortality from a Cluster-Randomized Trial in Ethiopia. Am J Trop Med Hyg. 2011. Sep 1;85(3):518–523. doi: 10.4269/ajtmh.2011.10-0720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clasen T, Boisson S, Routray P, Torondel B, Bell M, Cumming O, et al. Effectiveness of a rural sanitation programme on diarrhoea, soil-transmitted helminth infection, and child malnutrition in Odisha, India: a cluster-randomised trial. Lancet Glob Health. 2014. Nov 1;2(11):e645–e653. doi: 10.1016/S2214-109X(14)70307-9 [DOI] [PubMed] [Google Scholar]
- 54.Crump JA, Otieno PO, Slutsker L, Keswick BH, Rosen DH, Hoekstra RM, et al. Household based treatment of drinking water with flocculant-disinfectant for preventing diarrhoea in areas with turbid source water in rural western Kenya: cluster randomised controlled trial. BMJ. 2005;331(7515):478. doi: 10.1136/bmj.38512.618681.E0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Emerson PM, Lindsay SW, Alexander N, Bah M, Dibba SM, Faal HB, et al. Role of flies and provision of latrines in trachoma control: cluster-randomised controlled trial. Lancet. 2004;363(9415):1093–1098. doi: 10.1016/S0140-6736(04)15891-1 [DOI] [PubMed] [Google Scholar]
- 56.Lule JR, Mermin J, Ekwaru JP, Malamba S, Downing R, Ransom R, et al. Effect of home based water chlorination and safe storage on diarrhea among persons with human immonodeficiency virus in Uganda. Am J Trop Med Hyg. 2005;73(5):926–933. [PubMed] [Google Scholar]
- 57.Jain S, Sahanoon OK, Blanton E, Schmitz A, Wannemuehler KA, Hoekstra RM, et al. Sodium dichloroisocyanurate tablets for routine treatment of household drinking water in periurban Ghana: a randomized controlled trial. Am J Trop Med Hyg. 2010;82(1):16. doi: 10.4269/ajtmh.2010.08-0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ercumen A, Naser AM, Unicomb L, Arnold BF, Colford JMJ, Luby SP. Effects of Source versus Household Contamination of Tubewell Water on Child Diarrhea in Rural Bangladesh: A Randomized Controlled Trial. PLoS ONE. 2015. Mar 27;10(3):e0121907. doi: 10.1371/journal.pone.0121907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boisson S, Kiyombo M, Sthreshley L, Tumba S, Makambo J, Clasen T. Field Assessment of a Novel Household-Based Water Filtration Device: A Randomised, Placebo-Controlled Trial in the Democratic Republic of Congo. PLoS ONE. 2010. Sep 10;5(9):e12613. doi: 10.1371/journal.pone.0012613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peletz R, Simunyama M, Sarenje K, Baisley K, Filteau S, Kelly P, et al. Assessing water filtration and safe storage in households with young children of HIV-positive mothers: a randomized, controlled trial in Zambia. Am J Trop Med Hyg. 2012;101(3):555–565. doi: 10.1371/journal.pone.0046548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morris JF, Murphy J, Fagerli K, Schneeberger C, Jaron P, Moke F, et al. A Randomized Controlled Trial to Assess the Impact of Ceramic Water Filters on Prevention of Diarrhea and Cryptosporidiosis in Infants and Young Children—Western Kenya, 2013. Am J Trop Med Hyg. 2018. May;98(5):1260–1268. doi: 10.4269/ajtmh.17-0731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Conroy RM, Meegan ME, Joyce T, McGuigan K, Barnes J. Solar disinfection of water reduces diarrhoeal disease: an update. Arch Dis Child. 1999;81(4):337–338. doi: 10.1136/adc.81.4.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.du Preez M, Conroy RM, Ligondo S, Hennessy J, Elmore-Meegan M, Soita A, et al. Randomized intervention study of solar disinfection of drinking water in the prevention of dysentery in Kenyan children aged under 5 years. Environ Sci Technol. 2011;45(21):9315–9323. doi: 10.1021/es2018835 [DOI] [PubMed] [Google Scholar]
- 64.Luby SP, Agboatwalla M, Painter J, Altaf A, Billhimer WL, Hoekstra RM. Effect of Intensive Handwashing Promotion on Childhood Diarrhea in High-Risk Communities in Pakistan: A Randomized Controlled Trial. JAMA. 2004. Jun 2;291(21):2547–2554. doi: 10.1001/jama.291.21.2547 [DOI] [PubMed] [Google Scholar]
- 65.Bowen A, Agboatwalla M, Luby S, Tobery T, Ayers T, Hoekstra RM. Association between intensive handwashing promotion and child development in Karachi, Pakistan: a cluster randomized controlled trial. Arch Pediatr Adolesc Med. 2012;166(11):1037–1044. doi: 10.1001/archpediatrics.2012.1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nicholson JA, Naeeni M, Hoptroff M, Matheson JR, Roberts AJ, Taylor D, et al. An investigation of the effects of a hand washing intervention on health outcomes and school absence using a randomised trial in Indian urban communities. Trop Med Int Health. 2014;19(3):284–292. doi: 10.1111/tmi.12254 [DOI] [PubMed] [Google Scholar]
- 67.Abou-Ali H, El-Azony H, El-Laithy H, Haughton J, Khandker S. Evaluating the impact of Egyptian social fund for development programmes. J Dev Eff. 2010;2(4):521–555. [Google Scholar]
- 68.Reese H, Routray P, Torondel B, Sclar G, Delea MG, Sinharoy SS, et al. Design and rationale of a matched cohort study to assess the effectiveness of a combined household-level piped water and sanitation intervention in rural Odisha, India. BMJ Open. 2017;7(3):e012719. doi: 10.1136/bmjopen-2016-012719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shuval HI, Tilden RL, Perry BH, Grosse RN. Effect of investments in water supply and sanitation on health status: a threshold-saturation theory. Bull World Health Organ. 1981;59(2):243. [PMC free article] [PubMed] [Google Scholar]
- 70.White H, Menon R, Waddington H. Community-driven development: Does it build social cohesion or infrastructure? A mixed-method evidence synthesis. Working Paper 30. International Initiative for Impact Evaluation, New Delhi; 2018. [Google Scholar]
- 71.Otu A, Nsutebu EF, Hirst JE, Thompson K, Walker K, Yaya S. How to close the maternal and neonatal sepsis gap in sub-Saharan Africa. BMJ Glob Health. 2020. Apr 1;5(4):e002348. doi: 10.1136/bmjgh-2020-002348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cumming O, Arnold BF, Ban R, Clasen T, Esteves Mills J, Freeman MC, et al. The implications of three major new trials for the effect of water, sanitation and hygiene on childhood diarrhea and stunting: a consensus statement. BMC Med. 2019. Aug 28;17(1):173. doi: 10.1186/s12916-019-1410-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–222. doi: 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 74.Curtis V, Cairncross S. Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. Lancet Infect Dis. 2003;3(5):275–281. doi: 10.1016/s1473-3099(03)00606-6 [DOI] [PubMed] [Google Scholar]
- 75.Cairncross S, Blumenthal U, Kolsky P, Moraes L, Tayeh A. The public and domestic domains in the transmission of disease. Trop Med Int Health. 1996;1(1):27–34. doi: 10.1046/j.1365-3156.1996.d01-9.x [DOI] [PubMed] [Google Scholar]
- 76.Butz WP, Habicht JP, DaVanzo J. Environmental factors in the relationship between breastfeeding and infant mortality: the role of sanitation and water in Malaysia. Am J Epidemiol. 1984;119(4):516–525. doi: 10.1093/oxfordjournals.aje.a113769 [DOI] [PubMed] [Google Scholar]
- 77.Clasen T, Schmidt WP, Rabie T, Roberts I, Cairncross S. Interventions to improve water quality for preventing diarrhoea: systematic review and meta-analysis. BMJ. 2007. Apr 12;334(7597):782. doi: 10.1136/bmj.39118.489931.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kawata K. Water and other environmental interventions—the minimum investment concept. Am J Clin Nutr. 1978;31(11):2114–2123. doi: 10.1093/ajcn/31.11.2114 [DOI] [PubMed] [Google Scholar]
- 79.Gautam OP, Schmidt WP, Cairncross S, Cavill S, Curtis V. Trial of a Novel Intervention to Improve Multiple Food Hygiene Behaviors in Nepal. Am J Trop Med Hyg. 2017;96(6):1415–1426. doi: 10.4269/ajtmh.16-0526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kunz R, Oxman AD. The unpredictability paradox: review of empirical comparisons of randomised and non-randomised clinical trials. BMJ. 1998;317(7167):1185–1190. doi: 10.1136/bmj.317.7167.1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Allcott H. Site Selection Bias in Program Evaluation. Q J Econ. 2015;130(3):1117–1165. [Google Scholar]
- 82.Esrey SA, Potash JB, Roberts L, Shiff C. Effects of improved water supply and sanitation on ascariasis, diarrhoea, dracunculiasis, hookworm infection, schistosomiasis, and trachoma. Bull World Health Organ. 1991;69(5):609. [PMC free article] [PubMed] [Google Scholar]
- 83.Clasen TF, Alexander KT, Sinclair D, Boisson S, Peletz R, Chang HH, et al. Interventions to improve water quality for preventing diarrhoea. Cochrane Database Syst Rev. 2015;10. doi: 10.1002/14651858.CD004794.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hunter PR. Household water treatment in developing countries: comparing different intervention types using meta-regression. Environ Sci Technol. 2009;43(23):8991–8997. doi: 10.1021/es9028217 [DOI] [PubMed] [Google Scholar]
- 85.Wolf J, Prüss-Ustün A, Cumming O, Bartram J, Bonjour S, Cairncross S, et al. Systematic review: assessing the impact of drinking water and sanitation on diarrhoeal disease in low-and middle-income settings: systematic review and meta-regression. Trop Med Int Health. 2014;19(8):928–942. [DOI] [PubMed] [Google Scholar]
- 86.Freeman MC, Stocks ME, Cumming O, Jeandron A, Higgins JP, Wolf J, et al. Systematic review: hygiene and health: systematic review of handwashing practices worldwide and update of health effects. Trop Med Int Health. 2014;19(8):906–916. [DOI] [PubMed] [Google Scholar]
- 87.Wolf J, Hunter PR, Freeman MC, Cumming O, Clasen T, Bartram J, et al. Impact of drinking water, sanitation and handwashing with soap on childhood diarrhoeal disease: updated meta-analysis and meta-regression. Trop Med Int Health. 2018;23(5):508–525. doi: 10.1111/tmi.13051 [DOI] [PubMed] [Google Scholar]
- 88.Ross I, Bick S, Ayieko P, Dreibelbis R, Allen E, Freeman M, et al. Impact of handwashing with soap on acute respiratory infections in low- and middle-income countries–a systematic review [Internet]. PROSPERO CRD42021231414; 2021. [cited 2022 Jul 25]. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021231414 [Google Scholar]
- 89.Morris SS, Black RE, Tomaskovic L. Predicting the distribution of under-five deaths by cause in countries without adequate vital registration systems. Int J Epidemiol. 2003;32(6):1041–1051. doi: 10.1093/ije/dyg241 [DOI] [PubMed] [Google Scholar]
- 90.Benova L, Cumming O, Campbell OM. Systematic review and meta-analysis: association between water and sanitation environment and maternal mortality. Trop Med Int Health. 2014;19(4):368–387. doi: 10.1111/tmi.12275 [DOI] [PubMed] [Google Scholar]
- 91.Institute for Resource Development. An assessment of DHS-I data quality. Institute for Resource Development/Macro Systems, Incorporated, Westinghouse, Columbia MD; 1990. [Google Scholar]
- 92.Pullum T, Becker S. Evidence of omission and displacement in DHS birth histories: methodological reports vol. 11. Rockv ICF Int. 2014 [Google Scholar]
- 93.Schmidt WP, Cairncross S, Barreto ML, Clasen T, Genser B. Recent diarrhoeal illness and risk of lower respiratory infections in children under the age of 5 years. Int J Epidemiol. 2009;38(3):766–772. doi: 10.1093/ije/dyp159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pickering AJ, Null C, Winch PJ, Mangwadu G, Arnold BF, Prendergast AJ, et al. The WASH Benefits and SHINE trials: interpretation of WASH intervention effects on linear growth and diarrhoea. Lancet Glob Health. 2019;7(8):e1139–e1146. doi: 10.1016/S2214-109X(19)30268-2 [DOI] [PubMed] [Google Scholar]
- 95.Howard G, Bartram J, Brocklehurst C, Colford JM, Costa F, Cunliffe D, et al. COVID-19: urgent actions, critical reflections and future relevance of ‘WaSH’: lessons for the current and future pandemics. J Water Health. 2020;18(5):613–630. doi: 10.2166/wh.2020.162 [DOI] [PubMed] [Google Scholar]
- 96.Cairncross S, Cliff JL. Water use and health in Mueda, Mozambique. Trans R Soc Trop Med Hyg. 1987. Jan 1;81(1):51–54. doi: 10.1016/0035-9203(87)90280-x [DOI] [PubMed] [Google Scholar]
- 97.Churchill AA, de Ferranti D, Roche R, Tager C, Walters A, Yazer A. Rural water supply and sanitation: time for a change. World Bank; 1987. [Google Scholar]
- 98.Macura B, Dickin S, Sharma Waddington H, Liera C, Soto A, Orlando A, et al. Gender and social outcomes of WASH interventions: synthesis of research evidence [Internet]. CEDIL Syntheses Working Paper 7, CEDIL, Oxford; 2023. [cited 2023 Mar 17]. Available from: 10.51744/CSWP7 [DOI] [Google Scholar]
- 99.Moher D. CONSORT: an evolving tool to help improve the quality of reports of randomized controlled trials. JAMA. 1998;279(18):1489–1491. [DOI] [PubMed] [Google Scholar]
- 100.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother. 2010;1(2):100–107. doi: 10.4103/0976-500X.72352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bose R. CONSORT Extensions for Development Effectiveness: guidelines for the reporting of randomised control trials of social and economic policy interventions in developing countries. Journal of Development Effectiveness, 2010;2(1);173–186. doi: 10.1080/19439341003624441 [DOI] [Google Scholar]
- 102.Kremer M, Luby S, Maertens R, Tan B. Water treatment and child mortality: a systematic review and meta-analysis [Internet]. [cited 2022 Jul 26]. Available from: https://cpb-us-w2.wpmucdn.com/voices.uchicago.edu/dist/0/2830/files/2022/02/Water-meta-analysis-manuscript-2022.02.20.docx.pdf
- 103.Gallandat K, Cumming O. Impact Evaluation of Urban Water Supply Improvements on Cholera and Other Diarrhoeal in Uvira, Democratic Republic of Congo [Internet]. clinicaltrials.gov; 2022. Aug [cited 2022 Sep 15]. Report No.: NCT02928341. Available from: https://clinicaltrials.gov/ct2/show/NCT02928341 [Google Scholar]
- 104.Cha S, Kang D, Tuffuor B, Lee G, Cho J, Chung J, et al. The Effect of Improved Water Supply on Diarrhea Prevalence of Children under Five in the Volta Region of Ghana: A Cluster-Randomized Controlled Trial. Int J Environ Res Public Health. 2015. Oct;12(10):12127–12143. doi: 10.3390/ijerph121012127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Devoto F, Duflo E, Dupas P, Parienté W, Pons V. Happiness on tap: Piped water adoption in urban Morocco. Am Econ J Econ Policy. 2012;4(4):68–99. [Google Scholar]
- 106.Waddington HS, Villar PF, Valentine JC. Can Non-Randomised Studies of Interventions Provide Unbiased Effect Estimates? A Systematic Review of Internal Replication Studies. Eval Rev. 2022:0193841X221116721. doi: 10.1177/0193841X221116721 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Table A. The global burden of infectious disease is mainly due to mortality (per 100,000). Table B. Global Burden of Disease: A moving target? Table C. Description of studies included in this systematic review by WASH technology. Table D. Meta-regression analysis of diarrhoea mortality in childhood by WASH intervention technology. Fig A. All-cause mortality. Fig B. All-cause mortality: Water supply interventions. Fig C. All-cause mortality: Sanitation interventions. Fig D. All-cause mortality: Hygiene interventions. Fig E. All-cause mortality: Drinking water treatment and storage interventions. Fig F. Diarrhoea mortality. Fig G. Effects on all-cause mortality for participants aged over 5 years. Fig H. Funnel graphs with regression lines: All-cause mortality. Fig I. Funnel graphs with regression lines: Diarrhoea mortality. Fig J. Effects on all-cause mortality for nonrandomised studies of interventions at “high risk of bias.” Fig K. Effects on all-cause mortality for randomised controlled trials at “high risk of bias.”
(DOCX)
(DTA)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.