Abstract
Introduction:
There are limited data available on the long-term presence of SARS-CoV-2-specific binding antibodies and neutralizing antibodies in circulation among the elderly population. This study aims to examine levels of anti-SARS-CoV-2 antibodies in vaccines who have completed at least 6 months since the second vaccine dose. A cross-sectional study was conducted from November 2021 to January 2022 among 199 vaccines aged 60 years and above residing in Belagavi city, who received two doses of the Covishield vaccine.
Methods:
Antibody response to SARS-COV-2 virus whole cell antigen was measured by a kit COVID KAWACH IgG Micro LISA (J Mitra and Company, India) in 199 participants who had completed at least 6 months after receiving the second dose of Covishield vaccine. The antibody response was measured as a ratio of optical density (OD) in the participant’s sample to the mean OD in negative control test by normal (T/N). Independent Kruskal–Wallis test was applied to test the difference between the T/N ratio by months of vaccination since the second dose and by the age group strata.
Results:
The median T/N values among participants who completed 6, 7, 8, and 9 months since the second vaccine dose were 14.17, 10.46, 7.93, and 5.11, respectively, and this decline in T/N values was statistically significant. Antibody response values showed a decline with increasing age for participants in the age strata 60–69, 70–79, and 80 and above, respectively.
Conclusions:
A significant decline was observed in antibody response over 9 months supporting the administration of booster dose of vaccine.
Keywords: Antibody waning, booster dose, COVID-19 vaccine, elderly population, immunoglobulin G antibodies, SARS-CoV-2
INTRODUCTION
India has the second highest number of COVID-19 cases reported globally. The aging population is at more risk of developing severe disease.[1-3]
The antibody levels in persons convalescing from natural infection and those who received two doses of BNT162b2 vaccination showed a decline in antibody response after 6 months, particularly among men above 65 years.[4-6]
Government of India initiated as a precaution administration of booster dose for senior citizens from January 10, 2022.[7] There is a paucity of data on longevity of antibody response in the Indian population. The present study examined levels of anti-SARS-CoV-2 antibodies in vaccinees who have completed at least 6 months since the second vaccine dose.
Covishield is a single recombinant, replication-deficient chimpanzee adenovirus vector encoding the S glycoprotein of the SARS-CoV-2 vaccine. SARS-CoV-2 S glycoprotein is expressed locally after administration, stimulating neutralizing antibody, and cellular immune responses.[8]
METHODS
A cross-sectional study was conducted from November 2021 to January 2022.
The sample size was calculated using the formula n = Z2 pq/d2 where p = Proportion of people above 60 years who were vaccinated, with the precision of 10% and 95% of confidence interval. Assuming 10% attrition the calculated sample size was 182.
Study participants and data collection
Four hundred and seventy-three individuals who had registered for vaccination were approached. Of these 114 had not taken the second vaccine dose and 136 had not completed 6 months after the second vaccine dose. One participant reported COVID-19 positivity as a result of breakthrough infection and 24 did not give consent for participation and hence did not meet the eligibility criteria. Therefore, 199 senior citizens aged 60 years and above residing in Belagavi city, Karnataka, India, who received two doses of COVID vaccine (Covishield) at least 6 months before recruitment were enrolled in the study. None of the participants were on immunosuppressive therapy.
A list of fully vaccinated people above 60 years of age was obtained from Primary health Centre of Rukmini Nagar, Ashok Nagar, and Vantamuri in Belagavi. Participants were informed over the telephone about the study. Home visits were scheduled; vaccination status and eligibility for enrolment were confirmed. After obtaining written informed consent, demographic data were collected and a 2 ml blood sample was withdrawn and sent to the laboratory on the same day.
Laboratory procedure
Clear serum sample was separated after centrifugation of the coagulated blood and stored at − 20°C till use. Antibody response to SARS-CoV-2 virus whole cell antigen was measured by commercially available kit COVID KAWACH IgG Micro LISA (J Mitra and Company, India) with reported specificity of 100% and sensitivity of 96.33%. In brief, 100 ml of 1 in 100 dilution of serum was incubated in a microwell plate coated with SARS-CoV-2 virus whole-cell antigen. After incubation at 37°C for 60 min, plates were washed and 100 ml of anti-human Immunoglobulin G (IgG) enzyme conjugate was added to the well and incubated for 60 min at 37°C. The plate was then washed and 100 ml of 3,3’,5,5’- Tetramethylbenzidine substrate was added to the well and incubated in dark at room temperature for 10 min. The reaction was stopped after 10 min by adding 100 ml of stop solution (1N sulfuric acid). The resultant color was measured in spectrophotometer at 450 nm (EPOCH, Biotech, USA). The assay was considered valid when the ratio of optical density (OD) in positive control and average OD in negative control was above 1.5. The result of each sample was expressed as the ratio of OD in an experimental sample (T) and OD value in the negative control (N). The T/N value was taken as a surrogate for antibody level.
Statistical analysis
Independent Kruskal–Wallis test was applied to test the difference between T/N ratio by months of vaccination since the second dose and by the age group strata.
RESULTS
The mean age of 199 enrolled eligible participants was 69.20 ± 6.53 years, more than half of the participants were in the age group of 60–70 years (56.3%) and were male (53.3%) [Table 1].
Table 1.
Demographic profile of the respondents
| Sociodemographic variables | n (%) |
|---|---|
| Gender | |
| Male | 106 (53.3) |
| Female | 93 (46.7) |
| Age group (years) | |
| 60-70 | 112 (56.3) |
| 70-80 | 74 (37.2) |
| ≥80 | 13 (6.5) |
| Religion | |
| Hindu | 190 (95.5) |
| Muslim | 4 (2.0) |
| Christian | 1 (0.5) |
| Jain | 4 (2.0) |
| Total | 199 (100.0) |
Among the participants included for analysis, 30 (15.1%), 35 (17.6%), 94 (47.2%), and 40 (20.1%) had completed 6, 7, 8, and 9 months, respectively, since the second dose of vaccination. T/N values of the participants were compared between different groups. Participants who had completed 6 months since the last vaccine dose showed the highest T/N value (median 14.17) followed by median values of 10.46, 7.93, and 5.18 on completion of 7, 8, and 9 months and beyond, respectively. A steady decline was observed in the median T/N ratio from 6 to 9 months and the month-wise difference in the T/N ratio was statistically significant and the P = 0.038 [Figure 1]. On post hoc test comparison, the difference in T/N ratio was found to be statistically significant between 6 and 9 months and the P = 0.033.
Figure 1.
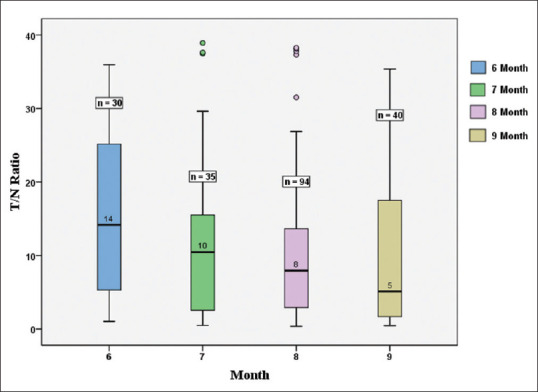
Month-wise T/N ratio distribution. T/N: Test by normal
Analysis by the age group showed although there was a decline in the mean and median values from the 60 to 70 years age group to the group above 80 years of age, the differences were not found to be significant [Table 2 and Figure 2].
Table 2.
Age group-wise T/N ratio distribution
| T/N ratio | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Age group | T/N ratio | ||||||
|
| |||||||
| n | Mean±SD | Median | Minimum | Maximum | Range | P | |
| 60-70 | 112 | 12.02±10.94 | 8.56 | 0.45 | 38.91 | 38.46 | 0.432 |
| 70-80 | 74 | 10.91±9.72 | 8.90 | 0.48 | 37.79 | 37.31 | |
| ≥80 | 13 | 7.19±5.22 | 7.48 | 0.36 | 19.96 | 19.60 | |
| Total | 199 | 11.29±10.25 | 8.03 | 38.55 | |||
SD: Standard deviation, T/N: Test by normal
Figure 2.
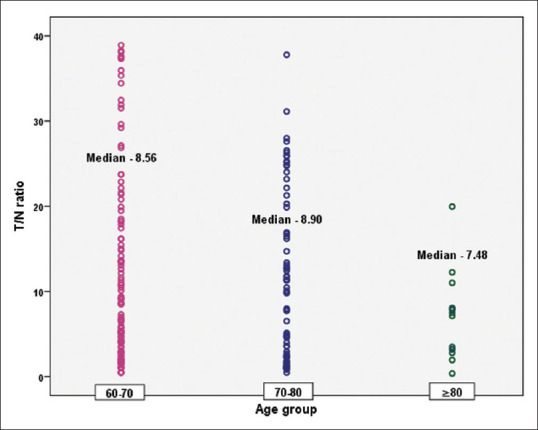
Scatter plot on antibody waning among different age group vaccinees
When comparing between genders, women had a more robust response (Median T/N value = 9.90) compared with men (Median T/N value = 6.48). However, the difference was not statistically significant.
DISCUSSION
Anti-SARS-CoV-2 vaccines were developed and made available to populations at a remarkable speed. Aging is accompanied by the decline in the function of lymphoid and nonlymphoid tissues involved in the host immune response.[3] However, a better understanding of the impact of age and age-related factors on the magnitude and durability of vaccine-induced immune responses is still evolving. The data will help to guide public health decisions about COVID-19 vaccine allocation. Although the Government of India has initiated a booster dose after 9 months of the second vaccine dose as a precaution, there is a lack of data on the sustenance of immune response post-vaccination. To the best of our knowledge, this is the first community-based study in India on SARS-CoV-2 vaccine-induced immune responses in the elderly population.
A study conducted in the UK reported that the waning of vaccine effectiveness was greater among 65+ years old compared to 40–64- years olds.[9] Another study conducted in Italy compared vaccine response among COVID-infected and naive subjects. It reported that antibodies generated after two doses of BTN162b2 vaccination rapidly diminished after 15 weeks, especially in infection-naive subjects.[10]
A longitudinal cohort study was conducted among 614 vaccinated health-care workers from three different districts of Odisha aimed to determine the dynamicity of vaccine-induced IgG antibodies against SARS-CoV-2. The results indicated that the antibody levels in the Covaxin and Covishield recipients dropped significantly after 2 months and 4 months, respectively. Age, gender, comorbidities, and blood types had no statistically significant effect on antibody titers.[11]
The present study findings showed waning of immunity levels in higher age groups and this finding is in agreement with past findings in the UK, where vaccination effectiveness against alpha and delta variants were evaluated over time after the two doses of Comirnaty, Vaxzevria, and Spikevax. The results indicated that waning was common in older adults and those in a clinical risk group, implying that these people should be given priority for booster doses.[9]
Results of this study showed a decline in response after 8 months, especially those in higher age groups, although the differences are not significant. This may be due to the outliers. The present data support the need for a booster dose after 9 months, especially in the older population that is at greater risk of developing severe disease.
Despite rigorous screening and implementation of lockdowns, there was a high prevalence of asymptomatic cases in the community.[12] It is possible that undetected asymptomatic infections are driving COVID-19 transmission in the community.[12-16] Some of the participants may have developed asymptomatic breakthrough infections resulting in higher responses. However, it is not possible to eliminate this confounder. This may be a limitation of this study. We have used the T/N ratio of optical densities as a surrogate marker for the quantitation of immune response. This may introduce some misclassification of quantitation. Another limitation was nonavailability of seroconversion data since the participants were vaccinated under the national program and it did not have a provision of testing for seroconversion after vaccination. Furthermore, the study’s strength is that it is a community-based study.
Hesitancy and resistance toward vaccination were the major reasons among 65 years and above elderly population for delayed acceptance of vaccines. Post-vaccination adverse effects, the adverse impact of the information on vaccines in the public domain, and the presence of comorbidities may be reasons for vaccine hesitancy. It was noted that about quarter of the individuals approached for the study had not taken the stipulated second vaccine dose.[17] There is a need for efforts to ensure the administration of both doses through the public health system.
CONCLUSIONS
The present data suggest that a booster dose is desirable after the completion of 9 months from the second dose of the COVID-19 vaccine, especially in higher age groups.
Research quality and ethics statement
This study was approved by the Institutional Review Board/Ethics Committee (JNMC Institutional Ethics Committee). The authors followed applicable EQUATOR Network (https://www.equator-network.org/) guidelines during the conduct of this research project.
Financial support and sponsorship
The study was supported by intramural funding by KAHER.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Coronavirus Disease (COVID-19) Situation Reports. [Last accessed on 2022 Apr 22]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports .
- 2.WHO Coronavirus (COVID-19) Dashboard. [Last accessed on 2022 Apr 30]. Available from: https://covid19.who.int .
- 3.Bajaj V, Gadi N, Spihlman AP, Wu SC, Choi CH, Moulton VR. Aging, immunity, and COVID-19: How age influences the host immune response to coronavirus infections? Front Physiol. 2020;11:571416. doi: 10.3389/fphys.2020.571416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall V, Foulkes S, Insalata F, Kirwan P, Saei A, Atti A, et al. Protection against SARS-CoV-2 after COVID-19 vaccination and previous infection. N Engl J Med. 2022;386:1207–20. doi: 10.1056/NEJMoa2118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fill Malfertheiner S, Brandstetter S, Roth S, Harner S, Buntrock-Döpke H, Toncheva AA, et al. Immune response to SARS-CoV-2 in health care workers following a COVID-19 outbreak: A prospective longitudinal study. J Clin Virol. 2020;130:104575. doi: 10.1016/j.jcv.2020.104575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guidelines for COVID19 Vaccination of Children between 15 to 18 years and Precaution Dose to HCWs FLWs &60 Population with Comorbidities. [Last accessed on 2022 Apr 30]. Available from: https://www.mohfw.gov.in/pdf/Guidelines for COVID19 Vaccination of Children between 15 to 18 years and Precaution Dose to HCWs FLWs &60 population with comorbidities.pdf .
- 8. [Last accessed on 2022 Apr 27]. Available from; https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/en/Smpcserum.pdf .
- 9.Andrews N, Tessier E, Stowe J, Gower C, Kirsebom F, Simmons R, Gallagher E, Chand M, Brown K, Ladhani SN, Ramsay M. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID-19 in the UK. medrxiv 2021 Sep 21 2021-09 [Google Scholar]
- 10.Siracusano G, Ruggiero A, Bisoffi Z, Piubelli C, Carbonare LD, Valenti MT, et al. Different decay of antibody response and VOC sensitivity in naïve and previously infected subjects at 15 weeks following vaccination with BNT162b2. J Transl Med. 2022;20:22. doi: 10.1186/s12967-021-03208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choudhary HR, Parai D, Chandra Dash G, Kshatri JS, Mishra N, Choudhary PK, et al. Persistence of antibodies against spike glycoprotein of SARS-CoV-2 in healthcare workers post double dose of BBV-152 and AZD1222 vaccines. Front Med (Lausanne) 2021;8:778129. doi: 10.3389/fmed.2021.778129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta M, Mohanta SS, Rao A, Parameswaran GG, Agarwal M, Arora M, et al. Transmission dynamics of the COVID-19 epidemic in India and modeling optimal lockdown exit strategies. Int J Infect Dis. 2021;103:579–89. doi: 10.1016/j.ijid.2020.11.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavezzo E, Franchin E, Ciavarella C, Cuomo-Dannenburg G, Barzon L, Del Vecchio C, et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo'. Nature. 2020;584:425–9. doi: 10.1038/s41586-020-2488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A Review. JAMA. 2020;324:782–93. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 15.Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar N, Shahul Hameed SK, Babu GR, Venkataswamy MM, Dinesh P, Kumar Bg P, et al. Descriptive epidemiology of SARS-CoV-2 infection in Karnataka state, South India: Transmission dynamics of symptomatic versus asymptomatic infections. EClinicalMedicine. 2021;32:100717. doi: 10.1016/j.eclinm.2020.100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umakanthan S, Patil S, Subramaniam N, Sharma R. COVID-19 vaccine hesitancy and resistance in India explored through a population-based longitudinal survey. Vaccines (Basel) 2021;9:1064. doi: 10.3390/vaccines9101064. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


