Annually, approximately one out of every 20 inpatients or an estimated 1.7 million individuals experience a hospital-acquired infection (HAI) in the US. In practice, this means that 5%–10% of admitted patients will develop an HAI and approximately 100,000 of these patients will die.[1,2] The estimated incidence of HAIs exceeds that of many other reportable diseases in the US, and the number of HAI-related deaths is greater than many of the leading causes of mortality.[1]
Yearly estimated costs attributable to HAIs in the US are between $35B and $88B, excluding the associated morbidity and mortality.[3] In 2014, the Hospital-Acquired Condition Reduction Program was created to reduce HAI rates. It has allowed the Centers for Medicare and Medicaid Services (CMS) to introduce financial accountability measures for institutions that report the lowest quartile performance in hospital-acquired conditions. When CMS pays a claim, such low-performing hospitals lose 1% of their Medicare payments for a 12-month period. These pay-for-performance programs are substantial, and in 2017 alone, 742 hospitals paid in $385 million in cumulative penalties.[4] Consequently, hospitals are constantly seeking to reduce their HAI rates to avoid penalties.
HAIs are typically categorized into 13 major types. The three most prevalent types include surgical site infections (SSI), urinary tract infections, and gastrointestinal infections. These major categories account for 26.9%, 22.3%, and 17.4% of all HAIs, respectively.[1,2] Although it has been historically accepted that pathogens responsible for HAIs originate primarily from surfaces, the recent literature indicates that airborne microbial burden constitutes a significant portion of the overall pathogens responsible for HAIs.[5] Of the pathogens responsible for SSIs, nearly 70% are potentially airborne.[6] These airborne pathogens are difficult to address effectively through traditional infection control protocols that have previous success in reducing HAI rates.
There are several national air filtration standards for hospitals that are applicable depending on the specific institutional/facility protocols.[7-10] The air delivered to clinical areas typically consists of a mixture of external and recirculated air. The exact composition of the delivered air varies depending on the particular clinical area. For example, the American National Standards Institute/American Society of Heating, Refrigerating, and Air-Conditioning Engineers/American Society for Healthcare Engineering Standard 170–2017, Ventilation of Health-care Facilities, requires a minimum total of 20 air changes per hour (ACH) for an operating room (OR) with a minimum of four outdoor ACH.[10] Externally sourced air brings in location-specific contamination, including microbial and chemical contaminants. Densely populated areas are characterized by higher contaminant loads compared to less populated locations. In addition, recirculated air, recycled from the space, typically has higher levels of biological and chemical contaminants than external air. It is important to note that even properly gowned health-care workers and patients have been documented to shed between 3,000 and 50,000 microorganisms per minute, which is equivalent to 0.12 colony forming units per cubic meter per minute.[6,11] An estimated 10% of the microorganisms shed have the potential to be infectious.[6] Based on this shedding rate, the standard OR would surpass the recommended microbial load for this space in as little as 10 min.[6,11] Airborne pathogens are among key contributors to SSIs. The very nature of an OR with many ACH, laminar flow, and constant personnel movement facilitates airborne pathogen sustainment. These airborne pathogens can remain viable and may circulate through the space for days or even weeks, depending on the unique characteristics of the specific infectious agent. It is vital to remediate all harmful pathogens in both the external and recirculated air to reduce the constant and high level of airborne microbes typically present in ORs.
Hospitals often use high-efficiency particulate air (HEPA) filters to process the air entering critical spaces. They are designed to remove particulates and reduce the levels of biological contamination within the protected space.[2] Because HEPA devices are designed to capture particulates, any retained particulates are likely to remain inside the filter matrix and the viable particulates (bacteria, viruses, and mold/fungi) may continue to grow and multiply. Subsequently, the force from sustained high air velocities across the filter can cause previously entrapped variables to separate from the HEPA matrix, thereby allowing them to become entrained in the airflow and enter the space.[12] In addition to HEPA filters, the use of ultraviolet germicidal irradiation (UVGI) has been employed in hospitals to further remove airborne pathogens and improve infection rates. Corresponding improvements in infection rates have been demonstrated in multiple studies.[13-15]
The LifeAire System (LAS) (LASs, Allentown, Pennsylvania) is installed within the hospital’s heating, ventilation, and air conditioning (HVAC) system. It replaces a section of ductwork and is located downstream of the air handling unit, which typically contains HEPA filtration. The LAS utilizes UVGI that has been designed and optimized to kill and inactivate infectious biologicals.[5,16] The high-dose UVGI has been mathematically and genomically modeled (and dosed) to destroy the RNA and DNA of infectious pathogens such as tuberculosis, influenza, coronavirus, and other infectious biologicals on a single pass.[5,16] The system design takes into account critical parameters to ensure that all pathogens in the air stream are remediated before entering the protected space, preventing harmful pathogens from coming into contact (and potentially infecting) the occupants. This application of UVGI within the airstream is markedly different from the application of UVGI to the cooling coil to prevent mold growth. Although UVGI applied to the cooling coil is effective in preventing mold growth, it is not capable of the comprehensive, single-pass remediation of airborne pathogens delivered by the LAS.
This technology has been previously installed and studied in the institutional review board-approved studies in an acute care hospital. The LAS was installed in the HVAC ductwork and protected a portion of a medical surgical floor.[5,16] The clinical and microbiological data were collected across three different air filtration zones as shown in Figure 1. The first zone (a) received comprehensive LifeAire remediation, the second zone (b) was an adjacent portion of the medical surgical floor that received a mixture of HEPA and LifeAire remediated return air, and the third zone (c) was a control floor with only HEPA remediation. Zone C was located below the zones A and B and had the same physical layout. All zones had similar standard operating procedures and staffing.[5,16]
Figure 1.
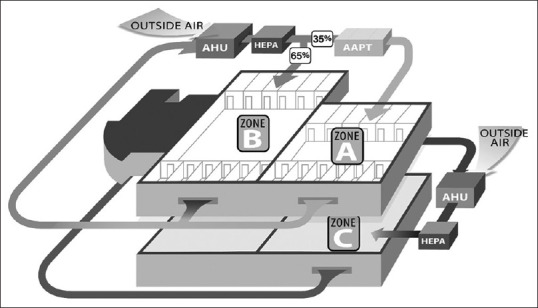
Schematic representing zones A, B, and C and the existing heating ventilation and air-conditioning layout for all zones.[16] Figures reproduced under the terms of byncnd4.0 international license
The environmental data were studied prospectively. Two active and occupied patient rooms were tested in each zone. The environmental testing included volatile organic compound (VOC) testing and airborne and surface bacterial and fungal testing. The airborne bacterial, fungal, and VOC levels decreased from zone C to B to A, which corresponded to the environmental purity in the zones – A was the purest and C the least. Similarly, measured surface pathogens decreased across the three zones.[5]
The key clinical parameters were studied retrospectively and included all surgical patients admitted to any zone who had a Case Mix Index included in their medical record at discharge. Sensitivity analyses excluded all unbalanced nonsurgical and bariatric patients, bringing the final number of studied patients to 1,002. Subsequent statistical analyses demonstrated that hospital charges and length of stay (LOS) decreased as environmental purity increased. There was a statistically significant 39.5% decrease in LOS, as well as 23% estimated cost savings between the zones A and C.[16]
The LAS installation has also been evaluated in a second, independent 15-month study within a long-term care facility’s (LTCF) memory support floor.[17] Two different resident floors were studied; a control floor with HEPA filtration and a study floor with complete LifeAire remediation. Statistical comparisons of HAI rates between the study floors were conducted with airborne and surface pathogen load measurements. There was an 88.43% statistically significant reduction in airborne pathogens on the study floor pre- and post-LifeAire installation with significant reductions in surface pathogens. The HAI rates for the LTCF were analyzed in two ways; first, a prospective comparison was made between the control and study floors during the study, which showed a 39.6% reduction in HAIs. A second retrospective analysis of the study floor pre and postinstallation resulted in a 54.5% HAI reduction on the study floor.
The hospital study has demonstrated that reduced airborne biologicals result in significant improvements in critical clinical metrics, namely, LOS and hospital charges.[16] The LTCF study has further confirmed these results and demonstrated a comparable reduction in HAIs (with surface and airborne pathogens) associated with the LAS installation. These findings can be applied to the OR environment because airborne microorganisms constitute a significant source of pathogens responsible for SSIs. It is further hypothesized that the reduction of airborne pathogens by the LAS within the OR environment may lead to an observed reduction in SSIs. Corresponding clinical studies are needed and warranted. Not only would a successful implementation of such system provide a safe and healthy environment for the patients and staff but it may also yield improvements in critical economic metrics and help hospitals reduce exposure to CMS penalties.
REFERENCES
- 1.Klevens RM, Edwards JR, Richards CL. Estimating health care-associated infections and deaths in U. S. hospitals. Public Health Rep. 2007;122:160–6. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kowalski WJ. Air-treatment systems for controlling hospital-acquired infections. HPAC Eng. 2007;79:2–22. [Google Scholar]
- 3.Scott RD. The Direct Medical Costs of Healthcare-Associated Infections in U. S. Hospitals and the Benefits of Prevention, National Center for Preparedness, Detection and Control of Infectious Diseases, Coordinating Center for Infectious Diseases, CDC. 2009 Mar [Google Scholar]
- 4.Vsevolozhskaya OA, Manz KC, Zephyr PM, Waters TM. Measurement matters: Changing penalty calculations under the hospital acquired condition reduction program (HACRP) cost hospitals millions. BMC Health Serv Res. 2021;21:131. doi: 10.1186/s12913-021-06108-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stawicki SP, Brisendine C, Levicoff L, Ford F, Snyder B, Eid S, et al. Vignettes in Patient Safety. Vol. 4. London: IntechOpen; 2019. [Last accessed on 2022 Mar 22]. Comprehensive and live air purification as a key environmental, clinical, and patient safety factor: A prospective evaluation. Available from: https://doi.org/10.5772/intechopen.84530 . [Google Scholar]
- 6.Kowalski W. Boca Raton, Florida: CRC Press; 2012. Hospital Airborne Infection Control. [Google Scholar]
- 7.American Institute of Architects (AIA). Guidelines for Design and Construction of Hospital and Health Care Facilities. Washington, D. C: American Institute of Architects (AIA); 2001. [Google Scholar]
- 8.Geshwiler M. Howard E, Helms C. Atlanta, GA: American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc; 2003. HVAC Design Manual for Hospitals and Clinics. [Google Scholar]
- 9.Centers for Disease Control (CDC) Guidelines for Environmental Infection Control in Healthcare Facilities. Atlanta, GA: Centers for Disease Control (CDC); 2003. [Google Scholar]
- 10.ANSI/ASHRAE/ASHE Standard 170-2017 Ventilation of Health Care Facilities; American Society of Heating, Refrigeration and Air-Conditioning Engineers: New York, NY, USA. 2017 [Google Scholar]
- 11.Bischoff WE, Tucker BK, Wallis ML, Reboussin BA, Pfaller MA, Hayden FG, et al. Preventing the airborne spread of Staphylococcus aureus by persons with the common cold: Effect of surgical scrubs, gowns, and masks. Infect Control Hosp Epidemiol. 2007;28:1148–54. doi: 10.1086/520734. [DOI] [PubMed] [Google Scholar]
- 12.Price DL, Simmons RB, Crow SA, Jr, Ahearn DG. Mold colonization during use of preservative-treated and untreated air filters, including HEPA filters from hospitals and commercial locations over an 8-year period (1996-2003) J Ind Microbiol Biotechnol. 2005;32:319–21. doi: 10.1007/s10295-005-0226-1. [DOI] [PubMed] [Google Scholar]
- 13.Lowell JD, Kundsin RB, Schwartz CM, Pozin D. Ultra-Violet Radiation and Reduction of Deep Wound Infections Following Hip and Knee Arthroplasty. Airborne Contagion, New York Academy of Sciences. 1980 doi: 10.1111/j.1749-6632.1980.tb18931.x. [DOI] [PubMed] [Google Scholar]
- 14.Brown IW, Jr, Moor GF, Hummel BW, Marshall WG, Jr, Collins JP. Toward further reducing wound infections in cardiac operations. Ann Thorac Surg. 1996;62:1783–9. doi: 10.1016/s0003-4975(96)00566-8. [DOI] [PubMed] [Google Scholar]
- 15.EPRI. UVGI for TB Infection Control in a Hospital. Palo Alto, CA: Electric Power Research Institute; 1997. [Google Scholar]
- 16.Stawicki SP, Wolfe S, Brisendine C, Eid S, Zangari M, Ford F, et al. The impact of comprehensive air purification on patient duration of stay, discharge outcomes, and health care economics: A retrospective cohort study. Surgery. 2020;168:968–74. doi: 10.1016/j.surg.2020.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Kelley KC, Schlener SD, Levicoff L, Stawicki SP. Economic effects of air purification technology on healthcare-acquired infection costs: A study of simulated implementation across low-and-middle-income regions of the globe. Int J Acad Med. 2021;7:285–6. [Google Scholar]


