Abstract
Background
Poly(ADP-ribose) polymerase inhibitors (PARPi) are increasingly used in oncology; their hematological toxicities affect classically red, platelet and neutrophil lineages, but some opportunistic infections have been reported concomitantly to deep lymphopenias.
Objective
This study was designed to provide an external and internal analysis of the crossed impacts of PARPi and age on lymphopenia risk.
Patients and Methods
A scoping review was performed on the PubMed and Embase databases to assess the reporting of lymphocyte rates in original studies on PARPi treatment for adult patients up to 1 April 2022. A retrospective cohort was extracted from the medical charts of all patients treated for gynecological cancer at our institution from 2015 to 2022 in accordance with ethical regulations.
Results
The scoping review research strategy retrieved 5840 abstracts; 225 studies were selected for full-text analysis. Lymphopenia was reported in 41.8% of the studies; frequency of all-grade and grade ≥ 3 lymphopenia reached 20.5% and 8.9%, respectively. Grade ≥ 3 lymphopenia was significantly higher in studies including older patients (median age ≥ 60 years vs. < 60 years), at 7.5% vs. 10.3% (p < 0.0001). PARIB-OLD-HCL included 46 patients, 19 of whom were aged < 70 years (median 44 years) and 27 of whom were aged ≥ 70 years (median 79 years); the frequency of all-grade and grade ≥ 3 lymphopenia reached 67% (< 70 years: 63%; ≥ 70 years: 70%) and 13% (< 70 years: 5%; ≥ 70 years: 19%), respectively.
Conclusion
Lymphopenia events were much more frequent in real-life than in previously reported studies, particularly in older patients. Future work is needed to improve patient follow-up and discuss prophylactic strategies.
Key Points
| Frequency of all-grade and grade ≥ 3 lymphopenia reached 20.5% and 8.9%, respectively. |
| Grade ≥ 3 lymphopenia was significantly higher in studies including older patients. |
Introduction
The poly(ADP-ribose) polymerase (PARP) superfamily of enzymes is involved in the synthesis of ADP-ribose polymers [1], which can be further processed and bound to damaged DNA [2, 3] to enable recruitment of DNA repair effectors. The practical medical implications of such enzymes were only recently proven with the advent of PARP inhibitors (PARPi).
PARPi are small molecules able to trap PARP1 onto damaged DNA, preventing the DNA repair process and leading to cell death, and initially developed to improve the cytotoxic effect of chemotherapy on cancer cells through DNA damage enhancement. Breast cancer (BRCA) type 1/2 proteins are key proteins for the homologous recombination (HR) repair process, allowing the cell to overcome DNA double-strand breaks. In the case of BRCA deficiency, as is the case in some neoplastic cells, alternative repair mechanisms, implying PARPs, are implemented to overcome such damage. PARPi prevent these mechanisms, leading to targeted BRCA-deficient cell death, which is now referred to as synthetic lethality [4, 5].
The impressive results of olaparib (first-in-class oral PARPi) in germline BRCA1/2-mutated ovarian, breast, pancreatic and prostate cancers, led to subsequent US FDA approval in several indications [6, 7]. Since then, various other PARPi have been approved, in a wider range of cancers, both in HR-deficient and HR-proficient settings [8–11]. Although PARPi were found to have an acceptable toxicity profile, some common class effects were reported, such as hematological toxicities [12]. Among these, anemia was the most frequently reported adverse event (AE), followed by thrombocytopenia and neutropenia [12]. Such toxicity was predictable since various studies demonstrated that hematopoietic stem cells (HSC) require PARPs to ensure their hematopoietic functions and proper renewal [13, 14]. Having been exposed in our practice to iterative Pneumocystis pneumonia in an octagenarian while under PARPi treatment, we revealed that these events arose concomitantly to deep lymphopenia events.
A very similar case was reported by Himeji et al. [15]. However, available data on PARPi-induced lymphopenia remain scarce in comparison with that of other blood lineage.
Considering the expected connections between age, PARPi use, and lymphopenia, we decided to explore the available evidence regarding PARPi-induced lymphopenia, particularly in older adults. Given that the current knowledge on PARPi-induced lymphopenia is insufficient to motivate a prospective trial, we decided to realize a comprehensive study on this issue, exploring not only the available evidence in the literature through a scoping review but also real-life data using a single-center retrospective study and a vigilance database. The aim was to provide sound evidence regarding age and PARPi effect on lymphocyte lineage that could be the groundwork of further studies. Leukopenia is frequently arising from neutropenia, which is a known AE of PARPi, and for this reason it was decided to restrict this study to lymphopenia events. In fact, it would have been impossible to determine if a reported leukopenia event resulted from neutropenia, lymphopenia, or both.
Methods
A scoping review of the literature from the PubMed/MEDLINE and Embase databases was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using the following queries: “Poly(ADP-ribose) polymerase inhibitors/therapeutic use*[MeSH Terms] AND (clinicaltrial[Filter] OR randomizedcontrolledtrial[Filter])” and “nicotinamide adenine dinucleotide adenosine diphosphate ribosyltransferase inhibitor AND (‘clinical article’/de OR ‘clinical study’/de OR ‘clinical trial’/de OR ‘clinical trial topic’/de OR ‘comparative effectiveness’/de OR ‘comparative study’/de OR ‘controlled clinical trial’/de OR ‘controlled study’/de OR ‘double blind procedure’/de OR ‘exploratory research’/de OR ‘feasibility study’/de OR ‘major clinical study’/de OR ‘multicenter study’/de OR ‘multicenter study topic’/de OR ‘open study’/de OR ‘phase 1 clinical trial’/de OR ‘phase 1 clinical trial topic’/de OR ‘phase 2 clinical trial’/de OR ‘phase 2 clinical trial topic’/de OR ‘phase 3 clinical trial’/de OR ‘phase 3 clinical trial topic’/de OR ‘preclinical study’/de OR ‘prospective study’/de OR ‘randomized controlled trial’/de OR ‘randomized controlled trial topic’/de OR ‘retrospective study’/de) AND (‘article’/it OR ‘article in press’/it)” using Medical Subject Heading (MeSH) and Emtree terms to 1 April 2022. We included all original studies reporting therapeutical use of PARPi in adult patients and providing information on toxicity. Early clinical studies with a very small sample size (number of patients < 10) were excluded to minimize bias. Regarding the Emtree search engine, it was decided to retrieve every clinical study on PARPi and then to remove those not fulfilling the inclusion criteria (retrospective, ancillary, etc.) to ensure no records were omitted. Data regarding lymphopenia, cancer type, patients’ age, and number of patients included in each study were collected. Patients were then grouped for statistical analysis. Ancillary studies were not included to avoid a particular patient being included several times. Considering the exclusion of scoping reviews from PROSPERO registration, this scoping analysis was not registered in any registration platform. The World Health Organization VigiAccess (VA) and the FDA Adverse Event Reporting System (FAERS) pharmacovigilance databases were then queried to assess reports on lymphopenia events.
In addition, we performed a retrospective study entitled ‘PARIB-OLD-HCL’ on patients treated with PARPi for gynecological cancer at our institution (Hôpital Lyon Sud-Hospices Civils de Lyon) between 2015 and 2022. We included eligible patients with a confirmed diagnosis of gynecological cancer for whom treatment with a PARPi was initiated at our institution, either in the ‘older’ group of patients if aged ≥ 70 years or in the ‘younger’ group of patients if aged ≤ 60 years at PARPi initiation. Exclusion criteria were known hematological disease and/or concomitant lympho/myelosuppressive treatment. The patient characteristics collected were age at PARPi introduction and longitudinal follow-up of hematologic lineages, including lymphocytes. The study was approved by the Scientific and Ethical Committee of Hospices Civils de Lyon (N° 22-5014) and registered in accordance with local guidelines.
Statistics
Endpoints of interest were any grade and grade ≥ 3 lymphopenia. For the retrospective study, these events were defined following the Common Terminology Criteria for Adverse Event (CTCAE) version 5. Comparisons were made using the Chi2 test for count data and Fisher’s exact test for small samples (< 5) using Python v3.10, Python Software Foundation.
Results
Scoping Review
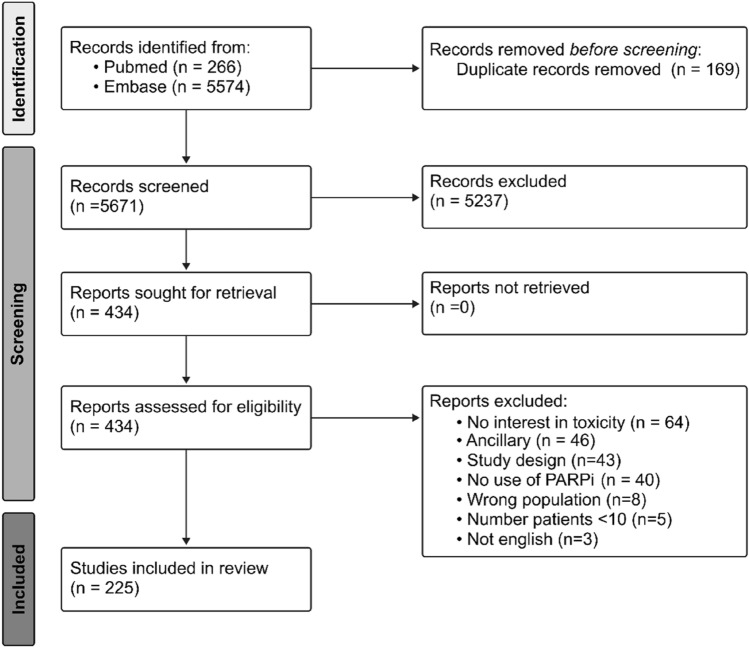
The search strategy retrieved 5840 records. After the removal of 169 duplicates, the titles of the 5671 remaining records were screened for eligibility. After exclusion of 5237 records, 434 were sought for retrieval and were fully read; a total of 225 studies were eventually included (Fig. 1). Two authors (GA, CF) independently performed this process and no disagreements were found. These 225 studies included a total of 22,743 patients. Among the studies, 92 were phase I studies (4002 patients, 17.6%), 1 was a pilot study (20 patients, < 1%), 82 were phase II studies (6562 patients, 28.9%), 40 were phase III studies (11,467 patients, 50.4%), and 12 were retrospective/real-world studies (692 patients, 3.0%).
Fig. 1.
Scoping review. PARPi poly(ADP-ribose) polymerase inhibitors
The reference list of included studies is presented in the electronic supplementary material (ESM). The data reported for each study were then gathered by a single author (GA), double-checked (CF), and pooled together before statistical analysis. The authors worked independently to minimize reporting bias. Data used for analyses are available upon request to the corresponding author.
Lymphopenia rates were reported in 94 (41.8%) studies, and most of the studies used the CTCAE criteria for AE reporting, although some of the studies did not clearly mention the chosen definition. Of the 178 (79.1%) studies that included patients aged ≥ 70 years of age, 77 (43.3%) provided data on lymphopenia; however, no study was specific to older patients. Olaparib and veliparib were the most frequently investigated PARPi (94 and 67 studies [41.8% and 29.8%], respectively), and lymphopenia was reported in 38/94 (40%) and 32/67 (48%) of these studies, respectively (Table 1). Tumor types were more frequently gynecological cancers (115 articles [51.1%], of which 42 reported data on lymphopenia [37%]), and lymphopenia rates were the most frequently reported in studies investigating digestive cancers (12/18 [67%]).
Table 1.
Descriptive analysis of the 225 studies included in the scoping review
| Lymphopenia available [n (%)] | ||
|---|---|---|
| Studies | 225 | 94 (41.8) |
| Patients | 22,743 | 9141 (40.2) |
| Inclusion of older patients (age ≥ 70 years)a | ||
| Studies | 178 | 77 (43.3) |
| PARPi studied | ||
| Olaparib | ||
| Studies | 94 | 38 (40) |
| Veliparib | ||
| Studies | 67 | 32 (48) |
| Niraparib | ||
| Studies | 24 | 8 (33) |
| Rucaparib | ||
| Studies | 11 | 5 (45) |
| Talazoparib | ||
| Studies | 10 | 5 (50) |
| Iniparib | ||
| Studies | 8 | 2 (25) |
| Fluzoparib | ||
| Studies | 3 | 2 (67) |
| Pamiparib | ||
| Studies | 3 | 1 (33) |
| Othersb | ||
| Studies | 2 | |
| Cancer type | ||
| Gynecological | ||
| Studies | 115 | 42 (37) |
| Multiple | ||
| Studies | 43 | 18 (42) |
| Digestive | ||
| Studies | 18 | 12 (67) |
| Lung | ||
| Studies | 17 | 7 (41) |
| Prostate | ||
| Studies | 11 | 6 (55) |
| Hematologic | ||
| Studies | 6 | 2 (33) |
| Brain | ||
| Studies | 4 | 2 (50) |
| Sarcoma | ||
| Studies | 4 | 3 (75) |
| Head and neck | ||
| Studies | 2 | 1 (50) |
| Melanoma | ||
| Studies | 2 | 1 (50) |
| Otherc | ||
| Studies | 3 | 0 (0) |
PARPi poly(ADP-ribose) polymerase inhibitors
a1 or more patients aged ≥ 70 years were included
bABT-767 and CEP-9722
cEwing sarcoma (n = 1), sarcoma (n = 1), germ line (n = 1)
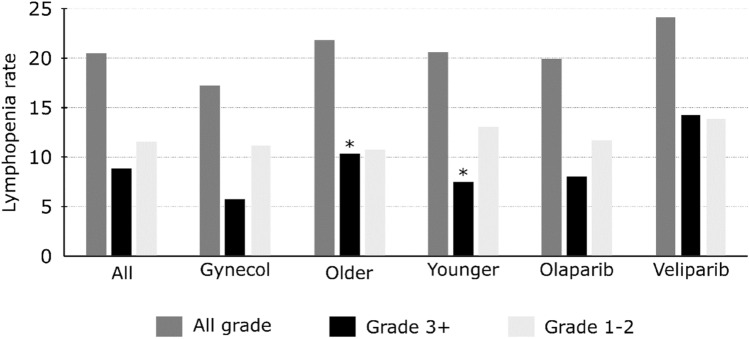
The 94 studies where lymphopenia is reported accounted for 9141 patients, including 7237 females (79.2%). Among these patients, all-grade lymphopenia was reported in 1445 patients (20.5%), grade ≥ 3 lymphopenia was reported in 782 (8.9%) patients, and grade 1–2 lymphopenia was reported in 736 (11.5%) patients. Grade ≥ 3 lymphopenia rates were significantly lower in studies that included younger patients (median age < 60 years: n = 362 [7.5%]) than older patients (median age ≥ 60 years: n = 303 [10.3%]; p < 0.0001); there was no significant difference in the rate of all-grade lymphopenia between these two groups (n = 681 [20.6%] vs. n = 617 [21.8%]; p = 0.242). In studies investigating gynecological cancers, all-grade and grade ≥3 lymphopenia rates were lower than in other cancers, at 17.2% (788 patients) vs. 26.2% (657 patients), and 5.7% (336 patients) vs. 15.9% (446 patients), respectively. Considering the impact of the different PARPi, reported rates of all-grade and grade ≥3 lymphopenia were 20% (566 patients) and 8% (314 patients) with olaparib, respectively, and 24.2% (355 patients) and 14.3% (209 patients) with veliparib, respectively (Fig. 2, electronic supplementary Table S1). Data regarding the impact of age on dose exposures and their impact on treatment toxicities is very scarcely reported in the studies included in the scoping review, therefore it was not possible to provide a conclusion regarding this point.
Fig. 2.
Lymphopenia rates in various settings among the 94 studies reporting data on lymphopenia. *Significant difference between the two groups (p < 0.0001). Gynecol gynecological cancer, Older median age ≥ 60 years, Younger median age < 60 years
Pharmacovgilance Database Queries
The VA and FAERS databases were queried on 4 February 2023 for the two most frequently used PARPi (olaparib and niraparib), at which point there were 10,600/10,334 reports for olaparib and 15,160/15,907 reports for niraparib (VA/FAERS databases, respectively). Among these, 24 (0.23%)/21 (0.20%) and 5 (0.03%)/1 (0.07%) were related to lymphopenia (VA/FAERS databases, respectively).
Retrospective Analysis
Patient Characteristics
Between 2015 and 2021, 46 patients were treated with PARPi and were included in the PARIB-OLD-HCL study. The ‘older’ group included 27 patients for whom PARPi were initiated between 2015 and 2021; the median age was 79 years (range 73–93) and the median follow-up was 11.9 months (range 1.8–61.6). Of these, 23 (85%) patients were treated for ovarian cancer and 4 (15%) were treated for endometrial cancer; 24 (89%) had been treated with one or two prior lines of treatment; 17 (63%) were treated with olaparib, 8 (30%) were treated with niraparib, and 2 (7%) were treated with rucaparib (Table 2).
Table 2.
Characteristics of patients included in the retrospective study
| Older patients [n = 27] | Younger patients [n = 19] | |
|---|---|---|
| Age, years | ||
| Median | 79 | 44 |
| Range | 73–93 | 25–59 |
| Localization of the primary tumor | ||
| Ovarian | 23 (85) | 14 (74) |
| Endometrial | 4 (15) | 0 (0) |
| Breast | 0 (0) | 5 (26)a |
| Prior lines of therapy | ||
| 1 | 12 (44) | 14 (74) |
| 2 | 12 (44) | 3 (16) |
| 3 | 1 (4) | 2 (10) |
| 4 | 2 (7) | 0 (0) |
| PARPi | ||
| Olaparib | 17 (63) | 14 (74) |
| Niraparib | 8 (30) | 5 (26) |
| Rucaparib | 2 (7) | 0 (0) |
| Relative dose intensity [median (SD)]b | 67% (± 24%) | 88% (± 23%) |
| Known hematological disease | ||
| None | 27 (100) | 19 (100) |
| Concomitant hematotoxic treatment | 0 (0) | 0 (0) |
Data are expressed as n (%) unless otherwise specified
PARPi poly(ADP-ribose) polymerase inhibitors, SD standard deviation
ap = 0.009
bRelative dose intensity was computed considering the full dose described in the treatment approval
The ‘younger’ group included 19 patients for whom PARPi were initiated between 2017 and 2021; the median age was 44 years (range 25–59) and the median follow-up was 5.9 months (range 2.3–23.6). Of these, 14 (74%) were treated for ovarian cancer and 5 (26%) were treated for breast cancer; 17 (89%) had been treated with one or two prior lines of treatment; 14 (74%) received olaparib and 5 (26%) received niraparib. There was no significant difference between the two groups in terms of patient characteristics, except for age and treatment for breast cancer (p = 0.009) (Table 2).
Lymphopenia
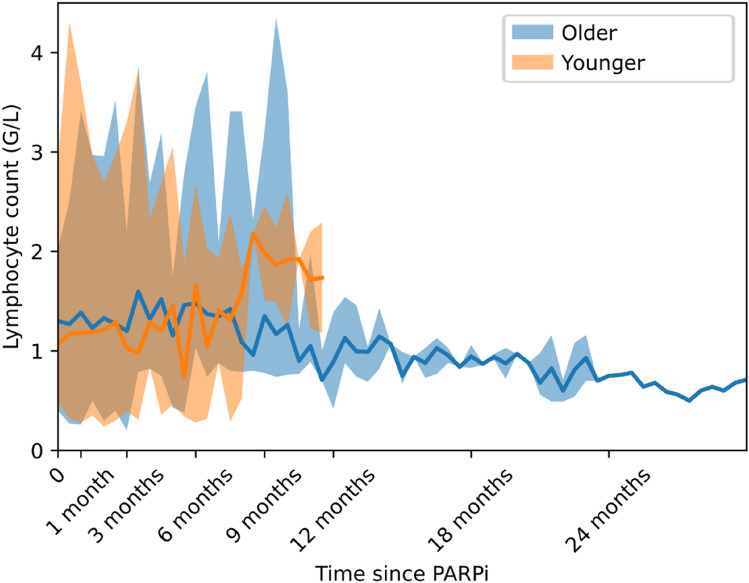
All-grade lymphopenia during PARPi treatment occurred in 19 (70%) patients in the ‘older’ group and 12 (63%) patients in the ‘younger’ group (p = 0.75). Furthermore, grade ≥ 3 lymphopenia occurred in 5 (19%) patients in the ‘older’ group and 1 (5%) patient in the ‘younger’ group (p = 0.37) (Fig. 3). The median nadir value of the lymphocyte count was 0.79 G/L (range 0.2–1.72) in the ‘older’ group and 1 G/L (range 0.24–2.1) in the ‘younger’ group. The median interval from PARPi introduction to lymphocyte nadir was 6.4 months (range 0.5–44) for the ‘older’ group and 2.9 months (range 0.4–8.3) for the ‘younger’ group. Evolution of lymphocyte count during treatment in the ‘older’ and ‘younger’ groups is presented Fig. 4.
Fig. 3.
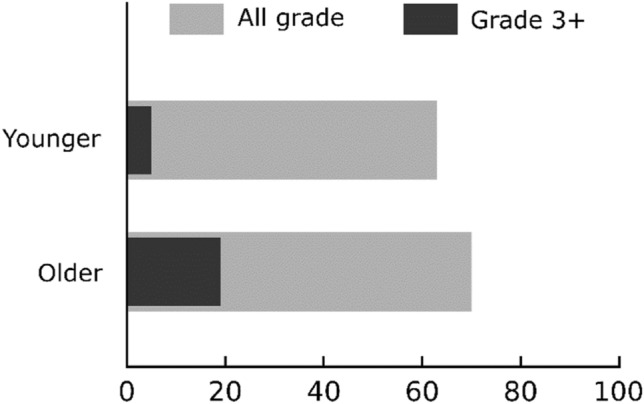
All-grade and grade ≥ 3 lymphopenia among the PARIB-OLD-HCL groups
Fig. 4.
Lymphocyte count evolution under PARPi treatment. Continuous line represents the median value on 2-week sliding time windows, and the shaded area depicts the maximum/minimum values for the same time period. PARPi poly(ADP-ribose) polymerase inhibitors
Discussion
In this study, we performed an external and internal analysis of lymphopenia events in patients treated with PARPi. To our knowledge, this study is the first dedicated to PARPi-induced lymphopenia, with a specific interest in the older population. For the first time, it sheds light on an additional hematologic cytopenia that should be more systematically reported in prospective trials and follow-up in everyday practice, along with neutropenia and thrombocytopenia, especially in older patients.
Noteworthy, in the WHO VigiAccess database (the international pharmacovigilance database), lymphopenia events are almost non-existent, whereas this toxicity, when reported, was frequent in patients treated with the same PARPi in clinical trials. This highlights the poor consideration of lymphopenia by the medical community in current practice, despite its known prognostic impact on different outcomes in both the oncological and geriatric settings [16–19]. Even in clinical trials, where hematologic toxicities are almost always reported, specific data on lymphopenia were only available in fewer than half of the trials. More strikingly, when available, reported all-grade lymphopenia rates were much lower than in our retrospective study. The same applies when only considering studies dedicated to gynecological cancers (17.20%) or including older patients (median age ≥60 years). When comparing older patients with younger patients, PARPi-induced lymphopenia was more frequent in older patients, a tendency that seemed more pronounced for grade ≥ 3 lymphopenia. This was noticed both in the scoping review and in the retrospective study. In addition, a trend towards a later and deeper nadir of lymphocyte count after PARPi initiation in older patients could illustrate an impaired hematopoietic resilience compared with younger patients. A similar observation was reported regarding the impact of age on neutrophil nadir after one or four courses of adjuvant chemotherapy in patients treated for breast cancer [20]. The impact of PARPi on lymphopenia events has a biological rationale considering the known impact of PARP pathways on various hematological functions. Yélamos et al. reported a direct deleterious effect of PARP2 gene deletion in mouse T lymphocytes, with PARP2 −/− mice displaying a decreased number of post-thymic T lymphocytes, which was explained by a higher sensitivity to the Bcl2 homology 3 (BH3)-mediated apoptosis pathway (with PARP being a key player in DNA repair), leading to T-cell death after CD3 stimulation and VJ segment recombination attempt [21]. Farrés et al. reported a broader impact at the HSC level, since PARP2-deficient HSC were shown to suffer more oxidative stress and apoptosis through TP53/BH3 pathway activation when urged to proliferate [13, 22]. Eventually, a direct PARPi-induced cytotoxic effect on HSC was observed by Hopkins et al. However, the concentrations of PARPi reported in this study are higher than those commonly obtained with the standard dosage of PARPi, therefore the direct toxicity of PARPi remains an exploratory hypothesis [14].
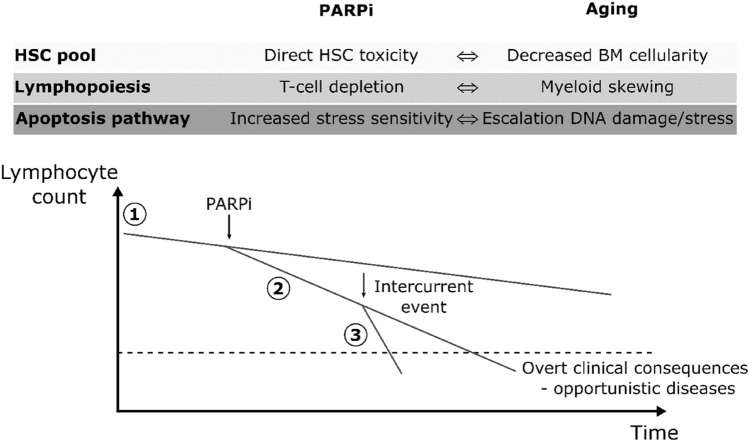
The reported lower resilience of the lymphocyte pool in response to PARPi exposure in the older population also has a robust biological rationale. First, the overall HSC pool is well known to decrease with age, leading to greater sensitivity to medullar treatment toxicity [23]. Moreover, an aging hematopoietic system is responsible for immune function losses and a shift towards myeloid lineage cell production [24, 25]. This decrease in lymphoid cell production in favor of myeloid cells is well described and is known as myeloid skewing [25]. More than simple myeloid shifting, recent studies have shown that aging hematopoiesis is mostly dedicated to platelet production through a significant increase in a specific class of HSCs [26]. Finally, aged HSC have been reported to accumulate DNA damage [27], leading to an increase in tumor suppressor pathway activation to avoid the onset of leukemia [28]. This process likely escalates with PARP inhibition, which is responsible for greater sensitivity to the apoptosis pathway, as explained earlier. Lymphocyte lineage, which may decline due to age-related hematopoietic changes [29], could thus be influenced by various intercurrent events, such as PARPi, possibly leading to profound lymphopenia and infectious-related consequences. The authors suggest a possible explanation for lymphopenia occurring in older patients, as summarized in Fig. 5.
Fig. 5.
Synergistic and deleterious effects of PARPi and aging on the HSC pool, lymphopoiesis and apoptosis pathway in HSC leading to lymphopenia. Schematic evolution of lymphocyte count in older patients: age-related hematopoietic changes lead to lymphocyte count decrease (1), initiation of PARPi treatment might escalate this decrease (2), which can be further worsened by other intercurrent events (3), possibly leading to lymphopenia-related complications. PARPi poly(ADP-ribose) polymerase inhibitors, HSC hematopoietic stem cell, BM bone marrow
Considering that PARPi treatment seems to be related to clonal hematopoiesis expansion, potentially leading to overt myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) [6, 10, 30], and that lymphopenia is reported to be linked to poor survival and cancer, especially in the case of underlying altered erythropoiesis [31, 32], and to an increased risk of myeloid malignancy [33], the relationship between PARPi-induced lymphopenia and treatment-related MDS/AML should be explored.
The present study has certain limitations; regarding the scoping review, a meta-analysis with heterogeneity and sensitivity analysis would have provided more accurate results. Reports of selection and data collection were independently performed by two authors to minimize various bias. Moreover, results obtained in this review were then compared with real-life data obtained in our center. Considering the real-life study, its single-center and retrospective design, along with its modest size, might have limited its power and likely explain its non-significant results. However, the use of automatic requests from medical charts allowed to limit inclusion bias; the availability of laboratory reports was not prone to subjectivity. Patients included in the retrospective analysis only had gynecological cancer (due to drug approval during the study period) and all were female, which might partly account for the differences in the results of the scoping review. For the same reasons, only three PARPi were prescribed in our center. Moreover, the median follow-up was shorter for the ‘young’ group, which might account for the difference in grade ≥ 3 lymphopenia onset.
Further study would be of first interest to investigate the real impact of PARPi on lymphopenia. This issue is even more crucial for older patients, since, for decades, their immunity has been known to be disturbed, as described by Wallford et al. [34]. In fact, if the consequences of lymphopenia are more vague than other cytopenias, it remains a key prognostic factor, especially in ovarian cancer [18, 19], and would thus deserve more consideration.
Conclusion
PARPi are responsible for a significant rate of all-grade and grade ≥ 3 lymphopenias, especially in the older population, possibly due to the synergic effect of PARPi and aging on hematopoietic function. Lymphocyte count should be reported in trials, as is already done for platelet count and hemoglobin. This article urges the design of clinical trials dedicated to older patients, who account for the largest part of cancer patients and deserve specific studies to better understand age-related toxicities. Such work would be of great use to identify the most vulnerable population and improve the management of related toxicity, such as trimethoprim/sulfamethoxazole (TMP-MSX) prophylaxis, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination schedule, etc.
Acknowledgements
The authors thank Drs Nathalie Bonnin, Amandine Bruyas, Aurélie Comte, Marianne Lorcet, and Julien Peron, and Prof. Benoît You for their help towards collecting patient information, as well as David Dayde and Camille Boin for their help during the regulatory procedures.
Declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Claire Falandry reported personal fees from Leo Pharma, Pfizer, MSD Oncology, Teva, AstraZeneca, Baxter, Eisai, Janssen Oncology, Novartis, Chugai Pharma and Astellas Pharma outside the submitted work; grants from Chugai Pharma, Pfizer, Pierre Fabre and Astellas Pharma outside the submitted work; and non-financial support from Janssen Oncology, Pierre Fabre, AstraZeneca and Leo Pharma outside the submitted work. Gabriel Antherieu, Maël Heiblig, Hervé Ghesquieres, and Gilles Freyer declare they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
All patients included in this study provided informed consent regarding data collection and publication of the corresponding results. This study was approved by the Scientific and Ethical Committee of Hospices Civils de Lyon (N° 22-5014) and was registered in accordance with local guidelines.
Availability of data and material
The datasets generated during and/or analyzed during the current study are not publicly available due to ethical and privacy reasons but are available from the corresponding author on reasonable request.
Author contributions
Conceptualization: GA, CF, MH. Methodology: GA, CF, MH. Validation: GA, CF, MH. Formal analysis: GA, CF. Investigation: GA, CF. Resources: CF. Data curation: GA, CF. Writing—original draft preparation: GA, CF. Writing—review and editing: GA, CF, GF, HG, MH. Supervision: CF, MH. Project administration: GA, CF. Funding acquisition: Not applicable. All authors have read and agreed to the published version of this manuscript. Every person involved in this analysis was offered authorship.
References
- 1.Schreiber V, Dantzer F, Ame J-C, et al. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 2.Satoh MS, Lindahl T. Role of poly(ADP-ribose) formation in DNA repair. Nature. 1992;356:356–358. doi: 10.1038/356356a0. [DOI] [PubMed] [Google Scholar]
- 3.Valerie K, Povirk LF. Regulation and mechanisms of mammalian double-strand break repair. Oncogene. 2003;22:5792–5812. doi: 10.1038/sj.onc.1206679. [DOI] [PubMed] [Google Scholar]
- 4.Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim G, Ison G, McKee AE, et al. FDA approval summary: olaparib monotherapy in patients with deleterious germline BRCA-mutated advanced ovarian cancer treated with three or more lines of chemotherapy. Clin Cancer Res. 2015;21:4257–4261. doi: 10.1158/1078-0432.CCR-15-0887. [DOI] [PubMed] [Google Scholar]
- 8.González-Martín A, Pothuri B, Vergote I, et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. 2019;381:2391–2402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 9.Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 10.Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416–2428. doi: 10.1056/NEJMoa1911361. [DOI] [PubMed] [Google Scholar]
- 11.Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949–1961. doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaFargue CJ, Dal Molin GZ, Sood AK, et al. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019;20:e15–28. doi: 10.1016/S1470-2045(18)30786-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farreś J, Martín-Caballero J, Martínez C, et al. Parp-2 is required to maintain hematopoiesis following sublethal γ-irradiation in mice. Blood. 2013;122:44–54. doi: 10.1182/blood-2012-12-472845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopkins TA, Ainsworth WB, Ellis PA, et al. PARP1 trapping by PARP inhibitors drives cytotoxicity in both cancer cells and healthy bone marrow. Mol Cancer Res. 2019;17:409–419. doi: 10.1158/1541-7786.MCR-18-0138. [DOI] [PubMed] [Google Scholar]
- 15.Himeji D, Tanaka G, Shiiba R, et al. Pneumocystis pneumonia in a patient with ovarian cancer receiving olaparib therapy. Intern Med. 2022;61:7485–7521. doi: 10.2169/internalmedicine.7485-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreu-Ballester JC, Pons-Castillo A, González-Sánchez A, et al. Lymphopenia in hospitalized patients and its relationship with severity of illness and mortality. PLoS ONE. 2021;16:e0256205. doi: 10.1371/journal.pone.0256205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ménétrier-Caux C, Ray-Coquard I, Blay J-Y, et al. Lymphopenia in cancer patients and its effects on response to immunotherapy: an opportunity for combination with cytokines? J Immunother Cancer. 2019;7:85. doi: 10.1186/s40425-019-0549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshino Y, Taguchi A, Takao M, et al. Lymphopenia after induction chemotherapy correlates with incomplete surgical resection in patients with advanced ovarian cancer. Int J Clin Oncol. 2019;24:428–436. doi: 10.1007/s10147-018-1374-4. [DOI] [PubMed] [Google Scholar]
- 19.Lee YJ, Chung YS, Lee J-Y, et al. Pretreatment lymphocytopenia is an adverse prognostic biomarker in advanced-stage ovarian cancer. Cancer Med. 2019;8:564–571. doi: 10.1002/cam4.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dees EC, O’reilly S, Goodman SN, et al. A prospective pharmacologic evaluation of age-related toxicity of adjuvant chemotherapy in women with breast cancer. Cancer Invest. 2000;18:521–529. doi: 10.3109/07357900009012191. [DOI] [PubMed] [Google Scholar]
- 21.Yélamos J, Monreal Y, Saenz L, et al. PARP-2 deficiency affects the survival of CD4+CD8+ double-positive thymocytes. EMBO J. 2006;25:4350–4360. doi: 10.1038/sj.emboj.7601301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farrés J, Llacuna L, Martin-Caballero J, et al. PARP-2 sustains erythropoiesis in mice by limiting replicative stress in erythroid progenitors. Cell Death Differ. 2015;22:1144–1157. doi: 10.1038/cdd.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaiswal S, Ebert BL. Clonal hematopoiesis in human aging and disease. Science. 2019;366:eaan4673. doi: 10.1126/science.aan4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woolthuis CM, de Haan G, Huls G. Aging of hematopoietic stem cells: Intrinsic changes or micro-environmental effects? Curr Opin Immunol. 2011;23:512–517. doi: 10.1016/j.coi.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Rossi DJ, Bryder D, Zahn JM, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grover A, Sanjuan-Pla A, Thongjuea S, et al. Single-cell RNA sequencing reveals molecular and functional platelet bias of aged haematopoietic stem cells. Nat Commun. 2016;7:11075. doi: 10.1038/ncomms11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rübe CE, Fricke A, Widmann TA, et al. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS ONE. 2011;6:e17487. doi: 10.1371/journal.pone.0017487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jonge HJM, de Bont ESJM, Valk PJM, et al. AML at older age: age-related gene expression profiles reveal a paradoxical down-regulation of p16INK4A mRNA with prognostic significance. Blood. 2009;114:2869–2877. doi: 10.1182/blood-2009-03-212688. [DOI] [PubMed] [Google Scholar]
- 29.Sun H, Kang X, Chen X, et al. Immunosenescence evaluation of peripheral blood lymphocyte subsets in 957 healthy adults from 20 to 95 years old. Exp Gerontol. 2022;157:111615. doi: 10.1016/j.exger.2021.111615. [DOI] [PubMed] [Google Scholar]
- 30.Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–1284. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 31.Biondini D, Semenzato U, Bonato M, et al. Lymphopenia is linked to an increased incidence of cancer in smokers without COPD. Eur Respir J. 2019;54:PA2586. [Google Scholar]
- 32.Zidar DA, Al-Kindi SG, Liu Y, et al. Association of lymphopenia with risk of mortality among adults in the US general population. JAMA Netw Open. 2019;2:e1916526. doi: 10.1001/jamanetworkopen.2019.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niroula A, Sekar A, Murakami MA, et al. Distinction of lymphoid and myeloid clonal hematopoiesis. Nat Med. 2021;27:1921–1927. doi: 10.1038/s41591-021-01521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walford RL. The immunologic theory of aging. Gerontologist. 1964;4:195–197. doi: 10.1093/geront/4.4.195. [DOI] [PubMed] [Google Scholar]