Abstract
Background
Primary cardiac lymphoma is an extremely rare malignancy involving the heart and pericardium. It is a disease that most commonly effects the right atrium and right ventricle. Left untreated it carries a very poor prognosis. Recent advancements in therapy including early recognition and initiation of chemotherapy has led to improved survival.
Case summary
A 78 year old female presented with weight loss, abdominal pain and distension. An ultrasound abdomen pre admission showed abdominal ascites. An echocardiogram performed during admission showed a large pericardial effusion with asymmetrical increase in left ventricular wall thickness and a new left atrial mass. Pericardial fluid analysis led to the diagnosis of diffuse large B cell non-Hodgkin's lymphoma. Positron Emission Tomography CT (PET-CT) shows avid fluorodeoxyglucose (FDG) uptake in cardiac muscle. Prompt treatment was initiated with a chemotherapy regimen involving Rituximab, Cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP). An echocardiogram 3 months post initiation of treatment showed resolution of the pericardial effusion and left atrial mass as well as an improvement in left ventricular wall thickness. A PET-CT following completion of treatment showed complete metabolic response.
Discussion
The diagnosis of primary cardiac lymphoma is uncommon and the presence of left rather than right heart involvement is rarer still. Multimodality imaging is key in diagnosis. Early recognition and treatment is vital in improving associated morbidity and mortality.
Keywords: Cardiac lymphoma, Cardiac mass, Cardiac malignancy, Cancer, Echocardiography, Case report
Learning points.
Early diagnosis is important in primary cardiac lymphoma and can lead to improved clinical outcomes and survivability.
Echocardiography is an important tool that can be used for response to treatment in patients with primary cardiac lymphoma.
Background
Primary cardiac tumours are extremely rare. A pooled autopsy series showed a frequency of primary cardiac tumours of approximately 0.02%.1 Primary cardiac tumours are further subdivided into benign and malignant tumours, with benign tumours accounting for over 75%.2,3 Primary cardiac lymphoma (PCL) therefore is an exceedingly rare malignant tumour of the myocardium, with a reported incidence of 1% of cardiac malignancies.4 Few cases of PCL have been cited5 with a literature review identifying only 197 cases of PCL between 1949 and 2009.6 A further study in 2016 used the National Cancer Database and identified 305 patients diagnosed with PCL between the years of 2004 and 2016.4 This condition usually carries with it poor prognosis. Timely diagnosis and treatment is a key. We present the case of a 78-year-old female who presented with non-specific symptoms and was diagnosed with PCL.
Timeline
| Day 1 | Patient presented with weight loss, malaise, and abdominal pain. An ultrasound (US) ordered as an outpatient shows abdominal ascites. Patient is admitted under a general medicine team for further investigation |
| Day 2 | Computed tomography thorax abdomen and pelvis shows large pericardial effusion and thickening of the left ventricle (LV) along with features of hepatic congestion and periportal oedema. No solid organ malignancy identified |
| Day 3 | US abdomen shows moderate ascites and simple hepatic cysts Echocardiogram shows large pericardial effusion with no sonographic evidence of tamponade. Increased wall thickness was noted in the basal posterior and inferior wall of the LV in addition to a new left atrial mass |
| Day 4 | Pericardiocentesis performed under echo guidance, histopathology concerning for a diagnosis of lymphoma |
| Day 5 | Bone marrow aspirate obtained |
| Day 10 | Positron emission tomography CT (PET-CT) shows avid fluorodeoxyglucose uptake in cardiac muscle with adenopathy in the mediastinum and right supraclavicular fossa, there is no uptake below the diaphragm |
| Day 11–14 | Patient initiated on high dose dexamethasone and placed on chemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone |
| Month 3 | Repeat echo showed resolution of pericardial effusion and left atrial mass and improvement of LV thickness from 2.9 cm to 1.2 cm |
| Month 6 | A repeat PET-CT shows metabolic resolution of lymphoma |
Case presentation
A 78-year-old Caucasian female presented to the emergency department with a 4-week history of unintentional 7 kg weight loss, malaise, increasing abdominal distension, and a 1-week history of dysuria, nausea, and left flank pain. A pre-admission ultrasound abdomen was organized by the patients general practitioner given her history of abdominal pain. This showed pelvic and abdominal ascites which prompted a referral to the emergency department. She had no history of chest pain, dyspnoea, palpitations, fever, or night sweats. She had a background history of psoriatic arthritis for which she was on Abatacept. Her history also included osteoporosis, hyperlipidaemia, and a minimally invasive parathyroidectomy. She is an ex-smoker with a 20 pack-year history and drank alcohol very rarely.
On arrival to hospital, she was haemodynamically stable. Her physical examination was unremarkable with the exception of small volume clinical ascites. Her chest X-ray showed cardiomegaly. A urine dipstick was positive for red blood cells but negative for nitrates and leukocytes. An admission electrocardiogram showed normal sinus rhythm with no evidence of electrical alternans. Her presentation to the emergency department was concerning for a possible underlying malignancy.
A computed tomography thorax abdomen and pelvis showed a large pericardial effusion with thickening of the lateral aspect of the left atrium and left ventricle (LV), small volume ascites, a heterogenous liver with periportal oedema, and mild hyper-attenuation of the common bile duct with borderline dilatation of the pancreatic duct. The abdominal ascites was deemed not amenable to drainage given its size.
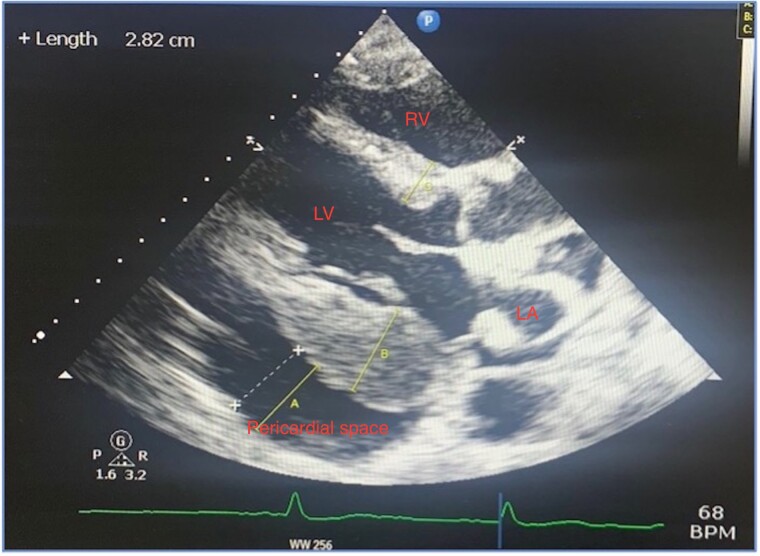
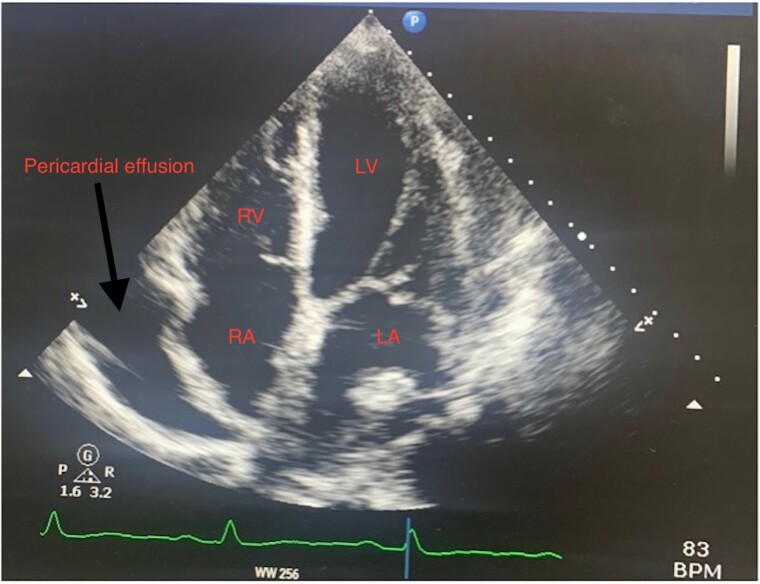
A transthoracic echocardiogram (TTE) showed a large pericardial effusion measuring 31 mm at its largest over the right atrium and basal to mid right ventricle. There was no collapse of the right atrium or right ventricle with no significant variation of mitral or tricuspid inflow Doppler to signify the presence of cardiac tamponade. There was severe thickening of the basal posterior/inferior cardiac wall measuring 29 mm with a low normal ejection fraction of 50%. There was also an echogenic fixed mass in the left atrium measuring 13 mm × 13 mm The right atrium and ventricle were of normal size with normal right ventricular function (Figures 1 and 2; see Supplementary material online, Figure S1). These findings were new compared to a routine outpatient echocardiogram performed in our institution 4 months prior which was normal and demonstrated no effusion or abnormal wall thickness. These findings were highly concerning for an acute primary cardiac tumour. A transoesophageal echocardiogram was not deemed necessary at the time.
Figure 1.
Transthoracic echocardiogram in parasternal long axis view showing large pericardial effusion measuring 31 mm with increased thickness of the wall of the left ventricle with a left atrial mass.
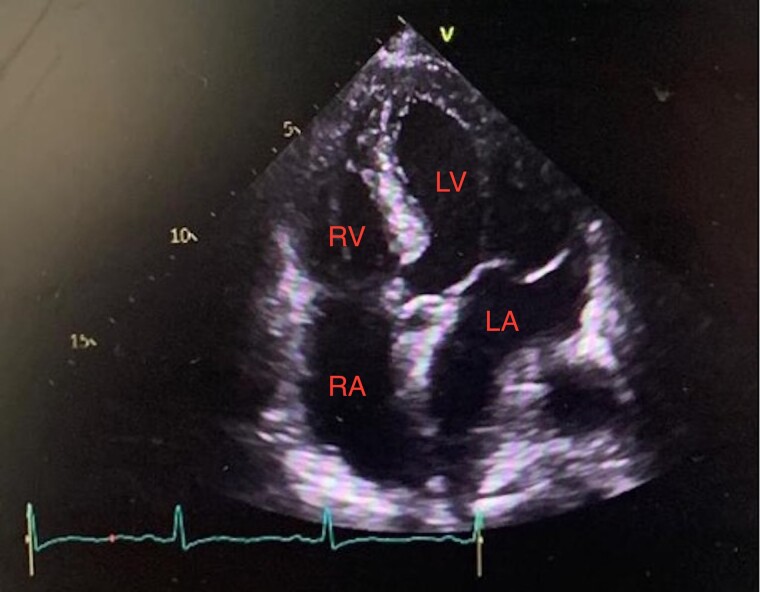
Figure 2.
Transthoracic echocardiogram in apical four-chamber showing left atrial mass measuring 13 mm × 13 mm with a normal right ventricle and right atrium.
Echo-guided pericardiocentesis was performed to obtain sample for histopathology. Pericardial fluid analysis confirmed the diagnosis of diffuse large B-cell lymphoma. A bone marrow aspirate was also performed to ascertain if there was any bone marrow invasion. Results from this were negative for marrow involvement.
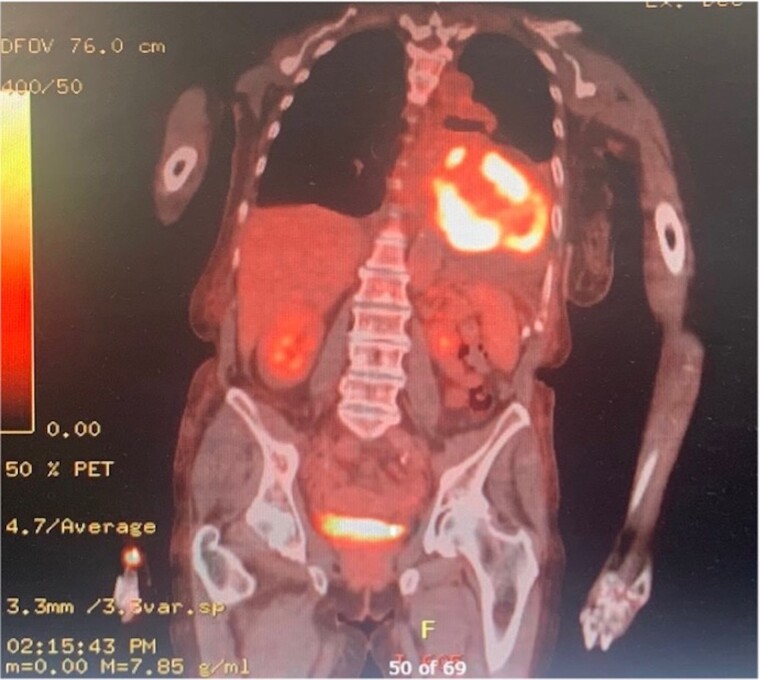
A PET-CT (Figure 3; see Supplementary material online, Figures S2 and S3) was performed for further evaluation of the extent of disease. This showed a large pericardial effusion and highly fluorodeoxyglucose (FDG) avid tissue, with a maximum standardized uptake value (SUV) of 25.3 infiltrating throughout the cardiac muscle involving the right and LV walls and the interventricular septum with thickening along the right atrial wall. There was also FDG-avid adenopathy in the mediastinum and right supraclavicular fossa with a maximum SUV of 16. There was no uptake below the diaphragm. Bilateral pleural effusions and ascites were identified with no FDG uptake. Our patients liver function tests were normal, and there was no concern on imaging to indicate a metastatic process or the presence of liver cirrhosis. Imaging and laboratory findings combined led us to the conclusion that her ascites was likely to be a consequence of cardiac failure.
Figure 3.
Positron emission tomography CT images showing fluorodeoxyglucose uptake throughout the cardiac muscle with infiltration involving the left and right ventricle and interventricular septum.
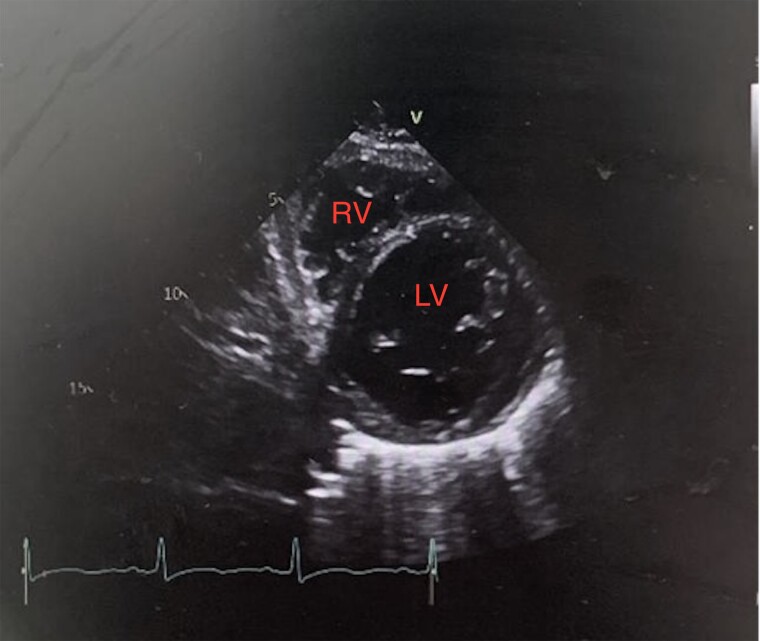
Figure 5.
Transthoracic echocardiogram in parasternal short axis view showing improvement in left ventricular wall thickness to a maximum of 12 mm.
The patient was commenced on oral chemotherapy treatment with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP) protocol and has completed six cycles of treatment. Her treatment has been complicated by an inpatient stay with urosepsis. She was treated with intravenous antibiotics for this, and no changes were made to her chemotherapy regimen. A repeat TTE was performed 3 months post-initiation of chemotherapy. This showed complete resolution of the pericardial effusion and the left atrial mass. It also showed only mild basal posterior wall thickening of 12 mm compared to 29 mm on her pre-treatment TTE (Figures 4 and 5). A post treatment positron emission tomography CT (PET-CT) was carried out after six cycles of chemotherapy. This showed interval resolution of the abnormal FDG uptake related to the heart as well as significant interval decrease in the mediastinal lymph nodes and level of FDG uptake (see Supplementary material online, Figures S4–S6).
Figure 4.
Transthoracic echocardiogram in apical four-chamber view showing resolution of left atrial mass, normal right atrium, ventricle, and interval improvement of left ventricular wall thickness.
Discussion
PCL is a non-Hodgkin lymphoma, often diffuse large B-cell lymphoma. PCL is a rare cardiac neoplasm thought to more prevalent in males and in immunocompromized patients.
Our patient presented with a large pericardial effusion, an intra-cardiac mass, and weight loss which alerted us to the possiblity of a diagnosis of cardiac lymphoma. This disease can present with a variety of non-specific symptoms including: B symptoms (fever, weight loss, and night sweats), chest pain, dyspnoea and, in rare occasions, SVC obstruction.6
Initial diagnosis remains a challenge due to the non-specific clinical manifestation of the disease. Detection of PCL involves multimodality imaging using echocardiogram, magnetic resonance imaging, CT, and PET-CT. Invasive testing to obtain samples for pathological examination is required. Endomyocardial biopsy (EMB) is, in most cases, necessary for diagnosis. A challenge for diagnosis therefore is availabilty of non-invasive and invasive testing, which may require referral to a tertiary or quaternary centre.
Endomyocardial biopsy is the gold-standard method for the diagnosis of many cardiac disorders including, cardiac amyloidosis, cardiac sarcoidosis, and various cardiac malignancies. It is however an invasive procedure that carries risks and limitations. A cardiac pathologist is required to interpret histological findings. Repeated procedures may be necessary to get sufficient samples, and rarely results may not be accurate. Contraindications to the procedure include the presence of intracacrdiac thrombus, severe valvular stenosis (tricuspid, aortic, or pulmonary) as well as tricuspid or aortic prosthesis. With regards to cardiac masses, EMB is not indicated in tumours with a high embolic potential such as those that are left sided.7,8
In the case of our patient, a diagnosis was made using pericardial fluid analysis.
Bone marrow biopsies are considered in patients with a possible underlying lymphoma to excluse systemic disease. While PET-CT can has been found to be highly specific for detecting bone marrow involvement, a negative PET scan does not fully rule out bone marrow involvement. A meta-analysis performed on 654 patients with diffuse large B-cell lymphoma showed that 3% of patients with proven bone marrow involvement on biopsy had a negative PET scan.9
A systematic review performed in 2011 showed that the right atrium and right ventricle were the most common sites of involvement with only 7% of cases citing left heart involvement in the absence of right heart involvement. Our patient was one of these rare cases with exclusive left heart involvement. The right atrium is the most commonly involved chamber of the heart. Pericardial effusions were present in 58% of cases.6 The same study showed a median survival rate in immunocompromised patients of 3.5 months, and they were unable to quantify the survival rate in immunocompetent patients. They found patients who had an associated arrhythmia had an improved survival rate, likely due to these patients presenting to hospital earlier.6 Lack of LV involvement was also associated with a more favourable outcome.6
The most common causes of death in these patients are progression of lymphoma, cardiac arrhythmias, intractable cardiac failure, and sepsis.6,10
There are currently no established guidelines for treatment of PCL given the paucity of data associated with the condition. If left untreated, the survival rate of PCL can be less than 1 month. Standard treatment is six cycles of R-CHOP, similar to other subtypes of non-Hodgkin’s lymphoma.4,11 Studies on patients diagnosed with diffuse large B-cell lymphoma have shown an increase in event-free survival rate with the use of R-CHOP when compared to CHOP (P < 0.001); overall survival rate was also improved with R-CHOP (P = 0.007) with no significant increase in drug toxicitity.12 A survival study analysis preformed from 2009 to 2019 has shown increase in overall survival and has found that surgery does not improve long-term outcome.13 Surgery, while unlikely to confer any survival benefit, can be helpful in relieving mass obstruction effects.
Conclusion
Early diagnosis and subsequent treatment with appropriate chemotherapy is crucial in cases of cardiac lymphoma. Increasing awareness and recognition of this disease play a crucial part in timely diagnosis and therefore improved survival benefit.
Supplementary Material
Acknowledgements
Cardiology Department at Cork University Department.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including the images and associated text have been obtained from the patient in line with COPE guidance.
Funding: None declared.
Contributor Information
Saghie Ellen, Department of Cardiology, Cork University Hospital, Wilton Road, Co Cork, Ireland.
Higgisson Emma, Department of Cardiology, Cork University Hospital, Wilton Road, Co Cork, Ireland.
Lead author biography
 Dr Ellen Saghie graduated medicine from Royal College of Surgeons in Ireland and has an undergraduate degree in Pharmacology from University College Dublin Ireland. She is a Registrar in Cardiology in Cork University Hospital.
Dr Ellen Saghie graduated medicine from Royal College of Surgeons in Ireland and has an undergraduate degree in Pharmacology from University College Dublin Ireland. She is a Registrar in Cardiology in Cork University Hospital.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports.
Data availability
The data underlying this article are available in the article and in its online Supplementary material.
References
- 1. Reynen K. Frequency of primary tumors of the heart. Am J Cardiol 1996;77:107. [DOI] [PubMed] [Google Scholar]
- 2. Odim J, Reehal V, Laks H, Mehta U, Fishbein M. Surgical pathology of cardiac tumors. Cardiovasc Pathol 2003;12:267–270. [DOI] [PubMed] [Google Scholar]
- 3. Kamiya H, Yasuda T, Nagamine H, Sakakibara N, Nishida S, Kawasuji M, et al. Surgical treatment of primary cardiac tumors. Jpn Circ J 2001;65:315–319. [DOI] [PubMed] [Google Scholar]
- 4. Sultan I, Aranda-Michel E, Habertheuer A, Kilic A, Arnaoutakis G, Bianco V, et al. Long-term outcomes of primary cardiac lymphoma. Circulation 2020;142:2194–2195. [DOI] [PubMed] [Google Scholar]
- 5. Ikeda H, Nakamura S, Nishimaki H, Masuda K, Takeo T, Kasai K, et al. Primary lymphoma of the heart: case report and literature review. Pathol Int 2004;54:187–195. [DOI] [PubMed] [Google Scholar]
- 6. Petrich A, Cho S, Billett H. Primary cardiac lymphoma. Cancer 2011;117:581–589. [DOI] [PubMed] [Google Scholar]
- 7. Porcari A, Baggio C, Fabris E, Merlo M, Bussani R, Perkan A, et al. Endomyocardial biopsy in the clinical context: current indications and challenging scenarios. Heart Fail Rev 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seferović P, Tsutsui H, McNamara D, Ristić A, Basso C, Bozkurt B, et al. Heart failure association of the ESC, Heart Failure Society of America and Japanese heart failure society position statement on endomyocardial biopsy. Eur J Heart Fail 2021;23:854–871. [DOI] [PubMed] [Google Scholar]
- 9. Adams HJA, Kwee TC. Increased bone marrow FDG uptake at PET/CT is not a sufficient proof of bone marrow involvement in diffuse large B-cell lymphoma. Am J Hematol 2015;90. [DOI] [PubMed] [Google Scholar]
- 10. Tanaka Y, Yamabe H, Yamasaki H, Tsuda H, Nagayoshi Y, Kawano H, et al. A case of reversible ventricular tachycardia and complete atrioventricular block associated with primary cardiac B-cell lymphoma. Pacing Clin Electrophysiol 2009;32:816–819. [DOI] [PubMed] [Google Scholar]
- 11. Lyon A, López-Fernández T, Couch L, Asteggiano R, Aznar M, Bergler-Klein J, et al. 2022. ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022;43:4229–4361. [Google Scholar]
- 12. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP Chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002;346:235–242. [DOI] [PubMed] [Google Scholar]
- 13. Chen H, Qian S, Shi P, Liu L, Yang F. A presentation, treatment, and survival analysis of primary cardiac lymphoma cases reported from 2009 to 2019. Int J Hematol 2020;112:65–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary material.