Abstract
Juvenile open-angle glaucoma (JOAG) is an uncommon subset of primary glaucoma with an onset before the age of 40 years. In this case report, we describe the cosegregation of MYOC , p.Pro370Leu and LTBP2 , p.Pro432Leu mutations in a family with JOAG. The family with autosomal dominant JOAG belonged to Northern India. The samples of proband and her parents were evaluated by whole exome sequencing. Sanger sequencing was conducted in all the study participants to check the mutations identified. Both MYOC and LTBP2 mutations were found to cosegregate in affected individuals leading to a severe JOAG phenotype, thereby suggesting a digenic inheritance of MYOC with LTBP2 in this family.
Keywords: glaucoma, digenic, inheritance, mutation, phenotype
Introduction
Juvenile open-angle glaucoma (JOAG) is primary open-angle glaucoma (POAG) with age of onset before 40 years, associated with high intraocular pressure (IOP), advanced glaucomatous optic neuropathy, and severe visual disability, if left untreated. Myocilin ( MYOC ) mutations have been associated with this form of glaucoma. Rarely, latent transforming growth factor-β-binding protein 2 ( LTBP2 ) mutations have also been associated with JOAG and adult-onset open-angle glaucoma. 1 2 In the past two decades, genetic loci associated with primary congenital glaucoma (PCG), JOAG, and POAG have been found to be overlapping. 3 The fact that some individuals with MYOC mutations never develop the disease and others without MYOC mutation, in a family with MYOC mutation, develop the disease, points toward the possible influence of other genetic variants in affected individuals, suggesting multiallelic inheritance. 3 4 5 6 7 8 9 An earlier study by Vincent et al showed occurrence of a digenic inheritance (DI) of CYP1B1 and MYOC in a JOAG family of East Indian descent. 10 Next-generation sequencing technologies have helped in identifying cosegregation of pathogenic variants, thus providing greater evidence for multiallelic inheritance. In this report, we identified a JOAG family with both MYOC and LTBP2 gene mutations.
Case Report
The JOAG family presented in this report is of North Indian descent. The study was approved by institute ethics committee of the All India Institute of Medical Sciences, New Delhi, India, and was conducted following the Declaration of Helsinki. Informed consent was obtained from all investigated individuals. Two milliliter of blood was collected in an EDTA vial from the family members available and DNA was isolated by salting out method. Using 1 µg genomic DNA, whole exome sequencing was performed in proband and her parents using SureSelectXT2 Human All Exon v5 + UTRs (Agilent Technologies; Santa Clara, California, United States) on an Illumina HiSeq 2000 System with 100-bp paired-end sequencing. The paired reads were aligned to GR.ch37 reference genome and the GATK Software ( https://www.broadinstitute.org/gatk/ ) was used for base quality calibration. The variant analysis was performed using GATK-3.6 toolkit with Unified Genotyper. The variant call files generated were analyzed using Golden Helix VarSeq Software v.1.2.1 (Bozeman; Montana, United States). False-positive detection of variants were minimized by keeping the cutoff read depth as 15 and variants were annotated with information from Ensembl ( http://www.ensembl.org ), 1000 Genomes Project ( http://www.1000genomes.org/ ), RefSeq ( http://www.ncbi.nlm.nih.gov/RefSeq/ ), and ExAC browser ( http://exac.broadinstitute.org/ ). To remove the common variants, minor allele frequency was kept as less than 1% or unknown.
The analysis focused on previously known genes for JOAG, PCG, and adult-onset POAG, namely, MYOC , CYP1B1 , LTBP2 , FOXC1 , PITX2 , TEK , COL15A1 , OPTN , and TMCO1 . For validating the genetic variants, Sanger sequencing with both forward and reverse primers on the amplified template was performed. The functional impact of variants was analyzed using tools such as PROVEAN, DEOGEN2, FATHMM-MKL, Polyphen, and SIFT. Protein–protein interactions (PPI) and RNA coexpression were checked by an online resource tool, namely, STRING (Search Tool for the Retrieval of Interacting Genes, version 11.2). The confidence score limit set for the PPI network generation was ≥ 0.4 and a cutoff value of p ≤ 0.01 was considered for significance of prediction/interaction. For RNA coexpression, a score above ≥ 0.15 was regarded as robust evidence.
All the affected individuals had onset of glaucoma or ocular hypertension (OHT) before the age of 15 years ( Fig. 1 ). The father had been diagnosed with glaucoma at a young age, the proband presented to us with advanced glaucomatous optic neuropathy while her younger sister and younger brother were detected, by us to have OHT, when we screened the siblings. The father had poor vision in both eyes, and the proband had a vision of only 3/60 in one eye due to advanced glaucomatous optic neuropathy while the other eye had advanced glaucomatous optic neuropathy but maintained a central visual acuity of 6/6. The affected sister and younger brother of proband had 6/6 vision in both eyes. The clinical characteristics of the affected members are given in Table 1 . None of the members had enlarged corneal diameters that would suggest a congenital glaucoma. Iris and lens morphology were normal and goniophotograph showed wide open angles. The proband showed advanced glaucomatous optic neuropathy in both eyes ( Fig. 2 ). Her perimetry was evaluated only in the left eye, which showed severe visual field loss. The siblings with OHT had normal thickness of their retinal nerve fiber layer on ocular coherence tomography.
Fig. 1.
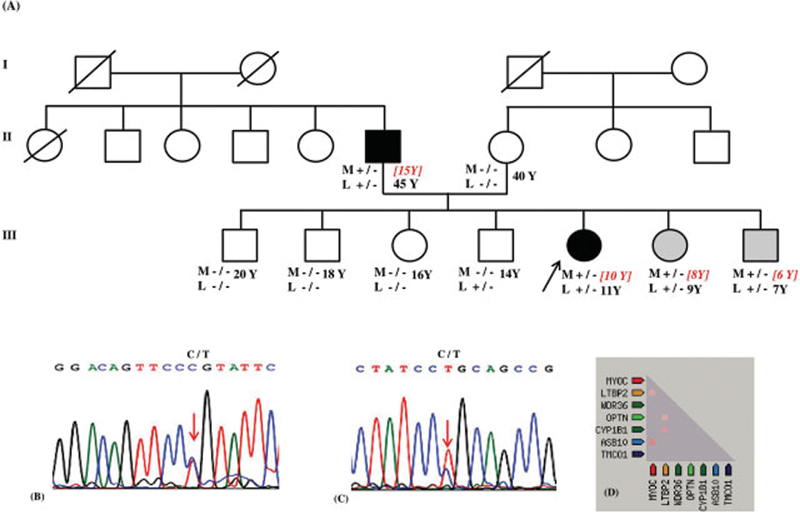
Pedigree of JOAG family, with MYOC (Pro370Leu) and LTBP2 (Pro432Leu) mutations. ( A ) Affected status is shaded, black is for JOAG and gray for ocular hypertension. M and L below each symbol indicates MYOC and LTBP2 , respectively. +/− indicates heterozygous mutation and −/− indicates homozygous wild genotype. Y indicates age in years at the time of study. Age indicated in square brackets with red colored italics denotes age of onset, while the age in black color indicates the age at the time of the study. ( B ) Chromatogram showing change at c.1109C > T of MYOC causing heterozygous p.Pro370Leu mutation. ( C ) Chromatogram showing change at c.1295C >T of LTBP2 causing heterozygous p.Pro432Leu mutation. ( D ) Heat map of coexpression of MYOC and LTBP2 in Homo sapiens .
Table 1. Clinical characteristics of the affected members in the family.
| Clinical parameters | Father | Proband | Sister | Brother |
|---|---|---|---|---|
| Visual acuity: RE, LE | 1/60, 6/12 | 3/60, 6/6 | 6/6, 6/6 | 6/6, 6/6 |
| Highest untreated IOP: RE, LE (mm Hg) | 40, 38 | 44, 32 | 26, 26 | 32, 36 |
| Central corneal thickness: RE, LE (µ) | 550, 550 | 470, 500 | 520, 500 | 560, 580 |
| Axial length: RE, LE (mm) | 23.3, 23.6 | 24.8, 24.7 | 21.9, 22 | 24, 23.9 |
| Cup disc ratio: RE, LE | Near total cup BE | Near total cup BE | 0.3:1, 0.3:1 | 0.4:1, 0.4:1 |
| Mean deviation: RE, LE (dB) | NP, −28.5 | NP, −27.8 | Not done | Not done |
| Treatment | BE trabeculectomy | BE trabeculectomy | Medical | Medical |
Abbreviations: BE, both eyes; IOP, intraocular pressure; LE, left eye; NP, not possible; RE, right eye.
Fig. 2.
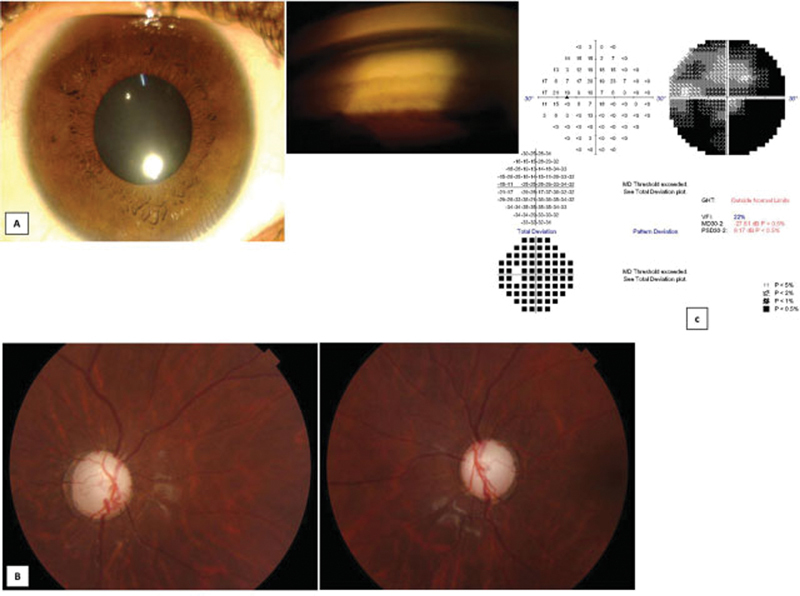
Anterior segment, optic disc photograph, and visual field of proband. ( A ) Anterior segment photograph of the proband along with goniophotograph showing wide open angles. ( B ) Optic disc photograph of the proband showing near total glaucomatous optic neuropathy in both eyes. ( C ) Visual field of the left eye of the proband showing severe visual field loss.
We identified a MYOC variant (chr1:171605471G > AGRch37; NM_000261.2:c.1109C > T; p.Pro370Leu) and LTBP2 variant (chr14:75018994G > A GRch37; NM_000428.3:c.1295C > T; p.Pro432Leu) in the proband and affected father. Both these variants are nonsynonymous, missense, and are highly conserved in the evolutionary process. The Genomic Evolutionary Rate Profiling (GERP + +) rejected substitutions scores were 4.55 and 2.68 for the MYOC and LTBP2 variants, respectively, indicative of a significant evolutionary constraint at these sites. The score values of the deleterious effect of MYOC and LTBP2 variants were: SIFT (0.05, 0.15), Polyphen (1, 0.011), PROVEAN (−8.44, −2.96), DEOGEN2 (0.92, 0.55), and FATHMM-MKL (0.80, 0.67), respectively.
The mutations were coinherited by the proband's younger brother and sister who were detected to have OHT ( Fig. 1A ). Sanger sequencing confirmed MYOC , p.Pro370Leu and LTBP2 , p.Pro432Leu heterozygous variations ( Fig. 1B, 1C ). Both variants were absent in three other unaffected siblings and mother of proband. However, in one unaffected sibling, only LTBP2 , p.Pro432Leu mutation was observed. The IOP of the carrier with the LTBP2 variant was 12 mm Hg in both eyes and his cup disc ratio was 0.3 in both eyes.
For PPI network generation, both genes were imported to STRING tool, to identify the network of interaction among them based on databases of experimental and predicted protein interaction. The PPI network view summarizes the network based on text mining, experiments, databases, coexpression, neighborhood gene fusion, and co-occurrence. Each of these interactions is assigned a score between 0 and 1 which is the probability that the interaction really exists given the available evidence. In our analysis, the PPI enrichment, p-value was 0.002 for a combined interaction score of 0.68 for MYOC and LTBP2. In the PPI network generated for MYOC and LTBP2 , the interaction sources that contributed to the combined interaction/prediction score were from text mining and RNA coexpression. Based on this, we concluded a tangible PPI between the two genes. The RNA coexpression score of 0.08, however, indicated a weak evidence of coexpression of the two genes ( Fig. 1D ).
Discussion
Our study describes cosegregation of MYOC , p.Pro370Leu and LTBP2 , p.Pro432Leu pathogenic variants in a family with JOAG. Both these mutations have been associated to be disease causing in POAG. 1 11 The allele-specific penetrance for MYOC , p.Pro370Leu is known to be more than 75% by the age of 25 years which is higher than other reported MYOC mutations. 12 The MYOC , p.Pro370Leu mutation has been described among JOAG families. 13 14 In the family described here, the penetrance was almost 100% by the age of 15 years. The much earlier penetrance and greater severity, points to the role of LTBP2 in causing an increased pathogenicity of MYOC , p.Pro370Leu mutation. The LTBP2 , p.Pro432Leu variant was found to be deleterious by PROVEAN, DEOGEN2, and FATHMM-MKL but not by the SIFT and Polyphen scores. The DEOGEN2 and FATHMM-MKL values score better as indices of deleteriousness compared with SIFT and Polyphen. 15 16
Both MYOC and LTBP2 genes have been shown to be coexpressing in trabecular meshwork (TM). 17 MYOC in TM plays an important role in regulation of IOP. The mutant MYOC aggregates in TM, which could deregulate the physiological function of TM, leading to IOP rise in the eye. The precise mechanism of LTBP2 contributing to glaucoma pathogenesis is not known, but it is presumed that the presence of defective LTBP2 in the ciliary body can alter the elasticity of Schlemm's canal and scleral spur (the aqueous humor drainage facilitating pathways) which in turn raises the IOP. A STRING analysis showed the two genes: LTBP2 and MYOC , to have an indirect interaction through other genes, though evidence of a direct interaction was not observed. While the values for PPI were indicative of a protein level interaction between the two genes, the RNA coexpression was weak given the value of 0.08. However, even if the score value is low (as was between the MYOC and LTBP2 genes), there is no way to rule out a nonoverlapping type of evidence for RNA coexpression.
While the primary disease association of biallelic LTBP2 variants is microspherophakia and/or ectopia lentis, LTBP2 mutations have been associated with wide range of phenotypes including PCG and recessively inherited JOAG. 2 17 18 19 Ours is the second report of an LTBP2 mutation in JOAG. An unaffected sibling, in this family, also carried LTBP2 , p.Pro432Leu mutation, but he did not have MYOC , p.Pro370Leu variant. In his case, probably both variants were required for disease manifestation or there is a possibility of later development of glaucoma in this sibling. It is also possible that MYOC , p.Pro370Leu may have the “dominator” effect, while LTBP2 , p.Pro432Leu may be having a “regulatory” effect.
DIgenic diseases DAtabase (DIDA), the first comprehensive resource for digenic diseases which includes 44 digenic diseases currently involving 213 digenic combinations, suggests that for two genetic variants to cause DI, it is essential to determine whether there is some relationship between genes in terms of direct or indirect interaction, coexpression, pathway membership, or similar function. In DIDA, almost 41% cases showed either one or two types of evidence/relationship favoring DI. 20 In this family, heterozygous mutations were present in the two genes, already known as glaucoma loci, with both genes having interaction with already known glaucoma genes. Since this is only a single small family, in which a statistical evidence of linkage of phenotype with genotype cannot be ascertained, we can only hypothesize the existence of DI and suggest both MYOC and LTBP2 genes could be screened for mutations, especially involving a severe JOAG phenotype. We believe that MYOC – LTBP2 interactions need to be addressed in larger samples of mutant carriers which may further provide clinical insight into the causal relationship among the variants in these genes, as also looking at the protein interactions of these two variants at a molecular level.
Funding Statement
Funding This study was supported by funding from the Indian Council of Medical Research (Grant Number: 5/3/8/339/2017-ITR), New Delhi, India.
Conflict of Interest None declared.
Data Availability Statement
Data supporting the findings of the study are available from the corresponding author (V.G.) on request.
Ethical Approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional ethics committee and with the Declaration of Helsinki 1964 and its later amendments or comparable ethical standards.
Authors' Contributions
Study conceptualization, analysis of clinical manifestations, and manuscript preparation were done by V.G. and B.I.S. Analysis and interpretation of genetic analysis data were done by B.I.S. Analysis of clinical manifestations was done by K.M., S.G., and K.A. Manuscript revision and study supervision were done by V.G.
References
- 1.Jelodari-Mamaghani S, Haji-Seyed-Javadi R, Suri F. Contribution of the latent transforming growth factor-β binding protein 2 gene to etiology of primary open angle glaucoma and pseudoexfoliation syndrome. Mol Vis. 2013;19:333–347. [PMC free article] [PubMed] [Google Scholar]
- 2.Saeedi O, Yousaf S, Tsai J, Palmer K, Riazuddin S, Ahmed Z M. Delineation of novel compound heterozygous variants in LTBP2 associated with juvenile open angle glaucoma. Genes (Basel) 2018;9(11):E527. doi: 10.3390/genes9110527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiggs J L. Genetic etiologies of glaucoma. Arch Ophthalmol. 2007;125(01):30–37. doi: 10.1001/archopht.125.1.30. [DOI] [PubMed] [Google Scholar]
- 4.Craig J E, Baird P N, Healey D L. Evidence for genetic heterogeneity within eight glaucoma families, with the GLC1A Gln368STOP mutation being an important phenotypic modifier. Ophthalmology. 2001;108(09):1607–1620. doi: 10.1016/s0161-6420(01)00654-6. [DOI] [PubMed] [Google Scholar]
- 5.Fingert J H, Héon E, Liebmann J M. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8(05):899–905. doi: 10.1093/hmg/8.5.899. [DOI] [PubMed] [Google Scholar]
- 6.Gupta V, Somarajan B I, Gupta S. The inheritance of juvenile onset primary open angle glaucoma. Clin Genet. 2017;92(02):134–142. doi: 10.1111/cge.12906. [DOI] [PubMed] [Google Scholar]
- 7.Gupta V, Somarajan B I, Gupta S.The mutational spectrum of myocilin gene among familial versus sporadic cases of juvenile onset open angle glaucomaEye (Lond)2020 [DOI] [PMC free article] [PubMed]
- 8.Hogewind B F, Gaplovska-Kysela K, Theelen T. Identification and functional characterization of a novel MYOC mutation in two primary open angle glaucoma families from The Netherlands. Mol Vis. 2007;13:1793–1801. [PubMed] [Google Scholar]
- 9.Iliev M E, Bodmer S, Gallati S. Glaucoma phenotype in a large Swiss pedigree with the myocilin Gly367Arg mutation. Eye (Lond) 2008;22(07):880–888. doi: 10.1038/sj.eye.6702745. [DOI] [PubMed] [Google Scholar]
- 10.Vincent A L, Billingsley G, Buys Y. Digenic inheritance of early-onset glaucoma: CYP1B1, a potential modifier gene. Am J Hum Genet. 2002;70(02):448–460. doi: 10.1086/338709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukhopadhyay A, Acharya M, Mukherjee S. Mutations in MYOC gene of Indian primary open angle glaucoma patients . Mol Vis. 2002;8:442–448. [PubMed] [Google Scholar]
- 12.Hewitt A W, Mackey D A, Craig J E. Myocilin allele-specific glaucoma phenotype database. Hum Mutat. 2008;29(02):207–211. doi: 10.1002/humu.20634. [DOI] [PubMed] [Google Scholar]
- 13.Adam M F, Belmouden A, Binisti P. Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Hum Mol Genet. 1997;6(12):2091–2097. doi: 10.1093/hmg/6.12.2091. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki Y, Shirato S, Taniguchi F, Ohara K, Nishimaki K, Ohta S. Mutations in the TIGR gene in familial primary open-angle glaucoma in Japan. Am J Hum Genet. 1997;61(05):1202–1204. doi: 10.1086/301612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassan M S, Shaalan A A, Dessouky M I, Abdelnaiem A E, ElHefnawi M. Evaluation of computational techniques for predicting non-synonymous single nucleotide variants pathogenicity. Genomics. 2019;111(04):869–882. doi: 10.1016/j.ygeno.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Raimondi D, Gazzo A M, Rooman M, Lenaerts T, Vranken W F. Multilevel biological characterization of exomic variants at the protein level significantly improves the identification of their deleterious effects. Bioinformatics. 2016;32(12):1797–1804. doi: 10.1093/bioinformatics/btw094. [DOI] [PubMed] [Google Scholar]
- 17.Ali M, McKibbin M, Booth A. Null mutations in LTBP2 cause primary congenital glaucoma. Am J Hum Genet. 2009;84(05):664–671. doi: 10.1016/j.ajhg.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Désir J, Sznajer Y, Depasse F. LTBP2 null mutations in an autosomal recessive ocular syndrome with megalocornea, spherophakia, and secondary glaucoma. Eur J Hum Genet. 2010;18(07):761–767. doi: 10.1038/ejhg.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan A O, Aldahmesh M A, Alkuraya F S. Congenital megalocornea with zonular weakness and childhood lens-related secondary glaucoma - a distinct phenotype caused by recessive LTBP2 mutations. Mol Vis. 2011;17:2570–2579. [PMC free article] [PubMed] [Google Scholar]
- 20.Gazzo A M, Daneels D, Cilia E.DIDA: A curated and annotated digenic diseases database Nucleic Acids Res 201644(D1):D900–D907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings of the study are available from the corresponding author (V.G.) on request.


