Abstract
Purpose
Immigrants are susceptible to marginalization due to social isolation, economic disadvantage and systemic bias. Our goal was to compare symptom burden between immigrant and long-term resident women undergoing breast cancer surgery in Ontario, Canada.
Methods
A population-level retrospective cohort-study using administrative databases was conducted. Women who underwent surgery for newly diagnosed breast cancer and were treated at regional cancer centers between 2010 and 2016 were included. The primary outcome was a moderate or severe (≥ 4) symptom score on the Edmonton Symptom Assessment System Scale (ESAS).
Results
There were 12,250 (87.2%) long-term Canadian residents and 1,806(12.8%) immigrants. Immigrants were younger (mean age 53 vs. 61 years); had a higher proportion residing in a lowest income quintile neighbourhood (22.2% vs 15.4%); were less often on a primary-care physician roster (83.7% vs. 90.4%); and were less often diagnosed with Stage I/II disease (80.9% vs. 84.6%) (all p < 0.01). The proportion of women with scores ≥ 4 was significantly higher amongst immigrant women for 7/9 symptom categories; with the largest differences for depression (24.9% vs. 20.2%, p < 0.01) and pain (28.0% vs. 22.4%, p < 0.01). On multivariable regression analysis, immigration status was associated with scores ≥ 4 for pain (OR 1.13, 95% CI 1.02–1.23). There was an association between moderate/severe pain and region of origin, but not length of stay in Canada or immigration class.
Conclusions
This is the first study comparing symptom burden amongst immigrant and non-immigrant women with breast cancer at a population-level. Immigrant women with breast cancer undergoing surgery were found to have a higher burden of pain.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10549-023-06938-8.
Keywords: Breast cancer, Symptom management, Disparities, Pain
Introduction
Disparities in breast cancer outcomes between segments of the population are well documented with a burgeoning body of literature shedding light on the interplay of multiple factors [1–4]. Social determinants have a direct effect on health [5]. Factors such as poverty, neighborhood disadvantage, access to health services, and systemic bias have been shown to adversely affect breast cancer outcomes [5]. Immigrants are people living in a country other than that of their birth. While there are cross-cutting social determinants of health that impact both immigrants and non-immigrants, immigrants may be particularly susceptible to marginalization because of challenges with health systemic navigation, absence of family or community support, financial stability and language fluency, amongst other issues. First-generation immigrants, those born outside of Canada, compromise 21.9% of the Canadian population. It is expected that this proportion will increase to 34% by 2041 for a number of reasons including: population growth, increasing connectivity, political situations such as conflict and climate change [6].
Differences in breast cancer screening rates [7] and stage at diagnosis [8] have been demonstrated between immigrant and non-immigrant Canadian women. Amongst immigrant women in Ontario, new immigrants, refugee status women, and women from South Asia have the lowest rates of screening [7]. Immigrants from low and middle income countries compared to high income countries have also been shown to be less likely to be diagnosed with early stage breast cancer [8]. However, breast cancer outcomes in the immigrant population have not been well studied to date. Measuring outcomes, including patient reported symptoms is important to develop a better understanding of the patient experience. Patients with breast cancer are known to experience many physical and psychosocial symptoms that negatively affect their quality of life [9, 10]. Work in the sphere of palliative care has demonstrated that immigrant patients with other cancers (lung, gastrointestinal, central nervous system and urologic) have higher symptom burden in the last 6 months of life compared to Canadian long-term residents [11]. This study focused on patients undergoing treatment at the end of life, while patients with early-stage disease and those under going multi-modality care were not included in the cohort. Qualitative methods examining survivorship have revealed certain immigrant groups with breast cancer report unmet physical and emotional needs [12]. However, assessment of patient reported symptom burden amongst immigrant women undergoing curative intent treatment at a population level or using quantitative methods is lacking.
The purpose of the present study is to compare symptom burden and trajectories between immigrant and Canadian long-term resident women who underwent breast cancer surgery at regional cancer centers in Ontario. The focus on patients undergoing breast cancer surgery was chosen as these patients typically have early-stage disease and are undergoing multi-modality care. We hypothesized that immigrant women with breast cancer would have a higher unmet symptom burden compared to long-term Canadian residents independent of co-morbidity, demographic, stage and treatment variables, due to the unique challenges faced by this population.
Methods
Study design and cohort
A population-level retrospective cohort-study using linked Ontario administrative databases held at ICES was conducted. ICES is an independent, non-profit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement. Women ≥ 18 years of age with newly diagnosed breast cancer between January 1, 2010 and March 1, 2016 and undergoing treatment at regional cancer centers in Ontario were included. Regional cancer centers are responsible for implementing programs for cancer care that meet the requirements and targets set out in their partnership agreements with Cancer Care Ontario [13]. We limited the cohort to women undergoing breast cancer surgery as part of their treatment. Women were excluded if they had in-situ disease only, missing staging data, another cancer diagnosed within the last five years, or did not have provincial health care plan coverage. In Ontario, universal healthcare plan coverage is provided by through the Ontario Health Insurance Plan (OHIP). The vast majority of residents of Ontario have OHIP coverage.
Data sources and covariates
Incident cancer cases were identified from the Ontario Cancer Registry (OCR) along with their stage and histology information. The Immigration, Refugees and Citizenship Canada’s Permanent Resident (IRCC-PR) database was used to determine immigration status. The IRCC-PR database contains immigration data on people who immigrated to Canada from 1985 onward. Women not in the IRCC-PR database were classified as “long-term residents/Canadian-born”, as some of these women would be immigrants who had arrived prior to 1985 and others would be Canadian born. Immigrant women were subclassified by immigrant class (economic, family, refugee or other), region of origin and length of stay in Canada. Region of origin was classified based on previously described modified World Bank Regions [7]. The Ontario Health Insurance Plan (OHIP) physician billing claims, Discharge Abstract Database (DAD), Same Day Surgery Database (SDS), Cancer Activity Level Reporting (ALR), and the National Ambulatory Care Reporting System (NACRS) were used to obtain treatment data including receipt of surgery, radiation and chemotherapy. Pre-existing comorbidities were categorized using the Johns Hopkins ACG® System Version 10 Aggregated Diagnosis Groups (ADGs) (defined using hospitalizations and outpatient visits occurring in the 2 years before the index date, while excluding the malignancy diagnosis group from the score) [14, 15]. The Ontario Marginalization (ON-Marg) index was used to assess for confounding based on the neighbourhood demographics [16]. Being enrolled on a primary physician roster was also captured through the Client Agency Program Enrolment (CAPE) database. These datasets were linked using unique encoded identifiers and analyzed at ICES.
Outcome measures
The primary outcome was the proportion of patients reporting moderate/severe (≥ 4) Edmonton Symptom Assessment System Scale (ESAS) scores for each of 9 individual symptom categories over time. The Edmonton Symptom Assessment System (ESAS) was first developed in 1991 as a simple and comprehensive method for symptom assessment in patients with advanced cancer admitted to palliative care units. Since its inception, it has been validated in patients undergoing curative intent treatment, has been translated into over 20 languages, is open source, and has now been implemented across regional cancer centers in Ontario [17]. The ESAS has several strengths including the ability to be completed quickly, it can be used to monitor symptom progression over time, and it allows for symptom clusters to be identified [18, 19]. Completion of the ESAS is voluntary. The ESAS is scored from 0 to 10, with higher scores indicating a higher symptom burden. A dichotomous outcome measure was used as a moderate-to-severe score which has been previously shown to identify patients with a clinically significant symptom burden [20]. Data points are reported at 3-month intervals for the first year after diagnosis, and then 6-month intervals until data cut-off in March 2020, prior to the COVID-19 pandemic. When more than one data point was available within a time interval, the highest reported score was taken. If scores for one of the symptoms for a patient was missing in a time interval, the score would be imputed to be clinically insignificant (< 4) [21].
Statistical analysis
The study cohort is presented using descriptive statistics. Baseline variables were described using means and frequencies for continuous and categorical variables, respectively. Total proportion of patients reporting ESAS scores ≥ 4 at any point in time as well as patient trajectories of these proportions were presented graphically, with a denominator given by the total number of ESAS assessments recorded in the time interval. Significance between study groups was determined by a Chi-Squared test. Multivariable logistic regression models were constructed to assess the association between immigration status and moderate/severe ESAS scores for all 9 symptom categories with covariates selected a priori. Generalized estimating equations with exchangeable correlation structure were applied to account for repeated measures for each patient. Surgery patients who reported ESAS scores were compared to those with missing ESAS score in order to assess generalizability. All analyses were conducted using SAS Version 9.4 (SAS Institute, Cary, NC).
Results
Baseline characteristics
The cohort included 14,056 women, of which 12,250 (87.2%) were categorized as Canadian long-term residents and 1806 (12.8%) as immigrants (Table 1). Immigrant women had a younger mean age of diagnosis (53 vs. 61 years), more often resided in a lowest income quintile neighbourhood (22.2% vs 15.4%) and were less likely to be on a primary care physician roster within the last 2 years (83.7% vs. 90.4%) (all p < 0.01). Immigrant women were less often diagnosed with Stage I/II disease (80.9% vs. 84.6%) and had a higher proportion of Grade 3 tumours (29.7 vs. 26.1%) (both p < 0.01). The characteristics of the immigrant cohort with respect to immigration specific variables including region of origin, length of stay in Canada and immigration class are displayed in Table 2.
Table 1.
Baseline variables of study cohort
| Variable | Total n = 14,056 |
Canadian long-term resident n = 12,250 |
Immigrant n = 1806 |
p-value | |
|---|---|---|---|---|---|
| Age at diagnosis | Mean (SD) | 60.08 (12.99) | 61.12 (12.85) | 53.01 (11.68) | < 0.01 |
| Neighborhood Income Quintile | 1–n (%) | 2291 (16.3%) | 1890 (15.4%) | 401 (22.2%) | < 0.01 |
| 2 | 2575 (18.3%) | 2242 (18.3%) | 333 (18.4%) | ||
| 3 | 2641 (18.8%) | 2279 (18.6%) | 362 (20.0%) | ||
| 4 | 3090 (22.0%) | 2680 (21.9%) | 410 (22.7%) | ||
| 5 | 3459 (24.6%) | 3159 (25.8%) | 300 (16.6%) | ||
| Johns Hopkins ADG Comorbidity score | 0–n (%) | 105 (0.7%) | 94 (0.8%) | 11 (0.6%) | 0.77 |
| 1–5 | 5684 (40.4%) | 4967 (40.5%) | 717 (39.7%) | ||
| 6–9 | 5741 (40.8%) | 4989 (40.7%) | 752 (41.6%) | ||
| 10 + | 2526 (18.0%) | 2200 (18.0%) | 326 (18.1%) | ||
| Geographic location | Rural—n (%) | 852 (6.1%) | 839 (6.8%) | 13 (0.7%) | < 0.01 |
| Urban | 13,204 (93.9%) | 11,411 (93.2%) | 1793 (99.3%) | ||
| Neighborhood dependency quintile | 1–n (%) | 3296 (23.4%) | 2541 (20.7%) | 755 (41.8%) | < 0.01 |
| 2 | 2731 (19.4%) | 2354 (19.2%) | 377 (20.9%) | ||
| 3 | 2628 (18.7%) | 2337 (19.1%) | 291 (16.1%) | ||
| 4 | 2339 (16.6%) | 2151 (17.6%) | 188 (10.4%) | ||
| 5 | 3062 (21.8%) | 2867 (23.4%) | 195 (10.8%) | ||
| Neighborhood deprivation quintile | 1–n (%) | 3113 (22.1%) | 2858 (23.3%) | 255 (14.1%) | < 0.01 |
| 2 | 3148 (22.4%) | 2803 (22.9%) | 345 (19.1%) | ||
| 3 | 2936 (20.9%) | 2548 (20.8%) | 388 (21.5%) | ||
| 4 | 2602 (18.5%) | 2208 (18.0%) | 394 (21.8%) | ||
| 5 | 2257 (16.1%) | 1833 (15.0%) | 424 (23.5%) | ||
| Neighborhood ethnic diversity quintile | 1–n (%) | 2217 (15.8%) | 2171 (17.7%) | 46 (2.5%) | < 0.01 |
| 2 | 2619 (18.6%) | 2529 (20.6%) | 90 (5.0%) | ||
| 3 | 3239 (23.0%) | 3044 (24.8%) | 195 (10.8%) | ||
| 4 | 3147 (22.4%) | 2711 (22.1%) | 436 (24.1%) | ||
| 5 | 2834 (20.2%) | 1795 (14.7%) | 1039 (57.5%) | ||
| Neighborhood instability quintile | 1–n (%) | 2769 (19.7%) | 2249 (18.4%) | 520 (28.8%) | < 0.01 |
| 2 | 2555 (18.2%) | 2245 (18.3%) | 310 (17.2%) | ||
| 3 | 2703 (19.2%) | 2478 (20.2%) | 225 (12.5%) | ||
| 4 | 2595 (18.5%) | 2344 (19.1%) | 251 (13.9%) | ||
| 5 | 3434 (24.4%) | 2934 (24.0%) | 500 (27.7%) | ||
| Cancer stage | I–n (%) | 6351 (45.2%) | 5636 (46.0%) | 715 (39.6%) | < 0.01 |
| II | 5471 (38.9%) | 4725 (38.6%) | 746 (41.3%) | ||
| III | 2081 (14.8%) | 1760 (14.4%) | 321 (17.8%) | ||
| IV | 153 (1.1%) | 129 (1.1%) | 24 (1.3%) | ||
| Presence on primary physician roster within 2 years of diagnosis | No–n (%) | 1474 (10.5%) | 1179 (9.6%) | 295 (16.3%) | < 0.01 |
| Yes | 12,582 (89.5%) | 11,071 (90.4%) | 1511 (83.7%) | ||
| HR status | Negative—n (%) | 1915 (13.6%) | 1658 (13.5%) | 257 (14.2%) | 0.60 |
| Positive | 10,068 (71.6%) | 8792 (71.8%) | 1276 (70.7%) | ||
| Unknown | 2073 (14.7%) | 1800 (14.7%) | 273 (15.1%) | ||
| HER2 status | Negative—n (%) | 7133 (50.7%) | 6235 (50.9%) | 898 (49.7%) | < 0.01 |
| Positive—n (%) | 1293 (9.2%) | 1078 (8.8%) | 215 (11.9%) | ||
| Unknown | 5630 (40.1%) | 4937 (40.3%) | 693 (38.4%) | ||
| Grade | 1–n (%) | 2112 (15.0%) | 1926 (15.7%) | 186 (10.3%) | < 0.01 |
| 2 | 5202 (37.0%) | 4562 (37.2%) | 640 (35.4%) | ||
| 3 | 3737 (26.6%) | 3201 (26.1%) | 536 (29.7%) | ||
| Unknown | 3005 (21.4%) | 2561 (20.9%) | 444 (24.6%) | ||
| Surgery | BCS—n (%) | 8976 (63.9%) | 7905 (64.5%) | 1071 (59.3%) | < 0.001 |
| Mastectomy | 5080 (36.1%) | 4345 (35.5%) | 735 (40.7%) | ||
| Received radiation therapy within 12 months of surgery | No—n (%) | 3730 (26.5%) | 3286 (26.8%) | 444 (24.6%) | 0.04 |
| Yes | 10,326 (73.5%) | 8964 (73.2%) | 1362 (75.4%) | ||
| Received chemotherapy within 6 months of diagnosis | No—n (%) | 6371 (45.3%) | 5686 (46.4%) | 685 (37.9%) | < 0.01 |
| Yes | 7685 (54.7%) | 6564 (53.6%) | 1121 (62.1%) |
ADG aggregated diagnosis groups, HR hormone receptor, BCS breast conserving surgery
Table 2.
Baseline variables specific to immigrant cohort
| Variable | Total = 1806 n (%) |
|---|---|
| Immigration class | |
| Economic | 905 (50.1%) |
| Family | 637 (35.3%) |
| Refugee | 231 (12.8%) |
| Other | 33 (1.8%) |
| Length of stay in Canada (years) | |
| Established (11 +) | 1244 (68.9%) |
| Recent (6–10) | 320 (17.7%) |
| New (0–5) | 242 (13.4%) |
| Region of origin | |
| East Asia and Pacific Islands | 465 (25.7%) |
| Eastern Europe and Central Asia | 335 (18.5%) |
| Latin America and the Caribbean | 193 (10.7%) |
| Middle East and North Africa | 242 (13.4%) |
| South Asia | 311 (17.2%) |
| Sub Saharan Africa | 82 (4.5%) |
| USA, Australia and New Zealand | 56 (3.1) |
| Western Europe | 122 (6.8%) |
| Canadian Language ability | |
| Bilingual | 60 (3.3%) |
| English | 1193 (66.1%) |
| French | 16 (0.9%) |
| Neither | 537 (29.7%) |
Outcomes
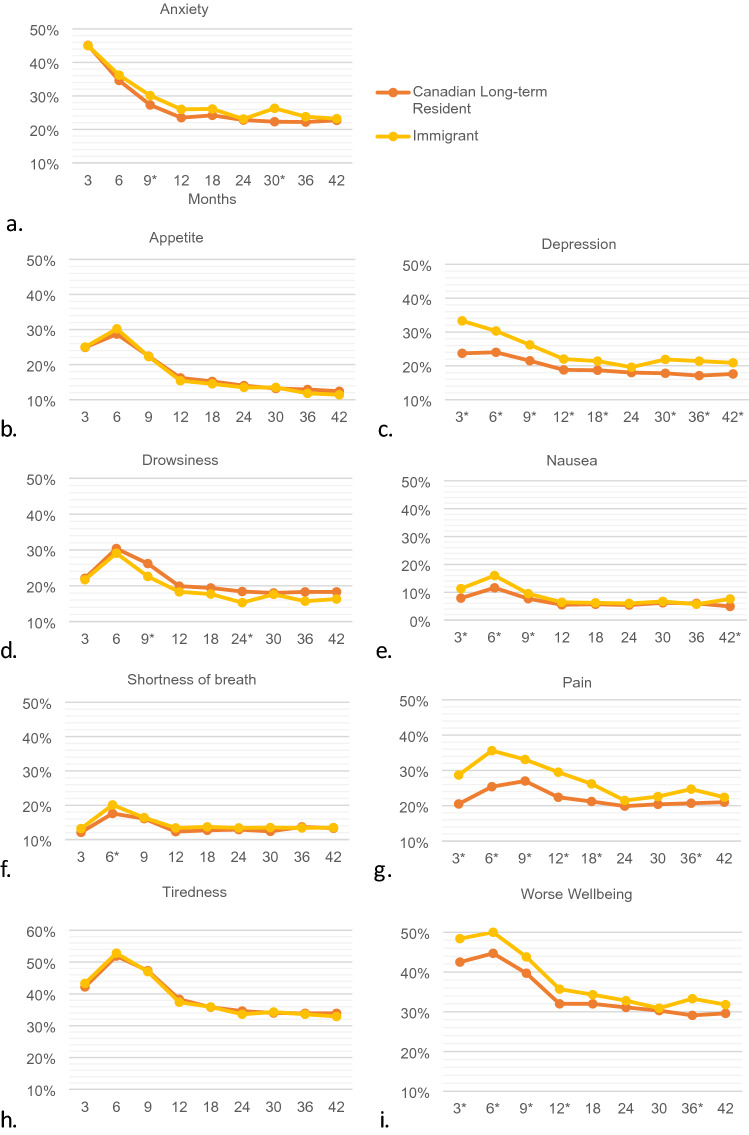
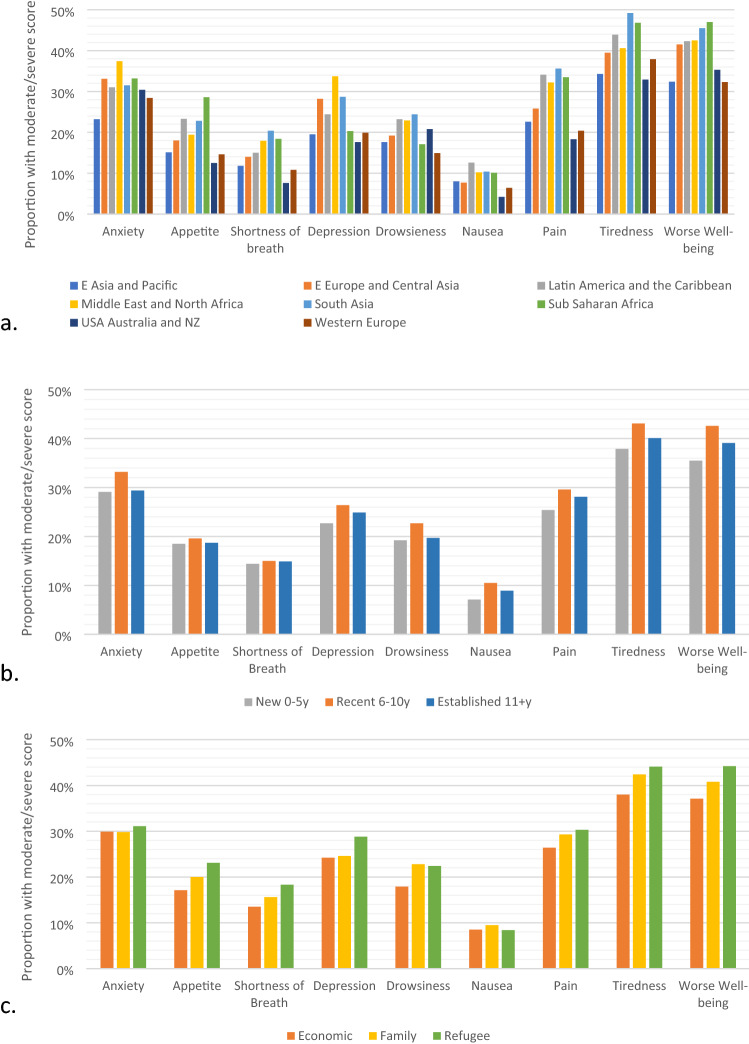
During the study time period there were 67,466 unique ESAS assessments measured within the follow-up period. Of these scores, 58,915 (87.3%) were from Canadian long-term residents and 8,551 (12.7%) were from immigrant women. Median number of scores reported per patient was the same in both groups [5 (IQR 3–7)]. Comparison of patients with and without ESAS assessments are presented in Online Appendix 1. Those without ESAS assessments includes those treated outside of a regional cancer center where ESAS measures are not routinely collected. The proportion of women reporting moderate/severe scores was significantly different between immigrant women and Canadian-long term residents for all symptoms categories except appetite and tiredness (Fig. 1). For the remaining 7 symptom categories, immigrant women reported more moderate/severe scores on all scales except drowsiness. The largest magnitude of difference was found for depression and pain. For depression, the proportion of Canadian long-term residents reporting an ESAS score ≥ 4 at 3 months was 20.2% vs 24.9% for immigrant women (p < 0.01). For pain, the proportion of Canadian long-term residents reporting an ESAS score ≥ 4 at 6 months was 22.4% vs 28.0% for immigrant women (p < 0.01). Symptom trajectories for each of the 9 individual symptom scales comparing immigrant and long-term resident women are presented in Fig. 2. The proportion of immigrant women with ESAS score ≥ 4 for each symptom category at any time point by region of origin, immigration class and length of stay is displayed in Fig. 3. For overall worse-well-being women from USA/Australia/New Zealand, Western Europe and East Asia had the lowest scores, whereas women from South Asia and Sub-Saharan Africa had the highest scores. A similar pattern was seen for tiredness, pain, shortness of breath and appetite.
Fig. 1.
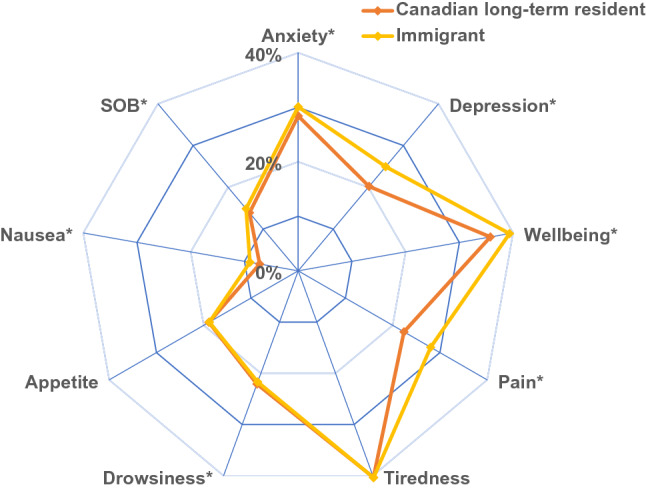
Proportion with ESAS ≥ 4 at any time point for each symptom scale comparing Canadian long-term resident and immigrant women. *denotes statistically significant difference between groups
Fig. 2.
Proportion with ESAS score ≥ 4 in all eligible patient visits over time comparing Canadian long-term residents and immigrant women for symptom scales: a anxiety, b appetite, c depression, d drowsiness, e nausea, f pain, g tiredness, h shortness of breath, i wellbeing. *Statistically significant difference between groups
Fig. 3.
Proportion of immigrant women with ESAS score ≥ 4 for each symptom at any time point based on a. region of origin b. length of stay and c. immigration class
Multivariable logistic regression modelling of the probability of a moderate/severe ESAS score adjusting for baseline variables comparing immigrant versus Canadian long-term resident women identified significant differences for anxiety, drowsiness, pain and tiredness (Table 3). Immigrant status was associated with a protective effect for anxiety (OR, 95% CI: 0.86, 0.78–0.94), drowsiness (OR 95% CI 0.81, 0.73–0.89) and tiredness (OR 95% CI 0.86, 0.79–0.94). Immigrant status was associated with worse pain (OR 95% CI 1.13, 1.03–1.23). While on univariate analysis immigrant women reported more depression, there was no difference found between immigrant and Canadian-long term residents on multivariable analysis. Higher odds of reporting a moderate/severe depression score was seen amongst women from the lowest income quintile neighbourhood, women from neighbourhoods with the highest deprivation and ethnic diversity quintiles, women with the highest co-morbidity scores, Grade 3 tumours, more advanced stage, those receiving chemotherapy and those having mastectomy on multivariable analysis (Online Appendix 2). In the multivariable model, type of surgery (mastectomy vs. BCS) was a significant predictor of higher scores for anxiety, appetite, depression, pain and worse well-being (Online Appendix 2).
Table 3.
Multivariable logistic regression comparing immigrant women and Canadian long-term resident women (Reference) for proportion of scores with ESAS ≥ 4 for individual symptom scales
| ESAS symptom | OR (95%CI) | p-value |
|---|---|---|
| Anxiety | 0.86 (0.78–0.94) | < 0.01 |
| Appetite | 0.91 (0.83–1.00) | 0.06 |
| Depression | 1.05 (0.95–1.16) | 0.40 |
| Drowsiness | 0.81 (0.73–0.89) | < 0.01 |
| Nausea | 1.09 (0.96–1.24) | 0.19 |
| Pain | 1.13 (1.03–1.23) | 0.01 |
| Tiredness | 0.86 (0.79–0.94) | < 0.01 |
| Shortness of breath | 1.07 (0.95–1.20) | 0.24 |
| Wellbeing | 0.98 (0.90–1.07) | 0.68 |
Variables included in the model: age, geographic location, neighbourhood income quintile, neighbourhood dependency quintile, neighbourhood deprivation quintile, neighbourhood ethnic diversity quintile, neighbourhood residential instability quintile, Johns Hopkins ADG comorbidity score, cancer stage, hormone receptor status, Her2 receptor status, grade, type of surgery, receipt of radiation, receipt of chemotherapy, presence on physician roster
Bold indicates statistical significance (p < 0.05)
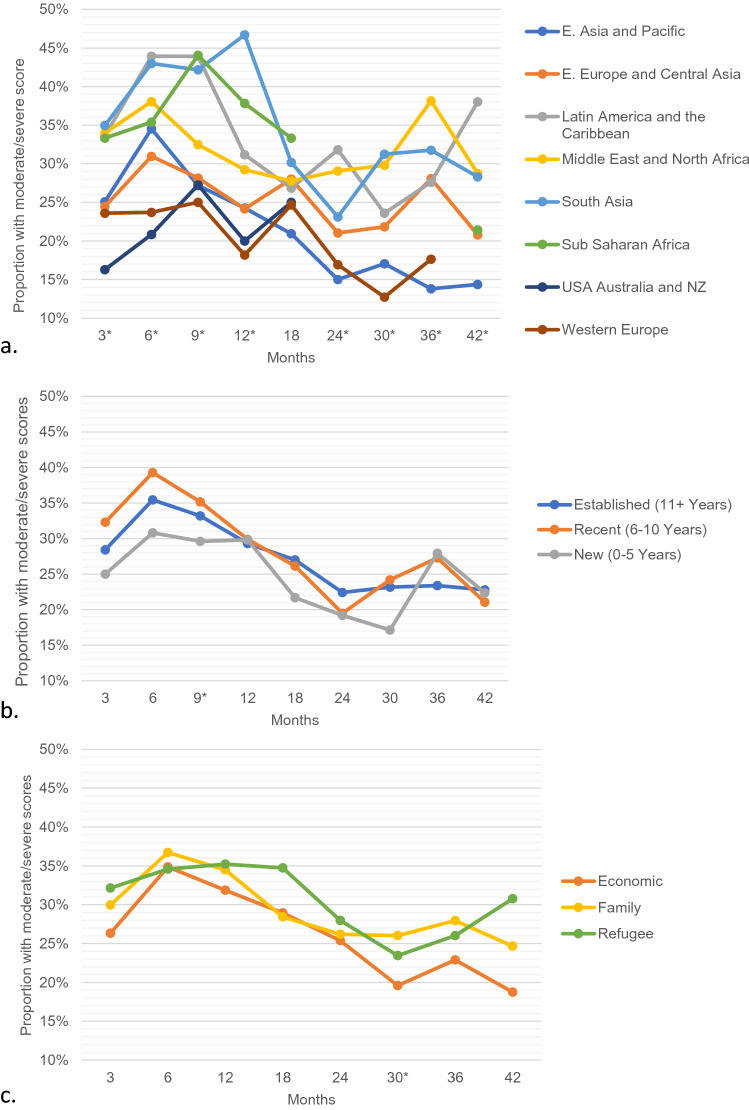
The proportion of immigrant women reporting moderate/severe pain over time was compared based on region of origin, length of stay in Canada and immigration class (Fig. 4). Statistically significant differences based on region of origin were found at all time points except 18 months. At 12 months, 46.7% of women from South Asia reported moderate/severe pain compared to 18.2% of women from Western Europe. Women from the USA/Australia/New Zealand, Western Europe, and East Asia had the lowest reported moderate/severe pain, whereas women from South Asia, Sub-Saharan Africa, Middle East/North Africa and Latin America and the Caribbean had the highest. Statistically significant differences were not observed at most time points when comparing based on length of stay or immigration class.
Fig. 4.
Proportion of immigrant women with ESAS score ≥ 4 for pain over time by a. region of origin b. length of stay and c. immigration class. *Statistically significant difference between groups. Values with fewer than 50 responses are censored
Discussion
The number of international migrants has been steadily rising over the last two decades, driven by economic, environmental, and political factors [22]. To our knowledge, this is the first study exploring symptom burden amongst immigrant women undergoing breast cancer surgery at the population level. This study included a large cohort of immigrant women from diverse countries of origin, length of stay in Canada and immigration class.
This study demonstrates that amongst immigrant women undergoing breast cancer surgery, there is a higher burden of symptoms of anxiety, depression, nausea, shortness of breath, pain and overall worse well-being compared to long-term residents/Canadian-born women. However, the higher burden of symptoms was no longer present when adjusting for confounders for all the symptom categories except for pain. Factors including stage, adjuvant chemotherapy, neighbourhood income quintile and deprivation quintile, and having a primary care physician were significant in the models.
Immigration status was found to be protective for anxiety amongst women with breast cancer on multi-variable analysis. This finding is in keeping with the literature which demonstrates that first generation immigrant groups have a lower prevalence of anxiety disorders [23]. The reason for this is likely multifactorial, but in part related to the resilience required for the migration experience [23]. Similar findings were seen for measures of fatigue—tiredness and drowsiness. Pain amongst women undergoing breast cancer treatment is a prevalent and complex issue that can have many detrimental effects [24]. In our study, immigration status was associated with worse pain in patients undergoing breast cancer surgery on multivariable analysis. Significant differences existed consistently between immigrant and Canadian-long term resident women until 18 months from the time of diagnosis. After 18 months, there was not significant difference seen for the majority of remaining time points, perhaps indicative of inadequate management of pain during active treatment rather than a baseline difference between groups. The magnitude of the difference was most pronounced at 6 months, where the difference in proportion of women reporting moderate/severe symptoms, exceeded 10%. Other factors linked to worse pain included age, neighbourhood income quintile, higher neighbourhood deprivation quintile, more advanced stage and receipt of chemotherapy. Mastectomy compared to breast conserving surgery was also associated with more pain on multivariable analysis.
The reason for the increased self-reported burden of pain amongst immigrant women is not clear, and likely multi-faceted. Pain in marginalized populations can be poorly recognized and unsuccessfully managed [25]. Immigrant women with language barriers may have challenges in adequately communicating their pain and response to treatment, and providers may have difficulty recognizing pain symptoms [25]. There may be cultural differences in reporting pain and response to treatment [26]. There is also the possibility of unconscious bias and stereotyping influencing health care providers clinical decisions [27]. Under treatment of pain can be related to under prescribing pain medication and/or patients’ being less willing to take medication [28]. A previous US study that used administrative data demonstrated that foreign-born women with breast cancer are less likely to use opioid pain medication [29]. We did also identify differences in proportion of women with moderate/severe pain based on region of origin, but not length of stay or immigration class. Women from South Asia and Sub-Saharan Africa had the highest reported scores, whereas women from Western Europe, the US, Australia and New Zealand had the lowest reported scores. The observed difference raises the possibility of the effect of unconscious bias and inadequate cultural competence. Women born in Sub-Saharan Africa and South Asia are often racialized, whereas women born in Western Europe, USA, Australia and New Zealand are less often racialized (though of course this is not true for every woman). We did not have race data in our cohort, but this finding suggests race may play a role in the difference we observed. Furthermore, fewer cultural similarities exist between Sub-Saharan Africa, South Asia and Canada compared to Western Europe, USA, Australia, New Zealand and Canada. When healthcare providers interact with women who dress or speak in a way unfamiliar to them, they may make unfounded assumptions about these women’s healthcare needs [30].
There are limitations to this study which warrant mention. Patients without provincial health care coverage are not included in the dataset. This group may experience more marginalization than the remainder of the cohort. With a large administrative database study, missing data, including missing ESAS measures, is prevalent. The cohort completing the ESAS assessments differed from those not completing the ESAS assessments on baseline characteristics. Those completing the ESAS assessments came from higher income neighbourhoods, resided in urban areas and in neighbourhoods with low levels of deprivation, dependency, and instability. Those receiving chemotherapy and radiation therapy more often completed the ESAS, likely related to more frequent health care visits. The impact on generalizability is difficult to interpret, but there may be a difference in symptom burden in the cohort not studied. In our study, all patients were cared for at regional academic cancer centers where uniform practice guidelines would be expected. The differences at other centers may be more pronounced. With a large database, it is possible to observe statistically significant differences that are not necessarily clinically meaningful. However, with equity research, even small differences which are otherwise unexplained warrant further investigation into potentially biased systems and processes.
Conclusion
Immigrant women in Ontario undergoing breast cancer surgery report more moderate/severe pain than Canadian long-term residents independent of other sociodemographic and treatment variables. Differences in pain were observed between women based on region of origin, but not length of stay or immigration class. Further research to understand approaches to more optimal pain management in these women is needed. This work highlights the need of symptom support services to be accessible and delivered by providers with cultural competence.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Dr. Parvez is supported by a career grant through the Hamilton Health Sciences Foundation W.E. Noonan Fellowship. Funding for this research was provided through the McMaster Buffet Taylor Chair in Breast Cancer Research.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by DK and EP. The first draft of the manuscript was written by EP and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). This study also received funding from the McMaster Buffet Taylor Chair in Breast Cancer Research. This document used data adapted from the Statistics Canada Postal CodeOM Conversion File, which is based on data licensed from Canada Post Corporation, and/or data adapted from the Ontario Ministry of Health Postal Code Conversion File, which contains data copied under license from ©Canada Post Corporation and Statistics Canada. Parts of this material are based on data and/or information compiled and provided by CIHI, the Ontario Ministry of Health, Ontario Health (OH), and Immigration, Refugees and Citizenship Canada (IRCC) current to May 2017. The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. We thank the Toronto Community Health Profiles Partnership for providing access to the Ontario Marginalization Index.
Data availability
The datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
All authors have no relevant conflicts of interest to disclose.
Ethical approval
The use of the data in this project is authorized under section 45 of Ontario’s Personal Health Information Protection Act (PHIPA) and does not require review by a Research Ethics Board.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yu AYL, et al. Disease characteristics and mortality among Asian women with breast cancer. Cancer. 2022;128(5):1024–1037. doi: 10.1002/cncr.34015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.San Miguel Y, et al. Age-related differences in breast cancer mortality according to race/ethnicity, insurance, and socioeconomic status. BMC Cancer. 2020;20(1):1–9. doi: 10.1186/s12885-020-6696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dreyer MS, Nattinger AB, McGinley EL, Pezzin LE. Socioeconomic status and breast cancer treatment. Breast cancer Res Treat. 2018;167(1):1–8. doi: 10.1007/s10549-017-4490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daly B, Olopade OI. A perfect storm: how tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65(3):221–238. doi: 10.3322/caac.21271. [DOI] [PubMed] [Google Scholar]
- 5.Coughlin SS. Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res Treat. 2019;177(3):537–548. doi: 10.1007/s10549-019-05340-7. [DOI] [PubMed] [Google Scholar]
- 6.Statistics Canada: Immigration and ethnocultural diversity statistics [Internet] (2022). Available from: https://www.statcan.gc.ca/en/subjects-start/immigration_and_ethnocultural_diversity
- 7.Vahabi M, Lofters A, Kumar M, Glazier RH. Breast cancer screening disparities among immigrant women by world region of origin: a population-based study in Ontario, Canada. Cancer Med. 2016;5(7):1670–1686. doi: 10.1002/cam4.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iqbal J, et al. A population-based cross-sectional study comparing breast cancer stage at diagnosis between immigrant and Canadian-born women in Ontario. Breast J. 2017;23(5):525–536. doi: 10.1111/tbj.12785. [DOI] [PubMed] [Google Scholar]
- 9.Chow S, et al. Symptoms predictive of overall quality of life using the edmonton symptom assessment scale in breast cancer patients receiving radiotherapy. Clin Breast Cancer. 2019;19(6):405–410. doi: 10.1016/J.CLBC.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Corkum JP, Butler K, Zhong T. higher distress in patients with breast cancer is associated with declining breast reconstruction. Plast Reconstr Surg Glob Open. 2020 doi: 10.1097/GOX.0000000000002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bubis LD, et al. Patient-reported symptom severity among 22,650 cancer outpatients in the last six months of life. J Pain Symp Manag. 2020;59(1):58–66.e4. doi: 10.1016/j.jpainsymman.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Wen K-Y, Fang CY, Ma GX. Breast cancer experience and survivorship among Asian Americans: a systematic review. J Cancer Surv. 2014;8(1):94–107. doi: 10.1007/s11764-013-0320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancer Care Ontario: Regional Cancer Programs. https://www.cancercareontario.ca/en/cancer-care-ontario/programs/regional-cancer-programs accessed 17 Mar 2023
- 14.Cheung MC, et al. Impact of immigration status on cancer outcomes in Ontario, Canada. J Oncol Pract. 2017;13(7):e602–e609. doi: 10.1200/JOP.2016.019497. [DOI] [PubMed] [Google Scholar]
- 15.Austin PC, Shah BR, Newman A, Anderson GM. Using the Johns Hopkins’ Aggregated Diagnosis Groups (ADGs) to predict 1-year mortality in population-based cohorts of patients with diabetes in Ontario, Canada. Diab Med. 2012;29(9):1134–1141. doi: 10.1111/j.1464-5491.2011.03568.x. [DOI] [PubMed] [Google Scholar]
- 16.van Ingen T, Matheson FI. The 2011 and 2016 iterations of the Ontario Marginalization Index: updates, consistency and a cross-sectional study of health outcome associations. Can J Public Health. 2022;113(2):260–271. doi: 10.17269/s41997-021-00552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkova J, et al. Cancer symptom assessment instruments: a systematic review. J Clin Oncol. 2006;24(9):1459–1473. doi: 10.1200/JCO.2005.02.8332. [DOI] [PubMed] [Google Scholar]
- 18.Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: past, present, and future developments. J Pain Symp Manag. 2017;53(3):630–643. doi: 10.1016/J.JPAINSYMMAN.2016.10.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson LA, Jones GW. A review of the reliability and validity of the Edmonton Symptom Assessment System. Curr Oncol. 2009;16(1):53–64. doi: 10.3747/CO.V16I1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selby D, et al. A single set of numerical cutpoints to define moderate and severe symptoms for the Edmonton Symptom Assessment System. J Pain Symp Manag. 2010;39(2):241–249. doi: 10.1016/J.JPAINSYMMAN.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Bubis LD, et al. Symptom burden in the first year after cancer diagnosis: an analysis of patient-reported outcomes. J Clin Oncol. 2018;36(11):1103–1111. doi: 10.1200/JCO.2017.76.0876. [DOI] [PubMed] [Google Scholar]
- 22.“Policy Brief No. 133: Migration Trends and Families,” United Nations Department of Economic and Social Affairs, 2021. https://www.un.org/development/desa/dpad/publication/un-desa-policy-brief-no-133-migration-trends-and-families/ accessed 27 Oct 2021
- 23.Alegria M, Alvarez K, DMarzio K. Immigration and mental health. Soc Epidemiol. 2017;4:145–155. doi: 10.1007/978-94-010-1430-4_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang K, et al. Prevalence of pain in patients with breast cancer post-treatment: a systematic review. Breast. 2018;42(2018):113–127. doi: 10.1016/j.breast.2018.08.105. [DOI] [PubMed] [Google Scholar]
- 25.Craig KD, et al. Pain in persons who are marginalized by social conditions. Pain. 2020;161(2):261–265. doi: 10.1097/j.pain.0000000000001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma S, Ferreira-Valente A, Williams ACDC, Abbott JH, Pais-Ribeiro J, Jensen MP. Group differences between countries and between languages in pain-related beliefs, coping, and catastrophizing in chronic pain: a systematic review. Pain Med (United States) 2020;21(9):1847–1862. doi: 10.1093/PM/PNZ373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wyatt R. Pain and ethnicity. Am Med Assoc J Ethics. 2013;15(5):449–454. doi: 10.1001/virtualmentor.2013.15.5.pfor1-1305. [DOI] [PubMed] [Google Scholar]
- 28.Kawi J, Reyes AT, Arenas RA. Exploring pain management among Asian immigrants with chronic pain: self-management and resilience. J Immigr Minor Health. 2019;21(5):1123–1136. doi: 10.1007/s10903-018-0820-8. [DOI] [PubMed] [Google Scholar]
- 29.Pinheiro LC, Check DK, Rosenstein D, Reeder-Hayes KE, Dusetzina S. Examining potential gaps in supportive medication use for US and foreign-born Hispanic women with breast cancer. Support Care Cancer. 2019;27(5):1639–1646. doi: 10.1007/s00520-018-4406-8. [DOI] [PubMed] [Google Scholar]
- 30.Epner DE, Baile WF. Patient-centered care: the key to cultural competence. Ann Oncol. 2012;23:33–42. doi: 10.1093/annonc/mds086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.