Graphical Abstract
Graphical Abstract.
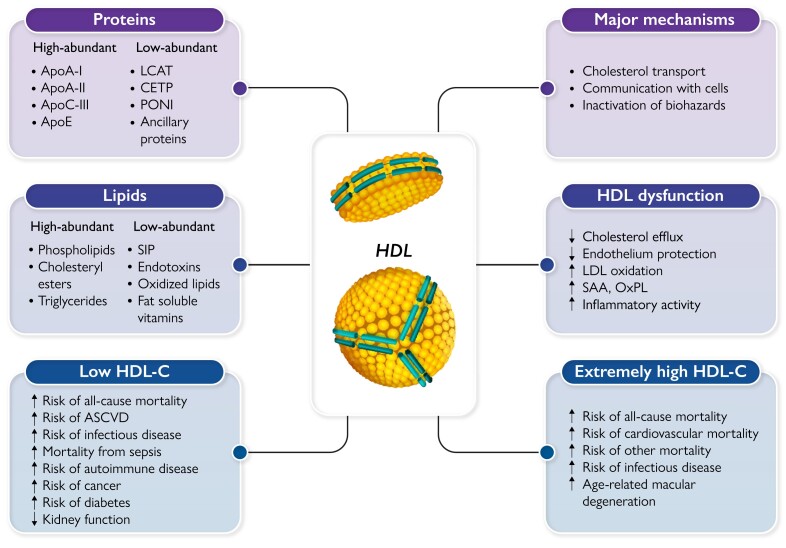
The great complexity of HDL. High-density lipoprotein (HDL) particles carry a large number of proteins and lipids, which contribute to define their compositional and functional complexity. HDLs exert multiple protective activities, essentially by three major mechanisms. HDLs, however, can lose their protective functions and even gain adverse functions in chronic diseases or during infections. U-shaped relationships between HDL-cholesterol (HDL-C) levels and several conditions have been reported, being both low and extremely high HDL-C levels associated with an increased risk of several pathologies and mortality. LCAT, lecithin:cholesterol acyltransferase; CETP, cholesteryl ester transfer protein; PONI, paraoxonase 1; S1P, sphingosine-1-phosphate; ASCVD, atherosclerotic cardiovascular disease; LDL, low-density lipoprotein; SAA, serum amyloid A; OxPL, oxidized phospholipids
Keywords: Cholesterol efflux, Evolution, Remnants, Triglycerides, Infectious disease, Cancer, Age-related macular degeneration, Autoimmune disease
Abstract
Previous interest in high-density lipoproteins (HDLs) focused on their possible protective role in atherosclerotic cardiovascular disease (ASCVD). Evidence from genetic studies and randomized trials, however, questioned that the inverse association of HDL-cholesterol (HDL-C) is causal. This review aims to provide an update on the role of HDL in health and disease, also beyond ASCVD. Through evolution from invertebrates, HDLs are the principal lipoproteins, while apolipoprotein B-containing lipoproteins first developed in vertebrates. HDLs transport cholesterol and other lipids between different cells like a reusable ferry, but serve many other functions including communication with cells and the inactivation of biohazards like bacterial lipopolysaccharides. These functions are exerted by entire HDL particles or distinct proteins or lipids carried by HDL rather than by its cholesterol cargo measured as HDL-C. Neither does HDL-C measurement reflect the efficiency of reverse cholesterol transport. Recent studies indicate that functional measures of HDL, notably cholesterol efflux capacity, numbers of HDL particles, or distinct HDL proteins are better predictors of ASCVD events than HDL-C. Low HDL-C levels are related observationally, but also genetically, to increased risks of infectious diseases, death during sepsis, diabetes mellitus, and chronic kidney disease. Additional, but only observational, data indicate associations of low HDL-C with various autoimmune diseases, and cancers, as well as all-cause mortality. Conversely, extremely high HDL-C levels are associated with an increased risk of age-related macular degeneration (also genetically), infectious disease, and all-cause mortality. HDL encompasses dynamic multimolecular and multifunctional lipoproteins that likely emerged during evolution to serve several physiological roles and prevent or heal pathologies beyond ASCVD. For any clinical exploitation of HDL, the indirect marker HDL-C must be replaced by direct biomarkers reflecting the causal role of HDL in the respective disease.
Historical perspective
The first observation of an inverse relationship between high-density lipoprotein-cholesterol (HDL-C) levels and the risk of developing coronary heart disease (CHD) dates back to the 1950s.1 Since the 1970s, results from many other studies have reinforced this strong inverse relationship,2–8 conferring to HDL-C the appellative of ‘good cholesterol’, as opposed to the low-density lipoprotein-cholesterol (LDL-C) referred to as ‘bad cholesterol’. These early observations paved the road for interventional clinical trials testing the hypothesis that increasing HDL-C levels using pharmacological approaches would reduce the cardiovascular (CV) risk.
Drugs that increase circulating HDL-C levels, including niacin, fibrates, and cholesteryl ester transfer protein (CETP) inhibitors except anacetrapib,9 have essentially failed to demonstrate any CV benefit, at least if added to state-of-the-art treatment with statins.10–13 However, it is important to reconcile that, with the exception of dalcetrapib, these drugs also alter plasma levels of other lipoproteins so that the futility of these interventions in reducing ASCVD may reflect limitations of the drugs or the study design (e.g. patient selection, combination with statins) rather than the role of HDL in atherosclerotic CV disease (ASCVD). Of note, the benefit shown with anacetrapib was directly proportional to the reduction of non-HDL-C. Genetic studies provided controversial evidence that HDL-C levels are causally associated with CV risk, also because most genetic determinants of HDL-C also affect other lipid traits, notably triglycerides but also LDL-C. Rare variants in genes which cause low HDL-C without altering other lipid traits, namely APOA1, ABCA1, and LCAT, were not associated with any increase of risk of ASCVD in general population studies,14–16 but associated with a higher prevalence of ASCVD in studies of families with low HDL-C17 or in a large lipid clinic registry.18 Variants in genes determining higher HDL-C levels also yielded equivocal results. Some like LIPC19,20 and SCARB121,22 are associated with normal to increased risk of ASCVD. Others like LIPG23,24 or CETP25,26 are associated with normal to reduced risk.23 Data from a genetic score combining 14 variants exclusively related to HDL-C showedno significant association with the risk of CHD.23 Also, more recently, even larger Mendelian randomization studies failed to show any significant genetic association of HDL-C levels with the risk of ASCVD.27 Finally, the findings in genetic animal models indicate the importance of specific genes and metabolic pathways as determinants of HDL’s role in ASCVD. For example, interferences with apoA-I expression show the expected inverse effects on HDL-C and atherosclerosis, whereas knock-out of Scarb1 increases both HDL-C and atherosclerosis.28
Apart from pleiotropic effects of gene variants, an important reason for this controversy is the non-continuous relationship of HDL-C with the risk of ASCVD. Data from six community-based cohorts showed an inverse and linear relationship between HDL-C and CHD risk up to a value of ≈90 mg/dL but for HDL-C values >90 mg/dL, no further reduction in CHD risk was observed.29 In a meta-analysis of 68 studies, this threshold was the 80th and 60th percentile for unadjusted and adjusted HDL-C levels, respectively.8 More recently, observational studies have shown a U-shaped relationship, with both low and very high levels of HDL-C being associated with an increased risk of all-cause mortality, CV mortality, infections, and dementia in the general population.30–32 These new findings have challenged the longstanding premise that raising HDL-C would reduce CV risk, but also suggested that, perhaps, HDL functionality rather than HDL-C levels may be more relevant in terms of drug development and as an ASCVD biomarker.
This new paradigm has reinforced the idea that HDL is not merely a cholesterol transporter, but, rather, possesses several additional functional properties [including cholesterol efflux capacity (CEC), anti-oxidant, anti-inflammatory, and immune-regulating activities].33 In addition, HDL is a rather complex family of different particles, being composed of sub-species differing in size, density, shape, charge, and composition, undergoing continuous remodelling processes in the circulation.33 This remarkable heterogeneity of the HDL particle family may explain why HDL cannot always be considered ‘anti-atherogenic’, but can sometimes become dysfunctional or even ‘pro-atherogenic’. Thus, it is not surprising that recent studies have shown that measures of various possible HDL functions, such as CEC and HDL inflammatory index (i.e. the capacity of HDL to inhibit the oxidation of LDL), or the number of circulating HDL particles are better predictors of CV events than just the cholesterol content of HDL.34–41
Is high-density lipoprotein-cholesterol all we need to measure?
High-density lipoprotein structure and composition
HDL is the smallest circulating lipoprotein and is found near all cells. HDL contains both proteins and lipids (Figure 1), but the unique arrangement of its two main constituents in different HDL particle sub-species provides insights into understanding its various physiological roles. Lipids in HDL are arranged in a micelle-like configuration, with the abundant amphipathic lipids (phospholipids and free cholesterol) forming a surface monolayer and the more hydrophobic or neutral lipids (cholesteryl esters and triglycerides) in the particle core. Particles with a hydrophobic neutral lipid core form spherical-like structures approximately 8–11 nm in diameter (referred to as α-migrating HDL, based on their migration on agarose gel), whereas particles depleted of neutral lipids form disc-like structures.42
Figure 1.
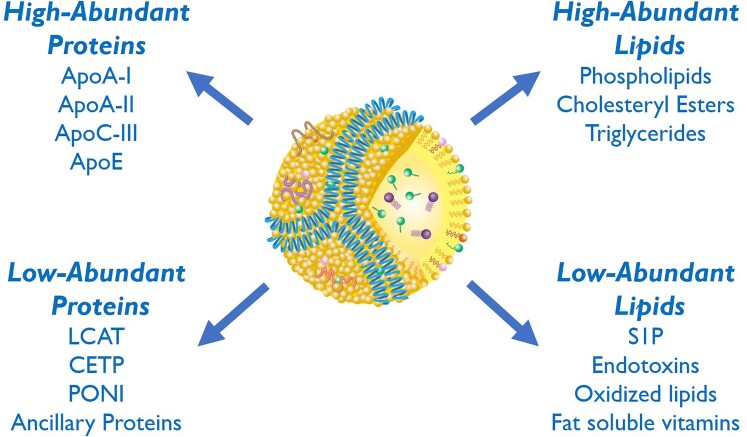
High and low abundant lipids and proteins in high-density lipoprotein particles. Apo, apolipoprotein; LCAT, lecithin:cholesterol acyltransferase; CETP, cholesteryl ester transfer protein; PONI, paraoxonase 1; S1P, sphingosine-1-phosphate.
Among the two major components of HDL, proteins and lipids, some are abundant whereas others are present in small amounts (Figure 1), with concentrations spanning 4–5 orders of magnitude and ranging from sub-micromolar ([ipid transfer proteins, apolipoprotein (apo) L1, sphingosine-1-phosphate, bile acids] to millimolar (cholesterol, phosphatidylcholine).42 Based on the average plasma concentration of 20 µmol/L, a representative HDL particle carries 50–100 molecules of esterified or unesterified cholesterol or phosphatidylcholine and 2–3 molecules of apoA-I. However, <5% of the particles each carry one molecule of minor constituents.42
The most abundant apolipoprotein on HDL is apoA-I, which contributes to the maintenance of HDL structure and to the removal of excess cellular cholesterol through the ATP-binding cassette transporter-1 (ABCA1).42 The typical large spherical form of HDL has at least three molecules of apoA-I in a trefoil-like configuration,43 whereas discoidal HDL has two copies of apoA-I wrapped around the side of the disc, shading the hydrophobic acyl chains of its phospholipid bilayer.44 The low-abundant proteins on HDL can be further divided into lipoprotein-specific proteins and ancillary proteins. Lipoprotein-specific proteins include lecithin:cholesterol acyltransferase (LCAT), CETP, and paraoxonase-1 (less than one copy per particle). Most of the ancillary HDL proteins, which now number over 200,45,46 are even less abundant and, for the most part, are only loosely associated with HDL. Although low in abundance, these ancillary proteins may, nevertheless, have important biological functions: as an example, haptoglobin or haptoglobin-related protein enables HDL to act as a potent trypanosome-lytic factor,47 and alpha-1 antitrypsin (an acute-phase protein)48 may enable a more efficient HDL delivery to sites of tissue damage where it suppresses inflammation.
Phospholipids are abundant, key structural components of HDL and often have been considered just structural, but this is an overly simplistic view. For example, in the absence of HDL due to LCAT deficiency, excess phospholipids generated during lipolysis of apoB-containing lipoproteins reorganize as multi-lamellar vesicles called lipoprotein-X (Lp-X).49 Lp-X particles get trapped in the glomerulus and can cause end-stage kidney disease, thus one potentially important role of HDL is to prevent this outcome. Moreover, they are substrates for enzymes (e.g. LCAT or endothelial lipase) that generate lysophospholipids and hence bioactive molecules. Although not as abundant as triglycerides on apoB-containing lipoproteins, the transfer of triglycerides to HDL by CETP may extend the time for the delivery of triglycerides for lipolysis in the post-prandial state and, because of the small size of HDL and its ability to enter extracellular fluid, it may enhance the delivery of triglycerides on HDL to peripheral tissues.50 Low-abundant lipids (or lipid-like substances) on HDL, like sphingosine-1 phosphate (S1P),51 as will be discussed below, may also have important effects because they are potent biological signalling molecules. HDL can even bind to miRNAs and other types of short nucleic acid fragments but the pathophysiological significance of this is not clear.52
High-density lipoprotein sub-fractions
Given the compositional complexity of HDL, it is not surprising that there are numerous sub-fractions or ways to further sub-divide HDL into different structural or functional categories. The main impetus behind this effort was to identify sub-fractions of HDL that may be diagnostically important for predicting CV disease risk, but it also has obvious implications for developing drugs that modulate HDL for the prevention of CV disease or other diseases. Historically, HDL was first sub-fractionated based on the density of lighter (and larger) HDL2 and heavier (and smaller) HDL3 sub-fractions.53 Another early classification of HDL sub-fractions was based on the presence or absence of apoA-II, the second most abundant protein in HDL. These types of classifications had, however, limited impact on routine diagnostic testing, because of lacking evidence for superiority. Separation of HDL into discrete HDL size fractions can now be readily done by nuclear magnetic resonance (NMR) spectroscopy in clinical laboratories, providing the ratio of large-to-small HDL, which may be useful for assessing not only CV risk but also insulin resistance and other conditions.53 Recent advances in mass spectrometry allow the comprehensive quantification of the proteome in total HDL as well as distinct sub-classes.45,46,54,55 At this time, there is only limited commercial availability of these advanced HDL sub-fractionation tests and hence they are not widely used but they are being actively investigated for their clinical utility.
High-density lipoprotein function
Recent proteomic analyses and metabolic turnover studies provided evidence that distinct HDL sub-classes have a pre-defined core-protein composition that remains relatively stable throughout their lifecycle.56,57 Interestingly, these HDL sub-classes frequently contain specialized proteins that fulfil related or complementary functions, for example, in haemostasis, protease inhibition, or the complement system.45,46,56 One notable example is the complex consisting of apoA-I, haptoglobin-related protein, and apoL1 by which haptoglobin-related protein provides the binding to Trypanosoma and the internalized apoL1 elicits the lysosomal swelling, ultimately killing Trypanosoma.58 By contrast, the lipid composition of HDL particles is highly dynamic. ABCA1 fluxes glycerophospholipids and cholesterol from cell membranes, especially to lipid-free apoA-I as well as to small, lipid-poor HDL. LCAT generates cholesteryl esters and lysophosphatidylcholines by the transfer of sn-2 fatty acids from phosphatidylcholines to the 3-OH group of cholesterol. Endothelial lipase and hepatic lipase hydrolyse phosphatidylcholines and triglycerides of HDL, respectively, generating free fatty acids, lysophosphatidylcholines, and diacylglycerols, which also are bioactive molecules. CETP exchanges cholesteryl esters of HDL for triglycerides from apoB-containing lipoproteins and phospholipid transfer protein (PLTP) transfers phospholipids from apoB-containing lipoproteins to HDL as well as between different HDLs.59 In addition, lipids are fluxed between HDL and cells and between HDL and other lipoproteins following concentration gradients or affinity. For example, unesterified cholesterol is readily accepted by HDL from both lipolysed triglyceride-rich lipoproteins (TGRLs) or cell membranes but also transferred from HDL to LDL or cells60,61 (Figure 2). S1P is readily effluxed from erythrocytes or endothelial cells, especially by HDLs that contain its chaperone apoM.62,63
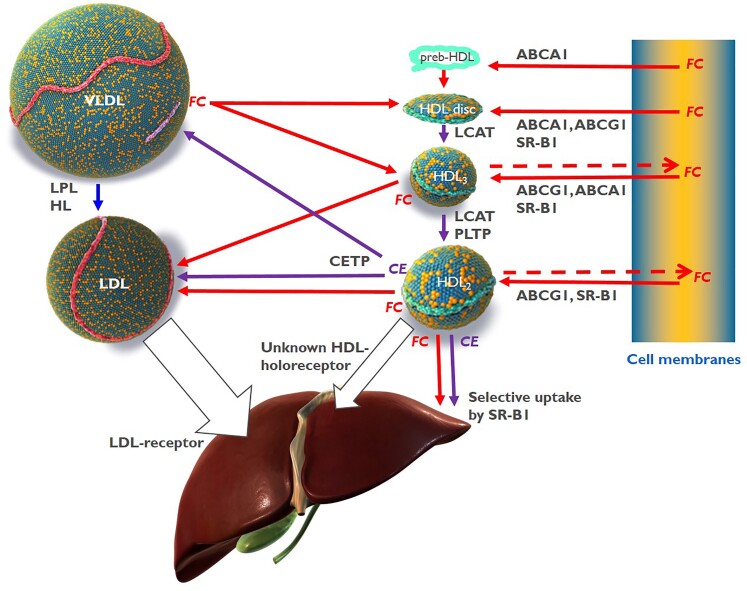
Figure 2.
Cholesterol transfers between high-density lipoprotein, very-low-density lipoprotein, low-density lipoprotein, and cells. ABCA1, ATP-binding cassette transporter A1; ABCG1, ATP-binding cassette transporter G1; CE, cholesteryl ester; CETP, cholesteryl ester transfer protein; FC, free cholesterol, unesterified cholesterol; HDL, high-density lipoprotein; HL, hepatic lipase; LCAT, lecithin:cholesterol acyltransferase; LDL, low-density lipoprotein; LPL, lipoprotein lipase; PLTP, phospholipid transfer protein; SR-B1, scavenger receptor B1.
Thus, HDLs are modular scaffolds that combine structural specificity with plasticity. Thereby, HDLs exert multiple functions that protect the organism from chemical or biological harm or help to repair the tissue damage caused by noxious agents. HDLs do so by three principal mechanisms.
Cholesterol transport
The most intensively investigated function of HDL is reverse cholesterol transport (RCT). According to this model, HDLs elicit cholesterol efflux from macrophage foam cells of atherosclerotic plaques either specifically, via sequential interactions with ABCA1 and ABCG1, or by aqueous diffusion, through a process facilitated by scavenger receptor BI (SR-BI) (Figure 2).64 Virtual HDL-deficiency in Tangier disease as well as in mice with systemic or hepatocyte-specific knock-out of ABCA1, illustrates the rate-limiting importance of ABCA1 for the biogenesis of HDL.59,65 Free cholesterol is then esterified by LCAT and cleared by the liver, either directly, by selective uptake through SR-BI, or indirectly after CETP-mediated transfer to apoB-containing lipoproteins which are then internalized by the LDL receptor.65 In addition, HDL particles as such are taken up by hepatocytes through a yet poorly understood mechanism (Figure 2).66
Of note, HDL-C levels reflect neither the capacity nor the intensity of RCT.67 Neither do increased HDL-C levels upon treatment with CETP inhibitors indicate enhanced RCT.68 Especially in the context of LDL receptor activation through the concomitant statin therapy, the interference with cholesteryl ester transfer from HDL to LDL blocks RCT by preventing the removal of cholesterol by the LDLR pathway. The doubling of HDL-C levels upon CETP inhibition indicates that the blockage of this indirect pathway is not compensated by the direct removal of HDL and its cholesterol cargo.
RCT is of special relevance for the removal of cholesterol from macrophages: following cholesterol accumulation, macrophages exert several pro-inflammatory activities which are dampened by HDL-mediated cholesterol efflux.69 Also, adipocytes are enriched with cholesterol to form the biomembranes surrounding lipid droplets. Hydrolysis of triglycerides in white adipocytes upon fasting or in brown adipocytes upon heat production is accompanied by a breakdown of these membranes and the release of considerable amounts of cholesterol for RCT. At least in mouse models, HDL or ABC transporters were shown to play an important role in this process.70,71
HDLs also accept unesterified cholesterol from TGRLs, a process enhanced upon hydrolysis of triglycerides by lipoprotein lipase and independent of phospholipids (Figure 2).72
Communication with cells
HDLs regulate the differentiation, proliferation, migration, survival, and function of many cell types. HDLs modulate the inflammatory action of innate and adaptive immune cells,69 support the integrity and functionality of endothelial barriers, stimulate angiogenesis,73 and secure energy homeostasis by stimulating insulin synthesis and secretion by pancreatic beta cells as well as glucose uptake by adipocytes and myocytes.74 Principally, these cellular responses result from either altered cholesterol homeostasis due to fluxes of cholesterol between cells and HDL, specific molecular interactions between HDL and cells, or combinations thereof.
First, cholesterol efflux alters the cholesterol content of specific plasma membrane domains, so-called rafts, that are enriched with signalling molecules, resulting in different effects depending on the cell type. Examples are the activation of endothelial nitric oxide synthase (eNOS) by HDL/SR-BI interaction in caveolae of endothelial cells75 as well as the dampening of toll-like receptor-4 response in monocytes76 or T-cell receptor signalling in lymphocytes;77 cholesterol efflux also alters the transcription of sterol-regulated genes.78
Second, the interactions of HDL with SR-BI or apoA-I with ABCA1 induce signal transduction via the recruitment of intracellular proteins, which in turn activate different cellular responses such as eNOS activation in endothelial cells or glucose uptake into myocytes.79,80
Third, HDLs transport agonists of specific signalling receptors; the interaction of S1P with S1P receptors activates diverse signalling cascades and results in many protective effects on endothelial functions, including the induction of nitric oxide (NO) production and the maintenance of the endothelial barrier integrity.73,81 Furthermore, S1P facilitates the trans-endothelial transport of HDL and thereby entry into extravascular tissues and spaces, where HDL exerts its protective activities.82 S1P also modulates inflammatory effects on macrophages and lymphocytes and promotes the survival of hearts and kidneys exposed to hypoxia, ischemia-reperfusion injury, or toxic drugs.81
Fourth, HDLs deliver cargo into cells either by selective uptake, i.e. independently of the entire particle, or via holoparticle uptake.66 SR-BI mediates the selective uptake not only of lipids but also microRNAs (miR) carried by HDL.83 HDL-holoparticle uptake in the liver plays an important role in the metabolism of HDL.84 In addition, monocyte-derived macrophages and enterocytes also internalize entire HDL particles, but the mechanism or the consequence of this is not understood.85,86
In summary, HDLs elicit a plethora of cellular responses by employing several modes of communication. Some mechanisms, for example, S1P receptor activation or cholesterol efflux, lead to many diverse responses in different cell types. Vice versa, identical responses can be evoked by several modes of action.
Inactivation of biohazards
By its amphiphilic structure and its cycling between extravascular and intravascular compartments, HDLs bind potentially toxic substances, such as bacterial lipopolysaccharides, oxidized lipids, as well as some lipophilic xenobiotics.87,88 In plasma, potentially hazardous molecules are either eliminated by reverse transport to the liver or inactivated directly on the surface of HDLs. The best-investigated example for the latter situation is the hydrolysis of oxidized phospholipids by paraoxonase 1, lipoprotein-associated phospholipase A2, and LCAT.89–91 HDLs also exert direct antimicrobial effects on viruses and even protozoa.58,88,92 At least in vitro, HDL or apoA-I interfere with the entry or fusion of viruses with target cells.92 Of note, SR-BI is an entry route of several viruses, including SARS-CoV-2, into cells93 and this process may be competed by HDLs.94
Finally, the proteome of HDLs is enriched with proteases and protease inhibitors which modulate platelet aggregation, coagulation, fibrinolysis, complement activation, and tissue degradation. They help to counteract downstream adverse effects of injuries, infections, and inflammation and support wound healing.48 Of note, functionally related proteins tend to cluster within distinct sub-populations of HDL.45,46
High-density lipoprotein dysfunction
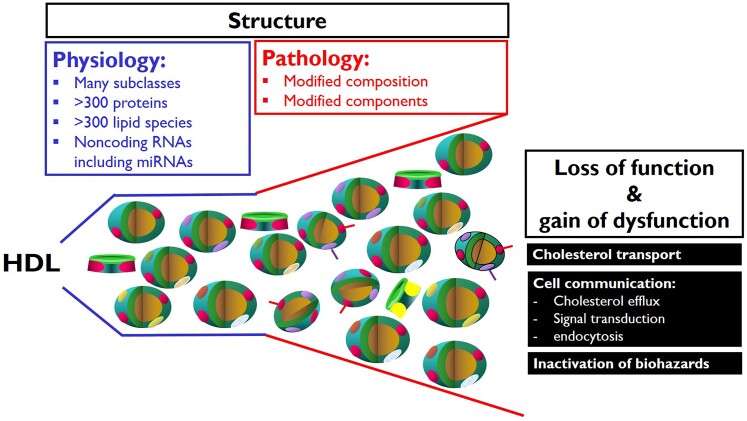
HDLs can lose protective functions and even gain adverse functions in chronic diseases, such as rheumatic and autoimmune diseases, CHD, diabetes, chronic kidney disease, or in the course of infectious diseases.42,95 HDL dysfunctions include reduced capacities to stimulate cholesterol efflux from macrophages, inhibit LDL oxidation, and regulate apoptosis, NO production, monocyte chemotactic protein-1, or vascular cell adhesion molecule expression in endothelial cells (Figure 3). A systematic investigation of HDLs’ structure–function relationships in CHD and diabetes showed that the different functionalities of HDL are not correlated with each other and are determined by different features and molecules of HDLs.96 HDLs of patients with CHD or chronic kidney disease inhibit rather than stimulating NO production because upon interaction with the lectin-like oxidized LDL receptor LOX-1 and the toll-like receptors TLR2 and TLR4 they induce the phosphorylation of inhibitory rather than activating sites in eNOS. The gain of these adverse receptor binding properties results from the accumulation of oxidized phospholipids and apoA-I, serum amyloid protein A (SAA), or symmetric dimethylarginine.97,98
Figure 3.
Structure–function relationships of high-density lipoprotein in health and disease. RNA, ribonucleic acid.
Biomarkers of high-density lipoproteins’ function or dysfunction
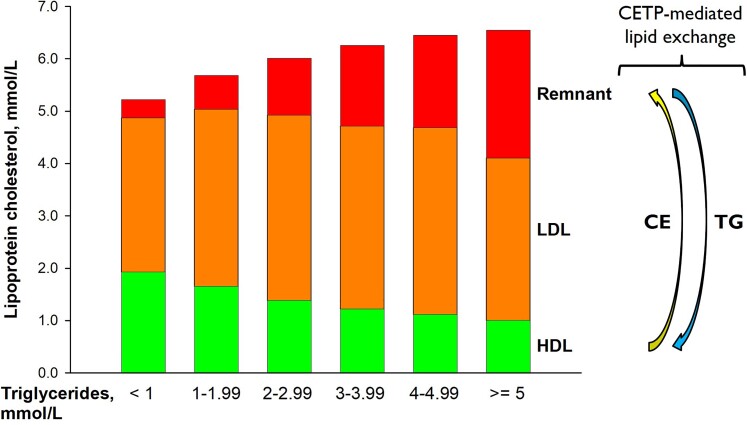
From a functional point of view, HDL-C is not a causal marker because the many functions of HDL are exerted either by entire particles or specific components other than cholesterol. Moreover, low HDL-C is strongly associated with increased levels of TGRL (Figure 4). Therefore and because HDL-C levels decrease upon disturbed lipolysis-induced transfer of unesterified cholesterol from TGRL to HDL100 as well as enhanced CETP-mediated transfer of cholesteryl esters from HDL to TGRL (Figure 4), low HDL-C levels are nowadays widely considered as an indirect and non-causal biomarker of elevated ASCVD risk reflecting the atherogenicity of elevated plasma levels of TGRL and their remnants.101 Any chance for future exploitation of HDL as a therapeutic target will depend on the availability of direct biomarker(s) reflecting a causal contribution of HDL to the pathogenesis of atherosclerosis and other diseases.
Figure 4.
Lipoprotein-cholesterol as a function of increasing levels of non-fasting triglycerides. Based on 60 000 individuals from the Copenhagen General Population Study. CE, cholesteryl ester; CETP, cholesteryl ester transfer protein; TG, triglycerides. Adapted from Chapman et al.99
A recent meta-analysis reported that the total number of HDL particles, as well as numbers of small and medium, but less so large HDL particles, are associated with incident ASCVD.102 A recent Mendelian randomization study found genetically causal associations of coronary artery disease with the concentrations of medium and small HDL particles, but not with large HDL.103 This heterogeneity is potentially important since lipid-modifying drugs cause diverse changes in HDL particle size, number, and composition: treatment with nicotinic acid and CETP inhibitors increases HDL-C levels more profoundly than HDL-P, while treatment with fibrates increases HDL-P more strongly than HDL-C.104
The plasma concentration of apoA-I is the most obvious candidate as a direct biomarker of HDL function, because it is an essential structural HDL component, but also exerts several biological activities. In epidemiological and clinical studies, apoA-I levels show inverse associations with ASCVD events, which however are not stronger than those of HDL-C.8,105 Neither did a Mendelian randomization study, based on a single SNP of APOA1, unravel any causal genetic relationship between apoA-I levels and ASCVD.106 Nevertheless, we believe it is still worthwhile to further validate apoA-I as a biomarker through observational and more comprehensive Mendelian randomization studies, also concerning endpoints other than ASCVD.
Cross-sectional studies identified several proteins and lipid species in HDL, which differ quantitatively between patients with various diseases (including CHD) and healthy control subjects.42,95,96 However, only a few of them were validated in prospective studies.42,45 The presence or absence of distinct proteins was found to determine the association of apoA-I levels with incident CV events. For example, apoA-I levels in particles that contain apoE or apoC-I but not their apoE- or apoC-I-free counterparts, or apoA-I levels in apoC-III-free particles but not in apoC-III-containing particles are inversely associated with incident ASCVD events.54 Enrichment of HDL with SAA was associated with mortality in CHD patients as well as patients with diabetic end-stage nephropathy.107,108 However, it is not clear whether the enrichment of HDL with apoC-III or SAA are direct measures of HDL dysfunction or indirect reporters of apoC-III’s adverse role in the metabolism of TGRL or the presence of inflammation. In support of the latter, apoC-III interferes with the capacity of HDL to inhibit the apoptosis of endothelial cells and to promote efflux from macrophages and SAA disturbs HDL’s ability to activate eNOS.107,109
As integrative measures of HDL function, also bioassays were validated in population and clinical studies. CEC was investigated most extensively, using apoB-depleted plasma or serum as a surrogate of HDL. Despite the heterogeneity of results, a recent meta-analysis revealed that CEC is inversely associated with ASCVD events independently of HDL-C.110 However, the assay is difficult to standardize and is not suitable for clinical routine.111 Moreover, CEC is no overall proxy of HDL functionality, because other functions of HDL neither correlate nor share molecular determinants with CEC.96 Although increasing CEC either as monotherapy or as a combination therapy with statins, treatment with evacetrapib did not prevent ASCVD events.112 Despite these limitations, CEC has been used as a reference to develop molecular biomarkers that can be measured in clinical laboratories. One example is the derivation of an algorithm that integrates the information of differently sized HDL particles as measured by NMR. The estimated NMR-based CEC correlated very well with the in vitro measured CEC. However, in contrast to initial encouraging results, the validation of the CEC estimation algorithm failed in large multicentric replication studies.113 Another example is a proteomic score integrating the information of apolipoproteins A-I, C-I, C-II, C-III, and C-IV, which showed a good correlation with CEC as well as significant association with the presence of coronary artery disease and CV mortality independently of clinical risk factors including conventionally measured concentrations of apoA-I and apoB.114 Replication studies are needed to validate these surrogate scores of CEC.
High-density lipoprotein in mortality
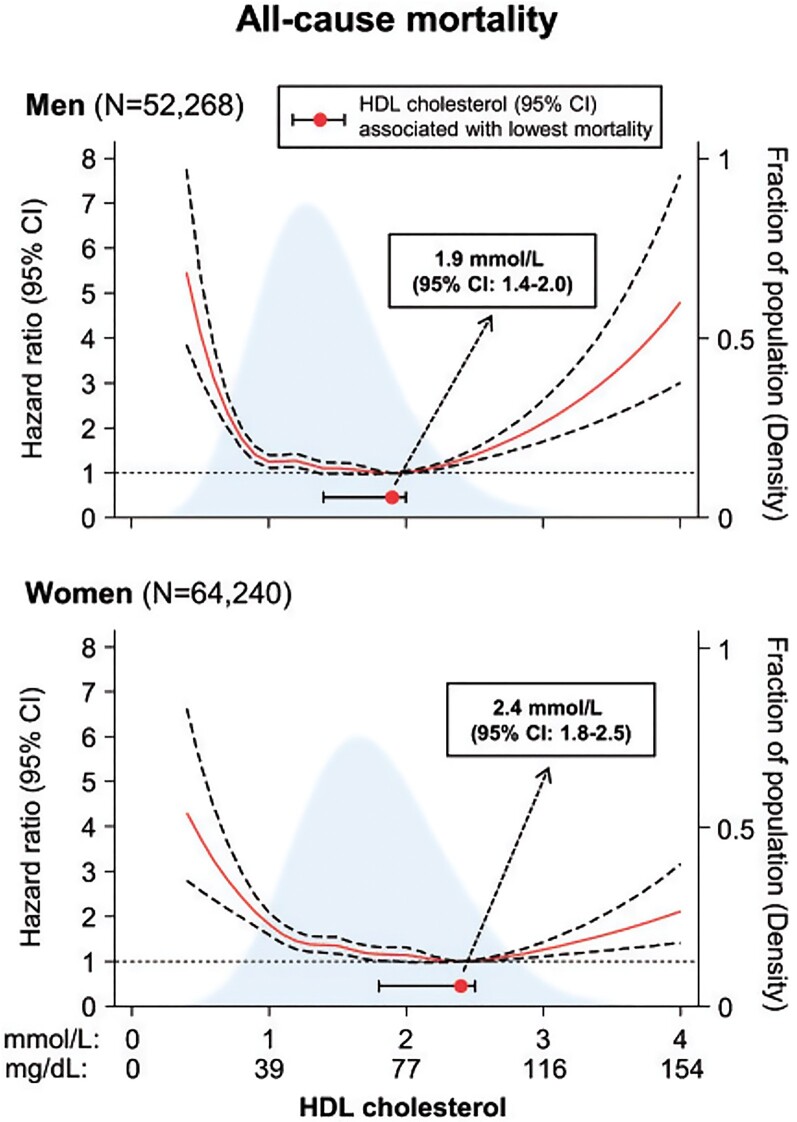
Both low and extremely high HDL-C levels are associated with an increased risk of all-cause mortality30 (Figure 5). Whereas the association with low HDL-C levels concurs with the repeatedly observed increased risk of ASCVD,8 the increased mortality at extremely high HDL-C levels is less easily understood. This finding, however, derives from many studies30,115–117 and raises the question of whether extremely high HDL-C levels have deleterious effects. Demonstrating a causal effect is very difficult, as conventional Mendelian randomization studies assume linear effects (the higher the level, the higher the risk over the entire concentration range, or vice versa). Non-linear Mendelian randomization designs can be used for U-shaped observational associations, but such studies need even more statistical power, and hitherto no such studies have examined whether extremely high HDL-C levels are causally related to increased mortality. Naturally, this limitation is also valid for low HDL-C associated with increased mortality, as well as for the other U-shaped relationships described in the following.
Figure 5.
High-density lipoprotein levels on a continuous scale and risk of all-cause mortality in men and women from the Copenhagen General Population Study. Adapted from Madsen et al.30
The largest observational studies showed that extremely high HDL-C levels were not associated with a higher risk of cancer mortality, but rather with CV and/or other mortalities.30,116,117 There are several possible explanations behind these associations. First, 11% of individuals with high HDL-C carry rare genetic variants that not only have a strong effect on HDL-C levels,118 but may also have concomitant detrimental health effects possibly through dysfunctional HDL, including an altered ability to remove excess cholesterol from cells.115 Second, the association could be driven by confounding, e.g. extremely high HDL-C levels are found in people with very high alcohol consumption, which could be the actual cause of high mortality.30,115 Third, high HDL-C levels result from delayed catabolism. On the one hand, this may indicate disturbed delivery of cholesterol to the liver for excretion. On the other hand, like for LDL, the prolonged residence time will promote modifications of the molecular composition and components of HDL, ultimately resulting in HDL dysfunction. At high HDL-C levels, the particle size is larger than normal. Therefore, it is at least theoretically possible that these large, likely dysfunctional HDL particles become trapped in the arterial intima, leading to cholesterol accumulation and eventually to atherosclerosis and ASCVD.115 Finally, in observational analyses, it is never possible to rule out reverse causation, that is, poor health leading to early death could also lead to extremely high HDL-C levels.
High-density lipoprotein in non-cardiovascular morbidity: from epidemiology to genetic and trial evidence
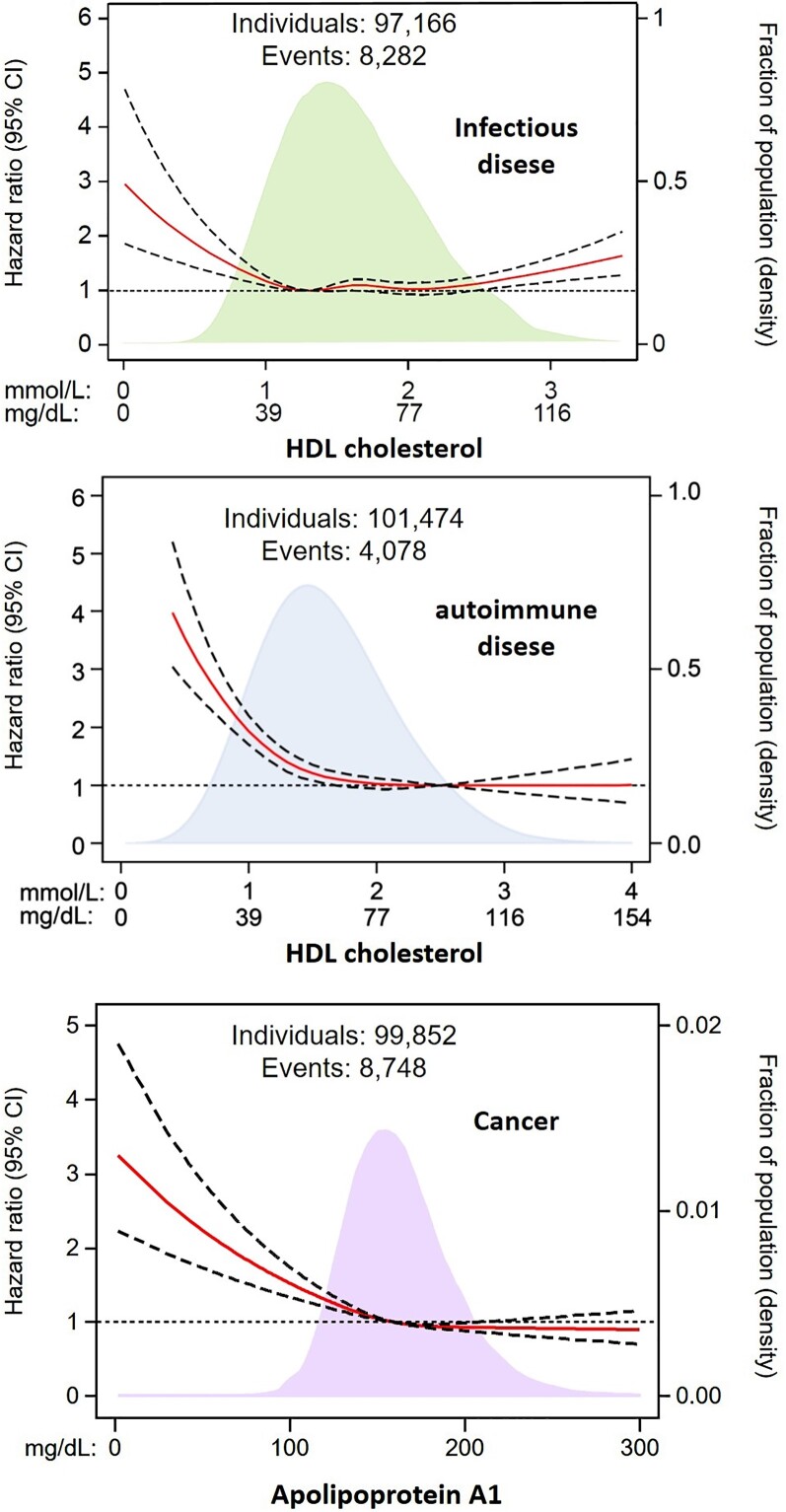
Both low and high HDL-C levels are associated with an increased risk of infectious disease31,119 (Figure 6, upper panel) as well as mortality from sepsis.88,92,121,122 There is genetic support for this function of HDL from gain-of-function variants in CETP associated with both lower HDL-C levels and higher mortality in sepsis.123 Possible mechanisms include the inactivation of bacterial lipopolysaccharides,88,92 but also beneficial effects of HDL on multiple organs and systemic responses, for example, in haemostasis and complement activation which are dysfunctional in hyperinflammation.48,69,73
Figure 6.
High-density lipoprotein levels on a continuous scale and risk of infectious disease, autoimmune disease, and cancer in individuals from the Copenhagen General Population Study. Adapted from Madsen et al.31 and Pedersen et al.120
Low HDL-C levels are associated with an increased risk of autoimmune disease124 (Figure 6, middle panel) and cancer115,120,125,126 (Figure 6, lower panel); for cancer, the risk increase was more pronounced for low apoA-I than for low HDL-C,120 suggesting that HDL particles rather than their cholesterol content drive this association. Currently, there is no convincing genetic evidence linking low HDL-C levels causally to the risk of autoimmune disease or cancer.115
Low HDL-C is not only frequently found in individuals with manifest diabetes mellitus Type 2 but is also associated with an increased risk of developing diabetes. There is genetic evidence that low HDL-C levels may be causally related to an increased risk of diabetes in two large studies,127,128 but not in a third.129 In randomized trials, CETP inhibition, leading to a 28–132% increase in HDL-C levels, improved glycaemic control and/or reduced the risk of new-onset diabetes.130,131 The beneficial glycaemic effects could, however, be due to pleiotropic effects of CETP inhibition beyond HDL-C increases. However, infusion of artificial HDL acutely improved glycaemia in patients with diabetes.132 Moreover, both in vitro and in animal experiments, HDL was found to exert potentially anti-diabetic effects on pancreatic beta cells, insulin signalling and glucose metabolism.74
Observational133–135 and genetic136–138 data show that low HDL-C is associated with decreased kidney function.115 Finally, smaller studies have linked lower HDL-C levels with asthma; however, there are no genetic studies to support any claim for causality,115 although overexpression of ApoA1 or treatment with reconstituted HDL (rHDL) showed beneficial effects in several animal models of lung diseases.139
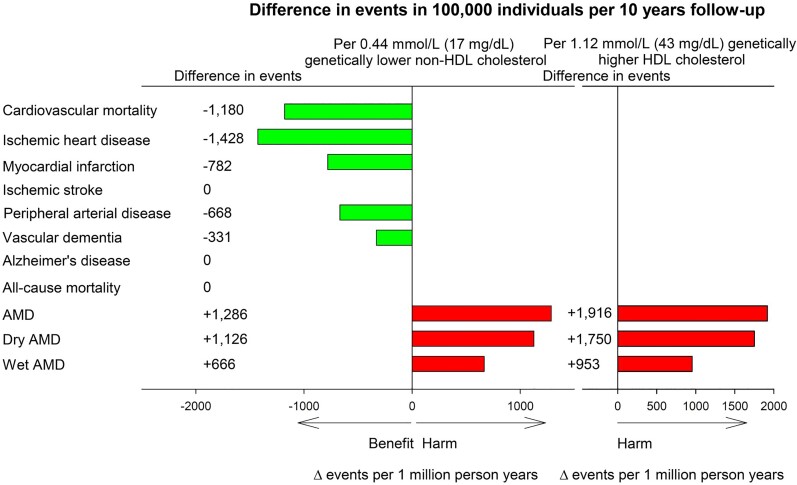
The risk of age-related macular degeneration also increases with elevated HDL-C and apoA1, according to both observational and genetic studies.140–144 Genetically, well-known genes involved in HDL metabolism (ABCA1, LIPC, CETP, and APOE) are drivers of the increased risk of age-related macular degeneration,140 the most common cause of blindness in the elderly. The use of drugs that specifically increase HDL levels is therefore of concern, and a recent genetic study estimated that the number of individuals who are potentially harmed by developing age-related macular degeneration via HDL-C increases due to CETP inhibition was of the same order of magnitude as the number of individuals who may have benefited from reduced ASCVD, as a consequence of lowering of non-HDL-C144 (Figure 7). However, in the REVEAL study treatment with anacetrapib did not cause any significant increase in the incidence of age-related macular degeneration (AMD), loss of visual acuity, or blindness.9 That said, REVEAL participants were followed for 4 years from a median age of 67 years during which time 298 cases of AMD were diagnosed, that is, the study had limited power to exclude an increased risk of AMD if these participants were treated beyond the median age of 72 years where AMD typically develops.144 Since the retina like the brain is separated from the bloodstream by a tight barrier, one must envisage that HDL-C levels in peripheral blood are only indirectly related to the functions of the HDL genes in the pathogenesis of AMD. For example, the targeted knock-out of ABCA1 and ABCG1 in retinal pigment epithelium led to retinal degeneration in mice as seen in human AMD.139 As interference with LDL metabolism in the liver for example by PCSK9 inhibition does not affect brain function, interference with HDL metabolism in the periphery may have no impact on retina function.
Figure 7.
Estimated benefit and harm due to genetically lower non-high-density lipoprotein-cholesterol and genetically higher high-density lipoprotein-cholesterol due to inhibition of cholesteryl ester transfer protein. Based on individuals in the Copenhagen General Population Study. Lower non-high-density lipoprotein-cholesterol by 0.44 mmol/L (17 mg/dL) and higher high-density lipoprotein-cholesterol by 1.12 mmol/L (43 mg/dL) correspond to the changes observed through anacetrapib treatment compared with placebo in the REVEAL trial.9 AMD, age-related macular degeneration; HDL, high-density lipoprotein; Δ=difference. Adapted from Nordestgaard et al.144
High-density lipoprotein across species, in search for a role through evolution
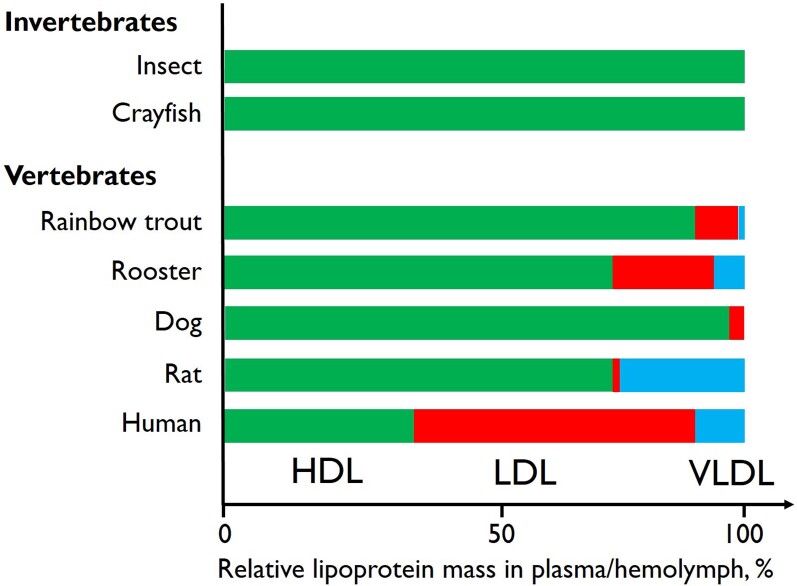
HDL is present in essentially all living species: in invertebrates including insects, crabs, and lobsters, different types of HDL-like lipoproteins represent the bulk of lipoproteins in haemolymph (the equivalent of human blood)145–147 (Figure 8). In contrast to vertebrates, invertebrates use only HDL-like lipoproteins for both exogenous and endogenous lipid transport: while circulating in haemolymph between different cells in different tissues, HDL alternately delivers and takes up lipids without being internalized or degraded. Besides lipid transport, haemolymph HDL is involved in bacterial lipopolysaccharide binding, clot formation, and wound healing, and oocyte maturation in females.145–147
Figure 8.
Examples of relative mass distribution of different lipoprotein fractions in different invertebrate and vertebrate species. HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein. Values adapted from Chapman145 and Van der Horst and Rodenburg.147
The lipoprotein systems of the most primitive vertebrates approach those of mammals and humans, including both HDL and the larger apolipoprotein B-containing lipoproteins like human chylomicrons, very-low-density lipoproteins (VLDLs), and LDL.145–147 The relative plasma content of HDL, LDL, and VLDL (including chylomicrons) differs between different vertebrates; however, in many species, HDL is the dominant lipoprotein145–147 (Figure 8).
This suggests that, through evolution, HDL particles appeared early, while chylomicrons, VLDL, and LDL first developed in vertebrates. HDL transports lipids to and from different cells like a reusable ferry, while the larger chylomicrons, VLDL, and LDL mediate more targeted delivery including triglyceride hydrolysis via lipases and cholesterol via receptor-mediated lipoprotein uptake. In this regard, it is important to remember that LDL plays an important role in RCT by accepting both unesterified and esterified cholesterol from HDL for LDL-receptor-mediated removal by the liver.60,148 However, for some organs and functions, for example the steroidogenesis in adrenals, the delivery of cholesterol by HDL rather than by LDL appears to be rate-limiting.149 As CV disease occurs after reproductive age and mainly in humans, it is unlikely that HDL and RCT, the most intensively investigated function of HDL particles, have developed during evolution to protect from atherosclerosis. It is more likely that HDL evolved as a multimolecular and multifunctional platform and part of the innate host defence to overcome acute crises in early life, such as infection and wounding. In this regard also RCT plays an important role, as tissue degradation, but also the physiological turnover of erythrocytes and platelets as well as lipolysis in the adipose tissue upon prolonged fasting mobilize cholesterol, which is taken up and transported by HDL for biliary excretion.
Therapies modulating high-density lipoprotein
Based on the earlier observations from epidemiological studies, it was assumed that increasing HDL-C levels would produce CV protection. As a consequence, many clinical trials have been conducted with drugs capable of increasing significantly the plasma levels of HDL-C. The results of such clinical trials have been, however, disappointing. Adding niacin to statin therapy did not provide any incremental clinical benefit among patients with ASCVD, despite a 25% increase in HDL-C levels.10 Several CETP inhibitors have now been tested in clinical trials, most of which failed to show a reduction in CV risk despite significant increases in HDL-C levels,12,13,150 except for anacetrapib; the latter significantly reduced CV events by 9%,9 although such reduction was attributed to the observed LDL-C lowering rather than the increase in HDL-C levels.151 Several studies have also aimed to find further explanations for these results, but neither changes in CEC of HDL13,112,152 nor variations in HDL sub-fractions,112,153,154, could provide clear explanations for the trial results. In addition, different patient populations and different quantification methods were used in these studies, thus further complicating this picture. A recent study showed that the treatment with torcetrapib and evacetrapib increases HDL sub-fractions that are associated with an increased CHD risk, such as those containing apoC-III, suggesting that the pharmacological increase of HDL-C would not be beneficial if an increase in dysfunctional HDL particles is achieved.155 In contrast with these observations, genetic variations in the CETP gene determining higher HDL-C levels were associated with a reduced risk of 28-day mortality from sepsis, and inhibition of CETP with anacetrapib preserved HDL-C levels decreased the severity of endotoxemia, and improved survival after caecal ligation and puncture in mouse models of sepsis.123 In post hoc analyses of large trials, CETP inhibitors were also found to improve glycaemic control and delay the onset of diabetes.130,131
Although pharmacologically increasing HDL-C levels has so far not shown any clinical benefit, several efforts have been launched to develop rHDL that are expected to improve specifically the HDL-mediated RCT rather than increasing its level. These were based on the observation that HDL infusion, as well as the overexpression of apoA-I in experimental animal models, were associated with the prevention or regression of atherosclerosis.156–158 Over time, three HDL mimetics have been developed and tested in humans.
ApoA-IMilano is a naturally occurring variant of apoA-I determining very low levels of HDL-C and apoA-I and high triglyceride levels but associated with a very low prevalence of CV disease.159,160 Sera from apoA-IMilano carriers exhibit a higher CEC compared with wild-type apoA-I161; this observation has led to the development of a complex (MDCO-216) consisting of purified apoA-IMilano and phospholipids. In one study, MDCO-216 produced significant regression of coronary atherosclerosis, in the absence of any demonstrable change in HDL-C levels, suggesting an improvement in HDL function;162 this observation, however, was not confirmed in another study.163 The development of MDCO-216 has now been halted.
Following an acute coronary syndrome (ACS) event, cholesterol efflux is significantly reduced, showing the lowest levels at 2–5 days post-event and returning to baseline approximately after 30 days.164 Thus, an approach that acutely increases apoA-I and CEC might be beneficial among post-acute myocardial infarction patients. CSL-111 was an early formulation of rHDL consisting of human apoA-I with soybean phosphatidylcholine; although it did not produce significant reductions in coronary atheroma volume in post-ACS patients, improvements in plaque characterization index and coronary score by quantitative coronary angiography were observed.165 The development of CSL-111 was halted due to adverse hepatic events. CSL-112 is a modified formulation of rHDL. It has threefold less phospholipid than CSL-111, did not show any major organ toxicity or immunogenicity,166 and is capable of increasing substantially ABCA1-mediated cholesterol efflux from cells.167 Compared with placebo, CSL-112 was associated with an improvement in measures of CEC (>3-fold).168 Similar results were reported in another Phase 2a trial.169 The ongoing Phase 3 AEGIS-II trial is evaluating 4 weekly infusions of CSL-112 can lower the short-term rates of recurrent events among post-AMI patients.170
CER-001 is a negatively charged lipoprotein complex, consisting of phosphatidylcholine, sphingomyelin, and recombinant human apoA-I. Although CER-001 promoted the regression of diet-induced atherosclerosis in a mouse model,171 no changes in coronary atherosclerosis were observed among patients with a recent ACS.172 It is conceivable that the infusion with HDL mimetics cannot reduce plaque burden beyond the effect induced by intensive statin therapy. Of note, CER-001 was proven to be effective in protecting kidneys in patients with familial LCAT deficiency,173,174 and intravenously administration of CER-001 in a severe COVID-19 patient increased apoA-I levels while HDL-C levels decreased, accompanied by significant decreases in many inflammatory markers and cytokines,175 suggesting potential utilization for diseases other than CV disease.
As LCAT plays a key role in HDL metabolism and RCT, ongoing studies are currently evaluating the effect of increasing LCAT activity not only for CV disease but also for other conditions such as familial LCAT deficiency and fish-eye disease. A Phase 2a study in subjects with stable CHD showed that recombinant human LCAT (MEDI6012) added to statin therapy increased HDL-C and apoA-I levels, increased non-ABCA1-mediated cholesterol efflux while reducing apoB levels and total and small LDL particle number.176 A Phase 2b trial is currently evaluating the safety and efficacy of MEDI6012 in patients presenting with an acute ST-elevation myocardial infarction (clinicaltrials.gov/ct2/show/NCT03578809).
Final considerations
Taken together, HDL is a dynamic multifaceted lipoprotein that can serve several physiological roles, most of which have been preserved throughout evolution for other reasons than protection from atherosclerosis (Graphical Abstract). Clinically, low HDL-C remains a strong and important risk marker for increased risk of ASCVD, likely due to the inverse association with increased levels of triglyceride-rich remnant lipoproteins. For patients with extremely high HDL-C, the documented increased risk of infectious disease and all-cause mortality should inform patients and doctors alike of the possible negative prognostic consequences of high HDL-C. Finally, the observed increased risk of AMD observationally and causal, genetically for any increase in HDL levels is of concern. Future efforts to pharmacologically modulate HDL should likely focus on functional metrics of HDL function rather than HDL-C and other clinical indications besides ASCVD.
Acknowledgements
We thank the staff and the participants of the Copenhagen City Heart Study for their valuable contributions. We thank Liv T. Nordestgaard for creating Figure 7.
Contributor Information
Arnold von Eckardstein, Institute of Clinical Chemistry, University Hospital Zurich and University of Zurich, Zurich, Switzerland.
Børge G Nordestgaard, Department of Clinical Biochemistry, Copenhagen University Hospital, Herlev and Gentofte Hospital, Herlev, Denmark; The Copenhagen General Population Study, Copenhagen University Hospital, Herlev and Gentofte Hospital, Herlev, Denmark; Institute of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Alan T Remaley, Lipoprotein Metabolism Section, Translational Vascular Medicine Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA.
Alberico L Catapano, Department of Pharmacological and Biomolecular Sciences, University of Milan, Milan, Italy; IRCCS MultiMedica, Sesto S. Giovanni, Milan, Italy.
Funding
Supported by the Swiss National Science Foundation (A.v.E.) and by Herlev Hospital, Copenhagen University Hospital (to B.G.N.). The work of A.L.C. is supported in part by the Ricerca Corrente from Ministero della Salute.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Gofman JW, Glazier F, Tamplin A, Strisower B, De Lalla O. Lipoproteins, coronary heart disease, and atherosclerosis. Physiol Rev 1954;34:589–607. [DOI] [PubMed] [Google Scholar]
- 2. Jenkins PJ, Harper RW, Nestel PJ. Severity of coronary atherosclerosis related to lipoprotein concentration. Br Med J 1978;2:388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller GJ, Miller NE. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet 1975;305:16–19. [DOI] [PubMed] [Google Scholar]
- 4. Wilson PW, Abbott RD, Castelli WP. High density lipoprotein cholesterol and mortality. The framingham heart study. Arteriosclerosis 1988;8:737–741. [DOI] [PubMed] [Google Scholar]
- 5. Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The framingham study. Am J Med 1977;62:707–714. [DOI] [PubMed] [Google Scholar]
- 6. Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The framingham study. Jama 1986;256:2835–2838. [PubMed] [Google Scholar]
- 7. Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, et al. . Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: the atherosclerosis risk in communities (ARIC) study. Circulation 2001;104:1108–1113. [DOI] [PubMed] [Google Scholar]
- 8. Emerging Risk Factors Collaboration, Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, et al. . Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. HPS3/TIMI55–REVEAL Collaborative Group, Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, et al. . Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med 2017;377:1217–1227. [DOI] [PubMed] [Google Scholar]
- 10. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, et al. . Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–2267. [DOI] [PubMed] [Google Scholar]
- 11. Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, Linz P, et al. . Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. . Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 2012;367:2089–2099. [DOI] [PubMed] [Google Scholar]
- 13. Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, et al. . Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med 2017;376:1933–1942. [DOI] [PubMed] [Google Scholar]
- 14. Frikke-Schmidt R, Nordestgaard BG, Stene MC, Sethi AA, Remaley AT, Schnohr P, et al. . Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA 2008;299:2524–2532. [DOI] [PubMed] [Google Scholar]
- 15. Haase CL, Tybjaerg-Hansen A, Grande P, Frikke-Schmidt R. Genetically elevated apolipoprotein A-I, high-density lipoprotein cholesterol levels, and risk of ischemic heart disease. J Clin Endocrinol Metab 2010;95:E500–E510. [DOI] [PubMed] [Google Scholar]
- 16. Haase CL, Tybjaerg-Hansen A, Qayyum AA, Schou J, Nordestgaard BG, Frikke-Schmidt R. LCAT, HDL cholesterol and ischemic cardiovascular disease: a Mendelian randomization study of HDL cholesterol in 54,500 individuals. J Clin Endocrinol Metab 2012;97:E248–E256. [DOI] [PubMed] [Google Scholar]
- 17. Tietjen I, Hovingh GK, Singaraja R, Radomski C, McEwen J, Chan E, et al. . Increased risk of coronary artery disease in caucasians with extremely low HDL cholesterol due to mutations in ABCA1, APOA1, and LCAT. Biochim Biophys Acta 2012;1821:416–424. [DOI] [PubMed] [Google Scholar]
- 18. Geller AS, Polisecki EY, Diffenderfer MR, Asztalos BF, Karathanasis SK, Hegele RA, et al. . Genetic and secondary causes of severe HDL deficiency and cardiovascular disease. J Lipid Res 2018;59:2421–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johannsen TH, Kamstrup PR, Andersen RV, Jensen GB, Sillesen H, Tybjaerg-Hansen A, et al. . Hepatic lipase, genetically elevated high-density lipoprotein, and risk of ischemic cardiovascular disease. J Clin Endocrinol Metab 2009;94:1264–1273. [DOI] [PubMed] [Google Scholar]
- 20. Silbernagel G, Scharnagl H, Kleber ME, Delgado G, Stojakovic T, Laaksonen R, et al. . LDL Triglycerides, hepatic lipase activity, and coronary artery disease: an epidemiologic and Mendelian randomization study. Atherosclerosis 2019;282:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Helgadottir A, Sulem P, Thorgeirsson G, Gretarsdottir S, Thorleifsson G, Jensson BO, et al. . Rare SCARB1 mutations associate with high-density lipoprotein cholesterol but not with coronary artery disease. Eur Heart J 2018;39:2172–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zanoni P, Khetarpal SA, Larach DB, Hancock-Cerutti WF, Millar JS, Cuchel M, et al. . Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science 2016;351:1166–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. . Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet 2012;380:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thomas DG, Wei Y, Tall AR. Lipid and metabolic syndrome traits in coronary artery disease: a Mendelian randomization study. J Lipid Res 2021;62:100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nomura A, Won HH, Khera AV, Takeuchi F, Ito K, McCarthy S, et al. . Protein-Truncating variants at the cholesteryl ester transfer protein gene and risk for coronary heart disease. Circ Res 2017;121:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ference BA, Kastelein JJP, Ginsberg HN, Chapman MJ, Nicholls SJ, Ray KK, et al. . Association of genetic variants related to CETP iInhibitors and statins with lipoprotein levels and cardiovascular risk. JAMA 2017;318:947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Richardson TG, Sanderson E, Palmer TM, Ala-Korpela M, Ference BA, Davey Smith G, et al. . Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: a multivariable Mendelian randomisation analysis. PLoS Med 2020;17:e1003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoekstra M, Van Eck M. Mouse models of disturbed HDL metabolism. Handb Exp Pharmacol 2015;224:301–336. [DOI] [PubMed] [Google Scholar]
- 29. Wilkins JT, Ning H, Stone NJ, Criqui MH, Zhao L, Greenland P, et al. . Coronary heart disease risks associated with high levels of HDL cholesterol. J Am Heart Assoc 2014;3:e000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J 2017;38:2478–2486. [DOI] [PubMed] [Google Scholar]
- 31. Madsen CM, Varbo A, Tybjaerg-Hansen A, Frikke-Schmidt R, Nordestgaard BG. U-shaped relationship of HDL and risk of infectious disease: two prospective population-based cohort studies. Eur Heart J 2018;39:1181–1190. [DOI] [PubMed] [Google Scholar]
- 32. Kjeldsen EW, Thomassen JQ, Juul Rasmussen I, Nordestgaard BG, Tybjaerg-Hansen A, Frikke-Schmidt R. Plasma high-density lipoprotein cholesterol and risk of dementia: observational and genetic studies. Cardiovasc Res 2022;118:1330–1343. [DOI] [PubMed] [Google Scholar]
- 33. Pirillo A, Catapano AL, Norata GD. Biological consequences of dysfunctional HDL. Curr Med Chem 2019;26:1644–1664. [DOI] [PubMed] [Google Scholar]
- 34. Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, et al. . HDL Cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 2014;371:2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, et al. . Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol 2015;3:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ajala ON, Demler OV, Liu Y, Farukhi Z, Adelman SJ, Collins HL, et al. . Anti-Inflammatory HDL function, incident cardiovascular events, and mortality: a secondary analysis of the JUPITER randomized clinical trial. J Am Heart Assoc 2020;9:e016507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. El Harchaoui K, Arsenault BJ, Franssen R, Despres JP, Hovingh GK, Stroes ES, et al. . High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med 2009;150:84–93. [DOI] [PubMed] [Google Scholar]
- 38. Mackey RH, Greenland P, Goff DC Jr., Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: mESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol 2012;60:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parish S, Offer A, Clarke R, Hopewell JC, Hill MR, Otvos JD, et al. . Lipids and lipoproteins and risk of different vascular events in the MRC/BHF heart protection study. Circulation 2012;125:2469–2478. [DOI] [PubMed] [Google Scholar]
- 40. Chandra A, Neeland IJ, Das SR, Khera A, Turer AT, Ayers CR, et al. . Relation of black race between high density lipoprotein cholesterol content, high density lipoprotein particles and coronary events (from the Dallas heart study). Am J Cardiol 2015;115:890–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuller LH, Grandits G, Cohen JD, Neaton JD, Prineas R. Multiple risk factor intervention trial research G. Lipoprotein particles, insulin, adiponectin, C-reactive protein and risk of coronary heart disease among men with metabolic syndrome. Atherosclerosis 2007;195:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rohatgi A, Westerterp M, von Eckardstein A, Remaley A, Rye KA. HDL In the 21st century: a multifunctional roadmap for future HDL research. Circulation 2021;143:2293–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang R, Silva RA, Jerome WG, Kontush A, Chapman MJ, Curtiss LK, et al. . Apolipoprotein A-I structural organization in high-density lipoproteins isolated from human plasma. Nat Struct Mol Biol 2011;18:416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Davidson WS, Silva RA. Apolipoprotein structural organization in high density lipoproteins: belts, bundles, hinges and hairpins. Curr Opin Lipidol 2005;16:295–300. [DOI] [PubMed] [Google Scholar]
- 45. Davidson WS, Shah AS, Sexmith H, Gordon SM. The HDL proteome watch: compilation of studies leads to new insights on HDL function. Biochim Biophys Acta Mol Cell Biol Lipids 2022;1867:159072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goetze S, Frey K, Rohrer L, Radosavljevic S, Krutzfeldt J, Landmesser U, et al. . Reproducible determination of high-density lipoprotein proteotypes. J Proteome Res 2021;20:4974–4984. [DOI] [PubMed] [Google Scholar]
- 47. Coker MM, Akinyemi RO, Bakare AA, Owolabi MO. Genetic epidemiology and associated diseases of APOL1: a narrative review. West Afr J Med 2021;38:511–519. [PubMed] [Google Scholar]
- 48. Gordon SM, Remaley AT. High density lipoproteins are modulators of protease activity: implications in inflammation, complement activation, and atherothrombosis. Atherosclerosis 2017;259:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ossoli A, Neufeld EB, Thacker SG, Vaisman B, Pryor M, Freeman LA, et al. . Lipoprotein X causes renal disease in LCAT deficiency. PLoS One 2016;11:e0150083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wuni R, Kuhnle GGC, Wynn-Jones AA, Vimaleswaran KS. A nutrigenetic update on CETP gene-diet interactions on lipid-related outcomes. Curr Atheroscler Rep 2022;24:119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bisgaard LS, Christoffersen C. The apoM/S1P Complex-A mediator in kidney biology and disease? Front Med (Lausanne) 2021;8:754490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vickers KC, Michell DL. HDL-small RNA export, transport, and functional delivery in atherosclerosis. Curr Atheroscler Rep 2021;23:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rosenson RS, Brewer HB Jr., Chapman MJ, Fazio S, Hussain MM, Kontush A, et al. . HDL Measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin Chem 2011;57:392–410. [DOI] [PubMed] [Google Scholar]
- 54. Sacks FM, Liang L, Furtado JD, Cai T, Davidson WS, He Z, et al. . Protein-Defined subspecies of HDLs (high-density lipoproteins) and differential risk of coronary heart disease in 4 prospective studies. Arterioscler Thromb Vasc Biol 2020;40:2714–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Karathanasis SK, Freeman LA, Gordon SM, Remaley AT. The changing face of HDL and the best way to measure it. Clin Chem 2017;63:196–210. [DOI] [PubMed] [Google Scholar]
- 56. Furtado JD, Yamamoto R, Melchior JT, Andraski AB, Gamez-Guerrero M, Mulcahy P, et al. . Distinct proteomic signatures in 16 HDL (high-density lipoprotein) subspecies. Arterioscler Thromb Vasc Biol 2018;38:2827–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mendivil CO, Furtado J, Morton AM, Wang L, Sacks FM. Novel pathways of apolipoprotein A-I metabolism in high-density lipoprotein of different sizes in humans. Arterioscler Thromb Vasc Biol 2016;36:156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wheeler RJ. The trypanolytic factor-mechanism, impacts and applications. Trends Parasitol 2010;26:457–464. [DOI] [PubMed] [Google Scholar]
- 59. Zannis VI, Fotakis P, Koukos G, Kardassis D, Ehnholm C, Jauhiainen M, et al. . HDL Biogenesis, remodeling, and catabolism. Handb Exp Pharmacol 2015;224:53–111. [DOI] [PubMed] [Google Scholar]
- 60. Cedo L, Metso J, Santos D, Garcia-Leon A, Plana N, Sabate-Soler S, et al. . LDL Receptor regulates the reverse transport of macrophage-derived unesterified cholesterol via concerted action of the HDL-LDL axis: insight from mouse models. Circ Res 2020;127:778–792. [DOI] [PubMed] [Google Scholar]
- 61. Pownall HJ, Rosales C, Gillard BK, Gotto AM Jr. High-density lipoproteins, reverse cholesterol transport and atherogenesis. Nat Rev Cardiol 2021;18:712–723. [DOI] [PubMed] [Google Scholar]
- 62. Sutter I, Park R, Othman A, Rohrer L, Hornemann T, Stoffel M, et al. . Apolipoprotein M modulates erythrocyte efflux and tubular reabsorption of sphingosine-1-phosphate. J Lipid Res 2014;55:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Christensen PM, Bosteen MH, Hajny S, Nielsen LB, Christoffersen C. Apolipoprotein M mediates sphingosine-1-phosphate efflux from erythrocytes. Sci Rep 2017;7:14983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Phillips MC. Molecular mechanisms of cellular cholesterol efflux. J Biol Chem 2014;289:24020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ouimet M, Barrett TJ, Fisher EA. HDL And reverse cholesterol transport. Circ Res 2019;124:1505–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zanoni P, Velagapudi S, Yalcinkaya M, Rohrer L, von Eckardstein A. Endocytosis of lipoproteins. Atherosclerosis 2018;275:273–295. [DOI] [PubMed] [Google Scholar]
- 67. Bashore AC, Liu M, Key CC, Boudyguina E, Wang X, Carroll CM, et al. . Targeted deletion of hepatocyte Abca1 increases plasma HDL (high-density lipoprotein) reverse cholesterol transport via the LDL (low-density lipoprotein) receptor. Arterioscler Thromb Vasc Biol 2019;39:1747–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Brousseau ME, Diffenderfer MR, Millar JS, Nartsupha C, Asztalos BF, Welty FK, et al. . Effects of cholesteryl ester transfer protein inhibition on high-density lipoprotein subspecies, apolipoprotein A-I metabolism, and fecal sterol excretion. Arterioscler Thromb Vasc Biol 2005;25:1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bonacina F, Pirillo A, Catapano AL, Norata GD. HDL In immune-inflammatory responses: implications beyond cardiovascular diseases. Cells 2021;10:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bartelt A, John C, Schaltenberg N, Berbee JFP, Worthmann A, Cherradi ML, et al. . Thermogenic adipocytes promote HDL turnover and reverse cholesterol transport. Nat Commun 2017;8:15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cuffe H, Liu M, Key CC, Boudyguina E, Sawyer JK, Weckerle A, et al. . Targeted deletion of adipocyte Abca1 (ATP-binding cassette transporter A1) impairs diet-induced obesity. Arterioscler Thromb Vasc Biol 2018;38:733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Darabi M, Kontush A. High-density lipoproteins (HDL): novel function and therapeutic applications. Biochim Biophys Acta Mol Cell Biol Lipids 2022;1867:159058. [DOI] [PubMed] [Google Scholar]
- 73. Robert J, Osto E, von Eckardstein A. The endothelium is both a target and a barrier of HDL’s protective functions. Cells 2021;10:1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Manandhar B, Cochran BJ, Rye KA. Role of high-density lipoproteins in cholesterol homeostasis and glycemic control. J Am Heart Assoc 2020;9:e013531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yuhanna IS, Zhu Y, Cox BE, Hahner LD, Osborne-Lawrence S, Lu P, et al. . High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med 2001;7:853–857. [DOI] [PubMed] [Google Scholar]
- 76. De Nardo D, Labzin LI, Kono H, Seki R, Schmidt SV, Beyer M, et al. . High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat Immunol 2014;15:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ito A, Hong C, Oka K, Salazar JV, Diehl C, Witztum JL, et al. . Cholesterol accumulation in CD11c(+) immune cells is a causal and targetable factor in autoimmune disease. Immunity 2016;45:1311–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Price NL, Zhang X, Fernandez-Tussy P, Singh AK, Burnap SA, Rotllan N, et al. . Loss of hepatic miR-33 improves metabolic homeostasis and liver function without altering body weight or atherosclerosis. Proc Natl Acad Sci U S A 2021;118:e2006478118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Linton MF, Tao H, Linton EF, Yancey PG. SR-BI: a multifunctional receptor in cholesterol homeostasis and atherosclerosis. Trends Endocrinol Metab 2017;28:461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tang S, Tabet F, Cochran BJ, Cuesta Torres LF, Wu BJ, Barter PJ, et al. . Apolipoprotein A-I enhances insulin-dependent and insulin-independent glucose uptake by skeletal muscle. Sci Rep 2019;9:1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cartier A, Hla T. Sphingosine 1-phosphate: lipid signaling in pathology and therapy. Science 2019;366:eaar5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Velagapudi S, Rohrer L, Poti F, Feuerborn R, Perisa D, Wang D, et al. . Apolipoprotein M and sphingosine-1-phosphate receptor 1 promote the transendothelial transport of high-density lipoprotein. Arterioscler Thromb Vasc Biol 2021;41:e468–e479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cuesta Torres LF, Zhu W, Ohrling G, Larsson R, Patel M, Wiese CB, et al. . High-density lipoproteins induce miR-223-3p biogenesis and export from myeloid cells: role of scavenger receptor BI-mediated lipid transfer. Atherosclerosis 2019;286:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Martinez LO, Najib S, Perret B, Cabou C, Lichtenstein L. Ecto-F1-ATPase/P2Y pathways in metabolic and vascular functions of high density lipoproteins. Atherosclerosis 2015;238:89–100. [DOI] [PubMed] [Google Scholar]
- 85. Gerster R, Eloranta JJ, Hausmann M, Ruiz PA, Cosin-Roger J, Terhalle A, et al. . Anti-inflammatory function of high-density lipoproteins via autophagy of IkappaB kinase. Cell Mol Gastroenterol Hepatol 2015;1:171–187.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lucero D, Islam P, Freeman LA, Jin X, Pryor M, Tang J, et al. . Interleukin 10 promotes macrophage uptake of HDL and LDL by stimulating fluid-phase endocytosis. Biochim Biophys Acta Mol Cell Biol Lipids 2020;1865:158537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zheng A, Dubuis G, Georgieva M, Mendes Ferreira CS, Serulla M, Del Carmen Conde Rubio M, et al. . HDLs extract lipophilic drugs from cells. J Cell Sci 2022;135:jcs258644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Meilhac O, Tanaka S, Couret D. High-Density lipoproteins are bug scavengers. Biomolecules 2020;10:598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mackness M, Mackness B. Human paraoxonase-1 (PON1): gene structure and expression, promiscuous activities and multiple physiological roles. Gene 2015;567:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rallidis LS, Tellis CC, Lekakis J, Rizos I, Varounis C, Charalampopoulos A, et al. . Lipoprotein-associated phospholipase A(2) bound on high-density lipoprotein is associated with lower risk for cardiac death in stable coronary artery disease patients: a 3-year follow-up. J Am Coll Cardiol 2012;60:2053–2060. [DOI] [PubMed] [Google Scholar]
- 91. Ossoli A, Simonelli S, Varrenti M, Morici N, Oliva F, Stucchi M, et al. . Recombinant LCAT (lecithin:cholesterol acyltransferase) rescues defective HDL (high-density lipoprotein)-mediated endothelial protection in acute coronary syndrome. Arterioscler Thromb Vasc Biol 2019;39:915–924. [DOI] [PubMed] [Google Scholar]
- 92. Pirillo A, Catapano AL, Norata GD. HDL In infectious diseases and sepsis. Handb Exp Pharmacol 2015;224:483–508. [DOI] [PubMed] [Google Scholar]
- 93. Wei C, Wan L, Yan Q, Wang X, Zhang J, Yang X, et al. . HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry. Nat Metab 2020;2:1391–1400. [DOI] [PubMed] [Google Scholar]
- 94. Cho KH, Kim JR, Lee IC, Kwon HJ. Native high-density lipoproteins (HDL) with higher paraoxonase exerts a potent antiviral effect against SARS-CoV-2 (COVID-19), while glycated HDL lost the antiviral activity. Antioxidants (Basel) 2021;10:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Annema W, von Eckardstein A. Dysfunctional high-density lipoproteins in coronary heart disease: implications for diagnostics and therapy. Transl Res 2016;173:30–57. [DOI] [PubMed] [Google Scholar]
- 96. Cardner M, Yalcinkaya M, Goetze S, Luca E, Balaz M, Hunjadi M, et al. . Structure-function relationships of HDL in diabetes and coronary heart disease. JCI Insight 2020;5:e131491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Krankel N, et al. . Abnormal high-density lipoprotein induces endothelial dysfunction via activation of toll-like receptor-2. Immunity 2013;38:754–768. [DOI] [PubMed] [Google Scholar]
- 98. Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, et al. . Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest 2011;121:2693–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Boren J, Catapano AL, et al. . Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J 2011;32:1345–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Feng M, Darabi M, Tubeuf E, Canicio A, Lhomme M, Frisdal E, et al. . Free cholesterol transfer to high-density lipoprotein (HDL) upon triglyceride lipolysis underlies the U-shape relationship between HDL-cholesterol and cardiovascular disease. Eur J Prev Cardiol 2020;27:1606–1616. [DOI] [PubMed] [Google Scholar]
- 101. Langsted A, Jensen AMR, Varbo A, Nordestgaard BG. Low high-density lipoprotein cholesterol to monitor long-term average increased triglycerides. J Clin Endocrinol Metab 2020;105:e1657-e1666. [DOI] [PubMed] [Google Scholar]
- 102. Wu Y, Fan Z, Tian Y, Liu S, Liu S. Relation between high density lipoprotein particles concentration and cardiovascular events: a meta-analysis. Lipids Health Dis 2018;17:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhao Q, Wang J, Miao Z, Zhang NR, Hennessy S, Small DS, et al. . A Mendelian randomization study of the role of lipoprotein subfractions in coronary artery disease. Elife 2021;10:e58361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Masana L, Cabre A, Heras M, Amigo N, Correig X, Martinez-Hervas S, et al. . Remarkable quantitative and qualitative differences in HDL after niacin or fenofibrate therapy in type 2 diabetic patients. Atherosclerosis 2015;238:213–219. [DOI] [PubMed] [Google Scholar]
- 105. Boekholdt SM, Arsenault BJ, Hovingh GK, Mora S, Pedersen TR, Larosa JC, et al. . Levels and changes of HDL cholesterol and apolipoprotein A-I in relation to risk of cardiovascular events among statin-treated patients: a meta-analysis. Circulation 2013;128:1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Karjalainen MK, Holmes MV, Wang Q, Anufrieva O, Kahonen M, Lehtimaki T, et al. . Apolipoprotein A-I concentrations and risk of coronary artery disease: a Mendelian randomization study. Atherosclerosis 2020;299:56–63. [DOI] [PubMed] [Google Scholar]
- 107. Zewinger S, Drechsler C, Kleber ME, Dressel A, Riffel J, Triem S, et al. . Serum amyloid A: high-density lipoproteins interaction and cardiovascular risk. Eur Heart J 2015;36:3007–3016. [DOI] [PubMed] [Google Scholar]
- 108. Kopecky C, Genser B, Drechsler C, Krane V, Kaltenecker CC, Hengstschlager M, et al. . Quantification of HDL proteins, cardiac events, and mortality in patients with type 2 diabetes on hemodialysis. Clin J Am Soc Nephrol 2015;10:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Riwanto M, Rohrer L, Roschitzki B, Besler C, Mocharla P, Mueller M, et al. . Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation 2013;127:891–904. [DOI] [PubMed] [Google Scholar]
- 110. Soria-Florido MT, Schroder H, Grau M, Fito M, Lassale C. High density lipoprotein functionality and cardiovascular events and mortality: a systematic review and meta-analysis. Atherosclerosis 2020;302:36–42. [DOI] [PubMed] [Google Scholar]
- 111. Schachtl-Riess JF, Coassin S, Lamina C, Demetz E, Streiter G, Hilbe R, et al. . Lysis reagents, cell numbers, and calculation method influence high-throughput measurement of HDL-mediated cholesterol efflux capacity. J Lipid Res 2021;62:100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nicholls SJ, Ruotolo G, Brewer HB, Kane JP, Wang MD, Krueger KA, et al. . Cholesterol efflux capacity and Pre-Beta-1 HDL concentrations are increased in dyslipidemic patients treated with evacetrapib. J Am Coll Cardiol 2015;66:2201–2210. [DOI] [PubMed] [Google Scholar]
- 113. Kuusisto S, Karjalainen MK, Tillin T, Kangas AJ, Holmes MV, Kahonen M, et al. . Genetic and observational evidence: no independent role for cholesterol efflux over static high-density lipoprotein concentration measures in coronary heart disease risk assessment. J Intern Med 2022;292:146-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Natarajan P, Collier TS, Jin Z, Lyass A, Li Y, Ibrahim NE, et al. . Association of an HDL apolipoproteomic score with coronary atherosclerosis and cardiovascular death. J Am Coll Cardiol 2019;73:2135–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Madsen CM, Varbo A, Nordestgaard BG. Novel insights from human studies on the role of high-density lipoprotein in mortality and noncardiovascular disease. Arterioscler Thromb Vasc Biol 2021;41:128–140. [DOI] [PubMed] [Google Scholar]
- 116. Ko DT, Alter DA, Guo H, Koh M, Lau G, Austin PC, et al. . High-Density lipoprotein cholesterol and cause-specific mortality in individuals without previous cardiovascular conditions: the CANHEART study. J Am Coll Cardiol 2016;68:2073–2083. [DOI] [PubMed] [Google Scholar]
- 117. Bowe B, Xie Y, Xian H, Balasubramanian S, Zayed MA, Al-Aly Z. High density lipoprotein cholesterol and the risk of all-cause mortality among U. S. Veterans. Clin J Am Soc Nephrol 2016;11:1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Dron JS, Wang J, Low-Kam C, Khetarpal SA, Robinson JF, McIntyre AD, et al. . Polygenic determinants in extremes of high-density lipoprotein cholesterol. J Lipid Res 2017;58:2162–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Trinder M, Walley KR, Boyd JH, Brunham LR. Causal inference for genetically determined levels of high-density lipoprotein cholesterol and risk of infectious disease. Arterioscler Thromb Vasc Biol 2020;40:267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Pedersen KM, Çolak Y, Bojesen SE, Nordestgaard BG. Low high-density lipoprotein and increased risk of several cancers: 2 population-based cohort studies including 116,728 individuals. J Hematol Oncol 2020;13:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Trinder M, Boyd JH, Brunham LR. Molecular regulation of plasma lipid levels during systemic inflammation and sepsis. Curr Opin Lipidol 2019;30:108–116. [DOI] [PubMed] [Google Scholar]
- 122. Cirstea M, Walley KR, Russell JA, Brunham LR, Genga KR, Boyd JH. Decreased high-density lipoprotein cholesterol level is an early prognostic marker for organ dysfunction and death in patients with suspected sepsis. J Crit Care 2017;38:289–294. [DOI] [PubMed] [Google Scholar]
- 123. Trinder M, Wang Y, Madsen CM, Ponomarev T, Bohunek L, Daisely BA, et al. . Inhibition of cholesteryl ester transfer protein preserves high-density lipoprotein cholesterol and improves survival in sepsis. Circulation 2021;143:921–934. [DOI] [PubMed] [Google Scholar]
- 124. Madsen CM, Varbo A, Nordestgaard BG. Low HDL cholesterol and high risk of autoimmune disease: two population-based cohort studies including 117341 individuals. Clin Chem 2019;65:644–652. [DOI] [PubMed] [Google Scholar]
- 125. Pirro M, Ricciuti B, Rader DJ, Catapano AL, Sahebkar A, Banach M. High density lipoprotein cholesterol and cancer: marker or causative? Prog Lipid Res 2018;71:54–69. [DOI] [PubMed] [Google Scholar]
- 126. Chandler PD, Song Y, Lin J, Zhang S, Sesso HD, Mora S, et al. . Lipid biomarkers and long-term risk of cancer in the women’s health study. Am J Clin Nutr 2016;103:1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. White J, Swerdlow DI, Preiss D, Fairhurst-Hunter Z, Keating BJ, Asselbergs FW, et al. . Association of lipid fractions with risks for coronary artery disease and diabetes. JAMA Cardiol 2016;1:692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Fall T, Xie W, Poon W, Yaghootkar H, Mägi R, Knowles JW, et al. . Using genetic variants to assess the relationship between circulating lipids and type 2 diabetes. Diabetes 2015;64:2676–2684. [DOI] [PubMed] [Google Scholar]
- 129. Haase CL, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. HDL Cholesterol and risk of type 2 diabetes: a Mendelian randomization study. Diabetes 2015;64:3328–3333. [DOI] [PubMed] [Google Scholar]
- 130. Dangas K, Navar AM, Kastelein JJP. The effect of CETP inhibitors on new-onset diabetes: a systematic review and meta-analysis. Eur Heart J Cardiovasc Pharmacother 2022;8:622-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Masson W, Lobo M, Siniawski D, Huerin M, Molinero G, Valero R, et al. . Therapy with cholesteryl ester transfer protein (CETP) inhibitors and diabetes risk. Diabetes Metab 2018;44:508–513. [DOI] [PubMed] [Google Scholar]
- 132. Drew BG, Duffy SJ, Formosa MF, Natoli AK, Henstridge DC, Penfold SA, et al. . High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation 2009;119:2103–2111. [DOI] [PubMed] [Google Scholar]
- 133. Bowe B, Xie Y, Xian H, Balasubramanian S, Al-Aly Z. Low levels of high-density lipoprotein cholesterol increase the risk of incident kidney disease and its progression. Kidney Int 2016;89:886–896. [DOI] [PubMed] [Google Scholar]
- 134. Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. Jama 2004;291:844–850. [DOI] [PubMed] [Google Scholar]
- 135. Muntner P, Coresh J, Smith JC, Eckfeldt J, Klag MJ. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int 2000;58:293–301. [DOI] [PubMed] [Google Scholar]
- 136. Coassin S, Friedel S, Köttgen A, Lamina C, Kronenberg F. Is high-density lipoprotein cholesterol causally related to kidney function? Evidence from genetic epidemiological studies. Arterioscler Thromb Vasc Biol 2016;36:2252–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lanktree MB, Thériault S, Walsh M, Paré G. HDL Cholesterol, LDL cholesterol, and triglycerides as risk factors for CKD: a Mendelian randomization study. Am J Kidney Dis 2018;71:166–172. [DOI] [PubMed] [Google Scholar]
- 138. Liu HM, Hu Q, Zhang Q, Su GY, Xiao HM, Li BY, et al. . Causal effects of genetically predicted cardiovascular risk factors on chronic kidney disease: a two-sample Mendelian randomization study. Front Genet 2019;10:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Gordon EM, Figueroa DM, Barochia AV, Yao X, Levine SJ. High-density lipoproteins and apolipoprotein A-I: potential new players in the prevention and treatment of lung disease. Front Pharmacol 2016;7:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Burgess S, Davey Smith G. Mendelian Randomization implicates high-density lipoprotein cholesterol-associated mechanisms in etiology of age-related macular degeneration. Ophthalmology 2017;124:1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Colijn JM, den Hollander AI, Demirkan A, Cougnard-Gregoire A, Verzijden T, Kersten E, et al. . Increased high-density lipoprotein levels associated with age-related macular degeneration: evidence from the EYE-RISK and European eye epidemiology consortia. Ophthalmology 2019;126:393–406. [DOI] [PubMed] [Google Scholar]
- 142. Han X, Ong JS, Hewitt AW, Gharahkhani P, MacGregor S. The effects of eight serum lipid biomarkers on age-related macular degeneration risk: a Mendelian randomization study. Int J Epidemiol 2021;50:325–336. [DOI] [PubMed] [Google Scholar]
- 143. Nordestgaard LT, Tybjaerg-Hansen A, Frikke-Schmidt R, Nordestgaard BG. Elevated apolipoprotein A1 and HDL cholesterol associated with age-related macular degeneration: 2 population cohorts. J Clin Endocrinol Metab 2021;106:e2749–e2758. [DOI] [PubMed] [Google Scholar]
- 144. Nordestgaard LT, Christoffersen M, Lauridsen BK, Afzal S, Nordestgaard BG, Frikke-Schmidt R, et al. . Long-term benefits and Harms associated with genetic cholesteryl ester transfer protein deficiency in the general population. JAMA Cardiol 2022;7:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Chapman MJ. Animal lipoproteins: chemistry, structure, and comparative aspects. J Lipid Res 1980;21:789–853. [PubMed] [Google Scholar]
- 146. Hoeger U, Schenk S. Crustacean hemolymph lipoproteins. Subcell Biochem 2020;94:35–62. [DOI] [PubMed] [Google Scholar]
- 147. Van der Horst DJ, Rodenburg KW. Lipoprotein assembly and function in an evolutionary perspective. Biomol Concepts 2010;1:165–183. [DOI] [PubMed] [Google Scholar]
- 148. von Eckardstein A. LDL Contributes to reverse cholesterol transport. Circ Res 2020;127:793–795. [DOI] [PubMed] [Google Scholar]
- 149. Hoekstra M. Identification of scavenger receptor BI as a potential screening candidate for congenital primary adrenal insufficiency in humans. Am J Physiol Endocrinol Metab 2020;319:E102–E104. [DOI] [PubMed] [Google Scholar]
- 150. Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, et al. . Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med 2007;357:2109–2122. [DOI] [PubMed] [Google Scholar]
- 151. Tall AR, Rader DJ. Trials and tribulations of CETP inhibitors. Circ Res 2018;122:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Metzinger MP, Saldanha S, Gulati J, Patel KV, El-Ghazali A, Deodhar S, et al. . Effect of anacetrapib on cholesterol efflux capacity: a substudy of the DEFINE trial. J Am Heart Assoc 2020;9:e018136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Chen Y, Dong J, Zhang X, Chen X, Wang L, Chen H, et al. . Evacetrapib reduces prebeta-1 HDL in patients with atherosclerotic cardiovascular disease or diabetes. Atherosclerosis 2019;285:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. van Capelleveen JC, Kastelein JJ, Zwinderman AH, van Deventer SJ, Collins HL, Adelman SJ, et al. . Effects of the cholesteryl ester transfer protein inhibitor, TA-8995, on cholesterol efflux capacity and high-density lipoprotein particle subclasses. J Clin Lipidol 2016;10:1137–1144.e3. [DOI] [PubMed] [Google Scholar]
- 155. Furtado JD, Ruotolo G, Nicholls SJ, Dullea R, Carvajal-Gonzalez S, Sacks FM. Pharmacological inhibition of CETP (cholesteryl ester transfer protein) increases HDL (high-density lipoprotein) that contains ApoC3 and other HDL subspecies associated with higher risk of coronary heart disease. Arterioscler Thromb Vasc Biol 2022;42:227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest 1990;85:1234–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Plump AS, Scott CJ, Breslow JL. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci U S A 1994;91:9607–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature 1991;353:265–267. [DOI] [PubMed] [Google Scholar]
- 159. Sirtori CR, Calabresi L, Franceschini G, Baldassarre D, Amato M, Johansson J, et al. . Cardiovascular status of carriers of the apolipoprotein A-I(Milano) mutant: the limone sul garda study. Circulation 2001;103:1949–1954. [DOI] [PubMed] [Google Scholar]
- 160. Franceschini G, Sirtori CR, Bosisio E, Gualandri V, Orsini GB, Mogavero AM, et al. . Relationship of the phenotypic expression of the A-IMilano apoprotein with plasma lipid and lipoprotein patterns. Atherosclerosis 1985;58:159–174. [DOI] [PubMed] [Google Scholar]
- 161. Franceschini G, Calabresi L, Chiesa G, Parolini C, Sirtori CR, Canavesi M, et al. . Increased cholesterol efflux potential of sera from ApoA-IMilano carriers and transgenic mice. Arterioscler Thromb Vasc Biol 1999;19:1257–1262. [DOI] [PubMed] [Google Scholar]
- 162. Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, et al. . Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. Jama 2003;290:2292–2300. [DOI] [PubMed] [Google Scholar]
- 163. Nicholls SJ, Puri R, Ballantyne CM, Jukema JW, Kastelein JJP, Koenig W, et al. . Effect of infusion of high-density lipoprotein mimetic containing recombinant apolipoprotein A-I Milano on coronary disease in patients with an acute coronary syndrome in the MILANO-PILOT trial: a randomized clinical trial. JAMA Cardiol 2018;3:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Soares AAS, Tavoni TM, de Faria EC, Remalay AT, Maranhao RC, Sposito AC, et al. . HDL Acceptor capacities for cholesterol efflux from macrophages and lipid transfer are both acutely reduced after myocardial infarction. Clin Chim Acta 2018;478:51–56. [DOI] [PubMed] [Google Scholar]
- 165. Tardif JC, Gregoire J, L’Allier PL, Ibrahim R, Lesperance J, Heinonen TM, et al. . Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. Jama 2007;297:1675–1682. [DOI] [PubMed] [Google Scholar]
- 166. Easton R, Gille A, D’Andrea D, Davis R, Wright SD, Shear C. A multiple ascending dose study of CSL112, an infused formulation of ApoA-I. J Clin Pharmacol 2014;54:301–310. [DOI] [PubMed] [Google Scholar]
- 167. Diditchenko S, Gille A, Pragst I, Stadler D, Waelchli M, Hamilton R, et al. . Novel formulation of a reconstituted high-density lipoprotein (CSL112) dramatically enhances ABCA1-dependent cholesterol efflux. Arterioscler Thromb Vasc Biol 2013;33:2202–2211. [DOI] [PubMed] [Google Scholar]
- 168. Michael Gibson C, Korjian S, Tricoci P, Daaboul Y, Yee M, Jain P, et al. . Safety and tolerability of CSL112, a reconstituted, infusible, plasma-derived apolipoprotein A-I, after acute myocardial infarction: the AEGIS-I trial (ApoA-I event reducing in ischemic syndromes I). Circulation 2016;134:1918–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Tricoci P, D’Andrea DM, Gurbel PA, Yao Z, Cuchel M, Winston B, et al. . Infusion of reconstituted high-density lipoprotein, CSL112, in patients with atherosclerosis: safety and pharmacokinetic results from a phase 2a randomized clinical trial. J Am Heart Assoc 2015;4:e002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Gibson CM, Kastelein JJP, Phillips AT, Aylward PE, Yee MK, Tendera M, et al. . Rationale and design of ApoA-I event reducing in ischemic syndromes II (AEGIS-II): a phase 3, multicenter, double-blind, randomized, placebo-controlled, parallel-group study to investigate the efficacy and safety of CSL112 in subjects after acute myocardial infarction. Am Heart J 2021;231:121–127. [DOI] [PubMed] [Google Scholar]
- 171. Tardy C, Goffinet M, Boubekeur N, Ackermann R, Sy G, Bluteau A, et al. . CER-001, a HDL-mimetic, stimulates the reverse lipid transport and atherosclerosis regression in high cholesterol diet-fed LDL-receptor deficient mice. Atherosclerosis 2014;232:110–118. [DOI] [PubMed] [Google Scholar]
- 172. Tardif JC, Ballantyne CM, Barter P, Dasseux JL, Fayad ZA, Guertin MC, et al. . Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: a randomized trial. Eur Heart J 2014;35:3277–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Pavanello C, Turri M, Strazzella A, Tulissi P, Pizzolitto S, De Maglio G, et al. . The HDL mimetic CER-001 remodels plasma lipoproteins and reduces kidney lipid deposits in inherited lecithin:cholesterol acyltransferase deficiency. J Intern Med 2022;291:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Faguer S, Colombat M, Chauveau D, Bernadet-Monrozies P, Beq A, Delas A, et al. . Administration of the high-density lipoprotein mimetic CER-001 for inherited lecithin-cholesterol acyltransferase deficiency. Ann Intern Med 2021;174:1022–1025. [DOI] [PubMed] [Google Scholar]
- 175. Tanaka S, Begue F, Veeren B, Tran-Dinh A, Robert T, Tashk P, et al. . First recombinant high-density lipoprotein particles administration in a severe ICU COVID-19 patient, a multi-omics exploratory investigation. Biomedicines 2022;10:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. George RT, Abuhatzira L, Stoughton SM, Karathanasis SK, She D, Jin C, et al. . MEDI6012: recombinant human lecithin cholesterol acyltransferase, high-density lipoprotein, and low-density lipoprotein receptor-mediated reverse cholesterol transport. J Am Heart Assoc 2021;10:e014572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.