Abstract
Purpose of Review
Genetic variants in GBA1 and LRRK2 genes are the commonest genetic risk factor for Parkinson disease (PD); however, the preclinical profile of GBA1 and LRRK2 variant carriers who will develop PD is unclear. This review aims to highlight the more sensitive markers that can stratify PD risk in non-manifesting GBA1 and LRRK2 variant carriers.
Recent Findings
Several case–control and a few longitudinal studies evaluated clinical, biochemical, and neuroimaging markers within cohorts of non-manifesting carriers of GBA1 and LRRK2 variants.
Summary
Despite similar levels of penetrance of PD in GBA1 and LRRK2 variant carriers (10–30%), these individuals have distinct preclinical profiles. GBA1 variant carriers at higher risk of PD can present with prodromal symptoms suggestive of PD (hyposmia), display increased α-synuclein levels in peripheral blood mononuclear cells, and show dopamine transporter abnormalities. LRRK2 variant carriers at higher risk of PD might show subtle motor abnormalities, but no prodromal symptoms, higher exposure to some environmental factors (non-steroid anti-inflammatory drugs), and peripheral inflammatory profile. This information will help clinicians tailor appropriate screening tests and counseling and facilitate researchers in the development of predictive markers, disease-modifying treatments, and selection of healthy individuals who might benefit from preventive interventions.
Keywords: Parkinson disease, GBA1, Glucocerebrosidase, LRRK2, Carrier, Prodromal
Introduction
In the last 20 years, we have witnessed an exponential and relentless growth of Parkinson disease (PD) cases, with more than 6 million people currently affected worldwide. Based on population projections, it is estimated that this number will double by 2040 [1]. The negative impact that the global burden of PD will have on those individuals primarily affected (patients and caregivers), economics and societies, is substantial [2]. Research efforts should therefore focus on interventions designed to prevent disease occurrence in healthy individuals and slow down progression in the early stages of manifest disease [3].
Individuals with a genetic predisposition for PD could be the first to be directed towards these treatments. Variants in the glucocerebrosidase (GBA1) gene, causing the lysosomal storage disorder Gaucher disease (GD) when present on both alleles, and leucine-rich repeat kinase 2 (LRRK2) gene are found in 10–15% and 1–2% of sporadic PD cases, respectively [4, 5], constituting the most important genetic risk factor for PD. The frequency of GBA1 variants and LRRK2 G2019S variant—the commonest—is even higher in specific populations. For instance, GBA1 variants and LRRK2-G2019S are found in up to 30% of individuals of Ashkenazi Jewish ancestry and north African Arabs, respectively [4, 6]. On the contrary, LRRK2-G2019S is found in only 0.1% of individuals of Asian background [4]. As a whole, PD patients carrying GBA1 variants are characterized by a more severe clinical phenotype compared with patients negative for variants in GBA1 and LRRK2 (hereinafter, referred to as sporadic PD, sPD) [7, 8]. GBA1-PD patients tend to present symptoms at earlier age and develop more severe non-motor symptoms (olfactory dysfunction, dysautonomia, cognitive decline, and psychiatric symptoms), particularly those carrying complex or severe pathogenic variants as compared to those carrying mild pathogenic or risk variants (the latter increasing PD risk but not being pathogenic for GD), supporting a genotype–phenotype association [8, 9]. Conversely, PD patients carrying LRRK2 variants tend to show a milder phenotype and a more benign disease course [4, 10, 11]. A possible genotype–phenotype association has been observed also for LRRK2, with pathogenic variants being associated with a milder disease compared with risk variants [12]. Several pathogenic mechanisms linked to GBA1 and LRRK2 variants might be responsible for the neurodegenerative changes seen in PD. Among others, reduced enzymatic activity of glucocerebrosidase and its complex interplay with α-synuclein pathology, alterations in the autophagy-lysosomal pathway (ALP), unfolded protein response (UPR), and endoplasmic reticulum (ER) stress have been implicated in GBA1-PD pathogenesis [12]. More recently, lipid dyshomeostasis has been linked to specific GBA1 risk variants [13]. Alterations in ALP, ER stress, and UPR have also been proposed as pathogenic mechanisms linked to LRRK2 variants [12].
Despite the increasing knowledge surrounding GBA1- and LRRK2-PD, a main question related to the penetrance of these variants remains unsolved. In fact, penetrance of GBA1 and LRRK2 variants in PD is low and incomplete (10–30%) [4, 14], meaning that only a minority of carriers will develop the condition over their lifetime. Investigating which factors can modulate PD risk in these individuals is, therefore, imperative to (1) provide the more appropriate genetic counseling and (2) address only the high-risk individuals to preventive treatments.
Herein, we present and critically discuss the more recent evidence related to preclinical/prodromal markers associated with increased risk of PD, classified as clinical, genetic, environmental, biochemical, and neuroimaging, in GBA1 and LRRK2 non-manifesting carriers (NMC). The purpose is to highlight information that can help guide the design of future research studies assessing cohorts of GBA1- and LRRK2-NMC.
Clinical Markers
Non-motor Symptoms
Prodromal non-motor symptoms may help determine PD risk. Current evidence suggests that certain non-motor symptoms, particularly if clustered together, could represent predictive factors for PD in GBA1-NMC but not in LRRK2-NMC.
REM Sleep Behavior Disorder (RBD).
RBD remains one of the strongest prognostic factors for PD conversion. A recent meta-analysis concluded that GBA1-PD patients carrying pathogenic variants display higher risk of RBD and higher RBD Screening Questionnaire (RBDSQ) scores, whereas LRRK2-PD patients show no difference or reduced risk compared with sPD [15]. This could suggest an increased prevalence of RBD within GBA1-NMC carrying pathogenic variants; however, current evidence is conflicting. Pooled analysis of cross-sectional studies showed similar rates of RBD between GBA1-NMC and healthy individuals who were negative for GBA1 or LRRK2 variants (hereinafter, referred to as healthy controls, HC) [15]. Nevertheless, a few longitudinal studies conducted on a group of GBA1-NMC based in the UK showed deterioration in RBDSQ scores compared with HC over time, despite similar values at baseline [16•, 17]. GBA1 variants were also significantly more common (9.5%) in patients with a diagnosis of idiopathic RBD (iRBD) made by video-polysomnography (PSG), compared with controls (4.1%), and GBA1-positive iRBD patients showed significantly increased rate of conversion to neurodegenerative conditions (mainly PD) compared with GBA1-negative iRBD [18]. No association was found between LRRK2 variants and iRBD [19].
Screening for RBD by PSG is superior in terms of diagnostic accuracy than clinical questionnaires [20] and should therefore be considered in GBA1-NMC. The recently proposed classification of PD subtypes into “body-first” and “brain-first” suggests that premotor RBD represents a highly predictive marker only of the body-first subtype [21]. Whether and how this classification applies to genetically stratified cohorts, such as GBA1-NMC, is an intriguing question for future studies.
Hyposmia and Cognition.
Several longitudinal studies have consistently reported that olfactory function deteriorates more rapidly in GBA1-NMC and GD patients—carrying biallelic pathogenic variants in GBA1—compared with HC [16•, 22, 23]. Reduced olfactory function was also found to correlate with worse motor outcome over time and was associated with poorer cognition scores [24] and was the only premotor sign showed by one GD patient of the UK cohort, who later developed PD [16•]. However, in case–control studies conducted on large cohorts of GBA1-NMC, no significant differences in olfactory function between GBA1-NMC, LRRK2-NMC, and HC were found [24–26].
Similar results have been obtained for cognitive function. Large case–control studies failed to identify significant differences across groups (GBA1-NMC, LRRK2-NMC, and HC) [25–27]. However, a trend towards lower Montreal Cognitive Assessment scores in GBA1-NMC compared with HC, LRRK2-NMC, and double mutant LRRK2-GBA1-NMC [28] or subtle alterations in specific cognitive domains, such as verbal memory or executive functioning, in GBA1-NMC [29], were detected in a few studies.
Overall, hyposmia or cognitive deficits may not be present in most GBA1-NMC. However, in a subset, subtle deficits in these domains, especially if clustered together [23], can represent an enhanced risk for PD conversion.
Other Non-motor Symptoms.
Psychiatric features and mood disorders have been reported at increased frequency in GBA1-PD compared to sPD [8]. In cross-sectional studies, increased rate of apathy and anxiety was reported in GBA1-NMC vs. HC, but not in LRRK2-NMC [30]. Longitudinal studies also reported deterioration in mood in GBA1-NMC and GD patients, which was found to be correlated with worse olfactory function at baseline [16•].
Data regarding incidence of other prodromal symptoms, such as constipation, in GBA1- or LRRK2-NMC is still lacking. A few studies detected constipation in cohorts of GD patients [31] but the significance of these results is unclear.
Motor Profile
As opposed to non-motor symptoms, current evidence suggests that motor function could be an important predictive factor of PD conversion in LRRK2-NMC, especially those individuals carrying the LRRK2-G2019S variant—the most studied population. Many studies have, in fact, consistently reported higher MDS-UPDRS part III scores in LRRK2-NMC compared to HC [32, 33•]. In a few studies using sensory-based gait analysis, the G2019S mutation was associated with increased arm swing asymmetry and variability during dual tasking or fast walking in cohorts of either NMC or PD patients [34, 35]. However, since these abnormalities were found in variant carriers, regardless of disease status, they could simply mirror the presence of LRRK2-G2019S, and not represent a true prodromal sign suggesting phenoconversion [34]. A subsequent study, conducted on a small cohort of individuals (27 LRRK2-G2019S-NMC and 36 HC), also found some differences in step time on the non-dominant side during fast-walking in NMC vs. controls and a negative correlation with dopamine transporter (DAT) uptake [36].
That said, are only LRRK2-G2019S-NMC the ones showing motor abnormalities, or is this a shared feature with other LRRK2 variants-NMC? These questions have been addressed by a few studies with discordant results. One report suggested a variant-specific phenotype, with G2019S associated with pure motor symptoms and R1441G associated with non-motor alterations such as dysautonomia, depression, and sleep disorders [32]. Conversely, other studies detected increased autonomic dysfunction in LRRK2-G2019S-NMC, not accompanied by other prodromal symptoms [33•, 37]. Two longitudinal studies have focused on PD risk in LRRK2-G2019S or G2385R carriers. In a study conducted on healthy first-degree relatives of Ashkenazi Jewish LRRK2-G2019S-PD patients (120 G2019S carriers, 111 G2019S non-carriers), over 5 years, 4.3% of the individuals were diagnosed with PD, and all were G2019S carriers. Prodromal markers such as higher UPDRS-III scores, somnolence, constipation, and erectile dysfunction, were statistically more frequent in newly diagnosed PD cases [38••]. In another 10-year longitudinal study conducted on Chinese communities composed by carriers of LRRK2-G2385R variant (329 subjects) or non-carriers (345 subjects), an increased PD risk for LRRK2-G2385R-NMC above 50 years of age (7.9%) compared with non-carriers (2.6%) was detected; however, no differences in motor or non-motor symptoms were observed between the two groups [39].
In conclusion, at present, it remains unclear whether there is a variant-specific profile for LRRK2-NMC variants. Subtle motor changes seem to precede manifest motor onset at least in LRRK2-G2019S-NMC, and therefore, we recommend including sensory-based gait analysis as part of screening evaluation of healthy carriers.
Genetic Markers Beyond GBA1 and LRRK2
Whether other genetic factors might act as modifiers of disease penetrance among carriers of GBA1 variants has been investigated by recent large genome-wide association studies. Interestingly, Straniero and colleagues found that deleterious variants in lysosomal genes can modulate the risk of GBA1-PD, in particular a second variant in GBA1 [40]. In another study, Blauwendraat and colleagues identified that variants near SNCA and CTSB contribute to increased PD risk and decreased age at onset, but the contribution of common genetic variants was small overall [41]. The occurrence of additional co-segregating rare variants in lysosomal genes was also found in families segregating GBA1 variants [42].
Similarly, for LRRK2 carriers, it has been suggested that non-LRRK2 genetic factors may influence PD penetrance [43]. Polygenic risk score (PRS), for instance, was associated with higher penetrance of PD in large cohorts of LRRK2-G2019S carriers [44, 45], and the combination of MDS Research Criteria for Prodromal PD with PRS has been proposed for LRRK2-NMC to identify subjects at higher risk [3].
Environmental Markers
In recent years, it has become evident that specific environmental factors can play an important role as modulators of PD penetrance.
Non-steroid anti-inflammatory drugs (NSAIDs) exposure has been proposed as marker of PD resistance [46]. A retrospective case–control study found that regular use (defined as two or more pills per week for 6 months or more) of NSAIDs (both aspirin and ibuprofen) may lower the risk for PD with OR of 0.34 among LRRK2 variant carriers, including pathogenic (such as G2019S) and risk variants [47••]. Different hypotheses have been proposed to explain the inverse association between NSAIDs and LRRK2-PD. On the one hand, NSAIDs could play a role as modifier factors of inflammation secondary to LRRK2 variants; on the other hand, NSAIDs intake could represent a marker of another determinant of reduced PD risk (for instance, caffeine intake or smoking) which is associated to NSAIDs use [48]. In support of the latter scenario, there is now increasing evidence suggesting a protective effect of caffeine intake and smoking towards PD development, particularly in LRRK2 carriers. In fact, plasma metabolomic analysis showed that PD patients (70 sPD, 118 LRRK2-PD) had lower levels of plasma caffeine concentration compared with healthy individuals (65 HC, 115 LRRK2-NMC), more so in LRRK2-positive (by 76%) compared with LRRK2-negative carriers (by 31%), with significant interaction between LRRK2 and PD status [49•]. Similar results were also found for caffeine demethylation metabolites (paraxanthine, theophylline, 1-methylxanthine) and trigonelline, a nonxanthine marker of coffee consumption [49•]. In this study, LRRK2-PD patients also reported reduced dietary caffeine intake compared with asymptomatic LRRK2-NMC [49•]. Similarly, two recent studies conducted on LRRK2-G2019S-PD from different geographical areas (Tunisia: 142 LRRK2-G2019S-PD, 200 sPD, 57 LRRK2-G2019S-NMC; Israel: 65 LRRK2-G2019S-PD, 100 sPD) showed an association between older PD age at onset and tobacco use and coffee and tea consumption [50, 51], suggesting a protective effect of these factors.
Overall, this evidence suggests that questions related to lifestyle habits (e.g., NSAIDs use, caffeine intake, and smoking) should become part of the initial screening of LRRK2-NMC, eventually followed by more refined analyses such as metabolomics, to further stratify individuals. Future studies should consider evaluating whether the association with these environmental factors is specific for LRRK2 or they can play a role in other genetically stratified cohorts (e.g., GBA1-NMC).
Biochemical Markers
To increase our ability to detect high-risk individuals, clustering clinical and historical data with biochemical markers represents a reasonable option [3].
Alfa-Synuclein.
Levels of α-synuclein in plasma or peripheral blood mononuclear cells (PMBCs) have been found to be increased in GBA1-PD patients compared to sPD [52, 53]. Recently, Emelyanov et al. showed significantly increased CD45 + α-synuclein levels in a group of 12 GBA1-NMC when compared with HC, sPD, and GBA1-PD [54]. Despite the small sample size, a spectrum of increased α-synuclein levels was observed from GBA1-PD, to sPD, HC, and GBA1-NMC, suggesting that either this increase represents a compensatory mechanism that GBA1-NMC exert, or an early, pathological marker that can decompensate later.
Evaluation of α-synuclein levels within LRRK2 carriers is more controversial. This may reflect the different neuropathological substrates associated with distinct LRRK2 variants, with G2019S showing Lewy body pathology in 64% of cases and R1441G only in a few [55, 56]. In a former study, CSF total α-synuclein levels were found similar in LRRK2-NMC, LRRK2-PD, and HC but higher than in sPD [57]. Conversely, a more recent study showed lower levels of CSF total α-synuclein and increased levels of oligomeric α-synuclein in LRRK2-NMC than HC, but similar levels compared with PD patients with and without LRRK2 variants [58]. The combination of total-α-synuclein, oligomeric-α-synuclein, and TNF-α could discriminate LRRK2-NMC from HC with an area under the curve of 0.843 [58]. Evaluation of CSF α-synuclein aggregates through real-time quaking-induced conversion (RT-QuIC) has also been proposed as a marker of possible phenoconversion in LRRK2 carriers. In one study, three of 16 LRRK2-G2019S-NMC (18.8%) were positive for RT-QuIC reaction; none of them developed manifest signs of PD during a 3-year follow-up; however, this subgroup tended to present altered DAT SPECT and fit the criteria for prodromal PD more frequently than NMC without positive RT-QuIC response [59]. Although the discriminant power of CSF α-synuclein measurements is promising, lumbar puncture is an invasive procedure and therefore not applicable as a large-scale screening tool.
Inflammation.
Due to highly expressed levels of LRRK2 protein in peripheral immune cells, in particular neutrophil and classical monocytes, and its recognized central role in several immune and infection processes [60], the peripheral and central inflammatory profiles have been evaluated not only in LRRK2-NMC but also in GBA1-NMC, as potential factors modulating disease risk.
Initial studies evaluating LRRK2-G2019S-NMC, found higher levels of serum (not CSF) cytokines, especially IL-1β, when compared with HC. Interestingly, a subset of G2019S carriers displayed IL-1β levels similar to people with manifest PD, but these levels did not correlate with any early prodromal PD symptom [61]. A subsequent study comparing large cohorts of LRRK2-G2019S and GBA1 variant carriers vs. non-carriers (either with or without PD) did not find any difference in serum or CSF cytokine levels between groups [26]. A separate study evaluating GBA1-NMC also did not show any difference in plasma cytokines levels in these individuals compared with HC [27]. The interpretation of these inconsistent results is challenging. It may be that for LRRK2, a peripheral, initial inflammation process is present at least for a subset of high-risk individuals, without any clear prodromal symptoms associated. For LRRK2-NMC, longitudinal studies evaluating peripheral cytokines as predictive markers might be worth pursuing, whereas, at this stage, invasive CSF analysis does not seem to be informative in the preclinical phase.
Blood urate, an endogenous antioxidant and end product of purine metabolism, is another anti-inflammatory factor which is considered a marker of resistance to PD [62], especially in the context of LRRK2-PD. A cross-sectional study evaluating LRRK2-PD vs. LRRK2-NMC showed that LRRK2-NMC had higher urate levels compared with LRRK2-PD [63]. These results were validated across three independent case–control datasets, and odds of developing PD were reduced by approximately 50% (OR = 0.48) among LRRK2 carriers for each 2 mg/dl increment in blood urate concentration [63]. Although a recent randomized controlled trial failed to show any benefit from use of urate-elevating inosine treatment in early PD [64], urate measurement in LRRK2-NMC remains a promising prognostic marker of resistance/susceptibility to PD development, and application of urate-modifying treatments should be considered in early LRRK2-PD cases. Moreover, since women show reduced levels of plasma urate compared with men [65], urate levels could contribute to the similar penetrance of PD in women and men carrying a LRRK2 variant [66], thus making it an important test to perform within LRRK2-NMC for prognostic purposes.
Lipid Metabolism.
Various markers linked to lipid metabolism have recently become the focus of many groups working on GBA1-PD. First, the enzymatic activity of the lysosomal enzyme gene product glucocerebrosidase (GCase) was evaluated as potential marker of PD conversion. Although a stepwise increase in GCase activity was noticed between severe, mild, risk variant GBA1-NMC, and HC, no difference was detected between mild GBA1-PD and mild GBA1-NMC or between severe GBA1-PD and severe GBA1-NMC, indicating that GCase activity reflects genotype status but cannot be used as prognostic biomarker for PD conversion [28]. Second, one of the sphingolipids accumulating in GD, glucosylsphingosine (GluSph), was compared in 20 subjects with and without PD, carrying or not the mild N370S GBA1 variant. Plasma GluSph levels were higher in the GBA1 groups, regardless of PD status; other lipids such as the GCase substrate glucosylceramide, or GCase product ceramide, did not show any difference across groups [67].
Significant alterations in sphingolipids and glycerolipids profile were recently shown in CSF of PD patients and LRRK2-NMC studied by untargeted high-performance liquid chromatography-tandem mass spectrometry; however, there was no correlation between CSF and serum lipidomes [68]. Lipidomic profile could therefore find its niche as a marker in clinical trials targeting LRRK2, but its applicability as prognostic marker of phenoconversion is unsure.
Routine Blood Markers.
In a study evaluating general biochemical markers (full blood count, renal function, C-reactive protein, and vitamin D) in GBA1-NMC, LRRK2-NMC, and HC, a positive association between sub-clinical renal impairment and higher likelihood for prodromal PD was detected among all non-manifesting subjects. Moreover, higher probability for PD was found to be associated with lower levels of vitamin D and hemoglobin in LRRK2-NMC, only [25]. The same group also found that LRRK2-NMC had higher triglyceride levels compared to GBA1-NMC and HC; irrespective of genotype, those NMC with probability rates for prodromal PD above 50% had higher frequencies of hypertriglyceridemia and prediabetes [69]. Overall, these findings suggest that these measurements might be useful to identify subjects at higher risk of PD, but they are not specific for genetically defined cohorts.
Pathogenic Mechanisms.
Use of analyses such as transcriptomics or proteomics can provide insight into the pathogenic mechanisms implicated in PD development in genetically stratified cohorts; however, their applicability in clinical setting is difficult. For instance, transcriptomic profile of monocytes in a cohort of GBA1-PD, sPD, GBA1-NMC, and HC, did not find any specific gene target or biological process related to GBA1 signature, but gene-based outlier analysis in GBA1-NMC showed involvement of mitochondrial function [70]. When the authors performed the same analysis in an independent and larger cohort of whole blood samples, they only marginally replicated the results suggesting cell-specificity [70]. Another recent study applied unbiased mass-spectrometry phospho-proteomic study in PBMCs to a cohort of LRRK2-G2019S and LRRK2-R1441G carriers (with and without PD). The authors detected specific protein differences between LRRK2-G2019S-PD, LRRK2-G2019S-NMC, and HC, with the largest protein phosphorylation differences between LRRK2-G2019S-PD and LRRK2-G2019S-NMC, suggesting the presence of specific phosphorylation events associated with changes from asymptomatic to manifest PD [71].
Bis(monoacylglycerol) phosphate (BMP) isoforms, which contribute to the multivesicular/lamellar morphology of the endolysosomal network, were higher in urine of carriers of LRRK2-G2019S than in non-carriers, independent of PD status [72]. Only the 2,2′-di-18:1-BMP isoform was marginally higher in LRRK2-G2019S-PD when compared with LRRK2-G2019S-NMC; therefore, whether these markers may be useful to predict PD conversion is unclear [72].
Neuroimaging Markers
Conventional and advanced imaging techniques constitute another interesting tool used to provide objective in vivo measurements of brain dysfunction, which may predate clinical manifestations of disease within genetically stratified cohorts [73]. For a comprehensive review on the topic, the reader is directed elsewhere [73]. Below, we will present the most recent neuroimaging studies evaluating GBA1-NMC and LRRK2-NMC, highlighting the successful/unsuccessful application of such imaging techniques as prediction markers.
Structural and Functional MRI (fMRI)
No structural brain changes (subcortical volumes and cortical thickness) have been reported in GBA1-NMC and LRRK2-NMC compared with HC [74, 75]. Conversely, alterations in resting state fMRI in GBA1-NMC have been reported in a few studies. An increased resting state striato-cortical functional connectivity (FC) between left posterior putamen and left postcentral gyrus and hyper-connectivity between left caudate and right parietal operculum and right planum temporale were detected in a small group of GBA1-NMC compared with HC, suggesting a possible early impairment of sensory system in these individuals [74]. In another fMRI study, despite similar performances on the Stroop test, GBA1-NMC showed increased task-related activity in the right medial frontal gyrus and reduced task-related activity in the left lingual gyrus compared with LRRK2-NMC and HC and higher activation patterns in the incongruent task in the left medial frontal gyrus and bilateral precentral gyrus compared with HC. These findings were interpreted as a compensatory mechanism allowing adequate cognitive performance [76], and therefore, they could simply reflect GBA1 status, and not be associated with increased risk of PD.
123-I Ioflupane DAT Imaging
Similar to fMRI, studies evaluating DAT striatal-binding ratios (SBRs) have found little and inconsistent alterations across studies. In a large cohort of GBA1- and LRRK2-NMC, only 3% of GBA1-NMC and 11% of LRRK2-NMC displayed DAT deficit. Interestingly, GBA1-NMC, but not LRRK2-NMC, showed increased DAT SBRs in the caudate, putamen, and striatum compared with HC, and this was interpreted as a compensatory mechanism [33•]. Conversely, in another study comparing iRBD, individuals with hyposmia, and GBA1-NMC and LRRK2-NMC, a lower mean SBR was observed in iRBD compared with hyposmia and NMC cohorts, and no differences were found between GBA1-NMC and LRRK2-NMC [77]. Using baseline and annual change rates in longitudinal data obtained using DAT SPECT from sPD, LRRK2-G2019S-PD, and GBA1-N370S-PD patients, Lee et al. tried to predict the temporal trajectory of putaminal dopaminergic dysfunction during the premotor phase. These authors suggested that the presence of dopaminergic degeneration in GBA1-PD was present 10 years before motor onset and that GBA1-PD patients had faster deterioration of dopaminergic function compared with both LRRK2-PD and sPD [78].
To increase the sensitivity of these imaging data at predicting PD, a recent study combined the evaluation of FC patterns and presynaptic striatal dopamine uptake DAT SPECT, in a group of 26 GBA1-NMC, 25 LRRK2-NMC, and 34 HC. Despite similar clinical features across groups, LRRK2-NMC showed reduced SBRs in the right putamen compared with HC, while no significant differences in SBRs were detected between GBA1-NMC and HC [79]. LRRK2-NMC also showed higher right putamen FC compared with GBA1-NMC. Within the LRRK2-NMC, higher striatal FC was associated with increased risk for PD. Within 3 years of follow-up, three individuals (2 GBA1-NMC and 1 LRRK2-NMC) converted to PD, and they were found to be 2 or 1 SD below their respective groups measured intra-striatal mean FC level [79].
Miscellaneous
Microglial activation has been measured in vivo using 11C-(R)-PK11195 positron emission tomography (PET) imaging on a small cohort of GBA1 variant carriers (including GD and heterozygous GBA1-NMC), showing that 11C-(R)-PK11195-binding potential was increased in the substantia nigra of GBA1 variant carriers compared with HC and correlated with hyposmia [80]. Mean striatal 18F-fluorodopa (F-DOPA) PET was similar to HC, and therefore, it was proposed that GBA1 variant carriers might show increased microglial activity in brain regions susceptible to neuropathological changes, either as a result of cytotoxic or neuroprotective process [80]. F-DOPA PET imaging was also investigated in a longitudinal study evaluating GBA1-NMC (both heterozygous and homozygous). No significant difference in striatal tracer uptake was observed compared with HC after a maximum of 9-year-follow-up [81].
Recent studies using [18F]-fluorodeoxyglucose (FDG) PET demonstrated altered brain metabolism in GBA1-PD compared with LRRK2-PD and sPD, underlying a more aggressive disease in GBA1-PD patients [82, 83]. Although no data are available to date in NMC, [18F]-FDG PET may be a promising tool to detect impaired brain metabolism in genetically at-risk individuals as recently shown in other PD at-risk groups like iRBD [84].
Overall, these neuroimaging data suggest that differential neuroimaging patterns can be observed among genetically at-risk individuals and that combined DAT and FC assessments might increase the ability to detect high-risk subjects [79]. Other imaging techniques such as fMRI or F-DOPA PET might not be sensitive markers to predict PD conversion in these populations.
Conclusions
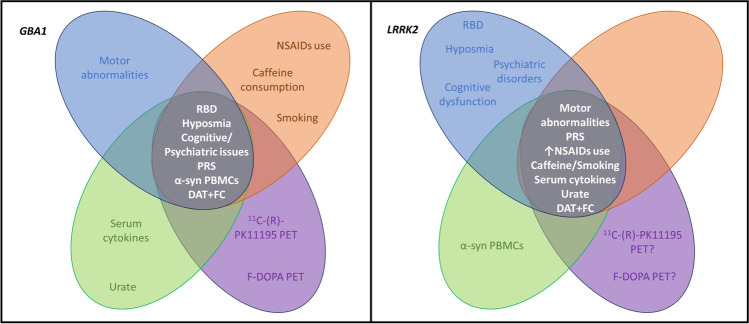
Over the last few decades, our understanding of clinical and pathological differences in GBA1-PD and LRRK2-PD has greatly increased; however, the issue of identifying those at greatest risk of conversion to PD remains beyond our capabilities. Review of all the potential prodromal markers of PD phenoconversion in GBA1 and LRRK2 variant carriers leads us to the conclusion that at present no single clinical, biochemical, or imaging feature, or combination thereof can either identify those most likely to convert or when. This represents an important challenge as the ability to do so would enable those individuals at risk to be offered preventative therapy that should ever become available. In Fig. 1, we suggest which markers might be relevant, or not, for GBA1 and LRRK2 variant carriers and should therefore be assessed in individuals carrying these variants. To validate whether these proposed preclinical/prodromal markers can reliably distinguish those individuals that are truly premotor PD, we need longitudinal studies of large preclinical cohorts, using a combination of clinical, historical, and genetic information, together with biochemical/imaging data, and artificial intelligence approaches to create trajectory models. These studies will pave the way for personalized therapeutic approaches.
Fig. 1.
Summary of clinical (blue), genetic/environmental (orange), biochemical (green), and imaging (purple) markers that could be more relevant to define higher risk of PD conversion in GBA1-NMC and LRRK2-NMC. FC, functional connectivity; PBMCs, peripheral blood mononuclear cells; PRS, polygenic risk score
Funding
Open access funding provided by Università degli Studi di Pavia within the CRUI-CARE Agreement. This research was funded in part by the Aligning Science Across Parkinson’s (grant number: ASAP-000420) through the Michael J. Fox Foundation for Parkinson’s Research (MJFF) and by the EU Joint Programme—Neurodegenerative Research (JPND) through the MRC grant code MR/T046007/1. For the purpose of open access, the author has applied a CC BY 4.0 public copyright license to all Author Accepted Manuscripts arising from this submission.
Declarations
Conflict of Interest
E. Menozzi and A. HV. Schapira report grants from Aligning Science Across Parkinson’s (grant number: ASAP-000420) through the Michael J. Fox Foundation for Parkinson’s Research (MJFF), grants from EU Joint Programme—Neurodegenerative Research (JPND) through the MRC grant code MR/T046007/1, during the conduct of the study. E. Menozzi is supported by a Royal Free Charity Fellowship. M. Avenali and F. Blandini have nothing to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Dorsey ER, Bloem BR. The Parkinson pandemic-a call to action. JAMA Neurol. 2018;75(1):9–10. doi: 10.1001/jamaneurol.2017.3299. [DOI] [PubMed] [Google Scholar]
- 2.Yang W, Hamilton JL, Kopil C, Beck JC, Tanner CM, Albin RL, et al. Current and projected future economic burden of Parkinson’s disease in the U.S. NPJ Parkinsons Dis. 2020;6:15. doi: 10.1038/s41531-020-0117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niotis K, West AB, Saunders-Pullman R. Who to enroll in Parkinson disease prevention trials? The case for genetically at-risk cohorts. Neurol. 2022;99(7 Suppl 1):10–18. doi: 10.1212/WNL.0000000000200812. [DOI] [PubMed] [Google Scholar]
- 4.Healy DG, Falchi M, O'Sullivan SS, Bonifati V, Durr A, Bressman S, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol. 2008;7(7):583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Shu L, Sun Q, Zhou X, Pan H, Guo J, et al. Integrated genetic analysis of racial differences of common GBA variants in Parkinson’s disease: a meta-analysis. Front Mol Neurosci. 2018;11:43. doi: 10.3389/fnmol.2018.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson’s disease in Ashkenazi Jews. N Engl J Med. 2004;351(19):1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- 7.Neumann J, Bras J, Deas E, O'Sullivan SS, Parkkinen L, Lachmann RH, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain. 2009;132(Pt 7):1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrucci S, Ginevrino M, Trezzi I, Monfrini E, Ricciardi L, Albanese A, et al. GBA-related Parkinson’s disease: dissection of genotype-phenotype correlates in a large Italian cohort. Mov Disord. 2020 doi: 10.1002/mds.28195. [DOI] [PubMed] [Google Scholar]
- 9.Liu G, Boot B, Locascio JJ, Jansen IE, Winder-Rhodes S, Eberly S, et al. Specifically neuropathic Gaucher’s mutations accelerate cognitive decline in Parkinson’s. Ann Neurol. 2016;80(5):674–685. doi: 10.1002/ana.24781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alcalay RN, Mirelman A, Saunders-Pullman R, Tang MX, Mejia Santana H, Raymond D, et al. Parkinson disease phenotype in Ashkenazi Jews with and without LRRK2 G2019S mutations. Mov Disord. 2013;28(14):1966–1971. doi: 10.1002/mds.25647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saunders-Pullman R, Mirelman A, Alcalay RN, Wang C, Ortega RA, Raymond D, et al. Progression in the LRRK2-asssociated Parkinson disease population. JAMA Neurol. 2018;75(3):312–319. doi: 10.1001/jamaneurol.2017.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith LJ, Lee CY, Menozzi E, Schapira AHV. Genetic variations in GBA1 and LRRK2 genes: biochemical and clinical consequences in Parkinson disease. Front Neurol. 2022;13:971252. doi: 10.3389/fneur.2022.971252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith LJ, Bolsinger MM, Chau KY, Gegg ME, Schapira AHV. The GBA variant E326K is associated with alpha-synuclein aggregation and lipid droplet accumulation in human cell lines. Hum Mol Genet. 2022 doi: 10.1093/hmg/ddac233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riboldi GM, Di Fonzo AB. GBA, Gaucher disease, and Parkinson’s disease: from genetic to clinic to new therapeutic approaches. Cells. 2019;8(4):364. 10.3390/cells8040364. [DOI] [PMC free article] [PubMed]
- 15.Huang J, Cheng Y, Li C, Shang H. Genetic heterogeneity on sleep disorders in Parkinson’s disease: a systematic review and meta-analysis. Transl Neurodegener. 2022;11(1):21. doi: 10.1186/s40035-022-00294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avenali M, Toffoli M, Mullin S, McNeil A, Hughes DA, Mehta A, et al. Evolution of prodromal parkinsonian features in a cohort of GBA mutation-positive individuals: a 6-year longitudinal study. J Neurol Neurosurg Psychiatry. 2019;90(10):1091–1097. doi: 10.1136/jnnp-2019-320394. [DOI] [PubMed] [Google Scholar]
- 17.Beavan M, McNeill A, Proukakis C, Hughes DA, Mehta A, Schapira AH. Evolution of prodromal clinical markers of Parkinson disease in a GBA mutation-positive cohort. JAMA Neurol. 2015;72(2):201–208. doi: 10.1001/jamaneurol.2014.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krohn L, Ruskey JA, Rudakou U, Leveille E, Asayesh F, Hu MTM, et al. GBA variants in REM sleep behavior disorder: a multicenter study. Neurol. 2020;95(8):e1008–e1016. doi: 10.1212/WNL.0000000000010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barber TR, Lawton M, Rolinski M, Evetts S, Baig F, Ruffmann C, et al. Prodromal Parkinsonism and neurodegenerative risk stratification in REM sleep behavior disorder. Sleep. 2017;40(8):zsx071. 10.1093/sleep/zsx071. [DOI] [PMC free article] [PubMed]
- 20.Heinzel S, Berg D, Gasser T, Chen H, Yao C, Postuma RB, et al. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2019;34(10):1464–1470. doi: 10.1002/mds.27802. [DOI] [PubMed] [Google Scholar]
- 21.Horsager J, Andersen KB, Knudsen K, Skjaerbaek C, Fedorova TD, Okkels N, et al. Brain-first versus body-first Parkinson’s disease: a multimodal imaging case-control study. Brain. 2020;143(10):3077–3088. doi: 10.1093/brain/awaa238. [DOI] [PubMed] [Google Scholar]
- 22.McNeill A, Duran R, Proukakis C, Bras J, Hughes D, Mehta A, et al. Hyposmia and cognitive impairment in Gaucher disease patients and carriers. Mov Disord. 2012;27(4):526–532. doi: 10.1002/mds.24945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullin S, Beavan M, Bestwick J, McNeill A, Proukakis C, Cox T, et al. Evolution and clustering of prodromal parkinsonian features in GBA1 carriers. Mov Disord. 2019;34(9):1365–1373. doi: 10.1002/mds.27775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moran EE, Bressman SB, Ortega RA, Raymond D, Nichols WC, Palmese CA, et al. Cognitive functioning of glucocerebrosidase (GBA) non-manifesting carriers. Front Neurol. 2021;12:635958. doi: 10.3389/fneur.2021.635958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thaler A, Omer N, Giladi N, Gurevich T, Bar-Shira A, Gana-Weisz M, et al. Biochemical markers for severity and risk in GBA and LRRK2 Parkinson’s disease. J Neurol. 2021;268(4):1517–1525. doi: 10.1007/s00415-020-10325-4. [DOI] [PubMed] [Google Scholar]
- 26.Thaler A, Omer N, Giladi N, Gurevich T, Bar-Shira A, Gana-Weisz M, et al. Mutations in GBA and LRRK2 are not associated with increased inflammatory markers. J Parkinsons Dis. 2021;11(3):1285–1296. doi: 10.3233/JPD-212624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galper J, Balwani M, Fahn S, Waters C, Krohn L, Gan-Or Z, et al. Cytokines and Gaucher biomarkers in glucocerebrosidase carriers with and without Parkinson disease. Mov Disord. 2021;36(6):1451–1455. doi: 10.1002/mds.28525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omer N, Giladi N, Gurevich T, Bar-Shira A, Gana-Weisz M, Glinka T, et al. Glucocerebrosidase activity is not associated with Parkinson’s disease risk or severity. Mov Disord. 2022;37(1):190–195. doi: 10.1002/mds.28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moran EE, Wang C, Katz M, Ozelius L, Schwartz A, Pavlovic J, et al. Cognitive and motor functioning in elderly glucocerebrosidase mutation carriers. Neurobiol Aging. 2017;58(239):e1–e7. doi: 10.1016/j.neurobiolaging.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pachi I, Koros C, Simitsi AM, Papadimitriou D, Bougea A, Prentakis A, et al. Apathy: an underestimated feature in GBA and LRRK2 non-manifesting mutation carriers. Parkinsonism Relat Disord. 2021;91:1–8. doi: 10.1016/j.parkreldis.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Gatto EM, Etcheverry JL, Sanguinetti A, Cesarini M, Fernandez Escobar N, Drelichman G. Prodromal clinical markers of Parkinson disease in Gaucher disease individuals. Eur Neurol. 2016;76(1–2):19–21. doi: 10.1159/000447510. [DOI] [PubMed] [Google Scholar]
- 32.Pont-Sunyer C, Tolosa E, Caspell-Garcia C, Coffey C, Alcalay RN, Chan P, et al. The prodromal phase of leucine-rich repeat kinase 2-associated Parkinson disease: clinical and imaging Studies. Mov Disord. 2017;32(5):726–738. doi: 10.1002/mds.26964. [DOI] [PubMed] [Google Scholar]
- 33.• Simuni T, Uribe L, Cho HR, Caspell-Garcia C, Coffey CS, Siderowf A, et al. Clinical and dopamine transporter imaging characteristics of non-manifest LRRK2 and GBA mutation carriers in the Parkinson’s Progression Markers Initiative (PPMI): a cross-sectional study. Lancet Neurol. 2020;19(1):71–80. 10.1016/S1474-4422(19)30319-9. This study reports the analysis of both clinical and imaging characteristics in a large group of non-manifesting carriers ofGBA1andLRRK2variants. [DOI] [PMC free article] [PubMed]
- 34.Mirelman A, Bernad-Elazari H, Thaler A, Giladi-Yacobi E, Gurevich T, Gana-Weisz M, et al. Arm swing as a potential new prodromal marker of Parkinson’s disease. Mov Disord. 2016;31(10):1527–1534. doi: 10.1002/mds.26720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirelman A, Gurevich T, Giladi N, Bar-Shira A, Orr-Urtreger A, Hausdorff JM. Gait alterations in healthy carriers of the LRRK2 G2019S mutation. Ann Neurol. 2011;69(1):193–197. doi: 10.1002/ana.22165. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez-Rodriguez A, Tirnauca C, Salas-Gomez D, Fernandez-Gorgojo M, Martinez-Rodriguez I, Sierra M, et al. Sensor-based gait analysis in the premotor stage of LRRK2 G2019S-associated Parkinson’s disease. Parkinsonism Relat Disord. 2022;98:21–26. doi: 10.1016/j.parkreldis.2022.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Mirelman A, Alcalay RN, Saunders-Pullman R, Yasinovsky K, Thaler A, Gurevich T, et al. Nonmotor symptoms in healthy Ashkenazi Jewish carriers of the G2019S mutation in the LRRK2 gene. Mov Disord. 2015;30(7):981–986. doi: 10.1002/mds.26213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.•• Mirelman A, Saunders-Pullman R, Alcalay RN, Shustak S, Thaler A, Gurevich T, et al. Application of the Movement Disorder Society prodromal criteria in healthy G2019S-LRRK2 carriers. Mov Disord. 2018;33(6):966–73. 10.1002/mds.27342. This study provides longitudinal data about conversion to Parkinson disease within a large cohort ofLRRK2-G2019S non-manifesting carriers. [DOI] [PMC free article] [PubMed]
- 39.Wang P, Pan J, Luo Q, Chen J, Tang H, Chen S, et al. A 10-Year Community-based study of leucine-rich repeat kinase 2 G2385R carriers’ conversion to Parkinson’s disease. Mov Disord. 2022;37(8):1767–1772. doi: 10.1002/mds.29127. [DOI] [PubMed] [Google Scholar]
- 40.Straniero L, Rimoldi V, Monfrini E, Bonvegna S, Melistaccio G, Lake J, et al. Role of lysosomal gene variants in modulating GBA-associated Parkinson’s disease risk. Mov Disord. 2022;37(6):1202–1210. doi: 10.1002/mds.28987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blauwendraat C, Reed X, Krohn L, Heilbron K, Bandres-Ciga S, Tan M, et al. Genetic modifiers of risk and age at onset in GBA associated Parkinson's disease and Lewy body dementia. Brain. 2020;143(1):234–48. doi: 10.1093/brain/awz350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aslam M, Kandasamy N, Ullah A, Paramasivam N, Ozturk MA, Naureen S, et al. Putative second hit rare genetic variants in families with seemingly GBA-associated Parkinson’s disease. NPJ Genom Med. 2021;6(1):2. doi: 10.1038/s41525-020-00163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mestre TA, Pont-Sunyer C, Kausar F, Visanji NP, Ghate T, Connolly BS, et al. Clustering of motor and nonmotor traits in leucine-rich repeat kinase 2 G2019S Parkinson’s disease nonparkinsonian relatives: a multicenter family study. Mov Disord. 2018;33(6):960–965. doi: 10.1002/mds.27272. [DOI] [PubMed] [Google Scholar]
- 44.Iwaki H, Blauwendraat C, Makarious MB, Bandres-Ciga S, Leonard HL, Gibbs JR, et al. Penetrance of Parkinson’s disease in LRRK2 p.G2019S carriers is modified by a polygenic risk score. Mov Disord. 2020;35(5):774–80. doi: 10.1002/mds.27974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai D, Alipanahi B, Fontanillas P, Schwantes-An TH, Aasly J, Alcalay RN, et al. Genomewide association studies of LRRK2 modifiers of Parkinson’s disease. Ann Neurol. 2021;90(1):76–88. doi: 10.1002/ana.26094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wahner AD, Bronstein JM, Bordelon YM, Ritz B. Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurol. 2007;69(19):1836–1842. doi: 10.1212/01.wnl.0000279519.99344.ad. [DOI] [PubMed] [Google Scholar]
- 47.•• San Luciano M, Tanner CM, Meng C, Marras C, Goldman SM, Lang AE, et al. Nonsteroidal anti-inflammatory use and LRRK2 Parkinson’s disease penetrance. Mov Disord. 2020;35(10):1755–64. 10.1002/mds.28189. This study analyses the effect of exposure to common drugs such as non-steroid anti-inflammatory drugs on Parkinson disease risk in a large cohort of LRRK2non-manifesting carriers. [DOI] [PMC free article] [PubMed]
- 48.Crotty GF, Lo RY, Schwarzschild MA. If LRRK2 set the fire, can nonsteroidal anti-inflammatory drugs wet the flames? Mov Disord. 2020;35(10):1727–1730. doi: 10.1002/mds.28240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.• Crotty GF, Maciuca R, Macklin EA, Wang J, Montalban M, Davis SS, et al. Association of caffeine and related analytes with resistance to Parkinson disease among LRRK2 mutation carriers: a metabolomic study. Neurol. 2020;95(24):e3428–37. 10.1212/WNL.0000000000010863. This study reports the potential inverse association between caffeine consumption and development of Parkinson disease within carriers ofLRRK2variants. [DOI] [PMC free article] [PubMed]
- 50.Luth T, Konig IR, Grunewald A, Kasten M, Klein C, Hentati F, et al. Age at onset of LRRK2 p.Gly2019Ser is related to environmental and lifestyle factors. Mov Disord. 2020;35(10):1854–8. doi: 10.1002/mds.28238. [DOI] [PubMed] [Google Scholar]
- 51.Yahalom G, Rigbi A, Israeli-Korn S, Krohn L, Rudakou U, Ruskey JA, et al. Age at onset of Parkinson’s disease among Ashkenazi Jewish patients: contribution of environmental factors, LRRK2 p.G2019S and GBA p.N370S mutations. J Parkinsons Dis. 2020;10(3):1123–32. doi: 10.3233/JPD-191829. [DOI] [PubMed] [Google Scholar]
- 52.Pchelina S, Emelyanov A, Baydakova G, Andoskin P, Senkevich K, Nikolaev M, et al. Oligomeric alpha-synuclein and glucocerebrosidase activity levels in GBA-associated Parkinson’s disease. Neurosci Lett. 2017;636:70–76. doi: 10.1016/j.neulet.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 53.Avenali M, Cerri S, Ongari G, Ghezzi C, Pacchetti C, Tassorelli C, et al. Profiling the biochemical signature of GBA-related Parkinson’s disease in peripheral blood mononuclear cells. Mov Disord. 2021;36(5):1267–1272. doi: 10.1002/mds.28496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Emelyanov A, Usenko T, Nikolaev M, Senkevich K, Kulabukhova D, Lavrinova A, et al. Increased alpha-synuclein level in CD45+ blood cells in asymptomatic carriers of GBA mutations. Mov Disord. 2021;36(8):1997–1998. doi: 10.1002/mds.28688. [DOI] [PubMed] [Google Scholar]
- 55.Kalia LV, Lang AE, Hazrati LN, Fujioka S, Wszolek ZK, Dickson DW, et al. Clinical correlations with Lewy body pathology in LRRK2-related Parkinson disease. JAMA Neurol. 2015;72(1):100–105. doi: 10.1001/jamaneurol.2014.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marti-Masso JF, Ruiz-Martinez J, Bolano MJ, Ruiz I, Gorostidi A, Moreno F, et al. Neuropathology of Parkinson’s disease with the R1441G mutation in LRRK2. Mov Disord. 2009;24(13):1998–2001. doi: 10.1002/mds.22677. [DOI] [PubMed] [Google Scholar]
- 57.Vilas D, Shaw LM, Taylor P, Berg D, Brockmann K, Aasly J, et al. Cerebrospinal fluid biomarkers and clinical features in leucine-rich repeat kinase 2 (LRRK2) mutation carriers. Mov Disord. 2016;31(6):906–914. doi: 10.1002/mds.26591. [DOI] [PubMed] [Google Scholar]
- 58.Majbour NK, Aasly JO, Hustad E, Thomas MA, Vaikath NN, Elkum N, et al. CSF total and oligomeric alpha-synuclein along with TNF-alpha as risk biomarkers for Parkinson’s disease: a study in LRRK2 mutation carriers. Transl Neurodegener. 2020;9(1):15. doi: 10.1186/s40035-020-00192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garrido A, Fairfoul G, Tolosa ES, Marti MJ, Green A, Barcelona LSG. alpha-synuclein RT-QuIC in cerebrospinal fluid of LRRK2-linked Parkinson’s disease. Ann Clin Transl Neurol. 2019;6(6):1024–1032. doi: 10.1002/acn3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmadi Rastegar D, Dzamko N. Leucine rich repeat kinase 2 and innate immunity. Front Neurosci. 2020;14:193. doi: 10.3389/fnins.2020.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dzamko N, Rowe DB, Halliday GM. Increased peripheral inflammation in asymptomatic leucine-rich repeat kinase 2 mutation carriers. Mov Disord. 2016;31(6):889–897. doi: 10.1002/mds.26529. [DOI] [PubMed] [Google Scholar]
- 62.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 2016;15(12):1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 63.Bakshi R, Macklin EA, Logan R, Zorlu MM, Xia N, Crotty GF, et al. Higher urate in LRRK2 mutation carriers resistant to Parkinson disease. Ann Neurol. 2019;85(4):593–599. doi: 10.1002/ana.25436. [DOI] [PubMed] [Google Scholar]
- 64.Parkinson Study Group S-PDI. Schwarzschild MA, Ascherio A, Casaceli C, Curhan GC, Fitzgerald R, et al. Effect of urate-elevating inosine on early Parkinson disease progression: the SURE-PD3 randomized clinical trial. JAMA. 2021;326(10):926–39. doi: 10.1001/jama.2021.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anton FM, Garcia Puig J, Ramos T, Gonzalez P, Ordas J. Sex differences in uric acid metabolism in adults: evidence for a lack of influence of estradiol-17 beta (E2) on the renal handling of urate. Metabolism. 1986;35(4):343–348. doi: 10.1016/0026-0495(86)90152-6. [DOI] [PubMed] [Google Scholar]
- 66.Marder K, Wang Y, Alcalay RN, Mejia-Santana H, Tang MX, Lee A, et al. Age-specific penetrance of LRRK2 G2019S in the Michael J. Fox Ashkenazi Jewish LRRK2 Consortium. Neurology. 2015;85(1):89–95. doi: 10.1212/WNL.0000000000001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Surface M, Balwani M, Waters C, Haimovich A, Gan-Or Z, Marder KS, et al. Plasma glucosylsphingosine in GBA1 mutation carriers with and without Parkinson’s disease. Mov Disord. 2022;37(2):416–421. doi: 10.1002/mds.28846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galper J, Dean NJ, Pickford R, Lewis SJG, Halliday GM, Kim WS, et al. Lipid pathway dysfunction is prevalent in patients with Parkinson’s disease. Brain. 2022 doi: 10.1093/brain/awac176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thaler A, Shenhar-Tsarfaty S, Shaked Y, Gurevich T, Omer N, Bar-Shira A, et al. Metabolic syndrome does not influence the phenotype of LRRK2 and GBA related Parkinson’s disease. Sci Rep. 2020;10(1):9329. doi: 10.1038/s41598-020-66319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Riboldi GM, Vialle RA, Navarro E, Udine E, de Paiva LK, Humphrey J, et al. Transcriptome deregulation of peripheral monocytes and whole blood in GBA-related Parkinson’s disease. Mol Neurodegener. 2022;17(1):52. doi: 10.1186/s13024-022-00554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garrido A, Santamaria E, Fernandez-Irigoyen J, Soto M, Simonet C, Fernandez M, et al. Differential phospho-signatures in blood cells identify LRRK2 G2019S carriers in Parkinson’s disease. Mov Disord. 2022;37(5):1004–1015. doi: 10.1002/mds.28927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alcalay RN, Hsieh F, Tengstrand E, Padmanabhan S, Baptista M, Kehoe C, et al. Higher urine bis(monoacylglycerol)phosphate levels in LRRK2 G2019S mutation carriers: implications for therapeutic development. Mov Disord. 2020;35(1):134–141. doi: 10.1002/mds.27818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Filippi M, Balestrino R, Basaia S, Agosta F. Neuroimaging in glucocerebrosidase-associated Parkinsonism: a systematic review. Mov Disord. 2022;37(7):1375–1393. doi: 10.1002/mds.29047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sezgin M, Kicik A, Bilgic B, Kurt E, Bayram A, Hanagasi H, et al. Functional connectivity analysis in heterozygous glucocerebrosidase mutation carriers. J Parkinsons Dis. 2021;11(2):559–568. doi: 10.3233/JPD-202295. [DOI] [PubMed] [Google Scholar]
- 75.Thaler A, Kliper E, Maidan I, Herman T, Rosenberg-Katz K, Bregman N, et al. Cerebral imaging markers of GBA and LRRK2 related Parkinson’s disease and their first-degree unaffected relatives. Brain Topogr. 2018;31(6):1029–1036. doi: 10.1007/s10548-018-0653-8. [DOI] [PubMed] [Google Scholar]
- 76.Bregman N, Thaler A, Mirelman A, Helmich RC, Gurevich T, Orr-Urtreger A, et al. A cognitive fMRI study in non-manifesting LRRK2 and GBA carriers. Brain Struct Funct. 2017;222(3):1207–1218. doi: 10.1007/s00429-016-1271-4. [DOI] [PubMed] [Google Scholar]
- 77.Chahine LM, Urbe L, Caspell-Garcia C, Aarsland D, Alcalay R, Barone P, et al. Cognition among individuals along a spectrum of increased risk for Parkinson’s disease. PLoS One. 2018;13(8):e0201964. doi: 10.1371/journal.pone.0201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee MJ, Pak K, Kim HK, Nudelman KN, Kim JH, Kim YH, et al. Genetic factors affecting dopaminergic deterioration during the premotor stage of Parkinson disease. NPJ Parkinsons Dis. 2021;7(1):104. doi: 10.1038/s41531-021-00250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Droby A, Artzi M, Lerman H, Hutchison RM, Bashat DB, Omer N, et al. Aberrant dopamine transporter and functional connectivity patterns in LRRK2 and GBA mutation carriers. NPJ Parkinsons Dis. 2022;8(1):20. doi: 10.1038/s41531-022-00285-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mullin S, Stokholm MG, Hughes D, Mehta A, Parbo P, Hinz R, et al. Brain microglial activation increased in glucocerebrosidase (GBA) mutation carriers without Parkinson’s disease. Mov Disord. 2021;36(3):774–779. doi: 10.1002/mds.28375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lopez G, Eisenberg DP, Gregory MD, Ianni AM, Grogans SE, Masdeu JC, et al. Longitudinal positron emission tomography of dopamine synthesis in subjects with GBA1 mutations. Ann Neurol. 2020;87(4):652–657. doi: 10.1002/ana.25692. [DOI] [PubMed] [Google Scholar]
- 82.Schindlbeck KA, Vo A, Nguyen N, Tang CC, Niethammer M, Dhawan V, et al. LRRK2 and GBA variants exert distinct influences on Parkinson’s disease-specific metabolic networks. Cereb Cortex. 2020;30(5):2867–2878. doi: 10.1093/cercor/bhz280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Greuel A, Trezzi JP, Glaab E, Ruppert MC, Maier F, Jager C, et al. GBA variants in Parkinson’s disease: clinical, metabolomic, and multimodal neuroimaging phenotypes. Mov Disord. 2020;35(12):2201–2210. doi: 10.1002/mds.28225. [DOI] [PubMed] [Google Scholar]
- 84.Carli G, Caminiti SP, Galbiati A, Marelli S, Casoni F, Padovani A, et al. In-vivo signatures of neurodegeneration in isolated rapid eye movement sleep behaviour disorder. Eur J Neurol. 2020;27(7):1285–1295. doi: 10.1111/ene.14215. [DOI] [PubMed] [Google Scholar]