Abstract
Prior studies on anterior circulation stroke have demonstrated that the benefits of endovascular treatment (EVT) may be absent in patients with poor collaterals. Our study focused on patients with basilar artery occlusion (BAO) to investigate time-dependent EVT effects according to the posterior circulation collateral score (PC-CS). The BASILAR study was a nationwide prospective Chinese registry of consecutive BAO patients. Patients were divided into groups receiving standard medical therapy alone (SMT group) or SMT plus EVT (EVT group). Restricted cubic spline analyses (RCSA) were performed to explore the nonlinear and linear relationships between EVT time and outcomes for different PC-CS. We included 828 patients with acute BAO. Compared with the poor collateral (PC-CS 0–3), the adjusted odds ratio of favorable outcome was 1.311 in patients with moderate (PC-CS 4–5) (95% CI, 0.781–2.201) and 1.899 with good (PC-CS 6–10) collateral (1.125–3.207) for EVT. RCSA revealed that in patients with PC-CS 0–3, the favorable outcome probability after EVT significantly decreased to 10% within 6 h and stabilized thereafter (Pnonlinearity = 0.035), while in patients with moderate and good collateral, the probability was maintained at approximately 30% and 40% respectively, even beyond 6 h (all Pnonlinearity > 0.05). Among patients with BAO, good collateral circulation was independently associated with improved outcomes along with the usage of thrombectomy. Patients with poor collaterals should receive EVT as early as possible, especially within 6 h of symptom onset, while the time window may be extended in patients with moderate and good collaterals. Unique identifier: ChiCTR1800014759.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-022-01301-z.
Keywords: Basilar artery occlusion, Computed tomography angiography, Collateral circulation, Thrombectomy, Stroke
Introduction
Acute basilar artery occlusion (BAO) is a rare subtype of stroke with a high risk of morbidity and mortality. Collateral circulation plays an important role in predicting outcomes in patients with acute BAO [1–5]. Studies of large-vessel occlusion of the anterior circulation have indicated that collateral status at baseline is also an important treatment effect modifier [6]. One randomized trial even excluded patients with poor collaterals, assuming that these patients would not benefit from endovascular treatment (EVT) [7]. However, whether collateral circulation can be used to make decisions regarding treatment modality in patients with BAO, particularly whether patients with poor collaterals benefit from EVT compared to medical treatment, is unclear.
In a recent study, the time to treatment was only associated with favorable outcomes in patients with BAO and poor collaterals [1]. The association between time-to-treatment and outcome is weaker in patients with good collaterals who seem to tolerate ischemia for longer times [8]. These findings indicate the important role of collaterals in determining the therapeutic time window. Regrettably, the time window of EVT for patients with different collateral circulations has not yet been systematically investigated.
The baseline posterior circulation collateral score (PC-CS) is a semi-quantitative computed tomography angiography (CTA)-based grading system that has been shown to be associated with outcomes in BAO [4]. Herein, we investigated whether the treatment modality is decided by PC-CS in patients with acute BAO. Furthermore, we aimed to depict how and to what extent the benefit of EVT varies with treatment delay in patients with different PC-CS.
Methods
Patient Selection
The BASILAR registry enrolled patients who presented with acute, symptomatic, radiologically confirmed BAO within 24 h of symptom onset from 47 comprehensive stroke centers in China between January 2014 and May 2019. Details of the study protocol have been published previously [9]. All consecutive patients from each participating center included to avoid selection bias. All consecutive patients with the following criteria were enrolled in the study: (1) age 18 years or older; (2) BAO confirmed by CTA, magnetic resonance angiography, or digital subtraction angiography; (3) presented within 24 h of the estimated initiation of BAO; (4) intravenous thrombolysis was performed within the therapeutic time window; and (5) informed consent could be obtained. For the EVT group, EVT also had to be initiated within 24 h of estimated time of BAO. Patients were excluded from the study in case of (1) evidence of intracranial hemorrhage on presentation; (2) a premorbid modified Rankin Scale (mRS) score > 2; (3) a serious, advanced, or terminal illness; (4) current pregnancy or lactation; (5) a lack of follow-up information; or (6) incomplete critical baseline data (e.g., imaging and time metrics). The ethics committee of the participating centers approved the study protocol (ethics committee of Xinqiao Hospital, Army Medical University, in Chongqing, China, 201,308,701). Written informed consent was obtained from all the patients or their legal representatives.
Treatment Strategy
The patients were divided into two groups according to the treatment they received: the standard medical therapy alone (SMT group) and the SMT plus endovascular therapy (EVT group). In the SMT group, patients were administered intravenous thrombolysis with recombinant tissue plasminogen activator within 4.5 h, or urokinase within 6 h of the estimated time of BAO, antiplatelet drugs, systemic anticoagulation, or various combinations of these approaches, in accordance with guidelines for the management of acute ischemic stroke [10]. EVT included thrombus retraction, aspiration, and the use of a stent retriever device. Re-occlusion often occurred after thrombectomy in atherosclerotic disease; therefore, rescue therapy including balloon dilation, stenting, intra-arterial thrombolysis, and glycoprotein IIb/IIIa inhibitor might be utilized to retrieve recanalization. The specific treatment method was left to the discretion of a local interventionist. After recanalization of the target artery, most of the patients were transferred to the neuro-intensive care unit for at least 24 h with their systolic blood pressure maintained at 120–140 mmHg. Additionally, the patients who underwent extracranial or intracranial stent implantation were prescribed antithrombotic medication to prevent acute stent thrombosis. For the patients without prior intravenous alteplase, loading doses of clopidogrel (300 mg) and aspirin (300 mg) were given, or a low dose of glycoprotein IIb/IIIa inhibitor was bolus-injected intra-arterially and maintained for at least 24 h, while for those with prior intravenous alteplase, clopidogrel (75 mg) and aspirin (100 mg) were given after 24 h of alteplase administration, then all the patients were given clopidogrel (75 mg/day) and aspirin (100 mg/day) for 3 months. Recanalization was assessed using the modified Thrombolysis in Cerebral Infarction Scale (mTICI). Successful recanalization was defined as a 2b/3 flow as mTICI [11]. The presumed causative mechanism of stroke was assessed based on the Trial of ORG10172 in Acute Stroke Treatment (TOAST) classification [12].
Clinical and Imaging Analysis
Neurological deficit was assessed using the National Institutes of Health Stroke Scale (NIHSS) score at admission. The estimated time of BAO was the time of symptom onset, consistent with the clinical diagnosis of BAO. If the exact time was unclear, the last time the patient was seen well by any witness was considered the time of onset.
An independent core imaging laboratory blinded to clinical outcomes and treatment method assessed all available imaging data. PC-CS at baseline was graded as previously described [4]. Briefly, each patent posterior inferior cerebellar artery, anterior inferior cerebellar artery, and superior cerebellar artery was allocated 1 point. For a posterior communicating artery, 1 point was allocated if its diameter was smaller than the ipsilateral P1 segment or 2 points if its diameter was equal to or larger than the ipsilateral P1 segment. A higher PC-CS value indicates better collateral circulation. Each imaging scan was separately reviewed by two trained neuroradiologists (Dr. Li and Dr. Qiu) who were unaware of the treatment-group assignments, clinical data, and outcomes. If the assessment was discrepant, the final decision was made by a third neuroradiologist (Dr. Zi). The PC-CS was then categorized into subgroups: 0–3, poor status; 4–5, moderate status; and 6–10, good collateral status.
Outcome Measurement
The primary efficacy outcome was a favorable functional outcome, defined as a modified Rankin score (mRS) of 0–3 at 90 days. A good outcome (defined as an mRS 0–2, functional independence) at 90 days was measured as the secondary outcome. Safety outcomes included all-cause mortality within 90 days and symptomatic intracerebral hemorrhage (SICH) within 48 h. Intracerebral hemorrhage confirmed on CT or magnetic resonance imaging was assessed according to the Heidelberg Bleeding Classification [13].
Statistical Analysis
Continuous variables were expressed as medians with interquartile ranges, while categorical data were summarized as counts and percentages. Univariate comparisons were performed using Fisher’s exact test or χ2 test for categorical variables and the Kruskal–Wallis test or Mann–Whitney U test for continuous variables. In this study, missing data of baseline covariates and outcomes were few, and missing values for select key variables were imputed to the mode or median; however, outcomes were analyzed with complete cases.
We compared the treatment effect of EVT with that of SMT in patients with different PC-CS categories using binary logistic regression analysis, adjusting for age, history of diabetes, baseline NIHSS score, and occlusion location. We further introduced an interaction term between the treatment modality and trichotomized PC-CS (0–3 versus 4–5 versus 6–10) into the model to investigate the effect modification of trichotomized PC-CS. The associations between PC-CS (as a continuous variable) and outcomes, and the interaction between treatment and numerical PC-CS were tested. The outcome-specific predicted probabilities for continuous PC-CS values in both treatment modalities were visually displayed using the marginsplot command, with setting other variables in the model to mean values.
To examine the relationships between onset to puncture time (OPT) and favorable outcomes in trichotomized PC-CS subgroups for EVT, we used restricted cubic splines with three knots at the 10th, 50th, and 90th centiles to flexibly model, adjusting for age, history of diabetes, baseline NIHSS, and location of occlusion. The test for nonlinearity was first checked; if nonlinearity was not significant, the test for linearity was checked.
Statistical analyses were performed using SPSS version 26 (IBM Corp, Armonk, NY, USA), STATA version 16.0 (StataCorpLLC, TX), and RStudio software (version 1.3.1093). Statistical significance was defined as P < 0.05.
Results
Baseline Characteristics
Applying the inclusion and exclusion criteria, we initially screened 1254 patients from 51 comprehensive stroke centers in China recorded in the BASILAR registry. Among them, 4 centers and 22 individual patients were excluded from participation in the registry because not all pertinent data on consecutive patients were recorded. Seventy-one patients were excluded from the analysis due to they had a BAO accompanied by anterior circulation LVO (large vascular occlusion), 121 patients due to chronic BAO, 187 patients due to missing critical baseline data (92 without records of time and 95 with poor quality of images), 11 survivors due to lack of available 90-day mRS scores, and one patient without a CTA at baseline. Finally, 828 patients were included in this study. A flowchart of patient selection is shown in Supplement eFig. 1. Among the 828 analyzed patients, 217 (26.2%) were women, and the median (interquartile range) age, NIHSS score, and PC-CS were 65 (57–74) years, 27 (16–33), and 4 (3–6), respectively. There were 234 patients with PC-CS 0–3 (46 patients with SMT and 188 patients with EVT), 317 patients with PC-CS 4–5 (68 patients with SMT and 249 patients with EVT), and 277 patients with PC-CS 6–10 (68 patients with SMT and 209 patients with EVT). Patients with higher PC-CS scores had lower baseline NIHSS scores. The occlusion site appeared more often in the proximal basilar artery and V4 segment of the vertebral artery in patients with PC-CS 0–3, but in the middle and distal parts of the basilar artery in patients with PC-CS 4–5 and 6–10 (Supplemental Table I). The baseline characteristics of the patients treated with SMT and EVT in the trichotomized PC-CS subgroups are shown in Table 1.
Table 1.
Baseline characteristics of the cohort by treatment modality in the trichotomized PC-CS subgroup
| Variables | PC-CS 0–3 | PC-CS 4–5 | PC-CS 6–10 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SMT (n = 46) | EVT (n = 188) | P value | SMT (n = 68) | EVT (n = 249) | P value | SMT (n = 68) | EVT (n = 209) | P value | |
| Age, years, median (IQR) | 73 (65–77) | 63 (57–71) | < 0.001 | 67 (59–76) | 66 (56–74) | 0.230 | 65 (56–73) | 64 (55–73) | 0.640 |
| Female, n (%) | 15 (32.6) | 38 (20.2) | 0.072 | 23 (33.8) | 62 (24.9) | 0.141 | 15 (22.1) | 64 (30.6) | 0.174 |
| Baseline NIHSS, median (IQR) | 30 (16–35) | 31 (23–34) | 0.301 | 29 (17–33) | 27 (18–34) | 0.952 | 23 (10–32) | 20 (14–30) | 0.729 |
| Medical history, n (%) | |||||||||
| Ischemic stroke | 17 (37.0) | 43 (22.9) | 0.05 | 15 (22.1) | 56 (22.5) | 0.94 | 16 (23.5) | 41 (19.6) | 0.488 |
| Hypertension | 35 (76.1) | 138 (73.4) | 0.710 | 47 (69.1) | 170 (68.3) | 0.894 | 52 (76.5) | 142 (67.9) | 0.182 |
| Hyperlipidemia | 16 (34.8) | 67 (35.6) | 0.913 | 24 (35.3) | 65 (26.1) | 0.135 | 29 (42.6) | 82 (39.2) | 0.618 |
| Diabetes mellitus | 9 (19.6) | 50 (26.6) | 0.325 | 19 (27.9) | 51 (20.5) | 0.189 | 12 (17.6) | 48 (23) | 0.355 |
| Coronary heart disease | 11 (23.9) | 29 (15.4) | 0.17 | 11 (16.2) | 53 (21.3) | 0.352 | 5 (7.4) | 23 (11.0) | 0.386 |
| Atrial fibrillation | 4 (8.7) | 20 (10.6) | 1.00* | 10 (14.7) | 73 (29.3) | 0.015 | 10 (14.7) | 43 (20.6) | 0.285 |
| IVT, n (%) | 9 (19.6) | 38 (20.2) | 0.922 | 24 (35.3) | 50 (20.1) | 0.009 | 14 (20.6) | 31 (14.8) | 0.264 |
| Occlusion site, n (%) | 0.004 | 0.129 | < 0.001 | ||||||
| Distal BA | 10 (21.7) | 30 (16) | 24 (35.3) | 116 (46.6) | 11 (16.2) | 76 (36.4) | |||
| Middle BA | 20 (43.5) | 40 (21.3) | 31 (45.6) | 76 (30.5) | 49 (72.1) | 79 (37.8) | |||
| Proximal BA | 6 (13) | 55 (29.3) | 5 (7.4) | 26 (10.4) | 3 (4.4) | 25 (12) | |||
| VA–V4 | 10 (21.7) | 63 (33.5) | 8 (11.8) | 31 (12.4) | 5 (7.4) | 29 (13.9) | |||
| Time metrics, min, median (IQR) | |||||||||
| Onset-puncture time | 449 (149–591) | 315 (203–537) | 0.575 | 300 (228–674) | 320 (218–490) | 0.451 | 425 (261–803) | 345 (240–472) | 0.392 |
| Onset-imaging time | 179 (71–400) | 196 (81–386) | 0.764 | 195 (103–304) | 211 (91–357) | 0.676 | 217 (74–401) | 219 (88–329) | 0.799 |
| Puncture-recanalization time | NA | 107 (77–151) | NA | NA | 101 (65–151) | NA | NA | 107 (70–154) | NA |
| Door-needle time | 96 (40–108) | 7 2(47–110) | 0.564 | 60 (47–87) | 78 (50–97) | 0.309 | 56 (47–108) | 58 (45–109) | 0.953 |
| mTICI 2b/3, n (%) | NA | 142 (75.5) | NA | NA | 204 (81.9) | NA | NA | 175 (83.7) | NA |
The missing door-needle time data were 2 (22%), 3 (12.5%), and 2 (14.3%) in the SMT and 11 (28.9%), 10 (20%), and 11 (35.5%) in the EVT of the trichotomized PC-CS subgroup. There was one missing data point with OPT and puncture-recanalization time in each EVT group of the trichotomized PC-CS subgroup
BA basilar artery, EVT endovascular treatment plus standard medical therapy, IQR interquartile range, mRS modified Rankin Scale, mTICI Modified Thrombolysis in Cerebral Infarction score, NIHSS National Institutes of Health Stroke Scale, PC-CS posterior circulation collateral score, SMT standard medical therapy, VA-V4 V4 of vertebral artery, IVT intravenous thrombolysis, NA not applicable
*Fisher exact test
Outcome per Treatment Modality Associated with PC-CS
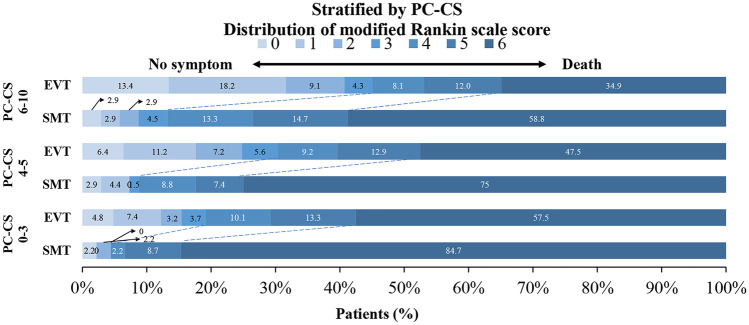
Overall, favorable outcomes were observed in 38 (16.2%) patients with PC-CS 0–3, 82 (25.9%) patients with PC-CS 4–5, and 103 (37.2%) patients with PC-CS 6–10 (Supplemental Table II). The 90-day mRS distribution for both treatment modalities in the PC-CS subgroups is illustrated in Fig. 1. Compared with SMT, EVT was associated with higher rates of favorable outcomes (mRS 0–3) in the PC-CS 0–3 subgroup (adjusted OR, 7.02; 95% CI, 1.48–33.29), PC-CS 4–5 subgroup (adjusted OR, 4.84; 95% CI, 1.82–12.87), and PC-CS 6–10 subgroup (adjusted OR, 6.16; 95% CI, 2.69–14.08; Table 2, Supplemental Table III). The ORs for decreases in mortality with EVT were 0.16 (95% CI, 0.06–0.44) for PC-CS 0–3, 0.25 (95% CI, 0.13–0.50) for PC-CS 4–5, and 0.36 (95% CI, 0.20–0.68) for PC-CS 6–10. In addition, the rate of functional independence was higher in the EVT group than in the SMT group in each PC-CS subgroup. In the EVT group, compared with the PC-CS 0–3, favorable outcome was more likely to occur in patients with PC-CS 4–5 (adjusted OR, 1.311; 95% CI, 0.781–2.201) and patients with PC-CS 6–10 (adjusted OR, 1.899; 95% CI, 1.125–3.207) (Table 3). However, the interaction terms between trichotomized PC-CS and treatment modality were not significant for all outcomes (all P > 0.05).
Fig. 1.
The 90-day mRS distribution for endovascular therapy vs standard medical treatment in patients stratified by the posterior circulation collateral score (PC-CS). mRS, modified Rankin Scale; EVT, endovascular treatment; SMT, standard medical treatment
Table 2.
EVT treatment effect by trichotomized PC-CS
| PC-CS 0–3 | PC-CS 4–5 | PC-CS 6–10 | P value for interaction 0–3 vs 6–10 | P value for interaction 4–5 vs 6–10 | ||||
|---|---|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |||
| Primary outcomes | ||||||||
| mRS 0–3 at 90 days | 7.02 (1.48–33.29) | 0.014 | 4.84 (1.82–12.87) | 0.002 | 6.16 (2.69–14.08) | 0.001 | 0.930 | 0.707 |
| Mortality within 90 days | 0.16 (0.06–0.44) | < 0.001 | 0.25 (0.13–0.50) | < 0.001 | 0.36 (0.20–0.68) | 0.002 | 0.258 | 0.581 |
| Secondary clinical outcomes | ||||||||
| mRS 0–2 at 90 days | 5.14 (1.06–25.02) | 0.042 | 4.17 (1.47–11.79) | 0.007 | 8.55 (3.28–22.28) | < 0.001 | 0.564 | 0.345 |
| SICH within 48 h | * | * | 3.16 (0.36–27.90) | 0.300 | 0.998 | 0.997 | ||
EVT endovascular treatment, PC-CS posterior circulation collateral score, mRS modified Rankin Scale, SICH symptomatic intracranial hemorrhage, OR odds ratio
*Not enough data are available to calculate the statistical parameters
Table 3.
Clinical outcomes in trichotomized PC-CS subgroups for EVT
| PC-CS 0–3 | PC-CS 4–5 | PC-CS 6–10 | P value | |
|---|---|---|---|---|
| Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | ||
| Primary outcomes | ||||
| mRS 0–3 at 90 days | Reference | 1.311 (0.781–2.201) | 1.899 (1.125–3.207) | 0.043 |
| Mortality within 90 days | Reference | 0.819 (0.528–1.270) | 0.662 (0.413–1.060) | 0.226 |
| Secondary clinical outcomes | ||||
| mRS 0–2 at 90 days | Reference | 1.245 (0.715–2.168) | 1.992 (1.149–3.454) | 0.024 |
| SICH within 48 h | Reference | 0.664 (0.306–1.441) | 0.709 (0.295–1.704) | 0.557 |
EVT endovascular treatment, PC-CS posterior circulation collateral score, mRS modified Rankin Scale, SICH symptomatic intracranial hemorrhage, OR odds ratio
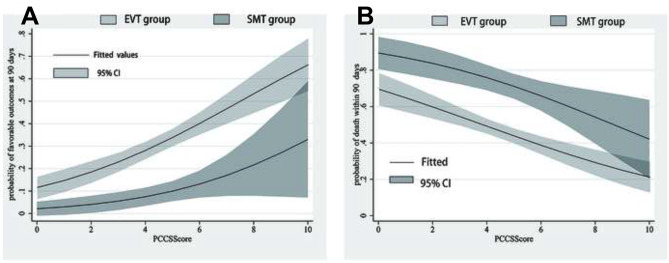
Figure 2A shows the predicted probabilities of favorable outcomes in the two treatment modalities, with an increase in PC-CS set as a continuous variable. The odds of favorable outcomes increased by 13% in the EVT group (adjusted OR, 1.13 [95% CI, 1.01–1.27], P = 0.028) for a 1-point increase in PC-CS. The odds of favorable outcome also increased as the PC-CS increased in the SMT group, but this difference was not statistically significant (adjusted OR, 1.24 [95% CI, 0.90–1.71], P = 0.185). Moreover, the probability of a favorable outcome was consistently higher in the EVT group than in the SMT group (Pinteraction = 0.605). In contrast, the mortality associated with both treatment modalities decreased with an increase in PC-CS (Fig. 2B). The probability of mortality was consistently lower in the EVT group than that in the SMT group (Pinteraction = 0.484).
Fig. 2.
A, B The predicted probability of favorable outcome and mortality for each level of the posterior circulation collateral score (PC-CS) in patients treated with endovascular therapy (EVT) and patients treated with standard medical treatment (SMT)
Outcomes of EVT Associated with PC-CS and Treatment Time
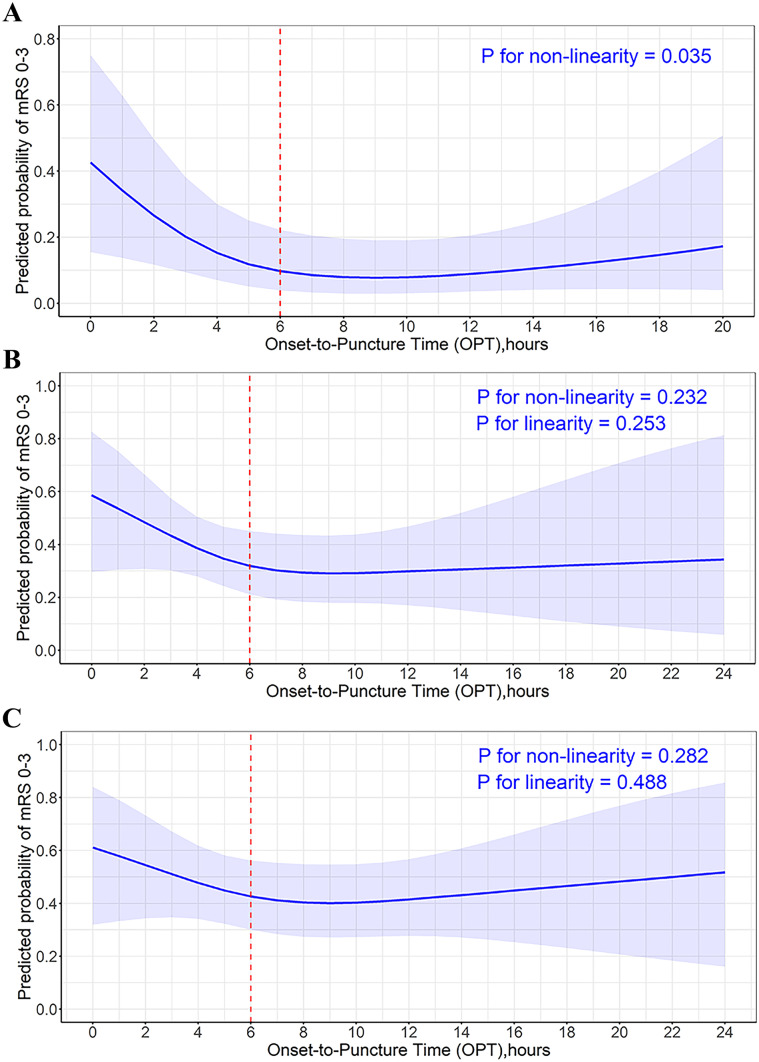
Continuous time-benefit predicted probability curves showing the relationship between OPT and clinical outcomes in patients with different PC-CS categories are shown in Fig. 3. For patients with PC-CS 0–3 score, the time-benefit relationship was non-linear (P for non-linearity = 0.035). The benefit of EVT exhibited a steep loss as the OPT delay within the 6-h time window, with a probability of favorable outcome decreasing from 42.6 to 10.0%, but this stabilized in the period beyond 6 h. The odds of favorable outcome decreased by 41% within 6 h (adjusted OR, 0.59 [95% CI, 0.38–0.91], P = 0.018) for a 1-h increase in OPT. The odds of favorable outcome decreased by 3% beyond 6 h after onset, but the difference was not statistically significant (adjusted OR, 0.97 [95% CI, 0.82–1.16], P = 0.76) (Table 4). Patients with PC-CS scores of 4–5 or 6–10 showed a consistent trend towards better outcomes with earlier treatment within 6 h; however, the differences were not statistically significant (Pnonlinearity = 0.232 vs Pnonlinearity = 0.282). When treatment was beyond 6 h after onset, the predicted probability of favorable outcome was maintained at approximately 30% and 40% in patients with PC-CS 4–5 and 6–10 scores, respectively. Moreover, we explored the linear relationship between OPT and favorable outcomes using a binary logistic regression analysis, adjusted for age, history of diabetes, baseline NIHSS score, and occlusion location. The association between OPT and favorable outcomes was also not significant in patients with PC-CS 4–5 and PC-CS 6–10 (OR, 1.000; 95% CI 0.999–1.001; Plinearity = 0.935 vs 1.000 (0.999–1.001); Plinearity = 0.444 (Supplement Table IV).
Fig. 3.
Relationship of onset-to-puncture time with the probability of favorable outcome at 90 days in patients stratified by the posterior circulation collateral score (PC-CS). The figures depict the probability of favorable outcome (mRS 0–3) and 95% CI with continuous change of onset-to-puncture time in patients with PC-CS 0–3 A, patients with PC-CS 4–5 B, and patients with PC-CS 6–10 C. Models are fitted with restricted cubic spline with 3 knots and are adjusted for age, history of diabetes, baseline National Institutes of Health Stroke Scale (NIHSS), and occlusion site. mRS, modified Rankin Scale; CI, confidence interval
Table 4.
Favorable outcome of different time dependence in patients with different collateral status for EVT
| PC-CS 0–3 | PC-CS 4–5 | PC-CS 6–10 | ||||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
| OPT0–6 | 0.59 (0.38–0.91) | 0.018 | 0.90 (0.64–1.27) | 0.563 | 1.04 (0.77–1.40) | 0.801 |
| OPT > 6 | 0.97 (0.82–1.16) | 0.758 | 1.07 (0.93–1.24) | 0.344 | 1.07 (0.94–1.20) | 0.307 |
EVT endovascular treatment, PC-CS posterior circulation collateral score, OPT onset-to-puncture time, OR odds ratio
Discussion
This study showed that, among patients with acute BAO, good collateral circulation was independently associated with improved outcomes along with the usage of thrombectomy. We also showed that the evaluation of collateral status could be a useful marker to determine a timeframe with a probable favorable clinical outcome for EVT.
Collaterals have previously been recognized to influence neurological outcomes in patients with acute BAO treated with endovascular therapy (EVT) [2, 14]. Our results are in accordance with those of a previous study, which showed that better collaterals were independently associated with favorable outcomes in patients with acute BAO for EVT. Furthermore, we found that in regard to outcome and safety, EVT was consistently superior to medical treatment, irrespective of the PC-CS scores. In addition, the interaction between the treatment and trichotomized PC-CS subgroups was also not significant. These results demonstrated that PC-CS score is not used to making decision for treatment modality in patients with BAO. Therefore, in BAO, patients with poor collaterals should not be excluded from EVT. This is consistent with the post hoc analysis by HERMES which suggested that EVT achieves better outcomes at 90 days than standard medical therapy in patients with large infarct (infarcts affecting more than 33% of middle cerebral artery territory or ASPECTS less than 6) across all strata of collateral circulation status [15].
Although our study is inconsistent with the results of Langezaal et al. [16], which reported that endovascular and medical therapy did not differ significantly with respect to a favorable functional outcome in BAO patients, considering that the study lasted for a long time, the change in the operation method and the equipment are likely to affect the outcome. Another possible explanation could be that our patients with EVT had a higher proportion of achieving mTICI 2b/3 (80.7%) than theirs (72%), which is a well-known factor for better prognosis [3, 14, 17].
The speed of infarct progression varies widely among patients with acute ischemic stroke owing to inherent differences in collateral circulation and tissue tolerance [18]. Therefore, individualizing the optimal timeframe is crucial to improving the clinical outcomes of EVT. In our study, the time-benefit curve of EVT in patients with PC-CS 0–3 scores showed a steeply decreased probability of favorable outcomes from 42.6 to 10% within 6 h of OPT. This was in accordance with a previous study which showed that favorable outcome was highly dependent on early treatment in patients with poor collaterals [1]. Furthermore, for those treated beyond 6 h, the probability of a favorable outcome did not further decrease below 10% over time. This represents a marginal potential benefit compared with the increased risk of SICH (10.9%) and the high cost of EVT. A previous study demonstrated that infarct progression was faster in patients with poor collaterals, and the salvageable brain tissue or ischemic penumbra was too limited to benefit from EVT in the delayed time window [19], thus supporting our results. Of note, patients treated with standard medical treatment were not analyzed in relation to treatment time due to the limited sample size, and thus, the 6 h after onset was not the timeframe for the absolute benefit of EVT. However, we believe that the 6-h time point could still provide a reference framework for interventionists to treat patients with poor collaterals, during which a beneficial effect may be achieved through accelerated treatment.
For patients with PC-CS scores of 4–5 and 6–10, the negative impact of treatment time on the outcomes was considerably blunted. Worthwhile clinical recovery was still observed even when EVT was started beyond 6 h after onset, with a probability of favorable outcomes of approximately 30% and 40%, respectively. Alemseged et al. [1] reported that EVT beyond 6 h was associated with worse outcomes in BAO patients with PC-CS 0–5, but not in patients with PC-CS ≥ 6, suggesting that only patients with good collaterals may achieve a good outcome in delayed time windows. However, combining PC-CS 4–5 with PC-CS 0–3 may have underestimated the potential beneficial effects of EVT in patients with PC-CS 4–5. As enrolled in our study and others [4], these patients with a PC-CS of 4–5 made up almost two-fifths of all patients. Excluding these patients from EVT will lose many patients with potential benefit from EVT.
Clinical trials in the anterior circulation have shown the value of whole-brain CT perfusion in assisting patient selection in a delayed time window; however, routine application remains challenging. The CTA-derived PC-CS for the quantification of collateral flow is simple, accessible, and provides significant advantages in terms of the speed of pretreatment workup [4]. Therefore, PC-CS 4–10 scores seem to be a promising marker for identifying eligible patients for EVT in delayed time windows.
This study has several limitations. First, as it was an observational study, it carried all the inherent limitations of its nonrandomized design. However, our study had the most diverse inclusion criteria with regard to stroke severity, time to treatment, and techniques and devices used, thus representing an extensive experience with EVT for patients with acute BAO. Second, the relatively small number of patients in each PC-CS subgroup, particularly in the delayed time window, may have affected the accuracy of the results. Further studies with larger prospective series or randomized trials are required to confirm these findings. Third, all scores were rated in an independent core laboratory and performed by two experienced neuroradiologists without time pressure. This decreased the interrater variability, but probably overestimated the reliability of the PC-CS in a real-world setting.
Conclusion
In our study, good collateral circulation was independently associated with improved outcomes along with the usage of thrombectomy in patients with acute BAO. Compared to medical treatment, the benefit of EVT was its robustness in patients with acute BAO. The chance of a favorable outcome for EVT in patients with poor collateral flow treated within 6 h of BAO is highly time-dependent. The time window for BAO patients with poor collateral may be reasonably set to 6 h. Conversely, outcomes in patients with moderate and good collaterals appear less time-dependent and could be extended up to 24 h after onset. Performing EVT as soon as possible in all patients with BAO is desirable in clinical practice.
Supplementary Information
Below is the link to the electronic supplementary material.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Abbreviations
- BAO
Basilar artery occlusion
- EVT
Endovascular treatment
- SMT
Standard medical therapy
- PC-CS
Posterior circulation collateral score
- CTA
Computed tomography angiography
- mRS
Modified Rankin Scale
- mTICI
Modified Thrombolysis in Cerebral Infarction
- NIHSS
National Institute of Health Stroke Scale
- SICH
Symptomatic intracerebral hemorrhage
- OPT
Onset to puncture time
- OR
Odds ratio
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (No. 82071323), Chongqing Natural Science Foundation (cstc2020jcyj-msxmX0926), Chongqing Science and Health Joint Project (No. 2019ZDXM002), and the Army Medical University Clinical Medical Research Talent Training Program (No. 2018XLC3039, No. 2019XLC2008, and No. 2019XLC3016).
Declarations
Competing Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wenjie Zi, Email: ziwenjie@126.com.
Pengfei Wang, Email: wpf5287598@163.com.
References
- 1.Alemseged F, Van der Hoeven E, Di Giuliano F, Shah D, Sallustio F, Arba F, et al. Response to late-window endovascular revascularization is associated with collateral status in basilar artery occlusion. Stroke. 2019:Strokeaha118023361. 10.1161/strokeaha.118.023361 [DOI] [PubMed]
- 2.Antunes Dias F, Castro-Afonso LH, Zanon Zotin MC, Alessio-Alves FF, Martins Filho R, Camilo MR, et al. Collateral scores and outcomes after endovascular treatment for basilar artery occlusion. Cerebrovasc Dis. 2019;47:285–290. doi: 10.1159/000502083. [DOI] [PubMed] [Google Scholar]
- 3.oBouslama M, Haussen DC, Aghaebrahim A, Grossberg JA, Walker G, Rangaraju S, et al. Predictors of good outcome after endovascular therapy for vertebrobasilar occlusion stroke. Stroke. 2017;48:3252–3257. 10.1161/STROKEAHA.117.018270 [DOI] [PubMed]
- 4.van der Hoeven EJ, McVerry F, Vos JA, Algra A, Puetz V, Kappelle LJ, et al. Collateral flow predicts outcome after basilar artery occlusion: the posterior circulation collateral score. Int J Stroke. 2016;11:768–775. doi: 10.1177/1747493016641951. [DOI] [PubMed] [Google Scholar]
- 5.Ribo M, Flores A, Rubiera M, Pagola J, Sargento-Freitas J, Rodriguez-Luna D, et al. Extending the time window for endovascular procedures according to collateral pial circulation. Stroke. 2011;42:3465–3469. doi: 10.1161/STROKEAHA.111.623827. [DOI] [PubMed] [Google Scholar]
- 6.Berkhemer OA, Jansen IG, Beumer D, Fransen PS, van den Berg LA, Yoo AJ, et al. Collateral status on baseline computed tomographic angiography and intra-arterial treatment effect in patients with proximal anterior circulation stroke. Stroke. 2016;47:768–776. doi: 10.1161/strokeaha.115.011788. [DOI] [PubMed] [Google Scholar]
- 7.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 8.Lindsberg PJ, Pekkola J, Strbian D, Sairanen T, Mattle HP, Schroth G. Time window for recanalization in basilar artery occlusion: speculative synthesis. Neurol. 2015;85:1806–1815. doi: 10.1212/wnl.0000000000002129. [DOI] [PubMed] [Google Scholar]
- 9.Writing Group for the BG, Zi W, Qiu Z, Wu D, Li F, Liu H, et al. Assessment of endovascular treatment for acute basilar artery occlusion via a nationwide prospective registry. JAMA Neurol. 2020;77:561–573. 10.1001/jamaneurol.2020.0156 [DOI] [PMC free article] [PubMed]
- 10.Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 american Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:3020–3035. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 11.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke. Stroke. 2013;44:2650–2663. doi: 10.1161/strokeaha.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams HP, Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. 10.1161/01.str.24.1.35 [DOI] [PubMed]
- 13.von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46:2981–2986. doi: 10.1161/STROKEAHA.115.010049. [DOI] [PubMed] [Google Scholar]
- 14.Kwak HS, Park JS. Mechanical thrombectomy in basilar artery occlusion: clinical outcomes related to posterior circulation collateral score. Stroke. 2020;51:2045–2050. doi: 10.1161/STROKEAHA.120.029861. [DOI] [PubMed] [Google Scholar]
- 15.Román LS, Menon BK, Blasco J, Hernández-Pérez M, Dávalos A, Majoie C, et al. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol. 2018;17:895–904. 10.1016/s1474-4422(18)30242-4 [DOI] [PubMed]
- 16.Langezaal LCM, van der Hoeven E, Mont'Alverne FJA, de Carvalho JJF, Lima FO, Dippel DWJ, et al. Endovascular therapy for stroke due to basilar-artery occlusion. N Engl J Med. 2021;384:1910–1920. doi: 10.1056/NEJMoa2030297. [DOI] [PubMed] [Google Scholar]
- 17.Deb-Chatterji M, Flottmann F, Leischner H, Alegiani A, Brekenfeld C, Fiehler J, et al. Recanalization is the key for better outcome of thrombectomy in basilar artery occlusion. Clin Neuroradiol. 2020;30:769–775. doi: 10.1007/s00062-019-00850-9. [DOI] [PubMed] [Google Scholar]
- 18.Wheeler HM, Mlynash M, Inoue M, Tipirnini A, Liggins J, Bammer R, et al. The growth rate of early dwi lesions is highly variable and associated with penumbral salvage and clinical outcomes following endovascular reperfusion. International journal of stroke : official journal of the International Stroke Society. 2015;10:723–729. doi: 10.1111/ijs.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wouters A, Dupont P, Christensen S, Norrving B, Laage R, Thomalla G, et al. Association between time from stroke onset and fluid-attenuated inversion recovery lesion intensity is modified by status of collateral circulation. Stroke. 2016;47:1018–1022. doi: 10.1161/strokeaha.115.012010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.