Abstract
Lafora disease is a fatal form of progressive myoclonic epilepsy caused by mutations in the EPM2A or NHLRC1/EPM2B genes that usually appears during adolescence. The Epm2a−/− and Epm2b−/− knock-out mouse models of the disease develop behavioral and neurological alterations similar to those observed in patients. The aim of this work is to analyze whether early treatment with metformin (from conception to adulthood) ameliorates the formation of Lafora bodies and improves the behavioral and neurological outcomes observed with late treatment (during 2 months at 10 months of age). We also evaluated the benefits of metformin in patients with Lafora disease. To assess neurological improvements due to metformin administration in the two mouse models, we evaluated the effects on pentylenetetrazol sensitivity, posturing, motor coordination and activity, and memory. We also analyzed the effects on Lafora bodies, neurodegeneration, and astrogliosis. Furthermore, we conducted a follow-up study of an initial cohort of 18 patients with Lafora disease, 8 treated with metformin and 10 untreated. Our results indicate that early metformin was more effective than late metformin in Lafora disease mouse models improving neurological alterations of both models such as neuronal hyperexcitability, motor and memory alterations, neurodegeneration, and astrogliosis and decreasing the formation of Lafora bodies. Moreover, patients receiving metformin had a slower progression of the disease. Overall, early treatment improves the outcome seen with late metformin treatment in the two knock-out mouse models of Lafora disease. Metformin-treated patients exhibited an ameliorated course of the disease with slower deterioration of their daily living activities.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-022-01304-w.
Keywords: Lafora disease, Epilepsy, Mouse models, Metformin, Drug repurposing, Early treatment
Introduction
Lafora disease (OMIM 254780; ORPHA501) is a rare form of progressive myoclonic epilepsy that generally appears early in adolescence. The main symptoms are seizures, and a general neurological deterioration with dementia that leads to death, usually 5 to 15 years after the onset of the disease (median of 11 years) [1]. There is no specific therapy, and patients can only be treated with antiseizure medications to temporarily control epileptic seizures [2–4].
The main histopathologic hallmark of Lafora disease is the presence of Lafora bodies (LBs), aberrant glycogen inclusions positive to periodic acid-Schiff (PAS) staining, which accumulate in the brain, heart, liver, and other tissues [3]. Lafora disease is caused by mutations in EPM2A (OMIM 607566) [5, 6], which encodes laforin, a glucan phosphatase [7], or NHLRC1/EPM2B (OMIM 608072) [8] that encodes malin, an E3 ubiquitin ligase [9]. The Epm2a−/− and Epm2b−/− mouse models present most of the neurological alterations observed in patients, such as memory deterioration, alterations of spontaneous activity and motor coordination, abnormal postures, loss of neurons, and the presence of LBs in brain and other tissues [10–12]. They also exhibit increased sensitivity to the epileptogenic agent pentylenetetrazol (PTZ), a GABAA receptor antagonist [13], which has been widely used to measure the epileptic activity of mouse models and the efficacy of antiseizure drugs [14–16]. This reflects the existence of neuronal hyperexcitability in the two mouse models of Lafora disease.
Metformin is an AMPK activator [17] widely used as an anti-diabetic drug. Metformin can act as a neuroprotective agent [18] reducing oxidative stress, neuroinflammation, and mitochondrial dysfunction [19–22]. We and others have previously shown that treatment with metformin improves the histological picture and the neurological symptoms [23] and decreases susceptibility to PTZ in adult Epm2b−/− mice [24]. Subsequently, metformin obtained orphan drug designation for the treatment of Lafora disease from the FDA and the EMA. Furthermore, some positive results have been recently reported in the first retrospective study in patients [25].
To assess whether early treatment with metformin (E-MET) from conception to adulthood prevents or delays the formation of LBs and exceeds the improvements observed with late metformin treatment (L-MET), we administered metformin from conception to adulthood in the two mouse models of Lafora disease and analyzed the effect of metformin in a cohort of patients with Lafora disease, comparing metformin treated and untreated patients.
Methods
Experimental Animals
The Epm2a−/− and Epm2b−/− mouse models of Lafora disease were generated as previously described [10, 11], and age-matched wild-type animals (C57BL6) were used as controls. Mice colonies were bred in the Animal Facility Service of the Instituto de Investigación Sanitaria (IIS)-Fundación Jiménez Díaz, housed in isolated cages with a 12:12 light/dark cycle under constant temperature (23 °C), with free access to food and water. All experiments were carried out using and sacrificing the minimum number of animals and minimizing their suffering. The experiments were conducted in accordance with the “Principles of laboratory animal care” (NIH publication No. 86–23, revised 1985), as well as with the European Communities Council Directive (2010/63/EU) and the Ethical Review Board of the Instituto de Investigación Sanitaria-Fundación Jiménez Díaz.
Metformin Treatment
Metformin treatment (12 mM) (Sigma Chemicals, St Louis, MI, USA) was administered in drinking water as previously described [23, 24]. Considering that the C57BL/6 strain generally drinks around 6 ml per day and weighs approximately 25 g, the estimated dose of metformin provided was 372 mg/kg/day [26]. E-MET was supplied in drinking water to breeding pairs of WT, Epm2a−/−, and Epm2b−/− mice, since metformin crosses the placental barrier [27]. Since metformin is not present in human milk, breast-fed pups were daily treated with the same dose of metformin through one subcutaneous injection [28, 29]. Finally, metformin was supplied to the mice after weaning in drinking water ad libitum until the completion of the study. L-MET was dispensed to 10-month-old mice, diluted in drinking water at the same dose (12 mM) for 2 months.
Sensitivity to PTZ
PTZ (Merck, Darmstadt, Germany) was used to analyze neuronal hyperexcitability in mice. PTZ was administered intraperitoneally in a single injection. Two different doses were used: 30 mg/kg (subconvulsive dose—hardly produces GTC seizures in WT animals) and 50 mg/kg (convulsive dose) [30]. The subconvulsive dose was used to assess the percentage of mice that had myoclonic jerks. The convulsive dose, which has been described to induce GTC seizures in 50% of WT animals [30], was administered to evaluate the percentage of animals with GTC seizures, their length, and the latency to the first myoclonic or GTC seizure (from here referred as latency time). No animal presented more than a single GTC seizure after each injection. The lethality due to the administration of this dose was also calculated. Each animal was examined for 45 min by two different researchers.
Tail Suspension Test (TST)
This test evaluates the presence of abnormal posturing and dyskinesia by measuring the presence of abnormal hind limb clasping in response to vertical suspension of mice from their tails. Each animal was suspended vertically for 30 s and scored using a scale system. When both hind limbs were fully extended (normal posture), the score was “0.” When one or both hind limbs were discontinuously or persistently bent towards the body, the scale was scored “1” (abnormal posture).
Motor Coordination
The rotarod test (Harvard Apparatus, Holliston, MA, USA) was used to measure motor coordination and balance. Mice were trained for 2 consecutive days. On day 1, mice were placed on the rotarod for 60 s at a constant speed (4 rpm). On day 2, they were placed on the rotarod at 4 rpm and trained to stay at the cylinder at increasing speed (from 4 to 8 rpm). On the next 2 days, to minimize differences due to learning difficulties, only mice that were able to stay on the rod for 60 s were analyzed in the test sessions. The latency time to fall down from the cylinder at an increasing speed from 4 to 40 rpm was recorded in two sessions per day, for a maximum period of 5 min.
Object Recognition Task (ORT)
This test was applied to study episodic memory retention. Mice were individually habituated to a dark open field box for 10 min. After 2 h, two identical objects (A and B) were placed in the center of the box. Each isolated mouse freely explored both A and B objects, and time examining both objects (tA and tB) was measured. The test was performed 2 h later. A new object C was placed in the box instead of object B, and the exploration time of each object (tA and tC) was recorded. Exploration times were measured using several virtual timers generated by the XNote Stopwatch software and activated, while mice were inspecting the object from 2 cm or less. A discrimination index (D.I.) was calculated following this equation: D.I. = (tC-tA) / (tC + tA).
Spontaneous Locomotor Activity
The accumulated spontaneous movements were monitored with a computerized actimeter (Harvard Apparatus, Holliston, MA, USA). The device registers the number of times each mouse crosses the open field by measuring the number of infrared light beam breaks, and the SEDACOM 1.4 software (Harvard Apparatus, Holliston, MA, USA) analyzed the spontaneous, rearing, and stereotyped movements after 5, 10, 15, 30, 45, and 60 min.
Immunohistochemistry Assays and PAS Staining
Mice were anesthetized and transcardially perfused with 4% phosphate-buffered paraformaldehyde. Brains were removed, dehydrated, and embedded in paraffin. The blocks were sectioned into consecutive 5-μm-thick sections. For PAS-diastase (PAS-D) staining, the sections were rehydrated in decreasing graded alcohols, processed using porcine pancreas α-amylase (5 mg/ml in dH2O) (Merck, Darmstadt, Germany) and the PAS Kit (Merck, Darmstadt, Germany), and counterstained with Gill No. 3 hematoxylin (Merck, Darmstadt, Germany). For immunohistochemistry, rehydrated sections were incubated in boiling 0.1 M sodium citrate buffer, pH 6.0, for antigen retrieval. Samples were incubated with a blocking buffer (1% bovine serum albumin, 5% fetal bovine serum, 2% Triton X-100, diluted in PBS) and with primary antibodies diluted in blocking buffer. The primary antibodies used were the neuronal nuclei (NeuN) antibody (Cat. # MAB377) (1:100 dilution) (Millipore, Temecula, CA, USA) and the glial fibrillary acidic protein (GFAP) antibody (Cat. # MAB360) (1:1000 dilution) (Millipore, Temecula, CA, USA). Subsequently, the sections were stained with the Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA). Immunoreactivity was developed using diaminobenzidine (Dako Cytomation, CA, USA) and H2O2. The sections were counterstained with Carazzi hematoxylin (Panreac Quimica, Barcelona, Spain). For all histological assays, samples from 4 mice per experimental group were used.
Two consecutive sections were stained and analyzed per animal in order to assure reproducibility. Images from the same area of the CA1 region of each hippocampus were acquired with a Leica DMLB 2 (Leica, Wetzlar, Germany), connected to a Leica DFC320 FireWire Digital Microscope Camera (Leica, Wetzlar, Germany). Finally, LBs and NeuN- or GFAP-positive cells were quantified by two different researchers using the ImageJ software (NIH, Bethesda, MD, USA). Each value represented is the mean of those quantifications.
Evolution of Patients With and Without Metformin
We studied the benefits of metformin by comparing the evolution of treated and untreated patients. Clinical data were obtained at baseline, 6, 12, 18, and 24 months of follow-up from the LD-Registry (http://www.lafora.es/). LD-Registry is a dynamic prospective world-wide registry maintained at our center. This registry aims to recruit all patients with Lafora disease who are eligible and willing to participate with the goal of enrolling all the affected population. There are no restrictions of gender, age, ethnicity, or race. Informed consent from the participant or legal representative is a pre-requisite for participation in the registry. We include symptomatic patients with a clinical diagnosis of Lafora disease and biallelic mutations in the EPM2A or EPM2B genes. If one or both mutations are previously undescribed, we require a positive biopsy with LBs. We also include pre-symptomatic individuals if they are siblings of patients with a diagnosis of Lafora disease and have biallelic mutations in the EPM2A or EPM2B genes. These individuals were tested in preparation for a near future clinical trial. A scientific board formed by José M. Serratosa (MD, PhD), María Machío-Castello (MD), Beatriz G. Giráldez (MD), and Juan González-Fernández (PhD) provide clinical expertise and supervision. Patients are recruited in centers that treat patients affected by Lafora disease. The principal investigator at each site identifies potentially eligible participants and inquires them about their willingness to participate. Information about the LD-Registry is also disseminated through a website, support groups, and advocacy newsletters. Participants are free to withdraw from the study at any time. The registry includes 30-min follow-up visits every 6 months. Information is completed during in-person or teleconference interviews. In the follow-up visits, functional and clinical situation, seizure types and their frequency during the last 6 months, and treatment and diagnostic procedures performed since last visit are recorded. The data obtained are entered electronically in an electronic case report form (eCRF) via secure internet-based technology. All accounts are password protected. Permissions are carefully maintained to allow only the required level of access to registry data, and access to the eCRF is protected by password and can only be granted by the study administrator after authorization of the committee.
To grade the progression stage of Lafora disease, we designed a scale, the Lafora Epilepsy Severity Scale (LESS), where severity is graded from 0 (less severity) to 90 (more severity). This scale includes information on the frequency of GTC seizures, presence of rest and action myoclonus, gait, cognitive status, speech, and performance of basic activities of daily living (ADL). To analyze the evolution of patients treated with metformin, we excluded those who were in advanced stages (LESS score of 50 or above), patients with a slow-evolution type of Lafora disease, and carriers of mutations previously described in patients showing a slower course of the disease, since they usually remain stable for periods longer than 1 year. Further information about how the scale is graded is disclosed in Supplementary Table S1, and additional clinical data of patients are included in Table 1.
Table 1.
Clinical information of participants in our study. We analyzed the progression of Lafora disease in patients treated with metformin (a) and without metformin (b)
| a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Gene | DNA mutation | Protein mutation | Age of onset (years) | Duration of evolution (years) | Comorbidity | Additional treatments | Neuroimaging | Data published | Metformin dose | Side effects |
| P1 | EPM2A | c.877C > T Homozygous (exon 4) | Gln293Ter | 10 | 9 | - | VPA, CZP, PER | Normal | - | 850 mg/12 h | - |
| P2 | EPM2B | c.203G > A Homozygous | Cys68Tyr | 14 | 6 | - | LEV, CZP, VPA | Venous angioma (no related with Lafora disease) | - | 850 mg/12 h | - |
| P3 | EPM2B | c.203G > A Homozygous | Cys68Tyr | 13 | 3 | - | LEV (inicio M12) | Normal | - | 850 mg/12 h—> 850 mg/d | Diarrhea |
| P4 | EPM2B | c.348C > A, Homozygous | Cys116Ter | 13 | 5 | - | VPA, LEV, CZP, ZNS | Normal | - | 1000 mg/12 h | - |
| P5 | EPM2B | c.468_469del/c.203G > T | p.G158Rfs*17/p.C68F | 10 | 5 | - | BRV (started at M12) | Nonexistent | - | 850 mg/d | Diarrhea |
| P6 | EPM2A | non-sens c.721 C > T (exon4), del exon 2 | p.Arg241*/Ex2del | 15 | 9 | - | CBD, ZNS, CLN, PER | Normal | - | 500 mg/8 h | - |
| P7 | EPM2A | non-sens c.721 C > T (exon4), del exon 2 | p.Arg241*/Ex2del | 13 | 5 | - | VPA, PER, TPM, CBD | Normal | - | 500 mg/8 h | - |
| P8 | EPM2A | c.512G > A | Arg171His | 14 | 4 | - | LZP, BRV, PER, ZNS, CBD | Normal | - | 500 mg/d—> 500 mg/8 h since M6 | - |
| b | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Gene | DNA mutation | Protein mutation | Age of onset (years) | Duration of evolution (years) | Comorbidity | Additional treatments | Neuroimaging | Data published | Metformin dose | Side effects |
| P9 | EPM2A | c.508C > Tp Homozygous | Pro170Ser | 9 | 11 | PER, BRV, CLB | Nonexistent | On metformin for 3 weeks and stopped the treatment | |||
| P10 | ? (pending, positive biopsia) | ? | ? | 13 | 6 | PHT, CZP, LTG | Normal | ||||
| P11 | EPM2A | c.322 C > T Homozygous | Arg108Cys | 13 | 4 | PER, PB, VPA, ZNS, piracetam | Temporal arachnoid cysts (not related with Lafora disease, asymptomatic) | ||||
| P12 | EPM2B | c.656 G > A / c.451 G > T | Trp219Ter / Val151Phe | 10 | 10 | Febrile seizure at 12 months | LEV, CLB, ZNS, VPA, CZP, PER, risperidone | Mild brain atrophy | |||
| P13 | EPM2A | c.98_121del Homozygous | Glu33_Arg41delinsGly | 16 | 4 | CZP, BRV, citalopram | Nonexistent | ||||
| P14 | EPM2A | c.290 T > G Homozygous | Leu97Arg | 14 | 14 | LEV, BRV, CLB, VPA, stiripentol, CZP, aripiprazole | Normal | https://doi.org/10.1186/s42466-019-0040-2 | On metformin for 3–4 months | ||
| P15 | EPM2A | c.721 C > T / c.487 A > G | R241X / N163D | 14 | 10 | no | CLB, VPA, ZNS, LEV, PER | Normal | |||
| P16 | EPM2A | c.70 G > C Homozygous | Gly24Arg | 10 | 10 | Febrile seizure at 12 months | LEV | Normal | |||
| P17 | EPM2A | c.163 C > T Homozygous | p.Gln55Ter | 10 | 3 | no | VPA | Nonexistent | |||
| P18 | EPM2A | c.721 C > T Homozygous | R241X | 12 | 5 | no | PER, BRV, VPA, CLB, CLN | Normal |
BRV brivaracetam, CBD cannabidiol, CLB clobazam, CZP clozapine, LEV levetiracetam, LTG lamotrigine, LZP lorazepam, PER perampanel, PHT phenytoin, TPM topiramate, VPA valproic acid, ZNS zonisamide
Standard Protocol Approvals, Registrations, and Patient Consents
Animal maintenance and behavior experiments were performed in agreement with the European Union Council Directive Guidelines (86/609/European Economic Community) and the Guide for the Care and Use of Laboratory Animals of the National Research Council of the National Academies of the USA [31]. The protocols were approved by the IIS-Jimenez Diaz Foundation Ethical Review Board. The humane endpoints were established following these guidelines based on monitoring exploratory activity, weight, and behavioral abnormalities. A qualified technician performed a weekly exam of the animals, and given any indication of pain or distress, measures to terminate, reduce, or minimize those symptoms were taken. No adverse events were reported. Patients included in LD-Registry signed a consent form. The LD-Registry was approved by the IIS-Fundación Jiménez Díaz Ethical Review Board.
Data Collection
In order to ensure the experimental reproducibility and the reliability of the findings in the two mouse models, we followed the recommendations for the study design and the reporting of results presented in the ARRIVE guidelines of the National Centre for the Replacement, Refinement & Reduction of Animals in Research [32]. Thus, an experimental protocol was established before the beginning of the study. The number of animals for each experiment was considered by our experience and available resources. We performed our experiments when behavioral alterations are present in both models (12 months of age). For patients, we analyzed the course of the disease at 6 (n = 18), 12 (n = 18), 18 (n = 15), and 24 (n = 8) months of follow-up using clinical data obtained prospectively from the “LD Registry.” Not all patients had reached the 18- or 24-month follow-up point. Data are summarized in Table 2.
Table 2.
Summary of the number of mice and patients analyzed to assess the effect of metformin treatment in Lafora disease. We conducted our experiments when neurological and histological alterations are present in both mouse models (12 months). In patients, we evaluated the progression of Lafora disease in those with metformin (MET) and in patients without it (W/O MET)
| Mouse models of Lafora disease | ||||
|---|---|---|---|---|
| 12 months | ||||
| WT | Epm2a−/− | Epm2b−/− | ||
| w/o MET | n = 25 | n = 26 | n = 24 | |
| E-MET | n = 17 | n = 19 | n = 14 | |
| L-MET | n = 7 | n = 9 | ||
| Patients with Lafora disease | ||||
|---|---|---|---|---|
| 6 months | 12 months | 18 months | 24 months | |
| MET | n = 8 | n = 8 | n = 7 | n = 5 |
| W/O MET | n = 10 | n = 10 | n = 8 | n = 3 |
Statistical Analysis
Values are given as mean ± standard error of mean (SEM) or as percentages. Differences between experimental groups were analyzed by one- or two-way ANOVA, Fisher’s exact, Kruskal–Wallis non-parametric, or Mann–Whitney tests, as indicated in each case. Statistical results were obtained using GraphPad Prism 6.0 (San Diego, CA). Statistical significance thresholds were *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Data Availability
Data supporting the findings of this study are available from the corresponding author, upon reasonable request.
Results
E-MET Decreases Hypersensitivity to PTZ in Epm2a−/− and Epm2b−/− Mice and Improves L-MET Benefits
In this work, we evaluated the benefits of E-MET compared to L-MET on PTZ sensitivity in 12-month-old Epm2a−/− and Epm2b−/− mice. We used a subconvulsive dose of PTZ to assess the effect on the percentage of mice with myoclonic jerks. We also injected a convulsive dose of PTZ to analyze the latency to the first myoclonic jerk, the percentage of mice with GTC seizures, and the latency to and the length of the GTC seizures.
PTZ-Induced Myoclonic Seizures
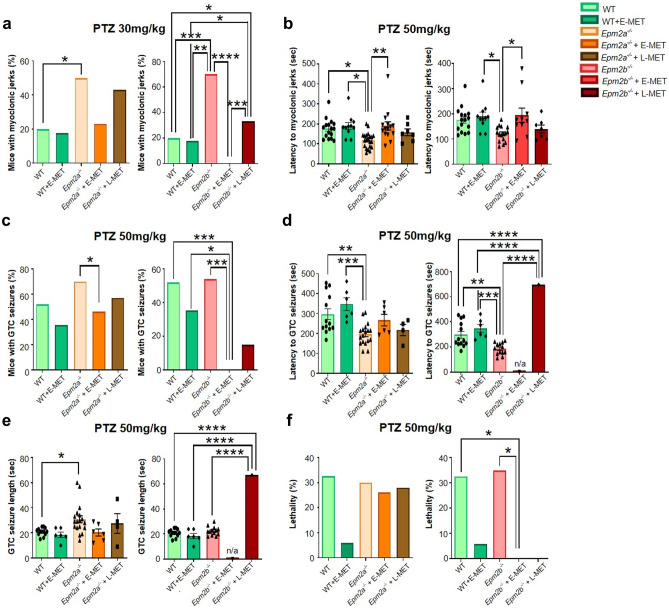
With subconvulsive doses of PTZ, we observed myoclonic seizures in a low percentage of WT animals with/without E-MET. In Epm2a−/− and Epm2b−/− mice, the same dose resulted in a higher proportion of animals with myoclonic jerks. After a convulsive dose, we observed a reduced latency to myoclonic jerks in the two Lafora disease mouse models compared to WT animals. E-MET decreased the percentage of mice with myoclonic seizures more efficiently than L-MET, whereas only E-MET increased the latency to myoclonic seizures in Epm2a−/− and Epm2b−/− mice (Fig. 1a, b). Remarkably, myoclonic jerks were absent in all 12-month-old Epm2b−/− mice with E-MET (Fig. 1a).
Fig. 1.
E-MET improves L-MET effects decreasing susceptibility of Epm2a−/− and Epm2b−/− mice to PTZ-induced seizures. We analyzed the percentage of animals with myoclonic jerks at a PTZ dose of 30 mg/kg (a) and the latency to myoclonic jerks at a PTZ dose of 50 mg/kg (b) in 12-month-old wild type (WT), Epm2a−/− and Epm2b−/− mice. We also evaluated the percentage of mice with PTZ-induced GTC seizures (c), their latency (d), length (e), and lethality (f), induced by a PTZ dose of 50 mg/kg in 12-month-old mice. Only one mouse treated with L-MET had a GTC seizure. When data are shown as a percentage, a Fisher’s exact test was performed between experimental groups. When data are shown as a mean ± SEM, a two-way ANOVA test was performed with Turkey’s multiple comparisons between the experimental groups. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
PTZ-Induced GTC Seizures
After a convulsive dose of PTZ, we observed GTC seizures in 50% of WT mice without E-MET and in 35% percent of WT with E-MET (non-statistically significant). Lafora disease mouse lines exhibited a higher percentage of animals with GTC seizures, longer seizure duration, and shorter latency to the first GTC seizure. After E-MET, PTZ did not induce GTC seizures in any of the Epm2b−/− mice (Fig. 1c), while L-MET only showed certain positive effects reducing the percentage of mice with GTC seizures (Fig. 1c) and increasing latency (Fig. 1d). Therefore, E-MET resulted in lower seizure susceptibility than L-MET in Epm2a−/− mice (Fig. 1c−e).
PTZ-Induced Lethality
The convulsive dose of PTZ was lethal for 30% of WT mice and for 5% of WT + E-MET animals. Lethality for Epm2a−/− and Epm2b−/−mice was 30%. E-MET and L-MET completely eliminated lethality in 12-month-old treated Epm2b−/− mice (Fig. 1f).
E-MET Enhances L-MET Benefits Reducing the Severity of Abnormal Posturing in Epm2a−/− and Epm2b−/− Mouse Models
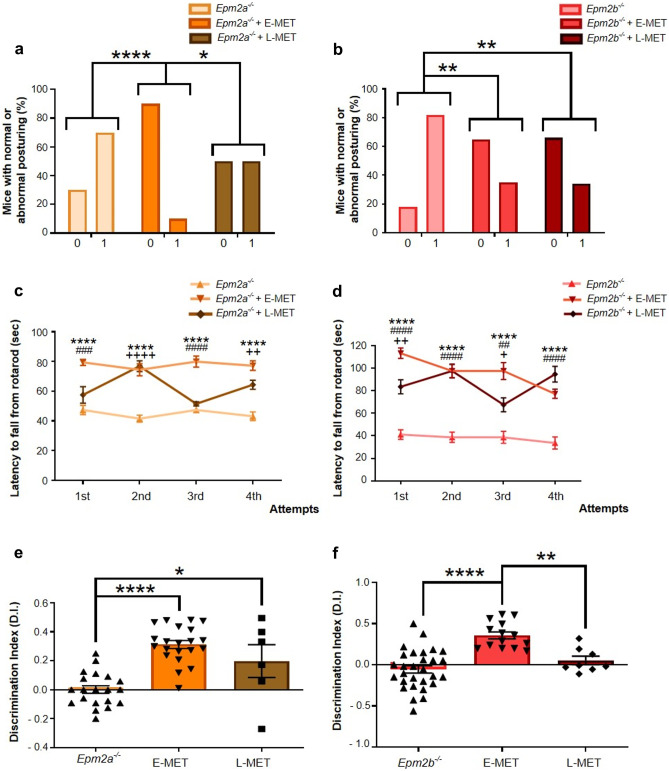
E-MET decreased the appearance of aberrant posturing in Lafora disease mice, diminishing the percentage of Epm2a−/− and Epm2b−/− with abnormal posturing (Fig. 2a, b). Whereas both treatments improved the score of Lafora disease mice, a higher percentage of Epm2a−/− and Epm2b−/− mice treated with E-MET did not show abnormal hind limb posture at 12 months of age (Fig. 2a, b).
Fig. 2.
E-MET outperforms L-MET effect in ameliorating the behavioral decline in adult Epm2a−/− and Epm2b−/− mice. We evaluated the improvements due to E-MET in the percentage of animals showing normal or altered posture (a, b), motor coordination (c, d), and memory deterioration (e, f). In the abnormal posturing test (TST), two different scores were assigned. The animal was graded as 0 when both hind limbs were fully extended, which is considered a normal posture. When one or both hind limbs were intermittently or continuously bent close to the body, the mouse was scored as 1, which is defined as an abnormal posture. In the motor coordination test (rotarod), only the latency to fall from the cylinder recorded during the four attempts in the test sessions is shown. When data are shown as a percentage, a Fisher’s exact test was performed between experimental groups. When data are shown as a mean ± SEM, a one-way ANOVA test was performed with a Turkey’s multiple comparisons test between experimental groups. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. Statistical differences between groups in figures c and d are shown as * for Epm2a−/− vs Epm2a−/− + E-MET or Epm2b−/− vs Epm2b−/− + E-MET; # for Epm2a−/− vs Epm2a−/− + L-MET or Epm2b−/− vs Epm2b−/− + L-MET; + for Epm2a−/− + E-MET vs Epm2a−/− + L-MET or Epm2b−/− + E-MET vs Epm2b−/− + L-MET
E-MET Produces Greater Benefits in the Prevention of Motor Coordination and Memory Impairments in Epm2a−/− and Epm2b−/− Mice
We studied the effect of E-MET and L-MET on motor coordination in Epm2a−/− and Epm2b−/− mice (Fig. 2c, d). E-MET improved the latency time to fall from the rod compared to untreated mice in all attempts (Fig. 2c, d), whereas L-MET increased the amount of time that the two mouse models spent in the cylinder only in some attempts (Fig. 2c, d). Regarding cognitive decline, Epm2a−/− and Epm2b−/− mice receiving E-MET presented a higher D.I. average than mice treated with L-MET (Fig. 2e, f). Moreover, both mouse models treated with E-MET showed no memory decline (Fig. 2e, f).
E-MET Outperforms the Positive Effect of L-MET in Spontaneous Locomotor Activity Observed in Epm2a−/− Mice and Shows Similar Benefits in Epm2b−/− Mice
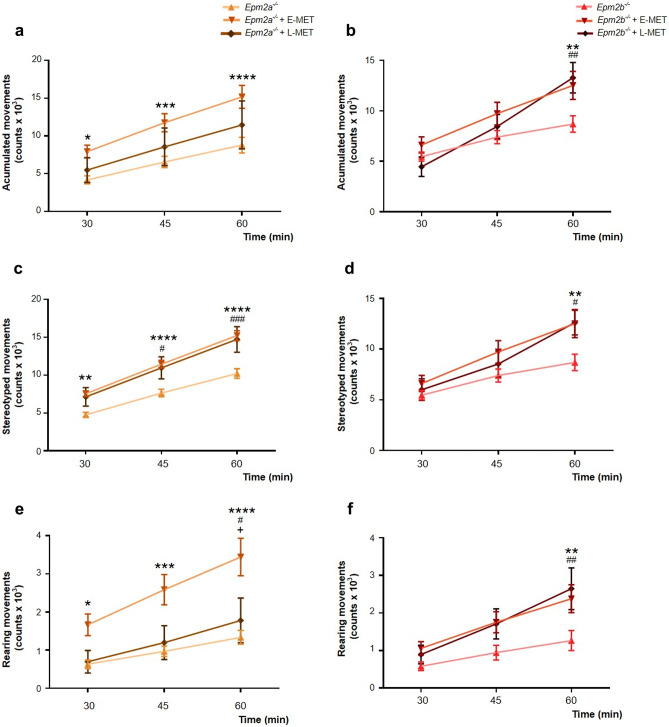
We also analyzed the beneficial outcome of both treatments on free movement in Epm2a−/− and Epm2b−/− mice (Fig. 3). Epm2a−/− mice treated with E-MET presented higher values than Epm2a−/− mice that received L-MET, although both experimental groups obtained a better score than untreated Epm2a−/− mice (Fig. 3a, c, e). E-MET and L-MET displayed almost identical positive results in Epm2b−/− mice (Fig. 3b, d, f).
Fig. 3.
E-MET enhances L-MET preventing the loss of spontaneous locomotor activity in 12-month-old Epm2a−/− and shows similar effect than L-MET in 12-month-old Epm2b−/− mice. We studied the benefits of E-MET in the appearance of spontaneous movement deterioration in 12-month-old mice. We evaluated the accumulated movement (a, b), the stereotyped movement (c, d), and the rearing movement (e, f) of both models. Data are shown as a mean ± SEM. A one-way ANOVA test was performed with a Turkey’s multiple comparisons test between experimental groups. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. Statistical differences between groups in figures c and d are shown as * for Epm2a−/− vs Epm2a−/− + E-MET or Epm2b−/− vs Epm2b−/− + E-MET; # for Epm2a−/− vs Epm2a−/− + L-MET or Epm2b−/− vs Epm2b−/− + L-MET; + for Epm2a−/− + E-MET vs Epm2a−/− + L-MET or Epm2b−/− + E-MET vs Epm2b−/− + L-MET
E-MET Ameliorates Formation of LBs in the Brain of Epm2a−/− and Epm2b−/− Mice and Improves L-MET Effect
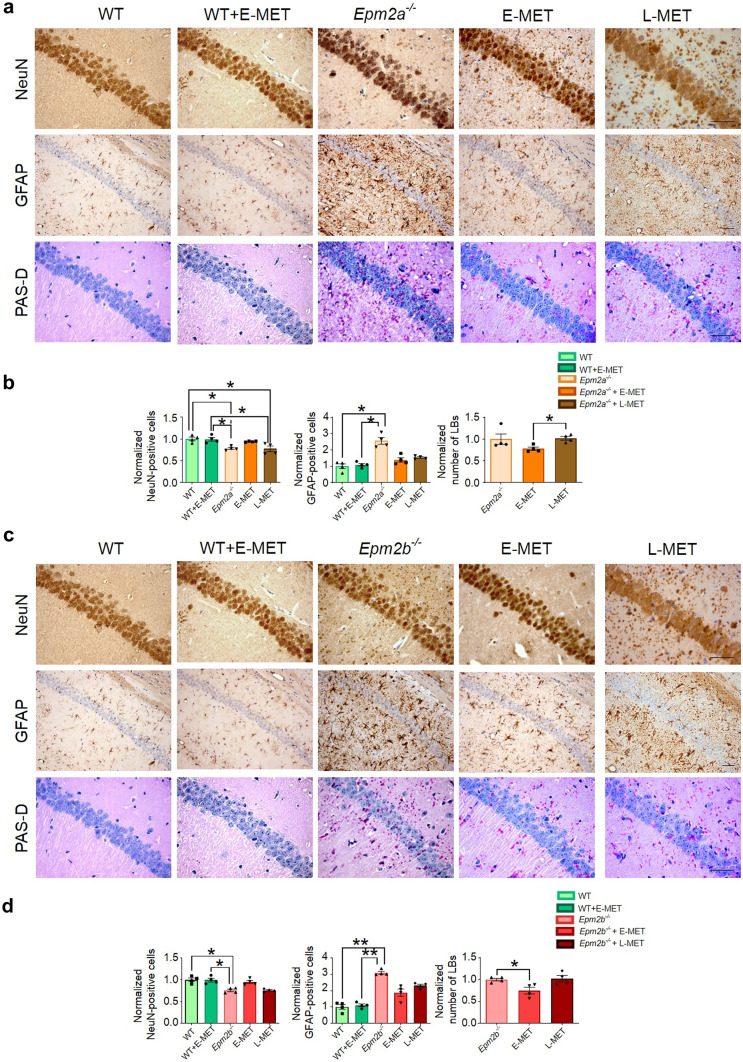
We analyzed the number of LBs detected in the CA1 region of the hippocampus using PAS staining. When we compared both treatments in 12-month-old Epm2a−/− and Epm2b−/− mice, we observed that brains from mice treated with E-MET showed a lower number of LBs in the CA1 region compared to animals treated with L-MET (Fig. 4).
Fig. 4.
E-MET improves the effect of L-MET on the reduction of neurodegeneration, astrogliosis, and formation of LBs in 12-month-old Epm2a−/− and Epm2b−/− mice. We studied the number of NeuN and GFAP positive cells and LBs positive to PAS-D staining, in the CA1 area of the hippocampus in WT, Epm2a−/− (a, b) and Epm2b−/− mice (c, d). Data are shown as a normalized mean ± SEM. Means were normalized using WT control values in NeuN and GFAP immunostaining assays, while the numbers of LBs detected in untreated Epm2a−/− or Epm2b−/− mice were used to normalize values in PAS-D staining. A Kruskal–Wallis non-parametric test followed by a Dunn’s multiple comparison test was performed. *p < 0.05; scale bar = 100 µm. NeuN, neuronal nuclear antigen; GFAP, glial fibrillary acidic protein; PAS-D, periodic acid–Schiff (PAS) stain used in combination with diastase
E-MET Decreases Neuronal Loss and Astrogliosis in Adult Epm2a−/− and Epm2b−/− Mice
We also evaluated the effect of E-MET in neurodegeneration and neuroinflammation using the NeuN antibody, a specific neuronal marker, and the GFAP antibody, a specific marker for astrogliosis. After quantifying the number of NeuN and GFAP-positive cells present in the CA1 field of Epm2a−/− and Epm2b−/− mice, we observed that brains from 12-month-old mice treated with E-MET showed a higher number of NeuN-positive cells and a lower quantity of GFAP-immunostained cells than mice treated with L-MET (Fig. 4). There were no significant differences between E-MET treated and non-treated wild-type animals.
Metformin in Patients with Lafora Disease
We analyzed the evolution of patients with Lafora disease at 6, 12, 18, and 24 months of follow-up, in order to study the clinical effect of metformin (Supplementary Table S2). We included 18 patients (10 females and 8 males) from 16 different families with mutations in the EPM2A or EPM2B genes. Mean age of disease onset was 11.5 years. The most frequently observed symptoms at onset were visual and GTC seizures. The initial number of patients receiving metformin was 8. Metformin was started a mean of 5.7 years after the onset of the disease. Mean dose was 1325 mg/day. The dose was defined considering the standard prescription dose of metformin for patients with diabetes (1000–2000 mg/day) and adjusted depending on the appearance of side effects and discomfort. Metformin was well tolerated, with no serious adverse events leading to treatment discontinuation. The most common side effects were diarrhea and mild hypoglycemia. If patients felt the side effects of their metformin dosage were too severe, physicians reduced this dose. More clinical information is provided in Table 1.
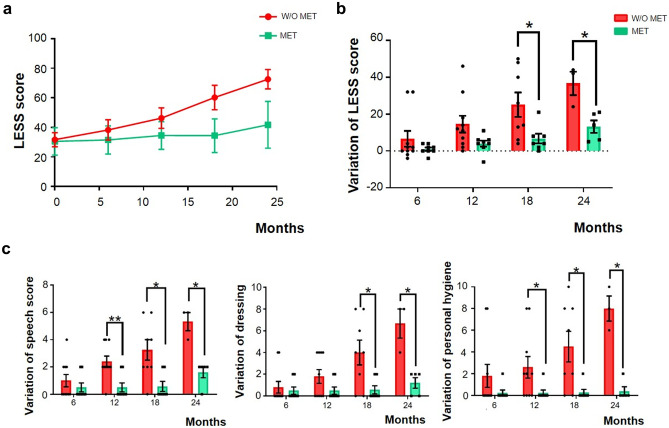
In the 12-month follow-up, the LESS score worsened a mean of 4 points in metformin-treated patients compared to 14.6 points in non-metformin-treated patients. When we used the LESS score to assess the variation from the initial examination to follow-ups at 18 and 24 months, we found significant differences between the two groups. The LESS score worsened at 18 months by an average of 6.7 points in patients receiving metformin compared to those not taking the treatment, who had a 25.1-point difference. Despite the fact that we only have data from 5 patients who received metformin and 3 patients who did not, the differences at 24 months were 13.2 points for the metformin group and 36.7 points for the non-metformin group. Therefore, patients who received metformin had a slower progression of the disease (Fig. 5 and Supplementary Table S2).
Fig. 5.
Metformin treatment slows the course of Lafora disease in patients. We compared the evolution of patients with Lafora disease who were receiving metformin (MET), to patients who were not (w/o MET) (a). We calculated the variation in the LESS score from the initial examination to 6, 12, 18, and 24 months of follow-up. Thus, to calculate all these variations, we always used the LESS score reported in the baseline follow-up as the reference (b). We show the most well-preserved alterations due to metformin administration (c). Data are shown as a mean of LESS score or a variation of these scores as received in the LESS ± SEM. A Student’s t-test was performed when we analyzed data at the initial examination and at 6, 12, and 18 months of follow-up. We used the Mann–Whitney non-parametric test when we studied data at 24 months. *p < 0.05; **p < 0.01
Finally, when we analyzed the benefits of metformin administration in each LESS category, we observed that it was more effective in preserving speech skills, dressing autonomy, and personal hygiene care (Fig. 5c and Supplementary Table S2). The effect of metformin in other categories is also reported (Supplementary Fig. S1).
Discussion
Currently, there is no specific treatment for Lafora disease. Patients can only be treated with antiseizure medications, with a low to moderate effect in controlling seizures. Death usually occurs 11 years after clinical onset [1]. While research and development of new drugs is a long, expensive, and high-risk procedure, most repurposed drugs can be used immediately and have a higher probability of obtaining an indication through expedited procedures [33, 34]. This can be appealing for rare diseases, as the number of patients is low and the return of investments in clinical trials and drug development is questionable.
In this work, we compare pre-symptomatic metformin treatment with treatment when neurological impairments have already manifested and show that E-MET improves the neurological outcomes of L-MET in mouse models of Lafora disease. In Lafora disease, seizures are one of the most severe symptoms and may lead to increased morbidity and mortality. If E-MET reduced their occurrence, patients would experience a significant improvement in their quality of life. Our experiments reveal that E-MET but not L-MET prevents the appearance of myoclonic and GTC seizures induced by PTZ injections in 12-month-old Epm2b−/− mice. In Epm2a−/− mice, E-MET reduced the hypersensitivity to PTZ. Unfortunately, we did not appreciate the same benefits for myoclonic or GTC seizures in our patients treated with metformin. We also show that E-MET but not L-MET prevents the onset of cognitive problems in 12-month-old Lafora disease mice. E-MET also resulted in a better outcome than L-MET in other neurological features, such as spontaneous locomotor activity, motor coordination, and postural impairments. E-MET also resulted in a lower amount of LBs in the hippocampus. Neuroinflammation and neurodegeneration were also reduced in mice receiving E-MET compared to L-MET.
We have confirmed that E-MET was safe from early development throughout life in mice. We did not observe developmental abnormalities or adverse reactions in mouse pups exposed to E-MET. In patients, safety of metformin in human embryos and placental passage has already been reported [28, 35]. Consequently, embryos are exposed to levels of metformin similar to those of adults. No teratogenic [27] or developmental abnormalities have been reported after in utero exposure [36]. In adults, only minor side effects have been reported, and these usually disappear after dose adjustments [37].
In our small cohort, patients treated with metformin had a slower progression than non-treated patients. When we analyzed the variation in the LESS score from the initial examination to the follow-ups at 18 and 24 months, we found significant differences between the two groups. Speech skills, dressing, and personal hygiene were the most preserved ADL in treated patients resulting in a slower deterioration of our patients’ functional status.
Since we aimed to evaluate the effect of chronic metformin treatment, patients that had received metformin for short periods before being included in the study were not considered receiving chronic metformin treatment. At the initiation of the study, the metformin-treated group had a faster progression of the disease, as for a similar LESS score (mean 30.5 treated vs 31.7 non-treated), evolution was shorter in the treated group (mean years after onset 5.7 vs 7.7 years). The faster evolution towards the same LESS score implies that patients treated with metformin were originally set on a more severe progression. Therefore, benefits reported in the metformin-treated group are likely related to the therapeutic effects of metformin.
In a recent retrospective study of patients with advanced Lafora disease, metformin showed a modest effect [25]. A clinical response, characterized by a reduction of seizure frequency and global clinical improvement, was reported in 3 out of 12 patients. The authors recommended that treatment with metformin may be attempted as early as possible in the course of Lafora disease. In our cohort, metformin resulted in a greater benefit, probably because the age at treatment initiation was much lower (5.7 years from onset in our study vs 8 years in the previous retrospective study). This finding is consistent with our findings in the two mouse models of Lafora disease. However, to reach more definitive conclusions, a longer follow-up study including a comparison to a historical control group is needed.
The mechanisms of action of metformin are poorly understood. It specifically inhibits mitochondrial respiratory-complex I, which decreases cellular respiration and activates AMPK. This kinase is a key factor that regulates energy metabolism, promoting catabolic pathways and deactivating anabolic signaling [38, 39]. AMPK also regulates glycogen turnover through the phosphorylation and deactivation of the glycogen synthase [40]. Reduction of glycogen synthase activity has been proposed as a therapy for Lafora disease. In fact, positive results have been reported in conditional mouse models of Lafora disease targeting the downregulation of glycogen synthase [41, 42]. AMPK activation through metformin intake may be a simple method to modulate glycogen synthase activity. It is also noteworthy that AMPK phosphorylates laforin, but does not interact with malin [43], which regulates laforin phosphatase activity and the ability to interact with its biological targets [44]. The presence of laforin in the Epm2b−/− mice but not in the Epm2a−/− mouse model may underlie the differences of the effect of metformin observed between these two models. Other molecular pathways have also been proposed to explain the effects of metformin in Lafora disease [23–25] and other neurodegenerative diseases [45–50]. Some of them could be responsible for the discrepancies in the benefits of metformin in Epm2a−/− and Epm2b−/− mice. We observed no variability in the response of patients treated with metformin carrying mutations in EPM2A or EPM2B.
In conclusion, our studies clearly support the hypothesis that future therapies should be started early in the course of Lafora disease or, if possible, in pre-symptomatic stages. E-MET enhanced the benefits of L-MET reducing behavioral, neurological, and epileptic impairments in two mouse models of Lafora disease. A slower evolution was also noticed in patients treated with metformin, although further controlled studies are required to sustain this observation. Our data supports the use of metformin in early stages of Lafora disease and in pre-symptomatic individuals such as siblings of Lafora disease patients. Starting to treat patients with metformin earlier than in this study may have a curative impact on myoclonic and GTC seizures, which remained unchanged during the study of the present patient cohort. Therefore, we recommend metformin in all pre-symptomatic patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Animal Facility of the IIS-Fundación Jiménez Díaz for their technical assistance.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author Contribution
D.F.B.: major role in data acquisition, data analysis, and wrote the manuscript. M.M.C.: data acquisition and analysis. N.I.C: data acquisition and analysis. B.G.G.: data acquisition. J.G.F.: data acquisition. G.S.M.: data acquisition. M.P.S.: design and conceptualization of the study, interpretation of the data, and revision of the manuscript. J.M.S.: design and conceptualization of the study, interpretation of the data, and revision of the manuscript.
Funding
This work was supported by grants from the Spanish Ministry of Economy (Rti2018-095784b-100SAF) to J.M.S. and M.P.S., from the Tatiana Perez de Guzman el Bueno Foundation (5258/002) to M.P.S. and J.M.S., from the CIBERER (ACCI 2020, 23—U744) to M.P.S., and a grant from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (P01NS097197), which established the Lafora Epilepsy Cure Initiative (LECI), to J.M.S. and M.P.S.
Declarations
Conflict of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article. We confirm that we have read the Journal’s position on issues involved in ethical publication and state that this report is consistent with those guidelines.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pondrelli F, Muccioli L, Licchetta L, Mostacci B, Zenesini C, Tinuper P, et al. Natural history of Lafora disease: a prognostic systematic review and individual participant data meta-analysis. Orphanet J Rare Dis. 2021;16(1):362. doi: 10.1186/s13023-021-01989-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van H, De Jager H. Progressive myoclonus epilepsy with lafora bodies. Clinical-Pathological Features. Epilepsia. 1963;4:95–119. doi: 10.1111/j.1528-1157.1963.tb05214.x. [DOI] [PubMed] [Google Scholar]
- 3.Berkovic SF, Andermann F, Carpenter S, Wolfe LS. Progressive myoclonus epilepsies: specific causes and diagnosis. N Engl J Med. 1986;315(5):296–305. doi: 10.1056/NEJM198607313150506. [DOI] [PubMed] [Google Scholar]
- 4.Berkovic SF, So NK, Andermann F. Progressive myoclonus epilepsies: clinical and neurophysiological diagnosis. J Clin Neurophysiol. 1991;8(3):261–274. doi: 10.1097/00004691-199107010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Minassian BA, Lee JR, Herbrick JA, Huizenga J, Soder S, Mungall AJ, et al. Mutations in a gene encoding a novel protein tyrosine phosphatase cause progressive myoclonus epilepsy. Nat Genet. 1998;20(2):171–174. doi: 10.1038/2470. [DOI] [PubMed] [Google Scholar]
- 6.Serratosa JM, Gómez-Garre P, Gallardo ME, Anta B, de Bernabé DB, Lindhout D, et al. A novel protein tyrosine phosphatase gene is mutated in progressive myoclonus epilepsy of the Lafora type (EPM2) Hum Mol Genet. 1999;8(2):345–352. doi: 10.1093/hmg/8.2.345. [DOI] [PubMed] [Google Scholar]
- 7.Minassian BA, Ianzano L, Meloche M, Andermann E, Rouleau GA, Delgado-Escueta AV, et al. Mutation spectrum and predicted function of laforin in Lafora’s progressive myoclonus epilepsy. Neurology. 2000;55(3):341–346. doi: 10.1212/WNL.55.3.341. [DOI] [PubMed] [Google Scholar]
- 8.Chan EM, Young EJ, Ianzano L, Munteanu I, Zhao X, Christopoulos CC, et al. Mutations in NHLRC1 cause progressive myoclonus epilepsy. Nat Genet. 2003;35(2):125–127. doi: 10.1038/ng1238. [DOI] [PubMed] [Google Scholar]
- 9.Gentry MS, Worby CA, Dixon JE. Insights into Lafora disease: malin is an E3 ubiquitin ligase that ubiquitinates and promotes the degradation of laforin. Proc Natl Acad Sci USA. 2005;102(24):8501–8506. doi: 10.1073/pnas.0503285102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganesh S, Delgado-Escueta AV, Sakamoto T, Avila MR, Machado-Salas J, Hoshii Y, et al. Targeted disruption of the Epm2a gene causes formation of Lafora inclusion bodies, neurodegeneration, ataxia, myoclonus epilepsy and impaired behavioral response in mice. Hum Mol Genet. 2002;11(11):1251–1262. doi: 10.1093/hmg/11.11.1251. [DOI] [PubMed] [Google Scholar]
- 11.Criado O, Aguado C, Gayarre J, Duran-Trio L, Garcia-Cabrero AM, Vernia S, et al. Lafora bodies and neurological defects in malin-deficient mice correlate with impaired autophagy. Hum Mol Genet. 2012;21(7):1521–1533. doi: 10.1093/hmg/ddr590. [DOI] [PubMed] [Google Scholar]
- 12.García-Cabrero AM, Marinas A, Guerrero R, de Córdoba SR, Serratosa JM, Sánchez MP. Laforin and malin deletions in mice produce similar neurologic impairments. J Neuropathol Exp Neurol. 2012;71(5):413–421. doi: 10.1097/NEN.0b013e318253350f. [DOI] [PubMed] [Google Scholar]
- 13.García-Cabrero AM, Sánchez-Elexpuru G, Serratosa JM, Sánchez MP. Enhanced sensitivity of laforin- and malin-deficient mice to the convulsant agent pentylenetetrazole. Front Neurosci. 2014;8(291). [DOI] [PMC free article] [PubMed]
- 14.Sansig G, Bushell TJ, Clarke VR, Rozov A, Burnashev N, Portet C, et al. Increased seizure susceptibility in mice lacking metabotropic glutamate receptor 7. J Neurosci Off J Soc Neurosci. 2001;21(22):8734–8745. doi: 10.1523/JNEUROSCI.21-22-08734.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy DS, Castaneda DC, O'Malley BW, Rogawski MA. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther. 2004;310(1):230–239. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Li Z, Sakurai E, Izadi Mobarakeh J, Ohtsu H, Watanabe T, et al. Chemical kindling induced by pentylenetetrazol in histamine H1 receptor gene knockout mice (H1KO), histidine decarboxylase-deficient mice (HDC−/−) and mast cell-deficient W/Wv mice. Brain Res. 2003;968(1):162–166. doi: 10.1016/S0006-8993(03)02229-7. [DOI] [PubMed] [Google Scholar]
- 17.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Investig. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patil SP, Jain PD, Ghumatkar PJ, Tambe R, Sathaye S. Neuroprotective effect of metformin in MPTP-induced Parkinson’s disease in mice. Neuroscience. 2014;277:747–754. doi: 10.1016/j.neuroscience.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 19.Ashabi G, Khalaj L, Khodagholi F, Goudarzvand M, Sarkaki A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab Brain Dis. 2015;30(3):747–754. doi: 10.1007/s11011-014-9632-2. [DOI] [PubMed] [Google Scholar]
- 20.Lu M, Su C, Qiao C, Bian Y, Ding J, Hu G. Metformin prevents dopaminergic neuron death in MPTP/P-induced mouse model of Parkinson’s disease via autophagy and mitochondrial ROS clearance. Int J Neuropsychopharmacol. 2016;19(9). [DOI] [PMC free article] [PubMed]
- 21.Zhu XC, Jiang T, Zhang QQ, Cao L, Tan MS, Wang HF, et al. Chronic metformin preconditioning provides neuroprotection via suppression of NF-kappaB-mediated inflammatory pathway in rats with permanent cerebral ischemia. Mol Neurobiol. 2015;52(1):375–385. doi: 10.1007/s12035-014-8866-7. [DOI] [PubMed] [Google Scholar]
- 22.Pintana H, Apaijai N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life Sci. 2012;91(11–12):409–414. doi: 10.1016/j.lfs.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Berthier A, Payá M, García-Cabrero AM, Ballester MI, Heredia M, Serratosa JM, et al. Pharmacological interventions to ameliorate neuropathological symptoms in a mouse model of Lafora disease. Mol Neurobiol. 2016;53(2):1296–1309. doi: 10.1007/s12035-015-9091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sánchez-Elexpuru G, Serratosa JM, Sanz P, Sánchez MP. 4-Phenylbutyric acid and metformin decrease sensitivity to pentylenetetrazol-induced seizures in a malin knockout model of Lafora disease. NeuroReport. 2017;28(5):268–271. doi: 10.1097/WNR.0000000000000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bisulli F, Muccioli L, d'Orsi G, Canafoglia L, Freri E, Licchetta L, et al. Treatment with metformin in twelve patients with Lafora disease. Orphanet J Rare Dis. 2019;14(1):149. doi: 10.1186/s13023-019-1132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32(6):435–443. doi: 10.1023/A:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanky E, Zahlsen K, Spigset O, Carlsen SM. Placental passage of metformin in women with polycystic ovary syndrome. Fertil Steril. 2005;83(5):1575–1578. doi: 10.1016/j.fertnstert.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 28.Simmons D. Safety considerations with pharmacological treatment of gestational diabetes mellitus. Drug Saf. 2015;38(1):65–78. doi: 10.1007/s40264-014-0253-9. [DOI] [PubMed] [Google Scholar]
- 29.Hale T, Kristensen J, Hackett L, Kohan R, Ilett K. Transfer of metformin into human milk. Adv Exp Med Biol. 2004;554:435–436. doi: 10.1007/978-1-4757-4242-8_58. [DOI] [PubMed] [Google Scholar]
- 30.Pitkänen A, Buckmaster PS, Galanopoulou AS, Moshe S. Models of seizures and epilepsy: Second Edition. 2017. 1–1151 p.
- 31.National Research Council Committee for the Update of the Guide for the C, Use of Laboratory A. The National Academies Collection: Reports funded by National Institutes of Health. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press (US). Copyright © 2011, National Academy of Sciences. 2011.
- 32.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fetro C, Scherman D. Drug repurposing in rare diseases: myths and reality. Therapie. 2020;75(2):157–160. doi: 10.1016/j.therap.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discovery. 2004;3(8):673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 35.Priya G, Kalra S. Metformin in the management of diabetes during pregnancy and lactation. Drugs Context. 2018;7:212523. doi: 10.7573/dic.212523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glueck CJ, Goldenberg N, Pranikoff J, Loftspring M, Sieve L, Wang P. Height, weight, and motor-social development during the first 18 months of life in 126 infants born to 109 mothers with polycystic ovary syndrome who conceived on and continued metformin through pregnancy. Hum Reprod (Oxford, England) 2004;19(6):1323–1330. doi: 10.1093/humrep/deh263. [DOI] [PubMed] [Google Scholar]
- 37.Fujita Y, Inagaki N. Metformin: new preparations and nonglycemic benefits. Curr DiabRep. 2017;17(1):5. doi: 10.1007/s11892-017-0829-8. [DOI] [PubMed] [Google Scholar]
- 38.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25(18):1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bultot L, Guigas B, Von Wilamowitz-Moellendorff A, Maisin L, Vertommen D, Hussain N, et al. AMP-activated protein kinase phosphorylates and inactivates liver glycogen synthase. Biochem J. 2012;443(1):193–203. doi: 10.1042/BJ20112026. [DOI] [PubMed] [Google Scholar]
- 41.Nitschke S, Chown EE, Zhao X, Gabrielian S, Petković S, Guisso DR, et al. An inducible glycogen synthase-1 knockout halts but does not reverse Lafora disease progression in mice. J Biol Chem. 2021;296:100150. doi: 10.1074/jbc.RA120.015773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varea O, Duran J, Aguilera M, Prats N, Guinovart JJ. Suppression of glycogen synthesis as a treatment for Lafora disease: establishing the window of opportunity. Neurobiol Dis. 2021;147:105173. doi: 10.1016/j.nbd.2020.105173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solaz-Fuster MC, Gimeno-Alcaniz JV, Ros S, Fernandez-Sanchez ME, Garcia-Fojeda B, Criado Garcia O, et al. Regulation of glycogen synthesis by the laforin-malin complex is modulated by the AMP-activated protein kinase pathway. Hum Mol Genet. 2008;17(5):667–678. doi: 10.1093/hmg/ddm339. [DOI] [PubMed] [Google Scholar]
- 44.Romá-Mateo C, Solaz-Fuster Mdel C, Gimeno-Alcañiz JV, Dukhande VV, Donderis J, Worby CA, et al. Laforin, a dual-specificity phosphatase involved in Lafora disease, is phosphorylated at Ser25 by AMP-activated protein kinase. Biochem J. 2011;439(2):265–275. doi: 10.1042/BJ20110150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Curry DW, Stutz B, Andrews ZB, Elsworth JD. Targeting AMPK signaling as a neuroprotective strategy in Parkinson’s disease. J Parkinsons Dis. 2018;8(2):161–181. doi: 10.3233/JPD-171296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poels J, Spasić MR, Callaerts P, Norga KK. Expanding roles for AMP-activated protein kinase in neuronal survival and autophagy. BioEssays. 2009;31(9):944–952. doi: 10.1002/bies.200900003. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Zimmermann HR, Ma T. Therapeutic potential of AMP-activated protein kinase in Alzheimer’s disease. J Alzheimers Dis. 2019;68(1):33–38. doi: 10.3233/JAD-181043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang S, Li T, Ji T, Yi W, Yang Z, Wang S, et al. AMPK: potential therapeutic target for ischemic stroke. Theranostics. 2018;8(16):4535–4551. doi: 10.7150/thno.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rotermund C, Machetanz G, Fitzgerald JC. The therapeutic potential of metformin in neurodegenerative diseases. Front Endocrinol. 2018;9(400). [DOI] [PMC free article] [PubMed]
- 50.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20(6):953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author, upon reasonable request.