Abstract
INTRODUCTION:
Cognitive resilience (CR) has been defined as the continuum of better (or worse) than expected cognition, given the degree of neuropathology. To quantify this concept, existing approaches focus on either cognitive level at a single timepoint or slopes of cognitive decline.
METHODS:
In a prospective study of 1,215 participants, we created a continuous measure of CR defined as the mean of differences between estimated person-specific and marginal cognitive levels over time, after accounting for neuropathologies.
RESULTS:
Neuroticism and depressive symptoms were associated with all CR measures (P-values<.012); as expected, cognitive activity and education were only associated with the cognitive-level approaches (P-values<.0002). However, compared with the existing CR measures focusing on a single measure or slopes of cognition, our new measure yielded stronger relations with risk factors.
DISCUSSION:
Defining CR based on the longitudinal differences between person-specific and marginal cognitive levels is a novel and complementary way to quantify CR.
Keywords: Alzheimer’s dementia, cognitive resilience, mixed-effects model, neuropathology, neuropsychological tests, longitudinal study
1. BACKGROUND
Previous research with postmortem neuropathologic measures indicates that about one-third of older persons,1–4 and 50% of the oldest-old,5,6 meet neuropathological criteria for Alzheimer’s Disease (AD) at death, but do not have clinical dementia. Parallel findings have been reported in studies using CSF or neuroimaging biomarkers of AD pathology.7 These individuals with better-than-expected cognition given their degree of pathology have been termed “cognitively resilient”.8,9 More recently, our group10 and others11,12 have extended the definition of cognitive resilience to include the entire continuum spanning from worse-than-expected through better-than-expected cognition, given the degree of neuropathology; this yields a single continuous measure of CR and enables statistically powerful quantification of the continuum of resilience.8,9 Identifying risk factors contributing to CR represents an important pathway to understanding how to delay, and possibly prevent clinical Alzheimer’s dementia.13,14 However, research on cognitive resilience is relatively new, and limited work to date has adequately explored the possible range of approaches for creating a continuous measure of CR to fully accommodate this complex concept.
One existing method of calculating CR focuses on cognition at a single timepoint (i.e., near the assessment of levels of neuropathology), using residual approaches.10,11 In this existing method, (which we denote as ), cognitive resilience is calculated as the discrepancy between the observed and the estimated level of global cognition, after accounting for common pathology and demographics in a linear regression model (Figure 1–A).10 However, a single timepoint may not provide an adequate representation of cognitive trajectories. Over the last few years our group has also conceptualized CR based on cognitive decline over time.15 Using longitudinal measures of global cognition, this method has defined CR (denoted here as ) as the residual slope of cognitive decline; that is the person-specific deviation from the rate of cognitive decline expected at the marginal level (i.e., compared to individuals sharing a similar profile of covariates) estimated in a linear mixed-effects model adjusted for neuropathologies and demographic characteristics, and assuming linearity of cognitive trajectories to facilitate interpretation (Figure 1–B). However, slopes of cognitive decline are not the sole means of considering trajectories. In particular, individuals may maintain high levels of cognition over time, despite experiencing cognitive decline.
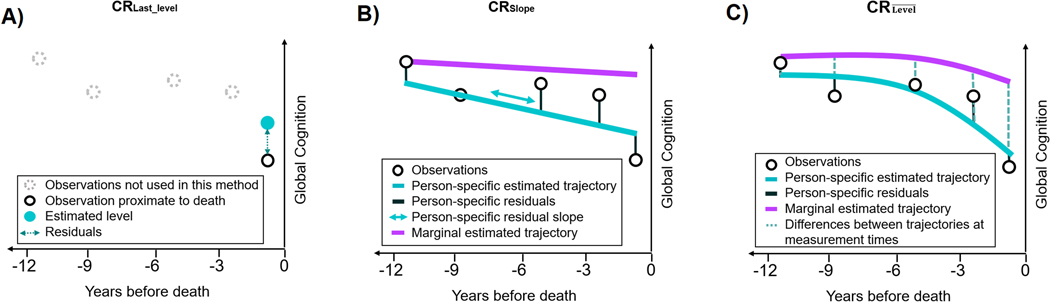
Figure 1.
Graphs illustrating the three strategies used to quantify cognitive resilience (CR) at the individual level.
The graphs each represent an individual with 5 repeated observations of global cognition collected over 12 years preceding death. The CRLast_level (panel A) is computed as the standardized difference between the observed and the estimated levels of global cognition (i.e., standardized residuals) proximate to death from a linear regression model accounting for demographics and neuropathologic indices. The (panel B) is defined as the person-specific deviation from the rate of cognitive decline expected at the population level (i.e., random slope), estimated in a latent process mixed-effects (LPM) model assuming linearity and adjusted for demographic and neuropathologic indices. Finally, (panel C) corresponds to the mean of the differences between the person-specific and the marginal estimations of global cognition at each measurement time estimated in a LPM model allowing non-linearity and adjusted for demographic and neuropathologic indices. For and (panels C–D), marginal trajectories represent the expected shape of cognition among individuals sharing similar profile of covariates (demographics and pathologic indices) as the theoretical individual.
Thus, here we extend our previous work by newly creating a calculation of CR (denoted here as ) considering longitudinal measures of cognitive level rather than slopes of cognitive decline. Specifically, the aim of the present study was to create a continuous measure of CR, focused on observed versus expected levels of cognitive function over multiple points in time (Figure 1–C). To further extend our previous research, we also considered departures from normality in cognitive outcomes and non-linearity of late-life cognitive decline in our models. Briefly, we will (i) define and calculate this new measure of CR, as well as the two existing measures (i.e., using a single cognitive assessment, and using slopes of decline) in two clinical-pathologic cohorts with annual cognitive testing and uniform neuropathologic examination at death. Then, we will (ii) contrast the three versions of CR, by examining associations between a range of risk factors (e.g., education level, neuroticism10,16) and our new and each of the two existing measures of CR.
2. METHODS
2.1. Study populations
We utilized data from two large clinical-pathologic cohort studies of aging and dementia.17 The Religious Orders Study (ROS) began in 1994 and enrolls older Catholic nuns, priests, and lay brothers from ~40 orders across the United States. The Rush Memory and Aging Project (MAP) started in 1997 and enrolls women and men from the metropolitan Chicago area. The design and operation of these two studies are identical in essential details; eligibility required older age, absence of known dementia, and agreement to annual clinical evaluations and brain donation at death. The follow-up rate exceeds 90% in both cohorts. All participants signed a consent form and an Anatomical Gift Act form for organ donation and both study protocols were approved by an Institutional Review Board of Rush University Medical Center.
2.2. Study participants
Of the 2,057 decedents (914 from ROS, 1,143 from MAP) who had completed the baseline clinical evaluation at the time of these analyses, we considered the 1,716 individuals who had undergone brain autopsy (813 ROS, 903 MAP) to date. We further excluded 175 participants who did not yet have data on all 9 neuropathologies of interest (118 ROS, 57 MAP), 107 without any complete cognitive evaluation during follow-up (32 ROS, 75 MAP) and 219 in whom the last clinical evaluation was >2 years before death (82 ROS, 137 MAP), resulting in an analytic sample of 1,215 participants (566 ROS, 649 MAP).
2.3. Global cognitive function assessment
In both cohorts, annual clinical evaluations included a uniform medical history, neurologic examination, and detailed neuropsychological performance testing. A battery of 17 psychometric tests was administered annually and used to create a global composite measure of cognitive function in both cohorts.17
We focus on a composite score of global cognition based on all 17 tests. Raw scores were converted on component tests to z-scores, using the baseline mean and standard deviation (SD) from the combined parent studies; at each assessment, the z-scores were averaged together, with higher scores indicating better function. In sensitivity analyses, we further examined MMSE score, one of the most common neuropsychological tests used in cohort studies.18
2.4. Neuropathologic evaluation
Details of the postmortem evaluations have been previously reported.19,20 Briefly, the brains of deceased participants were removed, weighed, and hemispheres were cut coronally into 1cm slabs. One hemisphere was fixed in 4% paraformaldehyde. After gross examination of both hemispheres, 9 brain regions of interest were dissected from the 1cm slabs of fixed tissue, processed and embedded in paraffin. Sections from the paraffin blocks were stained for assessment of pathology.
2.4.1. Neurodegenerative pathologies
Modified Bielschowsky silver stain was used to visualize neuritic plaques, diffuse plaques, and neurofibrillary tangles in 5 brain regions, with regional scores of each pathology standardized and averaged to yield a continuous composite measure of AD pathology.21 The presence of hippocampal sclerosis was determined using hematoxylin and eosin stain.22 The presence of neocortical Lewy body pathology was determined using antibodies to α-synuclein.23 The presence of transactive response DNA-binding protein 43 (TDP-43) cytoplasmic inclusions was assessed using monoclonal antibodies to phosphorylated TDP-43 (p5409/410; 1:100)24; TDP-43 localized at least in the hippocampus or entorhinal cortex was considered as positive.20,25
2.4.2. Vascular pathologies
Gross infarcts were identified by visually examining slabs and pictures from both cerebral hemispheres and confirmed histologically whereas microinfarcts were detected under microscopy using hematoxylin and eosin stain.26,27 Arteriolosclerosis was assessed histologically from hematoxylin and eosin-stained sections of the anterior basal ganglia.28 Assessment of atherosclerosis was based on visual inspection of vessels in the circle of Willis.28 Assessment of cerebral amyloid angiopathy was done using amyloid-beta immunostaining in 4 cortical regions.29 We considered the presence of moderate or severe arteriolosclerosis, atherosclerosis, and cerebral amyloid angiopathy.
2.5. Other covariates at cohort baseline
We utilized cohort data on age and sex as covariates in our models creating the CR variables. Further, we used several self-reported risk factors at baseline based on substantial previous work in our cohorts10,16,30 and others12,31 demonstrating clear associations with CR: cognitive activity in late-life, years of education, neuroticism, and depressive symptoms. Participation in cognitive activities was calculated based on reported time spent reading books, magazines, newspapers, and playing games; these four items were queried in both ROS and MAP.32,33 Neuroticism was measured using 12 items from the Revised NEO Personality Inventory (NEO-PI-R), and scores could range from 0 to 48, with higher scores indicating more neuroticism.34 Depressive symptoms were assessed using a modified, 10-item version of the Center for Epidemiologic Studies Depression scale (CES-D), with one point assigned for each reported depressive symptom.35 Further, we examined ApoE-ε4 genotype; this is an important risk factor for Alzheimer’s dementia and AD pathology, but not for CR, as a way to examine the function of our CR measures for null relations as well.36
2.6. Statistical methods
We computed three versions of the CR (Figure 1): the new CR, averaging cognitive levels over time () and the two previously-published CR scores that focus either on cross-sectional cognitive level proximate to death () or cognitive change over time (). The details of the calculation of CRs are provided in Supplementary Method.
Briefly, for all strategies, to address the departure from normality that we observed for the composite score of global cognition (skewness=−1.3, kurtosis=2.3; Supplementary Figure A), we relied on a flexible quadratic I-splines transformation.37,38 The optimal spline basis was selected according to the Akaike Information Criterion39 within two to five knots placed at the quantiles of the cognitive outcome distribution. For, the cognitive observations proximate to death were transformed using splines before inclusion in the linear regression model. In longitudinal frameworks (for and), we relied on a latent process mixed-effects (LPM) model for non-Gaussian outcomes.40 Models were all adjusted for age at death, sex, and pathologic indices as simple effects, and, for and , in interaction with the function of time (years before death). Although we use autopsy measures here to provide information on neuropathology, these covariates can be replaced in clinical cohorts with CSF or neuroimaging biomarker variables in the models.
was calculated as the standardized residuals obtained proximate to death (Figure 1–A). corresponded to the random slope parameter estimated in the LPM model (Figure 1–B). To facilitate interpretation, this strategy assumes linearity of cognitive trajectories, although some non-linearity in cognitive decline has been previously described.41,42 Finally, for , we focused on the level of cognition at each timepoint, rather than cognitive decline (Figure 1–C). Due to the observation of accelerated terminal decline near death in these cohorts,43 we incorporated in the LPM model a flexible function of time approximated by natural cubic splines (distinct from I-splines used above to transform the cognitive outcome).37 We defined as the mean of the differences between the person-specific and the marginal estimations of global cognition at each observation time. can be represented by:
where represents the total number of repeated scores of global cognition for the individual collected at the retrospective measurement times before death; is the function of time; and are the level (in the normalized scale) of cognition estimated at the person-specific and marginal level, respectively; and are correlated person-specific random intercept and slopes, respectively, with is indexed by as it represents the marginal value of cognition estimated for all individuals sharing a similar covariate profile.44 Note that, by considering person-specific estimated cognition instead of individual observations, reduces the person-specific variability that can be induced by measurement error inherent in any given administration of neuropsychological tests. In addition, as cognition was assessed annually, considering differences at discrete observation times is close to considering the area between the subject-specific and the marginal estimated cognition (i.e., integral).
Once we calculated the three versions of CR, we compared their distributions, and calculated Pearson correlations. Then, to assess the validity of our approach and contrast findings across strategies, we used separate linear regression models to estimate the associations of risk factors of CR to each of the standardized CR outcomes. Further, we investigated whether CR scores were related to ApoE genotype.
2.7. Sensitivity analyses: alternative computations of CR
First, we evaluated how our consideration of the non-normality of cognitive outcomes (in which we had used a quadratic I-splines transformation [see Section 2.6.]) influenced findings by re-calculating the three versions of CR using the untransformed composite score of global cognition in the models. Second, since many cohort studies administer the MMSE rather than an extensive cognitive battery, we re-calculated by considering MMSE as the cognitive outcome; we applied a normalizing pre-transformation of the MMSE score45 (Supplementary Figure B). Third, for , as non-linearity of cognitive decline occurs mostly near death in our cohorts (Supplementary Figure C), we examined how excluding terminal decline impacted our findings by recalculating after excluding cognitive data collected ≤3 years before death. Finally, since our complex flexible modeling approach for may not be necessary in all contexts, we examined how a simpler linear mixed model of cognitive trajectories would influence our results; for these sensitivity analyses, we recalculated assuming linearity (i.e., model used for CRSlope), both including and excluding terminal decline.
All analyses were conducted using R software version 4.0.3. We used the lcmm function of lcmm R package version 1.7.8. for LPM models44 and normMMSE function of NormPsy R package version 1.0.8. to pre-transform the MMSE score.45 Supplementary Appendix provides the code to replicate the analyses, including a function to obtain the integral of the differences over the observation period.
3. RESULTS
3.1. Participant characteristics
The analytic cohort included 1,215 individuals (Table 1), predominantly female (68%) with a mean age of 80 (standard deviation [SD]=7) years at enrollment and 90 years (SD=6) at death. During a mean follow-up of 9 years (SD=5, range=[0.8–26]), the median MMSE score decreased from 28 points (interquartile range [IQR]=[27–29]) at baseline to 25 (IQR=[17–28]) proximate to death. Alzheimer’s disease pathology was commonly observed, with 63% prevalence. Hippocampal sclerosis was identified in 8% of the participants, neocortical Lewy bodies in 12%, and TDP-43 pathology that extended beyond the amygdala in 31%. Each cerebrovascular pathology was present in approximately one-third of the participants, including macroscopic (36%) and microscopic infarcts (31%), moderate to severe amyloid angiopathy (35%), atherosclerosis (32%), and arteriolosclerosis (31%). The mean years of education was 16 (SD=4) and individuals reported participating in 3 (SD=1) cognitively stimulating activities at baseline on average. The mean level of neuroticism was 16 (SD=6), and 25% of participants reported >2 depressive symptoms (median=1, IQR=[1–2]). Overall, 23% of participants had the ApoE genotype (Table 1).
Table 1.
Characteristics of participants at baseline and proximate to death: Religious Orders Study and Rush Memory and Aging Project (N=1,215).
| Baseline | Proximate to death | |
|---|---|---|
|
| ||
| Demographics | ||
| Women, No. (%) | 820 (67.5) | – |
| Age, mean (SD), years | 80.4 (7.0) | 89.8 (6.4)a |
| Cognitive function | ||
| MMSE, mean (SD) | 27.7 (2.6) | 21.8 (7.9) |
| MMSE, median (IQR) | 28 (27; 29) | 25 (17; 28) |
| Global cognition, mean (SD) | −0.05 (0.59) | −0.90 (1.15) |
| Global cognition, median (IQR) | 0.03 (−0.39; 0.35) | −0.64 (−1.62; −0.01) |
| Time from last cognitive testing to death, mean (SD), months | – | 8.7 (5.3) |
| Neuropathology | ||
| Post-mortem interval, mean (SD), hoursb | – | 9.4 (8.3) |
| Neurodegenerative pathology | ||
| Global AD pathology, mean (SD) | – | 0.69 (0.58) |
| Pathologic AD, Reagan criteria, No. (%) | – | 760 (62.6) |
| Presence of hippocampal sclerosis, No. (%) | – | 100 (8.2) |
| Presence of cortical Lewy bodies, No. (%) | – | 148 (12.2) |
| Presence of TDP-43 cytoplasmic inclusions, No. (%) | – | 370 (30.5) |
| Vascular pathology, No. (%) | ||
| Presence of gross infarcts | – | 437 (36.0) |
| Presence of microscopic infarcts | – | 370 (30.5) |
| Moderate/severe cerebral amyloid angiopathy | – | 424 (34.9) |
| Moderate/severe atherosclerosis | – | 394 (32.4) |
| Moderate/severe arteriolosclerosis | – | 373 (30.7) |
| Risk factors of cognitive resilience | ||
| Years of education, mean (SD), years | 16.3 (3.7) | – |
| Cognitive activity, mean (SD)b | 3.4 (0.7) | – |
| Neuroticism, mean (SD)b | 16.3 (6.2) | – |
| Depressive symptoms, mean (SD)b | 1.1 (1.5) | – |
| Depressive symptoms, median (IQR)b | 1 (1; 2) | – |
| ApoE-ɛ4 carrier, ≥1 allele, No. (%) b | 277 (22.8) | – |
Abbreviations : AD, Alzheimer’s disease; ApoE, apolipoprotein E; CES-D, center for epidemiologic studies depression scale; IQR, interquartile range; MMSE, Mini-Mental State Examination; SD, standard deviation.
Age at death.
Summary measures given among non-missing values. Data were missing for 4 (0.3%) participants for post-mortem interval, 1 (0.1%) for cognitive activity, 116 (9.5%) for neuroticism, 2 (0.2%) for CES-D, and 25 (2.1%) for ApoE-ɛ4.
3.2. Illustration of the three CR strategies
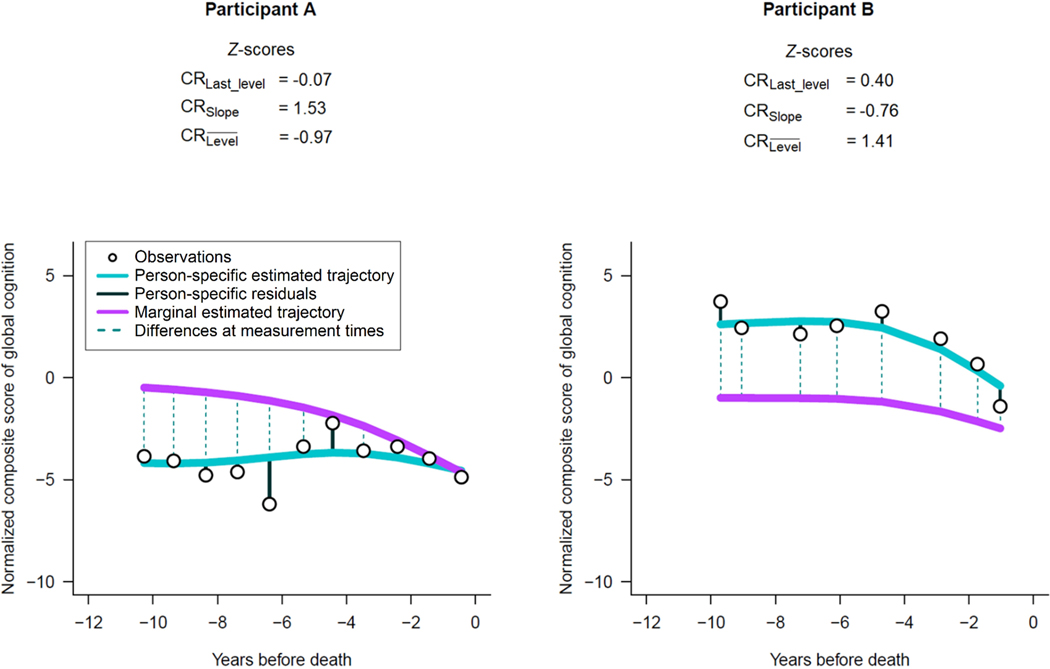
To help illustrate how specific contexts may lead to different CR scores across strategies, we identified participants with a variety of cognitive distributions and with z-scores of CR that differed substantially (Figure 2). For each individual, we show graphically their repeated observations of global cognition (in the normalized scale), as well as their person-specific and the marginal cognitive trajectories estimated using the flexible model described for . Participant A had slow cognitive decline, yet consistently lower estimated levels of cognition compared with the marginal trajectory; thereby leading to a high z-score for of 1.53 but a low of −0.97 and a near-zero of −0.07, due to the convergence of trajectories approaching death (Figure 2, left panel). Conversely, Participant B had steeper cognitive decline, yet consistently higher estimated levels of cognition compared with the marginal trajectory, resulting in a low z-score of of −0.76 but a high of 1.41 and a moderately high of 0.40 (Figure 2, right panel).
Figure 2.
Illustrative examples of two participants with varying z-scores of cognitive resilience (CR) and different estimated trajectories of cognition before death: Religious Orders Study and Rush Memory and Aging Project.
3.3. Distributions of CRs
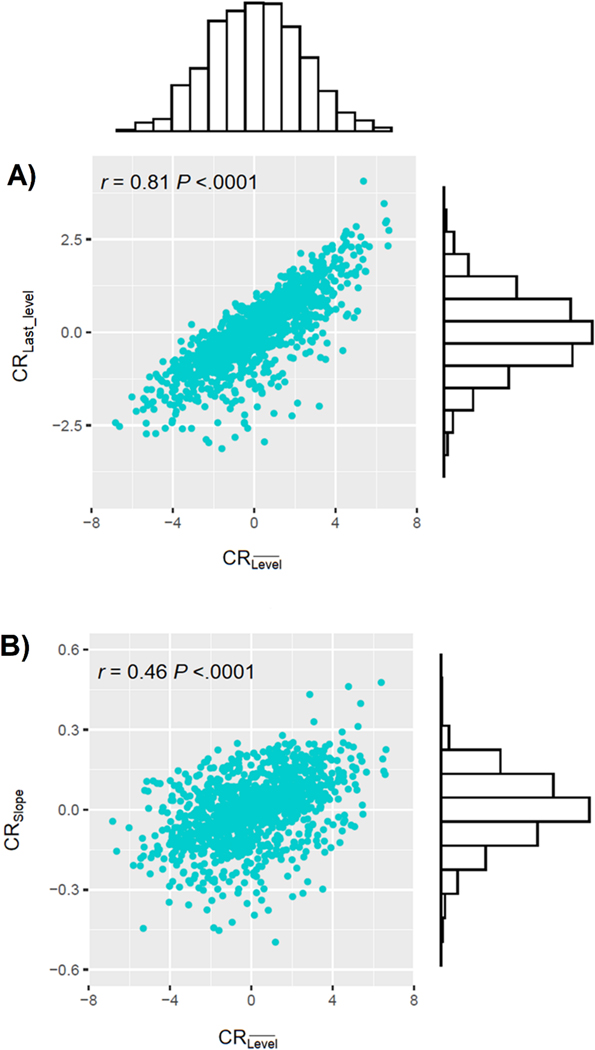
The score ranged from −3.1 to 4.1 (SD=1.0), from −0.5 to 0.5 (SD=0.1), and from −7.6 to 6.6 (SD=2.3) (Figure 3). The untransformed distributions of the CR statistics were all close to normal (skewness: =0.02, =−0.31, =−0.01; kurtosis: =0.34, =0.73, =−0.14). Moreover, was highly correlated with (Pearson’s correlation r=0.81) and moderately correlated with (r=0.46) (Figure 3).
Figure 3.
Distributions of , , and , and scatterplots of the relationship between and (panel A), and and (panel B); Religious Orders Study and Rush Memory and Aging Project (n=1,215).
3.4. Associations of risk factors at baseline with standardized CRs
Overall, for all three strategies, the effect estimates of the risk factors for cognitive resilience with CR z-score generally showed the same direction of association (Table 2), indicating that each provides largely consistent results. We found that provided consistently stronger magnitude of associations and smaller p-values across risk factors. For example, late-life cognitive activity was associated to (estimate=0.167, 95%CI=0.099;0.236, P<.2×10−6), (estimate=0.243, 95%CI=0.175;0.311, P<3×10−12), but not (estimate=0.041, 95%CI=−0.028;0.110, P=.2). When we considered education, which is generally more strongly related to cognitive level than cognitive decline, as expected, education was related to (estimate=0.029, 95%CI=0.013;0.044, P=.0002) and to (estimate=0.059, 95%CI=0.044;0.074, P<3×10−14), but not to (estimate=−0.002, 95%CI=−0.017;0.014, P=.8). In contrast, neuroticism and depressive symptoms were associated with the three CR measures, suggesting these risk factors are related to multiple dimensions of CR. Finally, ApoE genotype was not related to CR (Table 2).
Table 2.
Associations of established risk factors for cognitive resilience (CR) and ApoE-ɛ4 genotype with the three z-scores of Cognitive Resiliencea in separate linear regression models.
|
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 95%CI | P | 95%CI | P | 95%CI | P | ||||
|
| |||||||||
| Late-life cognitive activity, scoreb | 0.167 | (0.099; 0.236) | <2×10−6 | 0.041 | (−0.028; 0.110) | .244 | 0.243 | (0.175; 0.311) | <3×10−12 |
| Education, years | 0.029 | (0.013; 0.044) | .0002 | −0.002 | (−0.017; 0.014) | .844 | 0.059 | (0.044; 0.074) | <3×10−14 |
| Neuroticism, score | −0.025 | (−0.035; −0.016) | <2×10−7 | −0.012 | (−0.022; −0.003) | .012 | −0.031 | (−0.040; −0.022) | <5×10−11 |
| Nb. of depressive symptoms | −0.081 | (−0.117; −0.045) | <2×10−5 | −0.079 | (−0.115; −0.042) | <3×10−5 | −0.094 | (−0.131; −0.058) | <4×10−7 |
| ApoE ɛ4 carrier | 0.070 | (−0.070; 0.209) | .327 | 0.032 | (−0.108; 0.172) | .654 | 0.028 | (−0.111; 0.167) | .696 |
Abbreviations: 95%CI, 95% confidence interval; ApoE, apolipoprotein E.
After generating each CR variable, we standardized them to enable comparison of findings across the three strategies.
Frequency of participation in four common cognitively stimulating activities (e.g., read books, do puzzles), where a higher score indicates more activity.
3.5. Sensitivity analyses: alternative computations of CR
For each strategy, ignoring the non-normality of the composite score of global cognition generated alternative measures of CR highly equivalent to the original scores (all paired t-test P>.5) (Supplementary Table A). Additionally, the alternative based on MMSE did not differ meaningfully from the main (paired t-test P=.3) (Supplementary Table A). Additionally, when we recalculated after excluding terminal decline, the values of CR were slightly lower (paired t-test P=.011), reflecting the gap between the person-specific and marginal trajectories near-death (Supplementary Table A). Importantly however, these absolute differences in the CR values did not affect observed risk factor relations; as shown in Supplementary Table B, associations of risk factors to were similar when we excluded terminal decline from the calculations. Finally, when we applied linear mixed models for our calculation of , and tried both including and excluding terminal decline, then scores did not materially differ (all paired t-test P=.9) (Supplementary Table A); as expected, this assumption is limited and implied an increase in the variability of individual residuals as death approached (Supplementary Figure C). Moreover, associations of risk factors to CRs calculated assuming linearity were generally similar compared with the main findings (Supplementary Table B).
4. DISCUSSION
We propose here a novel, longitudinal method for quantifying cognitive resilience to Alzheimer’s disease and other neuropathologies. Our measure () represents the mean of differences, over repeated timepoints, of person-specific, estimated cognitive level minus the level of cognition that would be expected marginally, after controlling for age, sex, and neuropathologies. We defined this new measure as an extension and complement to two existing approaches – one () based on cognitive level at a single, cross-sectional timepoint proximate to death, and one () based on residual slopes of cognitive decline. In addition, the new measure further (i) incorporated a flexible fit to accommodate potential nonlinear cognitive trajectories, enabling the transformation of the new CR as needed, and (ii) accounted for measurement error in neuropsychological tests by focusing on estimated rather than observed cognition. When we examined several risk factors for CR, we generally found that, compared with the two existing cognitive resilience measures, the new measure yielded expected and generally stronger relations. Finally, we demonstrated that calculating using MMSE instead of our global composite score yielded similar results. Moreover, use of standard linear mixed-effects models for Gaussian outcomes instead of latent process mixed models, and consideration of sub-windows of observation (e.g., excluding terminal decline) produced similar results in these datasets as our more complex models incorporating all follow-up points. Thus, although the flexible modeling approaches we use could be of importance, in some settings simpler modeling approaches will be adequate (e.g., standard linear mixed model).
There are several advantages to adding our new to the arsenal of approaches for calculating CR. First, since some risk factors are associated with levels of cognition but not slopes (e.g., education is related to and but not ), then our longitudinal method of integrating cognitive levels into the calculation of CR provides a more powerful outcome than cognitive level proximate to death (as established by the much stronger association of education to than to CRLast_level). Additionally, with both and, we now have two complementary longitudinal approaches to calculating CR; future research on interventions may benefit by being able to consider risk factors which both help to maintain higher-than-expected levels of cognition over time (i.e., using the new CR measure) as well as flatter slopes of cognitive decline (i.e., using ), thereby very broadly promoting cognitive resilience and delayed onset of clinical dementia. Second, the design of our new allows calculation of resilience scores for distinct time-windows. For example, we were able to easily calculate over the entire observation period, as well as after excluding terminal decline (the last three years of observation prior to death). Further, it could be possible to examine CR in time periods before and after a specific event (e.g., a risk factor intervention), even if the time period of interest is fairly short (and therefore slopes of cognitive decline would be more challenging to estimate). Such future applications could help to better understand the determinants of cognitive resilience.
The new measure of CR has limitations that should be carefully considered. In particular, by focusing on the average of differences in cognitive levels over time, one possible limitation of our method is the classification of individuals in whom the difference between person-specific versus marginal cognitive levels may vary substantially in direction over time (i.e., sometimes above and sometimes below the marginal levels). Such variation over time could result in an estimate of CR that indicates the overall cognitive level is near the marginal level, despite large differences at specific timepoints. However, in our cohorts, it was rare to observe meaningful alterations from a positive to a negative difference (or vice-versa) within a given individual. Specifically, using a difference of ≥1 standard unit between the person-specific and the marginal level as our benchmark, less than 5% of participants had a large absolute difference from the marginal trajectory at one point accompanied by any level of opposing difference at another point. While we cannot predict exact findings in other cohorts, future studies using might also check for such fluctuations prior to conducting analyses.
Similarly, as we noted earlier, our new was designed in part to address a limitation of in that defines those with little change in cognitive function over time, yet overall poor cognition, as having good levels of resilience. Nonetheless, in our measure of , we have a parallel limitation in that those with steeper slopes of decline but consistently higher cognition are defined as having good levels of resilience. Ideally, in future research, methods for calculating CR might be developed which dually incorporate information on cognitive levels as well as the residual slope of cognitive decline.
Other limitations involve the structure of our model. The LPM model included demographics and nine common neuropathologies as a simple effect and in interaction with the function of time approximated by splines, resulting in a large number of parameters, that may lead to overfitting. However, in our data, the models converged.
Finally, a limitation across all three CR measures here is the use of postmortem neuropathology to estimate longitudinal antemortem cognitive resilience. Similarly, our nine neuropathologic indices used in calculating CR measures may not fully define brain pathology. In future research, we could adapt CR methodology to simultaneously incorporate in the model both AD biomarkers collected repeatedly during life (such as CSF proteins, or MRI and PET imaging) as well as postmortem neuropathologies, and we could test how further postmortem pathologic indices (e.g., postmortem imaging measures of brain structure) might influence our CR measure.
Data availability
The R code to replicate the analyses is provided in the supplementary material. All data in these analyses (and descriptions of the studies and variables) can be requested through the Rush Alzheimer’s Disease Center Research Resource Sharing Hub at www.radc.rush.edu.
Supplementary Material
RESEARCH IN CONTEXT.
SYSTEMATIC REVIEW:
Authors reviewed the existing literature via PubMed searches, as well as searches of conference presentations/abstracts. While research on cognitive resilience (CR) to Alzheimer’s disease pathology is relatively new, different residual approaches have been developed to continuously quantify this important concept. Existing methods are appropriately cited.
INTERPRETATION:
The proposed CR provides a single measure that reconciles the longitudinal differences between the level of cognition observed versus expected for a person with a similar profile of demographics and brain pathology. It offers a powerful way to quantify CR, and yielded consistently stronger associations across several key risk factors for CR than two existing measures focusing on cognitive level at a single point or on slopes of cognitive decline.
FUTURE DIRECTIONS:
The article proposes a novel measure of CR enabling future research on interventions directed at maintaining higher levels of cognition throughout old age, despite pathology.
ACKNOWLEDGMENTS
Dr. Wagner is supported by a post-doctoral fellowship from the French Foundation for Alzheimer’s Research (https://alzheimer-recherche.org/). We thank the Catholic nuns, priests, and brothers who participated in the Religious Orders Study and participants of the Rush Memory and Aging Project. We also thank key staff members; Traci Colvin, MPH, for coordination of the clinical data collection; Karen Skish, MS, for coordination of the pathologic data collection; and John Gibbons, MS, and Greg Klein, MS, for data management.
Funding
Study funded by NIH (R01AG17917, P30AG10161, R01AG15815, R01AG34374) and the Illinois Department of Public Health. In the past 36 months, and outside of the submitted work, SEL received financial support from NIH for travel and stipend to attend peer review meetings; DAB received consulting fees from the AbbVie DSMB Takeda adjudication committee Origent SBIR, financial support from academia or government for presentations or educational events, financial support from academia or government to attend meetings, financial support from AbbVie to participate in Advisory Board, and materials from the RADC; AWC received financial support to participate in Data Safety Monitoring Board at the Northwestern University of Chicago. The funding organizations had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the writing of the report or the decision to submit it for publication.
Footnotes
Conflicts of interest
The authors have no conflicts to declare.
REFERENCES
- 1.Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol. 1999;58(4):376–388. doi: 10.1097/00005072-199904000-00008 [DOI] [PubMed] [Google Scholar]
- 2.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55(3):370–376. doi: 10.1212/wnl.55.3.370 [DOI] [PubMed] [Google Scholar]
- 3.Iacono D, Markesbery WR, Gross M, et al. The Nun study: clinically silent AD, neuronal hypertrophy, and linguistic skills in early life. Neurology. 2009;73(9):665–673. doi: 10.1212/WNL.0b013e3181b01077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ewbank DC, Arnold SE. Cool with plaques and tangles. N Engl J Med. 2009;360(22):2357–2359. doi: 10.1056/NEJMe0901965 [DOI] [PubMed] [Google Scholar]
- 5.Price JL, McKeel DW, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30(7):1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corrada MM, Berlau DJ, Kawas CH. A population-based clinicopathological study in the oldest-old: the 90+ study. Curr Alzheimer Res. 2012;9(6):709–717. doi: 10.2174/156720512801322537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han SD, Nguyen CP, Stricker NH, Nation DA. Detectable Neuropsychological Differences in Early Preclinical Alzheimer’s Disease: A Meta-Analysis. Neuropsychol Rev. 2017;27(4):305–325. doi: 10.1007/s11065-017-9345-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arenaza-Urquijo EM, Vemuri P. Resistance vs resilience to Alzheimer disease: Clarifying terminology for preclinical studies. Neurology. 2018;90(15):695–703. doi: 10.1212/WNL.0000000000005303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2020;16(9):1305–1311. doi: 10.1016/j.jalz.2018.07.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Negash S, Wilson RS, Leurgans SE, et al. Resilient Brain Aging: Characterization of Discordance between Alzheimer’s Disease Pathology and Cognition. Curr Alzheimer Res. 2013;10(8):844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ossenkoppele R, Lyoo CH, Jester-Broms J, et al. Assessment of Demographic, Genetic, and Imaging Variables Associated With Brain Resilience and Cognitive Resilience to Pathological Tau in Patients With Alzheimer Disease. JAMA Neurol. 2020;77(5):632–642. doi: 10.1001/jamaneurol.2019.5154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bocancea DI, Loenhoud AC van, Groot C, Barkhof F, Flier WM van der, Ossenkoppele R. Measuring Resilience and Resistance in Aging and Alzheimer Disease Using Residual Methods: A Systematic Review and Meta-analysis. Neurology. Published online 2021. doi: 10.1212/WNL.0000000000012499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14(11):653–666. doi: 10.1038/s41582-018-0070-3 [DOI] [PubMed] [Google Scholar]
- 14.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu L, Tasaki S, Schneider JA, et al. Cortical Proteins Associated With Cognitive Resilience in Community-Dwelling Older Persons. JAMA Psychiatry. 2020;77(11):1172–1180. doi: 10.1001/jamapsychiatry.2020.1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham EK, James BD, Jackson KL, et al. Associations Between Personality Traits and Cognitive Resilience in Older Adults. The Journals of Gerontology: Series B. 2021;76(1):6–19. doi: 10.1093/geronb/gbaa135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2018;64(s1):S161–S189. doi: 10.3233/JAD-179939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 19.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66(2):200–208. doi: 10.1002/ana.21706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol. 2018;83(1):74–83. doi: 10.1002/ana.25123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61(3):378–384. doi: 10.1001/archneur.61.3.378 [DOI] [PubMed] [Google Scholar]
- 22.Nag S, Yu L, Capuano AW, et al. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol. 2015;77(6):942–952. doi: 10.1002/ana.24388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017;134(2):171–186. doi: 10.1007/s00401-017-1717-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumann M, Kwong LK, Lee EB, et al. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol. 2009;117(2):137–149. doi: 10.1007/s00401-008-0477-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson RS, Yu L, Trojanowski JQ, et al. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol. 2013;70(11):1418–1424. doi: 10.1001/jamaneurol.2013.3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24 [DOI] [PubMed] [Google Scholar]
- 27.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42(3):722–727. doi: 10.1161/STROKEAHA.110.595082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol. 2016;15(9):934–943. doi: 10.1016/S1474-4422(16)30029–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyle PA, Yu L, Nag S, et al. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology. 2015;85(22):1930–1936. doi: 10.1212/WNL.0000000000002175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson RS, Yu L, Lamar M, Schneider JA, Boyle PA, Bennett DA. Education and cognitive reserve in old age. Neurology. 2019;92(10):e1041–e1050. doi: 10.1212/WNL.0000000000007036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casaletto KB, Rentería MA, Pa J, et al. Late-Life Physical and Cognitive Activities Independently Contribute to Brain and Cognitive Resilience. J Alzheimers Dis. 2020;74(1):363–376. doi: 10.3233/JAD-191114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005;11(4):400–407. [PubMed] [Google Scholar]
- 33.Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb [DOI] [PubMed] [Google Scholar]
- 34.Capuano AW, Wilson RS, Leurgans SE, et al. Neuroticism, Negative Life Events and Dementia in Older White and Black Brazilians. Int J Geriatr Psychiatry. Published online 2020. doi: 10.1002/gps.5491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LS R The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 36.Buchman AS, Boyle PA, Wilson RS, Beck TL, Kelly JF, Bennett DA. Apolipoprotein E e4 allele is associated with more rapid motor decline in older persons. Alzheimer Dis Assoc Disord. 2009;23(1):63–69. doi: 10.1097/wad.0b013e31818877b5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eilers PHC, Marx BD, Durbán M. Twenty years of P-splines. 1. Published online 2015:149–186. [Google Scholar]
- 38.Proust-Lima C, Philipps V, Dartigues JF, et al. Are latent variable models preferable to composite score approaches when assessing risk factors of change? Evaluation of type-I error and statistical power in longitudinal cognitive studies. Stat Methods Med Res. 2019;28(7):1942–1957. doi: 10.1177/0962280217739658 [DOI] [PubMed] [Google Scholar]
- 39.Proust-Lima C, Amieva H, Jacqmin-Gadda H. Analysis of multivariate mixed longitudinal data: a flexible latent process approach. Br J Math Stat Psychol. 2013;66(3):470–487. doi: 10.1111/bmsp.12000 [DOI] [PubMed] [Google Scholar]
- 40.Proust-Lima C, Amieva H, Jacqmin-Gadda H. Analysis of multivariate mixed longitudinal data: a flexible latent process approach. Br J Math Stat Psychol. 2013;66(3):470–487. doi: 10.1111/bmsp.12000 [DOI] [PubMed] [Google Scholar]
- 41.Wilson RS, Wang T, Yu L, Bennett DA, Boyle PA. Normative Cognitive Decline in Old Age. Ann Neurol. 2020;87(6):816–829. doi: 10.1002/ana.25711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sliwinski MJ, Stawski RS, Hall CB, Katz M, Verghese J, Lipton R. Distinguishing preterminal and terminal cognitive decline. European Psychologist. 2006;11(3):172–181. doi: 10.1027/1016-9040.11.3.172 [DOI] [Google Scholar]
- 43.Wilson RS, Yu L, Leurgans SE, Bennett DA, Boyle PA. Proportion of cognitive loss attributable to terminal decline. Neurology. 2020;94(1):e42–e50. doi: 10.1212/WNL.0000000000008671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Proust-Lima C, Philipps V, Liquet B. Estimation of Extended Mixed Models Using Latent Classes and Latent Processes: The R Package lcmm. Journal of Statistical Software. 2017;78(1):1–56. doi: 10.18637/jss.v078.i02 [DOI] [Google Scholar]
- 45.Philipps V, Amieva H, Andrieu S, et al. Normalized Mini-Mental State Examination for assessing cognitive change in population-based brain aging studies. Neuroepidemiology. 2014;43(1):15–25. doi: 10.1159/000365637 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The R code to replicate the analyses is provided in the supplementary material. All data in these analyses (and descriptions of the studies and variables) can be requested through the Rush Alzheimer’s Disease Center Research Resource Sharing Hub at www.radc.rush.edu.